DOI:10.32604/biocell.2022.018967

| BIOCELL DOI:10.32604/biocell.2022.018967 |  |
| Article |
Cyclic biaxial tensile strain enhances osteogenic differentiation in rat bone marrow-derived mesenchymal stem cells via activating ERα-Wnt3a/β-catenin pathway
1Institute of Biomedical Engineering, School of Preclinical and Forensic Medicine, Sichuan University, Chengdu, 610041, China
2Laboratory Animal Center of Sichuan University, Chengdu, 610041, China
*Address correspondence to: Liang Li, lilianghx@163.com
#These authors contributed equally to this work
Received: 26 August 2021; Accepted: 11 October 2021
Abstract: The present study was designed to investigate the role of estrogen receptor α (ERα) in biaxial tensile strain (BTS) regulated osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells (rBMSCs). rBMSCs were derived from rats and overexpressed ERα. The rBMSCs were subjected to BTS at 1 Hz with a strain of 2% for 4 h per day, 3 days, with or without ERα inhibitor ICI 182,780 (ICI). Then, bone mineralization was performed by Alizarin Red Staining. The markers of osteogenic differentiation and downstream Wnt3a/β-catenin signaling were detected by western blotting. Results showed that BTS enhanced the osteogenic differentiation of rBMSCs, increased protein expression levels of alkaline phosphatase (ALP), runt-related transcription factor 2 (Runx2), collagen type I (Col I) and osteocalcin (OCN), and it increased the protein expression levels of estrogen receptor (ER) α (ERα), Wnt3a, and β-catenin. BTS The activated Wnt3a/β-catenin signaling pathway induced by BTS was abolished by ICI 182,780 (ICI). In addition, overexpressing ERα in rBMSCs promoted the osteogenic differentiation by BTS. Taken together, BTS induced osteogenic differentiation of rBMSCs via the ERα and downstream canonical Wnt3a/β-catenin pathway.
Keywords: BMSCs; BTS; Osteogenic differentiation; ERα; Wnt pathway
Osteoporosis is a systemic skeletal disorder characterized by reduced bone mass, increased bone fragility and fracture risk due to the destruction of bone microstructures. There are far more women than men in osteoporosis patients, especially postmenopausal women. The decrease in estrogen level is the main cause of postmenopausal osteoporosis (Sharma et al., 2018). The expression of estrogen receptor (ER), especially ERα, is regulated by the hormone levels in vivo (Khalid and Krum, 2016). With the decrease of estrogen level in postmenopausal women, the number and function of ERα on osteocytes reduce, which significantly increases the incidence of osteoporosis in postmenopausal women (Lanyon et al., 2004). Recent studies had also found that estrogen receptors could also interact with some signaling pathways in cells through phosphorylation to activate themselves in an estrogen-independent manner. Therefore, ERα and its downstream signaling pathway is an important mechanism of regulating osteoporosis.
Distraction osteogenesis is an important routine method for bone regeneration and bone bioengineering. Active osteoblasts are important mechanical stimulation receptor cells on the surface of bone, and they are also the ultimate effector cells of new bone formation, but osteoblasts do not have the ability of proliferation. Study showed that bone marrow mesenchymal stem cells (BMSCs) were the key cells affecting the balance of bone metabolism, which had the ability of proliferation and differentiation. There is comparatively less differentiation of BMSCs into osteoblast than adipocytes in osteoporosis. Such a shift in cell differentiation of BMSCs results in reduced bone formation, which contributes to osteoporosis (Infante and RodrÃguez, 2018).
Additionally, rat bone marrow mesenchymal stem cells (rBMSCs) are mechanosensitive cells and may be the main effector cells of bone tissue stretch (Zeng et al., 2017). In recent years, it has been confirmed that stretch can induce the osteogenic differentiation of BMSCs. However, the specific mechanism of stretch regulating the osteogenic differentiation of BMSCs is still not fully understood. Previous study found that ERα showed a response to mechanical traction in vivo and in vitro (Lee et al., 2003; Windahl et al., 2013). Our previous works also found that low magnitude vibration (LMV) promoted osteogenic differentiation of rBMSCs via the canonical Wnt pathway by up-regulating ERα in vivo (Li et al., 2019; Li et al., 2013), suggesting that ERα is a potential mediator for the mechanical response of bone tissue in the process of bone formation and metabolism.
Because the role of ERα in stretch-induced osteogenic differentiation of rBMSCs was still uncertain, to test whether BTS regulates the osteogenic differentiation of rBMSCs via ERα-canonical Wnt pathway, we evaluate the effects of ERα-canonical Wnt pathway on BTS-induced osteogenic differentiation of rBMSCs by ERα inhibitor and overexpressing ERα.
The BTS device was specifically designed for the cell type used in our study (Yan et al., 2013). This device mainly includes four parts: the control system (a regulator for parameters of strain, frequency, and time), motor diver, piston drive and culture chambers (φ = 3.5 cm, H = 3 cm). The culture chamber is divided into upper and lower parts by the flexible silicon membrane, the upper part is used for cell culture and the lower part is air chamber with a ventilated column. When the device is working, air in the column is sucked to make the silicon membrane oppress to the column and thus forming a fixed tensile strain, as showed in Fig. 1. The flexible silicone membranes had been precoated with type I collagen overnight before rBMSCs were seeded.
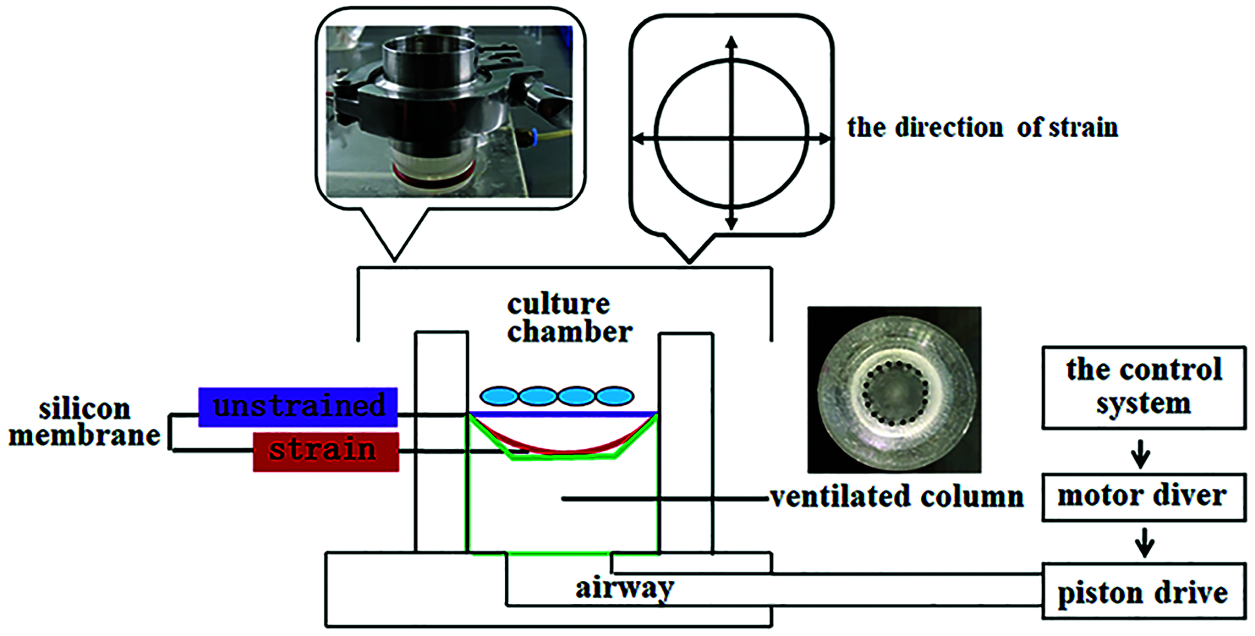
Figure 1: The biaxial tensile strain device. Schematic representation and images depicted the MechanoCulture device inside the cell culture incubator, as well as the in vitro model used to deliver cyclic tensile strain. Cells were seeded on a deformable silicone membrane puncture mounted on a ring designed to generate fixed tensile strain, resulting in the delivery of biaxial stretch.
RBMSCs were isolated as described previously (Yan et al., 2013). Briefly, the bone marrow was obtained from femur and tibia of 3-month-old Sprague-Dawley female rats (N = 6) and was suspended in DMEM-LG (Thermo Fisher Scientific) containing 15% fetal bovine serum (FBS; Hyclon), 2 mM L-glutamine (Thermo Fisher Scientific), 100 U/mL penicillin and 100 μg/mL streptomycin (Thermo Fisher Scientific). Cells were cultured at 37°C in a humidified 5% CO2 incubator. Non-adherent cells were removed by changing culture medium. The adherent cells were passaged upon reaching 80% confluences with 0.25% Trypsin/EDTA solution (Thermo Fisher Scientific) at room temperature, subcultured into T25 culture flasks. Immunophenotyping of BMSCs was analyzed by flow cytometry to make sure that the cells were positive for CD90, CD44, CD29, and negative for CD31, CD45, CD11b.
The cells (P3-P4) were seeded onto six-hole plate at a density of 3 × 104 cells/well and incubated in DMEM-LG containing 2% FBS overnight (12 h) before experiments. Through the selection of mechanical parameters, we chose 2% mechanical strain for 4 h as the mechanical stimulus parameters for the next study. Then, RBMSCs were sorted into the following groups based on the treatments: (1) BTS (BTS group): cells were subjected to BTS at frequency 1 Hz with a strain of 2%, 4 h every day for 3 days. (2) E2 (E2 group): cells were exposed to 17β-Estradiol (100 nM) for 3 days; (3) BTS with E2 supplements (BTS+E2 group): cells were subjected to BTS 4 h every day at frequency 1 Hz with a strain of 2% and treated with 17β-Estradiol (100 nM) for 3 days; (4) BTS with ICI supplements (BTS+ICI group): cells were treated with ICI 182780 (50 ng/mL) for 24 h before subjected to BTS 4 h every day at frequency 1 Hz with a strain of 2% for 3 days; (5) DMEM-LG medium (Control group): cells were cultured with DMEM-LG medium for 3 days. Samples of all cells were collected after treatment.
To overexpress the ERα in the rBMSCs, PcDNA3.1(+)-ERα DNA constructs were transfected before the load of the BTS. PcDNA3.1(+)-ERα DNA constructs used in this study were described previously (Zhu et al., 2014). For transient transfection, rBMSCs were grown in 6-well plates to 70%–80% confluent and transfected using Lipofectamine Stem transfection reagents (Thermo Fisher Scientific) were used according to the instructions of the manufacturer. Cells were analyzed as for the expression levels of different proteins.
Quantitative real-time RT-PCR (qRT-PCR) assays
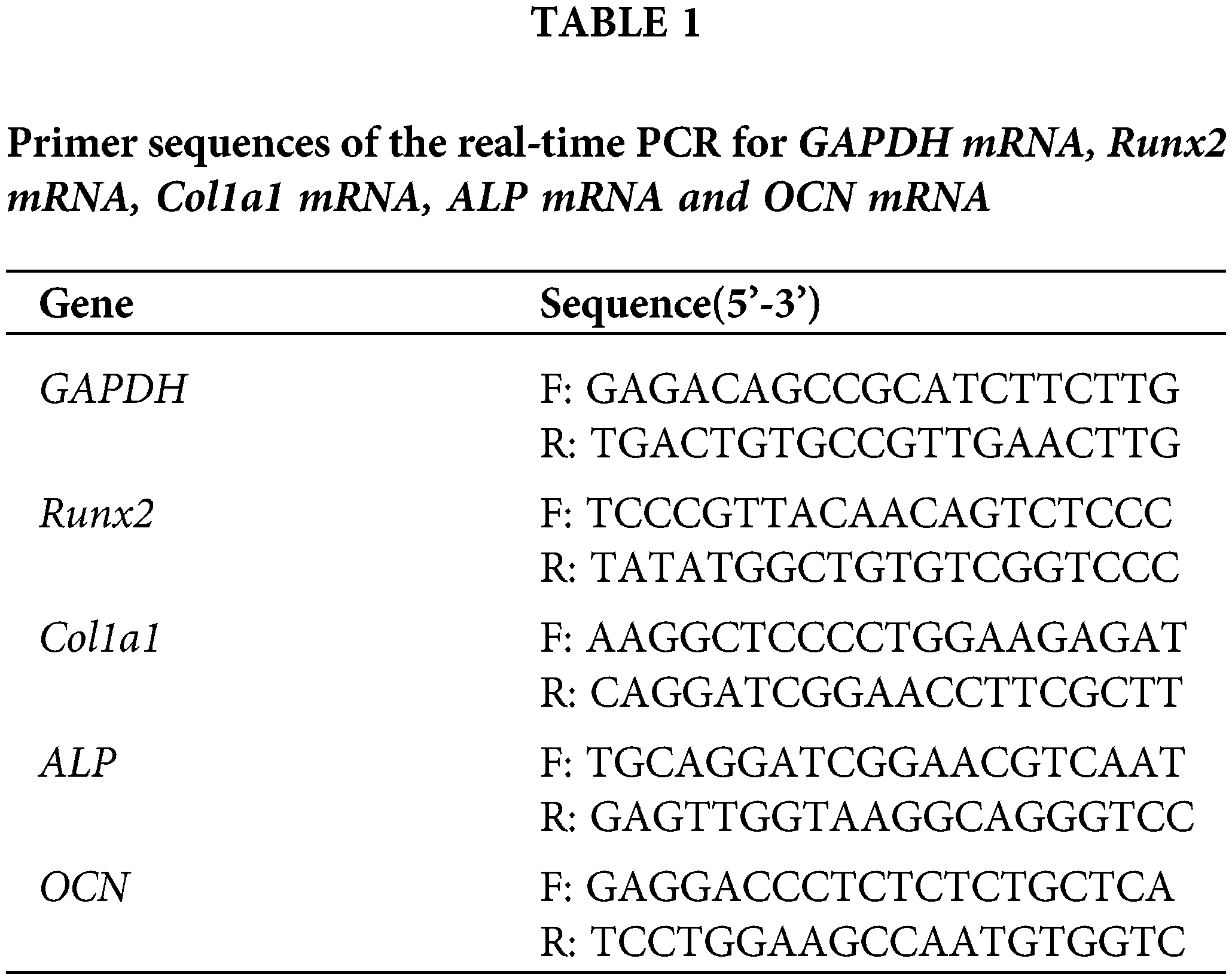
Total RNA was extracted using the Trizol Reagent (Thermo Fisher Scientific). One microgram of RNA was reverse transcribed using RNA PCR kit (Thermo Fisher Scientific). Complementary cDNA was synthesized from RNA using reverse transcription kit (Thermo Fisher Scientific). One microliter of cDNA was then used for PCR using primers for Runx2, Col I, ALP, OCN and glyceraldehyde 3-phosphatedehydrogenase (GAPDH), using TB Green Premix Ex Taq II (Tli RNaseH Plus) (Takara-Bio). Primers for osteogenic differentiation are shown in the Table 1. PCR was initiated at 95°C for 3 min, followed by 40 cycles of 15 s at 95°C, 10 s of primer-specific annealing, 72°C for 15 s, and a melt curve analysis at 65–96°C. PCR products were subjected to melting curve analysis to test if any nonspecific products were generated. The PCR of different amplicons had equal efficiency. Relative expression values were calculated using the comparative threshold cycle (2−ΔΔCT). GAPDH served as the housekeeping gene.
The cells were washed with ice-cold PBS and lysed in RIPA buffer (Thermo Fisher Scientific) for 30 min on ice. Samples were centrifuged at 12000 × g for 5 min at 4°C. Total cell protein was quantified by the BCA Protein Assay Kit (Beyotime, CHN). After that, 40 μg of total protein was separated by 10%–12% SDS-PAGE and then transferred onto 0.22-μm PVDF membranes (Millipore, USA). These membranes were blocked with freshly prepared TBS/T containing 5% non-fat dry milk. Primary antibodies directed against the following proteins were used: ERα, Wnt3a, β-catenin, Runx2, Col I, ALP, OCN, and β-actin (1:1000, Proteintech Group, Inc., USA). Membranes were washed with TBS/T and incubated with a secondary antibody conjugated with horseradish peroxidase (1:5000, Proteintech Group, Inc., USA) for 1 h at room temperature. Immunoreactive bands were visualized with the ECL kit (Thermo Fisher Scientific), and protein bands were quantified using Quantity One software (Bio-Rad).
The rBMSCs were plated in culture chambers at the density of 3 × 104 cells/well with the osteogenic inducing medium (DMEM containing 10% FBS, 50 μg/mL of ascorbic acid, and 5 mM β-phosphoglycerate, dexamethasone) (Cyagen, CHN), following the respective treatments (ERα, BTS, or BTS+ERα) for 3 days. The untreated cells with osteogenic medium served as control group. The medium was changed every 3 days with half volume. Then all cells were stained with Alizarin Red dye according to the manufacturer’s protocols to assess the osteogenic differentiation and bone mineralization. Briefly, the cells were fixed in 70% ethanol for 1 h and stained with Alizarin Red solution, pH 4.1 (Solarbio) at room temperature for 20 min with shaking. The cells were washed with PBS to remove unbound dye and observed under the microscope.
Three independent experiments in each test were performed. All data analysis involved estimation of means and SD using SPSS version 15.0 software (SPSS, Inc.). One-way analysis of variance (ANOVA) was used to compare the means of each group. The significance of between-group differences was evaluated with Dunnett’s multiple comparisons test. Herein, P < 0.05 (two-tailed) was considered to indicate statistical significance.
Mechanical strain induces the osteogenic differentiation of rBMSCs
Although our previous study has confirmed that tensile strain can up-regulate the mRNA expression levels of Runx2 and Col I of rBMSCs (Li et al., 2013), the optimal mechanical strain were ascertained in this study. The expression levels of mRNA and protein were detected by qRT-PCR and western blot, respectively. Firstly, compared with the control group, the mRNA expression levels of Runx2, ALP, Col I and OCN increased significantly in rBMSCs treated with E2 and 1% mechanical strain (P < 0.05) (Fig. 2a). The mRNA expression levels of ALP, Col I and OCN at 4 h and 6 h after treating with 1% mechanical strain were obviously higher than those at 2 h. The expression level of Runx2 was the most obvious at 4 h, while the mRNA expression level of Runx2 was significantly decreased at 6 h (P < 0.05). The mRNA expression levels of ALP, Col I and OCN at 2, 4, and 6 h after treating with 1% mechanical strain were significantly lower than those in E2 group.
The qRT-PCR results showed that the mRNA expression levels of Runx2, ALP, Col 1 and OCN were the most obvious at 4 h treating with 2% mechanical strain (P < 0.05). The mRNA expression levels of Runx2, Col 1 and ALP at 4 h in 2% mechanical strain group were significantly higher than those in E2 group (P < 0.05) (Fig. 2b). Similarly, the highest protein expression of Runx2 and Col I were also appeared at 4 h treating with 2% mechanical strain (Figs. 3a and 3b).

Figure 2: The effects of different BTS on mRNA expression levels of ALP, Col I, Runx2, and OCN of rat-derived BMSCs (mean ± SD, N = 3). (a) Comparison between control group, E2 group and 1% BTS group (2 h, 4 h, 6 h). (b) Comparison between control group, E2 group and 2% BTS group (2 h, 4 h, 6 h). (c) Comparison between control group, E2 group and 5% BTS group (2 h, 4 h, 6 h). (d) Comparison between 1%, 2% and 5% BTS at 4 h. *P < 0.05, compared to the control group; $P < 0.05, compared to the E2 group; #P < 0.05, compared to the 4 h group; &P < 0.05, Compared to the 2% BTS group.
Then, the rBMSCs were treated with 5% mechanical strain. We also found that the mRNA expression of Col 1, ALP, OCN, and Runx2 were reaching peak levels at 4 h (Fig. 2c). Similarly, the protein expression levels of ALP, Col 1, Runx2 and OCN were the highest at 4 h treated with 5% mechanical strain (Figs. 3c and 3d). Compared with 2% mechanical strain group, the ALP mRNA expression level in 5% mechanical strain group was clearly decreased by 17.78% (P < 0.05) (Fig. 2d). Similarly, the protein expression of Runx2 and OCN were clearly decreased by 57.70% (P < 0.05) and 57.14% (P < 0.05), respectively, compared with 2% mechanical strain group.
The above results showed that 2% mechanical strain was preferable to 5% mechanical strain in inducing the osteogenic differentiation of rBMSCs. Similarly, mechanical stimulation for 4 h was beneficial to increase the mRNA or protein expression levels of ALP, Col, Runx2, and OCN in rBMSCs (Fig. 3e). Therefore, we chose 2% mechanical strain for 4 h as the mechanical stimulus parameters for the next study.
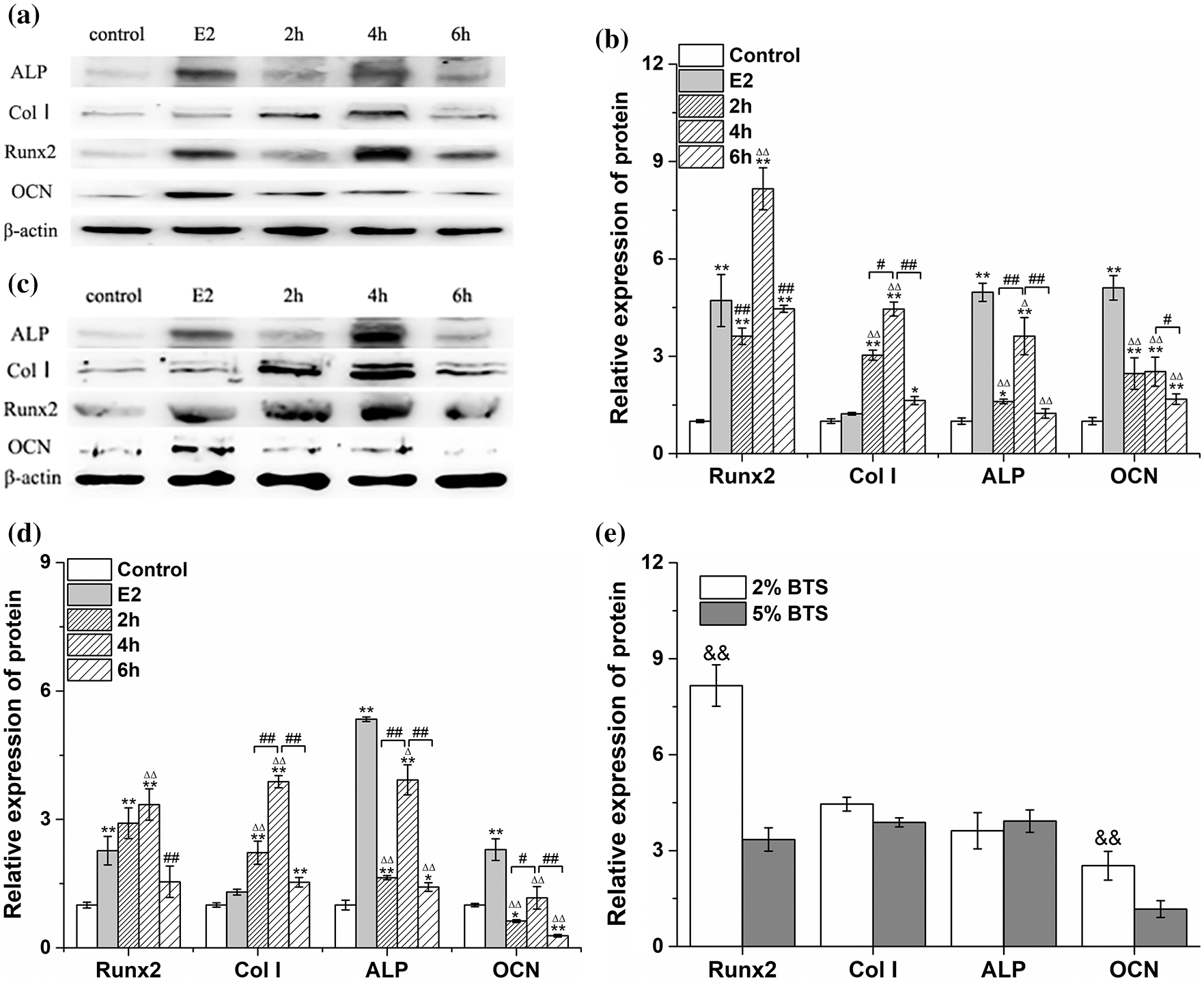
Figure 3: The effects of different BTS on the protein expression levels of ALP, Col I, Runx2, and OCN of rat-derived BMSCs (mean ± SD, N = 3). (a–b) Comparison between control group, E2 group and 2% BTS group (2 h, 4 h, 6 h). (c–d) Comparison between control group, E2 group and 5% BTS group (2 h, 4 h, 6 h). (e) Comparison between 2% and 5% BTS groups at 4 h. *P < 0.05, compared to the corresponding control group; ΔP < 0.05, compared to the E2 group; #P < 0.05, comparison to the 4 h; &P < 0.05, Compared to the 5% BTS group.
BTS can up-regulate the protein expression levels of ERα, Wnt3a, and β-catenin in rBMSCs
To investigate the effect of BTS on the ERα and Wnt3a/β-catenin signaling pathway. Western blotting results showed that the protein expression levels of ERα, Wnt3a and β-catenin were significantly increased in rBMSCs induced by BTS (P < 0.05). Moreover, the ERα, Wnt3a and β-catenin protein expression levels in BTS group were higher than those in E2 group (P < 0.05), and the results demonstrated the superposition effect of E2 and BTS on the ERα, Wnt3a and β-catenin protein expression levels (Fig. 4).
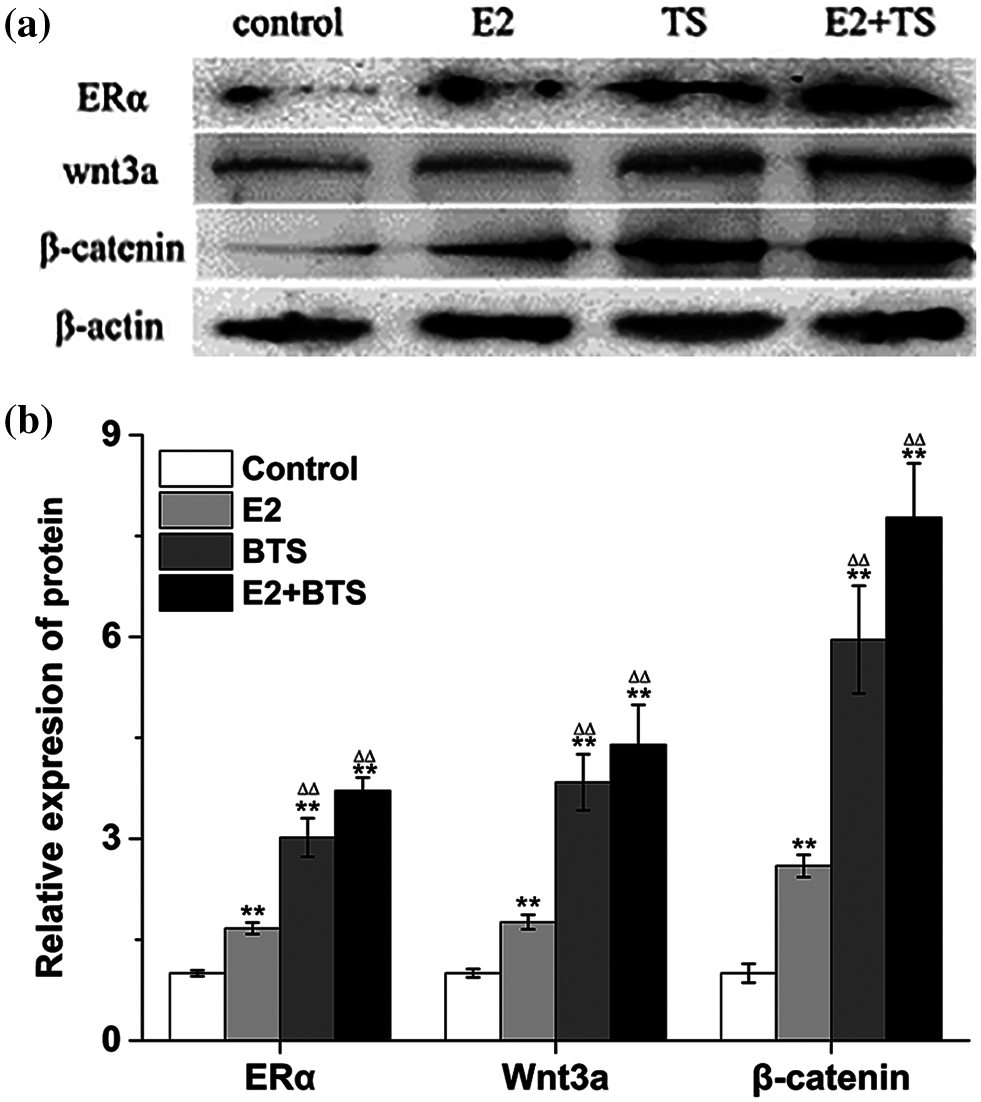
Figure 4: The BTS promoted the protein expression levels of ERα, Wnt3a and β-catenin of rat-derived BMSCs (mean ± SD, N = 3). (a) Protein electrophoresis strips of ERα, Wnt3a and β-catenin. (b) The relative expression levels of ERα, Wnt3a and β-catenin. *P < 0.05, compared to the corresponding control group; ΔP < 0.05, comparison between the two groups.
ICI can abolish the protein expression levels of ERα, Wnt3a, β-catenin and the osteogenic specific markers of rBMSCs induced by BTS
To further search the effect of ERα on the osteogenic differentiation of BMSCs induced by BTS, the BMSCs were treated with ICI. Interestingly, the increased protein expression levels of Wnt3a and β-catenin induced by BTS were abolished by ICI. The protein expression levels of ERα, Wnt3a and β-catenin were significantly downregulated (P < 0.05) (Figs. 5a and 5b). In addition, the protein expression levels of ALP, Col I, Runx2 and OCN were also significantly decreased by ICI (P < 0.05). What’s more, E2 could strengthen the osteogenic specific markers expression of BMSCs when treating with BTS (P > 0.05) (Figs. 5c and 5d).

Figure 5: The ICI inhibited the protein expression levels of Wnt3a, β-catenin, and the osteogenic specific markers of rat-derived BMSCs (mean ± SD, N = 3). (a) Protein electrophoresis strips of ERα, Wnt3a and β-catenin. (b) The relative expression levels of ERα, Wnt3a and β-catenin. (c) Protein electrophoresis strips of ALP, Col I, Runx2, and OCN. (d) The relative expression levels of ALP, Col I, Runx2, and OCN. *P < 0.05, compared to the corresponding control group; ΔP < 0.05, comparison between the two groups.
Overexpression of ERα can enhance the osteogenic differentiation of rBMSCs induced by BTS
To determine the potential effect of ERα involved in mechanical stimulation activating Wnt3a/β-catenin signaling pathway to promote osteogenic differentiation of rBMSCs, the PcDNA3.1(+)-ERα was successfully transfected into rBMSCs (Figs. 6a and 6b). Western blotting results showed that the protein expression levels of ERα, Wnt3a, β-catenin, ALP, Col, Runx2 and OCN in ERα and BTS+ERα groups were significantly increased (P < 0.05) (Figs. 6c–6f). These results prompted us to give further consideration to the effects of ERα on the calcium deposition by Alizarin Red staining. Compared with the control group, the ERα, BTS group, and BTS+ERα could obviously induce calcium deposition, furthermore, the calcium deposition effect in BTS group was enhanced by ERα (Fig. 7).
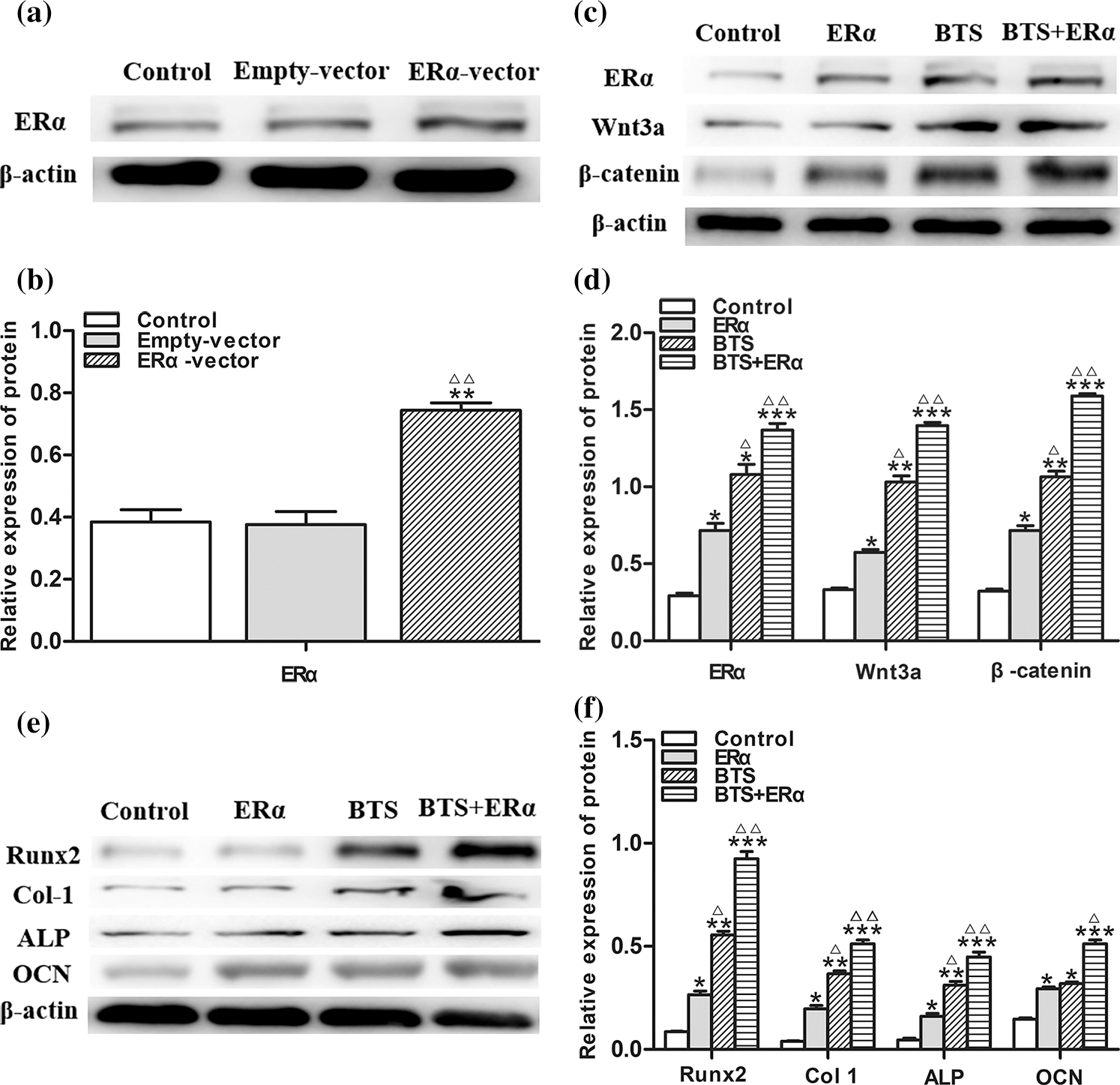
Figure 6: The ERα promoted the protein expression levels of Wnt3a, β-catenin and osteogenic specific marker of rat-derived BMSCs (mean ± SD, N = 3). (a) Protein electrophoresis strips of ERα. (b) the relative expression level of Wnt3a. (c) Protein electrophoresis strips of ERα, Wnt3a and β-catenin. (d) The relative expression levels of ERα, Wnt3a and β-catenin. (e) Protein electrophoresis strips of ALP, Col I, Runx2, and OCN. (f) The relative expression levels of ALP, Col I, Runx2, and OCN. *P < 0.05, compared to the corresponding control group; ΔP < 0.05, comparison between the two groups.
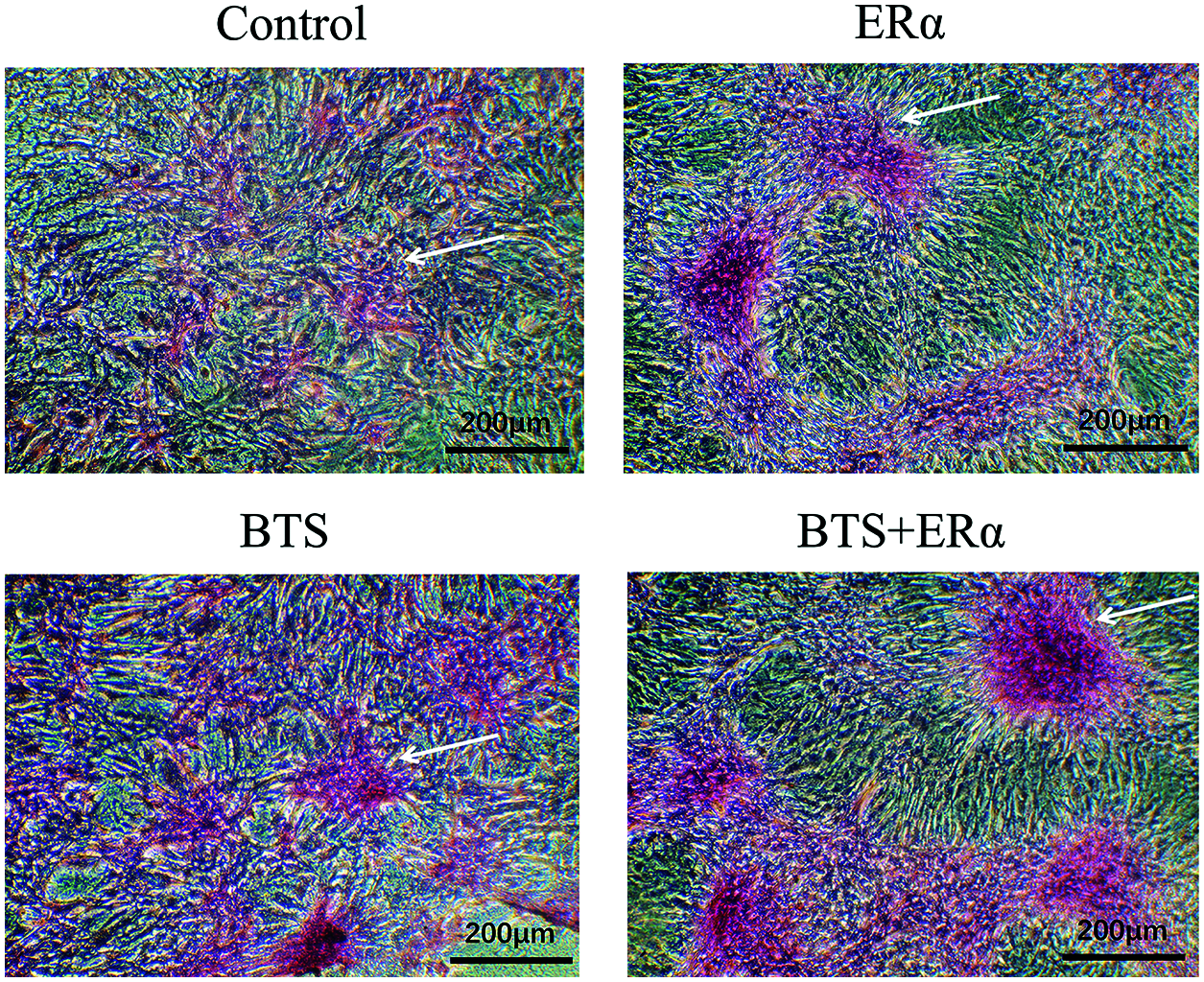
Figure 7: The ERα promoted BTS-induced calcium deposition in rat-derived BMSCs. The calcium deposition determined by Alizarin Red S staining. Compared with the control group, BTS could obviously induce calcium deposition, and the ERα can promote BTS-induced calcium deposition. The arrow points to the calcium deposition.
The effect of mechanical stimulation on bone formation has become one of the important means to treat osteoporosis, fracture, and other diseases. Studies have shown that mechanical stimulation promotes the reconstruction of bone tissue and the stabilization of the internal environment of bone tissue, which is related not only to the proliferation and metabolism of osteoblasts, but also to osteogenic differentiation ability of BMSCs (Li et al., 2003; Wang et al., 2017). Mechanical stretching can regulate many key functions of cells, such as proliferation and differentiation, as well as various tissues of regenerated mammals (Theodoropoulos et al., 2016; Zhang et al., 2011). Moderate distraction is particularly vital for the regeneration and reconstruction of bone tissue after bone injury (Liu et al., 2018). BMSCs are always in a mechanical environment in the bone marrow cavity, and this microenvironment is indispensable to the multidirectional differentiation potential for BMSCs. It has been found that lack of mechanical strain can inhibit the osteogenic differentiation of BMSCs and promote their adipogenesis (Li et al., 2015; Yamazaki et al., 2011). These studies found that low-magnitude mechanical stimulation could promote bone formation and inhibit adipogenesis and increase the expression of Runx2 in mouse mesenchymal stem cells. On the contrary, lack of physical activity due to spinals or brain injury can significantly reduce the mechanical strain of BMSCs, thus reducing the level of bone formation (Rosa et al., 2015). The low mechanical environment under aviation conditions can lead to an average of 1% to 2% bone loss per month, and ultimately induce osteoporosis (Vico et al., 2001). Additionally, cyclic tensile strain was also a mechanical stimulation which could modulate the differentiation of BMSCs into osteoblasts (Jiang et al., 2016). The expression of Runx2 in BMSCs was also increased by cyclic stretching strain (Song et al., 2018). In the present study, the BTS was used as a mechanical stimulation for rBMSCs, which can simulate the strain changes of BMSCs in bone marrow cavity in vivo.
We first explored the optimal parameters of BTS for promoting the differentiation of rBMSCs into osteoblasts. Different strains were applied to rBMSCs. The results showed the highest expression of osteogenic specific markers was found at 2% strain, 4 h/d, which was considerably higher than those in the E2 group. The expression of OCN, a late protein of osteoblasts, was lower than that of ALP, Col I and Runx2, suggesting that BTS might be more beneficial to the early protein or mRNA expression of osteoblasts. The expression of Runx2, Col I, ALP and OCN in rBMSCs with 5% strain loading for 6 h was significantly lower than that of 4 h, which may be due to cell injury by BTS overload. We found that both estradiol and BTS in vitro at 1 Hz for 4 h for 3 days are more favorable to the differentiation of rBMSCs into osteoblasts in this study. Thus, these parameters were utilized to explore the mechanism of BTS promoting differentiation of rBMSCs into osteoblasts.
ERα, a ligand-dependent nuclear transcription factor, is a receptor of estrogen in the target cells. ERα can protect bone by regulating the activity of osteoblasts and osteoclasts, rather than ERβ (Hertrampf et al., 2008). ERα not only mediates the estrogen effect in bone cells, but also participates in the transmission of mechanical signals (Jessop et al., 2004; Zaman et al., 2006). In addition, proliferation of BMSCs was inhibited by ER blockade (Melville et al., 2015). Our previously study also found that BTS can up-regulate the expression of ERα or β-catenin in rBMSCs, and ERα can functionally interact with β-catenin (Yao et al., 2014). These results suggested that ERα is an important mechanical receptor. Osteocytes differentiation and growth of osteocyte precursor cells were closely related to the activation of canonical Wnt/β-catenin signaling pathway (Korvala et al., 2012). Although these reports indicate that ERα and Wnt/β-catenin signaling pathway play important roles in osteoblasts differentiation, it was not clear whether ERα and Wnt/β-catenin signaling pathway mediate the BTS promoting osteoblasts differentiation.
Here, we detected ERα, Wnt3a and β-catenin in rBMSCs after loading BTS, the results showed that the protein expression levels of ERα, Wnt3a and β-catenin in rBMSCs in E2 and BTS groups increased significantly. Moreover, the protein expression levels of ERα, Wnt3a and β-catenin in rBMSCs in E2 combined with BTS group were considerably higher than that in E2 and BTS group, respectively (P < 0.05). ERα has been shown to regulate the stability of β-catenin by triggering the membrane or cytoplasmic signaling pathways. We also found that BTS can promote the activity of ERα and up-regulate the protein expression of β-catenin and Wnt3a in rBMSCs. However, less is known about the interaction between ERα and Wnt3a/β-catenin signaling pathway in mechano-transduction in rBMSCs.
In order to further confirm whether ERα is involved in BTS activating Wnt3a/β-catenin signaling pathway, and promoting osteogenic differentiation of rBMSCs, the rBMSCs were treated with ERα specific inhibitors (ICI 182780) or transfected with pcDNA-ERα. Interestingly, the results showed that Wnt3a and β-catenin in ICI+BTS group were sharply decreased (P < 0.05). Furthermore, ALP, Col, I, Runx2 and OCN in ICI+BTS group were significantly reduced. Similar results were found in the rBMSCs treated with LMV (Li et al., 2019). In the rBMSCs overexpressing ERα, the Wnt3a and β-catenin induced by BTS were further enhanced, and the osteogenic differentiation of rBMSCs was more obvious. In addition, BTS+ERα could obviously induce calcium deposition. These results suggested that BTS may up-regulate the protein expression of Wnt3a by promoting the activity of ERα, and then reduced the degradation of β-catenin in the cytoplasm. The activated β-catenin can translocate to the nucleus and trigger downstream signals to regulate osteogenic potential of rBMSCs.
In conclusion, ERα play a key role in osteogenic differentiation of rBMSCs induced by BTS via the canonical Wnt3a/β-catenin pathway.
Availability of Data and Materials: Requests for data, 12 months after publication of this article, will be considered by the corresponding author.
Author Contribution: Study design: Min Tang, Xueling He, Liang Li; data collection: Jirui Wen, Xinghong Yao; analysis and interpretation of results: Min Tang, Mingyue Bao, Xueling He; draft manuscript preparation: Min Tang, Xinghong Yao, Liang Li. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: This study and all experiments involved are under approval of The Ethical Committee of Sichuan University, with File Code: K2021015 in May 18, 2021.
Funding Statement: This work was supported by grants from the Nature Science Foundation of China (11572209, 11272225).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Hertrampf T, Schleipen B, Velders M, Laudenbach U, Fritzemeier KH et al. (2008). Estrogen receptor subtype-specific effects on markers of bone homeostasis. Molecular and Cellular Endocrinology 291: 104–108. DOI 10.1016/j.mce.2008.03.003. [Google Scholar] [CrossRef]
Infante A, RodrÃguez CI (2018). Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Research & Therapy 9: 244. [Google Scholar]
Jessop HL, Suswillo RF, Rawlinson SC, Zaman G, Lee K et al. (2004). Osteoblast like cells from estrogen receptor Î ± Knockout mice have deficient responses to mechanical strain. Journal of Bone & Mineral Research 19: 938–946. [Google Scholar]
Jiang Y, Wang Y, Tang G (2016). Cyclic tensile strain promotes the osteogenic differentiation of a bone marrow stromal cell and vascular endothelial cell co-culture system. Archives of Biochemistry & Biophysics 607: 37–43. [Google Scholar]
Khalid AB, Krum SA (2016). Estrogen receptors alpha and beta in bone. Bone 87: 130–135. DOI 10.1016/j.bone.2016.03.016. [Google Scholar] [CrossRef]
Korvala J, Löija M, Makitie O, Sochett E, Jüppner H et al. (2012). Rare variations in WNT3A and DKK1 may predispose carriers to primary osteoporosis. European Journal of Medical Genetics 55: 515–519. DOI 10.1016/j.ejmg.2012.06.011. [Google Scholar] [CrossRef]
Lanyon L, Armstrong V, Ong D, Zaman G, Price J (2004). Is estrogen receptor alpha key to controlling bones’ resistance to fracture? Journal of Endocrinology 182: 183–191. DOI 10.1677/joe.0.1820183. [Google Scholar] [CrossRef]
Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L (2003). Endocrinology: Bone adaptation requires oestrogen receptor-alpha. Nature 424: 389. [Google Scholar]
Li H, Wu W, He X, Cao C, Yu X et al. (2019). Applying vibration in early postmenopausal osteoporosis promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells and suppresses postmenopausal osteoporosis progression. Bioscience Reports 39: 168. DOI 10.1042/BSR20191011. [Google Scholar] [CrossRef]
Li L, Chen M, Deng L, Mao Y, Wu W et al. (2003). The effect of mechanical stimulation on the expression of alpha 2, beta 1, beta 3 integrins and the proliferation, synthetic function in rat osteoblasts. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 20: 187–192. [Google Scholar]
Li L, Yao XL, He XL, Liu XJ, Wu WC et al. (2013). Role of mechanical strain and estrogen in modulating osteogenic differentiation of mesenchymal stem cells (MSCs) from normal and ovariectomized rats. Cellular and Molecular Biology 59: 1889–1893. [Google Scholar]
Li R, Liang L, Dou Y, Huang Z, Mo H et al. (2015). Mechanical strain regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. BioMed Research International 2015: 1–10. DOI 10.1155/2015/873251. [Google Scholar] [CrossRef]
Liu C, Carrera R, Flamini V, Kenny L, Cabahug-Zuckerman P et al. (2018). Effects of mechanical loading on cortical defect repair using a novel mechanobiological model of bone healing. Bone 108: 145–155. DOI 10.1016/j.bone.2017.12.027. [Google Scholar] [CrossRef]
Melville KM, Kelly NH, Surita G, Buchalter DB, Schimenti JC et al. (2015). Effects of deletion of ERα in osteoblast-lineage cells on bone mass and adaptation to mechanical loading differ in female and male mice. Journal of Bone and Mineral Research 30: 1468–1480. DOI 10.1002/jbmr.2488. [Google Scholar] [CrossRef]
Rosa N, Simoes R, Magalhaes FD, Marques AT (2015). From mechanical stimulus to bone formation: A review. Medical Engineering & Physics 37: 719–728. DOI 10.1016/j.medengphy.2015.05.015. [Google Scholar] [CrossRef]
Sharma D, Larriera AI, Palacio-Mancheno PE, Gatti V, Fritton JC et al. (2018). The effects of estrogen deficiency on cortical bone microporosity and mineralization. Bone 110: 1–10. DOI 10.1016/j.bone.2018.01.019. [Google Scholar] [CrossRef]
Song Y, Tang Y, Song J, Lei M, Liang P et al. (2018). Cyclic mechanical stretch enhances BMP9-induced osteogenic differentiation of mesenchymal stem cells. International Orthopaedics 42: 947–955. [Google Scholar]
Theodoropoulos JS, Decroos AJ, Petrera M, Park S, Kandel RA (2016). Mechanical stimulation enhances integration in an in vitro model of cartilage repair. Knee Surgery, Sports Traumatology, Arthroscopy 24: 2055–2064. DOI 10.1007/s00167-014-3250-8. [Google Scholar] [CrossRef]
Vico L, Hinsenkamp M, Jones D, Marie PJ, Zallone A et al. (2001). Osteobiology, strain, and microgravity. Part II: Studies at the tissue level. Calcified Tissue International 68: 1–10. DOI 10.1007/BF02684996. [Google Scholar] [CrossRef]
Wang J, Leung KS, Chow SK, Cheung WH (2017). The effect of whole body vibration on fracture healing–A systematic review. European Cells and Materials 34: 108–127. DOI 10.22203/eCM.v034a08. [Google Scholar] [CrossRef]
Windahl SH, Saxon L, Borjesson AE, Lagerquist MK, Frenkel B et al. (2013). Estrogen receptor-alpha is required for the osteogenic response to mechanical loading in a ligand-independent manner involving its activation function 1 but not 2. Journal of Bone Miner Research 28: 291–301. [Google Scholar]
Yamazaki S, Mizumoto T, Nasu A, Horii T, Otomo K et al. (2011). Regulation of osteogenetic differentiation of mesenchymal stem cells by two axial rotational culture. Journal of Artificial Organs 14: 310–317. DOI 10.1007/s10047-011-0580-x. [Google Scholar] [CrossRef]
Yan Z, Yang G, Cui L, He X, Kuang W et al. (2013). Effects of electrical stimulation on the differentiation of mesenchymal stem cells into cardiomyocyte-like cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 30: 556–561. [Google Scholar]
Yao XL, Li L, He XL, Cui L, Kuang W et al. (2014). Activation of beta-catenin stimulated by mechanical strain and estrogen requires estrogen receptor in mesenchymal stem cells (MSCs). European Review for Medical & Pharmacological Sciences 18: 3149–3155. [Google Scholar]
Zaman G, Jessop HL, Muzylak M, de Souza RL, Pitsillides AA et al. (2006). Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. Journal of Bone Miner Research 21: 1297–1306. [Google Scholar]
Zeng Y, Wu J, He X, Li L, Liu X, Liu X (2017). Mechanical microenvironment regulation of age-related diseases involving degeneration of human skeletal and cardiovascular systems. Progress in Biophysics and Molecular Biology 148: 54–59. DOI 10.1016/j.pbiomolbio.2017.09.022. [Google Scholar] [CrossRef]
Zhang H, Landmann F, Zahreddine H, Rodriguez D, Koch M et al. (2011). A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 471: 99–103. DOI 10.1038/nature09765. [Google Scholar] [CrossRef]
Zhu Q, Wang J, Dong J, Shi LX, Zhang X et al. (2014). Construction of a recombinant eukaryotic expression vector of ERÎ ± gene and its expression in rat marrow mesenchymal stem cells. Journal of Medical Postgraduates 27: 34–37. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |