DOI:10.32604/biocell.2022.018441

| BIOCELL DOI:10.32604/biocell.2022.018441 |  |
| Article |
Transplanted choroidal plexus epithelial cells can integrate with organotypic spinal cord slices into a new system
1Department of Neurology, The Second Affiliated Hospital, College of Medicine, Xi’an Jiaotong University, Xi’an, 710004, China
2Department of Ophthalmology, Xi’an No. 3 Hospital, Xi’an, 710018, China
3Department of Orthopedics, The Second Affiliated Hospital, College of Medicine, Xi’an Jiaotong University, Xi’an, 710004, China
*Address correspondence to: Shengli Huang, hstudent@163.com
#These authors contributed equally to this work
Received: 25 July 2021; Accepted: 19 October 2021
Abstract: This study aimed to evaluate the integration of transplanted choroidal plexus epithelial cells with organotypic spinal cord slices. Organotypic spinal cord slices, normally cultured for 6 days, were divided into control group (Ctrl) and transplanted group (T). The choroidal plexus epithelial cells were dissociated and primary cultured (C group). The choroidal plexus epithelial cells cultured for 6–7 days were labeled by 1,1’-dioctadecyl-3,3,3’,3’-tetramethyl-indocarbocyanineperchlorate (CM-Dil), and were identified by transthyretin (TTR) in immunocytochemistry. They were adjusted to the density of 0.5–1 × 107/ml, then 2 μl cells suspension were transplanted to the spinal cord slices in the T group. The same amount of basal medium was dripped on the spinal cord slices in the Ctrl group. After 14 days of transplantation, the differentiations into neurons and astrocytes, and the synapses were identified by immunofluorescence histochemistry. At the same time, the ratios of cell differentiations and synapses in new system, and the changes of MAPK signaling pathway were tested by western blotting. The choroid plexus epithelial cells were well labeled by CM-Dil and were immune-stained by TTR in immunocytochemistry. The choroid plexus epithelial cells bodies were small when transplanted on the spinal cord slices, but big when transplanted on the polyester membrane inserts. The transplanted cells could differentiate into astrocytes, and possibly differentiate into neurons, and there were a large number of synaptophysin positive vesicles between transplanted cells and organotypic spinal cord slices in immunofluorescence histochemistry. The levels of GFAP, TUB-III and synaptophysin in the T group were higher than which in the Ctrl and C groups in western blotting (P < 0.05). And the ratios of p-JNK/JNK and p-P38/P38 in the T group were significantly lower than which in the Ctrl and C groups (P < 0.05). But the ratio of p-ERK/ERK in the three groups was of no significant difference. The transplanted choroidal plexus epithelial cells can integrate with organotypic spinal cord slices into a new system.
Keywords: Cell transplantation; Choroidal plexus epithelial cells; Integration; Organotypic spinal cord slices
Choroidal plexus epithelial cells have stem cells characteristics with markers of nestin (Huang et al., 2011; Huang et al., 2013a; Hashemi et al., 2017). They had the ability to differentiate into neurons (Itokazu et al., 2006; Bolos et al., 2013) and astrocytes (Kitada et al., 2001). Thus they can be used for repairing damaged central nervous system (Matsumoto et al., 2010; Ide et al., 2016; Aliaghaei et al., 2016; Kanekiyo et al., 2016; Xu et al., 2021). In addition, they can produce numerous growth factors and neurotrophic factors such as TGF-β, GDF-15, GDNF, BDNF, NGF, VEGF, etc. (Ikeda et al., 1999; Emerich et al., 2007; Huang et al., 2014; Ide et al., 2016; Zhao et al., 2018). As seed cells, they can change the host’s local microenvironment by secreting neurotrophic factors (Kitada et al., 2001; Borlongan et al., 2007; Ide et al., 2016; Eslami et al., 2021), which are helpful for axon regeneration and repairment. Therefore, choroidal plexus epithelial cells are a good choice for cell transplantation, and there is a potential value in the treatment of spinal cord injury and neurodegenerative diseases.
Microenvironment regulates seed cells survivals, differentiation, and synaptogenesis (Hofer et al., 2012; Shamloo et al., 2015). At the same time, the seed cells change the host’s microenvironment through their own activities. Thus, the seed cells and host tissue interact and integrate with each other to generate a new system (Jäderstad et al., 2010; Hofer et al., 2012). In the new system, there is not only behavioral and morphological change of seed cells, but also behavioral and microenvironmental changes of host’s tissue. Currently, studies about the change of new system are comparatively fewer.
The organotypic spinal cord slices preserve the basic tissue cytoarchitecture at utmost, and closely mimic complex tissue microenvironment (Kim et al., 2010; Cifra et al., 2012; Sypecka et al., 2015; Dionne et al., 2021). Importantly, it is advantageous for being host tissue, and it is an ideal platform to study the transplant new system. There is a growing body of literatures reporting the transplantation of choroidal plexus epithelial cells in several animal models or cell lines (Kitada et al., 2001; Matsumoto et al., 2010; Aliaghaei et al., 2016; Ide et al., 2016). However, there is no literature reporting the transplantation in organotypic spinal cord slices. The interaction and integration of choroidal plexus epithelial cells with organotypic spinal cord slices have not paid attention. The survivals, differentiations, synaptogenesis and intracellular signaling pathway changes in new system are not clear.
Thus, we conducted the current study to understand the above issues better. Our goal was to identify the changes of new system.
The Xi’an Jiaotong University Animal Experimentation Committee approved protocols for animal care and experimental management. Ethical approval for the study was obtained from the Medical Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University. Postnatal male Sprague-Dawley 5–7-day-old rats (N = 12) weighed 22–26 g and neonatal male Sprague-Dawley 1-day-old rats (N = 6) weighed 5–6 g were applied by the Center of Experimental Animals, Xi’an Jiaotong University. The 5–7-day-old rats were used for organotypic spinal cord slices, which were divided into control group (Ctrl) and transplanted group (T). In the T group, organotypic spinal cord slices were normally cultured for 6 days firstly, then were transplanted by choroidal plexus epithelial cells which were labeled by 1,1’-dioctadecyl-3,3,3’,3’-tetramethyl-indocarbocyanineperchlorate (CM-Dil). In the Ctrl group, organotypic spinal cord slices were normally cultured for 6 days, then the same amount of basal medium was dripped on the organotypic spinal slices. The organotypic spinal cord slices in the two groups both were cultured for 20 days in total, including 6 days before transplantation and 14 days after transplantation. The 1-day-old rats were used for dissociation and primary culture of choroidal plexus epithelial cells (C group).
Vibratome, razor blades, ophthalmic scissors, corneal scissors, microforceps and operating knife blades were used in this experiment. 0.4-μm-pore polyester membrane inserts (Transwell 3450) were provided by Corning Costar (New York, USA). Dulbecco’s modified Eagle’s medium (DMEM/F12, DMEM/low glucose), fetal bovine serum (FBS) and CM-Dil were purchased from Thermo Fisher Scientific (Waltham, Massachusetts, USA). Rabbit monoclonal anti-GFAP, anti-beta III Tubulin (TUB-III), anti-Synaptophysin (SYN) and anti-transthyretin (TTR) were purchased from Abcam (Cambridgeshire, UK). Rabbit polyclonal or monoclonal anti-caspase 3, anti-caspase 3 active, anti-ERK, anti-p-ERK, anti-P38, anti-p-P38, anti-JNK and anti-p-JNK were purchased from Cell Signaling Technology (Danvers, MA, USA). 4’,6-diamino-2-phenylindole (DAPI) was purchased from Roche (Basel, Switzerland). Anti-rabbit HRP, RIPA buffer, protease inhibitor cocktail, BCA assay and ECL were purchased from KPL (Milford, USA). Fluorescein-conjugated goat anti-rabbit IgG, biotin-conjugated goat anti-rabbit IgG and peroxidase streptavidin were provided by CWBIO (Beijing, China). Recombinant rat epidermal growth factor (EGF) was obtained from PeproTech (Rocky Hill, USA).
Preparation of organotypic spinal cord slices
The organotypic spinal cord slices were prepared according to the following method (Liu et al., 2017). Lumbar spinal cord was extracted because there were more neurons, and the meninges were removed. The spinal cord was embedded by two agarose blocks. Then they were transverse sectioned into 350-μm slices. The slices were placed on the surface of the membrane inserts and cultured for 20 days at 37°C in a 5% CO2 humidified incubator. The growth medium (DMEM/F12 supplemented with 10% FBS) was changed the day after plating, and then was changed every 3 days.
Choroidal plexus epithelial cells primary culture
The neonatal 1-day-old rats were used. The procedure is as follows (Huang et al., 2013b). Briefly, 6 rat brains were removed and kept in chilled DMEM/low glucose medium. The choroid plexus tissues were extracted from both lateral ventricles, transferred into a beaker containing chilled DMEM/low glucose medium. The tissue pieces were mechanically triturated by repeated passages through a 1 mL pipette. After centrifugation, the growth medium (DMEM/low glucose supplemented with 10% FBS and 10 ng/mL EGF) was added. Then, cells suspended homogeneously in the growth medium were seeded in Petri dishes. They were then cultured in a 5% humidified CO2 incubator at 37°C. The growth medium was changed 48–72 h later, and then changed every 3 days.
Transplantation of choroid plexus epithelial cells
Choroidal plexus epithelial cells cultured for 6–7 days were applied. The medium in Petri dishes was discarded, and CM-Dil (1 μg/mL) was added. The choroidal plexus epithelial cells and CM-Dil were incubated together at 37°C for 30 min, then at 4°C for 15 min. The choroidal plexus epithelial cells were washed by phosphate-buffered saline (PBS) for two times, then were mechanically dissociated into cells suspension. Next, they were centrifuged at 2000 × g for 5 min. The supernatant was discarded, and PBS was added to modulate cells density to 0.5–1 × 107/mL. A 2-μL cell suspension was dripped onto organotypic spinal cord slice cultured for 6 days in the T group. In the Ctrl group, the same amount of basal medium was dripped. The CM-Dil labeled choroidal plexus epithelial cells were observed by fluorescence microscopy (Olympus, Japan).
The choroidal plexus epithelial cells cultured for 6–7 days were fixed by 4% paraformaldehyde for 30 min, incubated with 0.5% Triton X for 15 min, blocked with 0.3% H2O2 for 15 min, and 10% normal goat serum for 40 min. They were subsequently incubated with rabbit monoclonal TTR (1:1000) at 4°C overnight. After 3 washes in PBS, they were incubated in the biotin-labeled secondary antibody followed by a further treatment of avidin-biotin-peroxidase complex. The nuclei were counterstained with Mayer’s hematoxylin. Microscopy was performed (Olympus, Japan). Negative controls were performed, with primary antibodies omitted.
Immunofluorescence histochemistry
The organotypic spinal cord slices cultured for 20 days in total in the T group were fixed with 4% paraformaldehyde for 30 min, incubated with 0.5% Triton X for 30 min and 10% normal goat serum for 40 min. Then they were incubated with rabbit monoclonal anti-GFAP (1:300), anti-beta III tubulin (1:500), and anti-synaptophysin (1:100), respectively, at 4°C overnight. Subsequently, they were incubated with fluorescein-conjugated goat anti-rabbit IgG for 2 h. Cell nuclei were counterstained using DAPI. The slices were then rinsed and cover-slipped with fluorescent mounting media. Laser confocal microscopy (Leica, Wetzlar, Germany) was performed. Negative controls were performed, with primary antibodies omitted.
There were 6 samples in each group. Samples in Ctrl, T and C groups were collected and centrifuged at 2000 × g. Then they were homogenized in ice-cold RIPA lysis buffer supplemented with protease inhibitors cocktail. Homogenates were centrifuged at 12,000 × g, and the supernatant were collected and measured using BCA protein assay. Protein samples were normalized and loaded for SDS-PAGE, then transferred to a PVDF membrane. Membranes were then incubated for 2 h in blocking buffer (5% skim milk, tris-buffered saline, 0.1% Tween 20). Subsequently, they were incubated in primary antibodies over night at 4°C: rabbit monoclonal or polyclonal anti-GFAP (1:5000), anti-beta III tubulin (1:5000), anti-synaptophysin (1:2000), anti-ERK (1:3000), anti-p-ERK (1:2000), anti-p-JNK (1:1000), anti-P38 (1:2000), anti-caspase 3 (1:3000), anti-caspase 3 active (1:1000), anti-JNK (1:1000) and anti-p-P38 (1:1000), followed by incubation with HRP coupled anti-rabbit IgG for 1 h. Then they were incubated in ECL solution. The images were taken using EPSON V300 camera system (Epson, Japan), and the immunoreactive bands were measured by Alphaview software (Alpha Innotech, San Leandro, CA).
SPSS 26.0 software package was used. All data were reported as means ± SD. Group data were compared using One-way ANOVA with the Dunnett’s post-hoc test. Statistical significance was assessed at P < 0.05.
The morphology of choroidal plexus epithelial cells
The choroidal plexus epithelial cells were well labeled by CM-Dil. The cytomembrane was emitted strong red fluorescence when observed by fluorescence microscopy (Fig. 1A). And they were well labeled by transthyretin (TTR) in immunohistochemistry. In which, the cytoplasm was manifested as brown (Fig. 1B). Because TTR is specifically expressed in choroidal plexus epithelial cells (Nilsson et al., 1992), in our experiment, the cells were identified definitely as choroidal plexus epithelial cells. The morphology of choroidal plexus epithelial cells transplanted at different positions was different. The cell bodies were small when transplanted on the organotypic spinal cord slices (Fig. 1D, white arrow), but were big when transplanted on the polyester membrane inserts (Fig. 1D, green arrow).
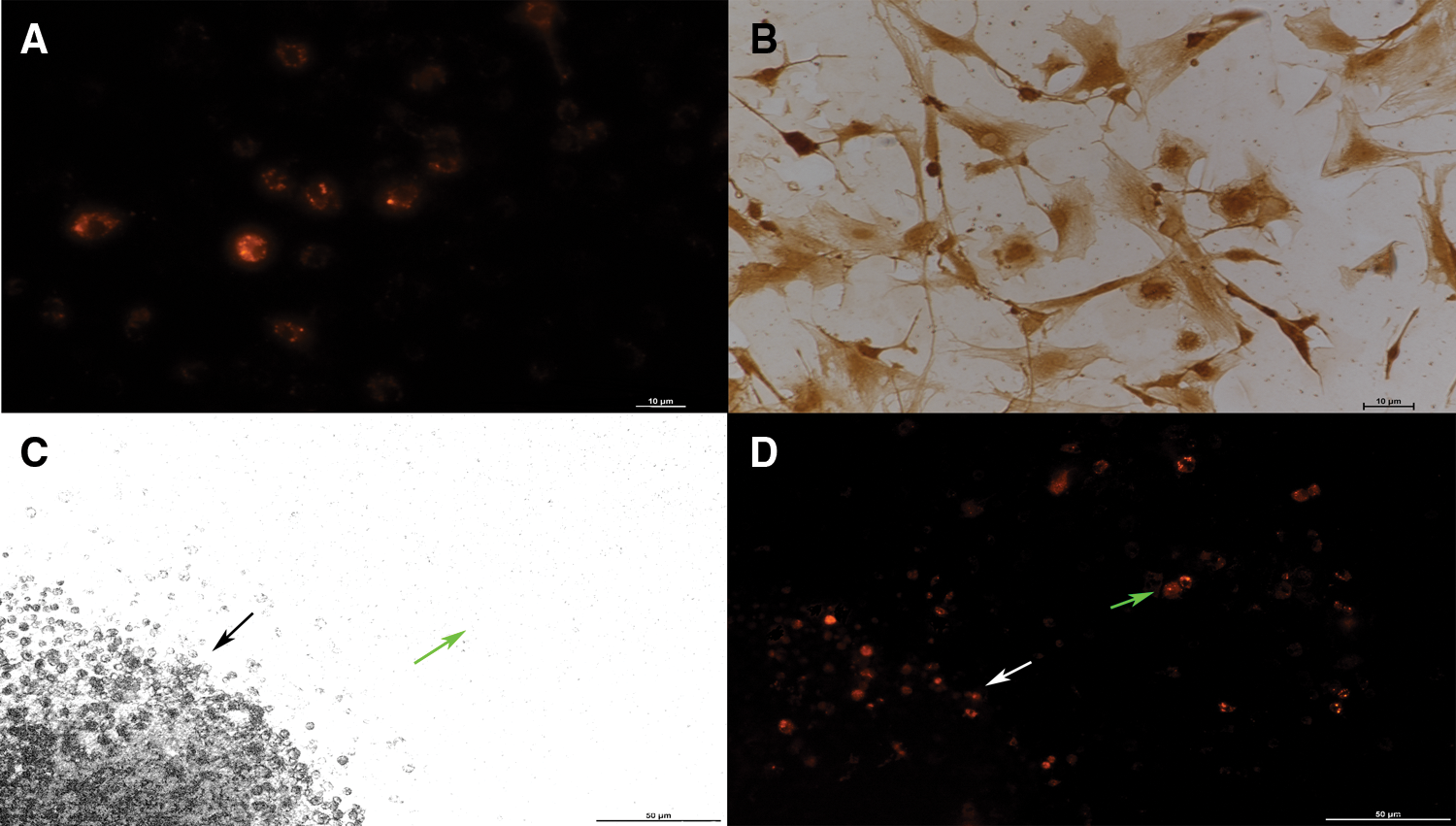
Figure 1: The morphology of choroidal plexus epithelial cells (A and B × 200, C and D × 100). (A) The cytomembrane of CM-Dil labeled choroidal plexus epithelial cells was emitted strong red fluorescence (Scale bar: 10 μm). (B) The primary-cultured choroidal plexus epithelial cells were immune-stained with an antibody against TTR in immunocytochemistry (Scale bar: 10 μm). (C) Organotypic spinal cord slices (black arrow) and polyester membrane inserts (green arrow). (D) Choroidal plexus epithelial cells transplanted into the organotypic spinal cord slices had small cell bodies (white arrow), and choroidal plexus epithelial cells transplanted to the polyester membrane inserts had big cell bodies (green arrow, Scale bar: 50 μm).
The differentiations of transplanted choroidal plexus epithelial cells
The differentiations of choroidal plexus epithelial cells which were transplanted into organotypic spinal cord slices for 14 days, were identified by immunofluorescence staining. The transplanted choroidal plexus epithelial cells could possibly differentiate into neurons (Fig. 2C, white arrow), and differentiate into astrocytes (Fig. 2F, white arrow).
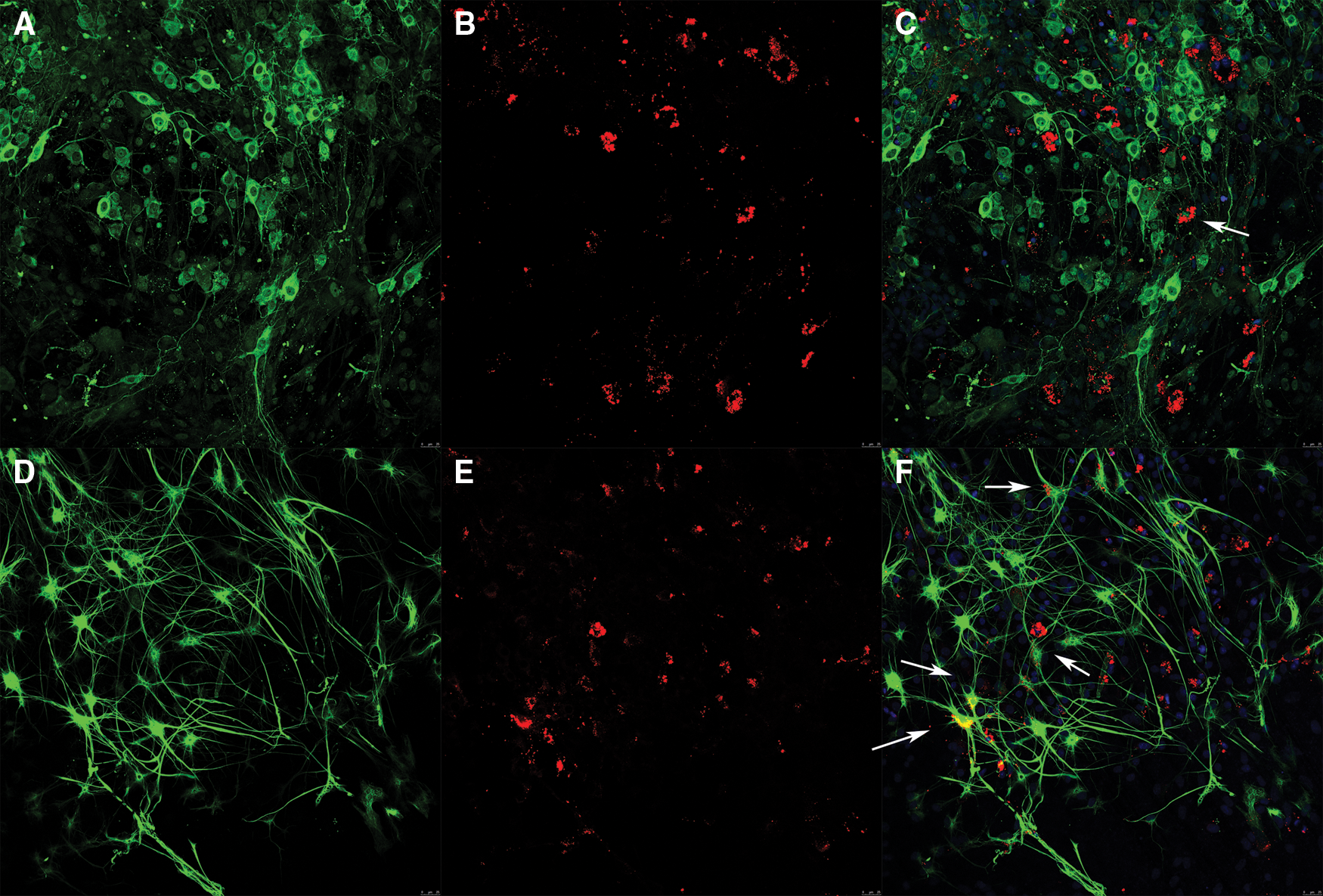
Figure 2: The differentiations of transplanted choroidal plexus epithelial cells (×200). (A) Green: neurons of the organotypic spinal cord slice. (B) Red: choroidal plexus epithelial cells transplanted onto the organotypic spinal cord slice. (C) Merged. Only one transplanted choroidal plexus epithelial cell possibly differentiated into neuron (white arrows). (D) Green: astrocytes of the organotypic spinal cord slice. (E) Red: choroidal plexus epithelial cells transplanted onto the organotypic spinal cord slice. (F) Merged. Part of transplanted choroidal plexus epithelial cells differentiated into astrocytes (white arrow, Scale bar: 25 μm).
The synapses in the transplant new system
The organotypic spinal cord slices and transplanted choroidal plexus epithelial cells formed a transplant new system. The synapses in the new system were tested by immunofluorescence staining. The synaptophysin positive vesicles were located between the neurons of organotypic spinal cord slice and transplanted choroidal plexus epithelial cells (Fig. 3D, white arrow).
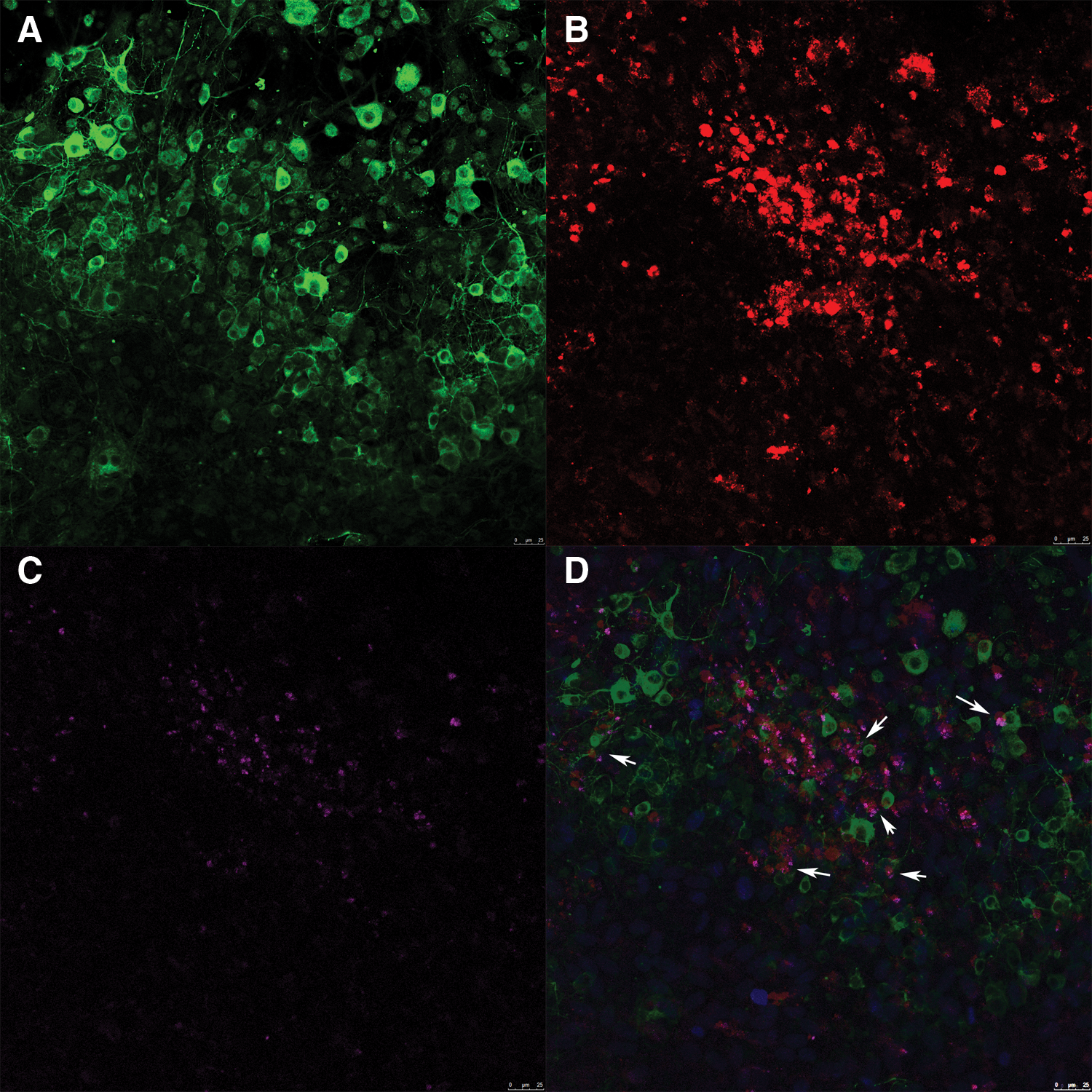
Figure 3: The synapses in the new system (×200). (A) Green: neurons of the organotypic spinal cord slice. (B) Red: choroidal plexus epithelial cells transplanted onto the organotypic spinal cord slice. (C) Pink: synaptophysin positive vesicles in the transplant new system. (D) The synaptophysin (white arrow) between neurons and choroidal plexus epithelial cells (Scale bar: 25 μm).
The cells differentiations and synaptogenesis in the new system
The ratios of different types of cells in the new system were tested by Western blotting. The levels of GFAP and TUB-III in the T group were higher than which in the Ctrl and C groups (Figs. 4A–4C). The new system promoted the cell differentiations into neurons and astrocytes. The synapses in the new system were also tested by Western blotting. The synaptophysin level in the T group was significantly higher as compared with in the Ctrl and C group (Figs. 4A and 4D). There were new synaptogenesis in the new system.
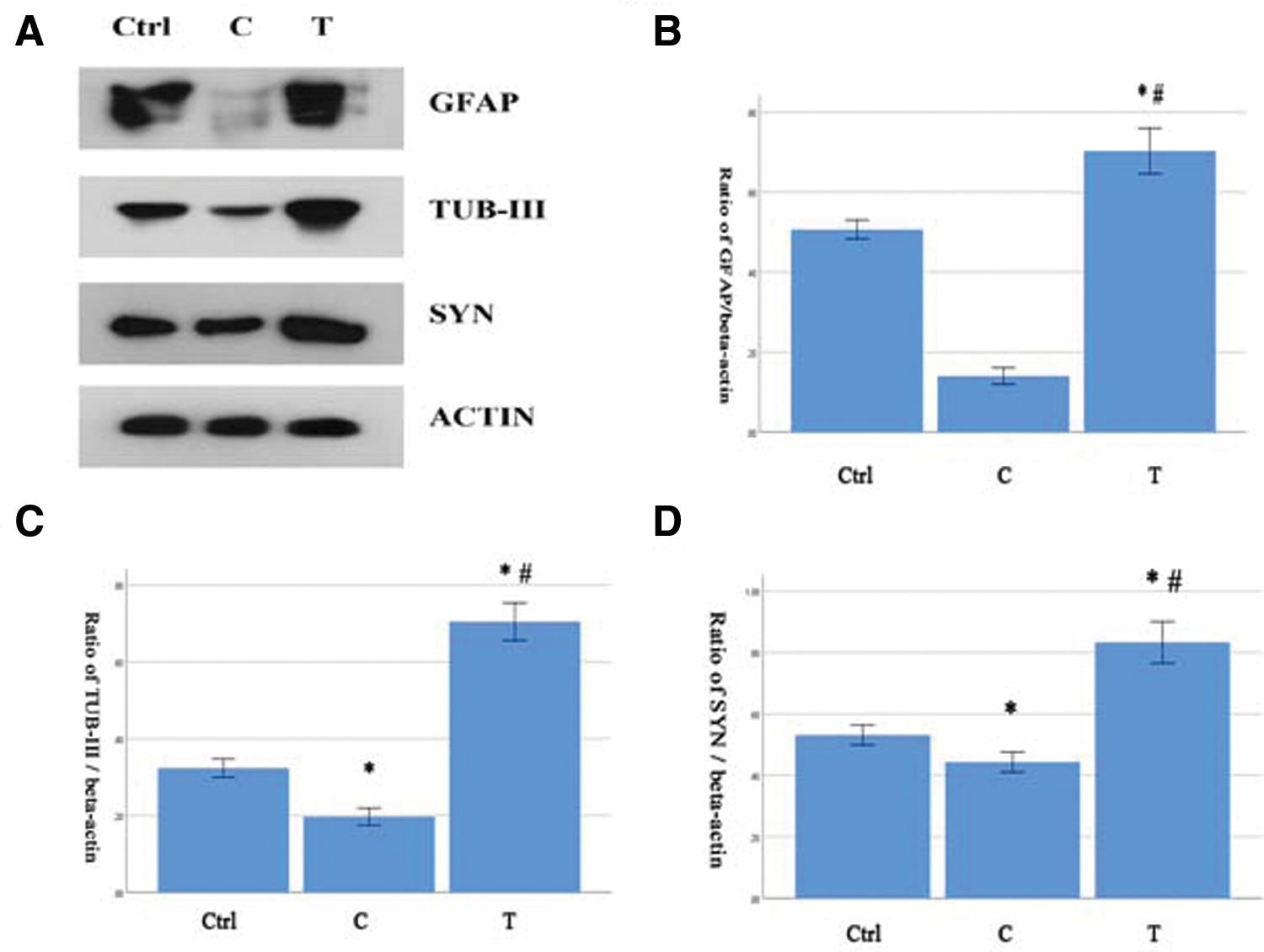
Figure 4: The cells differentiations and synaptogenesis in the new system (n = 6). (A–D) The levels of GFAP, TUB-III and synaptophysin were significantly higher in the T group. Compare with the Ctrl group, *P < 0.05. Compare with the C group, #P < 0.05.
The changes of MAPK signaling pathway in the new system
The ratios of p-JNK/JNK and p-P38/P38 in the T group were significantly lower than those in the Ctrl and C groups. But the ratios of p-ERK/ERK in the three groups were of no significant difference (Figs. 5A–5D).
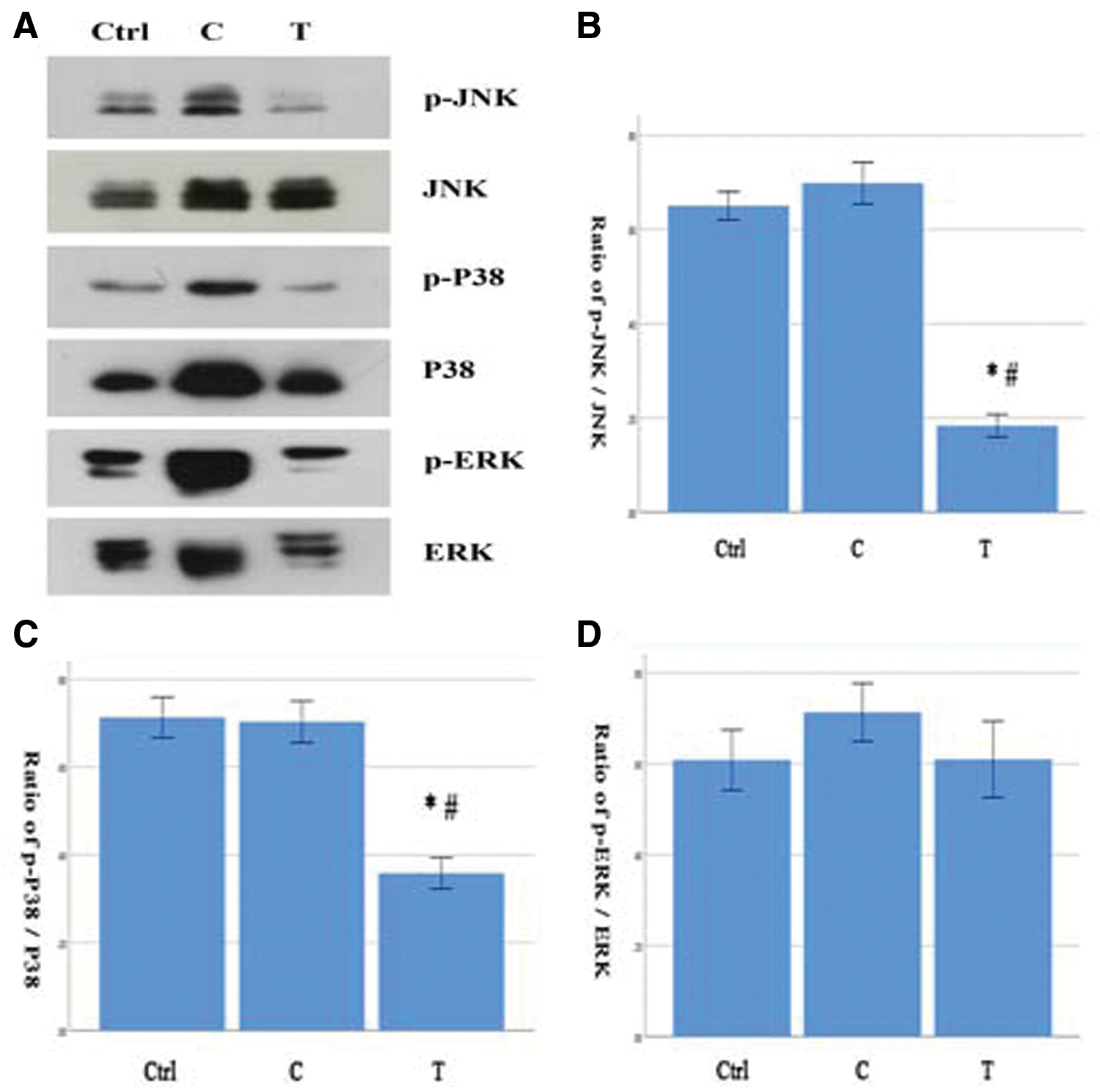
Figure 5: (A–D) The ratios of p-JNK/JNK and pP38/p38 were significantly lower in the T group and there was no significance in the ratio of p-ERK/ERK among the three groups (N = 6). Comparison with the Ctrl group, *P < 0.05. Comparison with the C group, #P < 0.05.
The choroidal plexus epithelial cells can be used as seed cells, and organotypic spinal cord slices can be used as host tissue. In the present experiment, we investigated the integration between choroidal plexus epithelial cells and organotypic spinal cord slices. We found that the transplanted choroidal plexus epithelial cells integrated excellently with organotypic spinal cord slices into a new system. The new system promotes transplanted cells differentiating into neurons and astrocytes, and it promotes the synaptogenesis. In the new system, there were changes of MAPK signaling pathway. The phosphorylation level of MAPK may be related with the cell survival, apoptosis, and differentiation.
Organotypic spinal cord slices leave the structural integrity essentially intact. This allows detailed studies of cellular responses and cell–cell interactions. The culture system has been successfully established in our previous work (Liu et al., 2017). So organotypic spinal cord slices as an excellent platform was used in our experiment.
CM-Dil is always used for fluorescent labeling. The cytomembranes which are dyed manifest stable red fluorescence. In addition, the cellular viability is not affected. Neurons labeled with CM-Dil can survive for up to 4 weeks in vitro and for up to one year in vivo (Honig and Hume, 1989). Along with it, the choroidal plexus epithelial cells we cultured for 20 days were dyed red fluorescence strongly and continuously, and their viability was excellent.
The external cues, such as polypeptides secreted by the surrounding tissue or adhesion molecules, and neuronal activity could modulate axonal growth as well as dendritic shape and complexity (Neuser et al., 2013). The local microenvironment is the key factor to affect the cellular morphology and biological behavior. It was reported that neural precursor cells transplanted on organotypic hippocampal slices had more mature morphology than those transplanted on membrane inserts (Morgan et al., 2012). Consistently, we found that the choroidal plexus epithelial cells transplanted on organotypic spinal cord slices had small cell bodies than which transplanted on membrane inserts.
The formation of functional contacts between the seed cells and the host tissue is a primary issue for cell transplantation. Several studies in vitro and in vivo have shown that the neural stem cells and brain tissue can influence each other, and functionally integrate into a new system (Jäderstad et al., 2010; Hofer et al., 2012; Shamloo et al., 2015). But the integration of transplanted choroidal plexus epithelial cells with organotypic spinal cord slices was unclear. In our experiment, the synaptophysin positive vesicles were located between neurons and transplanted choroidal plexus epithelial cells. Furthermore, the expression of synaptophysin increased sharply in western blotting. It indicated that there were new synaptogenesis in the new system, and the formation of morphological and functional junctions between transplanted cells and host’s cells.
The choroidal plexus epithelial cells possess the peculiarities of stem cells. As grafted cells, they have the excellent ability of survival, proliferation, and differentiation, which is dependent largely on the microenvironment of new system (Huang et al., 2013a). It was demonstrated that the choroidal plexus epithelial cells could differentiate into neurons (Itokazu et al., 2006; Bolos et al., 2013) and astrocytes (Kitada et al., 2001). In our experiment, the choroidal plexus epithelial cells transplanted into organotypic spinal cord slices could survive for a long time. Immunochemical study of GFAP (a marker for astrocyte) and TUB-III (a marker for neuron) showed the process of choroidal plexus epithelial cells differentiating into astrocytes, and possibly differentiating into neurons. Meanwhile, western blotting showed the expressions of GFAP and TUB-III increasing significantly. The results above indicated the differentiations of choroidal plexus epithelial cells into astrocytes and neurons in the new system.
The generic mitogen-activated protein kinases (MAPK) intracellular signaling pathway is associated with the cell inflammation, survival, proliferation, differentiation, and apoptosis (Sun et al., 2015). The MAPK is shared by four distinct cascades, including the extracellular signal-related kinases (ERK1/2), Jun amino-terminal kinases (JNK1/2/3), p38-MAPK and ERK5. ERK can be activated by mitotic factors such as EGF and b-FGF, which is associated with cell proliferation. JNK and P38 can be activated to regulate apoptosis and inflammation. The biological behavior of grafted cells and host tissue in the new system is also modulated by it. We found the MAPK pathway in the new system was different with which in the organotypic spinal cord slices without cells transplantation. In the new system, the phosphorylation level of JNK and P38 decreased, which indicated the decrease of apoptosis and inflammation. But the phosphorylation level of ERK was almost unchanged, which indicated the absence of cellular proliferation. Combined with western blotting results, the overall effect of the changes of MAPK pathway in the new system was the promotion of cells survival and differentiations into neurons and astrocytes.
Transplanted choroidal plexus epithelial cells can integrate with organotypic spinal cord slices into a new system. The cells can differentiate into neurons and astrocytes, and the new synaptic junction can be built in the new system. And there are changes of MAPK signaling pathway, which are related with cells survival, apoptosis, and differentiations. But our observation time for the new system, merely about two weeks, is relatively shorter. The long-term changes need to be fully and completely investigated furthermore. And how the transplanted choroidal plexus epithelial cells proliferate, adhere, spread, and differentiate in the new system also need to be deeply investigated furthermore.
Availability of Data and Materials: The data used to support the findings of this study are available from the corresponding author upon request.
Authors’ Contribution: JJL designed the experiments, performed the experiments, and wrote the manuscript. XYD performed the experiments and analyzed the data. LX performed data analysis and interpretation. SLH conceived the project and designed the experiments. All authors read and approved the final manuscript.
Ethics Approval: All experimental procedures were approved and supervised by the Xi’an Jiaotong University Animal Experimentation Committee. Ethical approval for the study was obtained from the Medical Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University (No. 2014-2118, March 08, 2014), Xi’an, 710004, China. All efforts were made to minimize animal suffering.
Funding Statement: This study was supported by National Natural Science Foundation of China (81471247).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Aliaghaei A, Gardaneh M, Maghsoudi N, Salehinejad P, Gharib E (2016). Dopaminergic induction of umbilical cord mesenchymal stem cells by conditioned medium of choroid plexus epithelial cells reduces apomorphine-induced rotation in parkinsonian rats. Archives of Iranian Medicine 19: 561–570. [Google Scholar]
Bolos M, Spuch C, Ordoñez-Gutierrez L, Wandosell F, Ferrer I, Carro E (2013). Neurogenic effects of β-amyloid in the choroid plexus epithelial cells in Alzheimer’s disease. Cellular and Molecular Life Sciences 70: 2787–2797. DOI 10.1007/s00018-013-1300-x. [Google Scholar] [CrossRef]
Borlongan CV, Thanos CG, Skinner SJ, Geaney M, Emerich DF (2007). Transplants of encapsulated rat choroid plexus cells exert neuroprotection in a rodent model of Huntington’s disease. Cell Transplantation 16: 987–992. DOI 10.3727/000000007783472426. [Google Scholar] [CrossRef]
Cifra A, Mazzone GL, Nani F, Nistri A, Mladinic M (2012). Postnatal developmental profile of neurons and glia in motor nuclei of the brainstem and spinal cord, and its comparison with organotypic slice cultures. Developmental Neurobiology 72: 1140–1160. DOI 10.1002/dneu.20991. [Google Scholar] [CrossRef]
Dionne KR, Tyler KL, Clarke P (2021). Slice culture modeling of CNS viral infection. Methods in Molecular Biology 2311: 109–130. DOI 10.1007/978-1-0716-1437-2. [Google Scholar] [CrossRef]
Emerich DF, Schneider P, Bintz B, Hudak J, Thanos CG (2007). Aging reduces the neuroprotective capacity, VEGF secretion, and metabolic activity of rat choroid plexus epithelial cells. Cell Transplantation 16: 697–705. DOI 10.3727/000000007783465145. [Google Scholar] [CrossRef]
Eslami M, Oryan SH, Rahnema M, Bigdeli MR (2021). Neuroprotective effects of normobaric hyperoxia and transplantation of encapsulated choroid plexus epithelial cells on the focal brain ischemia. Cell Journal 23: 303–312. [Google Scholar]
Hashemi E, Sadeghi Y, Aliaghaei A, Seddighi A, Piryaei A, Broujeni ME, Shaerzadeh F, Amini A, Pouriran R (2017). Neural differentiation of choroid plexus epithelial cells: Role of human traumatic cerebrospinal fluid. Neural Regeneration Research 12: 84–89. DOI 10.4103/1673-5374.198989. [Google Scholar] [CrossRef]
Hofer S, Magloire V, Streit J, Leib SL (2012). Grafted neuronal precursor cells differentiate and integrate in injured hippocampus in experimental pneumococcal meningitis. Stem Cells 30: 1206–1215. DOI 10.1002/stem.1097. [Google Scholar] [CrossRef]
Honig MG, Hume RI (1989). Dil and DiO: Versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends in Neurosciences 12: 333–341. DOI 10.1016/0166-2236(89)90040-4. [Google Scholar] [CrossRef]
Huang SL, He XJ, Li ZF, Yao L, Yuan GL, Shi W (2013a). Primary culture of choroid plexuses from neonate rats containing progenitor cells capable of differentiation. Balkan Medical Journal 30: 350–354. DOI 10.5152/balkanmedj.2013.8259. [Google Scholar] [CrossRef]
Huang SL, He XJ, Li ZF, Yao L, Shi W (2013b). A novel primary culture method for rat choroidal epithelial cells. Neurosciences 18: 27–32. [Google Scholar]
Huang SL, Shi W, Jiao Q, He XJ (2011). Change of neural stem cells in the choroids plexuses of developing rat. International Journal of Neuroscience 121: 310–315. DOI 10.3109/00207454.2011.556282. [Google Scholar] [CrossRef]
Huang SL, Wang J, He XJ, Li ZF, Pu JN, Shi W (2014). Secretion of BDNF and GDNF from free and encapsulated choroid plexus epithelial cells. Neuroscience Letters 566: 42–45. DOI 10.1016/j.neulet.2014.02.017. [Google Scholar] [CrossRef]
Ide C, Nakano N, Kanekiyo K (2016). Cell transplantation for the treatment of spinal cord injury—Bone marrow stromal cells and choroid plexus epithelial cells. Neural Regeneration Research 11: 1385–1388. DOI 10.4103/1673-5374.191198. [Google Scholar] [CrossRef]
Ikeda T, Xia XY, Xia YX, Ikenoue T, Choi BH (1999). Expression of glial cell line-derived neurotrophic factor in the brain and cerebrospinal fluid of the developing rat. International Journal of Developmental Neuroscience 17: 681–691. DOI 10.1016/S0736-5748(99)00057-X. [Google Scholar] [CrossRef]
Itokazu Y, Kitada M, Dezawa M, Mizoguchi A, Matsumoto N, Shimizu A, Ide C (2006). Choroid plexus ependymal cells host neural progenitor cells in the rat. Glia 53: 32–42. DOI 10.1002/(ISSN)1098-1136. [Google Scholar] [CrossRef]
Jäderstad LM, Jäderstad J, Herlenius E (2010). Graft and host interactions following transplantation of neural stem cells to organotypic striatal cultures. Regenerative Medicine 5: 901–917. DOI 10.2217/rme.10.80. [Google Scholar] [CrossRef]
Kanekiyo K, Nakano N, Noda T, Yamada Y, Suzuki Y, Ohta M, Yokota A, Fukushima M, Ide C (2016). Transplantation of choroid plexus epithelial cells into contusion-injured spinal cord of rats. Restorative Neurology and Neuroscience 34: 347–366. DOI 10.3233/RNN-150546. [Google Scholar] [CrossRef]
Kim HM, Lee HJ, Lee MY, Kim SU, Kim BG (2010). Organotypic spinal cord slice culture to study neural stem/progenitor cell microenvironment in the injured spinal cord. Experimental Neurobiology 19: 106–113. DOI 10.5607/en.2010.19.2.106. [Google Scholar] [CrossRef]
Kitada M, Chakrabortty S, Matsumoto N, Taketomi M, Ide C (2001). Differentiation of choroid plexus ependymal cells into astrocytes after grafting into the pre-lesioned spinal cord in mice. Glia 36: 364–374. DOI 10.1002/(ISSN)1098-1136. [Google Scholar] [CrossRef]
Liu JJ, Huang YJ, Xiang L, Zhao F, Huang SL (2017). A novel method of organotypic spinal cord slice culture in rats. NeuroReport 28: 1097–1102. DOI 10.1097/WNR.0000000000000892. [Google Scholar] [CrossRef]
Matsumoto N, Taguchi A, Kitayama H, Watanabe Y, Ohta M et al. (2010). Transplantation of cultured choroid plexus epithelial cells via cerebrospinal fluid shows prominent neuroprotective effects against acute ischemic brain injury in the rat. Neuroscience Letters 469: 283–288. DOI 10.1016/j.neulet.2009.09.060. [Google Scholar] [CrossRef]
Morgan PJ, Liedmann A, Hübner R, Hovakimyan M, Rolfs A, Frech MJ (2012). Human neural progenitor cells show functional neuronal differentiation and regional preference after engraftment onto hippocampal slice cultures. Stem Cells and Development 21: 1501–1512. DOI 10.1089/scd.2011.0335. [Google Scholar] [CrossRef]
Neuser F, Polack M, Annaheim C, Tucker KL, Korte M (2013). Region-specific integration of embryonic stem cell-derived neuronal precursors into a pre-existing neuronal circuit. PLoS One 8: e66497. DOI 10.1371/journal.pone.0066497. [Google Scholar] [CrossRef]
Nilsson C, Lindvall-Axelsson M, Owman C (1992). Neuroendocrine regulatory mechanisms in the choroid plexus-cerebrospinal fluid system. Brain Research Reviews 17: 109–138. DOI 10.1016/0165-0173(92)90011-A. [Google Scholar] [CrossRef]
Shamloo A, Heibatollahi M, Mofrad MR (2015). Directional migration and differentiation of neural stem cells within three-dimensional microenvironments. Integrative Biology 7: 335–344. DOI 10.1039/C4IB00144C. [Google Scholar] [CrossRef]
Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF (2015). Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. Journal of Receptors and Signal Transduction 35: 600–604. DOI 10.3109/10799893.2015.1030412. [Google Scholar] [CrossRef]
Sypecka J, Koniusz S, Kawalec M, Sarnowska A (2015). The organotypic longitudinal spinal cord slice culture for stem cell study. Stem Cells International 2015: 1–10. DOI 10.1155/2015/471216. [Google Scholar] [CrossRef]
Xu Z, Liu C, Wang R, Gao X, Hao C, Liu C (2021). A combination of lycopene and human amniotic epithelial cells can ameliorate cognitive deficits and suppress neuroinflammatory signaling by choroid plexus in Alzheimer’s disease rat. Journal of Nutritional Biochemistry 88: 108558. DOI 10.1016/j.jnutbio.2020.108558. [Google Scholar] [CrossRef]
Zhao F, Ding XY, Wu F, Li XH, Li YH, Huang SL (2018). Effects of passage and cryopreservation on neurotrophic factor secretion from choroid plexus epithelial cells. Biomedical Reports 8: 535–539. DOI 10.3892/br.2018.1087. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |