DOI:10.32604/biocell.2022.018442

| BIOCELL DOI:10.32604/biocell.2022.018442 |  |
| Article |
Characterization of three-dimensional multipotent adipose-derived stem cell spheroids
1Ophthalmology Department, First Affiliated Hospital of Jinan University, Guangzhou, 510632, China
2Ophthalmology Department, Guangdong Second Provincial General Hospital, Guangzhou, 510310, China
3Institute of Ophthalmology, Medical College, Jinan University, Guangzhou, 510632, China
4Key Laboratory for Regenerative Medicine of Ministry of Education, Jinan University, Guangzhou, 510632, China
*Address correspondence to: Yonglong Guo, guoyonglong@163.com; Jiansu Chen, chenjiansu2000@163.com
Received: 25 July 2021; Accepted: 29 October 2021
Abstract: Human adipose stem cells (hADSCs) are reliable sources for cell therapy. However, the clinical applications are limited by the decrease in activity during in vitro culture. We used a knockout serum replacement (KSR) medium, Eppendorf (EP) tube culture, and a simulated microgravity (SMG) culture system to establish hADSC spheroids. We found that hADSCs aggregated and formed spheroids in the KSR culture medium. The EP tube culture method revealed many biological cell characteristics, such as good cell viabilities, rough surfaces, polar growth, fusion phenomenon, and injectability. The findings show its advantages for hADSCs spherical cultures. When cultured in SMG, hADSC spheroids produced large-scale spheroids. Additionally, confocal examination and viability assays revealed that SMG-cultured hADSC spheroids had higher cell viabilities and looser spherical structures, relative to those cultured in EP tubes. hADSC spheroids in static EP tube culture had tighter structures and more dead cells with rough and irregular surfaces, while hADSC spheroids in dynamic SMG condition exhibited looser structures and better cell viabilities with flat and regular surfaces. Therefore, the KSR media promotes spherical formation by hADSCs, which showed polar growth, fusion, and injectability in vitro. The dynamic SMG culture enhances the formation of a looser structure and better cell viabilities for hADSC spheroids.
Keywords: hADSCs; EP tube; Spheroids; Microgravity
Human adipose stem cells (hADSCs) are multipotent adult stem cells with the ability to differentiate into various cell types, including bone, cartilage, neurons, myocardium, and smooth muscle endothelia (Gimble and Guilak, 2003; Mizuno, 2010). Due to their advantages, including multi-lineage differentiation, wide range of sources, ease of collection with limited damage, rapid proliferation, and low immunogenicity, hADSCs have numerous potential applications. However, hADSCs are a small proportion of adipose tissue, and low cell viability and poor engraftment after transplanted into ischemic region, it is a major limitation for hADSCs cell therapy (Lee et al., 2018). Thus, there is an urgent need to develop new in vitro culture methods to enhance cell viabilities and maintain hADSC stemness as they must be extensively expanded in vitro to obtain enough numbers for clinical applications (Fraser et al., 2006).
Most human cells interconnect with neighboring cells and the extracellular matrix (ECM), forming three-dimensional (3D) structures as well as complex networks, which is crucial for cells to execute their functions. 3D culture systems can mimic normal tissue architectures and direct the differentiation as well as functional assemblies of stem cells (Vunjak-Novakovic and Scadden, 2011). 3D spheroid cultures enhance cell viabilities, functional performance, and stemness (Hurrell et al., 2018; Szebeni et al., 2017). We previously found that hADSC spheroids produced in silicone microwells exhibit better viabilities and neural differentiation potentials (Guo et al., 2015a). Relative to conventional 2D cultures, 3D cultures of bovine corneal endothelial cell (B-CEC) spheroids exhibited higher stemness potentials (Guo et al., 2015b). We also found that rabbit corneal stromal cells (CSCs) on decellularized bovine cornea scaffolds under simulate microgravity (SMG) rotary cell culture systems (RCCS) exhibited spherical aggregate growth, while in 2D static culture, they formed monolayers (Li et al., 2013). SMG markedly enhanced rabbit keratocyte proliferation and facilitated growth into or on dehydrated bovine acellular corneal stroma (Chen et al., 2007; Dai et al., 2012). Thus, 3D spheroid cultures may be efficient at maintaining the viability and stemness of hADSCs.
Cells can be cultured into spheroids using various methods, including the use of low adhesion cultures, methylcellulose, Eppendorf (EP) tube cultures, hanging-drop, and dynamic culture methods (Lin and Chang, 2008; Laschke and Menger, 2017; Petrenko et al., 2017). Even though these methods are convenient at generating homogeneous spheroids, they are not amenable to large-scale spheroid production. SMG involves spheroidal microtissues, and is amenable to large-scale, high-density cell cultures. Moreover, it exhibits lower shear stress (Frith et al., 2010; Saleh et al., 2012). Studies have reported that SMG is useful in large-scale culture of stem cells (Li et al., 2009; Fridley et al., 2010) and may increase the viability of hADSCs (Zhang et al., 2013). In this study, we used the KSR medium, EP tube, as well as the SMG culture system to establish spheroids and evaluated their multipotent differentiation capacities, proliferative abilities, colony-forming efficiencies, spheroid viabilities, fusion abilities, as well as spheroid injectability. Moreover, we characterized hADSC spheroids grown in the EP tube and SMG in knockout serum replacement (KSR) medium and evaluated their viabilities as well as morphologies. 3D hADSC spheroid cultures will benefit stem cell growth, tissue engineering, and 3D bio-printing.
Culture reagents were purchased from Gibco (Grand Island, NY, USA). ADSCs were cultured in a conventional medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 100-U/mL penicillin G sodium, 100-mg/mL streptomycin sulfate, and 10% vol/vol FBS. Unless otherwise stated, all other reagents were obtained from Sigma (St. Louis, MO, USA). KnockOut™ Serum Replacement (KSR), Vybrant™ CM-DiI/DiO Cell-Labeling kit and Collagenase type I were acquired from Life technologies (San Francisco, USA). Cell Counting Kit-8 (CCK-8) was procured from Dojindo (Kyushu, Japan). Cell Cycle and Apoptosis Analysis Kit as well as Annexin VFITC/ PI apoptosis detection kit were bought from KeyGEN (Nanjing, China). Viability/Cytotoxicity Assay Kits were obtained from BIOTIUM (San Francisco, USA). The Cell-Light™ EdU Cell Proliferation Detection kit was purchased from Ribobio (Guangzhou, China). CD29, CD44, CD59, CD34, CD45, and HLA-DR antibodies were obtained from Abcam (Cambridge, UK).
This assay was performed as previously reported (Dai et al., 2014). Human adipose tissues were obtained via liposuction from the abdomens and thighs of 3 healthy female donors (mean age: 32), who had consented to the study. Ethical approval for this study was obtained from the institutional ethical review board of the First Affiliated Hospital of Jinan University, China. We adhered to the Declaration of Helsinki guidelines. Adipose tissues were repeatedly washed using PBS until the complete removal of blood, then, they were incubated with equal volumes of DMEM containing 0.1% type I collagenase in a shaking incubator at 110 rpm, 37°C for 1 h. The suspension was filtered through a 100-mesh sieve and centrifuged (300×g, 10 min). Then, re-suspension cells were rinsed in a culture medium composed of DMEM, centrifuged (300×g, 5 min), and suspended at a concentration of 104 cells/mL in a conventional medium supplemented with 100-U/mL penicillin G sodium, 100-mg/mL streptomycin sulfate, and 10% (vol/vol) FBS. Cells were seeded into a 25 cm2 plastic culture flask, supplemented with 4 mL medium, and incubated at 37°C in a 5% CO2 incubator. The culture medium was changed every second day (All cells used in this study were with three passage).
Analysis of hADSCs gene expressions by flow cytometry
Surface markers for cell type were quantified by flow cytometry using CD29, CD44, CD59, CD34, CD45, and HLA-DR antibodies. Then, hADSCs (Passage 0) were collected and counted, 1 × 106 cells/tube were washed twice using PBS, resuspended in 100 μL antibodies (diluted with 1:100 PBS), and incubated for 30 min at 4°C. Then the cells were then washed twice, incubated with FITC labeled secondary antibody (diluted with 1:100 PBS) for 20 min at dark, 4°C. After incubation, PBS washed 2–3 times. resuspended in 200 μL of PBS. Fluorescence analyzed by flow cytometry.
Multipotency differentiation of hADSCs
This assay was performed as we previously reported (Dai et al., 2014). Adipogenic differentiation: Monolayer of hADSCs (Passage 3) were cultured in an adipogenic induction medium (DMEM/F12, 10% FBS, 1 μM insulin, 200 μM indometacin, 1 μM dexamethasone, 0.5 mM isobutyl-methylxanthine and 1% antibiotic-antimycotic. After two weeks induction, adipogenic differentiation was verified by staining lipids with Oil red O. Osteogenic differentiation: Monolayer of hADSCs (Passage 3) were cultured in osteogenic induction medium (DMEM/F12, 10% FBS, 0.1 μM dexamethasone, 50 mg/L 2-phosphate ascorbic acid, 10 mM β-glycerophosphate, and 1% antibiotic-antimycotic. After two weeks induction, osteogenic differentiation was verified by staining calcium-rich deposits with Alizarin red.
hADSCs proliferation and apoptosis assay
These procedures were performed as in our previous reports, with some modifications (Dai et al., 2014). The CCK8 assay was used to assess hADSCs proliferation. Then 2.5 × 103, 5.0 × 103, 7.5 × 103, and 1 × 104 hADSCs (Passage 2) dissociated with 0.25% trypsin- ethylenediaminetetraacetate (EDTA) solution (Gibco) and then seeded in a 96-well plate and incubated at 37°C and 5% CO2 for 24 h, Then medium were removed, after which the experimental group (presented in Fig. 1) was cultured in 100 μL KSR medium (DMEM/F12, 1% non-essential Amino Acids (NEAA), 1% L-Glutamine, 4 ng/mL basic fibroblast growth factor (bFGF), 0.1 mM β-mercaptoethanol, 20% KSR, 1% penicillin/streptomycin (P/S), while the control group was cultured in 100 μL conventional complete medium (DMEM/F12, 10% FBS, 1% P/S). After 72 h of incubation, 10 μL of the CCK8 reagent was added into each well, after which cells were incubated at 37°C and 5% CO2 for 2 h. Then, absorbance was read at 450 nm using a microplate reader. To evaluate apoptosis, hADSCs (Passage 3) were seeded in a 6-well plate and cultured to 90% confluence. Cells in the experimental group were cultured in KSR medium, while the control group was cultured in conventional complete medium for 3 days. hADSCs dissociated with 0.25% trypsin-EDTA) solution (Gibco), washed with PBS and then incubated with Annexin V- FITC/PI (diluted with 1:100), for 20 min at dark in 4°C. After incubation, PBS washed 2–3 times. After which their apoptosis was evaluated by flow cytometry. Data were analyzed using the WinMDI software.
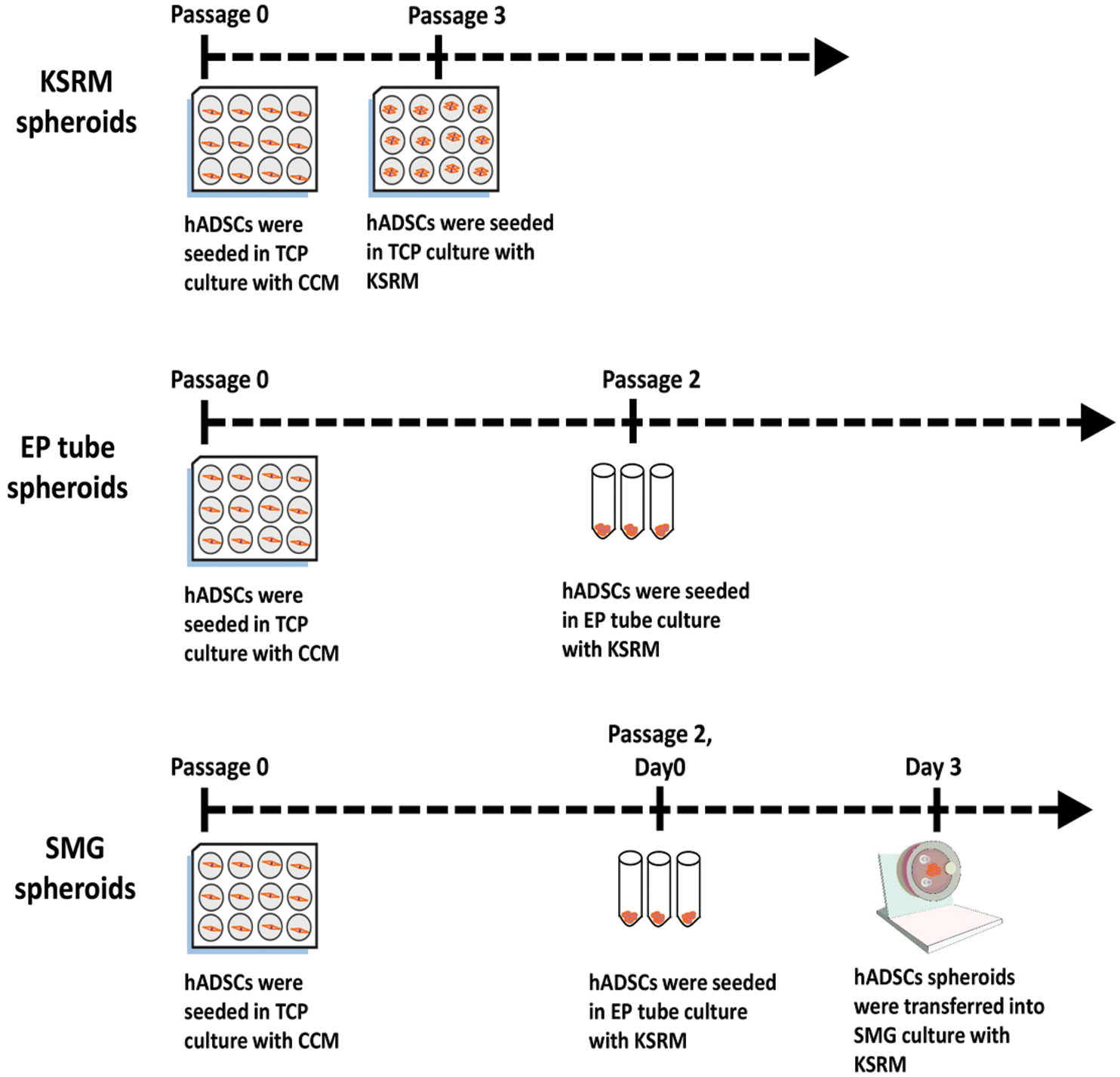
Figure 1: Flow diagram. A sketch of the process of spherical formation for KSRM spheroids, EP tube spheroids, SMG spheroids. CCM: conventional complement medium; KSRM: KSR medium; TCP: tissue culture plate.
Paraffin sections and H & E staining
hADSCs (Passage 2) were obtained and counted. Approximately, 2 × 104 hADSCs were seeded in EP tubes with KSR medium. Then, tubes were vertically placed in an incubator with 5% CO2 at 37°C for 48 h. In order for the dissociated hADSCs to form spheroids. The hADSC spheroids in EP tubes were fixed in alcohol/formaldehyde (AF, 40% formalin and 95% ethanol, 1:10), overnight, dehydrated in a gradient ethanol series and embedded in paraffin: The hADSC spheroids were soaked with PBS for 3 min three times treated with 70% ethanol overnight; then treated with 80% ethanol 1 h; treated with 90% ethanol 1 h; treated with 100% ethanol 1 h three times; and then treated with xylene 15 min twice, each time. The spheroids were soaked in melted paraffin and placed in an oven at 60°C for 1 h, then replaced with new paraffin and placed in an oven at 60°C for 1 h. The spheroids were removed from the oven, quickly placed in a stainless-steel embedding box, covered with plastic embedding lid, poured in melted paraffin wax, and cooled to room temperature. After that, the spheroids were cut into 5-μm thick tissue slices with a paraffin slicer, and the slices were pasted on the prepared slides coated with poly-lysine, labeled, and stored in the slicing box for later use.
H & E staining: The hADSC spheroids sections were dewaxed with xylene for 2 min three times, they were treated with 100% ethanol 2 min three times; then treated with 90% ethanol 2 min; and treated with 80% ethanol 2 min; soaked in distilled water for 2 min, then stained with hematoxylin for 2 min. Then they were washed with water and stained with eosin for 10 min. Then they were washed with water. The hADSC spheroids were treated with 80% ethanol for 30 s, 90% ethanol 30 s, and 100% ethanol for 30 s, three times. After dehydrated in a gradient ethanol series, Slices were treated with xylene for 30 s three times. Finally, the sheet was sealed with neutral gum, which can be preserved permanently, and then examined and imaged on a microscopic.
The experiment procedure is carried out according to the procedure of Viability/Cytotoxicity Assay Kit for Animal Live & Dead Cells. hADSCs (Passage 2) were obtained, counted after which, 2 × 104 hADSCs were seeded in EP tubes with KSR medium (presented in Fig. 1). The EP tube were vertically placed in an incubator 5% CO2 at 37°C for 48 h for cell spheroid formation. The hADSC spheroids were harvested, rinsed 2–3 times using PBS, resuspended in 2 mL PBS supplemented with 4 µL EthD-III (Ethidium Homodimer III 4.5 μM, BIOTIUM) and 1 µL of Calcein AM (Calcein acetoxymethyl ester 2 μM, BIOTIUM). Then, the mixture was incubated for 30–45 min in the dark. After discarding the staining solution, cells were rinsed 2–3 times using PBS. Nuclei were counterstained using DAPI after which spheroids were imaged by fluorescence microscope. Live cells were stained green, while dead cells were stained red.
5-Ethynyl-2'-deoxyuridine (EdU) cell proliferation stain
This procedure was performed as in our previous reports, with some modifications (Guo et al., 2020). Monolayer hADSCs and spheroids were plated in triplicates in 48-well plates, then based on the manual of the EdU labeling/detection kit, 50 μM EdU labeling medium was added to the cell culture after which incubation was performed for 24 h at 37°C in a 5% CO2 atmosphere. Cultured hADSC spheroids were fixed in 4% paraformaldehyde (pH 7.4) for 30 min and incubated with glycine for 5 min. Then, they were washed using PBS and stained with anti-EdU at room temperature for 30 min. Cells were washed using 0.5% Triton X-100 in PBS, and there after incubated with 5 μg/mL Hoechst 33342 dye at room temperature for 30 min. Samples were visualized and imaged under a fluorescence microscope.
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was performed to observe surface ultrastructural morphologies of hADSC spheroids. Samples were fixed in 2.5% glutaraldehyde, washed thrice for 30 min, each time in 0.1 M PBS, and postfixed in 1% osmium tetroxide for 30 min. Again, they were washed thrice using PBS after which they were passed through a graded series of alcohol (50%, 70%, 80%, 90%, and 100%). After three 5-min changes of 100% ethanol, samples were transferred to isoamyl acetate for 30 min, critical point drying, conductive coating, and mounted for viewing using ULTRA 55 SEM (Zeiss, Germany).
Vybrant™ CM-DiI/DiO cell-labeling assay
Monolayer hADSCs (Passage 2) were cultured in adhesive six-well plates, incubated to about 80% confluence and gently washed using PBS, 2–3 times before addition of the dye solution (1 mL serum-free medium + 5 μL DiI/DiO application liquid) to cover hADSCs. Then, they were incubated at 37°C, in a 5% CO2 atmosphere for 5–10 min, followed by incubation at 4°C in the dark for 15 min. They were washed thrice (5 min each) using PBS. The DiI/DiO labelled hADSCs (Passage 2) were obtained, counted, after which 2 × 104 cells were cultured in EP tubes with KSR medium (DMEM/F12, 1% NEAA, 1% L-Glutamine, 4 ng/mL bFGF, 0.1 mM β-mercaptoethanol, 20% KSR, and 1% P/S). Then EP tubes were vertically placed in an incubator at 37°C and 5% CO2 for 2 days. Next, DiI-labeled spheroids and DiO labeled spheroids were transferred to low-adhesion 24-wells (CORNING) with pipette for further incubation. They were observed and imaged by fluorescence microscopy at 2, 4, 6, 8, and 10 days.
Injectability of hADSC spheroids
Monolayer hADSCs (Passage 2) were obtained and counted, after which cells of different densities were transferred into EP tubes with KSR medium and incubated at 37°C, 5% CO2 for 2 days. Then hADSC spheroids were transferred to low-adhesion wells for further incubation for 6 days. Next, using pipettes, spheroids were injected into six-well plates, through the tip (4844, Corning Incorporated Life Sciences, USA; inner diameter ¼ 710 mm) for a 200 mL pipette, cultured with conventional complete medium and incubated. At different time point, they were observed and imaged by microscopy.
Cultured of hADSCs spheroids in simulated microgravity culture systems
To assess the growth of hADSC spheroids in simulated microgravity (SMG) cultures, hADSC spheroids at Passage 2 were cultured in EP tubes for 2 days, collected, resuspended in KSR medium (1 spheroid/mL) and seeded in a 25-mL microgravity reactor (presented in Fig. 1). The reactor was incubated for 5 days at 37°C, 15 r/min and 5% CO2. After 5 days cultured, hADSC spheroids suspensions were taken out for examined and imaged by microscopy.
All experiments were performed with at least 3–6 (N = 3–6) different times from each donor (there were 3 donors). All data are presented as the average standard deviations from 3–6 independent experiments. Statistical analyses were conducted using a two-sided unpaired Student’s t-test to compare differences between two groups. P < 0.05 was considered statistically significant.
Characterization and differentiation of human ADSCs
When cultured in conventional medium, hADSCs from fresh lipoaspirates exhibited typical fibroblast morphologies (presented in Figs. 2A–2C). Flow cytometry analysis of surface phenotypes of human ADSCs revealed that primary hADSCs were positive for CD29, CD44, and CD59, but not CD34, CD45, or HLA-DR (presented in Fig. 2H). After 2 weeks of culture in osteogenic and adipogenic induction media, cells were stained with Alizarin red and Oil red O (presented in Figs. 2D–2G).
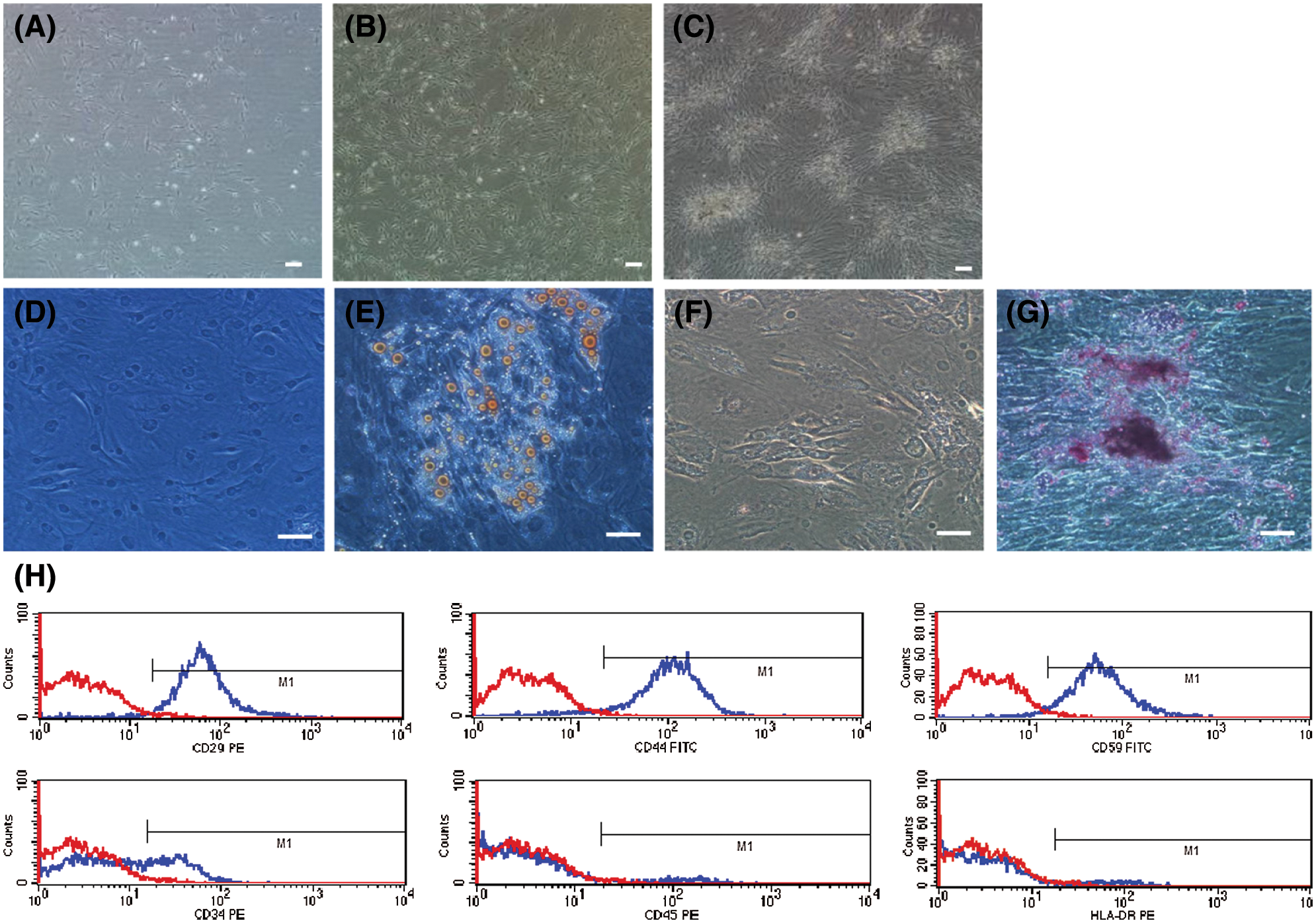
Figure 2: Characterization and differentiation of human ADSCs. hADSCs (Passage 0) were cultured on days 3 (A), 7 (B), 12 (C). (D–G) adipogenesis and osteogenesis induction of hADSCs: cells stained positive for Oil red O (E) and Alizarin red (G), D and F are control groups. (H) Flow cytometry analysis for surface phenotypes of hADSCs. (A–B) Scale bar: 100 µm, (D–G) Scale bar: 200 µm.
KSR medium was conducive for hADSC spheroids formation
When cultured in conventional media for a week, hADSCs exhibited fibroblast morphologies (presented in Fig. 3A). Gradually, in KSR culture media, hADSCs aggregated into multicellular spheroids within 7 days (presented in Figs. 3B–3E). The CCK-8 assay showed that the proliferation rate for hADSCs were significantly reduced in KSR media relative to controls (P = 0.001, presented in Fig. 3F). Annexin V and PI staining revealed markedly reduced apoptosis rates in hADSCs cultured in KSR media (P = 0.01, presented in Figs. 3G–3I). These findings imply that KSR medium promotes the formation of hADSC spheroids, and significantly down-regulates the proliferation and apoptosis rates of hADSCs.
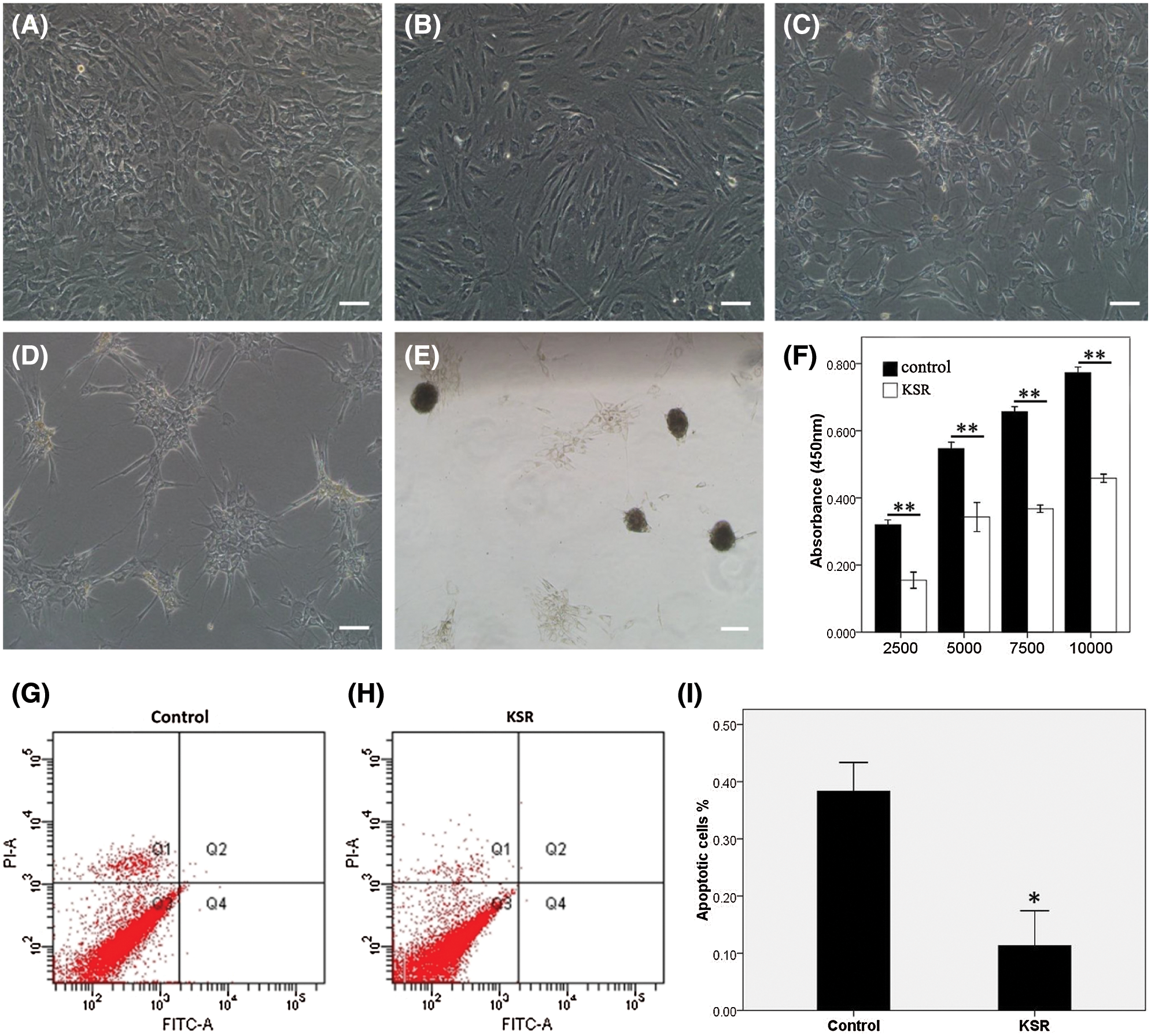
Figure 3: Spheroid formation of hADSCs cultured with KSR medium. The morphology of hADSCs cultured with KSR medium on days 1 (B), 3 (C), 7 (D), 12 (E), or without KSR medium on day 7 (A). (F) CCK-8 analysis of the proliferation in ADSCs cultured with or without KSR medium. (G–I) Apoptosis were significantly reduced in hADSCs cultured with KSR medium relative to controls. (A–E) Scale bar: 200 µm. (*P < 0.05 and **P < 0.01 KSR vs. Control, unpaired Student’s t test, n = 4).
EP tube and KSR medium promoted hADSC spheroids formation, spheroid fusion and polar growth characteristics
The hADSCs cultured in EP tubes developed floating aggregates (presented in Fig. 4A). H&E analysis showed that hADSCs were relatively round and evenly distributed in spheroids (presented in Fig. 4B). Cell viability analysis showed that spheroid viable while their surfaces were stained by Calcein AM (green) (presented in Fig. 4C). EdU staining revealed that relative to controls, spheroid proliferating cells were mainly concentrated on one side (presented in Figs. 4D and 4E). Scanning electron microscopy showed that spheroids were convex (presented in Figs. 4I–4K) while monolayer hADSCs exhibited a flat morphology (presented in Figs. 4F–4H). The hADSC spheroids were stained with DiI (red) or Dio (green). When two spheroids came in to contact, they underwent self-assembly, eventually fusing into single spheroidal microtissues (presented in Fig. 4L). hADSC spheroids were obtained and seeded to standard adherent culture plates containing a conventional complete medium. More adherent cells distribute on one side, when the polar growth characteristics observed at 48 h adherent culture (presented in Fig. 4M). These findings show that EP tube and KSR medium promote hADSC spheroids formation, which were characterized by polar growth and fusion.
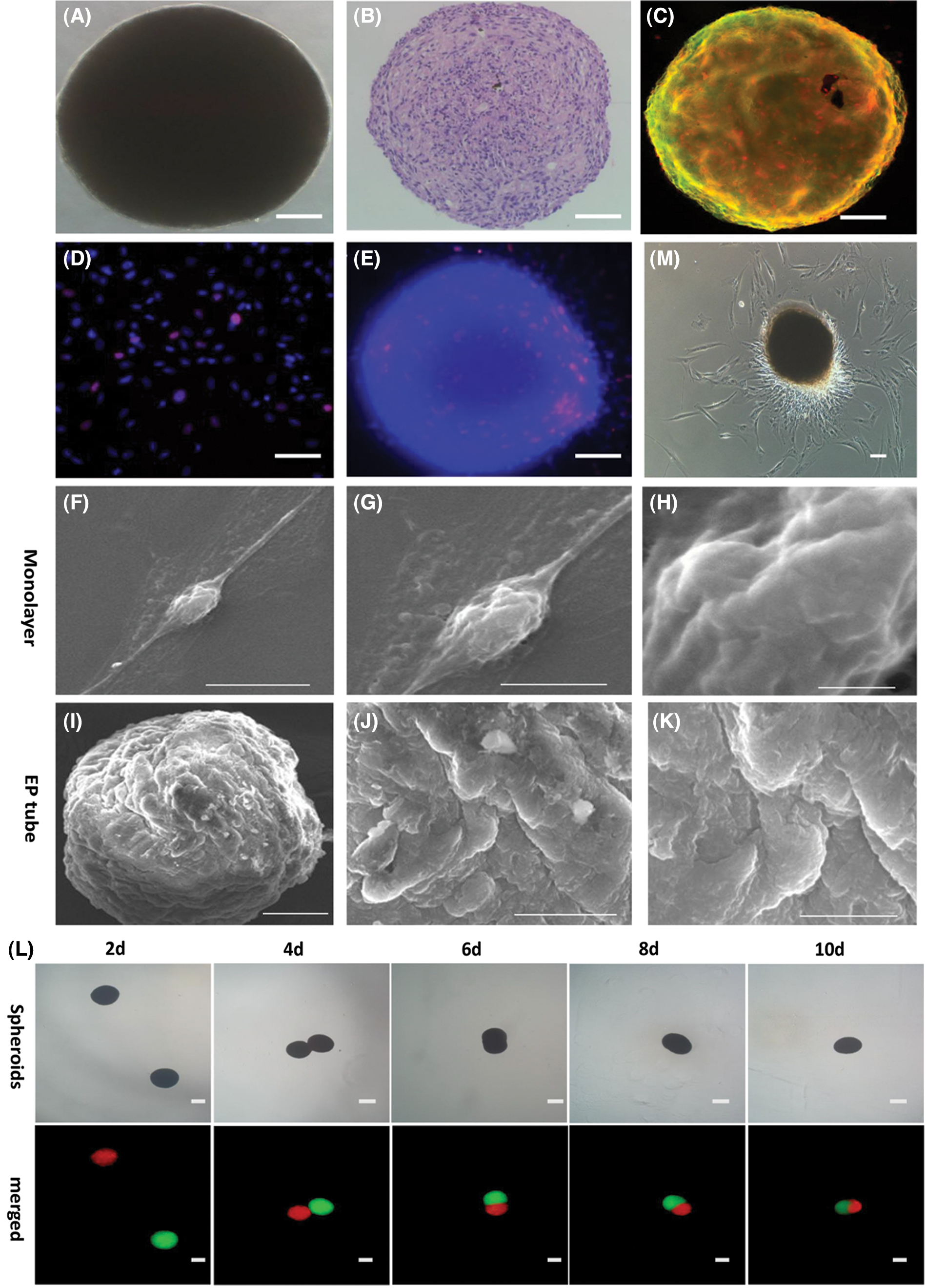
Figure 4: Characterization of viability and fusion of hADSCs spheroids formed in EP tubes. Representative bright image (A), H&E staining (B) and Calcein AM (green)/EthD-III (red) cell staining (C) for hADSCs spheroids formed in EP tubes. EdU staining in hADSCs spheroids formed in EP tube (E) and single cells (D). Scanning electron microscopy of hADSCs spheroids formed in EP tube (I–K) and single cells (F–H). (L) Representative images of two spheroids colliding and sticking together, and then fusing was marked by DiI (red) and DiO (green) staining, respectively. (M) Polar growth characteristics were observed when hADSCs spheroids were adhered onto standard adherent culture plates for 48 h. (A–E) Scale bar: 50 µm, (L–M) Scale bar: 100 µm, (F/K) Scale bar: 10 µm, (G) Scale bar: 5 µm, (H) Scale bar: 1 µm, (I) Scale bar: 50 µm, (J) Scale bar: 20 µm.
Injectability of hADSC spheroids in vitro simulation experiments
The hADSCs aggregation experiment in EP tubes was done at densities of 1 × 104, 2 × 104, 3 × 104, 4 × 104, 5 × 104, and 6 × 104 cells per tube. After 1 day in culture, cells at all densities quickly aggregated into single floating aggregates, with spheroid sizes increasing with cell densities. Spheroids exhibited a shrinking appearance over time, which was attributed to tissue compaction (presented in Fig. 5) (Lin et al., 2006). To determine if hADSC spheroids remained intact and viable after in vivo transplantation, we performed in vitro simulation experiments. Briefly, using a pipette, hADSC spheroids were obtained and injected into standard adherent culture plates containing conventional complete medium. After passing through the injector, spheroids remained aggregated. Moreover, at higher densities, spheroids were tightly packed, and they slowly passed through the pipettor. These findings show that at higher seeding densities, hADSCs assemble into bigger spheroids and that when injected onto culture plates, spheroids maintain their structural integrity and cellular viability.
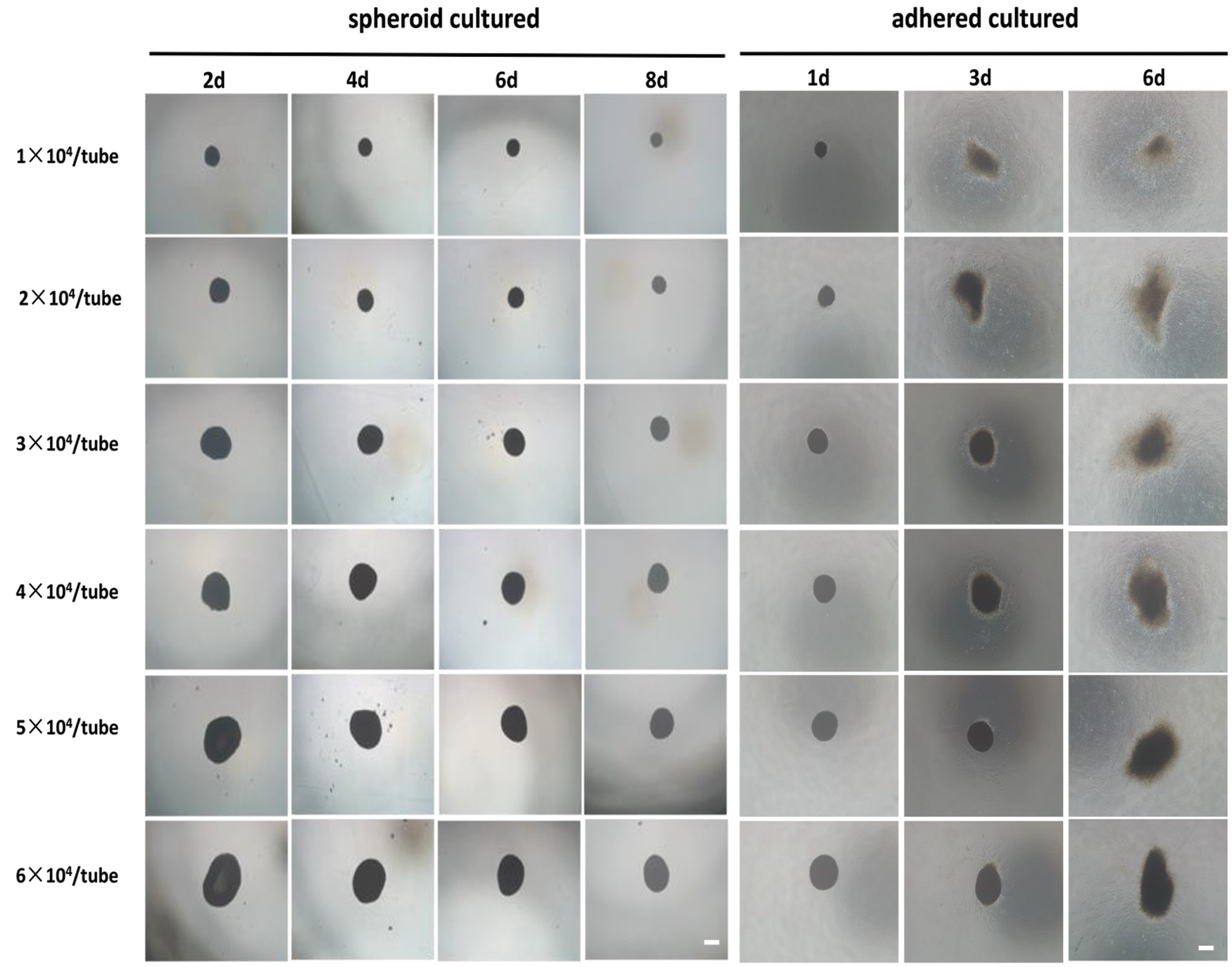
Figure 5: Injectability of hADSCs spheroids. hADSCs aggregation analysis in EP tubes was performed at densities of 1 × 104, 2 × 104, 3 × 104, 4 × 104, 5 × 104, and 6 × 104 cells per EP tube, respectively, and hADSCs spheroids collected and adhered onto culture plates. After passing through the injector, the spheroids retained their spheroidal morphology and viability. Scale bar: 100 µm.
Simulated microgravity culture system maintained the viability of hADSCs
Analysis of hADSC spheroids cultured in SMG vs EP tube culture systems revealed that those cultured in EP tube culture systems were fused together (presented in Fig. 6A), however, fusion was significantly less than for those in the SMG culture system (presented in Fig. 6D). Moreover, spheroids contained more live green cells compared to EP tube culture system, which the arrangement of cells was looser in the SMG culture system as examined by confocal microscopy (presented in Figs. 6C and 6F). Scanning electron microscopy revealed that SMG spheroids had smoother surfaces and more regular arrangement relative to EP spheroids (presented in Figs. 6G–6L). These data indicate that relative to the EP tube culture system, SMG exhibited better maintenance effects on hADSCs. The possible relevance of the differences in cell density and surface smoothness between the two methods, maybe double effect of rotational tangent force and gravity for SMG culture, while only gravity for EP culture.
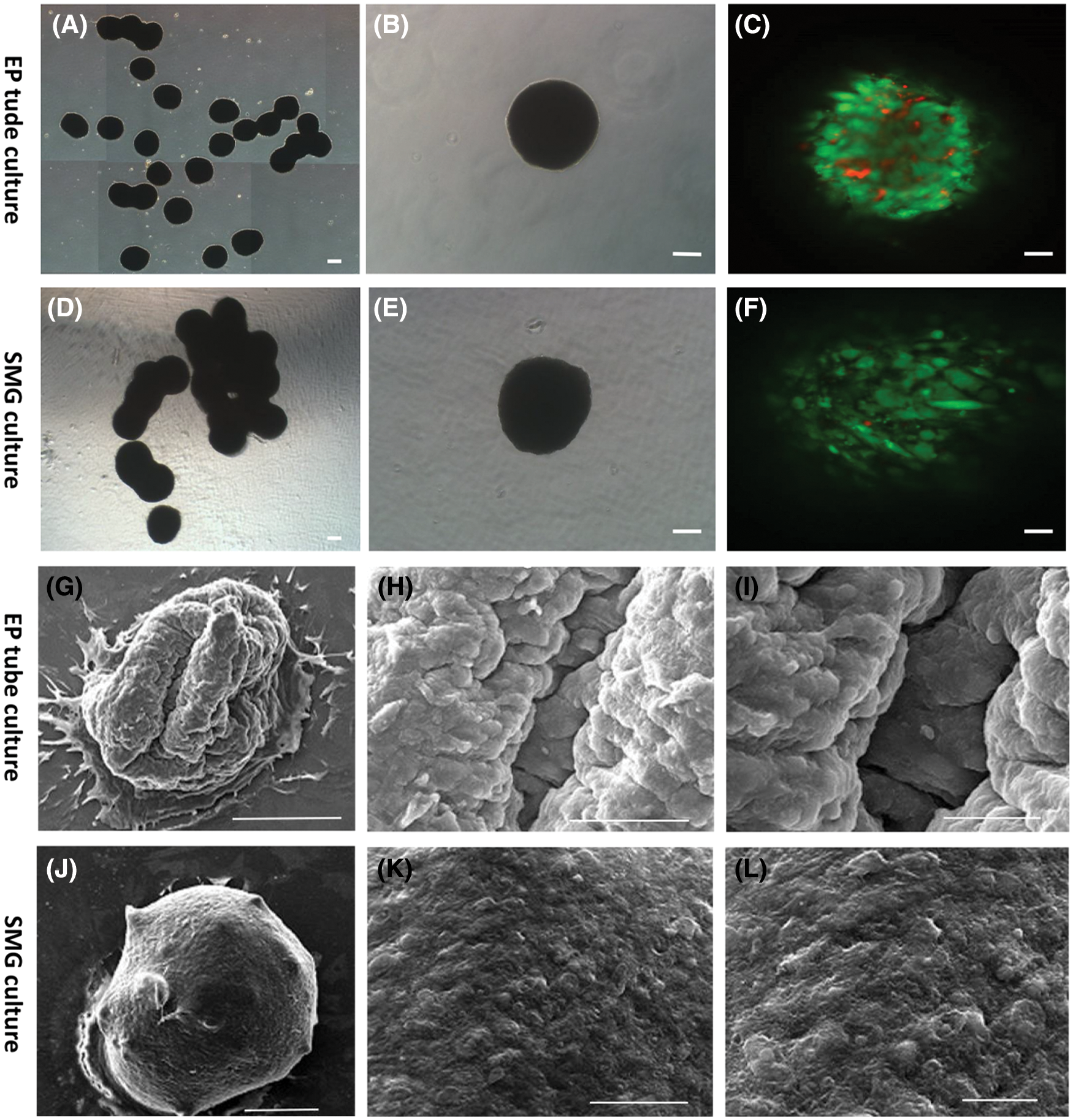
Figure 6: Characterization of viability in hADSCs spheroid formed by the simulated microgravity culture system. Representative bright image of hADSCs spheroid formed in EP tube (A and B) and SMG (D and E). Live/dead cells staining of hADSC spheroid formed in EP tube (C) and SMG (F). Scanning electron microscopy of hADSCs spheroid formed by EP tube (G–I) and SMG (J–L). (B–C) and (E–F) Scale bar: 100 µm, (A) and (D) Scale bar: 200 µm, (G/J) Scale bar: 200 µm, (H/K) Scale bar: 50 µm, (I/L) Scale bar: 20 µm.
We used the KSR medium, EP tube culture, and SMG culture systems to develop hADSC spheroids and found that KSR medium was conducive for hADSC spheroids formation. We characterized hADSC spheroids in EP tubes and developed a scalable EP tube spheroids culture strategy. In vitro simulation experiments showed that hADSC spheroids were injectable and viable. Moreover, the viability of hADSCs was improved by the SMG spheroid culture system, therefore, SMG is suitable for large-scale preparation of hADSC spheroids.
Stem cells have self-renewal, and proliferation potentials. hADSCs, adult multipotent stem cells from adipose tissues, can grow as clones and they exhibit multidirectional differentiation potentials (Gimble and Guilak, 2003). hADSCs are widely studied in heterologous tissue engineering (Boquest et al., 2006). After forming spheroids, cells exhibit a structure similar to real tissues, which is conducive for studying normal physiological and biochemical characteristics of cells, as well as tissue functions (Page et al., 2013). Therefore, 3D spherical cultures of hADSCs matche the actual state in vivo than monolayer cultures (Zhang et al., 2015). We compared the biological characteristics of hADSCs grown in KSR medium, EP tube culture, and SMG culture systems, in 3D (spherical) vs. 2D (conventional monolayer) cultures.
Studies (Dromard et al., 2011; Rajanahalli et al., 2012; Al-Saqi et al., 2014; Chen et al., 2014) reported that serum-free medium can maintain stem cells in an undifferentiated state. KSR, a serum-free medium, is used to culture ES cells (embryonic stem cells) and can slow down cell differentiation rates. 3D cultures of human mesenchymal stem cells (hMSCs) have been reported, however, most of them contains fetal bovine serum, and are therefore, not suitable for further clinical applications. The exclusion of serum enhances HMSC spheroid formation (Dong et al., 2019). We used serum-free KSR media to culture hADSCs and observed that during culture, hADSCs gradually condensed into spheroids. Relative to normal media, KSR slowed hADSCs proliferation without triggering apoptosis.
There are many ways in which cells form spheroids, including serum free culture (Dromard et al., 2011; Yoshida et al., 2005), low adhesion culture (Zhang et al., 2012; Napolitano et al., 2007), agarose culture (Ferro et al., 2011), methyl cellulose culture (Mi et al., 2010), centrifugal sedimentation culture (Ong et al., 2006), shaking table dynamic culture (Lee et al., 2011), mechanical curettage cell spheroidization (Bae et al., 2011), carrier spheroidization (Chen et al., 2009; Lei et al., 2011), hanging-drop spheroidization (Sakai et al., 2011; Yang et al., 2007), and suspension culture in bioreactors (Skardal et al., 2010). After forming spheres, some adult stem cells maintain and improve their stemness (Wang et al., 2009). Aggregated sphere cultures can induce non-stem cells into acquiring stem cell properties (Pastrana et al., 2011; Su et al., 2013). Additionally, cells have a higher survival rate after sphere formation which may enhance wound healing. This finding provides new ideas for clinical applications of 3D spheroids (Mimura et al., 2005). We found that EP tube culture promotes self-assembly of hADSCs into spheroidal microtissues, which have high cell viabilities. Spherical cultures facilitate cell-to-cell interactions and communication. Thus, we investigated the biological characteristics of hADSCs cultures in KSR media using the EP tube culture system. EP tubes do not allow cell adherence to tube’s inner walls and exert a gravity effect. This method can easily generate size- and cell number-controllable spheroids, but this may take long. We found that sizes of hADSC spheroids increased with increasing cell numbers. As culture time prolonged spheroids gradually condensed. We also found that hADSC spheroids have injectability and maintain better cell activity when cultured on EP tube.
We also found that spheroids developed polar growth in adherent culture. It has been reported that after spheroid formation, cell clusters suspended in serum-free medium exhibit polarity (Ferro et al., 2011). Polar growth represents a departure from growth models of typical decentralized cells. Cell polarity means the asymmetric spatial organization of cellular components within a cell (Chang et al., 2016). Polar growth also occurs in spherical culture of human cells. Our analyses revealed that hADSC spheroids exhibit polar growth, with spheroids on one side proliferating.
If two or more hADSC spheroids are placed together, they easily fuse, over time, forming cell clusters that may also fuse under certain conditions. Cell fusion is necessary for development of multicellular organisms. Skeletal muscles are composed of a large number of multinucleated muscle fibers fused by progenitor cells during development (Petrany and Millay, 2019). Cell fusion promotes the development, growth, and maintenance of tissues and organs throughout life. Cell-cell fusion occurs in various cell types, such as myoblasts, placental cells, osteoclasts, and stem cells. Thus, specific fusion events vary by cell types and species (Willkomm and Bloch, 2015). However, mechanism and significance of hADSCs fusion are unknown.
The EP tube culture method is a 3D static culture method that may affect the expressions, growth, and differentiation of cells. However, the EP tube culture system does not produce enough cell numbers for clinical application. In this study, we showed that hADSCs can form spheroids and remain viable when cultured in SMG, which allows for even exchange of nutrients and gases, with sufficient contacts among cells (Hammond and Hammond, 2001). However, SMG culture may be more conducive for differentiation of various types of cells, compared to the EP tube method, SMG has been shown to promote the development of ocular structures (Taibbi et al., 2013), mesenchymal stem cell differentiation (Grimm et al., 2014) and neural differentiation (Chen et al., 2011). Spheroid formation in SMG enhances stemness properties and therapeutic potentials of hADSCs, without involvement of any biomaterials, exogenous genes, or proteins (Zhang et al., 2015). Hence, hADSC spheroids after 2 days in the EP tube were transferred into the SMG system and further cultured for 5 days. Then, the viability assay showed that the number of dead cells in SMG spheroids was less than that of EP tube spheroids. Scanning electron microscopy showed that surfaces of SMG spheroids were relatively smooth and organized in a regular, granular shape, which may be beneficial for ever exchange of nutrients and gases evenly.
In conclusion, relative to hADSCs cultured in normal media, their proliferation and apoptosis were low in KSR media while spheroids formed in EP tubes condensed gradually. Our experiments revealed polar growth, fusion, and injectability of hADSC spheroids, providing important information on their spherical structure and transplantation capacity in vitro. Dynamic SMG culture enhances loose structure formation and better cell viabilities of hADSC spheroids. However, this study has some limitations. For example, the mechanisms mediating hADSC spheroids formation in SMG culture, and how to maintain or improve viability as well as stemness, are unclear. These questions should be addressed in future studies.
Acknowledgement: We would like to thank Peiyuan Wang for suggestions in writing.
Availability of Data and Materials: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions: JC conceived and designed the study. HL and CW conducted experiments. HL, CW, YG and SL analyzed the data. HL and CW drafted the first version of the manuscript. YG and SL revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Six women with a mean age of 35.1 ± 6.5 years were selected for the study after written informed consent was obtained. The institutional ethical review board of the First Affiliated Hospital of Jinan University approved the protocols (2020IRB001, 03/02/2020). A tumescent solution consisting of a mixture of 0.9% NaCl, 0.1% lidocaine, and 1:100000 epinephrine was injected using a 50 ml syringe into the fat donor sites of each patient’s abdomen. A 2.5 mm diameter cannula and 20 ml syringes were used to harvest 200 ml of adipose tissue from the left abdomen of each patient. All data used in this study was anonymized.
Funding Statement: This study was supported by the National Natural Science Foundation of China (21318261 and 3201101202 to JC), National Natural Science Foundation of China (82000943 to YG), the Joint Fund of Basic and Applied Basic Research Fund of Guangdong Province (2019A1515110355 to YG), and the Project funded by China Postdoctoral Science Foundation (2019M663391 and 2021T140273 to YG).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Al-Saqi SH, Saliem M, Asikainen S, Quezada HC, Ekblad A et al. (2014). Defined serum-free media for in vitro expansion of adipose-derived mesenchymal stem cells. Cytotherapy 16: 915–926. DOI 10.1016/j.jcyt.2014.02.006. [Google Scholar] [CrossRef]
Bae KS, Park JB, Kim HS, Kim DS, Park DJ et al. (2011). Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Medical Journal 52: 401–412. DOI 10.3349/ymj.2011.52.3.401. [Google Scholar] [CrossRef]
Boquest AC, Shahdadfar A, Brinchmann JE, Collas P (2006). Isolation of stromal stem cells from human adipose tissue. In: Methods in Molecular Biology, vol. 325, pp. 35–46. [Google Scholar]
Chang NC, Chevalier FP, Rudnicki MA (2016). Satellite cells in muscular dystrophy-Lost in polarity. Trends in Molecular Medicine 22: 479–496. DOI 10.1016/j.molmed.2016.04.002. [Google Scholar] [CrossRef]
Chen G, Yue A, Ruan Z, Yin Y, Wang R et al. (2014). Monitoring the biology stability of human umbilical cord-derived mesenchymal stem cells during long-term culture in serum-free medium. Cell and Tissue Banking 15: 513–521. DOI 10.1007/s10561-014-9420-6. [Google Scholar] [CrossRef]
Chen J, Chen R, Gao S (2007). Morphological characteristics and proliferation of keratocytes cultured under simulated microgravity. Artificial Organs 31: 722–731. DOI 10.1111/j.1525-1594.2007.00440.x. [Google Scholar] [CrossRef]
Chen J, Liu R, Yang Y, Li J, Zhang X et al. (2011). The simulated microgravity enhances the differentiation of mesenchymal stem cells into neurons. Neuroscience Letters 505: 171–175. DOI 10.1016/j.neulet.2011.10.014. [Google Scholar] [CrossRef]
Chen YH, Wang IJ, Young TH (2009). Formation of keratocyte spheroids on chitosan-coated surface can maintain keratocyte phenotypes. Tissue Engineering Part A 15: 2001–2013. DOI 10.1089/ten.tea.2008.0251. [Google Scholar] [CrossRef]
Dai Y, Chen J, Li H, Li S, Chen J et al. (2012). Characterizing the effects of VPA, VC and RCCS on rabbit keratocytes onto decellularized bovine cornea. PLoS One 7: e50114. [Google Scholar]
Dai Y, Guo Y, Wang C, Liu Q, Yang Y et al. (2014). Non-genetic direct reprogramming and biomimetic platforms in a preliminary study for adipose-derived stem cells into corneal endothelia-like cells. PLoS One 9: e109856. DOI 10.1371/journal.pone.0109856. [Google Scholar] [CrossRef]
Dong G, Wang S, Ge Y, Deng Q, Cao Q et al. (2019). Serum-free culture system for spontaneous human mesenchymal stem cell spheroid formation. Stem Cells International 2019: 6041816. DOI 10.1155/2019/6041816. [Google Scholar] [CrossRef]
Dromard C, Bourin P, André M, De Barros S, Casteilla L et al. (2011). Human adipose derived stroma/stem cells grow in serum-free medium as floating spheres. Experimental Cell Research 317: 770–780. DOI 10.1016/j.yexcr.2011.01.001. [Google Scholar] [CrossRef]
Ferro F, Spelat R, Falini G, Gallelli A, D’Aurizio F et al. (2011). Adipose tissue-derived stem cell in vitro differentiation in a three-dimensional dental bud structure. American Journal of Pathology 178: 2299–2310. DOI 10.1016/j.ajpath.2011.01.055. [Google Scholar] [CrossRef]
Fraser JK, Wulur I, Alfonso Z, Hedrick MH (2006). Fat tissue: An underappreciated source of stem cells for biotechnology. Trends in Biotechnology 24: 150–154. DOI 10.1016/j.tibtech.2006.01.010. [Google Scholar] [CrossRef]
Fridley KM, Fernandez I, Li MT, Kettlewell RB, Roy K (2010). Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors. Tissue Engineering Part A 16: 3285–3298. DOI 10.1089/ten.tea.2010.0166. [Google Scholar] [CrossRef]
Frith JE, Thomson B, Genever PG (2010). Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Engineering Part C: Methods 16: 735–749. DOI 10.1089/ten.tec.2009.0432. [Google Scholar] [CrossRef]
Gimble JM, Guilak F (2003). Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 5: 362–369. DOI 10.1080/14653240310003026. [Google Scholar] [CrossRef]
Grimm D, Wehland M, Pietsch J, Aleshcheva G, Wise P et al. (2014). Growing tissues in real and simulated microgravity: New methods for tissue engineering. Tissue Engineering Part B: Reviews 20: 555–566. DOI 10.1089/ten.teb.2013.0704. [Google Scholar] [CrossRef]
Guo X, Li S, Ji Q, Lian R, Chen J (2015a). Enhanced viability and neural differential potential in poor post-thaw hADSCs by agarose multi-well dishes and spheroid culture. Human Cell 28: 175–189. DOI 10.1007/s13577-015-0116-4. [Google Scholar] [CrossRef]
Guo Y, Liu Q, Yang Y, Guo X, Lian R et al. (2015b). The effects of ROCK inhibitor Y-27632 on injectable spheroids of bovine corneal endothelial cells. Cellular Reprogramming 17: 77–87. DOI 10.1089/cell.2014.0070. [Google Scholar] [CrossRef]
Guo Y, Xue Y, Wang P, Cui Z, Cao J et al. (2020). Muse cell spheroids have therapeutic effect on corneal scarring wound in mice and tree shrews. Science Translational Medicine 12: eaaw1120. DOI 10.1126/scitranslmed.aaw1120. [Google Scholar] [CrossRef]
Hammond TG, Hammond JM (2001). Optimized suspension culture: The rotating-wall vessel. American Journal of Physiology-Renal Physiology 281: F12–F25. DOI 10.1152/ajprenal.2001.281.1.F12. [Google Scholar] [CrossRef]
Hurrell T, Ellero AA, Masso ZF, Cromarty AD (2018). Characterization and reproducibility of HepG2 hanging drop spheroids toxicology in vitro. Toxicology in Vitro 50: 86–94. DOI 10.1016/j.tiv.2018.02.013. [Google Scholar] [CrossRef]
Laschke MW, Menger MD (2017). Life is 3D: Boosting spheroid function for tissue engineering. Trends in Biotechnology 35: 133–144. DOI 10.1016/j.tibtech.2016.08.004. [Google Scholar] [CrossRef]
Lee JI, Sato M, Kim HW, Mochida J (2011). Transplantation of scaffold-free spheroids composed of synovium-derived cells and chondrocytes for the treatment of cartilage defects of the knee. European Cells & Materials 22: 275–290. DOI 10.22203/eCM.v022a21. [Google Scholar] [CrossRef]
Lee TJ, Shim MS, Yu T, Choi K, Kim DI et al. (2018). Bioreducible polymer micelles based on acid-degradable poly(ethylene glycol)-poly(amino ketal) enhance the stromal cell-derived factor-1α gene transfection efficacy and therapeutic angiogenesis of human adipose-derived stem cells. International Journal of Molecular Sciences 19: 529. DOI 10.3390/ijms19020529. [Google Scholar] [CrossRef]
Lei XH, Ning LN, Cao YJ, Liu S, Zhang SB et al. (2011). NASA-approved rotary bioreactor enhances proliferation of human epidermal stem cells and supports formation of 3D epidermis-like structure. PLoS One 6: e26603. DOI 10.1371/journal.pone.0026603. [Google Scholar] [CrossRef]
Lin RZ, Chang HY (2008). Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnology Journal 3: 1172–1184. DOI 10.1002/biot.200700228. [Google Scholar] [CrossRef]
Lin RZ, Chou LF, Chien CC, Chang HY (2006). Dynamic analysis of hepatoma spheroid formation: Roles of E-cadherin and beta1-integrin. Cell Tissue Research 324: 411–422. DOI 10.1007/s00441-005-0148-2. [Google Scholar] [CrossRef]
Li S, Ma Z, Niu Z, Qian H, Xuan D et al. (2009). NASA-approved rotary bioreactor enhances proliferation and osteogenesis of human periodontal ligament stem cells. Stem Cells and Development 18: 1273–1282. DOI 10.1089/scd.2008.0371. [Google Scholar] [CrossRef]
Li S, Wang C, Dai Y, Yang Y, Pan H et al. (2013). The stimulatory effect of ROCK inhibitor on bovine corneal endothelial cells. Tissue and Cell 45: 387–396. DOI 10.1016/j.tice.2013.06.006. [Google Scholar] [CrossRef]
Mimura T, Yamagami S, Yokoo S, Yanagi Y, Usui T et al. (2005). Sphere therapy for corneal endothelium deficiency in a rabbit model. Investigative Ophthalmology & Visual Science 46: 3128–3135. DOI 10.1167/iovs.05-0251. [Google Scholar] [CrossRef]
Mi S, Chen B, Wright B, Connon CJ (2010). Ex vivo construction of an artificial ocular surface by combination of corneal limbal epithelial cells and a compressed collagen scaffold containing keratocytes. Tissue Engineering Part A 16: 2091–2100. DOI 10.1089/ten.tea.2009.0748. [Google Scholar] [CrossRef]
Mizuno H (2010). Adipose-derived stem and stromal cells for cell-based therapy: Current status of preclinical studies and clinical trials. Current Opinion in Molecular Therapeutics 12: 442–449. [Google Scholar]
Napolitano AP, Dean DM, Man AJ, Youssef J, Ho DN et al. (2007). Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques 43: 494–500. DOI 10.2144/000112591. [Google Scholar] [CrossRef]
Ong SY, Dai H, Leong KW (2006). Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials 27: 4087–4097. DOI 10.1016/j.biomaterials.2006.03.022. [Google Scholar] [CrossRef]
Page H, Flood P, Reynaud EG (2013). Three-dimensional tissue cultures: Current trends and beyond. Cell and Tissue Research 352: 123–131. DOI 10.1007/s00441-012-1441-5. [Google Scholar] [CrossRef]
Pastrana E, Silva-Vargas V, Doetsch F (2011). Eyes wide open: A critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 8: 486–498. DOI 10.1016/j.stem.2011.04.007. [Google Scholar] [CrossRef]
Petrany MJ, Millay DP (2019). Cell fusion: Merging membranes and making muscle. Trends in Cell Biology 29: 964–973. DOI 10.1016/j.tcb.2019.09.002. [Google Scholar] [CrossRef]
Petrenko Y, Syková E, Kubinová Š (2017). The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Research & Therapy 8: 94. DOI 10.1186/s13287-017-0558-6. [Google Scholar] [CrossRef]
Rajanahalli P, Meyer K, Zhu L, Wagner BD, Robinson ML et al. (2012). Conversion of mouse fibroblasts to sphere cells induced by AlbuMAXI-containing medium. Frontiers in Bioscience 1: 1813–1822. [Google Scholar]
Sakai Y, Yoshiura Y, Nakazawa K (2011). Embryoid body culture of mouse embryonic stem cells using microwell and micropatterned chips. Journal of Bioscience and Bioengineering 111: 85–91. DOI 10.1016/j.jbiosc.2010.08.014. [Google Scholar] [CrossRef]
Saleh FA, Frith JE, Lee JA, Genever PG (2012). Three-dimensional in vitro culture techniques for mesenchymal stem cells. 916: 31–45. [Google Scholar]
Skardal A, Sarker SF, Crabbé A, Nickerson CA, Prestwich GD (2010). The generation of 3-D tissue models based on hyaluronan hydrogel-coated microcarriers within a rotating wall vessel bioreactor. Biomaterials 31: 8426–8435. DOI 10.1016/j.biomaterials.2010.07.047. [Google Scholar] [CrossRef]
Szebeni GJ, Tancos Z, Feher LZ, Alfoldi R, Kobolak J et al. (2017). Real architecture for 3D Tissue (RAFTTM) culture system improves viability and maintains insulin and glucagon production of mouse pancreatic islet cells. Cytotechnology 69: 359–369. DOI 10.1007/s10616-017-0067-6. [Google Scholar] [CrossRef]
Su G, Zhao Y, Wei J, Han J, Chen L et al. (2013). The effect of forced growth of cells into 3D spheres using low attachment surfaces on the acquisition of stemness properties. Biomaterials 34: 3215–3222. DOI 10.1016/j.biomaterials.2013.01.044. [Google Scholar] [CrossRef]
Taibbi G, Cromwell RL, Kapoor KG, Godley BF, Vizzeri G (2013). The effect of microgravity on ocular structures and visual function: A review. Survey of Ophthalmology 58: 155–163. DOI 10.1016/j.survophthal.2012.04.002. [Google Scholar] [CrossRef]
Vunjak-Novakovic G, Scadden DT (2011). Biomimetic platforms for human stem cell research. Cell Stem Cell 8: 252–261. DOI 10.1016/j.stem.2011.02.014. [Google Scholar] [CrossRef]
Willkomm L, Bloch W (2015). State of the art in cell-cell fusion. Methods in Molecular Biology 1313: 1–19. DOI 10.1007/978-1-4939-2703-6. [Google Scholar] [CrossRef]
Wang W, Itaka K, Ohba S, Nishiyama N, Chung UI et al. (2009). 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials 30: 2705–2715. DOI 10.1016/j.biomaterials.2009.01.030. [Google Scholar] [CrossRef]
Yang MJ, Chen CH, Lin PJ, Huang CH, Chen W et al. (2007). Novel method of forming human embryoid bodies in a polystyrene dish surface-coated with a temperature-responsive methylcellulose hydrogel. Biomacromolecules 8: 2746–2752. DOI 10.1021/bm0704166. [Google Scholar] [CrossRef]
Yoshida S, Shimmura S, Shimazaki J, Shinozaki N, Tsubota K (2005). Serum-free spheroid culture of mouse corneal keratocytes. Investigative Ophthalmology & Visual Science 46: 1653–1658. DOI 10.1167/iovs.04-1405. [Google Scholar] [CrossRef]
Zhang Q, Nguyen AL, Shi S, Hill C, Wilder-Smith P et al. (2012). Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells and Development 21: 937–947. DOI 10.1089/scd.2011.0252. [Google Scholar] [CrossRef]
Zhang S, Liu P, Chen L, Wang Y, Wang Z et al. (2015). The effects of spheroid formation of adipose-derived stem cells in a microgravity bioreactor on stemness properties and therapeutic potential. Biomaterials 41: 15–25. DOI 10.1016/j.biomaterials.2014.11.019. [Google Scholar] [CrossRef]
Zhang S, Zhang Y, Chen L, Liu T, Li Y et al. (2013). Efficient large-scale generation of functional hepatocytes from mouse embryonic stem cells grown in a rotating bioreactor with exogenous growth factors and hormones. Stem Cell Research & Therapy 4: 145. DOI 10.1186/scrt356. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |