DOI:10.32604/biocell.2022.018530

| BIOCELL DOI:10.32604/biocell.2022.018530 |  |
| Review |
Ferroptosis molecular inducers: A future direction for malignant tumor chemotherapy
1State Key Laboratory of Oral Diseases, Chengdu, 610041, China
2West China School of Stomatology, Sichuan University, Chengdu, 610041, China
3National Clinical Research Center for Oral Diseases, Chengdu, 610041, China
4Chinese Academy of Medical Sciences Research Unit of Oral Medicine of Carcinogenesis and Management, Chengdu, 610041, China
5West China Hospital of Stomatology, Sichuan University, Chengdu, 610041, China
*Address correspondence to: Yingqiang Shen, shen@scu.edu.cn
#These two authors contribute equally to this work
Received: 31 July 2021; Accepted: 21 October 2021
Abstract: Iron-dependent ferroptosis is a form of cell death dependent on iron levels. Cells that undergo ferroptosis have glutathione (GSH) deficiency, reduced Glutathione peroxidase-4 (GPX4) activity and intracellular lipid peroxidation, Mitochondria, lysosomes and many signal pathways are involved in the regulation of ferroptosis. More importantly, many tumor cells resistant to other cell death methods exhibit sensitivity to ferroptosis. Moreover, over recent years, a number of ferroptosis-induced drugs have been recommended for the treatment of malignant tumors. Therefore, the study of ferroptosis is of great significance for future cancer treatments. In this review, we discussed the metabolic process of ferroptosis, the role of different organelles, the typical signaling pathways involved in ferroptosis, as well as natural and synthetic compounds that can induce ferroptosis, aiming to point out new conceptual avenues for utilizing ferroptosis in future cancer treatments.
Keywords: Ferroptosis; Cancer; Organelle; Pathways; Ferroptosis-induced drugs
Ferroptosis is a programmed cell death dependent on iron. It was first discovered by Stockwell Lab in 2012 and described as a form of cell death induced by erastin (Jiang et al., 2021). Ferroptosis is characterized by the accumulation of lipid peroxides and, reduced GPX4 activity within the cells. So far, it has been discovered that the process of ferroptosis can be inhibited by the depletion of iron inhibitors, lipophilic antioxidants, lipid peroxidation inhibitors, and polyunsaturated fatty acids (PUFAs) (Stockwell et al., 2017). Unlike traditional cell death methods, the morphology of erastin-induced ferroptotic cells is characterized by dysmorphic small mitochondria with decreased crista, as well as condensed, ruptured outer membranes (Tang et al., 2019). A variety of signaling pathways and organelles have also been found to have a significant role in regulating ferroptosis. Consequently, over the years, some molecules and cell structures have been recommended as targets for inducing ferroptosis, thus providing theoretical support for the development of new tumor treatment technologies through ferroptosis. This review discussed the metabolic processes of ferroptosis, the signaling pathways, and the role of organelles and cell structures involved in this programmed cell death process. In addition, we discussed the potential applications and prospects of different natural and synthetic compounds in the treatment of ferroptosis in tumor treatment, which may provide new ideas and new directions for malignant tumor treatment.
The Mechanism and Characteristics of Ferroptosis Initiation
Abnormal production of glutathione
Glutathione (GSH) is an antioxidant composed of glutamate, cysteine, and glycine, widely present in human cells (Jiang et al., 2021). Cells take up the raw materials for GSH synthesis through System Xc-, which consists of light chain subunit SLC7A11 and heavy chain subunit SLC3A2 (Li et al., 2020), ingesting cystine and excreting glutamate in equal proportions. The most classic ferroptosis inducer, erastin and its derivatives, can inhibit System Xc-, thus leading to a deficiency of cysteine, which reduces intracellular GSH. The tumor suppressor p53 (TP53) can inhibit the transcription of SLC7A11, thereby inhibiting System Xc- (Lin et al., 2020), which leads to the depletion of GSH, accumulation of lipid peroxides, protein or membrane damage, and subsequent cell death (Jiang et al., 2021). Yet, cells that are resistant to ferroptosis may take up or synthesize cysteine through some other ways, thereby avoiding the inhibition of System Xc- by ferroptosis. For example, some cells can use methionine (MET) to synthesize homocysteine (HCY) through the sulfur transfer pathway. Homocysteine can be converted to cystathionine under the catalysis of cystathionine b-synthase (CBS), which is then converted to cysteine by cystathionine glyase (CGL) (Lin et al., 2020). In addition, cysteine can also be derived from protein degradation in lysosomes (Fig. 1). After proteolysis, the cysteine used for GSH synthesis is released into the cytoplasm through cystine protein and H+-driven lysosomal transporter (SLC66A4) (Capelletti et al., 2020).
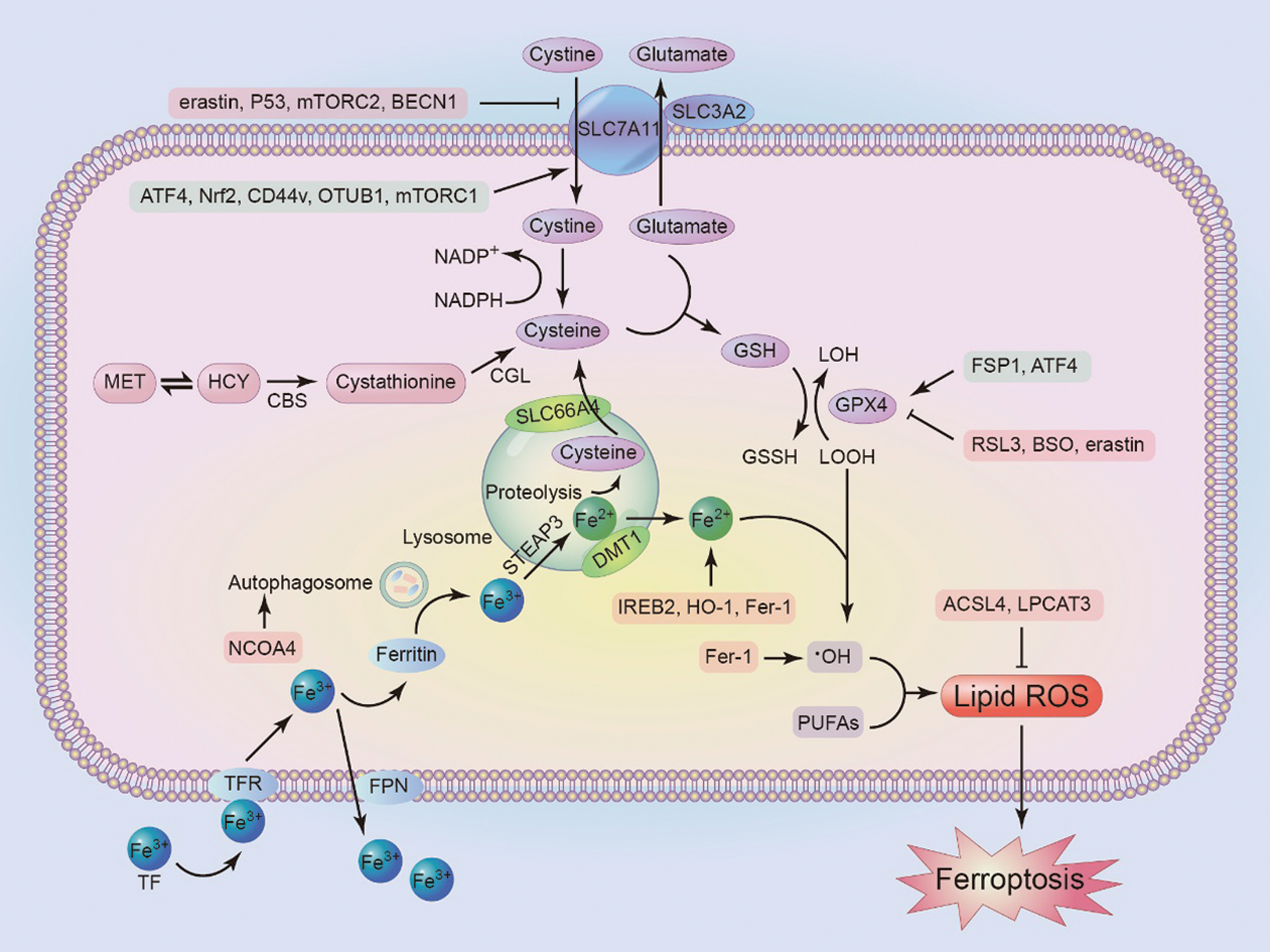
Figure 1: The mechanism of ferroptosis. Inhibiting antioxidant factors such as SLC7A11, GPX4, or increasing intracellular Fe3+ can induce ferroptosis. At the same time, the figure shows some factors that regulate these molecules. mTORC, rapamycin complex; ATF4, activating transcription factor 4; CBS, cystathionine b-synthase; CGL, cystathionine g-lyase; NCOA4, Nuclear receptor co-activator 4; TFR, transferrin receptor; TF, transferrin; FPN, ferroportin; DMT1, divalent metal transporter 1; IREB2, Iron response element binding protein 2; FSP1, ferroptosis suppressor protein 1; BSO, Butthionine sulfoxide amine; ACSL4, acyl-CoA synthase 4; LPCAT3, lysolecithin acyltransferase 3.
Glutathione peroxidases (GPXs) are evolutionarily highly conserved enzymes that use GSH as a cofactor to reduce peroxides (e.g., –OOH) to their corresponding alcohols (R–OH), thereby limiting the transition metal-dependent formation of toxic radicals (e.g., R–O•). Unlike other family members, GPX4 can act as a phospholipid hydroperoxide to reduce lipid peroxides to lipid alcohols. Thus, GPX4 activity is essential to maintain lipid homeostasis in the cell, preventing the accumulation of toxic lipid ROS (Forcina and Dixon, 2019). However, during ferroptosis, the activity of GPX4 is inhibited. The direct action site of the ferroptosis inducer RSL3 is GPX4, which covalently interacts with the nucleophilic active site Sec of GPX4, resulting in irreversible inactivation of the enzyme (Jiang et al., 2021; Yang et al., 2016). GSH is a cofactor for GPX4 to catalyze the conversion of peroxides to alcohols, GSH depletion caused by cysteine deprivation directly inactivates GPX4 and leads to subsequent ferroptosis. Buthionine sulfoxide amine (BSO) is the rate-limiting enzyme of GSH synthesis, which reduces the activity of GPX4 by inhibiting GSH synthesis (Yang et al., 2014). Other direct and indirect GPX4 inhibitors include FIN56, ML162 and ML210, and 1,2-dioxolane FINO2. In addition, besides inhibiting System Xc-, erastin promotes the degradation of GPX4 by increasing the level of lysosomal-associated membrane protein 2a (LAMP2a), promoting chaperone-mediated autophagy (Guan et al., 2020).
However, cells can clear peroxides through other pathways when GPX4 is inhibited. For example, ferroptosis inhibitor-1 (FSP1) can reduce coenzyme Q10 to lipophilic free radical scavenger panthenol by acting as an oxidoreductase. Panthenol can limit the accumulation of reactive oxygen species (ROS) without GPX4 so as to inhibit ferroptosis (Doll et al., 2019), which will be introduced in detail later on.
ROS and intracellular iron are necessary for the production of lipid peroxides
ROS, including superoxide anion, hydrogen peroxide, hydroxyl radical, singlet oxygen, etc. are free radicals with strong oxidizing ability. ROS are usually generated in the mitochondria, peroxisomes, cyclooxygenase, and lipoxygenase. Electron transport chain on the inner mitochondrial membrane is the most important site for ROS generation in the cell, producing 95% of ROS. During the electron transfer process, 1–2% of the electrons combine with oxygen molecules to form superoxide anions, which subsequently form hydrogen peroxide and oxygen. Hydrogen peroxide is oxidized into hydroxyl radicals in the presence of Fe2+ by the Fenton reaction catalyzed by iron (Bains and Hall, 2012), which then oxidizes with lipids and proteins in cells.
Nishida et al. (2014) proposed that a certain level of ROS is necessary for cell survival; yet it is also known that the overproduction of ROS can trigger cell death. The metabolic level of tumor cells is higher than that of normal cells, so their ROS levels are correspondingly higher. Increasing ROS can make tumor cells reach the death threshold earlier than normal cells.
Polyunsaturated fatty acids are the main target of lipid peroxidation
The lipids involved in lipid peroxidation in the ferroptosis process are a type of phospholipids containing polyunsaturated fatty acids (PUFAs), which are abundant in cell membranes (Kagan et al., 2017). They can increase the fluidity of cell membranes and are important for cell survival. The abundance of PUFAs determines the degree of lipid peroxidation in the cell and the process of ferroptosis.
There are two types of lipid peroxidation in cells, including non-enzymatic lipid peroxidation and enzymatic lipid peroxidation. Non-enzymatic lipid peroxidation is a free radical-mediated chain reaction. Fe2+ reacts with H2O2 to generate hydroxyl radicals by Fenton reaction, which then reacts with polyunsaturated fatty acids in a multi-step free radical chain reaction to generate lipids peroxide. Long-chain fatty acyl-CoA synthase 4 (ACSL4) and lysolecithin acyltransferase 3 (LPCAT3) are involved in the remodeling of polyunsaturated fatty acids and the insertion of membrane phospholipids (Dixon et al., 2015). Lack of ACSL4 and LPCAT3 can inhibit lipid peroxidation and the occurrence of ferroptosis (Fig. 1).
Enzymatic lipid peroxidation suggests that polyunsaturated fatty acids are catalyzed by lipoxygenase (LOX) to generate lipid hydrogen peroxide and alkoxy radicals to participate in the next lipid peroxidation chain reaction (Reis and Spickett, 2012). Lipid peroxidation consumes polyunsaturated fatty acids, changes the fluid-structure of cell membranes, increases membrane permeability, and ultimately leads to cell death.
Normally, extracellular Fe3+ can enter the cell through transferrin (TF) and transferrin receptors (TFR) in the circulatory system. After entering the cell, iron is stored and carried in the form of ferritin. Moreover, intracellular iron can be carried out of the cell by ferroportin (FPN), the only known outlet to control iron leakage in mammals (Trujillo-Alonso et al., 2019). Ferritin liberates Fe3+ through the formation of autophagosomes. The metallic reductase six-transmembrane epithelial antigen of the prostate-3 (STEAP3) in lysosomes can convert Fe3+ to Fe2+, which is released into the cytoplasmic labile iron pool (LIP) via divalent metal transporter 1 (DMT1) (Song et al., 2016).
As an important factor for lipid peroxidation and ferroptosis induction, the level of intracellular active iron (redox-active Fe2+) can be increased by TF-mediated iron uptake or ferritin autophagy/lysosomal degradation (ferritin autophagy). Nuclear receptor coactivator 4 (NCOA4) is a ferritin phagocytosis-specific cargo receptor that mediates the transport of ferritin to autophagosomes and promotes ferritin phagocytosis, thereby releasing Fe2+ (Mancias et al., 2014). The inhibition of NCOA4 has shown to reduce the sensitivity of human fibrosarcoma cells (HT-1080) and human pancreatic cancer cells to ferroptosis (Hou et al., 2016).
Iron response element-binding protein 2 (IREB2) is the master regulator of iron metabolism. Silencing IREB2 can significantly reduce the ferroptosis induced by erastin (Dixon et al., 2012). Moreover, Fer-1 is a ferroptosis suppressant that reduces free iron in LIP by forming a compound with Fe2+. Meanwhile, it can reduce free oxygen radicals and decrease lipid peroxidation (Miotto et al., 2020). In addition, in order to support rapid proliferation, cancer cells have a higher demand for iron than non-malignant cells. Down-regulation of FPN and up-regulation of TFR1 have been observed in many cancer cell lines. Also, strong iron dependence (known as iron addiction) makes cancer cells more susceptible to iron overload and ROS accumulation than non-cancerous cells, thus making tumor microenvironment (TME) targeted therapy and ferroptosis-mediated cancer treatment possible.
Pivotal Cell Organelles in the Process of Ferroptosis
The endoplasmic reticulum (ER) is a network of tubules and flat sacs connecting the nucleus. Such organelles include the rough and smooth ER, have a key role in the regulation of protein synthesis, folding and secretion, calcium homeostasis, and lipid biosynthesis. In the process of ferroptosis, excessive ROS can induce misfolded proteins and trigger ER stress. Under ER stress, protein kinase RNA-like ER kinase (PERK) is activated to repair ER homeostasis. PERK can phosphorylate eukaryotic translation initiation factor 2α (eIF2α), thereby increasing ATF4, which can induce the expression of SLC7A11 and increase the activity of GPX4 thus protecting cells from ferroptosis (Chen et al., 2019). In addition, Nrf2 can be stimulated by activated PERK to promote ARE expression (Fig. 2). CHAC-1 is located downstream of ATF4 and has been shown to promote the degradation of GSH and subsequent iron metabolism. PUMA is another downstream of ATF4. The iron metabolism reagent artemisinin (ART) induces ER stress through the PERK-eIF2α-ATF4-CHOP pathway mediated by ER stress and increases the expression of the pro-apoptotic molecule PUMA without inducing apoptosis (Jiang et al., 2021; Lee et al., 2018).
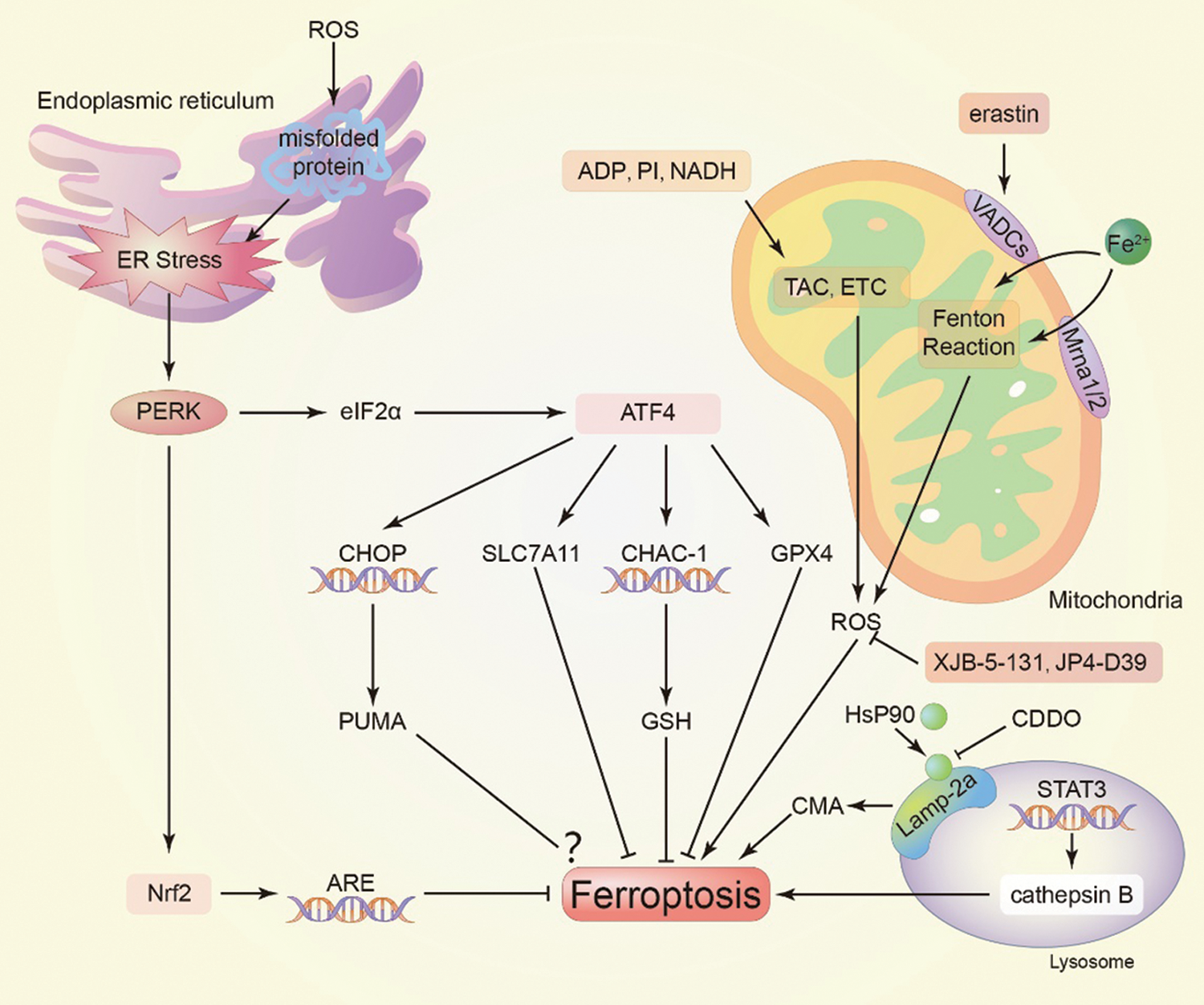
Figure 2: Function of organelles. In mitochondria, TAC is used to regulate intracellular ROS. The endoplasmic reticulum regulates the nuclear translocation of Nrf2 through endoplasmic reticulum stress to regulate the expression of ARE, thereby avoiding ferroptosis. However, CMA in the lysosome is involved in the process of ferroptosis. PERK, protein kinase RNA-like ER kinase; eIF2, eukaryotic translation initiation factor 2α; ARE, antioxidant response element; CMA, chaperone-mediated autophagy; TAC, tricarboxylic acid cycle; ETC, electron transport chain; VDACs, voltage-dependent anion channels; Mrna, membrane transporter mitomycin; CDDO, 2-amino-5-chloro-N,3-dimethylbenzamide.
Mitochondria are the energy suppliers of cells closely related to the programmed cell death process. Mitochondrial voltage-dependent anion channels (VDACs) have been shown to be potential targets of erastin. Erastin and its analogues antagonize the effect of free tubulin on VDACs and enable metabolites (such as respiratory substrates, ADP and phosphatidylinositol) to enter the mitochondria, thus increasing the opening of VDACs and the mitochondrial membrane potential, as well as the formation of ROS (Fig. 2) (Wang et al., 2020). The Fenton reaction is the main source of ROS that causes ferroptosis. Excessive cellular Fe2+ generates hydroxyl radicals by Fenton reaction, which transfer the electrons in the reducing compounds to H2O2. Studies have shown that VDAC1 oligomerization is the main cause of mitochondrial dysfunction and secondary ferroptosis (Niu et al., 2021). Intracellular antioxidant such as XJB-5-131 and JP4-039 can significantly affect the inhibition of ferroptosis. XJB-5-131 is a mitochondrial-targeted antioxidant with high effectiveness. In vitro and in vivo studies have suggested that XJB-5-131 can effectively reduce the expression of peroxides in kidney injured cells and inhibit the occurrence of ferroptosis, alleviating the hypokinesia caused by Huntington’s disease, respectively (Polyzos et al., 2016; Zhao et al., 2020). This highly targeted nitroxide exhibits a significant protective effect on ferroptosis induced by erastin or RSL3. In addition, XJB-5-131 has anti-erastin efficacy similar to the classic small-molecule Fer-1 (Wang et al., 2020). As mitochondrial iron metabolism primarily occurs in the mitochondrial matrix, iron must pass through the inner mitochondrial membrane (IMM), which is mainly involved in electron transfer and ATP synthesis. Also, cytochrome is the iconic protein on IMM, which needs iron to synthesize. Iron transport in IMM is an activation process that depends on the membrane transporter mitomycin 1 (Mrna 1) and its homologue mitomycin 2 (Mrna 2). The imbalance of Mfrn1/2 leads to mitochondrial iron accumulation and oxidative damage. At the same time, VDACs located in the outer mitochondrial membrane regulate the influx of iron into the mitochondria (Battaglia et al., 2020).
Lysosomes contain hydrolytic enzymes, which can degrade and circulate essential nutrients and maintain homeostasis in various ways. During ferroptosis, a proportion of ROS also originates from lysosomes. Lysosomes affect the intracellular iron intake by reducing the intracellular transport of TF or the intracellular degradation of ferritin (Kroemer and Jäättelä, 2005). Previous studies have found that inhibition of lysosomal cathepsin B (a cysteine protease) can reduce the sensitivity of cells to ferroptosis. Among them, the Signal Transducer and Activator of Transcription gene (STAT3) participates in ferroptosis by regulating the expression of cathepsin B through the MEK-ERK pathway in human pancreatic ductal adenocarcinoma (PDAC) cell lines, thus producing MDA (malondialdehyde), one of the final products of lipid peroxidation (Gao et al., 2018). Wei et al. (2020) found that the lysosomal inhibitor CQ can significantly block the apoptosis of MIN6 cells induced by NaAsO2. The down-regulated expression of GPX4 induced by NaAsO2 can be reversed by CQ, thus alleviating the impairment of GSH and superoxide dismutase activity, as well as malondialdehyde overload.
Chaperone-mediated autophagy (CMA) is another pathway that occurs in the lysosome during ferroptosis. It was found that the level of Lamp-2a increases, after which HSP90 binds to Lamp-2a on the lysosomal membrane, regulating the functional kinetics of Lamp-2a complex activation of CMA, and inhibiting GPX4 activity. Also, as autophagy-dependent cell death, ferroptosis is inseparable from the hydrolysis of enzymes. As a result of the lysosomal degradation of ferritin, the free iron released from lysosomes can be utilized by the cells, generating more ROS (Wang et al., 2021). As an effective iron metabolism inhibitor, 2-amino-5-chloro-N,3-dimethylbenzamide (CDDO) can inhibit HSP90 from blocking GPX4 degradation, lipid peroxidation, ROS accumulation and cell death (Fig. 2) (Wu et al., 2019).
In addition to the important organelles in the cell, the nucleus, also participates in the ferroptosis regulation processes. Suppressing of antioxidant genes can promote peroxides, inducing ferroptosis. Erastin treatment can induce growth inhibition in acute myeloid leukaemia (AML) cells with an NRASQ61L mutation (HL-60), but not in other cell lines harbouring RAS mutations or RAS wild type (WT), thus suggesting other genetic or non-genetic factors influencing ferroptosis sensitivity. Moreover, there is evidence suggesting that oncogenic KRAS mutations may protect cells from ferroptosis (Bebber et al., 2020). Furthermore, recent studies have shown that the α6β4/Src/STAT3 axis and PVRL4/α6β4/Src pathways can prevent cancer cells from iron death by inhibiting ACSL4 and maintaining the activity of GPX4. Therefore, inhibiting these pathways may become a new mode of tumor treatment in the future (Brown et al., 2017).
Regulatory Factors of Ferroptosis
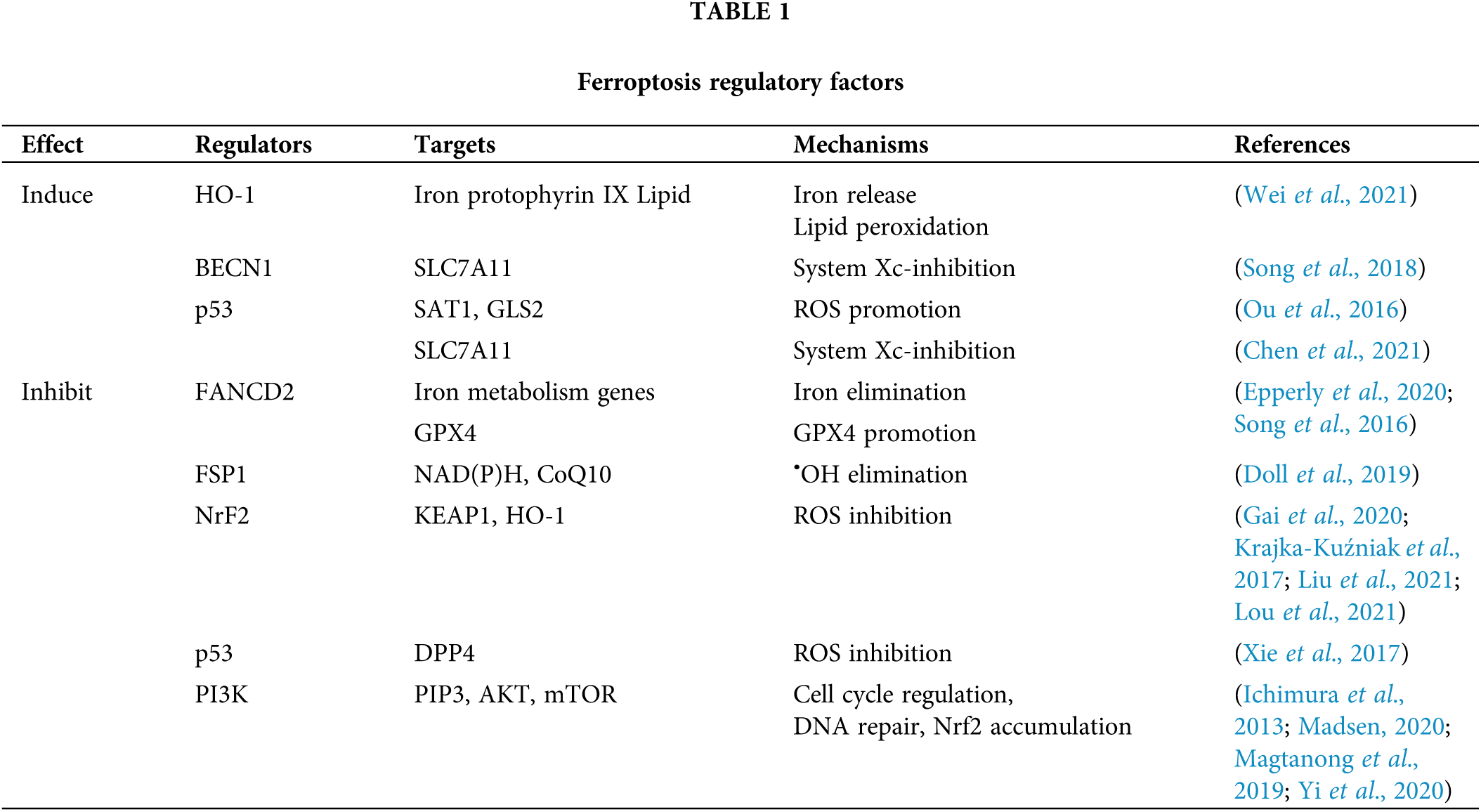
HO-1, also known as heat shock protein 32 (Hsp32), is the rate-limiting enzyme in the process of heme decomposition. The decomposition of heme produces biliverdin, carbon monoxide (CO), and Fe2+, while excess Fe2+ is stored in the form of ferritin (Lin et al., 2007). H0-1 has a higher expression in many malignant tumors, where it stabilizes the tumor microenvironment, promoting cancer cell growth, angiogenesis and metastasis, and resistance to chemotherapy and radiotherapy. Moreover, overexpression of HO-1 leads to excessive Fe2+ that causes damage to cells after it exceeds the buffering capacity of ferritin (Vítek and Schwertner, 2007).
Wei et al. (2021) found that as a downstream gene (effector) of Nrf2, upregulated HO-1 increases in the labile iron pool, which promotes lipid peroxidation. Several small molecules have also been found to trigger ferroptosis by regulating the expression and activity of HO-1. These small molecules have similar properties. Most of them can trigger the release of iron and generate a lot of ROS. Some molecules have been found to induce HO-1 related ferroptosis, including heme, erastin/sorafenib/RSL3, magnesium isoglycyrrhizinate, BAY117085, and withaferin A (Hassannia et al., 2018).
BECN1 is a key regulator of autophagy in macrophages involved in the production of the PtdIns3K complex that activates autophagy. Studies have confirmed that BECN1 promotes ferroptosis independently of the formation of PtdIns3K complexes (Kang et al., 2018). BECN1-dependent ferroptosis requires the formation of the BECN1-SLC7A11 complex. With the help of fluorescent biosensor C11-BODIPY or through the quantitative detection of oxidative stress marker malondialdehyde, it is found that BECN1 promotes lipid peroxidation. These findings suggest that BECN1 promotes ferroptosis by regulating lipid peroxidation (Table 1) (Song et al., 2018). Also, Hu et al. (2021) found that cytoplasmic HMGB1 acts as an autophagy promoter during ferroptosis by binding to BECN1, which is induced by an iron-binding nuclear protein pirin.
Post-translational modifications, especially phosphorylation, regulate the function of BECN1. During the ferroptosis process, the 1-150 amino acids of BECN1 are required to form the BECN1-SLC7A11 complex and lipid peroxidation, while only the s90,93,96a mutant restricts the binding of BECN1 to SLC7A11 and the production of malondialdehyde. This indicates that the phosphorylation of BECN1 at s90, s93, and s96 contributes to the formation of the BECN1-SLC7A11 complex and subsequent lipid peroxidation, which is an important part of ferroptosis (Song et al., 2018).
In addition, BECN1 phosphorylation requires AMP-activated protein kinase (AMPK) to trigger the formation of the BECN1-SLC7A11 complex. AMPK is a key regulator of energetic metabolism. AMPK-mediated phosphorylation of BECN1 at S90 and S93 not only promotes autophagy and cell trafficking, but also induces ferroptosis. Using compound C (compound C, AMPK selective inhibitor) to block PRKAA/AMPKα can inhibit BECN1 phosphorylation, BECN1-SLC7A11 complex formation, and subsequent lipid peroxidation during ferroptosis (Lee et al., 2016). It was also shown that regulating the BECN1 pathway may improve anti-cancer treatment by inducing ferroptosis.
To date, ferroptosis-suppressor-protein 1 (FSP1) has been considered as newly corroborated with the capacity of antagonizing the process of ferroptosis (Xie et al., 2021). Further research found that the inhibition of ferroptosis by FSP1 is mediated by ubiquitin (also known as coenzyme Q10, CoQ10); FSP1 can reduce coenzyme Q10 to lipophilic free radical scavenger panthenol by acting as an oxidoreductase. Panthenol can trap free radicals that mediate lipid peroxidation, while FSP1 can utilize NAD(P)H to regenerate CoQ10. The pharmacological targeting of FSP1 has a strong synergistic effect with GPX4 inhibitors, which can trigger ferroptosis in many cancer entities. Therefore, the FSP1-CoQ10-NAD (P)H pathway exists as an independent parallel system, which works with GPX4 and GSH to inhibit phospholipid peroxidation and ferroptosis (Doll et al., 2019).
Nrf2 belongs to CNC-BZIP (cap’nap’n’collar-basic leucine zipper) transcription activator family. Nrf2 is located in the cytoplasm and binds to KEAP1, a negative regulator of Nrf2. However, under endogenous stress (such as the increase of ROS), KEAP1 conformational changes and phosphorylation of Nrf2 allow Nrf2 to enter the nucleus and initiate transcription of the gene containing ARE (antioxidant element) (Krajka-Kuźniak et al., 2017), whose downstream target gene is involved in maintaining intracellular redox homeostasis.
Cancer cells with mutations in Nrf2 can increase the transcription of antioxidant genes. Lou et al. (2021) found that ginkgetin disrupts redox homeostasis caused by the inactivation of the Nrf2/HO-1 axis in non-small-cell lung cancer cells. Moreover, Liu et al. (2021) indicated that ferroptosis could be inhibited by activating the Nrf2/HO-1 signaling pathway. During erastin-induced ferroptosis, Nrf2 is prone to nuclear translocation, leading to gene silencing, which in turn leads to ferroptosis (Gai et al., 2020).
P53 protein is a tumor suppressor protein. In addition to regulating cell apoptosis, autophagy, and cell cycle, P53 also regulates the sensitivity of cells to ferroptosis by affecting transcription and post-translational mechanisms.
On the one hand, p53 can induce ferroptosis by inhibiting the expression of SLC7A11 (Chen et al., 2021). It also achieves this goal by enhancing the expression of SAT1 (spermidine/spermine n1-acetyltransferase 1) and GLS2 (glutaminase 2). SAT1 increases the level of intracellular ROS by increasing the expression of ALOX15 (arachidonic acid 15-lipoxygenase) (Ou et al., 2016). Moreover, GLS increases the level of ROS by reducing the level of GSH, thereby increasing the sensitivity of cells to ferroptosis.
On the other hand, p53 inhibits ferroptosis by directly inhibiting the activity of DPP4 (dipeptidyl peptidase 4). DPP4 binds to NOX1 (NADPH oxidase 1) to trigger the ROS generation reaction associated with the cell membrane. In addition, p53 can make DPP4 accumulate in the nucleus to prevent this reaction, thereby inhibiting the occurrence of ferroptosis (Xie et al., 2017).
PI3K belongs to phosphatidylinositol kinase, activated by interacting with protein tyrosine kinase-linked receptors and G protein-linked receptors. It can activate PIP3 (phosphatidylinositol triphosphate) on the plasma membrane. PIP3 can bind to, and activate AKT (serine/threonine protein kinase), and regulate the expression of downstream targets (such as mTOR, FoxO, GSK-3), thereby regulating cell cycle, DNA damage and repair and other life processes (Madsen, 2020).
Inhibition of the PI3K-AKT-mTOR pathway can induce the sensitivity of cells to ferroptosis. Mechanistically, AKT up-regulates the expression of mTORC1, which in turn up-regulates SREBP1 (central regulator of lipid synthesis). SREBP1 can regulate a variety of lipid synthesis-related genes (such as ACLY, FASN, and SCD). Studies have shown that SCD1 is the main target of the PI3K signaling pathway to protect cells from ferroptosis. After SCD1 is knocked out, the inhibition of PI3K, AKT and mTOR no longer induces the sensitivity of cells to ferroptosis (Yi et al., 2020). In addition, SCD1 is an enzyme that converts saturated fatty acids into monounsaturated fatty acids (MUFAs) that can inhibit ferroptosis (Magtanong et al., 2019), which further proves the role of the PI3K-AKT-mTOR signaling pathway in inhibiting ferroptosis.
In addition, mTORC1 promotes the binding of p62 and Keap1 through phosphorylation of p62, leading to the degradation of Keap1. This in turn leads to the accumulation of Nrf2 and inhibits the occurrence of ferroptosis (Ichimura et al., 2013).
Fanconi anemia (FA) is a genetically heterogeneous recessive disease caused by DNA repair defects. The destruction caused by iron metabolism plays a key role in the etiology of this disease (Kang and Tang, 2017). Fanconi Anemia Complementary Group D2 (FANCD2) is the central protein of the FA pathway, which is activated after a DNA damage response, limiting the DNA damage of bone marrow mesenchymal stem cells.
Bone marrow mesenchymal stem cells deficient in FANCD2 tend to exhibit iron overload and lipid peroxidation under the action of erastin. In particular, FANCD2 deletion has been associated with increased gene expression of iron uptake (such as transferrin, transferrin receptor, and HSPB1), and decreased gene expression of iron storage (such as FTH) and iron output (such as hepcidin antimicrobial peptide) in ferroptosis patients (Song et al., 2016). FANCD2 regulates the expression of genes and/or proteins related to iron metabolism (such as FTH1, TF, TFRC, HAMP, HSPB1, SLC40A1, and STEAP3) and lipid peroxidation (such as GPX4). In addition, in vivo experiments have confirmed that the drug is less effective at inducing ferroptosis in FA mice, whereas the effect on control mice has been improved (Epperly et al., 2020). These data indicate that FANCD2 has an important role in combating ferroptosis in bone marrow mesenchymal stem cells, and FANCD2 may be a viable target for the development of new anti-cancer therapies to reduce the side effects of ferroptosis inducers.
Potential Applications of Compounds in Ferroptosis
Potential ferroptosis inducers extracted from natural plants
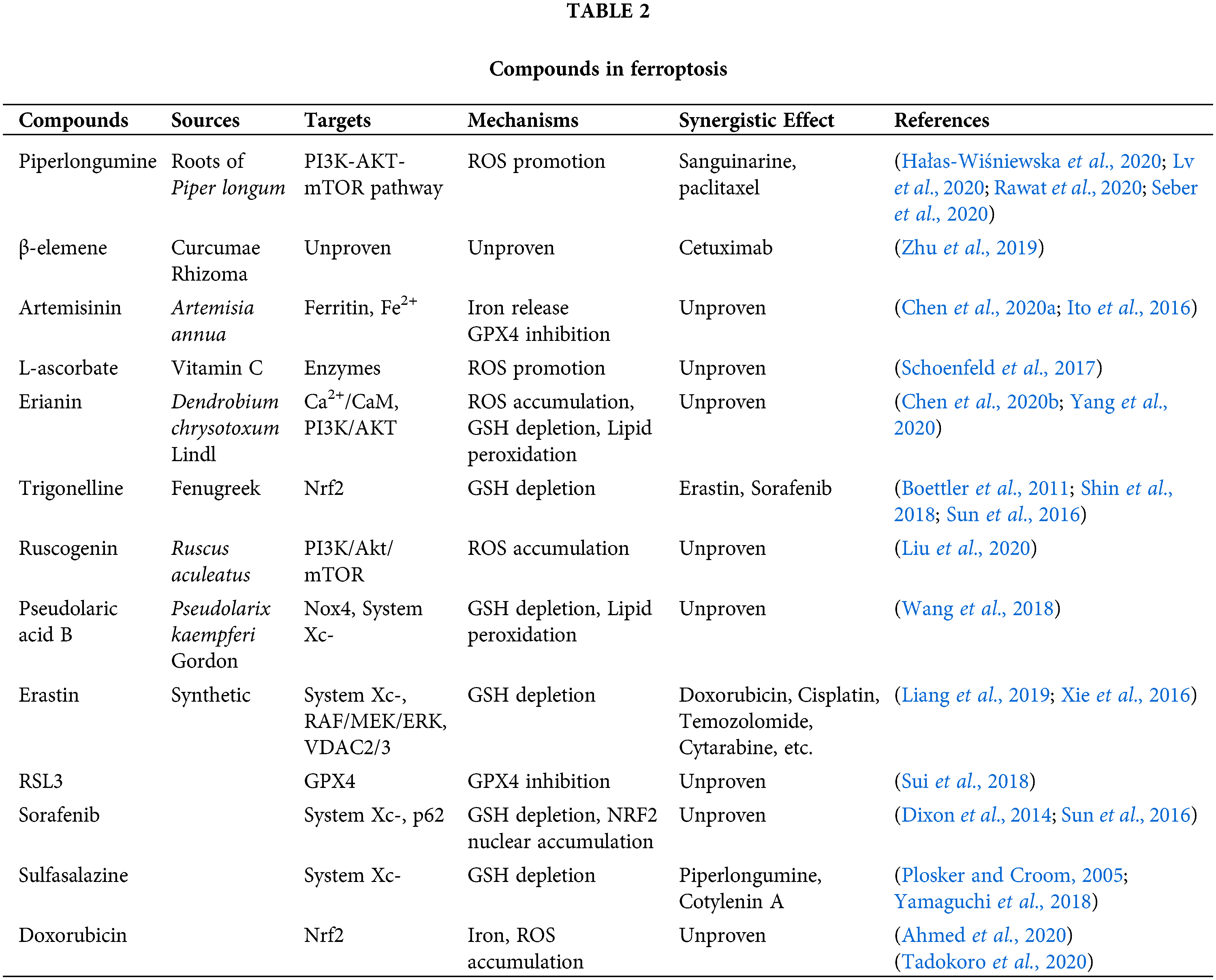
Piperlongumine (PL) is a biologically active alkaloid found in the roots of Piper longum. Existing studies have shown that PL exerts cytotoxicity on tumor cells through the accumulation of ROS in cells. Interestingly, PL has high selectivity; at a concentration, it does not affect healthy cells, while it is highly toxic to cancer cells, thus potentially representing a new drug for tumor treatment (Tripathi and Biswal, 2020).
PL can increase ROS in tumor cells and directly antagonize AKT and block the PI3K-AKT-mTOR pathway, thereby inducing ferroptosis. Lv et al. (2020) evaluated the molecular docking of PL and Akt by rearranging the crystal structure, and then calculating root mean square deviation (RMSD), thus confirming that PL directly binds to Akt and inhibits its phosphorylation.
At the same time, PL shows a synergistic effect with many anti-tumor drugs. Hałas-Wiśniewska et al. (2020) demonstrated that the combined effect of PL and Sanguinarine increased cell apoptosis. Moreover, Rawat et al. (2020) showed that PL and paclitaxel could produce a synergistic anti-tumor effect; PL was able to sensitize INT-407 and HCT-116 cancer cells to paclitaxel (drug dose, from 0.1 to 1 μM). Furthermore, Sirin and colleagues showed that PL in combination with doxorubicin and paclitaxel increases apopotosis in a cervical cancer cell compared to either chemotherapeutic agent alone (Seber et al., 2020).
β-elemene, a bioactive compound isolated from the Chinese herb Curcumae rhizoma, and a new type of ferroptosis inducer, exhibits a spectral anticancer effect and is used to treat various cancer. Zhu et al. (2019) found that a combinative treatment of β-elemene and cetuximab induces ferroptosis and inhibits EMT in KRAS mutant CRC cell HCT116, thus providing a prospective strategy for CRC patients with RAS mutations.
Artemisinins, which are extensively used as the first-line antimalarial drug, have also revealed potential efficacy in treating cancers (Bai et al., 2021). Dihydroartemisinin (DAT), a derivative of artemisinin, induces lysosomal degradation of ferritin in an autophagy-independent manner, increasing the cellular free iron; yet, it has no effect on cellular glutathione. Furthermore, it stimulates the binding of iron-regulatory proteins (IRPs) with mRNA molecules containing iron-responsive element (IRE) sequences, thus further increasing cellular free iron (Chen et al., 2020a). Li et al. (2021) developed ferrous-supply nano-carrier for ART based on tannic acid (TA) and ferrous ion (Fe(II)) coated on the zeolitic imidazolate framework-8 (ZIF) with ART encapsulated (TA-Fe/ART@ZIF) via coordination-driven self-assembly, providing a novel approach for enhancing the potency of ferroptotic nano-medicine.
Artemisinin has also been effective for drug-resistant tumor cells. For example, pancreatic cancer cells resistant to chemotherapy-induced apoptosis have shown great sensitivity to artemisinin-induced ferroptosis (Efferth, 2017). Moreover, DAT can augment GPX4 inhibition-induced ferroptosis in a cohort of cancer cells that are otherwise highly resistant to ferroptosis (Chen et al., 2020a).
It has been established that neoplastic cells harbor higher levels of Fe2+ in comparison with their non–tumorous counterparts (Ito et al., 2016; Schoenfeld et al., 2017; Shi et al., 2017). The abundance of high Fe2+ indicates a high level of oxidative stress. L-ascorbate, also known as vitamin C, is an essential reductant and cofactor in many enzymes. However, ascorbate works as a pro-oxidant in the presence of abundant Fe2+ to generate hydroxyl radicals. Thus, several clinical trials are currently testing the pharmacological level (~mmol/L) of ascorbate necessary to kill cancer cells in addition to a standard radio-chemotherapy in advanced-stage cancer patients (Schoenfeld et al., 2017). As a natural substance, vitamin C is not harmful to human body (Toyokuni et al., 2020).
Erianin, a natural product isolated from Dendrobium chrysotoxum Lindl, has been reported to exert antitumor effects on several cancer types. Chen et al. (2020b) found that ferroptosis contributes to erianin-induced cell death both in vitro and in vivo, accompanied by ROS accumulation, GSH depletion, and lipid peroxidation. The same study also showed that Ca2+/CaM signaling was a critical mediator of erianin-induced ferroptosis, thus suggesting that erianin may be a brand new medicine for ferroptosis. In addition, Yang et al. (2020) found that erianin promotes the apoptosis of HCC cells. Mechanistically, it has been discovered that erianin inhibited the activation of the PI3K/AKT pathway. Therefore, erianin can be a potential agent for tumor treatment through ferroptosis.
Trigonelline, the main pharmacological ingredient of a traditional Chinese herbal medicine Trigonella foenum-graecum L. (fenugreek), is an alkaloid present in coffee and fenugreek seed (Kamatani et al., 2011). Recently, studies have discovered that trigonelline acts against NRF2 (Boettler et al., 2011; Shin et al., 2018). Inhibition of NRF2 by the alkaloid trigonelline was found to significantly enhance the anticancer activity of erastin and sorafenib in HCC cells and tumor xenograft models. During the inhibition of NRF2 by trigonelline, the expression of MT-1G is blocked, which in turn reduces the content of GSH and further causes ferroptosis (Sun et al., 2016).
Ruscogenin was initially extracted from Ruscus aculeatus and is the main component of Ophiopogonis Radix (Ophiopogon japonicas). Previous studies have shown that ruscogenin inhibites hepatocellular carcinoma metastasis via the PI3K/Akt/mTOR signaling pathway (Hua et al., 2018). Furthermore, ruscogenin inhibits the activity of pancreatic cancer cells by inducing ferroptosis. Also, in vivo experiments have proved the anti-tumor effect of ruscogenin on pancreatic cancer xenografts without obvious toxicity (Song et al., 2020). Therefore, this compound is a promising anti-cancer candidate for the treatment of malignant cancer.
Pseudolaric acid B (PAB) is a diterpene-type acid isolated from the root and trunk bark of Pseudolarix kaempferi Gordon of the Pinaceae family, which has been used to treat eczema, fungal skin infections, and other skin diseases (Liu et al., 2017). As traditional Chinese medicine, people have been studying PAB for many years. Recently, it has been discovered that PAB can promote autophagy. Also, PAB has effective anti-tumor effects on human breast cancer, cervical cancer, melanoma, thyroid squamous cell carcinoma, and rhabdomyosarcoma by inducing apoptosis and autophagy (Wang et al., 2020). A recent study showed that PAB induced lipid peroxidation not only through activating Nox4 in an iron-dependent manner, but also by depleting GSH and cysteine via p53-regulated inhibition of System Xc- (Wang et al., 2018).
New synthetic ferroptosis inducers to treat malignant tumors
Erastin can directly inhibit System Xc- to reduce GSH levels. In 2012, Dixon et al. (2012) discovered that erastin triggers the accumulation of reactive oxygen species in NRAS mutant HT-1080 fibrosarcoma cells (Dixon et al., 2012). Further research proved the importance of the RAF/MEK/ERK signaling pathway in the ferroptosis triggered by erastin in RAS mutant cancers (Xie et al., 2016). Moreover, mitochondrial voltage-dependent anion channel (VDAC) is a molecular target of erastin, and its knockdown leads to erastin resistance. In addition to triggering ferroptosis for cancer therapy, erastin can enhance the chemotherapeutic effect of traditional anti-cancer drugs (e.g., doxorubicin, cisplatin, temozolomide, cytarabine, etc.) in certain cancer cell lines (Liang et al., 2019).
RSL3 can promote ferroptosis-induced cell death and directly inhibit GPX4 triggering ferroptosis (Yang et al., 2014). Sui et al. (2018) found elevated ROS levels and transferrin expression in colorectal cancer cells treated with RSL3 accompanied by a decrease in the expression of GPX4, which suggested that RSL3 induces ferroptosis. Also, treatment with RSL3 led to a significant decrease in prostate cancer cell growth and migration in vitro and significantly delayed the tumor growth of treatment-resistant prostate cancer in vivo, with no side effects (Ghoochani et al., 2021). These results demonstrate the potential of RSL3 as a novel strategy for cancer therapy.
Sorafenib is a (multikinase) inhibitor in cancer therapy that includes advanced renal cell carcinoma, thyroid carcinoma, and hepatocellular carcinoma. The cytotoxicity of sorafenib to hepatocellular carcinoma can be eliminated by using an iron chelator (Hou et al., 2019). Like erastin, sorafenib inhibits System Xc-mediated cystine import, leading to endoplasmic reticulum stress (ER stress), GSH depletion and the iron-dependent accumulation of lipid ROS (Dixon et al., 2014). Also, sorafenib promotes the expression of p62, causing p62 to bind to Keap1 and inactivate it, enhancing NRF2 nuclear accumulation and inducing ferroptosis (Sun et al., 2016).
Sulfasalazine (SAS) was originally used as a disease-modifying antirheumatic drug. It is generally well-tolerated in clinical trials because of its safety, convenience, and low cost. In recent years, more and more studies have confirmed that SAS exerts a powerful effect in tumor treatment, especially improving the sensitivity of tumors to chemotherapeutic drugs (Plosker and Croom, 2005). Sugiyama et al. (2020) found that the cytotoxic effect of SAS was stronger in paclitaxel-resistant cells compared with that sensitive cells, and that SAS-mediated cell death was induced through ferroptosis rather than apoptosis. In SAS-treated tumor cells, the expressions of GSH and System Xc- were reduced, proving that SAS induces ferroptosis. Moreover, because sulfasalazine is considered as non-toxic, it can be combined with traditional drugs to increase the efficacy of drugs and reduce the drug resistance of tumor cells. For instance, Yamaguchi et al. (2018) found that a triple combination treatment with piperlongumine, cotylenin A, and sulfasalazine was highly effective against pancreatic cancer.
Doxorubicin, as an anticancer drug, has a wide range of anticancer activities, but its clinical application has been limited due to its significant cardiotoxicity and nephrotoxicity. Ahmed et al. (2020) found that membrane lipid peroxidation in renal tissue increased significantly, and the levels of GSH and GPX4 decreased in mice after treated with DOX, indicating that DOX reduced the resistance of renal tissue to oxidative stress (Ahmed et al., 2020). Furthermore, Tadokoro et al. (2020) showed that DOX downregulated GPX4 and induced excessive lipid peroxidation through DOX-Fe2+ complex in mitochondria, leading to mitochondria-dependent ferroptosis. They also noticed that DOX upregulated HO-1 in an NRF2-dependent manner, leading to heme degradation and accumulation of non-heme iron.
Over the last decade, a great progress has been made in nano-targeted therapy for the treatment of cancer. For example, photodynamic therapy (PDT) involves delivering a photosensitizer to the tumor site and irradiating malign cells with the light of a specific wavelength to initiate a photochemical reaction. The cytotoxic ROS produced during PDT can destroy intracellular biological macromolecules through oxidative stress. A high concentration of ROS can lead to the opening of the mitochondrial membrane permeability transition pore (mPTP), causing a reduced the mitochondrial membrane potential, the release of cytochrome C, the triggering of the caspase cascade, and accelerate of cell apoptosis (Wang et al., 2021). However, due to the high intracellular oxygen consumption and the hypoxia of the tumor microenvironment (TME) caused by the distortion of tumor blood vessels, the production of ROS is too low, thus the application of PDT in clinical work is still very limited (Zhu et al., 2019).
Recently, a novel nanoparticle called SRF@FeIIITA (SFT) has shown the ability to inhibit tumor progression. By loading methylene blue (MB) into SFT through depositing tannic acid (TA) and Fe3+ onto SRF nanocrystal, a large amount of ROS can be generated during ferroptosis to support oxygen consumption in PDT (Liu et al., 2018). Nanomaterials can also induce ferroptosis through GSH metabolism. The arginine-capped manganese silicate nanobubbles (AMSNs) were developed with high efficiency of GSH depletion, based on the high ratio of surface area to volume. Further in vivo study indicated AMSNs could help suppress Huh7 xenograft tumor growth by downregulating GPX4 (Jiang et al., 2020). In addition, Guan et al. created a new iron-based nanomaterial in which sorafenib (SRF) and ultrasmall SPIO nanoparticles were loaded into the mesopores and onto the surface of MPDA NPs to form SRF@MPDA-SPIO nanoparticles. SPIO loading endowed the system with an iron-supply for ferroptosis and made the system MRI-visible. Furthermore, the heat generated by MPDA NPs upon laser irradiation offered a moderate PTT to boost the ferroptosis effect (Guan et al., 2020). Therefore, nanomaterials have good application prospects in ferroptosis, which deserves further explorations (Table 2).
Since the concept of ferroptosis was proposed in 2012, a large number of experimental studies have continuously updated people’s understanding of ferroptosis, including biochemical basis, signal pathways, and regulatory reagents. Moreover, in the study of drug-resistant tumor cells, ferroptosis has shown an excellent ability. Piperlongumine, artemisinins and other traditional Chinese medicine ingredients have also been found to induce ferroptosis, and some can even enhance the efficacy of chemotherapy drugs, which shows a promising method for malignant tumor treatment. However, we cannot deny that there are still some issues worthy of consideration, such as the development of targeted drugs that are more specific to the tumor area while minimizing systemic side effects. Moreover, in the future, there may be tumor cells that are resistant to ferroptosis. Is there a completely dominant ferroptosis inhibitor? These problems will prompt us to further research on ferroptosis and create ne
Authors’ Contribution: All authors made substantial contributions to conception and design and picture production; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was supported by the Innovation and Entrepreneurship Training Scheme for university students Program (No. C2021114631) from West China School/Hospital of Stomatology Sichuan University.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ahmed OM, Galaly SR, Raslan MMM (2020). Thyme oil and thymol abrogate doxorubicin-induced nephrotoxicity and cardiotoxicity in Wistar rats via repression of oxidative stress and enhancement of antioxidant defense mechanisms. BIOCELL 44: 41–53. DOI 10.32604/biocell.2020.08157. [Google Scholar] [CrossRef]
Bai G, Gao Y, Liu S, Shui S, Liu G (2021). pH-dependent rearrangement determines the iron-activation and antitumor activity of artemisinins. Free Radical Biology & Medicine 163: 234–242. DOI 10.1016/j.freeradbiomed.2020.12.024. [Google Scholar] [CrossRef]
Bains M, Hall ED (2012). Antioxidant therapies in traumatic brain and spinal cord injury. Biochimica et Biophysica Acta 1822: 675–684. DOI 10.1016/j.bbadis.2011.10.017. [Google Scholar] [CrossRef]
Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F (2020). Ferroptosis and cancer: Mitochondria meet the “iron maiden” cell death. Cells 9: 1505. DOI 10.3390/cells9061505. [Google Scholar] [CrossRef]
Bebber CM, Müller F, Prieto Clemente L, Weber J, von Karstedt S (2020). Ferroptosis in cancer cell biology. Cancers 12: 164. DOI 10.3390/cancers12010164. [Google Scholar] [CrossRef]
Boettler U, Sommerfeld K, Volz N, Pahlke G, Teller N, Somoza V, Lang R, Hofmann T, Marko D (2011). Coffee constituents as modulators of Nrf2 nuclear translocation and ARE (EpRE)-dependent gene expression. Journal of Nutritional Biochemistry 22: 426–440. DOI 10.1016/j.jnutbio.2010.03.011. [Google Scholar] [CrossRef]
Brown CW, Amante JJ, Goel HL, Mercurio AM (2017). The α6β4 integrin promotes resistance to ferroptosis. The Journal of Cell Biology 216: 4287–4297. [Google Scholar]
Capelletti MM, Manceau H, Puy H, Peoc’h K (2020). Ferroptosis in liver diseases: An overview. International Journal of Molecular Sciences 21: 4908. [Google Scholar]
Chen GQ, Benthani FA, Wu J, Liang D, Bian ZX, Jiang X (2020a). Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death and Differentiation 27: 242–254. [Google Scholar]
Chen P, Wu Q, Feng J, Yan L, Sun Y et al. (2020b). Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduction and Targeted Therapy 5: 51. [Google Scholar]
Chen W, Jiang L, Hu Y, Tang N, Liang N, Li XF, Chen YW, Qin H, Wu L (2021). Ferritin reduction is essential for cerebral ischemia-induced hippocampal neuronal death through p53/SLC7A11-mediated ferroptosis. Brain Research 1752: 147216. [Google Scholar]
Chen Y, Mi Y, Zhang X, Ma Q, Song Y et al. (2019). Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. Journal of Experimental & Clinical Cancer Research 38: 402. DOI 10.1186/s13046-019-1413-7. [Google Scholar] [CrossRef]
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM et al. (2012). Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072. DOI 10.1016/j.cell.2012.03.042. [Google Scholar] [CrossRef]
Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED et al. (2014). Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3: e02523. DOI 10.7554/eLife.02523. [Google Scholar] [CrossRef]
Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, Superti-Furga G, Stockwell BR (2015). Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chemical Biology 10: 1604–1609. DOI 10.1021/acschembio.5b00245. [Google Scholar] [CrossRef]
Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC et al. (2019). FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575: 693–698. DOI 10.1038/s41586-019-1707-0. [Google Scholar] [CrossRef]
Efferth T (2017). From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Seminars in Cancer Biology 46: 65–83. [Google Scholar]
Epperly MW, Fisher R, Zhang X, Hou W, Shields D, Wipf P, Wang H, Thermozier S, Greenberger JS (2020). Fanconi anemia mouse genotype-specific mitigation of total body irradiation by GS-nitroxide JP4-039. Vivo 34: 33–38. [Google Scholar]
Forcina GC, Dixon SJ (2019). GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 19: e1800311. [Google Scholar]
Gai C, Yu M, Li Z, Wang Y, Ding D, Zheng J, Lv S, Zhang W, Li W (2020). Acetaminophen sensitizing erastin-induced ferroptosis via modulation of Nrf2/heme oxygenase-1 signaling pathway in non-small-cell lung cancer. Journal of Cellular Physiology 235: 3329–3339. [Google Scholar]
Gao H, Bai Y, Jia Y, Zhao Y, Kang R, Tang D, Dai E (2018). Ferroptosis is a lysosomal cell death process. Biochemical and Biophysical Research Communications 503: 1550–1556. [Google Scholar]
Ghoochani A, Hsu EC, Aslan M, Rice MA, Nguyen HM, Brooks JD, Corey E, Paulmurugan R, Stoyanova T (2021). Ferroptosis inducers are a novel therapeutic approach for advanced prostate cancer. Cancer Research 81: 1583–1594. DOI 10.1158/0008-5472.CAN-20-3477. [Google Scholar] [CrossRef]
Guan Q, Guo R, Huang S, Zhang F, Liu J, Wang Z, Yang X, Shuai X, Cao Z (2020). Mesoporous polydopamine carrying sorafenib and SPIO nanoparticles for MRI-guided ferroptosis cancer therapy. Journal of Controlled Release 320: 392–403. DOI 10.1016/j.jconrel.2020.01.048. [Google Scholar] [CrossRef]
Hałas-Wiśniewska M, Zielińska W, Izdebska M, Grzanka A (2020). The synergistic effect of piperlongumine and sanguinarine on the non-small lung cancer. Molecules 25: 3045. DOI 10.3390/molecules25133045. [Google Scholar] [CrossRef]
Hassannia B, Wiernicki B, Ingold I, Qu F, van Herck S et al. (2018). Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. The Journal of Clinical Investigation 128: 3341–3355. DOI 10.1172/JCI99032. [Google Scholar] [CrossRef]
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ III, Kang R, Tang D (2016). Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12: 1425–1428. DOI 10.1080/15548627.2016.1187366. [Google Scholar] [CrossRef]
Hou WT, Xia HW, Zhou S, Fan ZH, Xu HJ, Gong QY, Nie YZ, Tang QL, Bi F (2019). The MEK inhibitors enhance the efficacy of sorafenib against hepatocellular carcinoma cells through reducing p-ERK rebound. Translational Cancer Research 8: 1224–1232. DOI 10.21037/tcr.2019.07.11. [Google Scholar] [CrossRef]
Hu N, Bai L, Dai E, Han L, Kang R, Li H, Tang D (2021). Pirin is a nuclear redox-sensitive modulator of autophagy-dependent ferroptosis. Biochemical and Biophysical Research Communications 536: 100–106. DOI 10.1016/j.bbrc.2020.12.066. [Google Scholar] [CrossRef]
Hua H, Zhu Y, Song YH (2018). Ruscogenin suppressed the hepatocellular carcinoma metastasis via PI3K/Akt/mTOR signaling pathway. Biomedicine & Pharmacotherapy 101: 115–122. DOI 10.1016/j.biopha.2018.02.031. [Google Scholar] [CrossRef]
Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J et al. (2013). Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Molecular Cell 51: 618–631. DOI 10.1016/j.molcel.2013.08.003. [Google Scholar] [CrossRef]
Ito F, Nishiyama T, Shi L, Mori M, Hirayama T, Nagasawa H, Yasui H, Toyokuni S (2016). Contrasting intra- and extracellular distribution of catalytic ferrous iron in ovalbumin-induced peritonitis. Biochemical and Biophysical Research Communications 476: 600–606. DOI 10.1016/j.bbrc.2016.06.003. [Google Scholar] [CrossRef]
Jiang M, Qiao M, Zhao C, Deng J, Li X, Zhou C (2020). Targeting ferroptosis for cancer therapy: Exploring novel strategies from its mechanisms and role in cancers. Translational Lung Cancer Research 9: 1569–1584. DOI 10.21037/tlcr-20-341. [Google Scholar] [CrossRef]
Jiang X, Stockwell BR, Conrad M (2021). Ferroptosis: Mechanisms, biology and role in disease. Nature Reviews Molecular Cell Biology 22: 266–282. DOI 10.1038/s41580-020-00324-8. [Google Scholar] [CrossRef]
Kagan VE, Mao G, Qu F, Angeli JP, Doll S et al. (2017). Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nature Chemical Biology 13: 81–90. DOI 10.1038/nchembio.2238. [Google Scholar] [CrossRef]
Kamatani N, Fujimori S, Hada T, Hosoya T, Kohri K, Nakamura T, Ueda T, Yamamoto T, Yamanaka H, Matsuzawa Y (2011). Placebo-controlled, double-blind study of the non-purine-selective xanthine oxidase inhibitor Febuxostat (TMX-67) in patients with hyperuricemia including those with gout in Japan: Phase 3 clinical study. Journal of Clinical Rheumatology 17: S19–26. DOI 10.1097/RHU.0b013e31821d36de. [Google Scholar] [CrossRef]
Kang R, Tang D (2017). Autophagy and ferroptosis—What’s the connection? Current Pathobiology Reports 5: 153–159. DOI 10.1007/s40139-017-0139-5. [Google Scholar] [CrossRef]
Kang R, Zhu S, Zeh HJ, Klionsky DJ, Tang D (2018). BECN1 is a new driver of ferroptosis. Autophagy 14: 2173–2175. DOI 10.1080/15548627.2018.1513758. [Google Scholar] [CrossRef]
Krajka-Kuźniak V, Paluszczak J, Baer-Dubowska W (2017). The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacological Reports 69: 393–402. DOI 10.1016/j.pharep.2016.12.011. [Google Scholar] [CrossRef]
Kroemer G, Jäättelä M (2005). Lysosomes and autophagy in cell death control. Nature Reviews Cancer 5: 886–897. DOI 10.1038/nrc1738. [Google Scholar] [CrossRef]
Lee Y, Park BH, Bae EJ (2016). Compound C inhibits macrophage chemotaxis through an AMPK-independent mechanism. Biochemical and Biophysical Research Communications 469: 515–520. DOI 10.1016/j.bbrc.2015.12.015. [Google Scholar] [CrossRef]
Lee YS, Lee DH, Choudry HA, Bartlett DL, Lee YJ (2018). Ferroptosis-induced endoplasmic reticulum stress: Cross-talk between ferroptosis and apoptosis. Molecular Cancer Research 16: 1073–1076. DOI 10.1158/1541-7786.MCR-18-0055. [Google Scholar] [CrossRef]
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G (2020). Ferroptosis: Past, present and future. Cell Death & Disease 11: 88. DOI 10.1038/s41419-020-2298-2. [Google Scholar] [CrossRef]
Li Z, Wu X, Wang W, Gai C, Zhang W, Li W, Ding D (2021). Fe(II) and tannic acid-cloaked MOF as carrier of artemisinin for supply of ferrous ions to enhance treatment of triple-negative breast cancer. Nanoscale Research Letters 16: 37. DOI 10.1186/s11671-021-03497-z. [Google Scholar] [CrossRef]
Liang C, Zhang X, Yang M, Dong X (2019). Recent progress in ferroptosis inducers for cancer therapy. Advanced Materials 31: e1904197. DOI 10.1002/adma.201904197. [Google Scholar] [CrossRef]
Lin Q, Weis S, Yang G, Weng YH, Helston R et al. (2007). Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. The Journal of Biological Chemistry 282: 20621–20633. DOI 10.1074/jbc.M607954200. [Google Scholar] [CrossRef]
Lin X, Ping J, Wen Y, Wu Y (2020). The mechanism of ferroptosis and applications in tumor treatment. Frontiers in Pharmacology 11: 1061. DOI 10.3389/fphar.2020.01061. [Google Scholar] [CrossRef]
Liu C, Wang F, Wang B, Wu T, Wang Y (2020). Pseudolaric acid B induces apoptosis in human rhabdomyosarcoma RD cells. Oncology Letters 20: 358. [Google Scholar]
Liu ML, Sun D, Li T, Chen H (2017). A systematic review of the immune-regulating and anticancer activities of pseudolaric acid B. Frontiers in Pharmacology 8: 394. [Google Scholar]
Liu T, Liu W, Zhang M, Yu W, Gao F, Li C, Wang SB, Feng J, Zhang XZ (2018). Ferrous-supply-regeneration nanoengineering for cancer-cell-specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano 12: 12181–12192. [Google Scholar]
Liu XJ, Lv YF, Cui WZ, Li Y, Liu Y, Xue YT, Dong F (2021). Icariin inhibits hypoxia/reoxygenation-induced ferroptosis of cardiomyocytes via regulation of the Nrf2/HO-1 signaling pathway. FEBS OpenBio 11: 2966–2976. [Google Scholar]
Lou JS, Zhao LP, Huang ZH, Chen XY, Xu JT, Tai WC, Tsim KWK, Chen YT, Xie T (2021). Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine 80: 153370. [Google Scholar]
Lv F, Deng M, Bai J, Zou D, Wang J, Li H, Zhang Y, Ji X (2020). Piperlongumine inhibits head and neck squamous cell carcinoma proliferation by docking to Akt. Phytotherapy Research 34: 3345–3358. DOI 10.1002/ptr.6788. [Google Scholar] [CrossRef]
Madsen RR (2020). PI3K in stemness regulation: From development to cancer. Biochemical Society Transactions 48: 301–315. DOI 10.1042/BST20190778. [Google Scholar] [CrossRef]
Magtanong L, Ko PJ, To M, Cao JY, Forcina GC et al. (2019). Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chemical Biology 26: 420–432. DOI 10.1016/j.chembiol.2018.11.016. [Google Scholar] [CrossRef]
Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC (2014). Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509: 105–109. DOI 10.1038/nature13148. [Google Scholar] [CrossRef]
Miotto G, Rossetto M, Di Paolo ML, Orian L, Venerando R et al. (2020). Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biology 28: 101328. DOI 10.1016/j.redox.2019.101328. [Google Scholar] [CrossRef]
Nishida N, Yasui H, Nagane M, Yamamori T, Inanami O (2014). 3-Methyl pyruvate enhances radiosensitivity through increasing mitochondria-derived reactive oxygen species in tumor cell lines. Journal of Radiation Research 55: 455–463. DOI 10.1093/jrr/rrt142. [Google Scholar] [CrossRef]
Niu B, Lei X, Xu Q, Ju Y, Xu D et al. (2021). Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell Biology and Toxicology 5: 58. DOI 10.1007/s10565-021-09624-x. [Google Scholar] [CrossRef]
Ou Y, Wang SJ, Li D, Chu B, Gu W (2016). Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proceedings of the National Academy of Sciences of the United States of America 113: E6806–E6812. DOI 10.1073/pnas.1607152113. [Google Scholar] [CrossRef]
Plosker GL, Croom KF (2005). Sulfasalazine: A review of its use in the management of rheumatoid arthritis. Drugs 65: 1825–1849. DOI 10.2165/00003495-200565130-00008. [Google Scholar] [CrossRef]
Polyzos A, Holt A, Brown C, Cosme C, Wipf P, Gomez-Marin A, Castro MR, Ayala-Peña S, McMurray CT (2016). Mitochondrial targeting of XJB-5-131 attenuates or improves pathophysiology in HdhQ150 animals with well-developed disease phenotypes. Human Molecular Genetics 25: 1792–1802. DOI 10.1093/hmg/ddw051. [Google Scholar] [CrossRef]
Rawat L, Hegde H, Hoti SL, Nayak V (2020). Piperlongumine induces ROS mediated cell death and synergizes paclitaxel in human intestinal cancer cells. Biomedicine & Pharmacotherapy 128: 110243. [Google Scholar]
Reis A, Spickett CM (2012). Chemistry of phospholipid oxidation. Biochimica et Biophysica Acta 1818: 2374–2387. [Google Scholar]
Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL et al. (2017). O2∙− and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell 31: 487–500. [Google Scholar]
Seber S, Sirin DY, Yetisyigit T, Bilgen T (2020). Piperlongumine increases the apoptotic effect of doxorubicin and paclitaxel in a cervical cancer cell line. Nigerian Journal of Clinical Practice 23: 386–391. [Google Scholar]
Shi L, Ito F, Wang Y, Okazaki Y, Tanaka H et al. (2017). Non-thermal plasma induces a stress response in mesothelioma cells resulting in increased endocytosis, lysosome biogenesis and autophagy. Free Radical Biology & Medicine 108: 904–917. [Google Scholar]
Shin D, Kim EH, Lee J, Roh JL (2018). Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radical Biology & Medicine 129: 454–462. DOI 10.1016/j.freeradbiomed.2018.10.426. [Google Scholar] [CrossRef]
Song X, Xie Y, Kang R, Hou W, Sun X, Epperly MW, Greenberger JS, Tang D (2016). FANCD2 protects against bone marrow injury from ferroptosis. Biochemical and Biophysical Research Communications 480: 443–449. DOI 10.1016/j.bbrc.2016.10.068. [Google Scholar] [CrossRef]
Song X, Zhu S, Chen P, Hou W, Wen Q et al. (2018). AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Xc-activity. Current Biology 28: 2388–2399. DOI 10.1016/j.cub.2018.05.094. [Google Scholar] [CrossRef]
Song Z, Xiang X, Li J, Deng J, Fang Z, Zhang L, Xiong J (2020). Ruscogenin induces ferroptosis in pancreatic cancer cells. Oncology Reports 43: 516–524. [Google Scholar]
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M et al. (2017). Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171: 273–285. DOI 10.1016/j.cell.2017.09.021. [Google Scholar] [CrossRef]
Sugiyama A, Ohta T, Obata M, Takahashi K, Seino M, Nagase S (2020). xCT inhibitor sulfasalazine depletes paclitaxel-resistant tumor cells through ferroptosis in uterine serous carcinoma. Oncology Letters 3: 2689–2700. DOI 10.3892/ol.2020.11813. [Google Scholar] [CrossRef]
Sui X, Zhang R, Liu S, Duan T, Zhai L et al. (2018). RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Frontiers in Pharmacology 9: 1371. DOI 10.3389/fphar.2018.01371. [Google Scholar] [CrossRef]
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D (2016). Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63: 173–184. DOI 10.1002/hep.28251. [Google Scholar] [CrossRef]
Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K, Tsutsui H (2020). Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight 5: e132747. DOI 10.1172/jci.insight.132747. [Google Scholar] [CrossRef]
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G (2019). The molecular machinery of regulated cell death. Cell Research 29: 347–364. DOI 10.1038/s41422-019-0164-5. [Google Scholar] [CrossRef]
Toyokuni S, Yanatori I, Kong Y, Zheng H, Motooka Y, Jiang L (2020). Ferroptosis at the crossroads of infection, aging and cancer. Cancer Science 111: 2665–2671. DOI 10.1111/cas.14496. [Google Scholar] [CrossRef]
Tripathi SK, Biswal BK (2020). Piperlongumine, a potent anticancer phytotherapeutic: Perspectives on contemporary status and future possibilities as an anticancer agent. Pharmacological Research 156: 104772. DOI 10.1016/j.phrs.2020.104772. [Google Scholar] [CrossRef]
Trujillo-Alonso V, Pratt EC, Zong H, Lara-Martinez A, Kaittanis C et al. (2019). FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nature Nanotechnology 14: 616–622. DOI 10.1038/s41565-019-0406-1. [Google Scholar] [CrossRef]
Vítek L, Schwertner HA (2007). The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Advances in Clinical Chemistry 43: 1–57. DOI 10.1016/S0065-2423(06)43001-8. [Google Scholar] [CrossRef]
Wang H, Liu C, Zhao Y, Gao G (2020). Mitochondria regulation in ferroptosis. European Journal of Cell Biology 99: 151058. DOI 10.1016/j.ejcb.2019.151058. [Google Scholar] [CrossRef]
Wang Z, Ding Y, Wang X, Lu S, Wang C et al. (2018). Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Letters 428: 21–33. DOI 10.1016/j.canlet.2018.04.021. [Google Scholar] [CrossRef]
Wang Z, Peng H, Shi W, Gan L, Zhong L et al. (2021). Application of photodynamic therapy in cancer: Challenges and advancements. BIOCELL 45: 489–500. DOI 10.32604/biocell.2021.014439. [Google Scholar] [CrossRef]
Wang ZX, Ma J, Li XY, Wu Y, Shi H, Chen Y, Lu G, Shen HM, Lu GD, Zhou J (2021). Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. British Journal of Pharmacology 178: 1133–1148. DOI 10.1111/bph.15350. [Google Scholar] [CrossRef]
Wei R, Zhao Y, Wang J, Yang X, Li S et al. (2021). Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. International Journal of Biological Sciences 17: 2703–2717. DOI 10.7150/ijbs.59404. [Google Scholar] [CrossRef]
Wei S, Qiu T, Yao X, Wang N, Jiang L et al. (2020). Arsenic induces pancreatic dysfunction and ferroptosis via mitochondrial ROS-autophagy-lysosomal pathway. Journal of Hazardous Materials 384: 121390. DOI 10.1016/j.jhazmat.2019.121390. [Google Scholar] [CrossRef]
Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M, Shan B, Pan H, Yuan J (2019). Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proceedings of the National Academy of Sciences of the United States of America 116: 2996–3005. DOI 10.1073/pnas.1819728116. [Google Scholar] [CrossRef]
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D (2016). Ferroptosis: Process and function. Cell Death and Differentiation 23: 369–379. DOI 10.1038/cdd.2015.158. [Google Scholar] [CrossRef]
Xie Y, Zhu S, Song X, Sun X, Fan Y et al. (2017). The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Reports 20: 1692–1704. DOI 10.1016/j.celrep.2017.07.055. [Google Scholar] [CrossRef]
Xie Z, Hou H, Luo D, An R, Zhao Y, Qiu C (2021). ROS-dependent lipid peroxidation and reliant antioxidant ferroptosis-suppressor-protein 1 in rheumatoid arthritis: A covert clue for potential therapy. Inflammation 44: 35–47. DOI 10.1007/s10753-020-01338-2. [Google Scholar] [CrossRef]
Yamaguchi Y, Kasukabe T, Kumakura S (2018). Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. International Journal of Oncology 52: 1011–1022. DOI 10.3892/ijo.2018.4259. [Google Scholar] [CrossRef]
Yang L, Hu Y, Zhou G, Chen Q, Song Z (2020). Erianin suppresses hepatocellular carcinoma cells through down-regulation of PI3K/AKT, p38 and ERK MAPK signaling pathways. Bioscience Reports 40: BSR20193137. DOI 10.1042/BSR20193137. [Google Scholar] [CrossRef]
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proceedings of the National Academy of Sciences of the United States of America 113: E4966–E4975. DOI 10.1073/pnas.1603244113. [Google Scholar] [CrossRef]
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R et al. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell 156: 317–331. DOI 10.1016/j.cell.2013.12.010. [Google Scholar] [CrossRef]
Yi J, Zhu J, Wu J, Thompson CB, Jiang X (2020). Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proceedings of the National Academy of Sciences of the United States of America 117: 31189–31197. DOI 10.1073/pnas.2017152117. [Google Scholar] [CrossRef]
Zhao Z, Wu J, Xu H, Zhou C, Han B et al. (2020). XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death & Disease 11: 629. DOI 10.1038/s41419-020-02871-6. [Google Scholar] [CrossRef]
Zhu T, Shi L, Yu C, Dong Y, Qiu F, Shen L, Qian Q, Zhou G, Zhu X (2019). Ferroptosis promotes photodynamic therapy: Supramolecular photosensitizer-inducer nanodrug for enhanced cancer treatment. Theranostics 9: 3293–3307. DOI 10.7150/thno.32867. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |