DOI:10.32604/biocell.2022.019681

| BIOCELL DOI:10.32604/biocell.2022.019681 |  |
| Review |
Tuning mesenchymal stem cell secretome therapeutic potential through mechanotransduction
1Laboratório de Plasticidade e Diferenciação de Células da Crista Neural, Departamento de Biologia Celular, Embriologia e Genética, Centro de Ciências Biológicas, Universidade Federal de Santa Catarina, Campus Universitário–Trindade, CEP, Florianópolis, 88040-900, Brazil
2Laboratório de Biologia Básica de Células-Tronco, Instituto Carlos Chagas, Fiocruz/PR, CEP, Curitiba, 81350-010, Brazil
*Address correspondence to: Giordano Wosgrau Calloni, giordano.calloni@ufsc.br; Marco Augusto Stimamiglio, marco.stimamiglio@fiocruz.br
Received: 08 October 2021; Accepted: 29 November 2021
Abstract: Mesenchymal stem cells (MSCs) and their byproducts have been widely validated as potential therapeutic products for regenerative medicine. The therapeutic effects result mainly from the paracrine activity of MSCs, which consists of the secretion of bioactive molecules, whether dispersed in medium conditioned by cell culture or encapsulated in extracellular vesicles. The composition of the MSC secretome, which represents the set of these secreted cellular products, is crucial for the performance of the desired therapeutic functions. Different cell culture strategies have been employed to adjust the secretome composition of MSCs to obtain the best therapeutic responses for different clinical contexts. However, the manipulation of culture conditions has focused mainly on the use of different biochemical elements for the preconditioning of MSCs and less on the physical conditions of the cell culture environment. Herein, we offer our point of view regarding the importance of the physical properties of cell culture substrates and their mechanotransduction responses in preconditioning the MSCs secretome. We highlight the relevance of studying mechanotransduction events associating cell morphology and the modulation of gene expression to customize and expand the use of MSCs secretomes.
Keywords: Mesenchymal stem cells; Mechanotransduction; Scaffolds; Secretome; Bioengineering
| Abbreviations | |
| CM: | Conditioned Medium |
| COX-2: | Cyclooxygenase 2 |
| hAd-MSCs: | human adipose mesenchymal stem cells |
| ICAM-1: | InterCellular Adhesion Molecule 1 |
| IGF-1: | Insulin-like Growth Factor 1 |
| IL-6: | Interleukin 6 |
| iMCP-1: | Chemokine Monocyte Chemoattractant Protein-1 |
| kPa: | Kilopascal |
| LINC: | Linker of Nucleoskeleton and Cytoskeleton |
| MSCs: | Mesenchymal Stem Cells |
| rAd-MSCs: | rat adipose mesenchymal stem cells |
| TEAD: | TEA domain |
| TSG-6: | Tumor necrosis factor stimulated gene-6 |
| YAP/TAZ: | Yes-associated protein and transcriptional co-activator with PDZ-binding motif |
Mesenchymal Stem Cells Secretomes
Advances in the study and application of technologies using stem cells have revolutionized the field of tissue engineering and regenerative medicine. The use of different populations of stem/progenitor cells for tissue engineering or its administration to injured tissues has allowed the construction of new functional tissues or tissue/organ repair and regeneration (Zakrzewski et al., 2019).
In recent decades, mesenchymal stem cells (MSCs) have been at the center of cell-based therapies. The reason for this lies in their ability to differentiate into mature end-stage cells but, above all, in their paracrine activity, which results in the production of a broad spectrum of bioactive signals with immunomodulatory and trophic/pro-regenerative activities (Murphy et al., 2013). These bioactive signals are conventionally defined as secretomes and have been identified in conditioned medium (CM) by cell culturing. Therefore, soluble proteins, lipid mediators, nucleic acid complexes and extracellular vesicles are secreted by cells in the extracellular environment, constituting the conditioned medium of the mesenchymal stem cells (MSCs-CM). These factors/molecules are considered to promote the pro-regenerative activity triggered by MSCs and have been observed in several biological systems, acting as modulators of the immune response (Brini et al., 2017; Legaki et al., 2016), inducing angiogenesis (De Luca et al., 2011), preventing apoptosis (Li et al., 2015), and generating healing effects (Park et al., 2018).
Due to this pro-regenerative potential, the MSCs secretome has attracted the interest of researchers, who envision their bioprocessing for use in cell-free therapies (Phelps et al., 2018). Considering that paracrine factors constitute the main mechanism of action of MSCs, the use of the MSCs secretome can be advantageous in several aspects when compared to cell therapy. First, there is no need to implant live cells, which avoids concerns about cell degeneration or senescence, as well as the risk of immune rejection, mutations and carcinogenesis. Furthermore, production and storage conditions are less demanding. Additionally, the prerogative that the secretome components can be customized through modifications in cell culture conditions—known as preconditioning—represents one of the greatest advantages of its use (Praveen Kumar et al., 2019).
Therefore, the preconditioning of MSCs has been widely explored through the application of a variety of stimuli and different culture conditions to target and enhance the therapeutic capacity of the MSCs secretomes. Over time, the manipulation of cell culture conditions has focused mainly on the incorporation of different chemical elements into the culture environment. Some of these manipulations, such as the reduction of oxygen tension, determined as hypoxia preconditioning, can increase the paracrine cytoprotective and immunomodulatory activity of MSCs (Hu et al., 2016; Lan et al., 2015). The addition of inflammatory cytokines and growth factors (mixed or individually; known as soluble factor preconditioning) to the culture medium is another widely used strategy to target the immunomodulatory and proangiogenic activity of MSCs (Gorin et al., 2016; Maffioli et al., 2017; Redondo-Castro et al., 2017). Similarly, pharmacological agents have also been used for preconditioning (Liu et al., 2015; Pourjafar et al., 2017). Please refer to (Ferreira et al., 2018) for an in-depth understanding of the various MSCs preconditioning strategies. In similar circumstances, the evaluation of biophysical aspects, such as the use of different biomaterials in cell culture for applications in tissue engineering, has also been explored as modulators of cell behavior and of the MSCs secretome.
Mechanotransduction at a Glance
Whatever the MSCs preconditioning strategy, the objective, as mentioned above, is to customize MSCs cultures and their secretome and, therefore, to regulate cell gene expression. However, few studies thus far have focused on understanding the mechanisms by which the physical properties of substrates or scaffolds can modulate gene expression to produce specific secretomes useful for different therapeutic applications.
Classically, gene activation or repression relies on a basic cellular circuit that initiates when a signaling molecule (hormone, cytokine, growth factor) binds to a specific receptor at the cell’s plasma membrane, activating a complex network of cytoplasmic proteins that ultimately flow into the cell nucleus promoting the activation or silencing of specific genes. In the early 1980s, a new proposal made independently by Donald Ingber and Mina Bissel began to gain attention through a bold and innovative postulation: physical forces of the environment applied to a cell membrane could promote changes in gene expression (Bissell et al., 1982; Ingber et al., 1981). The phenomenon was called mechanotransduction, which is the conversion of mechanical inputs into chemical signals and/or gene expression. It was a complete paradigm shift in which changes in cell morphology could influence gene activity. The discovery of the LINC (linker of nucleoskeleton and cytoskeleton) complex by the Donald Ingber group in 1997 (Maniotis et al., 1997) was the missing puzzle piece necessary to better understand the mechanism of mechanotransduction (Fig. 1). The LINC complex establishes a physical connection between the cytoplasmic cytoskeleton and the nucleoskeleton and is responsible for conducting the external physical forces generated in the cell membrane to the cell’s DNA.
With these observations in mind, in a short time, researchers began to design materials to control the behavior of cells. Thus, in 2006, the Dennis Discher group demonstrated that matrix elasticity can direct stem cell lineage specification (Engler et al., 2006). In this study, inert polyacrylamide gels were developed with different concentrations of bis-acrylamide, generating different crosslinks that defined the elasticity of the gels. Then, naive MSCs were seeded on these gels, and surprisingly, they differentiated with extreme sensitivity to tissue-level elasticity. For example, soft matrices that mimic the nervous system promoted neural differentiation, stiffer matrices that mimic muscle allowed the emergence of myogenic cells, and rigid matrices that mimic collagenous bone induced the appearance of cells of the osteogenic lineage (Engler et al., 2006). The only variable in this experiment involved gels of different rigidities so that the physical forces sensed by cells were able to modulate the entire gene network responsible for the differentiation of a specific cell type.
Yet, how is mechanotransduction able to direct stem cell fate choice? Essentially, as cells can sense differences in substrate stiffness through integrins, the mechanical stimulus can pass through the actin cytoskeleton that connects to the nuclear envelope. The nuclear envelope is physically tethered to the actin cytoskeleton via the LINC complex, which consists of KASH-domain proteins and SUN-domain proteins (Fig. 1). The latter connect to lamin proteins (part of the nucleoskeleton) that in turn anchor chromatin loops. This mechanical force can displace certain genes that were located in transcriptionally inactive regions to active regions (and vice versa). Therefore, substrates of different topologies, stiffnesses, and roughnesses can modulate gene transcription based on the spatial displacement of chromatin (Fig. 1). This must also be true in vivo, where fluids, neighboring cells, and extracellular matrices may constitute physical forces that influence cell behavior.
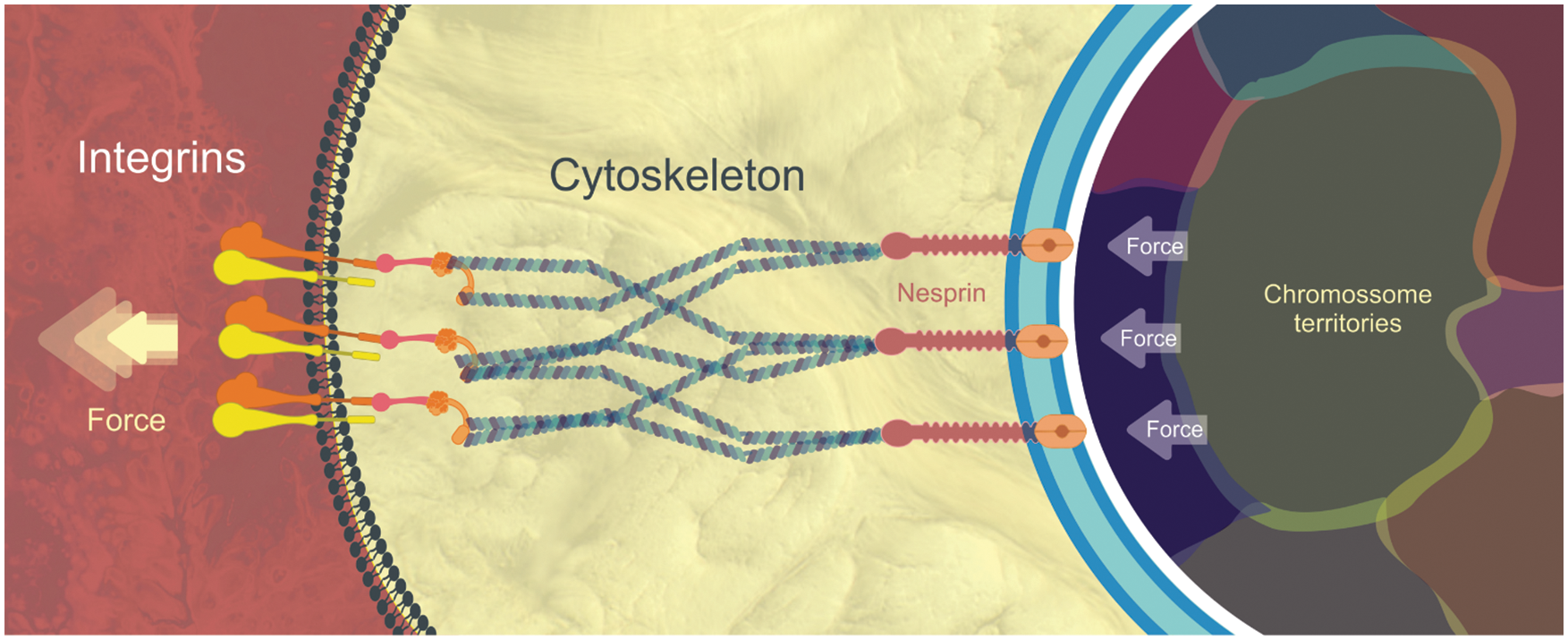
Figure 1: Simplified view of Mechanotransduction and LINC complex: Physical forces promote a “pull out” of the plasma membrane through interactions between integrins and the extracellular matrix. This force is transmitted through the cell’s cytoskeleton to the nesprins, whose terminal domain (Kash) inserts itself into the outer nuclear envelope. In the perinuclear space and crossing the inner nuclear membrane we find the SUN proteins (in orange). Kash and Sun make part of the LINC complex. Finally, SUN connects with nuclear lamina proteins (white line) which in turn anchor the chromatin fibers present in specific chromosomal territories. Physical forces applied in the opposite direction (against the plasma membrane) can also displace the chromosomal territories promoting a different pattern of gene activation and repression (Wang et al., 2009).
It is important to note that according to Tremblay et al. (2013), although the cytoskeleton is capable of force transmission from the extracellular matrix to the nuclear envelope, the forces it transmits to the nucleus is limited due to its significantly smaller cross-sectional area in comparison to the nucleus. This incapacity of the cytoskeleton to transmit all the stress to the nucleus is translated into the deformation of the cytoskeleton. Thus, it is expected that only a large microenvironmental strain is capable of inducing a deformation able to regulate the gene activity (Tremblay et al., 2013).
For this reason, mechanotransduction must currently be viewed as a multitude of coupled complex events, where application of a mechanical stimulus to the cell surface not only promotes deformation of the lipid bilayer but also triggers the activation of several types of membrane-associated signal-transduction molecules and mechanosensitive ion channels, like Piezo 1 activation, which very recently showed to be related to YAP nuclear translocation and regulation of its target genes (Liu et al., 2021).
In this line, the discovery of YAP/TAZ transcription factors that work as molecular “readers” of ECM elasticity (and geometry) added new complexities to mechanotransduction. Both proteins were shown to be relevant to mediating biological responses to mechanical inputs. The subcellular localization of these “sensors” is related to substrate stiffness and consequently to the fate adopted by MSCs. For example, YAP and TAZ are nuclear on hard substrates and translocate to cytoplasm on softer substrates. Thus, inhibition of YAP/TAZ (which mimics a soft environment) blocks osteogenic differentiation of MSCs on stiff matrices. Conversely, the knockdown of YAP/TAZ allows MSCs to differentiate into adipocytes when seeded on stiff substrates (Dupont et al., 2011). These results clearly show that YAP/TAZ are key elements that transmit important mechanical cues from the environment. They continually shuttle between the cytoplasm and nucleus, but the presence of a tense cytoskeleton promotes their nuclear retention. In the nucleus, YAP/TAZ act as coactivators for the TEAD family of DNA-binding proteins to regulate gene expression (Currey et al., 2021). Therefore, YAP/TAZ regulate gene expression by acting as dynamic sensors of mechanical forces conveyed by cytoskeletal tension.
Linking Mechanotransduction with Mesenchymal Stem Cell Secretomes
If mechanical forces can be so powerful to dictate the destiny of undifferentiated cells, could we imagine that physical forces could modulate the secretome profile of cells? The answer is affirmative, according to some works in the literature showing that stiffness, topology, and dimensionality are correlated with changes in the secretome profile produced by cells.
For example, Carter and colleagues analyzed the profile of secreted factors produced by human bone marrow-derived mesenchymal stem cells (hMSCs) as a function of their growth in 2D culture dishes and/or on 3D electrospun fiber scaffolds (composed of polycaprolactone and gelatin) (Carter et al., 2019). This 3D scaffold was optimized to mimic the mechanical properties of corneal tissue. A Luminex immunoassay showed that the concentrations of factors such as fibroblast growth factor beta (FGF-b), hepatocyte growth factor (HGF), and ICAM-1 were substantially elevated in 3D cultures compared to 2D cultures. The increase in these factors in the secretome under MSC-3D conditions may be related to the enhanced wound healing effects seen in the in vitro migration assay performed with corneal fibroblasts.
Su and colleagues correlated electrospun polycaprolactone fiber morphology and orientation with the paracrine secretion and function of rat adipose mesenchymal stem cells (rAd-MSCs) (Su et al., 2017). The scaffolds included electrospun fibers in random, aligned, and mesh organizations. The rAd-MSCs were cultured on these different scaffolds and in polystyrene microplates. They observed that all fibers enhanced the paracrine function of MSCs compared to plastic plates and that the cells exhibited differential secretome profiles that correlated with the specific fibrous topography and orientation. For example, cells cultured on aligned and mesh fibers secreted increased levels of the immunosuppressive mediators prostaglandin E2 (PGE2) and inducible nitric oxide synthase (iNOS) compared to cells cultured on random fibers. Moreover, cells cultured in mesh fibers secreted much more HGF and vascular endothelial growth factor (VEGF) than cells cultured on aligned and random fibers. In a functional assay, CM derived from cells cultured on mesh fibers showed potent effects on promoting the anti-inflammatory responses of macrophage cells compared to CM obtained from cells cultured on random fibers. The authors did not address the mechanisms by which the fibers can induce such differences in the secretome profile but they suggest that it may be related to differences in cell shape. Therefore, the authors noted that on random fibers, cells exhibited a round shape; on aligned fibers, they were fully stretched; and on mesh fibers, the cell shapes were in between. They compare their results to studies that demonstrate that stem cell differentiation can be alterable by substrates affecting cell orientation and shape.
Wan and colleagues advanced along and investigated the mechanism behind the effects of poly (L-lactic acid) (PLLA) electrospun fiber orientation on the secretory behavior of human adipose mesenchymal stem cells (hAd-MSCs) concerning immunomodulation. Here, they clearly showed the involvement of YAP/TAZ mechanotransducers in this phenomenon (Wan et al., 2018). The fibrous scaffolds were developed with two different orientations, random fibers (RF) vs. aligned fibers (AF). They observed the markedly elevated production of both TSG-6 and COX-2 in hAd-MSCs on AF. TSG-6 is considered a biomarker for predicting the immunosuppressive efficacy of MSCs. COX-2 is an essential enzyme for the synthesis of the immunosuppressive mediator PGE2. These data indicate that AF may promote the immunomodulatory efficacy of hAd-MSCs. The group observed a high immunofluorescence signal of YAP/TAZ in the nuclei of hAd-MSCs cultured on AF. Conversely, cells cultured on RF exhibited YAP/TAZ located mainly in the cytoplasm. Activated YAP/TAZ signaling was correlated with the enhanced immunomodulatory properties of hAd-MSCs on AF since specific inhibition of YAP/TAZ with verteporfin reduced the gene expression of the immunomodulatory factors COX-2, TSG-6, IL-1ra, and MCP-1. Thus, the authors suggest that aligned fibers must activate YAP/TAZ signaling, keeping hAd-MSCs in a multipotent status, which is also required for their immunomodulation properties through the secretion of immunomodulatory factors. This was the first study to demonstrate the involvement of YAP/TAZ signaling in mediating the physical cues provided by fiber orientation on the immunomodulation of hAd-MSCs.
Cadherins are cell-cell adhesion proteins that act as force transducers regulating cytoskeletal organization and signaling in response to changes in intercellular tension. The possibility of creating tailored biomaterials allowed the presentation of N-cadherin-engaging peptides, such as HAVDI, in combination with integrin-engaging ligand RGD, to modulate several cell behaviors, such as adhesion, proliferation, and differentiation. More recently, Qin and coworkers analyzed the effects of these modified substrates on the secretome profile of MSCs (Qin et al., 2020). The researchers tested three different immobilized N-cadherin-derived fragments and demonstrated that MSCs cultured on the full N-cadherin extracellular domain (EC1-5) exhibited stiffness-dependent changes in nuclear YAP/TAZ localization and significantly higher secretion of VEGF and IGF-1 compared to cells cultured on hydrogels displaying either EC1-2 or the HAVDI peptide. The increased paracrine secretion also enhanced myogenic differentiation. However, the authors did not establish a relationship between the nuclear accumulation of YAP/TAZ and paracrine secretion since N-cad EC1-2 and HAVDI bind MSCs with similar affinities and appear to elicit similar IGF-1 secretion.
Finally, in 2020, Molly Ogle and colleagues designed polyethylene glycol hydrogel substrates with tunable mechanical and biochemical properties to screen the effect of culture surfaces on the secretome profile of MSCs-CM (Ogle et al., 2020). The MSCs were cultured in two-dimensional materials with Young’s moduli spanning from 30–100 kPa (Young’s modulus measures the tensile or compressive stiffness of solid material when the force is applied lengthwise). Moreover, a variety of biochemical modifications, such as the integration of adhesive ligands (HAVDI, RGD, and the glycosaminoglycan heparin), were also accomplished on these substrates.
Importantly, the authors observed that the substrate stiffness exerted a greater impact on MSC secretory profiles than the biochemical composition of the biomaterial. Hydrogels of 30 kPa stiffness halted MSC proliferation but broadly enhanced the level of immunomodulatory factors versus either 100-kPa hydrogels or polystyrene/plastic culture dishes that were several orders of magnitude stiffer (GPa range). Despite changes in some individual factors (i.e., iMCP-1 and IL-6), MSCs cultured on 100-kPa hydrogels did not exhibit a broad distinct secretory profile from those cultured on plastic dishes. Moreover, 100-kPa gels promoted MSC proliferation with reduced replicative senescence compared to cells cultured on plastic dishes. Very importantly, unlike cells cultured on plastic dishes, MSCs serially passaged on 100-kPa RGD hydrogels are able to retain the ability to secrete many growth factors, cytokines, and chemokines when transferred to 30-kPa hydrogels (Ogle et al., 2020).
The group also performed functional assays to compare the effects of CM obtained from MSCs cultured on hydrogels of different stiffnesses. The CM from 30-kPa surfaces enhanced HUVEC network formation compared with 100-kPa surfaces. Therefore, CM from 30-kPa surfaces exhibited increased angiogenic potency. These results corroborated other studies showing that substrates promoting MSC aggregation and surfaces with stiffness of approximately 40 kPa enhanced VEGF secretion, improving proangiogenic signaling (Abdeen et al., 2014; Lee et al., 2012).
Our aim was not to perform an extensive review of the subject, and we apologize for the many scientific articles that were potentially left out. Our objective was to show that there are already some works, the vast majority of them very recent, that seek to correlate the physical properties of substrates influencing the secretome profile of cells. Unfortunately, to date, few studies have associated or addressed the real mechanism behind the effects observed. The evidence is that the vast majority of the studies described here did not perform a simple comparative analysis of the cell morphology cultured under different substrates.
Therefore, we propose that mechanotransduction must be the key mechanism that can explain why cells cultured under specific substrates produce different secretome profiles. Furthermore, we suggest that the possibility of creating substrates with different physical properties aiming at the production of specific secretome profiles should be the next step in biotechnological research for application in regenerative medicine using the secretome of MSCs.
For example, Fig. 2 exemplifies the main steps by which substrates with different rigidities can be employed to pre-conditionate MSCs to produce pro-angiogenic secretomes. Ideally, future studies should initially work with single cells placed on substrates with different physical properties. These single cells must undergo a rigorous and thorough imaging process that can correlate their morphology with the cytoplasmic cytoskeletal conformation and the consequent arrangement adopted by specific chromatin territories in the nucleus. This correlation between cellular morphology and chromatin territories, in turn, must be correlated to specific elicited gene transcription and inhibition events (transcriptomic profile). Finally, this should be correlated with a detailed proteomic analysis of the secretome produced by cells. In this sense, we envisage that different substrates using the same type of MSC (a strain or lineage) can allow the creation of a secretome bank, the profile of which can be standardized and used for specific therapeutic situations (i.e., angiogenesis, immunomodulation, tissue regeneration, etc.). Doubtless, it is increasingly necessary that single-cell analyses continue to progress, especially concerning the possibility of carrying out in-depth studies from small samples.
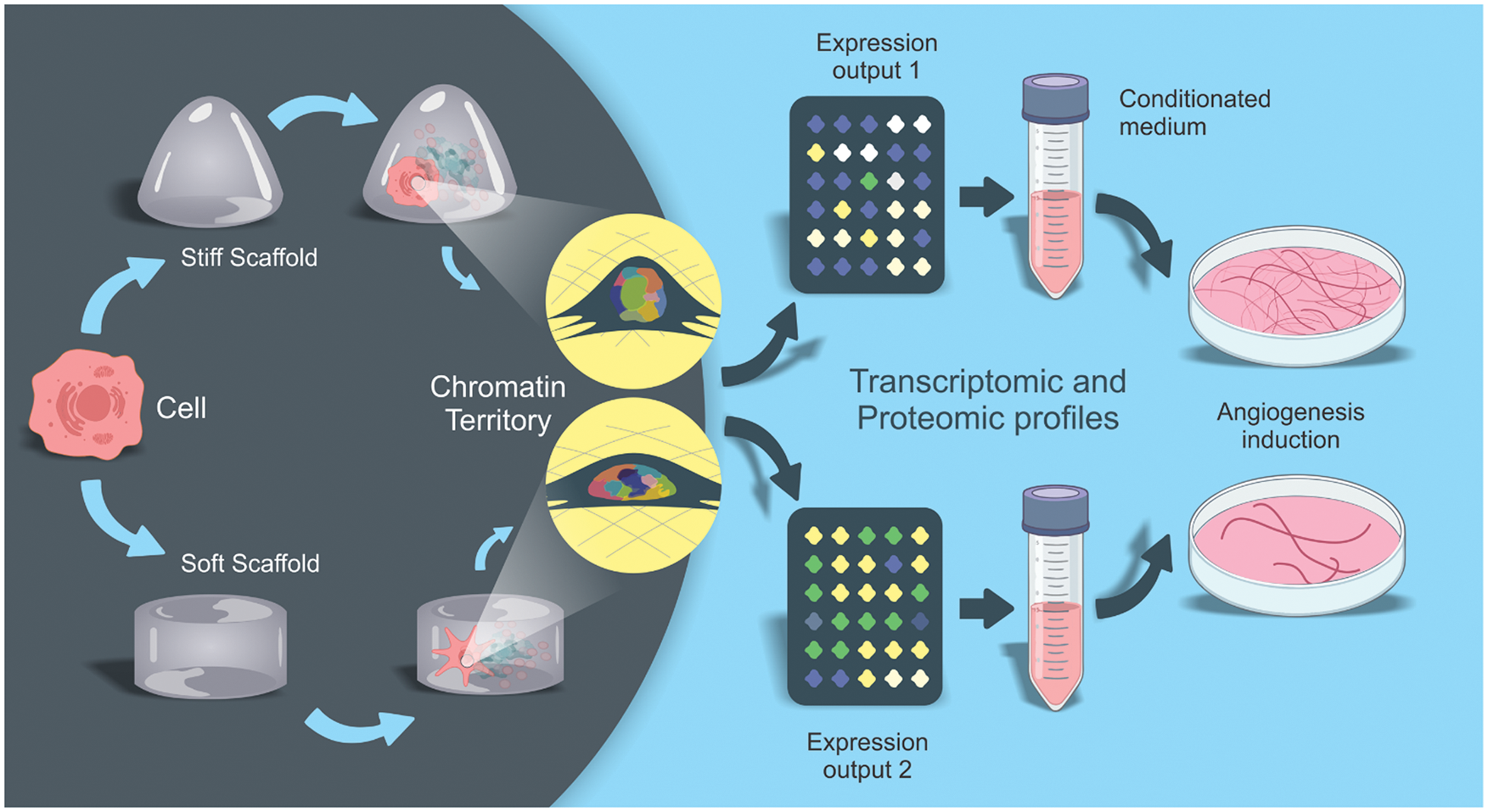
Figure 2: Using mechanotransduction to produce customized secretomes.
Recent studies, including those of our group, show the benefits of employing the harvested secreted products from cells compared to cell infusion/transplantation (Robert et al., 2019; Rode et al., 2018). The use of the cell secretome allows more convenient control of the dosage and storage of the therapeutic substance and avoids important immunological concerns. However, it is absolutely necessary to establish regulatory policies to address the quality, safety, and efficacy of this new category of therapeutics containing highly heterogeneous components. Effectively, a deep comprehension of the mechanism behind the generation of a specific secretome profile can not only give us the possibility to customize and expand the use of conditioned medium from cells but also facilitate reproducibility and consistency to ensure the quality of the products produced and, hopefully, affordable prices for public health systems.
Acknowledgement: The authors would like to thank Wagner Nagib from the Carlos Chagas Institute Communication Advisory for the scientific illustrations.
Authors’ Contribution: The authors contributed equally to study conception and design of this manuscript.
Ethics Approval: Not applicable.
Funding Statement: This work was supported by Brazilian National Council for Scientific and Technological Development (CNPq Grant No. 442411/2019-7).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abdeen AA, Weiss JB, Lee J, Kilian KA (2014). Matrix composition and mechanics direct proangiogenic signaling from mesenchymal stem cells. Tissue Engineering Part A 20: 2737–2745. DOI 10.1089/ten.tea.2013.0661. [Google Scholar] [CrossRef]
Bissell MJ, Hall HG, Parry G (1982). How does the extracellular matrix direct gene expression? Journal of Theoretical Biology 99: 31–68. DOI 10.1016/0022-5193(82)90388-5. [Google Scholar] [CrossRef]
Brini AT, Amodeo G, Ferreira LM, Milani A, Niada S et al. (2017). Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Scientific Reports 7: 9904. DOI 10.1038/s41598-017-09487-5. [Google Scholar] [CrossRef]
Carter K, Lee HJ, Na KS, Fernandes-Cunha GM, Blanco IJ, Djalilian A, Myung D (2019). Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomaterialia 99: 247–257. DOI 10.1016/j.actbio.2019.09.022. [Google Scholar] [CrossRef]
Currey L, Thor S, Piper M (2021). TEAD family transcription factors in development and disease. Development 148: 17225. DOI 10.1242/dev.196675. [Google Scholar] [CrossRef]
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature 474: 179–184. DOI 10.1038/nature10137. [Google Scholar] [CrossRef]
Engler AJ, Sen S, Sweeney HL, Discher DE (2006). Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689. DOI 10.1016/j.cell.2006.06.044. [Google Scholar] [CrossRef]
Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM (2018). Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Frontiers in Immunology 9: 2837. DOI 10.3389/fimmu.2018.02837. [Google Scholar] [CrossRef]
Gorin C, Rochefort GY, Bascetin R, Ying H, Lesieur J et al. (2016). Priming dental pulp stem cells with fibroblast growth factor-2 increases angiogenesis of implanted tissue-engineered constructs through hepatocyte growth factor and vascular endothelial growth factor secretion. Stem Cells Translational Medicine 5: 392–404. DOI 10.5966/sctm.2015-0166. [Google Scholar] [CrossRef]
Hu X, Xu Y, Zhong Z, Wu Y, Zhao J et al. (2016). A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: Paracrine activity without remuscularization. Circulation Research 118: 970–983. DOI 10.1161/CIRCRESAHA.115.307516. [Google Scholar] [CrossRef]
Ingber DE, Madri JA, Jamieson JD (1981). Role of basal lamina in neoplastic disorganization of tissue architecture. Proceedings of the National Academy of Science 78: 3901–3905. DOI 10.1073/pnas.78.6.3901. [Google Scholar] [CrossRef]
Praveen Kumar L, Kandoi S, Misra R, Vijayalakshmi S, Rajagopal K et al. (2019). The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine & Growth Factor Reviews 46: 1–9. DOI 10.1016/j.cytogfr.2019.04.002. [Google Scholar] [CrossRef]
Lan YW, Choo KB, Chen CM, Hung TH, Chen YB, Hsieh CH, Kuo HP, Chong KY (2015). Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Research & Therapy 6: 97. DOI 10.1186/s13287-015-0081-6. [Google Scholar] [CrossRef]
Lee EJ, Park SJ, Kang SK, Kim GH, Kang HJ, Lee SW, Jeon HB, Kim HS (2012). Spherical bullet formation via E-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Molecular Therapy 20: 1424–1433. DOI 10.1038/mt.2012.58. [Google Scholar] [CrossRef]
Legaki E, Roubelakis MG, Theodoropoulos GE, Lazaris A, Kollia A, Karamanolis G, Marinos E, Gazouli M (2016). Therapeutic potential of secreted molecules derived from human amniotic fluid mesenchymal stem/stroma cells in a mice model of colitis. Stem Cell Reviews and Reports 12: 604–612. DOI 10.1007/s12015-016-9677-1. [Google Scholar] [CrossRef]
Li B, Zhang H, Zeng M, He W, Li M, Huang X, Deng DYB, Wu J (2015). Bone marrow mesenchymal stem cells protect alveolar macrophages from lipopolysaccharide-induced apoptosis partially by inhibiting the Wnt/β-catenin pathway. Cell Biology International 39: 192–200. DOI 10.1002/cbin.10359. [Google Scholar] [CrossRef]
Liu J, Zhu P, Song P, Xiong W, Chen H et al. (2015). Pretreatment of adipose derived stem cells with curcumin facilitates myocardial recovery via antiapoptosis and angiogenesis. Stem Cells International 2015: 1–12. DOI 10.1155/2015/638153. [Google Scholar] [CrossRef]
Liu S, Xu X, Fang Z, Ning Y, Deng B, Pan X, He Y, Yang Z, Huang K, Li J (2021). Piezo1 impairs hepatocellular tumor growth via deregulation of the MAPK-mediated YAP signaling pathway. Cell Calcium 95: 102367. DOI 10.1016/j.ceca.2021.102367. [Google Scholar] [CrossRef]
De Luca A, Gallo M, Aldinucci D, Ribatti D, Lamura L, D’Alessio A, De Filippi R, Pinto A, Normanno N (2011). Role of the EGFR ligand/receptor system in the secretion of angiogenic factors in mesenchymal stem cells. Journal of Cell Physiology 226: 2131–2138. DOI 10.1002/jcp.22548. [Google Scholar] [CrossRef]
Maffioli E, Nonnis S, Angioni R, Santagata F, Calì B, Zanotti L, Negri A, Viola A, Tedeschi G (2017). Proteomic analysis of the secretome of human bone marrow-derived mesenchymal stem cells primed by pro-inflammatory cytokines. Journal of Proteomics 166: 115–126. DOI 10.1016/j.jprot.2017.07.012. [Google Scholar] [CrossRef]
Maniotis AJ, Chen CS, Ingber DE (1997). Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Science 94: 849–854. DOI 10.1073/pnas.94.3.849. [Google Scholar] [CrossRef]
Murphy MB, Moncivais K, Caplan AI (2013). Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Experimental & Molecular Medicine 45: e54. DOI 10.1038/emm.2013.94. [Google Scholar] [CrossRef]
Ogle ME, Doron G, Levy MJ, Temenoff JS (2020). Hydrogel culture surface stiffness modulates mesenchymal stromal cell secretome and alters senescence. Tissue Engineering Part A 26: 1259–1271. DOI 10.1089/ten.tea.2020.0030. [Google Scholar] [CrossRef]
Park SR, Kim JW, Jun HS, Roh JY, Lee HY, Hong IS (2018). Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Molecular Therapy 26: 606–617. DOI 10.1016/j.ymthe.2017.09.023. [Google Scholar] [CrossRef]
Phelps J, Sanati-Nezhad A, Ungrin M, Duncan NA, Sen A (2018). Bioprocessing of mesenchymal stem cells and their derivatives: Toward cell-free therapeutics. Stem Cells International 2018: 1–23. DOI 10.1155/2018/9415367. [Google Scholar] [CrossRef]
Pourjafar M, Saidijam M, Mansouri K, Ghasemibasir H, Karimi Dermani F, Najafi R (2017). All-trans retinoic acid preconditioning enhances proliferation, angiogenesis and migration of mesenchymal stem cell in vitro and enhances wound repair in vivo. Cell Proliferation 50: e12315. DOI 10.1111/cpr.12315. [Google Scholar] [CrossRef]
Qin EC, Ahmed ST, Sehgal P, Vu VH, Kong H, Leckband DE (2020). Comparative effects of N-cadherin protein and peptide fragments on mesenchymal stem cell mechanotransduction and paracrine function. Biomaterials 239: 119846. DOI 10.1016/j.biomaterials.2020.119846. [Google Scholar] [CrossRef]
Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E (2017). Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Research Therapy 8: 79. DOI 10.1186/s13287-017-0531-4. [Google Scholar] [CrossRef]
Robert AW, Azevedo Gomes F, Rode MP, Marques da Silva M, Veleirinho MBDR, Maraschin M, Hayashi L, Wosgrau Calloni G, Stimamiglio MA (2019). The skin regeneration potential of a pro-angiogenic secretome from human skin-derived multipotent stromal cells. Journal of Tissue Engineering 10: 204173141983339. DOI 10.1177/2041731419833391. [Google Scholar] [CrossRef]
Rode MP, Batti Angulski AB, Gomes FA, da Silva MM, da Jeremias TS et al. (2018). Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. Journal of Biomaterials Applications 33: 422–434. DOI 10.1177/0885328218795569. [Google Scholar] [CrossRef]
Su N, Gao PL, Wang K, Wang JY, Zhong Y, Luo Y (2017). Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials 141: 74–85. DOI 10.1016/j.biomaterials.2017.06.028. [Google Scholar] [CrossRef]
Tremblay D, Andrzejewski L, Leclerc A, Pelling AE (2013). Actin and microtubules play distinct roles in governing the anisotropic deformation of cell nuclei in response to substrate strain. Cytoskeleton 70: 837–848. DOI 10.1002/cm.21148. [Google Scholar] [CrossRef]
Wan S, Fu X, Ji Y, Li M, Shi X, Wang Y (2018). FAK- and YAP/TAZ dependent mechanotransduction pathways are required for enhanced immunomodulatory properties of adipose-derived mesenchymal stem cells induced by aligned fibrous scaffolds. Biomaterials 171: 107–117. DOI 10.1016/j.biomaterials.2018.04.035. [Google Scholar] [CrossRef]
Wang N, Tytell JD, Ingber D (2009). Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nature Reviews Molecular Cell Biology 10: 75–82. DOI 10.1038/nrm2594. [Google Scholar] [CrossRef]
Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z (2019). Stem cells: Past, present, and future. Stem Cell Research & Therapy 10: 68. DOI 10.1186/s13287-019-1165-5. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |