DOI:10.32604/biocell.2022.017609

| BIOCELL DOI:10.32604/biocell.2022.017609 |  |
| Review |
A mini-review on pharmacological effects of ginsenoside Rb3, a marked saponin from Panax genus
1College of Chinese Medicinal Materials, Jilin Agricultural University, Changchun, 130118, China
2National & Local Joint Engineering Research Center for Ginseng Breeding and Development, Changchun, 130118, China
3Maternity Diagnosis & Treatment Center, The Affiliated Hospital of Changchun University of Chinese Medicine, Changchun, 130021, China
*Address correspondence to: Meiling Fan, Fanmeiling1982@163.com; Zi Wang, wangzi8020@126.com
Received: 24 May 2021; Accepted: 07 July 2021
Abstract: Ginsenoside Rb3 (G-Rb3) is one of the primary active compounds isolated from Panax ginseng Meyer, which belongs to protopanaxadiol ginsenosides (PPD). Based on the structure-activity relationship (SAR) of ginsenosides, the pentose structure of G-Rb3 limited itself to possess more pharmacological activity to a certain extent. However, pharmacokinetics show that G-Rb3 is processed through deglycosylation in the intestinal tract and converted into more active rare saponins, such as Compound K, F2, etc. A series of studies focused on neuroprotection and the cardiovascular system demonstrating its therapeutic potentials, which was achieved by diminishing oxidative stress and apoptosis. Therefore, more systematic and in-depth studies are needed to complete the pharmaceutical value and to promote its clinical applications. This article highlights the multiple pharmacological effects and mechanisms of G-Rb3 and prospects for its development.
Keywords: Ginsenoside Rb3; Pharmacological effects; Review; Signaling pathway
List of Abbreviations
| AUC: | area under the plasma concentration vs. time curve; |
| MRT: | mean residence time; |
| Tmax, t1/2: | the elimination half-life. |
Ginseng, a highly valuable and special medicinal herb, has been proposed to be an adaptogen over 2000 years in China (Chen et al., 2008; Gao et al., 2016). The cognition of its “homology of medicine and food” was promoted and recognized in China, Korea, Japan, Europe, and America. Belonging to the Araliaceae family, ginseng is a perennial medicinal plant, mainly growing in the East Asia region, especially in cold zones. Evidence demonstrated that ginsenosides contribute primarily to the pharmacological function of ginseng (Choi, 2008), such as anti-tumors (Xia et al., 2014), anti-inflammatory (Chen et al., 2007; Liu et al., 2020a), antifatigue (Tang et al., 2008), etc. Among the series of ginsenoside candidates, researchers have suggested that G-Rb3 could be potential substitute medicines for diverse diseases, including not limited to anti-inflammatory (He et al., 2014), anti-diabetic (Bu et al., 2012), and antioxidant (Shi et al., 2011), but also neural (Cui et al., 2012) and cardiovascular protective effects (Shi et al., 2011).
As mentioned the pharmacological activity of ginsenoside, it is natural to explain its related structure–activity relationship (SAR), in which the position and number of sugars in ginsenoside determine their pharmacological activity (Wang et al., 2007). For example, the rare ginsenoside Rg3 is formed with special functions after being metabolized in the body owing to its crucial transformation in sugar. Different from Rg3, G-Rb3 is relatively richer in ginseng rhizomes, and mainly derived from Panax notoginseng/P. quinquefolius L./P. ginseng Meyer/Gynostemma pentaphyllum (Thunb.) Makino/P. japonicus C. A. Mey (Jia et al., 2019; Liu et al., 2018; Zhang et al., 2021; Zhang et al., 2019). For SAR, although G-Rb3 shows potentials for the prevention of neurological and cardiovascular diseases, the pentose structure (3 sugars) of G-Rb3 limits itself from possessing more pharmacological activity to a certain extent. Hence reviewing and summarizing research achievements on Rb3 contribute to its further application in clinical.
According to the different structures of aglycones, ginsenosides can be divided into three types: Dammarane (including Topazanediol and Topazanetriol), Oleanose, and Ocorol. G-Rb3 is a type of tetracyclic triterpenoid saponin, belongs to PPD. G-Rb3 possesses 1 xylose and 3 glucose moieties with 20(S)-protopanaxadiol (PPD) aglycone (Fig. 1), which is one of the major ginsenosides with a content of about 2.1 mg/g in P. ginseng Meyer (Liu et al., 2016). The molecular formula of G-Rb3 is C53H90O22, with a molecular weight of 1079.2844 g/mol. It is white powder and soluble in water, methanol, and ethanol. It was found that the content distribution order of G-Rb3 was Panax notoginseng > P. quinquefolius L. > P. ginseng Meyer > Gynostemma pentaphyllum (Thunb.) Makino > P. japonicus C. A. Mey. Recent studies revealed that the type of dammarane, the number of sugar moieties, and differences in the substituent groups were responsible for its anti-cancer effect owing to its biological SAR (Liu et al., 2003; Wang et al., 2007). Herein, we hypothesize that the structure of G-Rb3 (1 xylose and 3 glucose moieties) may affect its relevant pharmacological activities.
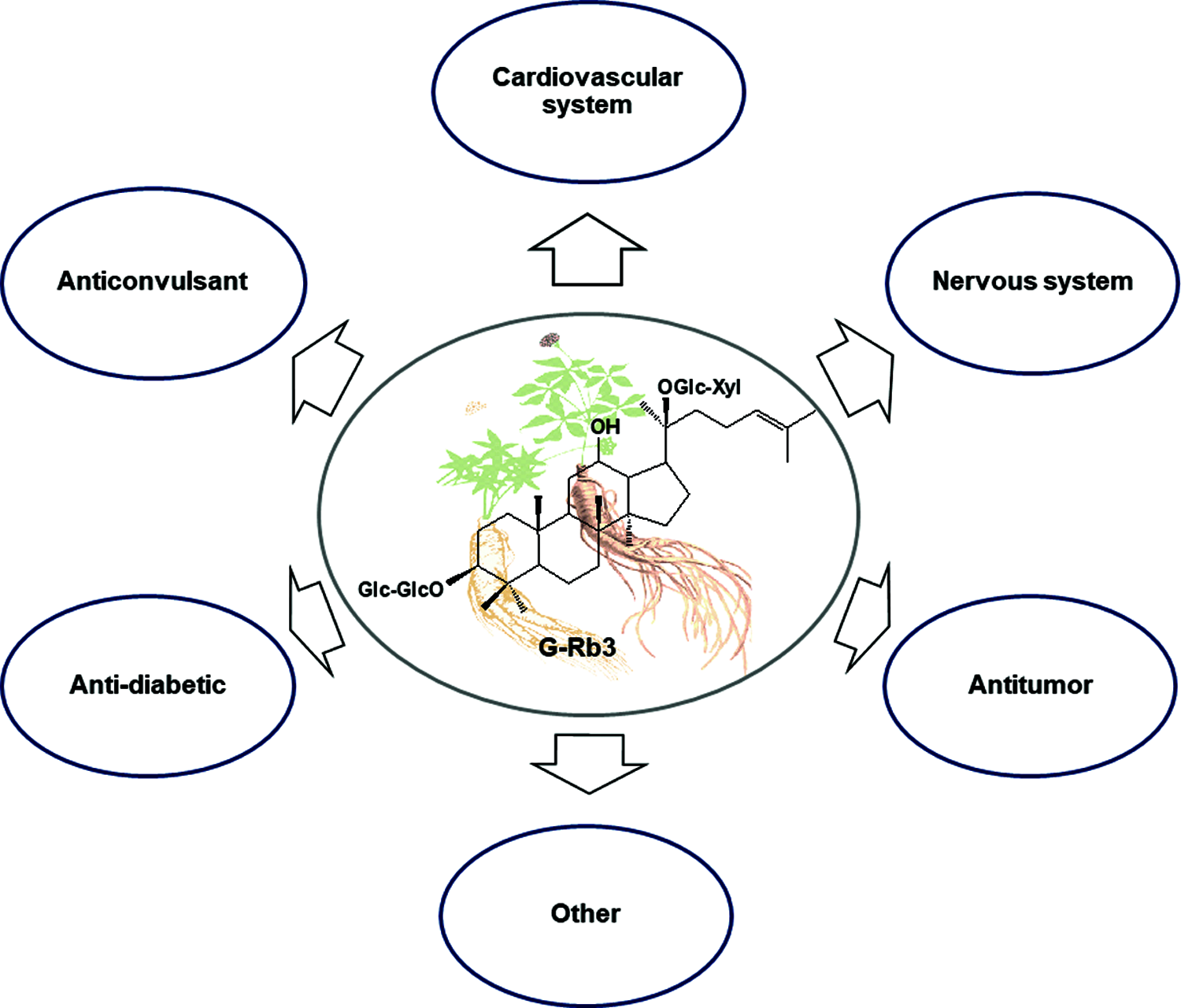
Figure 1: Summary of various pharmacological activities related to G-Rb3.
Pharmacokinetic Studies on G-Rb3
Understanding the pharmacokinetics of ginsenosides is crucial for designing an optimal dose regimen and avoiding the potential unwanted interactions between ginsenosides and other drugs in clinical application. It is logical to think that the pharmacokinetic behaviors of ginsenosides are based on molecular structures. However, G-Rb3 with one glucose-linked β-D-xylose is poorly absorbed than G-Rb1 by the oral administration tracked using liquid chromatography-tandem mass spectrometry (LC-MSn) analysis (Zhao et al., 2012) which may be attributed to its pentose groups. Pharmacokinetic parameters of G-Rb3 are shown in Table 1.
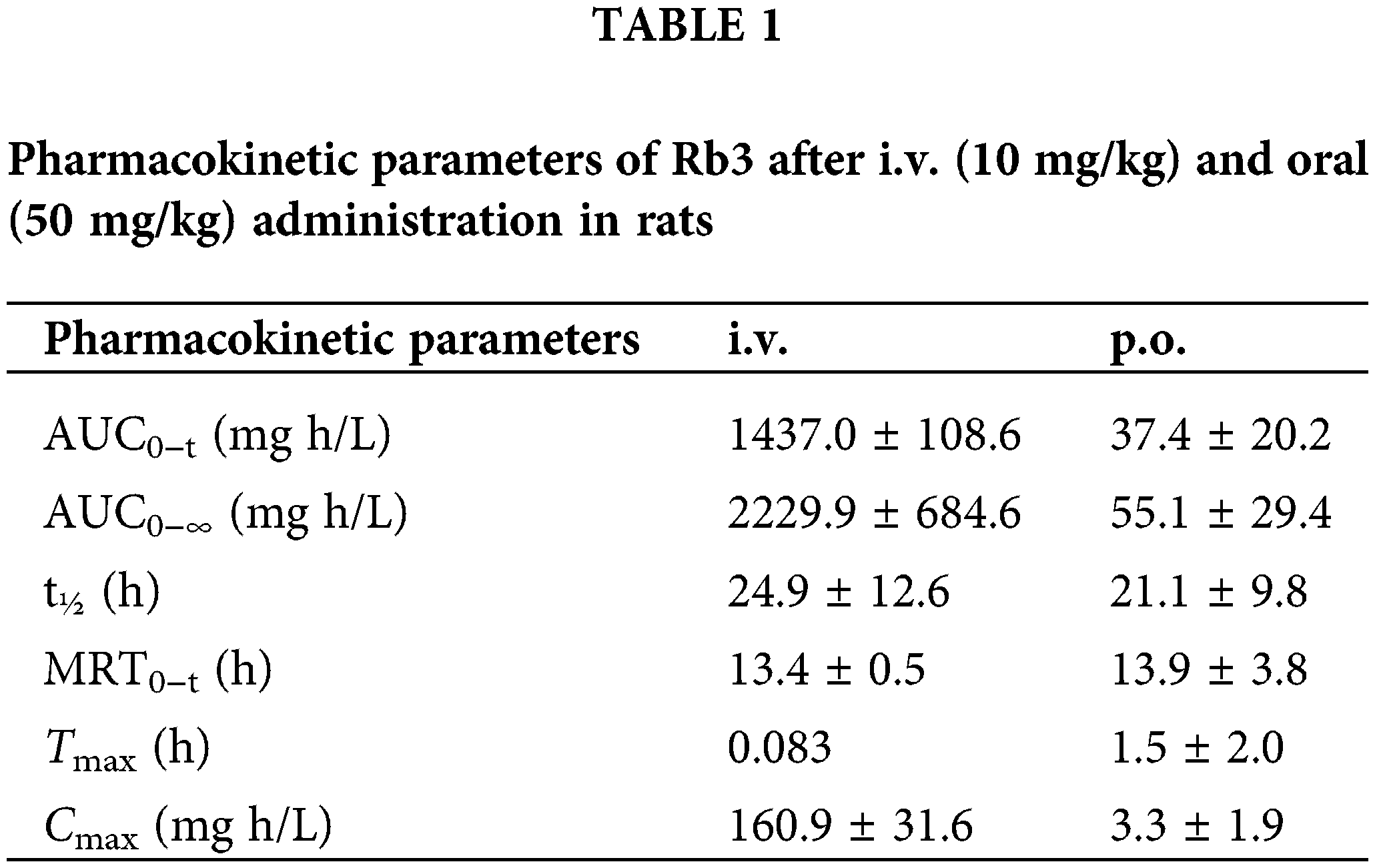
Comparatively, the elimination of i.v. is faster than p.o. The lengths of the ginsenoside sugar chain are closely but negatively related to their biological activities (Park et al., 2010; Tawab et al., 2003). Further study demonstrated that deglycosylation was the major metabolic pathway of G-Rb3 in rats, and the mean plasma elimination half-lives for distribution and exterminate phases t1/2α and t1/2β were 13.77 ± 1.23 min and 2045.70 ± 156.20 min (Zhao et al., 2018). Meanwhile, two major metabolites Mb1 and M2’ were tentatively identified in rat urine samples after intravenous administration, and the additional two metabolites were F2 and CK after oral administration, as depicted in Table 2. In summary, it is urgent to identify the effective delivery pathway of G-Rb3.
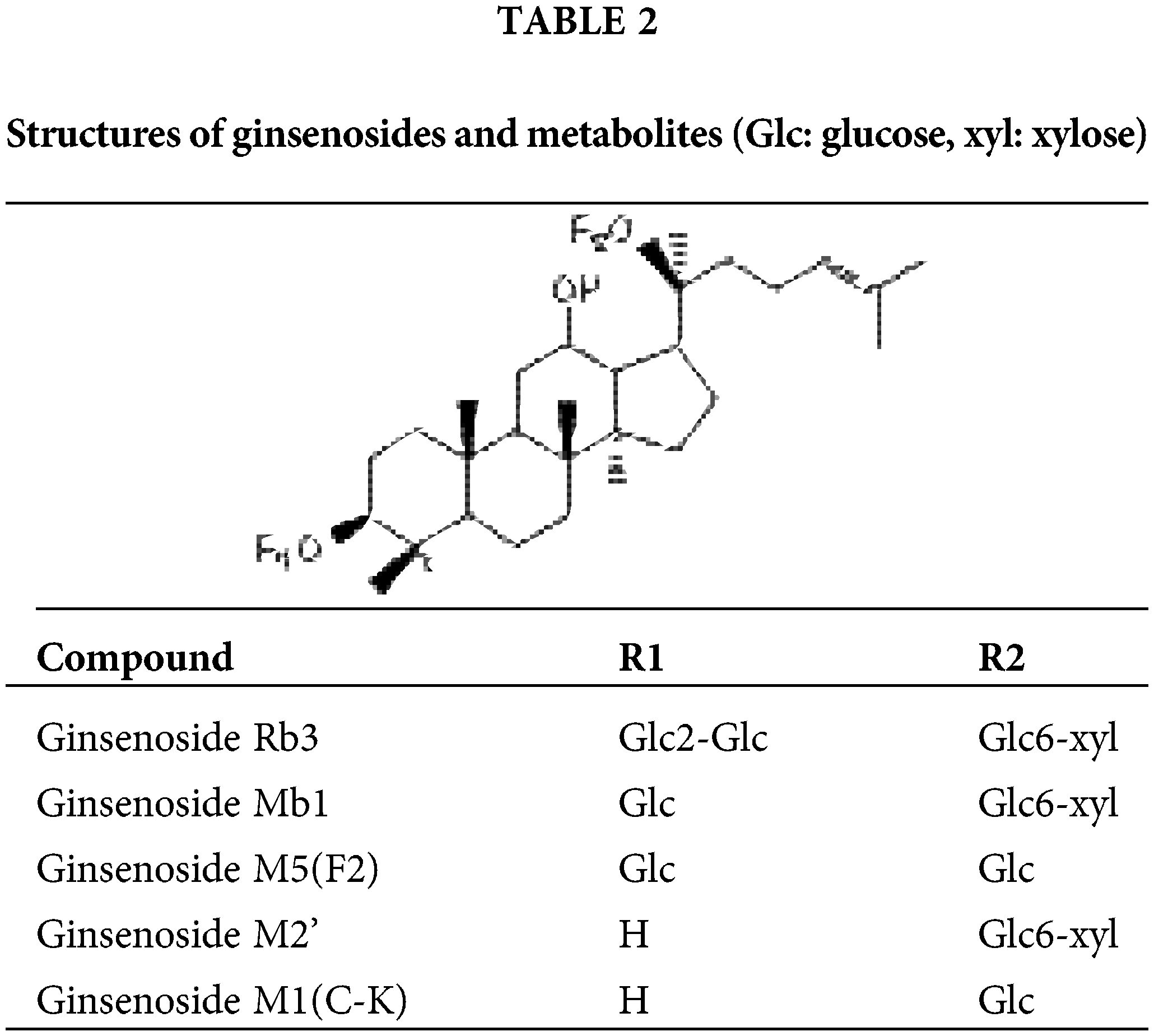
It is commonly accepted that orally ingested major ginsenosides (such as Rb1, Rb2, and Rb3), containing three to five sugars of these saponins, can be deglycosylated into active minor ginsenosides, also named “rare ginsenoside”, by the intestinal bacteria of the microflora. More specifically, major ginsenosides may act as pro-drugs when they are taken orally, and their deglycosylated derivatives are responsible for the in vivo effects. Given the biotransformation of G-Rb3, enzymology studies were performed to prepare rare ginsenosides through G-Rb3 (Liu et al., 2014a).
Protective effects for nervous system and possible mechanisms
According to pretreatment or posttreatment of G-Rb3 in different experimental reports, the potential effect of G-Rb3 on the central nervous system injury model is mainly aimed at hypoxic/ischemia brain injury and oxygen and glucose deprivation (OGD) stress. G-Rb3 has been proven to possess a neuroprotective effect on various cell lines in vitro and several animal models in vivo through multiple molecular mechanisms (Fig. 1).
In a cultured hippocampal neurons model tested with hypoxia, G-Rb3 stabilized the cell membrane and suppressed NOS, especially inducible nitric oxide synthase (iNOS) (Shen et al., 2006a). In glutamate-treated hippocampal neurons, G-Rb3 enhanced the hippocampal neuronal viability, decreased the lactate dehydrogenase (LDH) leakage, and elevated the nitric oxide synthase (eNOS). A report has provided vital evidence that G-Rb3 exhibits significant protective effects on glutamate excitotoxic injury (Shen et al., 2006b). The involved mechanism may include antagonizing the injury of neuron membrane, inhibiting the viability of iNOS, and increasing the activity of eNOS.
In further research related to OGD, the anti-ischemic activity of G-Rb3 was confirmed in ischemic and reperfusion injury model of PC12 cells by increasing cell viability, Bcl-2 protein expression and inhibiting LDH release, activities of cytosolic cytochrome c, cleaved-caspase 3, caspase-3, -8, -9 and Bax protein expression. The related mechanisms, at least partly, may be attributed to the effect of G-Rb3 to suppress the intracellular Ca2+ elevation and inhibiting mitochondria-mediated apoptosis pathway (Zhu et al., 2010).
Another study by Xu et al. (2005) showed that G-Rb3 inhibited strychnine-sensitive glycine receptors in acutely dissociated hippocampal neurons of rats. The GABAA receptor, a ligand-gated ion channel consisting of the chloride channel complex, activation increases the membrane conductance which further suppresses postsynaptic action potential discharge accompanied by blocked excitatory synaptic responses (Leidenheimer, 2008; Zhou et al., 2007). G-Rb3 exhibits neuroprotection via modulating GABAA receptor in the OGD model in vitro (Jiang et al., 2011). In addition to this receptor, the neuroprotective activity of G-Rb3 was reported through the inhibitory action on the NMDA receptor (Jiang et al., 2018). In NMDA receptor-treated rat hippocampal neurons cells, neuronal viability was reduced accompanied by the leakage of LDH and Ca2+ influx (Berliocchi et al., 2005; White et al., 2000). Nevertheless, NMDA receptor activation caused excitotoxicity can be reversed by the treatment of G-Rb3. The underlying protective mechanisms of G-Rb3 may attribute to inhibiting NMDA receptors induced by the acceleration of concentration-dependent Ca2+ levels accompanied with the reduced intracellular free Ca2+ in the pathological response of hypoxic/ischemic brain injury (Peng et al., 2009). Early studies (Cui et al., 2012) on G-Rb3 showed a protective effect against ischemic neurons, which might be achieved through the suppression of persistent Na+ by NMDA triggered imputation of Ca2+ levels (Peng et al., 2009) in vitro.
Further data in SK-N-SH cells suggest that G-Rb3 possesses a dammarane-type core structure, which may be responsible for its significant effect in promoting neurite outgrowth activity (Zou et al., 2002). Nevertheless, the underlying mechanism of G-Rb3 has not been fully elucidated. Additional evidence of a neuroprotective effect of G-Rb3 has been obtained in a rat neuro-damaging model induced by 3-nitropropionic acid (Lian et al., 2005a). The protective mechanism of G-Rb3 may attribute to preventing ischemia and hypoxia sodium channel allosteric, scavenging oxygen free radicals, inhibiting lipid peroxidation, antagonizing calcium ions, and reducing the neurotoxicity of glutamate and nitric oxide (NO).
Depression is a common mental disorder in the clinic. The prevention and treatment of depression have been sought by the medical profession and society (Bebbington, 2001). G-Rb3 exhibits antidepressant-like activity via regulating multiple signaling pathways and targets. A study by Cui et al. (2012) showed that G-Rb3 was shown to alleviated hypothermia, palpebral ptosis, and immobility in a reserpine-induced syndrome model; they further revealed that chronic G-Rb3 treatment increased the locomotor activity, food consumption, and restored sucrose preference in the chronic mild stress model (Cui et al., 2012), indicating that G-Rb3 produce antidepressant-like activity by involving the function of noradrenergic pathways. Another study demonstrated that treatment with G-Rb3 (10, 50 mg/kg) remarkably increased the level of mouse brain monoamine neurotransmitters (NA) via regulating noradrenergic pathways (Zhang et al., 2016). Additionally, the protective effects on the nervous system are also important, such as the regulation of neurotrophic factor expression in the nervous system.
Protective Effects on Cardiovascular System and Possible Mechanisms
It is worth noting that accumulating sights have been focused on the research relevant to ginseng on cardiovascular diseases, especially in protopanaxadiol. According to the hemodynamic index in rats, G-Rb3 has a cardiovascular protective effect, which was initially proved to be related to calcium channel blockade and anti-free-radicals (Chen et al., 1994; Zhong et al., 1995). G-Rb3 can prevent isoproterenol-induced cardiovascular damage and cardiac dysfunction. Ex vivo G-Rb3 treatment restored Ang II-stimulated endothelial dysfunction by reversing over-expression of NADPH oxidases, NOX-2 and NOX-4 levels, over-production of reactive oxygen species (ROS), and improving NO bioavailability (Wang et al., 2014). G-Rb3 suppressed angiotensin II (Ang II)-stimulated proliferation of vascular smooth muscle cells and inhibited experimentally induced myocardial dysfunction (Wang et al., 2010b; Wong et al., 2010). G-Rb3 administration significantly reduced the increased creatine kinase (CRE), LDH, and malondialdehyde (MDA), whereas restored the decreased superoxide dismutase (SOD) and catalase (CAT); suggesting that G-Rb3 might subdue oxygen free radical impairment and protect the antioxidant enzyme activity in cardiomyocytes (Wang et al., 2010a). During acute myocardial infarction (AMI), the main damage to myocardial tissue is caused by initial ischemia and subsequent reperfusion. The beneficial effect of G-Rb3 on myocardial ischemia-reperfusion injury (MIRI) was characterized by the decrease in plasma endothelin and Ang II levels and myocardial infarct size. The underlying mechanism was in part related to its antioxidant activity and functional microcirculatory improvement. Previous studies further revealed a novel mechanism of G-Rb3 to attenuates oxidative stress via activating the antioxidation signaling pathway of PERK/Nrf2/HMOX1 in vivo and in vitro (Sun et al., 2019). A similar study by Liu et al. (2014b) also confirmed that G-Rb3 administration (20 mg/kg) effectively attenuated MIRI-induced apoptosis and inflammation in vitro, accompanied by inhibition of B-cell lymphoma 2-associated X protein (BAX), ROS accumulation, oxidative stress, and elevated the level of B-cell lymphoma 2 (Bcl-2), an anti-apoptotic signaling. G-Rb3 regulated energy metabolism via activating the PPARα signaling pathway, which increased expressions of key enzymes involved in β-oxidation of fatty acids, exerting an anti-apoptosis effect (Chen et al., 2019). Liu et al. (2020b) also clarified the anti-apoptosis effect of G-Rb3 in myocardial ischemia–reperfusion injury. In another report using H9c2 cells subjecting to OGD followed by reperfusion (OGD-Rep), G-Rb3 suppressed the expression of NF-κB, phosphorylation of JNK, and many inflammatory cytokine releases, such as IL-6, TNF-α, monocyte chemotactic protein-1 (MCP-1), matrix metalloproteinase-2 (MMP-2) and MMP-9. This finding revealed the potential mechanism of the protective effect of G-Rb3 being attributed to the inhibition of the JNK-mediated NF-κB pathway (Ma et al., 2014). In another myocardial injury model by Yang et al. (2017) pretreatment with G-Rb3 was found to improve the cell viability and further inhibit endothelial-to-mesenchymal transition (EMT) caused by coxsackievirus B3 (CVB3) through the Pyk2-PI3K-AKT pathway, in the treatment of myocardial fibrosis (MF) in vitro. Above all, G-Rb3 may protect cardiomyocytes from damages through signaling pathways of inflammatory and cell proliferation, indicating that G-Rb3 may be a promising therapeutic drug to treat cardiovascular diseases. However, the establishment of a cardiovascular disease model to simultaneously track the protective effect of the drug in cardiac and vascular dysfunctions would be of great significance. By further elucidating the role of oxidative stress, apoptosis, and inflammation in the development of disease, clear guidance for the clinical application may be created accordingly.
Within the strategies for tumor treatment, the ability of drugs to inhibit tumor cells is usually investigated initially. The co-treatment of G-Rb3 and cisplatin enhanced tumoricidal with anti-proliferative action on human breast carcinoma MCF-7 cells. Therefore, combinations of G-Rb3 and chemotherapeutic agents may be a novel neoadjuvant agent in clinical cancer treatment (Aung et al., 2007). Another similar research by Huang et al. (2017) demonstrated that G-Rb3 treatment in mice diminished colorectal cancer-induced increase in expression of cancer-promoting signaling and pro-inflammatory markers.
Anti-Diabetic Effect and Possible Mechanisms
Diabetes is a metabolic disease with hyperglycemia attributed to the development of insulin-secreting or functional defects in all ages. Chronic hyperglycemia in diabetes mellitus leads to chronic impairment and dysfunction of various tissues, especially kidneys, heart, and nerves (Akkati et al., 2011). Early studies suggested that G-Rb3 may act as a potential therapeutic reagent in diabetic healing. Evidence obtained from in vitro and in vivo studies demonstrated that G-Rb3 treatment significantly decreased the level of postprandial blood glucose, reinstated oral glucose tolerance at a dose-dependent manner in the normoglycemic group and mice suffered from alloxan injection, and stimulated glucose consumption in the C2C12 myotubes (Bu et al., 2012).
Gluconeogenesis, the increase in liver glucose production, is an important factor in the progression of glucose disease. In physiological conditions, liver glycogen synthesis and gluconeogenesis remain in a dynamic equilibrium. However, when insulin resistance occurs in the liver, liver gluconeogenesis increases, and liver glycogen synthesis decreases. After the balance between gluconeogenesis and glycogen synthesis is disrupted, liver glycogen output increases, and then blood sugar rises. As a key regulator of energy metabolism, AMPK can reduce plasma and liver triglyceride levels and gluconeogenesis gene transcription (Cool et al., 2006). Forkhead transcription factor 1 (FOXO1) is another important factor in gluconeogenesis. AMPK can regulate FOXO1, which inhibiting liver gluconeogenesis (Zhang et al., 2009). G-Rb3, as an AMP-activated protein kinase (AMPK) activator, offers a promising clinical therapy for treating diabetes mellitus and its complications. Potential key targets, such as phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase, forkhead transcription factor 1 (FOXO1), and hepatic nuclear receptor 4α (HNF4α), have been explored (Meng et al., 2017).
The possibility that ginseng may have anticonvulsant activity was first suggested by Lee et al. (2002). Further, the underlying anticonvulsant activity of pretreatment with G-Rb3 has been studied on different chemicals causing convulsion, including kainic acid, pilocarpine, and pentylenetetrazol in rats. However, the involved mechanism for anticonvulsant action of G-Rb3 remains unclear (Lian et al., 2006; Lian et al., 2005b).
Beyond all, G-Rb3 presented obvious renal protection against cisplatin-induced renal injury through the intervention of increased ROS levels and decreased expressions of autophagy-related proteins (Xing et al., 2019). In UV-B radiation-induced photogate cells, the restorative activity of pretreated G-Rb3 has been proved by the reduced levels of ROS, pro-MMP-2, and proMMP-9, the increased total glutathione (GSH) content, SOD activity, and cell viability (Oh et al., 2015). In the MMPs-induced cartilage degradation model, treatment with G-Rb3 (100 µg/mL) significantly reduced MMP3 secretion compared to S12 murine articular cartilage cells treated with IL-1β, indicating that G-Rb3 offered a potential therapeutic approach for the modulation of collagen degradation (Shin et al., 2009). In another in vitro study, as an NF-κB inhibitor, G-Rb3 exerted anti-inflammatory activity through NF-kB deactivation (He et al., 2014). Furthermore, the authors of this study confirmed that G-Rb3 suppressed the expression of cyclooxygenase-2 (COX-2) and iNOS messenger ribonucleic acid (mRNA) in HepG2 cells exposure to TNF-α, suggesting a potential role of the inflammatory response (He et al., 2014). In 2, 2 V-azobis (2-amidinopropane hydrochloride) (APPH)-treated peroxidation of human erythrocytes, antioxidative properties of G-Rb3 are responsible for erythrocytes protection. However, the antioxidative mechanism of ginsenosides Rb3 in AAPH-induced hemolysis requires further study (Liu et al., 2002). A similar report by Li and Liu (2008) also demonstrated that G-Rb3 protected human erythrocytes against hemin-induced hemolysis.
This review first illuminates the chemical structure of G-Rb3 and then elucidates the pleiotropic protective role played by G-Rb3 in diversified diseases threatening human life. It aims to stimulate more preclinical investigations and studies on various molecular pathways employed by G-Rb3. Modern pharmacological studies have also validated intricate officinal uses of G-Rb3, though data regarding many aspects of the ginsenoside monomer, such as the mechanism of action, adverse effects, and toxicology research are still confined. Despite recent advances in nervous and cardiovascular systems, the exploration concentrated on other biological effects remains limited. Moreover, the pathways of G-Rb3 absorption, distribution, metabolism, and excretion need to be clarified by pharmacokinetic study with multifarious drug delivery. For existing research, one factor responsible for the poor in vivo bioavailability of G-Rb3 may be the sugar groups. In comparison to pentose groups in G-Rb3 ginsenosides, hexose and hydroxyl groups (Rb1) in the same glycosylation site may present better oral absorption.
Taken together, based on a broad perspective in nervous and cardiovascular systems, G-Rb3 may provide substitute treatment in further theoretical study and clinical application to ascertain the effective doses. Nevertheless, it is noteworthy that the accuracy and systematisms of both in vivo and in vitro models of various diseases are not yet definitive. Therefore, more detailed emerging studies need to be taken in the transition to clinical trials. In addition, available data on G-Rb3 are still limited and perplexed. (1) Differences exist in the chemical configuration of panaxadiol saponins (PDS). Is there a different function of G-Rb3 in targeted organs, tissues, cells, or intracellular parts? (2) Does the diverse structure require a specific drug delivery? Further investigations in pharmacological action/mechanism relationship and pharmacokinetics studies are highly recommended to provide more solid evidence for the multiple effective efficiency of G-Rb3.
Author Contribution: Mei-ling Fan and Zi Wang conceived the idea of the whole article and provided specific guidance; Yue-yang Duan and Xiao-tong Yan completed the literature search and collation of the chemistry section; Xiang-xiang Liu and Wei Li completed a compilation of pharmacological research literature, and Wei Li is responsible for compiling of the whole work.
Funding Statement: This work was funded by the grant of Jilin Science & Technology Development Plan (No. 20200301037RQ).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Akkati S, Sam KG, Tungha G (2011). Emergence of promising therapies in diabetes mellitus. Journal of Clinical Pharmacology 51: 796–804. [Google Scholar]
Aung HH, Mehendale SR, Wang CZ, Xie JT, Mcentee E et al. (2007). Cisplatin’s tumoricidal effect on human breast carcinoma MCF-7 cells was not attenuated by American ginseng. Cancer Chemotherapy and Pharmacology 59: 369–374. [Google Scholar]
Bebbington P (2001). The world health report 2001. Social Psychiatry and Psychiatric Epidemiology 36: 473–474. [Google Scholar]
Berliocchi L, Bano D, Nicotera P (2005). Ca2+ signals and death programmes in neurons. Philosopical Transactions of the Royal Society of London B: Biological Sciences 360: 2255–2558. [Google Scholar]
Bu Q, Zhang W, Chen Q, Zhang C, Gong X et al. (2012). Anti-diabetic effect of ginsenoside Rb(3) in alloxan-induced diabetic mice. Medicinal Chemistry 8: 934–941. [Google Scholar]
Cool B, Zinker B, Chiou W, Kifle L, Cao N et al. (2006). Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metabolism 3: 403–416. [Google Scholar]
Cui J, Jiang L, Xiang H (2012). Ginsenoside Rb3 exerts antidepressant-like effects in several animal models. Journal of Psychopharmacology 26: 697–713. [Google Scholar]
Chen CF, Chiou WF, Zhang JT (2008). Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacologica Sinica 29: 1103–1108. [Google Scholar]
Chen LW, Wang YQ, Wei LC, Shi M, Chan YS (2007). Chinese herbs and herbal extracts for neuroprotection of dopaminergic neurons and potential therapeutic treatment of Parkinson’s disease. CNS & Neurological Disorders-Drug Targets 6: 273–281. [Google Scholar]
Chen X, Wang Q, Shao M, Ma L, Guo D et al. (2019). Ginsenoside Rb3 regulates energy metabolism and apoptosis in cardiomyocytes via activating PPARα pathway. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 120: 109487. [Google Scholar]
Chen X, Yang SJ, Chen L, Ma XL, Chen YP et al. (1994). The effects of Panax quinquefolium saponin (PQS) and its monomer ginsenoside on heart. Zhongguo Zhong Yao Za Zhi 19: 617–620. [Google Scholar]
Choi KT (2008). Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacologica Sinica 29: 1109–1118. [Google Scholar]
Gao L, Wang XD, Niu YY, Duan DD, Yang X et al. (2016). Molecular targets of Chinese herbs: A clinical study of hepatoma based on network pharmacology. Scientific Reports 6: 24944. [Google Scholar]
He F, Ding Y, Liang C, Song S, Dou D et al. (2014). Antitumor effects of dammarane-type saponins from steamed Notoginseng. Pharmacognosy Magazine 10: 314–317. [Google Scholar]
Huang G, Khan I, Li X, Chen L, Leong W et al. (2017). Ginsenosides Rb3 and Rd reduce polyps formation while reinstate the dysbiotic gut microbiota and the intestinal microenvironment in ApcMin/+ mice. Scientific Reports 7: 12552. [Google Scholar]
Jia L, Zuo T, Zhang C, Li W, Wang H et al. (2019). Simultaneous profiling and holistic comparison of the metabolomes among the flower buds of Panax ginseng and by UHPLC/IM-QTOF-HDMS-based metabolomics analysis. Molecules 24: 2188. [Google Scholar]
Jiang S, Fang DF, Chen Y (2018). Involvement of N-methyl-D-aspartic acid receptor and DL-α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor in ginsenosides Rb1 and Rb3 against oxygen-glucose deprivation-induced injury in hippocampal slices from rat. Pharmacology 101: 133–139. [Google Scholar]
Jiang S, Miao B, Song X, Jiang Z (2011). Inactivation of GABA(A) receptor reduces ginsenoside Rb3 neuroprotection in mouse hippocampal slices after oxygen-glucose deprivation. Journal of Ethnopharmacology 133: 914–916. [Google Scholar]
Lee JH, Kim SR, Bae CS, Kim D, Hong H et al. (2002). Protective effect of ginsenosides, active ingredients of Panax ginseng, on kainic acid-induced neurotoxicity in rat hippocampus. Neuroscience Letters 325: 129–133. [Google Scholar]
Leidenheimer NJ (2008). Regulation of excitation by GABA(A) receptor internalization. Results & Problems in Cell Differentiation 44: 1–28. [Google Scholar]
Li GX, Liu ZQ (2008). The protective effects of ginsenosides on human erythrocytes against hemin-induced hemolysis. Food and Chemical Toxicology 46: 886–892. DOI 10.1016/j.fct.2007.10.020. [Google Scholar] [CrossRef]
Lian XY, Zhang Z, Stringer JL (2005a). Protective effects of ginseng components in a rodent model of neurodegeneration. Annals of Neurology 57: 642–648. DOI 10.1002/(ISSN)1531-8249. [Google Scholar] [CrossRef]
Lian XY, Zhang Z, Stringer JL (2006). Anticonvulsant and neuroprotective effects of ginsenosides in rats. Epilepsy Research 70: 244–256. DOI 10.1016/j.eplepsyres.2006.05.010. [Google Scholar] [CrossRef]
Lian XY, Zhang ZZ, Stringer JL (2005b). Anticonvulsant activity of ginseng on seizures induced by chemical convulsants. Epilepsia 46: 15–22. DOI 10.1111/j.0013-9580.2005.40904.x. [Google Scholar] [CrossRef]
Liu C, Jin Y, Yu H, Sun C, Gao P et al. (2014a). Biotransformation pathway and kinetics of the hydrolysis of the 3-O- and 20-O-multi-glucosides of PPD-type ginsenosides by ginsenosidase type I. Process Biochemistry 49: 813–820. DOI 10.1016/j.procbio.2014.02.011. [Google Scholar] [CrossRef]
Liu F, Ma N, He C, Hu Y, Li P et al. (2018). Qualitative and quantitative analysis of the saponins in Panax notoginseng leaves using ultra-performance liquid chromatography coupled with time-of-flight tandem mass spectrometry and high performance liquid chromatography coupled with UV detector. Journal of Ginseng Research 42: 149–157. [Google Scholar]
Liu J, Liu Y, Zhao L, Zhang ZH, Tang ZH (2016). Profiling of ginsenosides in the two medicinal Panax herbs based on ultra-performance liquid chromatography-electrospray ionization-mass spectrometry. Springerplus 5: 1770. [Google Scholar]
Liu W, Wang Z, Leng J, Wei H, Ren S et al. (2020a). 20(R)-ginsenoside Rg3, a product of high-efficiency thermal deglycosylation of ginsenoside Rd, exerts protective effects against scrotal heat-induced spermatogenic damage in mice. BIOCELL 44: 655–669. [Google Scholar]
Liu X, Jiang Y, Fu W, Yu X, Sui D (2020b). Combination of the ginsenosides Rb3 and Rb2 exerts protective effects against myocardial ischemia reperfusion injury in rats. International Journal of Molecular Medicine 45: 519–531. [Google Scholar]
Liu X, Jiang Y, Yu X, Fu W, Zhang H et al. (2014b). Ginsenoside-Rb3 protects the myocardium from ischemia-reperfusion injury via the inhibition of apoptosis in rats. Experimental and Therapeutic Medicine 8: 1751–1756. [Google Scholar]
Liu Z, Luo X, Sun Y, Chen Y, Wang Z (2002). Can ginsenosides protect human erythrocytes against free-radical-induced hemolysis? Biochimica et Biophysica Acta (BBA)-General Subjects 1572: 58–66. DOI 10.1016/S0304-4165(02)00281-7. [Google Scholar] [CrossRef]
Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC et al. (2003). In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. Journal of Agricultural and Food Chemistry 51: 2555–2558. DOI 10.1021/jf026228i. [Google Scholar] [CrossRef]
Ma L, Liu H, Xie Z, Yang S, Xu W et al. (2014). Ginsenoside Rb3 protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of JNK-mediated NF-κB pathway: A mouse cardiomyocyte model. PLoS One 9: e103628. DOI 10.1371/journal.pone.0103628. [Google Scholar] [CrossRef]
Meng F, Su X, Li W, Zheng Y (2017). Ginsenoside Rb3 strengthens the hypoglycemic effect through AMPK for inhibition of hepatic gluconeogenesis. Experimental and Therapeutic Medicine 13: 2551–2557. DOI 10.3892/etm.2017.4280. [Google Scholar] [CrossRef]
Oh SJ, Oh Y, Ryu IW, Kim K, Lim CJ (2015). Protective properties of ginsenoside Rb3 against UV-B radiation-induced oxidative stress in HaCaT keratinocytes. Bioscience, Biotechnology, and Biochemistry 80: 95–103. DOI 10.1080/09168451.2015.1075862. [Google Scholar] [CrossRef]
Park CS, Yoo MH, Noh KH, Oh DK (2010). Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Applied Microbiology and Biotechnology 87: 9–19. DOI 10.1007/s00253-010-2567-6. [Google Scholar] [CrossRef]
Peng L, Shen H, Jiang Z, Li X, Wang G et al. (2009). Inhibition of NMDA receptors underlies the neuroprotective effect of ginsenoside Rb3. American Journal of Chinese Medicine 37: 759–770. DOI 10.1142/S0192415X09007223. [Google Scholar] [CrossRef]
Shen H, Zhang Z, Jiang S, Jiang Z (2006a). Protective effects of ginsenoside RB3 on hypoxic/ischemic brain injury and involved mechanisms. Zhongguo Ying Yong Sheng Li Xue Za Zhi 22: 302–306. [Google Scholar]
Shen HM, Jiang ZL, Gu XS (2006b). Effects and mechanisms of ginsenoside Rb3 on glutamate excitotoxic injury in cultured neurons of rat hippocampus. Zhongguo Ying Yong Sheng Li Xue Za Zhi 22: 31–34. [Google Scholar]
Shi Y, Han B, Yu X, Qu S, Sui D (2011). Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury in rats. Pharmaceutical Biology 49: 900–906. DOI 10.3109/13880209.2011.554845. [Google Scholar] [CrossRef]
Shin JS, Park N, Ra J, Kim Y, Shin M et al. (2009). Panax ginseng C.A. Meyer modulates the levels of MMP3 in S12 murine articular cartilage cell line. Journal of Ethnopharmacology 124: 397–403. [Google Scholar]
Sun J, Yu X, Huangpu H, Yao F (2019). Ginsenoside Rb3 protects cardiomyocytes against hypoxia/reoxygenation injury via activating the antioxidation signaling pathway of PERK/Nrf2/HMOX1. Biomedicine & Pharmacotherapy 109: 254–261. [Google Scholar]
Tang W, Zhang Y, Gao J, Ding X, Gao S (2008). The anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by intranasally administration. Biological and Pharmaceutical Bulletin 31: 2024–2027. [Google Scholar]
Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M (2003). Degradation of ginsenosides in humans after oral administration. Drug Metabolism and Disposition 31: 1065–1071. [Google Scholar]
Wang T, Yu X, Qu S, Xu H, Han B et al. (2010a). Effect of ginsenoside Rb3 on myocardial injury and heart function impairment induced by isoproterenol in rats. European Journal of Pharmacology 636: 121–125. [Google Scholar]
Wang T, Yu XF, Qu SC, Xu HL, Sui DY (2010b). Ginsenoside Rb3 inhibits angiotensin II-induced vascular smooth muscle cells proliferation. Basic & Clinical Pharmacology & Toxicology 107: 685–689. [Google Scholar]
Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H et al. (2007). In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemotherapy and Pharmacology 59: 589–601. [Google Scholar]
Wang Y, Dong J, Liu P, Lau CW, Gao Z et al. (2014). Ginsenoside Rb3 attenuates oxidative stress and preserves endothelial function in renal arteries from hypertensive rats. British Journal of Pharmacology 171: 3171–3181. [Google Scholar]
White BC, Sullivan JM, Degracia DJ, O’neil BJ, Neumar RW et al. (2000). Brain ischemia and reperfusion: Molecular mechanisms of neuronal injury. Journal of the Neurological Sciences 179: 1–33. [Google Scholar]
Wong WT, Tian XY, Chen Y, Leung FP, Liu L et al. (2010). Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: Implications on hypertension. Circulation Research 107: 984–991. [Google Scholar]
Xia X, Jiang B, Liu W, Wang P, Mou Y et al. (2014). Anti-tumor activity of three novel derivatives of ginsenoside on colorectal cancer cells. Steroids 80: 24–29. DOI 10.1016/j.steroids.2013.11.018. [Google Scholar] [CrossRef]
Xing JJ, Hou JG, Ma ZN, Wang Z, Ren S et al. (2019). Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Proliferation 52: 37. DOI 10.1111/cpr.12627. [Google Scholar] [CrossRef]
Xu YX, Shi JS, Jiang ZL (2005). Inhibitory influence of ginsenoside Rb3 on activation of strychnine-sensitive glycine receptors in hippocampal neurons of rat. Brain Research 1037: 99–106. DOI 10.1016/j.brainres.2004.12.044. [Google Scholar] [CrossRef]
Yang L, Liu Q, Yu Y, Xu H, Chen S et al. (2017). Ginsenoside-Rb3 inhibits endothelial-mesenchymal transition of cardiac microvascular endothelial cells. Herz 44: 60–68. DOI 10.1007/s00059-017-4628-4. [Google Scholar] [CrossRef]
Zhang B, Zhou G, Li C (2009). AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metabolism 9: 407–416. DOI 10.1016/j.cmet.2009.03.012. [Google Scholar] [CrossRef]
Zhang H, Li Z, Zhou Z, Yang H, Zhong Z et al. (2016). Antidepressant-like effects of ginsenosides: A comparison of ginsenoside Rb3 and its four deglycosylated derivatives, Rg3, Rh2, compound K, and 20(S)-protopanaxadiol in mice models of despair. Pharmacology Biochemistry and Behavior 140: 17–26. [Google Scholar]
Zhang MM, Zheng W, Zhang J, Gao L, Liu XF et al. (2021). Qualitative analysis of Gynostemma longipes for medicinal usage. Zhongguo Zhong Yao Za Zhi 46: 951–965. [Google Scholar]
Zhang S, Sun H, Wang C, Zheng X, Jia X et al. (2019). Comparative analysis of active ingredients and effects of the combination of Panax ginseng and Ophiopogon japonicus at different proportions on chemotherapy-induced myelosuppression mouse. Food & Function 10: 1563–1570. [Google Scholar]
Zhao J, Su C, Yang C, Liu M, Tang L et al. (2012). Determination of ginsenosides Rb1, Rb2, and Rb3 in rat plasma by a rapid and sensitive liquid chromatography tandem mass spectrometry method: Application in a pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis 64-65: 94–97. [Google Scholar]
Zhao L, Ma Y, Chen C, Liu S, Wu W (2018). Pharmacokinetic and metabolic studies of ginsenoside Rb3 in rats using RRLC-Q-TOF-MS. Journal of Chromatographic Science 56: 480–487. [Google Scholar]
Zhong GG, Sun CW, Li YY, Qi H, Zhao CY et al. (1995). Calcium channel blockade and anti-free-radical actions of panaxadiol saponins Rb1, Rb2, Rb3, Rc, and Rd. Zhongguo Yao Li Xue Bao 16: 255–260. [Google Scholar]
Zhou C, Xiao C, Deng C, Hong Ye J (2007). Extracellular proton modulates GABAergic synaptic transmission in rat hippocampal CA3 neurons. Brain Research 1145: 213–220. [Google Scholar]
Zhu J, Tao Y, Lou S, Wu Z (2010). Protective effects of ginsenoside Rb(3) on oxygen and glucose deprivation-induced ischemic injury in PC12 cells. Acta Pharmacologica Sinica 31: 273–280. [Google Scholar]
Zou K, Zhu S, Meselhy MR, Tohda C, Cai S et al. (2002). Dammarane-type saponins from Panax japonicus and their neurite outgrowth activity in SK-N-SH cells. Journal of Natural Products 65: 1288–1292. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |