DOI:10.32604/biocell.2022.016564

| BIOCELL DOI:10.32604/biocell.2022.016564 |  |
| Article |
Intrauterine high androgen promotes obesity of the offspring of rats with polycystic ovarian syndrome via activating macrophage-angiogenesis-related androgen signaling
1Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, 200232, China
2The Obstetrics and Gynecology Hospital of Fudan University, Shanghai, 200232, China
*Address correspondence to: Chunlin Su, ceilingsu@163.com
#These authors contributed equally to this work
Received: 23 March 2021; Accepted: 02 June 2021
Abstract: The development of polycystic ovary syndrome (PCOS) is closely related to the chronic inflammatory and obese. Recent studies have found macrophages regulate the chronic inflammation and adipose tissue remodelling, but the underlying mechanisms have not been clarified. In this study, we established a model of PCOS in the offspring rats by high androgen exposure during late pregnancy in parental and established a female rat macrophage eliminating model by rejection of clodronate liposome. Then, the offspring rat macrophage phenotype in offspring female rat adipose tissue, and levels of testosterone, angiogenic factors (PDGF and VEGF) and inflammatory factors (TNF-α and MCP-1) were investigated. By coculture of RAW264.7 macrophage with adipocytes or C166 endothelial cells (ECs), the mobility of adipocytes, and the ECs function with associated signalling pathway were detected by using of androgen inhibitor Apalutamide, NF-κB inhibitor JSH-23 and ERK1/2 inhibitor LY3214996. It was found that high androgen exposure during late pregnancy led to increased testosterone levels and overweight and obesity, increased size and reduced number of subcutaneous and intra-abdominal adipocytes, and increased secretion of TNF-α and MCP-1 in female rats in the offspring. Eliminating macrophages significantly increased adipocytes and angiogenesis in offspring of rats with intrauterine high androgen, and reduced TNF-α and MCP-1. Macrophages promoted mobility of adipocytes, and inhibited proliferation, migration, tube formation of ECs under hyperandrogenic condition, which were significantly inhibited by Apalutamide, JSH-23 and LY3214996. Thus, intrauterine high androgen promotes obesity of the offspring of rats with polycystic ovarian syndrome through increasing M1 differentiation of pro-inflammatory macrophages and activating VEGF-related angiogenesis via androgen/NF-κB/ERK1/2 signalling pathway.
Keywords: Polycystic ovary syndrome; Androgen; Adipose tissue; Angiogenesis; Macrophages
Polycystic ovary syndrome (PCOS) is one of the most common reproductive endocrine and metabolic disorders in women. About 4–12% of adolescent and childbearing women suffer from PCOS. Long-term anovulation and hyperandrogenism are important endocrine and metabolic features of PCOS (Trikudanathan, 2015). Hyperandrogenemia (HA) generally refers to the increased content, production and activity of serum androgens such as testosterone (testosterone, T), free testosterone (FT) and androstenedione (DTA) (Brydon, 2011). The pathogenesis of PCOS is very complex. PCOS may cause a fetal disease (Raperport and Homburg, 2019), pregnant rats exposed to high doses of androgen shows abnormal ovarian cycle of female offspring, multiple cystic ovarian follicles of different sizes, obesity, and abnormal glucose and lipid metabolism (Parlee et al., 2014; Scheele and Wolfrum, 2020), suggesting intrauterine hyperandrogen environment plays an important role in the occurrence of obesity in offspring of rats with PCOS (Xu et al., 2019).
Overweight or obesity is a common symptom in PCOS patients, PCOS patients with combined obesity have more severe endocrine metabolic disorders and are at increased risk for long-term complications such as cardiovascular disease, hypertension, endometrial cancer, and diabetes mellitus (Legro, 2012). The onset of obesity is accompanied by a remodelling of the adipose tissue structure (Sun et al., 2011). Adipose tissue remodelling is due to the adipocytes size and cell number increase (Parlee et al., 2014). When obesity is occurring, due to the increasing cell size of adipose tissue, the growth rate of adipocytes exceeds the rate of neovascularization in adipose tissue, and the remodelling of adipose tissue is affected, the metabolism of the excess adipose tissue, obese patients have increased oxygen consumption and carbon dioxide production (Xu et al., 2019), which lead to cell lysis and apoptosis, followed by macrophages in a ring around the apoptotic adipocytes, leading to increased secretion of inflammatory factors, and the continuous increase of inflammatory factors exacerbates the inflammatory response, resulting in a chronic low inflammatory state. Chronic low-grade inflammation is a characteristic feature in patients with PCOS, which cross-talk with insulin signal transduction pathway through inflammatory signal transduction pathway to induce insulin resistance, which is the initial factor and central link of insulin resistance (Chiaverina et al., 2019). The level of inflammatory factors in patients with PCOS is often positively correlated with the level of androgen (Spiller et al., 2014; van Rooijen and Sanders, 1994). The levels of inflammatory factors such as TNF-α, IL-6 and MCP-1 in serum of patients with PCOS are increased, which is positively correlated with the concentration of testosterone in serum (Escobar-Morreale, 2018; Otto-Buczkowska et al., 2018).
Adipose tissue is heterogeneous and includes pre-adipocytes, endothelial cells, vascular smooth muscle cells, and macrophages, in addition to mature adipocytes (Brydon, 2011). In contrast to conventional inflammatory cells, adipocytes can also secrete numerous inflammatory factors and influence the inflammatory response and insulin resistance through endocrine or paracrine pathways (Scheele and Wolfrum, 2020). In adipose tissue, inflammation inhibits adipocyte expansion and differentiation, alters adipocyte endocrine secretion, and induces extracellular matrix remodelling (Khan et al., 2009). Macrophages play an important role in chronic low-grade inflammation (Hong et al., 2020; Li et al., 2019; Lima et al., 2018). During the development of PCOS, it has been found that hyperandrogenic conditions may lead to the recruitment macrophages under the influence of chemokines (Lima et al., 2018). In adipose tissue, M1 macrophages secrete pro-inflammatory cytokines necrosis factor alpha (TNF-α), monocyte chemoattractant factor 1 (MCP-1), interleukin 1 (IL-1), and interleukin 6 (IL-6) to promote insulin resistance, while macrophage polarization activates anti-inflammatory M2 macrophages that participate in the maintenance of tissue homeostasis and contribute to adipose tissue remodelling (Sun et al., 2011; Zhao et al., 2020). In obesity, M1 macrophages in adipose tissue was increased, which induced secretion of pro-inflammatory cytokines and in turn to facilitate the obesity (Otto-Buczkowska et al., 2018; Scheele and Wolfrum, 2020).
During the development of adipose tissue, angiogenesis often precedes the formation of adipose tissue. It has been found that the process of adipose tissue proliferation is accompanied by the neovascularization, expansion, and remodelling of the vascular system, and the process involves vascular endothelial cell and preadipocyte interactions. Angiogenesis refers to the generation of blood vessels in a budding or non-budding form through endothelial cell proliferation and differentiation on the basis of pre-existing blood vessels. Angiogenesis refers to the generation of blood vessels through endothelial cell proliferation and differentiation on the basis of existing blood vessels (Kliche and Waltenberger, 2001). The hallmark of vessel maturation is the formation of basement membrane (Jain, 2003), mainly the recruitment of endothelial cells to pericytes, resulting in the formation of stable vessels surrounded by pericytes (Chiaverina et al., 2019). Vascular endothelial growth factor (VEGF) is the factor known to induce neovascularization, but VEGF alone can induce neovascularization but cannot successfully recruit pericytes (Lane and Kelman, 2003), resulting in immature neovascularization. VEGF interacts with other factors such as PDGF to recruit pericytes and produce basement membrane, which can promote neovascular stability (Spiller et al., 2014). During the development of obesity, the production of PDGF may decrease due to the relative decrease of preadipocytes due to continuous differentiation into mature adipocytes, while the hypertrophic adipose tissue needs more neovascularization, and the infiltration of macrophages into adipose tissue can compensatingly increase the secretion of PDGF and promote neovascularization. However, at the same time, the inflammatory response in adipose tissue is also progressively increased, resulting in obese patients in a chronic low-grade inflammation status.
In this study, we hypothesized that macrophages may play an important role in adipose remodelling in PCOS patient. To validate our idea, we aimed to examine the adipose remodelling, sex hormone and inflammatory factor expression in the offspring female rats at different periods by establishing a rat model exposed to high androgen in late pregnancy; and we employed an in vitro macrophage cell and adipocyte and vascular endothelial cell co-culture model.
Testosterone injection in pregnancy SD rats
All SD rats were kept under standard laboratory conditions at room temperature 22 ± 2°C, humidity 55 ± 5%, 12 h light-dark cycles (light, 6:00–18:00; dark, 18:00–6:00), fed unrestrictedly with standard laboratory rat pellets chow and water. The SD rats were purchased from Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine. All animal care and handling were in accordance with the guidelines for the care and use of laboratory animals published by the National Research Institute of the United States and this study was approved by the ethics committee of Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine. After 1 week of acclimatization in the above environment, the SD rats females were mated with males (5 females with 1 male per cage), and the mating was considered successful on the second day (Gestational Day 0, GD0). In the late pregnancy (G16–G20), 5 Sprague–Dawley (SD) rats were injected subcutaneously with testosterone solution (1 mg/100 g body weight) per days, and the control group was injected with the same volume of normal saline in the same day. For evaluation of the effect of macrophage on adipose tissue remodelling, clodronate liposomes (0.15 mL/100 g body weight) or PBS liposomes (the same volume) were injected into the offspring by intraperitoneal injection. The injections were started on the first post-operative day after birth and then once a week. The body weight of pups was monitored regularly. Neonatal, adolescent and adult and were killed at postnatal day PD4, PD50, PD100. At least three offspring female rats were used in each experiment. The rats were anaesthetized with an intravenous injection of trichloroacetaldehyde hydrate (0.3 mL/kg, SigmaAldrich, USA), and then sacrificed by standard cervical dislocation method. Subcutaneous and intra-abdominal adipose tissue were isolated for further analysis.
The subcutaneous and intra-abdominal adipose tissue of neonatal, adolescent, and adult female rats were fixed with paraformaldehyde and embedded in paraffin. Paraffin sections were routinely dewaxed (xylene I for 10 min and xylene II for 10 min), hydration (anhydrous alcohol I for 5 min, anhydrous alcohol II for 5 min, 95% alcohol for 5 min, 80% alcohol for 5 min, 70% alcohol for 5 min) and then washed with distilled water for 5 min. The slices were stained with hematoxylin for 5 min, and washed with tap water, color separation with 1% hydrochloric acid alcohol for 5 s, rinsed with tap water, and dipped with 0.6% ammonia, and then rinsed with tap water. The slices were dyed for 3 min in eosin ethanol solution; dehydrated by gradient alcohol, cleared in xylene, sealed by neutral gum, and adipose tissue was observed under inverted microscope (Leica DMI3000 M, Leica, Wetzlar, Germany). Three experimental replicates were performed (n = 5).
The subcutaneous and intra-abdominal adipose tissue of neonatal, adolescent, and adult female rats were taken for frozen sections; the slices were fully washed with distilled water; the oil red dilution (Sigma-Aldrich) (6 mL oil red saturated solution added with 4 mL distilled water) was dyed for 10–15 min to avoid light and seal; 60% ethanol differentiates to clear stroma under ethanol microscope. Fully washed with distilled water three times, sealed with glycerol or glycerin gelatin, and observed the staining of adipose tissue under inverted microscope (Leica DMI3000 M, Leica, Wetzlar, Germany). Three experimental replicates were performed (n = 5).
The antigen used was diluted with 0.01 M PBST (0.2% Tween-20) to an appropriate concentration, 100 μL antigen diluent solution was added to each well, placed at 37°C for 4 h, and the liquid in the hole was discarded and then blocked with 5% BSA in PBST for 40 min at 37°C, and then each wells were washed with washing solution for 3 min, three times each; the diluted samples were added to the enzyme-labelled reaction hole, 100 μL per well, at 37°C, 40–60 min. Washed with detergent full hole for 3 min each time. According to the reference work dilution provided by the enzyme conjugate provider, carry out the dilution between 37°C and 30–60 min; add 100 μL per well and wash the same as before. 100 μL of substrate solution was added to each well, placed at 37°C and kept away from light for 3–5 min, and 50 μL of terminating solution was added to each well to terminate the reaction. Three experimental replicates were performed (5 offspring female rats per group). Human testosterone (Ab174569), TNF-α (Ab181421) and MCP-1 (Ab179886) Elisa kit were purchased from Abcam company (Cambridge, Britai, UK). The absorbance was recorded by a scanning multiwall spectrometer (Multiskan SkyHigh, Thermo, Courtaboeuf, France).
Establishment of cell co-culture system
The macrophages (RAW264.7) and adipocytes (3T3-L1) were purchased from Procell, Wuhan, China. Vascular endothelial cells (C166) were obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA). Macrophages (RAW264.7 cells), adipocytes (3T3-L1) and vascular endothelial cells (C166) growing in logarithmic phase were inoculated in Transwell chamber at 2 × 104 cells/mL and cultured at 37°C, 10% CO2 and 10 FBS for 12 h. The medium was removed, and serum-free medium was added for further culture for 24 h. Then the medium containing high concentration of testosterone (10−5 mol/L) was added to continue culture. Adipocytes or vascular endothelial cells were placed in the lower layer of the Transwell, and the chambers of macrophages were placed on the upper layer of the culture plate. The pore size of the filter membrane at the bottom of the Transwell chamber is 0.4 nm, and the giant follower cells cannot pass through, but the culture medium and cytokines between the two can pass through each other. Three experimental replicates were performed (5 offspring female rats per group).
Cell RAW264.7, 3T3-L1 and C166 cells were adjusted to 2 × 103 cells/mL in medium (DMEM with 10% FBS and 1% penicillin/streptomycin) and then inoculated in 96-well plate at 100 μL per well. Cell viability was detected by CCK-8 kit (Shanghai, China) at 0, 1, 2, 3, 4, and 5 days, respectively. After adding CCK8 reagent, for 2 h, the OD value of each hole at 450 nm was determined by Multifunctional enzyme marker. Three experimental replicates were performed.
The total RNA was extracted by Trizol method. Intra-abdominal and subcutaneous adipose tissue in neonatal, adolescent, and adult female rat offspring were grinded on ice. RNAs were transcribed into cDNA by reverse transcription system of TaKaRa reverse transcription kit (Japan). To evaluate the expression levels of PDGF, VEGF, CD16, CD206, CD31 and Actin, qRT-PCR was performed using SYBR green PCR Master mix kit (TaKaRa, Japan). All the relative expression levels were measured by using the 2−∆∆Ct method. The primers sequences were used in this study were list below: Actin (internal control), F: 5’-CATCACTGCCACCCAGAAGACTG-3’, R: 5’-ATGCCAGTGAGCTTCCCGTTCAG-3’; PDGF, F: 5’-TAACTCGAGAACCCACTGCTTACT-3’, R: 5’-CTAGAAGGCACACTCGAGGCTGAT-3’; VEGF, F: 5’-TCGAGTCCCTCACTGTTACCCTTG-3’, R: 5’-TAGATGACTTAAGCCTCAGCAGCA-3’; CD16, F: 5’- AGGGTGGCAGGCGGGTCTACC-3’, R: 5’-AGCTCGGCCCTTCGGCTTTAAA-3’; CD206, F: 5’-GGTGGCTACCGCTCCCGGCTT-3’, R: 5’-TGGTTAATGCCAATTAGCACTAT-3’; CD31, F:5’- ATGGACCTGCCCAGGGGCCTGG-3’, R: 5’-TCACTCCAGCACTTTGGGGGTG-3’.
Intra-abdominal and subcutaneous adipose tissue in neonatal, adolescent, and adult female rat offspring were grinded on ice. The total proteins were extracted by RIPA method (Beyotime, Shanghai, China). The proteins were quantified by BCA method (Beyotime, Shanghai, China). Total 50 μg proteins were loaded for electrophoresis on 12% SDS-polyacrylamide gel by TanonVE186 (Tanon, Shanghai, China), and then transferred the proteins to PVDF membranes (Merck Millipore, Billerica, MA) by TanonVE180 (Tanon, Shanghai, China). After transfer, the PVDF membranes was blocked at room temperature with 5% skimmed milk powder diluted in Tris-buffered saline/Tween 20 (TBST) for 1 h. After blocking, the membrane was incubated with primary antibody GSK-3 β (1:1000, Abcam), C/EBP α (1:500, Abcam), I κ B α (1:800, Abcam), I κ B α (1:500, Abcam), p-I κ B α (1:500, Abcam), p65 (1:1000, Abcam), p-p65 (1:500, Abcam), ERK1/2 (1:2000, Abcam), p-ERK1/2 (1:1000, Abcam) overnight at 4°C; and then the membrane was washed with TBST three times, each time 10 min. The membrane was incubated with secondary antibody HRP labelled sheep anti-rabbit IgG, 1:3000, Abcam) at room temperature for 1 h, and then the membrane was washed with TBST three times, each time 10 min. The blots were visualized by ECL reagent (Pierce). The western bolting results were analyzed by BIO-RAD ImageLab Software, Version 5.1.
A layer of Matrigel (diluted with serum-free medium at 1:4, 75 μL per well, incubated at 37°C for 30 min) was coated on the filter membrane of Corning chamber (pore diameter 8 μm). Macrophage and adipocyte co-culture system was treated with high concentration of testosterone (10−5 mol/L) in upper chamber foramen. RAW264.7 and 3T3-L1 adipocytes were used as control (4 × 105 cells/well, single cell suspension of serum-free medium). The plates were incubated at 37°C and 5% CO2 for 24 h. The upper chamber was removed with forceps and the cells were gently removed from the upper surface of the polycarbonate film with a wet cotton swab. The polycarbonate film was carefully removed from the upper chamber and the cells were fixed in pre-chilled 95% alcohol for 20 min. Then, the cells were stained with 0.1% crystal violet for 1 min, washed three times with PBS, and observed under the microscope. The cells were observed under inverted fluorescence microscope and photographed (×100), and the number of cells was calculated. Three experimental replicates were performed (n = 3).
First use a marker pen on the back of the 6-hole plate, compared it with a ruler, and draw a horizontal line evenly, about every 0.5–1 cm, across the hole. Each hole passed through at least 5 lines, and about 5 × 105 cells were seeded in each hole. The cell culture plate was gently shaken so that the cells were evenly spread at the bottom of the culture plate and continued to be cultured for 12 h. After the cells were adhered to the wall, a straight line was drawn across the plate with a pipette tip. After the scratches were finished, the cells were washed with PBS three times, and the serum-free medium was added and continue culture for 24 h at 37°C, 5% CO2. At the end of the experiment, the cells were observed and photographed under an inverted fluorescence microscope. Three experimental replicates were performed (n = 3).
All data are analyzed by SPSS22.0 software. The measurement data were expressed as the mean ± SD and analyzed by using Student’s t-test (differences between two groups) and one-way ANOVA with a post hoc Tukey’s test (comparison of >3 groups). The difference was statistically significant (P < 0.05).
Testosterone increases weight and enlargement of adipocytes, but reduces the number of adipocytes in neonatal and adolescent offspring of late pregnancy rats
The effects of high level of testosterone on weight and adipose tissue of the offspring of late pregnancy rats during different periods (neonatal, adolescence, and adulthood) were investigated (Fig. 1A). The weight of offspring increased significantly with lifetime (adulthood > adolescence > neonatal). In female neonatal offspring and female adolescence offspring, the weight in the testosterone group was significantly higher than that of the saline group (control group, P < 0.01, Fig. 1A). There were not significant different between control group and testosterone groups in adulthood offspring (Fig. 1A). These results suggested that the effect of high androgen exposure on body weight is mainly in the neonatal period and adolescence.
The subcutaneous and intra-abdominal adipose tissue in the female rats were taken from the neonatal, adolescence, and adulthood offspring, respectively. With increasing weeks of age, the size of adipocytes in the intra-abdominal and subcutis became larger and but the cells number were decreased (Figs. 1B and 1D). Compared to controls, the number of adipocytes in the intra-abdominal were significantly reduced in neonatal and adolescence offspring (P < 0.01, Fig. 1C), in subcutaneous adipocytes, the number of adipocytes were significantly reduced in neonates and adult offspring (P < 0.01, Fig. 1E). There was no significant difference in the number of adipocytes in the intra-abdominal of testosterone groups in adulthood compared to the control group (Fig. 1C) and in subcutaneous adipocytes, there was no significant difference in the number of adipocytes in testosterone group compared to the control group in adolescent offspring (P < 0.05, Fig. 1E).
Oil red O staining showed that the size of subcutaneous adipocytes increased while their number decreased, along with the week age increasing (Fig. 1F). Compared with control group, testosterone significantly decreased the subcutaneous adipocytes in female neonatal and adolescent offspring (P < 0.01, Fig. 1G). There were not significant different in the subcutaneous adipocytes between control group and testosterone groups in adulthood offspring (Fig. 1G).
The results suggested that the trend of changes in subcutaneous and intra-abdominal adipose tissues were the similar in the different stage of offspring female rats that developed PCOS.

Figure 1: Changes of body weight and adipose tissue in offspring female rats at different periods under high testosterone environment. (A) The body weight of offspring female rats. (B) The peritoneal adipose tissue was stained with HE and imaged. (C) The fat cell number of peritoneal adipose tissue. (D) The subcutaneous adipose tissue was stained with HE and imaged. (E) The fat cell number of subcutaneous adipose tissue. (F) The subcutaneous adipocytes were stained with Oil Red O and imaged. (G) The fat cell number of subcutaneous adipocytes. Data are presented as the mean ± SD; *P < 0.05 and **P < 0.01. Statistical analysis was performed by Student’s t-test (n = 20). All experiments were performed in triplicate.
Testosterone increases levels of serum testosterone, TNF-α and MCP-1 in offspring of late pregnancy rats
The serum in the female rats were collected from the neonatal, adolescence, and adulthood offspring, respectively. The serum testosterone, TNF-α and MCP-1 were detected (Fig. 2). As shown in Fig. 2A, compared with the saline group, serum testosterone was significantly increased in neonatal offspring rats by testosterone (P < 0.01), but was not significantly changed in adolescence and adulthood offspring.
The TNF-α and MCP-1 levels in the testosterone group was significantly increased by testosterone in the neonatal, adolescence, and adulthood offspring (P < 0.01, Figs. 2B and 2C). Thus, intrauterine high androgen increased the levels of testosterone, TNF-α and MCP-1 of the offspring of rats.
The results suggested that the parental hyperandrogenic exposure in the late pregnancy leads to HA in the offspring females mainly in the neonatal stage, but the expression of inflammatory factors is significantly higher in the offspring at different stage.
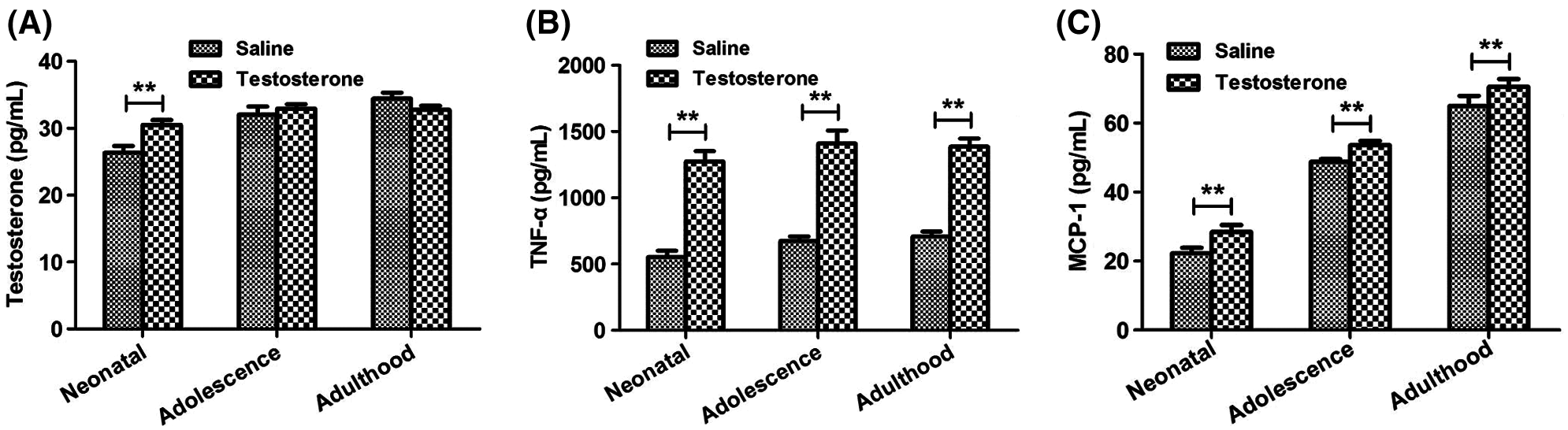
Figure 2: Changes of testosterone, TNF-α and MCP-1 expression levels in offspring female rats at different times. The testosterone (A), TNF-α (B) and MCP-1 (C) expression levels in serum of each group detected by ELISA assay. Data are presented as the mean ± SD; *P < 0.05 and **P < 0.01. Statistical analysis was performed by Student’s t-test (n = 20). All experiments were performed in triplicate.
Testosterone increases adipocytes and angiogenesis in adipose tissues in offspring of late pregnancy rats, which is associated with macrophage
The subcutaneous and intra-abdominal adipose tissues in the female rats were taken from the neonatal, adolescence, and adulthood offspring with clodronate liposomes or PBS liposomes injections, respectively. Clodronate liposomes, once phagocytosed by macrophages, accumulate intracellularly and lead to apoptosis, and are often used to model macrophage clearance in experimental studies, and clodronate liposomes have been successfully used for macrophage clearance in liver, spleen, lung, lymph nodes, joints, peritoneal cavity and testis (van Rooijen and Sanders, 1994).
As shown in Figs. 2A and 2B of HE-staining, the number of adipocytes in subcutaneous and intra-abdominal adipose tissues of neonatal, adolescence, and adulthood offspring were slight increase in clodronate group compared to PBS group (Figs. 3A and 3B). The EC markers CD16 and CD31 in adipose tissues were detected by Immunofluorescence assay (Figs. 3C–3F), and the results showed that the expression of CD16 and CD31 were significantly increased in clodronate treatment group (Figs. 3C–3F). The results suggested that macrophages may be involved in the remodeling of adipose tissue in PCOS patients, and the presence of macrophages may inhibit the differentiation of ECs.
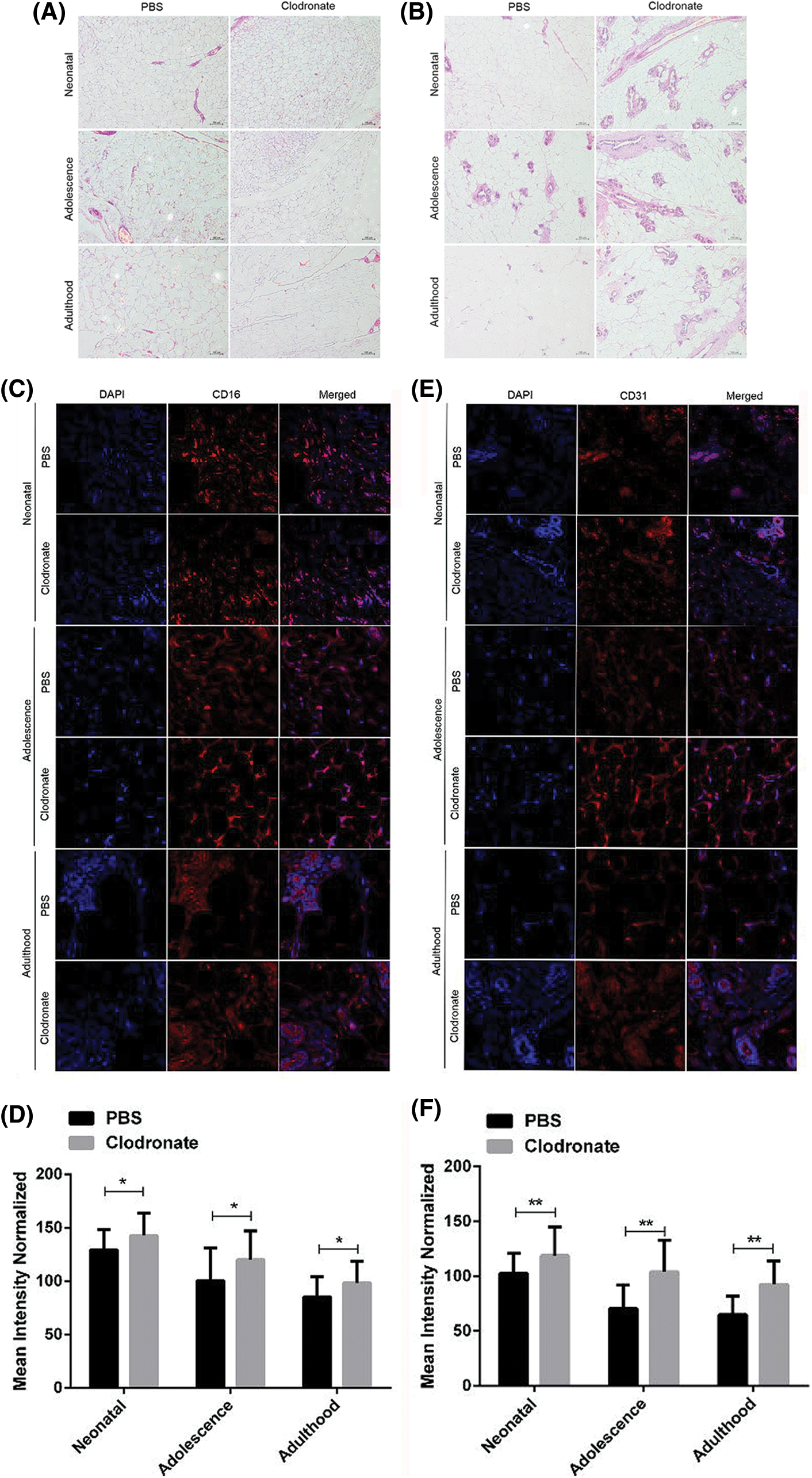
Figure 3: Effects of macrophages on adipose remodeling in offspring female rats at different periods under high androgen conditions. (A) The peritoneal adipose tissue was stained with HE and imaged. (B) The subcutaneous adipose tissue was stained with HE and imaged. (C–F) The CD16 and CD31 expression level in adipose tissue was confirmed by Immunofluorescence staining. Data are presented as the mean ± SD; *P < 0.05 and **P < 0.01. Statistical analysis was performed by Student’s t-test (n = 20). All experiments were performed in triplicate.
Depletion of macrophage by clodronate liposomes attenuates the levels of PDGF and inflammatory factors including TNF-α, IL-6, MCP-1 in female offspring of hyperandrogenic female rats
To further explore, the effect of macrophages on angiogenic and inflammatory factors during the development of PCOS, we used ELISA to detect the expression levels of angiogenic factors PDGF, VEGF and inflammatory factors TNF- α, IL-6, MCP-1 in offspring female rat under hyperandrogenic conditions (Fig. 4). The results showed that compared to the PBS group, both the PDGF and IL-6 expression levels of were significantly decreased (P < 0.01, Figs. 4A and 4B), and VEGF was not significantly changed (Fig. 4C) in neonatal, adolescence, and adulthood stages. The expression level of MCP-1 in neonatal and adulthood stage (P < 0.01, Fig. 4D) and the expression level of TNF-α in adolescence (P < 0.001) and adulthood (P < 0.05) stage were significantly reduced in clodronate group compared to the PBS group (Fig. 4E).
We also performed the qRT-PCR to detect the mRNA expression levels of PDGF, VEGF, CD16/CD32, CD206 and CD31 in the adipose tissue in offspring at different stages. We found that the mRNA level of PDGF was significantly reduced in different stages (P < 0.01, Fig. 4F), VEGF was significantly increased in adolescence (P < 0.01) and adulthood (P < 0.05) stages (Fig. 4G), CD16 and CD31 were all significantly increased different stages (P < 0.01, Figs. 4H and 4I), CD206 was significantly increased in neonatal stage (P < 0.05, Fig. 4J).
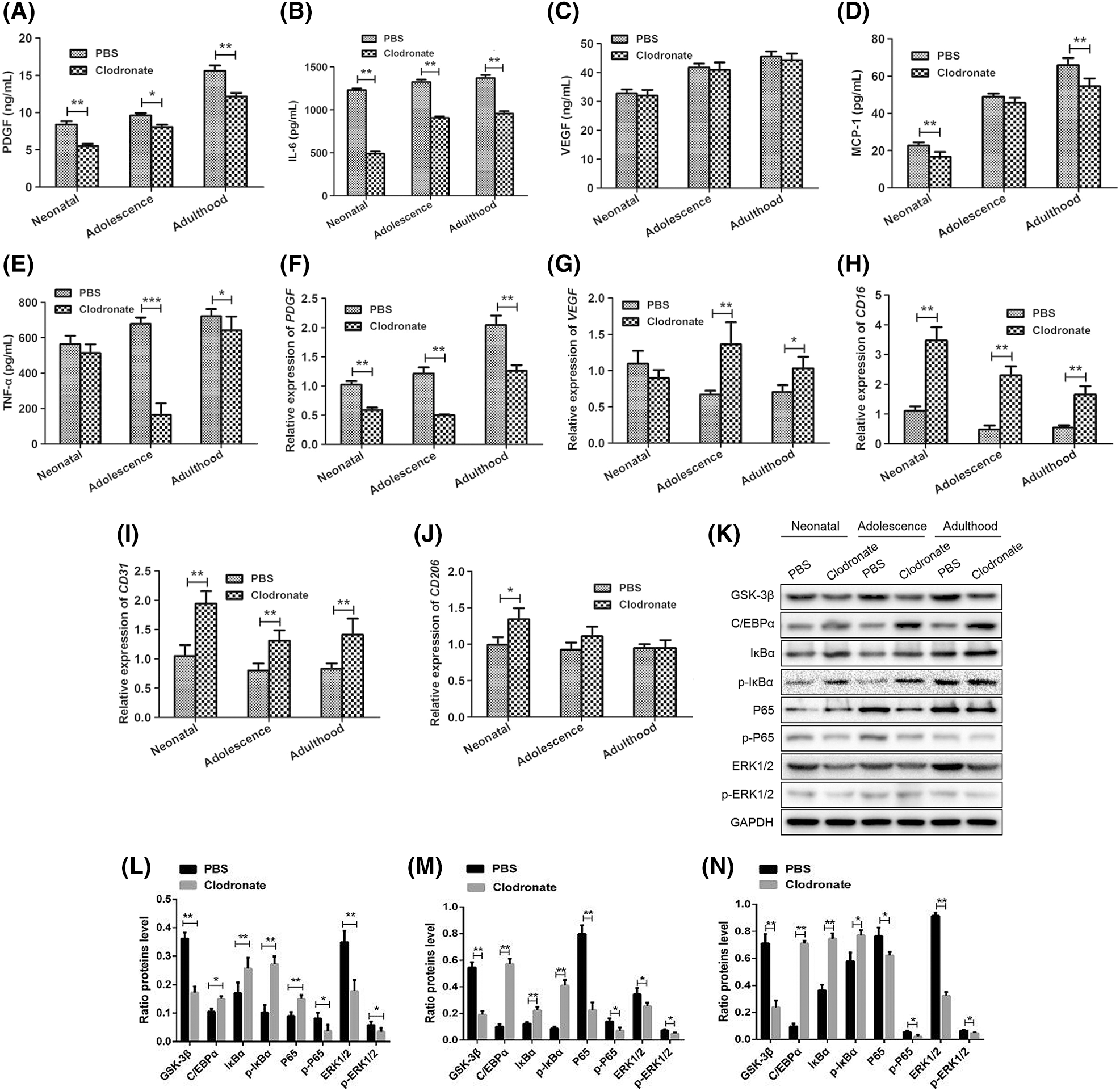
Figure 4: Expression of the angiogenic factors PDGF and VEGF and the inflammatory factors TNF-α, IL-6 and MCP-1 in serum and adipose tissues in female offspring of hyperandrogenic female rats. The PDGF (A), IL-6 (B), VEGF (C), MCP-1 (D), TNF-α (E), expression levels in serum of each group detected by ELISA assay. The PDGF (F), VEGF (G), CD16 (H), CD31 (I), CD206 (J) expression levels in adipose tissue of each group detected by qRT-PCR assay. (K–N) The protein expression levels of GSK-3β, p65, p-P65, ERK1/2, p-ERK1/2, C/EBP α, I κ B α and p-I κ B α was detected by Western blotting assay. Data are presented as the mean ± SD; *P < 0.05 and **P < 0.01. Statistical analysis was performed by Student’s t-test (n = 20). All experiments were performed in triplicate.
To further investigate the mechanism of macrophage effects on adipose remodeling in a hyperandrogenic environment, we examined the expression of the inflammatory response-related pathway NF-κB/ERK1/2 and the adipose tissue remodeling-related pathway GSK-3β/C/EBP α/I κ B α protein by western blot in adipose tissue at different stage. The results showed that compared to the PBS group, the protein expression levels of GSK-3β, p65, p-P65, ERK1/2 and p-ERK1/2 were significantly decreased in clodronate group at all stages (Figs. 4K–4N), while the protein expression levels of C/EBP α, I κ B α and p-I κ B α were significantly increased.
Thus, depletion of macrophage by clodronate liposomes attenuated the levels of pro-angiogenic and proinflammatory factors and disrupted related pathways in female offspring of hyperandrogenic female rats.
Macrophages promotes mobility of adipocytes under hyperandrogenic condition
To further confirm the effect of macrophages on the biological function of adipose tissue cells under hyperandrogenic conditions, we examined the effect of macrophages on the mobility of 3T3-L1 preadipocytes by Transwell and scratch assay in vitro study. RAW264.7 Macrophages co-cultured with 3T3-L1 adipocytes, and the macrophages and adipocytes were cultured separately as control (Fig. 5), and the culture media contain high testosterone concentration (10−5 mol/L). We found that the mobility ability of 3T3-L1 adipocytes induced by RAW264.7 macrophage were significant attenuated when Androgen inhibitor (Apalutamide), NF-κB inhibitor (JSH-23) and ERK1/2 inhibitor (LY3214996) were added (Figs. 5A–5D). These results suggested that androgen/NF-κB/ERK1/2 signal pathway may be involved in the regulated the motility in Adipose tissue.
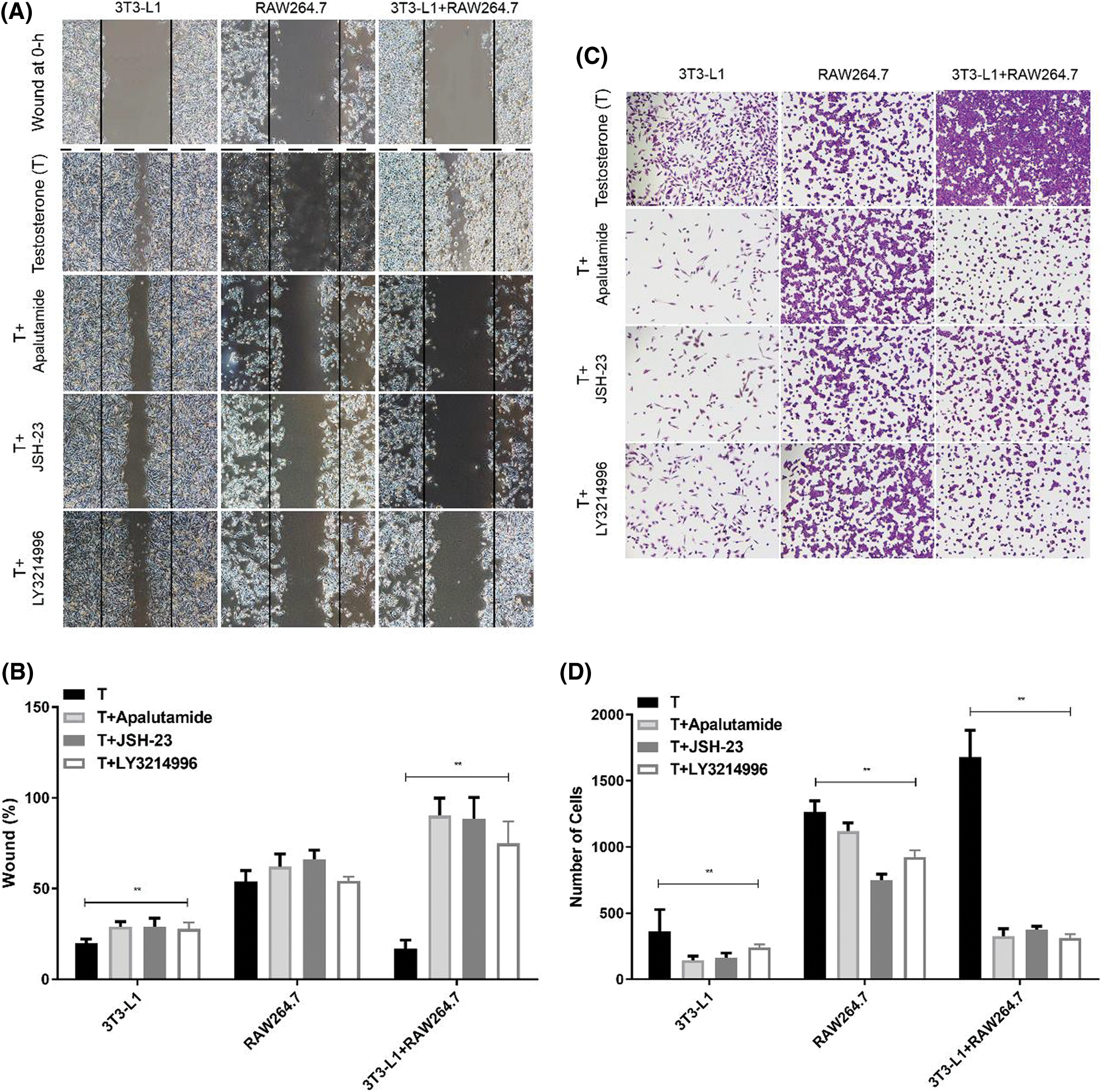
Figure 5: Effect of macrophages on the mobility of adipocytes in hyperandrogenic condition. Cells were treated with testosterone (T) with or without androgen inhibitor (apalutamide), NF-κB inhibitor (JSH-23) and ERK1/2 inhibitor (LY3214996). (A–B) The cell migration was detected by scratch test. Dark line indicated the wound at 0 h. The wound at 0 h-images were also shown. (C–D) The mobility was detected by Transwell coated with matrigel. Data are presented as the mean ± SD; **P < 0.01 vs. T. Statistical analysis was performed by one-way ANOVA (n = 3). All experiments were performed in triplicate.
Macrophages inhibits proliferation, migration, and tube formation of ECs under hyperandrogenic condition
To further confirm the effect of macrophages on the biological function of ECs under hyperandrogenic conditions. We observed the proliferation, migration, and tube formation ability of vascular endothelial cell C166 co-cultured with RAW264.7 macrophages, and RAW264.7 macrophages and C166 were cultured separately as control (Fig. 6). The results showed that the cell proliferation of C166 was significantly decreased induced by high androgen concentration in co-cultured with RAW264.7 macrophages (Fig. 6A).
In cell migration assay, we found that the cell ability of migration was significantly decreased induced by high androgen concentration in co-cultured with RAW264.7 macrophages when Androgen inhibitor (Apalutamide), NF-κB inhibitor (JSH-23) and ERK1/2 inhibitor (LY3214996) was added (Fig. 6B), and we found that the tube formation ability of C166 can be significant inhibited when co-cultured with RAW264.7 macrophages, but the inhibition effect were reversed when Androgen inhibitors, NF-κB inhibitors, ERK1/2 inhibitors were added (Fig. 6C).
These results suggest that under hyperandrogenic conditions, macrophages are able to significantly reduce the proliferation and migration capacity of vascular endothelial cells, as well as to significantly inhibit the angiogenic capacity of vascular endothelial cells.
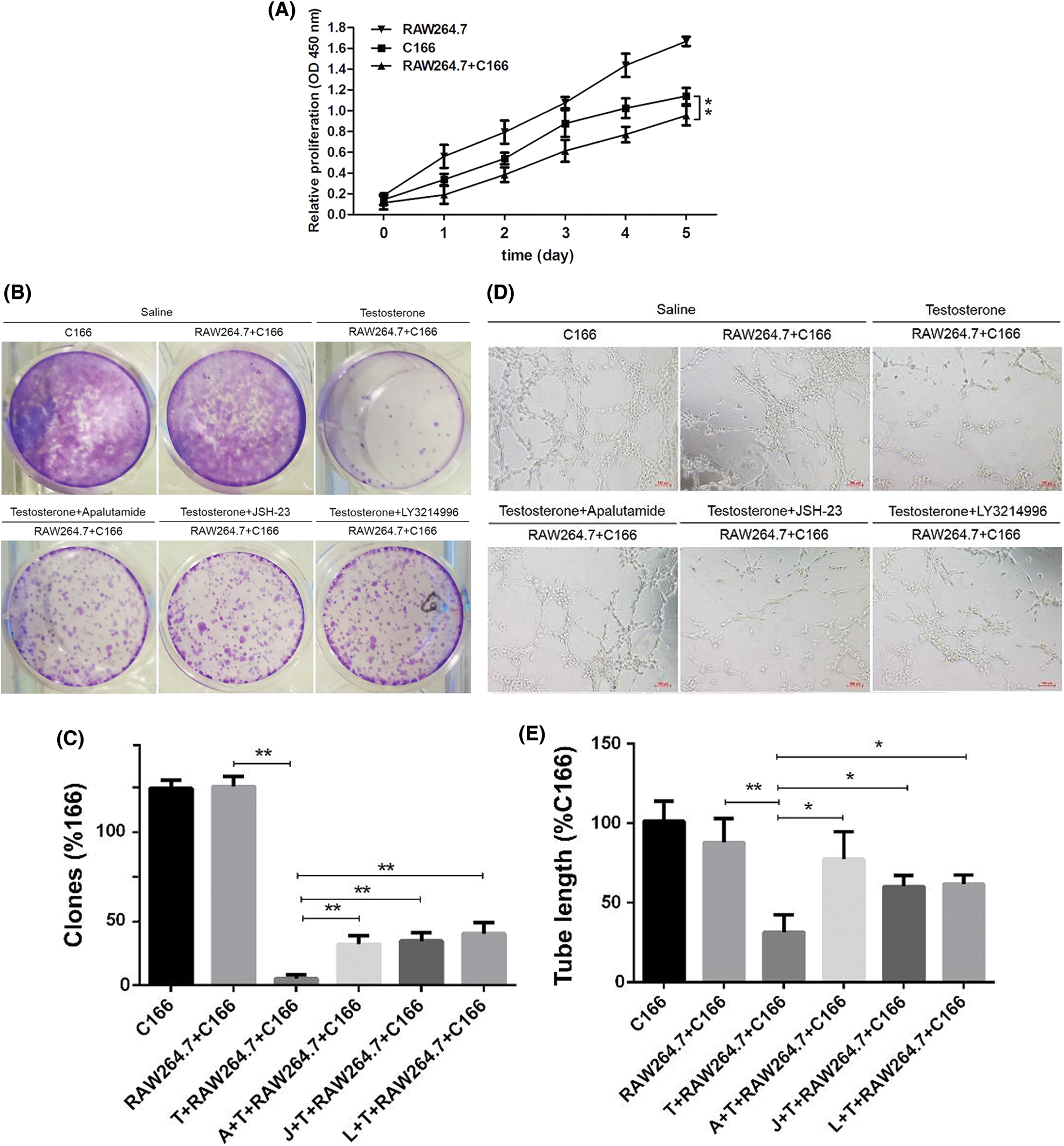
Figure 6: Effect of macrophages on the proliferation of endothelial cells in hyperandrogenic condition. (A) The ECs proliferation was detected by CCK8. (B–C) The colon formation of ECs and clones. (D–E) The ECs was cultured in matrigel and the tube length was quantified. * p < 0.05 and ** p < 0.01. Statistical analysis was performed by one-way ANOVA (n = 3).
Macrophages induced the levels of PDGF, VEGF, MCP-1, IL-6 and TNF-α, and activation of NF-κB/ERK1/2 in ECs
We further explored the mechanism of macrophage effect on vascular endothelial cells. Frist, we detected the expression of PDGF, VEGF, MCP-1, IL-6 and TNF-α in C166 co-culture with RAW 264.7 macrophages at high androgen concentration, and the results showed that after high concentration of testosterone treatment, and the results showed that the expression of PDGF, IL-6, MCP-1 and TNF-α were significantly increased in the co-culture system and C166 cultured alone; the expression of VEGF was slightly increased with no significant changes, while the expression of PDGF, IL-6, MCP-1 and TNF-α were significantly decreased after the addition of three inhibitors respectively; the expression of VEGF was slightly decreased with no significant changes (Figs. 7A–7E).
Next, we detected the NF-κB/ERK1/2 signal pathway proteins expression in the co-culture system to explore the mechanism of macrophages promote the vascular endothelial cell angiogenic capacity under hyperandrogenic conditions. We found that the expressions of IκBα and p-IκBα were significantly reduced, and P65, p-P65, ERK1/2 and p-ERK1/2 were significantly increased in the co-culture system, and these protein expression level can be reversed after the addition of three inhibitors respectively (Figs. 7F and 7G).
These results suggest that under hyperandrogenic conditions, macrophages are able to significantly promote the inflammation response by activating the NF-κB/ERK1/2 signal pathway and reduce the vascular endothelial cells angiogenic capacity.
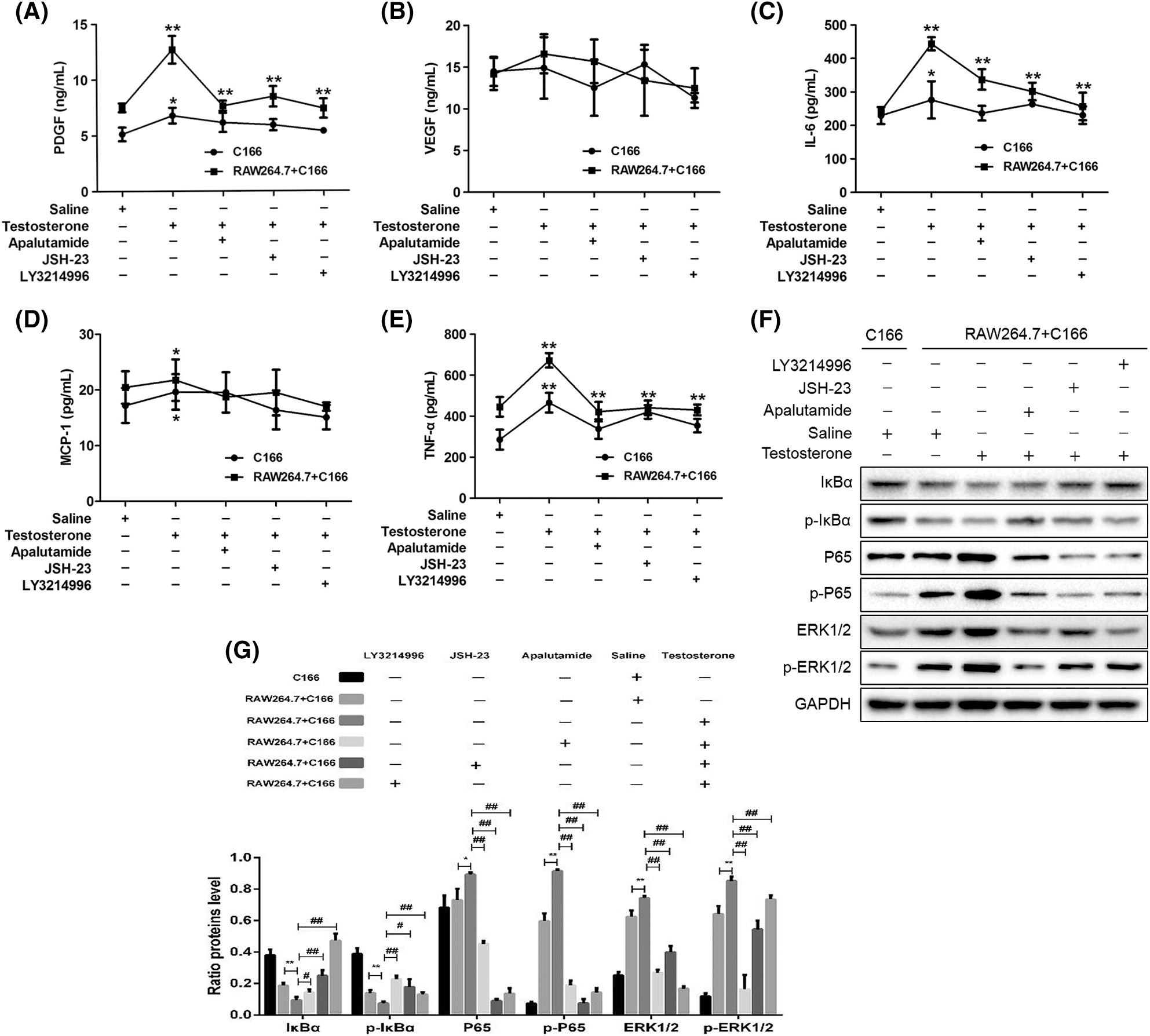
Figure 7: Effects of macrophages on the levels of PDGF, IL-6 and TNF-α, and activation of ERK1/2 in ECs. The PDGF (A), VEGF (B), IL-6 (C), MCP-1 (D), TNF-α (E) expression level was detected by ELISA. (F–G) The protein expression of GSK-3β, p65, p-P65, ERK1/2, p-ERK1/2, C/EBP α, I κ B α and p-I κ B α were detected by Western blot. *,#P < 0.05 and **,##P < 0.01. Statistical analysis was performed by one-way ANOVA (n = 3). All experiments were performed in triplicate.
Overweight or abdominal obesity is a common symptom in patients with PCOS (Escobar-Morreale, 2018). Patients with PCOS often have disorders of glucolipid metabolism, and obesity increases the risk of diabetes, hyperlipidemia, and coronary heart disease and cerebrovascular disease (Otto-Buczkowska et al., 2018). In overweight or obese patients with PCOS, excess fat-induced inflammation may trigger a cascade of events leading to increased insulin resistance (IR), and dyslipidemia. Recent studies have shown that the core etiology and main endocrine features of PCOS are HA and IR (Wang et al., 2019). In animal studies, the symptoms of PCOS can be induced by in vitro injection of testosterone, and intrauterine administration of high concentration testosterone to pregnant rhesus monkeys, the female offspring would exhibit all phenotypes of human PCOS patients with disorders of the reproductive and metabolic systems, including adult HA, anovulation, polycystic ovaries, central obesity, IR (Eisner et al., 2000). In the present study we induced the occurrence of PCOS in offspring females by intrauterine exposure to high concentrations of testosterone in late pregnant rats to explore the mechanisms of disorders of lipid metabolism leading to the development of overweight and obesity in patients with PCOS.
It was found that the body weight of neonatal female rat weight in offspring from hyperandrogenic female rats was higher than that from the control female rats. The third trimester of pregnancy is a critical period for fetal weight growth (Jain, 2003). We speculate that high androgen exposure in the uterus in the third trimester of pregnancy will cause disorders of fetal lipid metabolism, which will lead to an increase in body weight of neonatal rat and may be impair insulin sensitivity of peripheral tissue in offspring. It has been previously reported that hyperandrogenic exposure in the third trimester of pregnancy will lead to structural changes of ATP-sensitive potassium channels in islet β cells of offspring, increase the occurrence of insulin resistance in offspring, and lead to adipose accumulation in offspring (Abbott et al., 2005). The adipose tissue can be divided into intra-abdominal and subcutaneous adipose tissue according to the location of distribution (Ibrahim, 2010). Intra-abdominal adipose tissue, also known as visceral adipose tissue, mainly refers to the one located around internal organs and subcutaneous adipose tissue refer to the fat distributed beneath the skin (Mittal, 2019). In patients with PCOS, adipose tissue distribution and function are abnormally affected by hyperandrogenemia, and androgen receptors are expressed in both subcutaneous and visceral adipose tissue, and androgens have been found to stimulate adipocyte growth, disrupt the differentiation of preadipocytes from adipocytes, and reduce lipolysis of subcutaneous adipose tissue (Blouin et al., 2009; Chazenbalk et al., 2013). We observed that the changes of subcutaneous and intra-abdominal adipose tissue of offspring rats in different periods. The human subcutaneous adipose tissue is a natural fat reserve site, which stores excess free fatty acid (FFA) in the form of triacylglycerol (triglyceride, TG) in adipocytes, and the volume of adipose tissue depends on the size and number of adipocytes, adipocyte hypertrophy is characteristic of overweight and obese populations, and adipocyte hyperplasia is of greater relevance in obese populations (Spalding et al., 2008). When the body consumes more energy than the subcutaneous adipose tissue can handle, the excess fat would accumulate elsewhere, and leads to an increase in visceral adipose tissue, liver, and skeletal muscle (Arner, 2018; Danforth, 2000). In patients with PCOS due to the presence of high levels of insulin and androgens, individuals with larger adipocytes in subcutaneous adipose tissue have a reduced capacity to store lipids, leading to the development of metabolic disorders (de Zegher et al., 2009; Goodpaster et al., 2005). It was found that with the increase of age, the volume of adipocytes in the experimental group was significantly higher than that in the control group, while the number of adipocytes decreased significantly, suggesting that significant fat accumulation and lipid metabolism disorder occurred in puberty rats after hyperandrogenic exposure in the third trimester of pregnancy. The change trends in subcutaneous and visceral fat were similar, and we hypothesize that this trend is influenced by hyperandrogenemia.
PCOS patients with or without obesity have varyingly elevated levels of inflammatory factors, and obesity exacerbates this chronic low-grade inflammatory state (Ghowsi et al., 2018; Xu et al., 2014). In normal adipose tissue, there are about 5% macrophages, when the obesity occurring, the rate would rise to 50% and the cell polarization state also shifts from M2 to M1 type and releases large amounts of pro-inflammatory factors (Lumeng et al., 2007; Sárvári et al., 2015). It has been found that the progressive loss of anti-inflammatory capacity and the development of insulin resistance during the conversion of adipose tissue macrophages of type M2 to type M1 during obesity may occur by the mechanism by which MCP-1, TNF-α and free fatty acids through Toll-like receptor 4 (TLR4) make increased peripheral circulation of inflammatory monocytes to move to adipose tissue, leading to alterations in the macrophage microenvironment and inducing the infiltration of macrophages into adipose tissue to M1-type polarization (Murray and Wynn, 2011). Macrophage infiltration into adipose tissue is a major feature of obesity (Weisberg et al., 2003). Adipocytes and the macrophages in adipose tissue are the major source of inflammatory cytokines, TNF-α, IL-6, etc. (Ye and Keller, 2010). IL-6 is a multipotent cytokine, and adipose tissue is an important source of IL-6 secretion (Scheele and Wolfrum, 2020). In obese and type 2 diabetic patients, elevated IL-6 levels were found to be associated with elevated glucose, reduced glucose tolerance and decreased insulin sensitivity (IR) (Xu et al., 2019). TNF-α is a multifunctional cytokine that affects the germinal axis, and TNF-α may induce IR by activating insulin receptor substrate-1 (IRS-1) serine phosphorylation and inhibiting glucose carrier protein-4 expression in adipocytes (Khan et al., 2009). MCP-1 has an important role in macrophage infiltration of adipose tissue, and it was found that in high-fat-fed MCP-1 and its receptor chemokine receptor 2 (C-C motif chemokine receptor 2, CCR2) knockout mice compared to wild-type high-fat-fed mice macrophage infiltration was reduced and adipose tissue inflammatory response as well as systemic IR was improved (Lumeng et al., 2007). Studies in a diet-induced obese rat model also found significantly higher plasma MCP-1 levels, increased number of infiltrating macrophages in adipose tissue, and decreased insulin sensitivity (Kaneko et al., 2011). In our animal experiments, we clearly observed elevated expression of IL-6, TNF-α and MCP-1 in the serum of offspring female rats. We hypothesize that the onset of obesity in rats with PCOS-like symptoms in the offspring due to late parental exposure can exacerbate the chronic inflammatory response state in PCOS, and that adipose macrophages may play an important role in this.
In this study, we used clodronate liposomes to remove macrophages in the offspring of SD female rats, and to observe the remodeling of adipose tissue in offspring female rats at different periods and verify the effect of macrophages on adipose remodeling in PCOS. The fat cells in organs were not detected yet. We found that the number of adipocytes in the peritoneal adipose tissue and subcutaneous adipose tissue in the clodronate group increased significantly in offspring at different periods, and the expression of inflammatory factors IL-6, TNF-α and MCP-1 decreased. We hypothesize that the chronic inflammatory response state of PCOS patients can be alleviated by reducing the inflammatory response in adipose tissue of PCOS patients. There are many signal pathways involved in regulating the inflammation response, such as JAK-STAT pathway and JNK/AP-1 pathway. Evidence has shown that the inflammatory signal pathway of PCOS patients is in an activated state, for example, the activation state of NF-κB in peripheral blood mononuclear cells of PCOS patients is significantly higher than that of non-PCOS controls after taking sugar (Eisner et al., 2000). In the resting state, NF-κB binds to the inhibitory protein IκB in the form of dimer in the cytoplasm, when stimulated by pro-inflammatory, IκB was degraded and dissociated off from NF-κB, and NF-κB is activated and translocated to the nucleus, which can initiate the transcription of related genes, such as TNF-α and MCP-1 (Wang et al., 2019). In recent years, studies have found that there is a cross-talk between androgen signaling pathway and inflammatory signaling pathway, such as androgen forms a cross-talk with NF-κB at the transcriptional level through nuclear AR, activating NF-κB p65 signaling pathway in prostate cancer cells (Ibrahim, 2010), promoting the endothelial cell adhesion (Mittal, 2019). Androgen can also bind to its membrane AR, cross-talk with G protein, and then activate intracellular second messenger molecules such as ERK, P38, MAPK, etc., Ibrahim, 2010. ERKs is an important member of MAPK signal pathway in cellular inflammatory response. ERKs includes five subfamilies of ERK1-ERK5. The activation of ERK1 and ERK3 pathway can phosphorylate a series of intracellular signal transduction molecules and transcription factors, including NF-κB, AP-1 and so on. These transcription factors further regulate the transcription of their respective target genes and cause changes in the expression or activity of specific proteins (Spalding et al., 2008). Some studies have shown that the ERK1/2 of PCOS is in a state of over activation (Arner, 2018). In this study, we found that the expressions of NF-κB p65, NF-κB P65, ERK1/2 and p-ERK 1/2 were significantly decreased and the protein expression levels of IκBα and PTC IκBα were significantly increased in the offspring of rats at different stages when macrophages were removed by clodronate liposome. We hypothesize that the inflammatory response state in adipose tissue of PCOS patients is associated with androgen/NF-κB/ERK1/2 signaling pathway activation.
Glycogen synthase kinase-3 (GSK-3) is a multifunctional serine B-threonine protein kinase, which has two main subtypes: GSK-3α and GSK-3β, in which GSK-3β is an important component of many intracellular signal transduction pathways (Danforth, 2000). GSK-3β can inhibit glycogen synthesis and glucose transport, promote gluconeogenesis, and inhibit insulin secretion, thus increasing blood glucose. Studies in prostate cancer and other diseases have shown that GSK-3β is closely related to the synthesis and activity of androgens (Blouin et al., 2009). Chazenbalk et al. (2013) reported that GSK-3β can increase glucose uptake, glycogen synthesis, and improve insulin resistance (IR). It has been found that GSK3β activity regulates androgen receptor function in prostate cancer cell lines (Jain, 2003), and abnormal GSK3β activity in PCOS patients promotes androgen overproduction and enhances the 17-hydroxylase activity of P450c17 leading to the development of hyperandrogenism (Chiaverina et al., 2019). CCMT enhancer binding protein α (C/EBPα) is a member of the C/EBPs family and plays a key role in adipogenic differentiation (de Zegher et al., 2009; Goodpaster et al., 2005; Xu et al., 2014). We found that the expression of GSK-3β was up-regulated and the expression of C/EBP α was down-regulated in offspring at different periods, but the expression trend of GSK-3β and C/EBPα was opposite under the condition of macrophage removal. We hypothesize that in obese patients with PCOS, disruption of lipid metabolism occurs in adipose tissue as a result of activation of inflammatory response signaling pathways mediated through hyperandrogenemia.
Obesity leads to abnormal IR and to other metabolic disfunction, and affects the secretion of angiogenesis-related factors, leading to impaired adipose tissue remodeling. In patients with PCOS, there is a state of chronic low-grade inflammation. The process of adipose tissue proliferation is accompanied by the regeneration, expansion and remodeling of the vascular system in the tissue, while vascular endothelial cells and preadipocytes interact with each other during development (Lumeng et al., 2007). Recent studies have found that platelet-derived growth factor (PDGF) is closely related to diabetic microangiopathy (Tanii et al., 2006). The infiltration of macrophages into adipose tissue can compensatively increase the secretion of PDGF and promote angiogenesis. We found that in different stages of female rats without macrophages, compared with the control group, the expression of endothelial cell specific markers CD16 and CD31 in adipose tissue decreased, the expression of angiogenic factor PDGF decreased significantly, but the expression of VEGF was not significant; the expression of TNF-α, IL6 and MCP-1 decreased significantly, animal experiments suggested that macrophages inhibited endothelial cell differentiation and angiogenesis in adipose tissue with the condition hyperandrogenism. Through the co-culture of macrophages and adipocytes/vascular endothelial cells, we found that under the condition of hyperandrogenic, macrophages could promote the mobility of adipocytes; macrophages could significantly reduce the proliferation, migration, and tubular structure formation of vascular endothelial cells; the content of PDGF increased significantly, the content of VEGF increased slightly, and the expression of TNF-α, IL6 and MCP-1 increased significantly. Studies suggest that in PCOS, persistent low-degree inflammation reduces the ability of adipose tissue angiogenesis and affects adipocyte remodeling due to hyperandrogenemia.
To sum up, through the above experimental studies, we believe that in PCOS, obesity can exacerbate the inflammatory response state in PCOS, leading to abnormal lipid metabolism and abnormal adipose tissue remodeling, the mechanism of which may occur through hyperandrogenemia-induced increases the levels of TNF-α, IL-6 and MCP-1, while M2 macrophage polarization to M1 macrophage conditions increase TNF-α, IL-6 and MCP-1 in adipose tissue, and then the inflammatory response signaling pathway NF-κB/ERK1/2 were activated. The increase in inflammatory factors affects the expression of angiogenic factors such as VEGF and PDGF in adipose tissue, which leads to a decrease in adipose tissue remodeling capacity. This study elucidates the mechanism of macrophage effect on adipose tissue remodeling in PCOS and provides a direction for clinical intervention in PCOS patients to prevent the occurrence of obesity.
Availability of Data and Materials: Readers can access the data used in the study by contact corresponding author.
Authors’ Contribution: The authors confirm contribution to the paper as follows: study conception and design: M. Chen, C. Su; data collection: M. Chen, Y. Huang; analysis and interpretation of results: M. Chen, Y. Huang; draft manuscript preparation: M. Chen, Y. Huang, C. Su. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: All animal care and handling were in accordance with the guidelines for the care and use of laboratory animals published by the National Research Institute of the United States and this study was approved by the ethics committee of Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine (RENJI2018-06; JUNE 6th, 2018).
Funding Statement: This research was supported in by the National Natural Science Foundation of China (Grant No. 81701409) and Natural Science Foundation of Shanghai of China (Grant No. 12ZR441400).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abbott DH, Barnett DK, Bruns CM, Dumesic DA (2005). Androgen excess fetal programming of female reproduction: A developmental aetiology for polycystic ovary syndrome? Human Reproduction Update 11: 357–374. DOI 10.1093/humupd/dmi013. [Google Scholar] [CrossRef]
Arner P (2018). Fat tissue growth and development in humans. Recent Research in Nutrition and Growth 89: 37–45. DOI 10.1159/issn.1664-2147. [Google Scholar] [CrossRef]
Blouin K, Veilleux A, Luu-The V, Tchernof A (2009). Androgen metabolism in adipose tissue: Recent advances. Molecular Cell Endocrinology 301: 97–103. DOI 10.1016/j.mce.2008.10.035. [Google Scholar] [CrossRef]
Brydon L (2011). Adiposity, leptin and stress reactivity in humans. Biological Psychology 86: 114–120. DOI 10.1016/j.biopsycho.2010.02.010. [Google Scholar] [CrossRef]
Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA (2013). Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids 78: 920–926. DOI 10.1016/j.steroids.2013.05.001. [Google Scholar] [CrossRef]
Chiaverina G, di Blasio L, Monica V, Accardo M, Palmiero M et al. (2019). Dynamic interplay between pericytes and endothelial cells during sprouting angiogenesis. Cells 8: 1109. DOI 10.3390/cells8091109. [Google Scholar] [CrossRef]
Danforth EJr (2000). Failure of adipocyte differentiation causes type II diabetes mellitus? Nature Genetics 26: 13. DOI 10.1038/79111. [Google Scholar] [CrossRef]
de Zegher F, Lopez-Bermejo A, Ibáñez L (2009). Adipose tissue expandability and the early origins of PCOS. Trends in Endocrinology and Metabolism 20: 418–423. DOI 10.1016/j.tem.2009.06.003. [Google Scholar] [CrossRef]
Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH (2000). Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. Journal of Clinical Endocrinology & Metabolism 85: 1206–1210. DOI 10.1210/jc.85.3.1206. [Google Scholar] [CrossRef]
Escobar-Morreale HF (2018). Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nature Reviews Endocrinology 14: 270–284. DOI 10.1038/nrendo.2018.24. [Google Scholar] [CrossRef]
Ghowsi M, Khazali H, Sisakhtnezhad S (2018). Evaluation of TNF-α and IL-6 mRNAs expressions in visceral and subcutaneous adipose tissues of polycystic ovarian rats and effects of resveratrol. Iranian Journal of Basic Medical Sciences 21: 165–174. [Google Scholar]
Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB et al. (2005). Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Archives of Internal Medicine 165: 777–783. DOI 10.1001/archinte.165.7.777. [Google Scholar] [CrossRef]
Hong Y, Wan B, Li X (2020). Age-related modifications of macrophages influenced by “inflamm-ageing” in graft vs. host disease. BIOCELL 44: 237–246. DOI 10.32604/biocell.2020.08887. [Google Scholar] [CrossRef]
Ibrahim MM (2010). Subcutaneous and visceral adipose tissue: Structural and functional differences. Obesity Reviews 11: 11–18. DOI 10.1111/j.1467-789X.2009.00623.x. [Google Scholar] [CrossRef]
Jain RK (2003). Molecular regulation of vessel maturation. Nature Medicine 9: 685–693. DOI 10.1038/nm0603-685. [Google Scholar] [CrossRef]
Kaneko H, Anzai T, Horiuchi K, Morimoto K, Anzai A et al. (2011). Tumor necrosis factor-α converting enzyme inactivation ameliorates high-fat diet-induced insulin resistance and altered energy homeostasis. Circulation Journal 75: 2482–2490. DOI 10.1253/circj.CJ-11-0182. [Google Scholar] [CrossRef]
Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M et al. (2009). Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Molecular and Cellular Biology 29: 1575–1591. DOI 10.1128/MCB.01300-08. [Google Scholar] [CrossRef]
Kliche S, Waltenberger J (2001). VEGF receptor signaling and endothelial function. IUBMB Life 52: 61–66. DOI 10.1080/15216540252774784. [Google Scholar] [CrossRef]
Lane NE, Kelman A (2003). A review of anabolic therapies for osteoporosis. Arthritis Research & Therapy 5: 214–222. DOI 10.1186/ar797. [Google Scholar] [CrossRef]
Legro RS (2012). Obesity and PCOS: Implications for diagnosis and treatment. Seminars in Reproductive Medicine 30: 496–506. DOI 10.1055/s-00000072. [Google Scholar] [CrossRef]
Li W, Gao H, Tao R, Liu L, Shang S (2019). Association of TRIM22 with the type 1 interferon response during primary human cytomegalovirus infection in THP-1 macrophages. BIOCELL 43: 285–291. DOI 10.32604/biocell.2019.08177. [Google Scholar] [CrossRef]
Lima PDA, Nivet AL, Wang Q, Chen YA, Leader A et al. (2018). Polycystic ovary syndrome: Possible involvement of androgen-induced, chemerin-mediated ovarian recruitment of monocytes/macrophages. Biology of Reproduction 99: 838–852. DOI 10.1093/biolre/ioy096. [Google Scholar] [CrossRef]
Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR (2007). Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56: 16–23. DOI 10.2337/db06-1076. [Google Scholar] [CrossRef]
Mittal B (2019). Subcutaneous adipose tissue & visceral adipose tissue. Indian Journal of Medical Research 149: 571–573. DOI 10.4103/ijmr.IJMR_1910_18. [Google Scholar] [CrossRef]
Murray PJ, Wynn TA (2011). Protective and pathogenic functions of macrophage subsets. Nature Reviews Immunology 11: 723–737. DOI 10.1038/nri3073. [Google Scholar] [CrossRef]
Otto-Buczkowska E, Grzyb K, Jainta N (2018). Polycystic ovary syndrome (PCOS) and the accompanying disorders of glucose homeostasis among girls at the time of puberty. Pediatric Endocrinology, Diabetes & Metabolism 24: 40–44. DOI 10.18544/PEDM-24.01.0101. [Google Scholar] [CrossRef]
Parlee SD, Lentz SI, Mori H, Macdougald OA (2014). Quantifying size and number of adipocytes in adipose tissue. Methods in Enzymology 537: 93–122. DOI 10.1016/B978-0-12-411619-1.00006-9. [Google Scholar] [CrossRef]
Raperport C, Homburg R (2019). The source of polycystic ovarian syndrome. Clinical Medicine Insights: Reproductive Health 13: 1179558119871467. [Google Scholar]
Sárvári AK, Doan-Xuan QM, Bacsó Z, Csomós I, Balajthy Z, Fésüs L (2015). Interaction of differentiated human adipocytes with macrophages leads to trogocytosis and selective IL-6 secretion. Cell Death & Disease 6: 579. [Google Scholar]
Scheele C, Wolfrum C (2020). Brown adipose crosstalk in tissue plasticity and human metabolism. Endocrine Reviews 41: 53–65. DOI 10.1210/endrev/bnz007. [Google Scholar] [CrossRef]
Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA et al. (2008). Dynamics of fat cell turnover in humans. Nature 453: 783–787. DOI 10.1038/nature06902. [Google Scholar] [CrossRef]
Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR et al. (2014). The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35: 4477–4488. DOI 10.1016/j.biomaterials.2014.02.012. [Google Scholar] [CrossRef]
Sun K, Kusminski CM, Scherer PE (2011). Adipose tissue remodeling and obesity. Journal of Clinical Investigation 121: 2094–2101. DOI 10.1172/JCI45887. [Google Scholar] [CrossRef]
Tanii M, Yonemitsu Y, Fujii T, Shikada Y, Kohno R et al. (2006). Diabetic microangiopathy in ischemic limb is a disease of disturbance of the platelet-derived growth factor-BB/protein kinase C axis but not of impaired expression of angiogenic factors. Circulation Research 98: 55–62. DOI 10.1161/01.RES.0000197842.38758.45. [Google Scholar] [CrossRef]
Trikudanathan S (2015). Polycystic ovarian syndrome. Medical Clinics 99: 221–235. DOI 10.1016/j.mcna.2014.09.003. [Google Scholar] [CrossRef]
van Rooijen N, Sanders A (1994). Liposome mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. Journal of Immunolical Methods 174: 83–93. DOI 10.1016/0022-1759(94)90012-4. [Google Scholar] [CrossRef]
Wang J, Wu D, Guo H, Li M (2019). Hyperandrogenemia and insulin resistance: The chief culprit of polycystic ovary syndrome. Life Sciences 236: 116940. DOI 10.1016/j.lfs.2019.116940. [Google Scholar] [CrossRef]
Weisberg SP, Mccann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AWJr (2003). Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation 112: 1796–1808. DOI 10.1172/JCI200319246. [Google Scholar] [CrossRef]
Xu L, Nagata N, Chen G, Nagashimada M, Zhuge F et al. (2019). Empagliflozin reverses obesity and insulin resistance through fat browning and alternative macrophage activation in mice fed a high-fat diet. BMJ Open Diabetes Research and Care 7: e000783. DOI 10.1136/bmjdrc-2019-000783. [Google Scholar] [CrossRef]
Xu X, Du C, Zheng Q, Peng L, Sun Y (2014). Effect of metformin on serum interleukin-6 levels in polycystic ovary syndrome: A systematic review. BMC Womens Health 14: 1472–6874. DOI 10.1186/1472-6874-14-93. [Google Scholar] [CrossRef]
Ye J, Keller JN (2010). Regulation of energy metabolism by inflammation: A feedback response in obesity and calorie restriction. Sedentary Life and Nutrition 2: 361–368. DOI 10.18632/aging.100155. [Google Scholar] [CrossRef]
Zhao D, Ji Q, Zhu S, Zhu K, Wang C (2020). Decreased serum HMGB1 associated with M2 macrophage polarization and patients with calcific aortic valve disease. BIOCELL 44: 315–321. DOI 10.32604/biocell.2020.09169. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |