DOI:10.32604/biocell.2022.018921

| BIOCELL DOI:10.32604/biocell.2022.018921 |  |
| Viewpoint |
Applications of scaffolds: Tools for enhancing the immunomodulation of mesenchymal stromal cells
1Department of Anatomy and Cell Biology, College of Medicine, Chung-Ang University, Seoul, 06974, South Korea
2Department of Global Innovative Drugs, Graduate School of Chung-Ang University, Seoul, 06974, South Korea
3Severance Biomedical Science Institute, Yonsei Biomedical Research Institute, College of Medicine, Yonsei University, Seoul, 03722, South Korea
*Address correspondence to: Hyun Jung Lee, pluto38@cau.ac.kr
#Equally contributed as first authors
Received: 24 August 2021; Accepted: 08 November 2021
Abstract: Exogenously delivered mesenchymal stromal cells (MSCs) are therapeutically beneficial owing to their paracrine effect; they secrete various cytokines, nucleic acids, and proteins. Multiple bioengineering techniques can help MSC cultures to release secretomes by providing stem cell niche-like conditions (both structurally and functionally). Various scaffolds mimic the natural extracellular matrix (ECM) using both natural and synthetic polymers, providing favorable environments for MSC proliferation and differentiation. Depending on material properties, either topographically or elastically structured scaffolds can be fabricated. Three-dimensional scaffolds have tunable substrate rigidities and structures, aiding MSC cultivation. Decellularized ECM-derived hydrogels are similar to the natural ECM, thus improving the paracrine effects of MSCs. Here, we discuss recent research on the application of scaffolds to maximize the immunomodulatory function of MSCs.
Keywords: Extracellular matrix (ECM); Immunomodulation; Mesenchymal stromal cells (MSCs); Scaffolds; Stem cell niche
Mesenchymal stromal cells (MSCs) are defined by the International Society for Stem Cell Research (ISSCR) as fibroblast-like non-hematopoietic cells (Ullah et al., 2015). They are multipotent but are CD40, CD80, and CD86 negative and have low major histocompatibility complex (MHC) I/II values; thus, they are less immunogenic than other stem cells. MSCs are therefore increasingly being utilized for tissue engineering and immunotherapy applications. In particular, MSCs have the potential to modulate inflammatory or immune-activated circumstances by secreting immune modulation factors, nucleic acids or proteins (Li et al., 2019). These immune-modulating factors include indoleamine 2,3-dioxygenase (IDO), heme oxygenase-1 (HO-1), transforming growth factor-β (TGF-β), TNF-α stimulated gene/protein 6 (TSG-6), cyclooxygenease 2 (COX2), prostaglandin E2 (PGE2), hepatocyte growth factor (HepGF), galectins-1 (Gal-1), iNOS, interleukin-6 (IL-6), interleukin-1 receptor antagonist (IL-1Rag), interleukin-10 (IL-10) and human leukocyte antigen-G (HLA-G) (Pittenger et al., 2019). Additionally, secretome from MSCs can regulate the proliferation, differentiation, and activity of most immune cells, including T cells, B cells, natural killer (NK) cells, dendritic cells (DCs), and macrophages (Lee and Song, 2018). MSCs can therefore alleviate inflammatory or immune-related diseases. Emphasis regarding regenerative medicine using MSCs has been shifting toward either producing cytokines or other factors (termed the paracrine effect), rather than the differentiation and rebuilding of damaged tissues using MSCs themselves. The potentiation of the immunomodulatory function of MSCs means that they constitute an emerging and potentially important tool for cell therapy.
To enhance the capacity of the immunomodulatory function of MSCs, multiple bioengineering techniques can be employed during their cultivation. Scaffolds can be utilized during cell culturing to preserve the tissue architecture and provide a three-dimensional (3D) biomimetic milieu to MSCs, similar to a stem cell niche. It has been known that the dimensionality, physical characteristics, topographical cues, surface chemistry of biomaterials, and micro-structure of scaffolds can regulate the immunomodulatory function of MSCs. Here, we review the link between the immunomodulatory function of MSCs and the properties of scaffolds, particularly topographical cues or substrate elasticities of biomaterials.
Scaffolds with Topographical Features
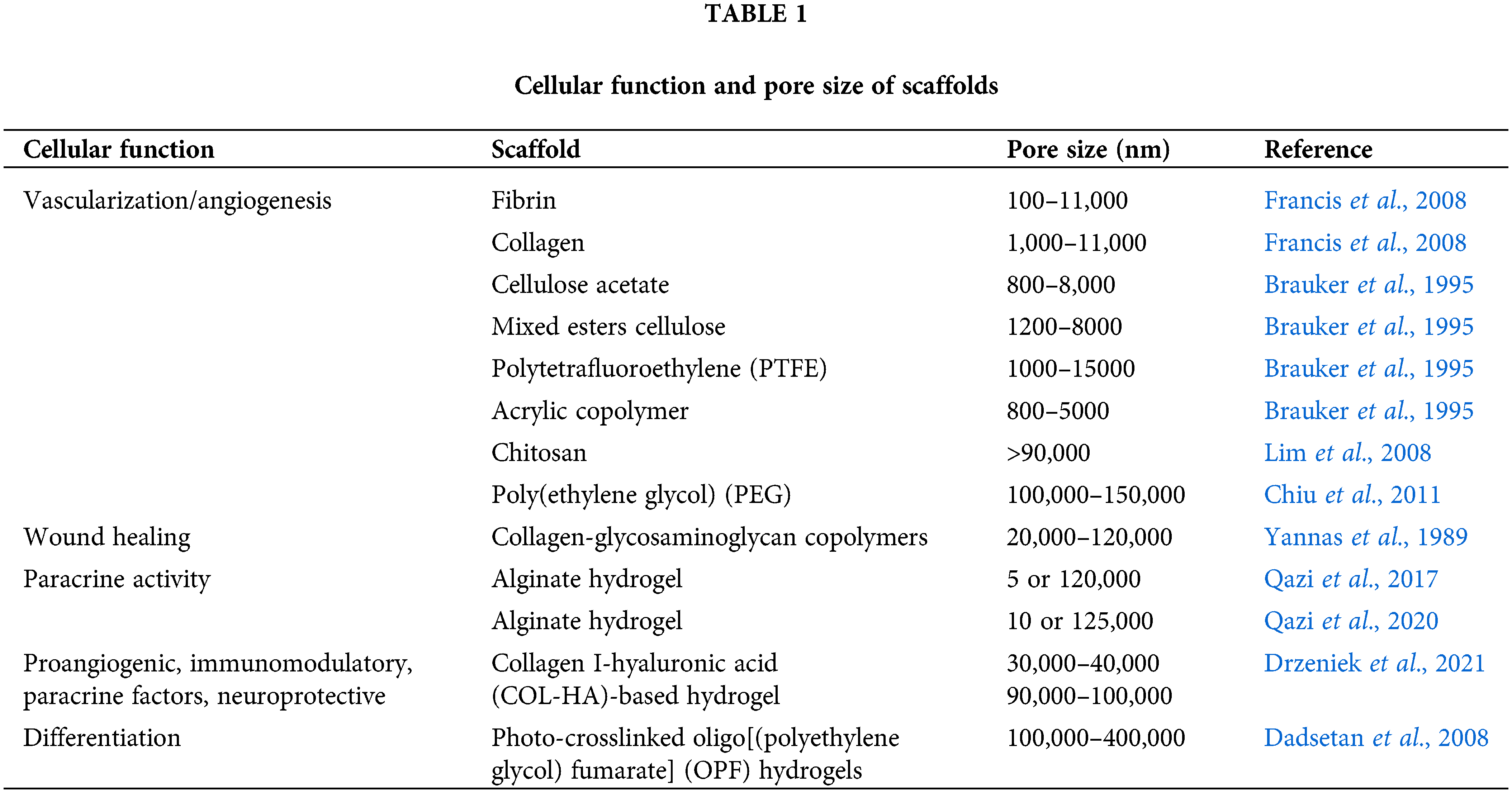
The ECM consists of networks of fibrous proteins, collagens, and elastic fibers, which are immersed in a viscoelastic gel that is rich in proteoglycans (Mecham, 2011). Thus, diverse synthetic polymers and natural polymers have been used to generate scaffolds with fibrous structures, so as to mimic the natural ECM. Synthetic polymers such as polycaprolactone (PCL), poly(lactic-co-glycolic acid) (PLGA), and polylactic acid (PLA) can be utilized to generate fine-tuned fibrous structures via electrospinning. Su et al. (2017) reported that electrospun PCL scaffolds promoted pro-angiogenic and anti-inflammatory paracrine factors in adipose-derived stem cells [MSCs(A)]. They also further analyzed the effects of aligned, randomized, or meshed patterns fabricated with PCL on MSCs(A), revealing that meshed scaffolds best enhanced their paracrine effects. This finding indicates that the orientations of the fibers and the substrate rigidity might also be important factors for controlling the functions of MSCs. biocompatible material that can be used to fabricate several forms, including fibers, gels, and films (Qi et al., 2017). A recent study demonstrated that the meshed scaffolds comprising silk fibroin nanofibers strongly elevated the levels of immunomodulatory factors such as IDO-1, COX2, and PGE2 in bone marrow-derived stem cells [MSCs(M)] (Kim et al., 2019a). Furthermore, MSCs(M) on mesh form of silk fibroin nanofibers reduced mouse mortality from polymicrobial sepsis through their improved immunomodulatory functions than MSCs(M) only (Kim et al., 2021). ECM like structured silk fibroin seems to provide a favorable environment for MSC cultivation through its structural and material properties. Vallés et al. also demonstrated the effect of topographical cue on immunomodulatory function of MSCs (Vallés et al., 2015). MSCs(M) on 3D polystyrene scaffolds showed smaller cell bodies, but secreted a higher level of soluble factors than MSCs(M) on two-dimensional (2D) polystyrene scaffolds, indicating that topographical cue affects cell function differently. The pore size in hydrogel-based scaffolds also can make a critical difference even in the same hydrogel. For example, sponge or foam porous scaffolds contain connected pore structures that favorably transport gas and nutrients into cells. Hydrogels made of natural materials such as fibrin, alginate, or silk fibroin have been used as scaffolds for cells to promote cell growth and function (He et al., 2020). However, if the pores are too small, the cellular penetration, ECM deposition, or neovascularization can be inhibited (Shruti et al., 2013). Meanwhile, pores that are excessively large can decrease the cell-to-cell contact ratio, as the cells exhibit 2D growth patterns on the substrate rather than adopting a 3D organization (Marrella et al., 2018). The effective pore sizes for cellular function in tissue engineering applications are listed in Table 1. The micro-architecture supporting MSCs seems to be an important cue to manage the paracrine effect of MSCs.
Substrate Stiffness and Biochemistry of Scaffolds
Since the effect of matrix elasticity on the MSC function and fate was demonstrated by Engler et al. (2016) for the first time, many studies have been performed to find the optimal substrate rigidity for MSC cultivation. Hydrogels are favorable for MSC cultivation as they can be tuned regarding to their substrate rigidity. Drzeniek et al. (2021) reported that a microporous 3D hydrogel composed of collagen significantly improved the paracrine effects of MSCs, compared with a 2D collagen-coated polystyrene. They used a customizable collagen I-hyaluronic acid (COL-HA)-based hydrogel to encapsulate MSCs for mimicking stem cell niches. Compared with a 2D surface coated with COL-HA, the 3D hydrogel comprising COL-HA elevated the secretion of angiogenesis-, immunomodulation-, hemostasis-, and ECM remodeling-related cytokines from MSCs. Similarly, Wong et al. (2020) also demonstrated that soft extracellular matrix (~2 kPa) mimicking bone marrow maximized the ability of MSCs(M) to produce paracrine factors and induce chemotaxis upon inflammatory stimulation. In addition to matrix elasticity, composite scaffolds that mimic natural conditions may greatly enhance the paracrine effect of MSCs. In order to maximize the similarity to the natural ECM, decellularized ECM (dECM) from various tissues and organs have been investigated and applied via a number of techniques that utilize chemical, enzymatic, or mechanical disruption. As the ECM is an essential non-cellular component of the tissue microenvironment, decellularized scaffolds composed entirely of ECM can help to reconstruct stem cell niches in vitro. Hydrogel forms of dECM can both affect stem cell differentiation and exert a functional effect, according to the origin of the organ or tissue in question. One disadvantage of ECM-derived hydrogels, however, is their poor self-supporting ability, which arises from their low viscosities and mechanical properties as scaffolds. Due to this characteristic, which hinders the possibility of making large and complex 3D structures with hydrogels (Yi et al., 2019), the combination with other ECM components, such as collagen, can be applied for enhancing the stiffness of hydrogels. There are, however, limitations to the use of dECM in standard clinical treatments, since there is no standard process for the dECM (Kim et al., 2019b) and the current process is unable to completely remove all cell materials in a practical setting (Gilbert et al., 2009). Thus, the general standard guideline for processing dECM will accelerate the clinical trials of MSCs and dECM-derived scaffolds.
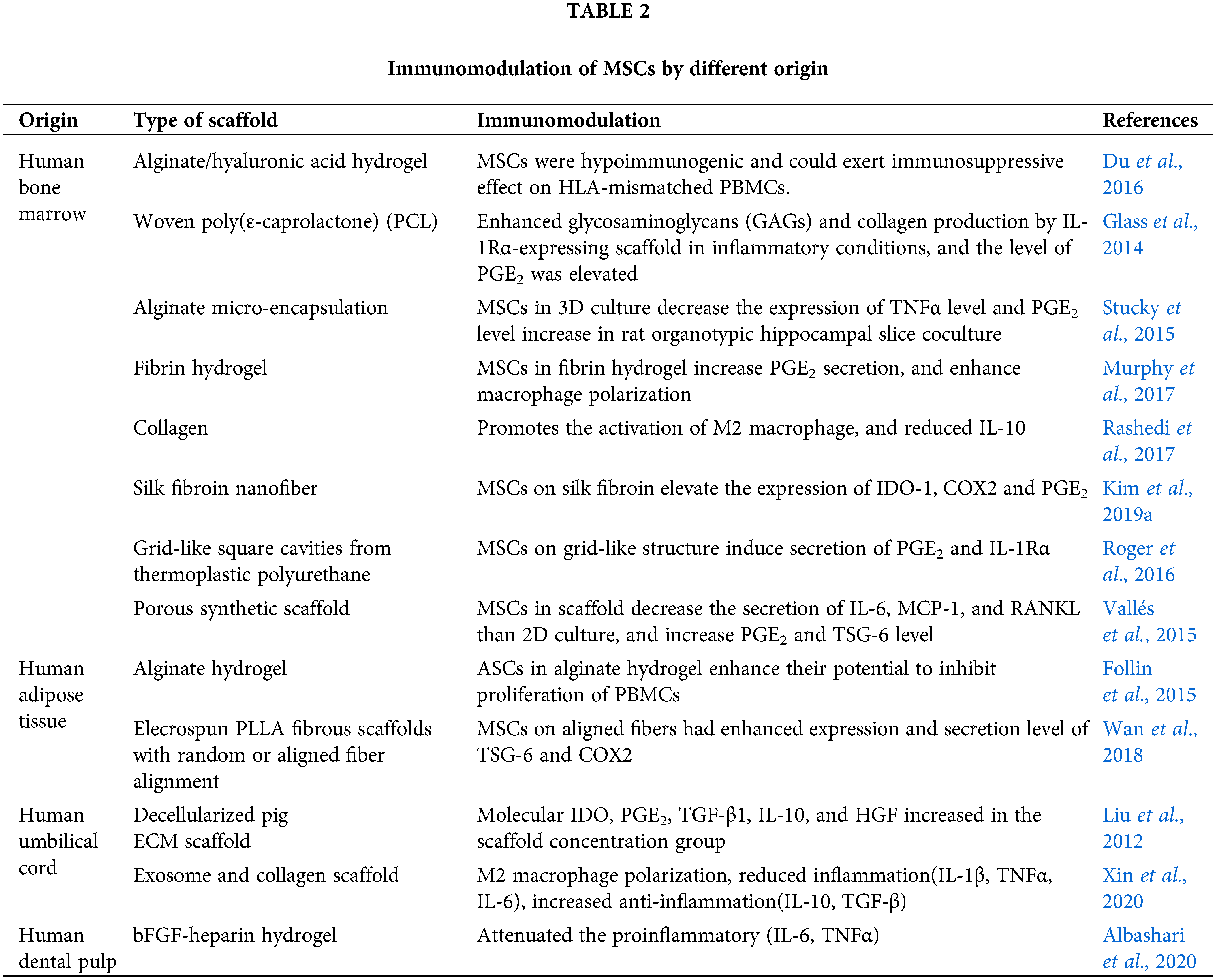
Apart from the above mentioned factors, some reports have indicated that the cell source might affect the immunomodulatory function of MSCs, as they can be harvested from various adult tissues as well as neonatal tissues. Although MSCs(M) seem to be designated as the gold standard of MSCs (Hall et al., 2013), MSCs(A) and cord blood-derived MSCs [MSCs(CB)] have also been widely used in clinical studies due to their easy accessibility. Although it is hard to conclude which MSCs are more appropriate to use, it has been reported that human MSCs(A) are more genetically and morphologically stable in long-term culture, display a lower senescence ratio, show a higher proliferative capacity, and retain differentiation potential for a longer period in culture compared with human MSCs(M) (Elman et al., 2014). Further, the yield of MSCs(A) is approximately 500-fold greater than that of MSCs(M) when isolated from an equivalent amount of adipose tissue and bone marrow stroma, respectively (Strioga et al., 2012). Nonetheless, more intensive studies about the cell source are needed for arriving at a conclusion. The functions of the MSCs from different sources are listed in Table 2.
In conclusion, many methods for preparing scaffolds with various materials have been developed. In particular, scaffolds with favorable properties for improving MSC functions are actively being fabricated. Furthermore, enhancing the similarity of these substrates (in terms of their structures and properties) for MSC cultivation can improve both the paracrine effect and the differentiation of stem cells, thus enhancing the feasibility and efficacy of MSC therapies for clinical applications (Fig. 1). Now, it is important to consider how the improved function of MSCs can be maintained sustainably within scaffolds or how long the injected MSCs can survive after in vivo transplantation. Thus, thermo-responsive or injectable hydrogels and adhesion-related ligand-containing hydrogels are being actively developed (Hong et al., 2019). In this regard, more biocompatible scaffolds need to be further developed to take advantage of the paracrine products of MSCs. Such studies could help to promote cell therapy and achieve efficient translational research.
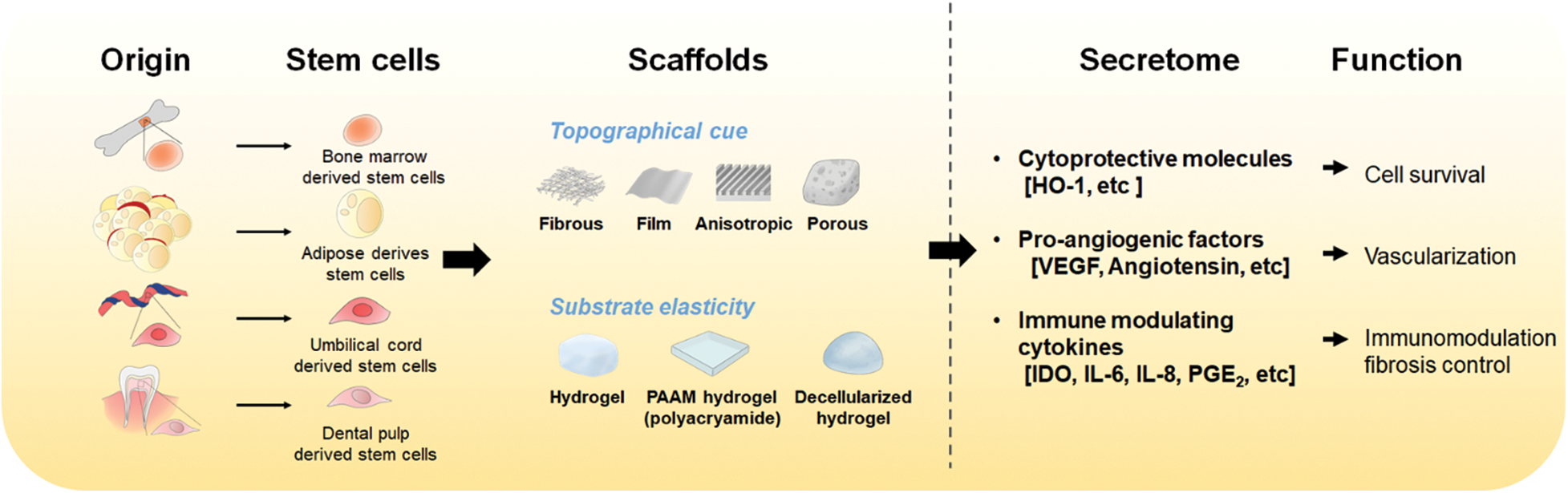
Figure 1: The effect of scaffolds on MSC cultivation and the function of secretome from MSCs. MSCs can be cultivated on various types of scaffolds. The optimal topographical cue and substrate elasticity of scaffolds provide a better environment than regular culture plates for MSCs to improve their paracrine effect. Secreted factors from MSCs include cytokines, nucleic acids, and proteins which play roles in improving cell survival, vascularization, or immunomodulation.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contribution: The authors confirm contribution to the paper as follows: conception and design: HJ Lee; draft manuscript preparation: OH Kim, ER Kim, JH Park, and HJ Lee. All authors reviewed the manuscript and approved the final version.
Funding Statement: This research is supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Grant No. 2020R1A2C2011617) and by a Chung-Ang University Research Scholarship Grants in 2019.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Albashari A, He Y, Zhang Y, Ali J, Lin F et al. (2020). Thermosensitive bFGF-modified hydrogel with dental pulp stem cells on neuroinflammation of spinal cord injury. ACS Omega 5: 16064–16075. DOI 10.1021/acsomega.0c01379. [Google Scholar] [CrossRef]
Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnson WD et al. (1995). Neovascularization of synthetic membranes directed by membrane microarchitecture. Journal of Biomedical Materials Research 29: 1517–1524. DOI 10.1002/(ISSN)1097-4636. [Google Scholar] [CrossRef]
Chiu YC, Cheng MH, Engel H, Kao SW, Larson JC et al. (2011). The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials 32: 6045–6051. DOI 10.1016/j.biomaterials.2011.04.066. [Google Scholar] [CrossRef]
Dadsetan M, Hefferan TE, Szatkowski JP, Mishra PK, Macura SI et al. (2008). Effect of hydrogel porosity on marrow stromal cell phenotype expression. Biomaterials 29: 2193–2202. DOI 10.1016/j.biomaterials.2008.01.006. [Google Scholar] [CrossRef]
Drzeniek NM, Mazzocchi A, Schlickeiser S, Forsythe SD, Moll G et al. (2021). Bio-instructive hydrogel expands the paracrine potency of mesenchymal stem cells. Biofabrication 13: 045002. DOI 10.1088/1758-5090/ac0a32. [Google Scholar] [CrossRef]
Du WJ, Reppel L, Leger L, Schenowitz C, Huselstein C et al. (2016). Mesenchymal stem cells derived from human bone marrow and adipose tissue maintain their immunosuppressive properties after chondrogenic differentiation: Role of HLA-G. Stem Cells and Development 25: 1454–1469. DOI 10.1089/scd.2016.0022. [Google Scholar] [CrossRef]
Elman JS, Li M, Wang F, Gimble JM, Parekkadan B (2014). A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. Journal of Inflammation 11: 1–8. DOI 10.1186/1476-9255-11-1. [Google Scholar] [CrossRef]
Engler AJ, Sen S, Sweeney HL, Discher DE (2006). Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689. DOI 10.1016/j.cell.2006.06.044. [Google Scholar] [CrossRef]
Follin B, Juhl M, Cohen S, Pedersen AE, Gad M et al. (2015). Human adipose-derived stromal cells in a clinically applicable injectable alginate hydrogel: Phenotypic and immunomodulatory evaluation. Cytotherapy 17: 1104–1118. DOI 10.1016/j.jcyt.2015.04.008. [Google Scholar] [CrossRef]
Francis ME, Uriel S, Brey EM (2008). Endothelial cell-matrix interactions in neovascularization. Tissue Engineering Part B: Reviews 14: 19–32. DOI 10.1089/teb.2007.0115. [Google Scholar] [CrossRef]
Gilbert TW, Freund JM, Badylak SF (2009). Quantification of DNA in biologic scaffold materials. Journal of Surgical Research 152: 135–139. DOI 10.1016/j.jss.2008.02.013. [Google Scholar] [CrossRef]
Glass KA, Link JM, Brunger JM, Moutos FT, Gersbach CA et al. (2014). Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 35: 5921–5931. DOI 10.1016/j.biomaterials.2014.03.073. [Google Scholar] [CrossRef]
Hall SRR, Tsoyi K, Ith B, Padera RFJr, Lederer JA et al. (2013). Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: The importance of neutrophils. Stem Cells 31: 397–407. DOI 10.1002/stem.1270. [Google Scholar] [CrossRef]
He W, Reaume M, Hennenfent M, Lee BP, Rajachar R (2020). Biomimetic hydrogels with spatial- and temporal-controlled chemical cues for tissue engineering. Biomaterials Science 8: 3248–3269. DOI 10.1039/D0BM00263A. [Google Scholar] [CrossRef]
Hong KH, Kim YM, Song SC (2019). Fine-tunable and injectable 3D hydrogel for on-demand stem cell niche. Advanced Science 6: 1900597. DOI 10.1002/advs.201900597. [Google Scholar] [CrossRef]
Kim OH, Park JH, Son JI, Yoon OJ, Lee HJ (2021). Bone marrow mesenchymal stromal cells on silk fibroin scaffolds to attenuate polymicrobial sepsis induced by cecal ligation and puncture. Polymers 13: 1433. DOI 10.3390/polym13091433. [Google Scholar] [CrossRef]
Kim OH, Yoon OJ, Lee HJ (2019a). Silk fibroin scaffolds potentiate immunomodulatory function of human mesenchymal stromal cells. Biochemical and Biophysical Research Communications 519: 323–329. DOI 10.1016/j.bbrc.2019.09.006. [Google Scholar] [CrossRef]
Kim YS, Majid M, Melchiorri AJ, Mikos AG (2019b). Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioengineering and Translational Medicine 4: 83–95. DOI 10.1002/btm2.10110. [Google Scholar] [CrossRef]
Lee DK, Song SU (2018). Immunomodulatory mechanisms of mesenchymal stem cells and their therapeutic applications. Cellular Immunology 326: 68–76. DOI 10.1016/j.cellimm.2017.08.009. [Google Scholar] [CrossRef]
Li H, Shen S, Fu H, Wang Z, Li X et al. (2019). Immunomodulatory functions of mesenchymal stem cells in tissue engineering. Stem Cells International 2019: 9671206–9671218. DOI 10.1155/2019/9671206. [Google Scholar] [CrossRef]
Lim TC, Bang CP, Chian KS, Leong KF (2008). Development of cryogenic prototyping for tissue engineering. Virtual and Physical Prototyping 3: 25–31. DOI 10.1080/17452750701799303. [Google Scholar] [CrossRef]
Liu S, Yuan M, Hou K, Zhang L, Zheng X et al. (2012). Immune characterization of mesenchymal stem cells in human umbilical cord Wharton’s jelly and derived cartilage cells. Cellular Immunology 278: 35–44. DOI 10.1016/j.cellimm.2012.06.010. [Google Scholar] [CrossRef]
Marrella A, Lee TY, Lee DH, Karuthedom S, Syla D et al. (2018). Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Materials Today 21: 362–376. DOI 10.1016/j.mattod.2017.10.005. [Google Scholar] [CrossRef]
Mecham RP (2011). The Extracellular Matrix: An Overview. Berlin, Germany: Springer. [Google Scholar]
Murphy KC, Whitehead J, Zhou D, Ho SS, Leach JK (2017). Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomaterialia 64: 176–186. DOI 10.1016/j.actbio.2017.10.007. [Google Scholar] [CrossRef]
Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM et al. (2019). Mesenchymal stem cell perspective: Cell biology to clinical progress. njp Regenerative Medicine 4: 22. DOI 10.1038/s41536-019-0083-6. [Google Scholar] [CrossRef]
Qazi TH, Mooney DJ, Duda GN, Geissler S (2017). Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials 140: 103–114. DOI 10.1016/j.biomaterials.2017.06.019. [Google Scholar] [CrossRef]
Qazi TH, Mooney DJ, Duda GN, Geissler S (2020). Niche-mimicking interactions in peptide-functionalized 3D hydrogels amplify mesenchymal stromal cell paracrine effects. Biomaterials 230: 119639. DOI 10.1016/j.biomaterials.2019.119639. [Google Scholar] [CrossRef]
Qi Y, Wang H, Wei K, Yang Y, Zheng RY et al. (2017). A review of structure construction of silk fibroin biomaterials from single structures to multi-level structures. International Journal of Molecular Sciences 18: 237. DOI 10.3390/ijms18030237. [Google Scholar] [CrossRef]
Rashedi I, Talele N, Wang XH, Hinz B, Radisic M et al. (2017). Collagen scaffold enhances the regenerative properties of mesenchymal stromal cells. PLoS One 12: e0187348. DOI 10.1371/journal.pone.0187348. [Google Scholar] [CrossRef]
Roger Y, Schack LM, Koroleva A, Noack S, Kurselis K et al. (2016). Grid-like surface structures in thermoplastic polyurethane induce anti-inflammatory and anti-fibrotic processes in bone marrow-derived mesenchymal stem cells. Colloids and Surface B: Biointerfaces 148: 104–115. DOI 10.1016/j.colsurfb.2016.06.024. [Google Scholar] [CrossRef]
Shruti S, Salinas AJ, Lusvardi G, Malavasi G, Menabue L et al. (2013). Mesoporous bioactive scaffolds prepared with cerium-, gallium- and zinc-containing glasses. Acta Biomaterialia 9: 4836–4844. DOI 10.1016/j.actbio.2012.09.024. [Google Scholar] [CrossRef]
Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J (2012). Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells and Development 21: 2724–2752. DOI 10.1089/scd.2011.0722. [Google Scholar] [CrossRef]
Stucky EC, Schloss RS, Yarmush ML, Shreiber DI (2015). Alginate micro-encapsulation of mesenchymal stromal cells enhances modulation of the neuro-inflammatory response. Cytotherapy 17: 1353–1364. DOI 10.1016/j.jcyt.2015.05.002. [Google Scholar] [CrossRef]
Su N, Gao PL, Wang K, Wang JY, Zhong Y et al. (2017). Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials 141: 74–85. DOI 10.1016/j.biomaterials.2017.06.028. [Google Scholar] [CrossRef]
Ullah I, Subbarao RB, Rho GJ (2015). Human mesenchymal stem cells-current trends and future prospective. Bioscience Reports 35: e00191. DOI 10.1042/BSR20150025. [Google Scholar] [CrossRef]
Vallés G, Bensiamar F, Crespo L, Arruebo M, Vilaboa N et al. (2015). Topographical cues regulate the crosstalk between MSCs and macrophages. Biomaterials 37: 124–133. DOI 10.1016/j.biomaterials.2014.10.028. [Google Scholar] [CrossRef]
Wan S, Fu X, Ji Y, Li M, Shi X et al. (2018). FAK- and YAP/TAZ dependent mechanotransduction pathways are required for enhanced immunomodulatory properties of adipose-derived mesenchymal stem cells induced by aligned fibrous scaffolds. Biomaterials 171: 107–117. DOI 10.1016/j.biomaterials.2018.04.035. [Google Scholar] [CrossRef]
Wong SW, Lenzini S, Cooper MH, Mooney DJ, Shin JW (2020). Soft extracellular matrix enhances inflammatory activation of mesenchymal stromal cells to induce monocyte production and trafficking. Science Advances 6: eaaw0158. DOI 10.1126/sciadv.aaw0158. [Google Scholar] [CrossRef]
Xin L, Lin X, Zhou F, Li C, Wang X et al. (2020). A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomaterialia 113: 252–266. DOI 10.1016/j.actbio.2020.06.029. [Google Scholar] [CrossRef]
Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF (1989). Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proceedings of the National Academy of Sciences USA 86: 933–937. DOI 10.1073/pnas.86.3.933. [Google Scholar] [CrossRef]
Yi HG, Choi YJ, Jung JW, Jang J, Song TH et al. (2019). Three-dimensional printing of a patient-specific engineered nasal cartilage for augmentative rhinoplasty. Journal of Tissue Engineering 10: 1–14. DOI 10.1177/2041731418824797. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |