DOI:10.32604/biocell.2022.018131

| BIOCELL DOI:10.32604/biocell.2022.018131 |  |
| Article |
Gene expression profile analysis reveals the effect of metformin treatment on HepG2 cells
1School of Pharmacy, Suzhou Vocational Health College, Suzhou, 215000, China
2Institute of Epigenetics & Epigenomics, Yangzhou University, Yangzhou, 225009, China
*Address correspondence to: Hengmi Cui, hmcui@yzu.edu.cn
Received: 01 June 2021; Accepted: 03 September 2021
Abstract: Metformin is a first-line drug in the fight against type 2 diabetes. In recent years, studies have shown that metformin has some preventive and therapeutic effects on liver cancer, but the effects of metformin on the gene expression of liver cancer cells are not fully known. This study focused on the differences in the gene expression profiles in liver cancer cells treated with or without metformin. A total of 153 differentially expressed genes (DEGs) (FC > 2 and q-values < 0.001) were found, including 77 upregulated genes and 76 downregulated genes. These DEGs are involved in mitogen-activated protein kinase (MAPK), nuclear factor-kappa B (NF-κB), cell adhesion molecules (CAMs), and leukocyte transendothelial migration signaling pathways. These findings reveal the effects of metformin treatment on gene expression profiles in liver cancer cells and provide new clues for unveiling the mechanism of the antitumor effects of metformin.
Keywords: Metformin; Gene expression profiles; Antitumor; HepG2 cells
In recent years, the incidence of hepatocellular carcinoma (HCC) has been increasing, seriously threatening human health (Gerbes et al., 2018). Currently, surgical treatment is still the main treatment of HCC. However, HCC usually lacks obvious clinical symptoms and specific serological diagnostic indicators; thus, it is difficult to detect HCC in the early stages (Lin et al., 2018). Therefore, most HCC patients have reached an advanced stage when they are diagnosed, and their 5-year survival rate is low (Ikeda et al., 2018). Most patients with advanced HCC choose nonsurgical treatment. Drug therapy is an important means of nonsurgical treatment for HCC. However, the current chemotherapeutic drugs do not achieve optimal effects. They can cause serious toxicity and side effects and trigger the cancer cells to develop drug resistance (Raoul et al., 2018). Therefore, there is a need to find effective and safe chemotherapeutic drugs for HCC treatment and prevention.
Metformin, as a very effective and safe hypoglycemic drug, has been widely used for decades. Recently, scientists have found that metformin also has antitumor effects (Chen et al., 2011). Through meta-analysis, researchers found that metformin can significantly reduce the incidence of HCC in diabetic patients (Singh et al., 2013; Zhang et al., 2013; Zhou et al., 2016). Metformin can also inhibit subcutaneous primary hepatocellular carcinoma (PHC) formation in nude mice, as well as in situ PHC tumor formation and metastasis induced by diethylnitrosamine (Bhalla et al., 2012; Cheng et al., 2014; Miyoshi et al., 2014). According to previous laboratory results and literature reports, metformin can effectively inhibit the growth of HepG2 cells in a dose-dependent manner in vivo and in vitro. Therefore, we believe that metformin might have a certain value in the prevention and treatment of hepatocellular carcinoma.
Some studies have attempted to investigate the mechanism of metformin through in vitro experiments on the basis of confirming that metformin inhibits hepatoma cell growth and promotes apoptosis (Sun et al., 2016; Zhang et al., 2017). Some studies have reported that metformin can affect the expression of some cancer-related genes in liver cancer cells (Saito et al., 2013). We speculated that the current research results may not be comprehensive. Therefore, we aimed to identify other oncogenes and signaling pathways related to metformin’s inhibition of liver cancer cell growth and prevention of HCC through gene chip analysis to provide data support for the clinical application of metformin in the precise treatment and prevention of liver cancer and its combined use with other anticancer drugs.
In this study, we detected the effects of metformin on the gene expression of HepG2 cells by RNA-seq technology and analyzed the differentially expressed genes (DEGs). Gene ontology (GO) was used to analyze the relationship between the differentially transcribed genes and molecular functions, cell components and biological processes, and Kyoto Encyclopedia of Genes and Genomes (KEGG) was used to analyze the signaling pathways involved in differentially transcribed genes. Through this research, our goal was to identify new targets of metformin that inhibited the proliferation of hepatocellular carcinoma cells and could prevent hepatocellular carcinoma, to provide a more theoretical basis for metformin treatment and approaches to the prevention of hepatocellular carcinoma.
Dulbecco’s modified Eagle’s medium (DMEM), 10% fetal bovine serum (FBS), penicillin and streptomycin were purchased from Gibco. Metformin was purchased from Sigma-Aldrich Co. (St Louis, MO, USA). TRIzol was obtained from Life Technologies (Carlsbad, CA). The HCC cell line HepG2 was obtained from the American Type Culture Collection (ATCC).
Cell culture and metformin treatments
HepG2 cells were seeded into 6-well plates in DMEM with 10% FBS, penicillin (100 U/mL) and streptomycin (100 μg/mL), cultured at 37°C in 5% CO2 and 95% humidity, and then untreated/treated with 1 mM metformin (dissolved in culture medium) for 48 h. The experiment was repeated three times independently. We used the MTT method to conduct a preliminary experiment on the effects of different concentrations of metformin on the proliferation of HepG2 cells. The experimental results showed that the relative viability of HepG2 cells was 80.6 ± 3.0%, 60.1 ± 4.1%, and 40.3 ± 5.3% after treatment with different concentrations of metformin (1, 5, 10 mM) for 48 h. We thought that differences in cell relative activity would affect the changes in the genes at the transcriptional level, so we chose a 1 mM concentration of metformin for these experiments.
Total RNA was extracted by the TRIzol method; the integrity of the total RNA was detected by the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 System (Agilent, USA). The RNA integrity numbers (RINs) of metformin Treatment Groups 1–3 and Control Groups 1–3 were 9.8, 7.9, 9.0, 9.4, 9.1 and 8.9, respectively. RNA sequencing was performed by BGI Technology Co., Ltd. (Beijing, China). mRNA was purified from total RNA using magnetic beads attached to oligos (dT). At an appropriate temperature, the mRNA molecules were fragmented into small pieces using fragment buffer. First-strand cDNA was generated by reverse transcription with random hexamer primers, followed by second-strand cDNA synthesis. Then, a-Tailing Mix and RNA Index Adapters were added for end repair. The cDNA fragments from the previous step were amplified by PCR and purified. The product was validated on an Agilent Technologies 2100 bioanalyzer. The double-stranded PCR products were heated, denatured, and circularized by the splint oligo sequence. Single-stranded circular DNA (ssCir DNA) was formatted as the final library. The final library was amplified with phi29 to make DNA nanoballs (DNBs), which had more than 300 copies of each molecule. DNBs were loaded into the patterned nanoarray, and single-end 50-base reads were generated on the BGISeq500 platform.
GO and KEGG enrichment analysis
According to the standard fold change >2 and q-values < 0.001, the genes with significant differences in transcription were screened. Then, gene ontology was established to analyze the molecular functions, cell components and biological processes of differentially transcribed genes, and KEGG pathway and GO term enrichment analyses were used to analyze the related signaling pathways of the differentially transcribed genes. The KEGG pathways and GO terms with adjusted P-values < 0.05 were considered to have significant enrichment of differentially expressed genes.
Reverse transcription and quantitative PCR (RT qPCR)
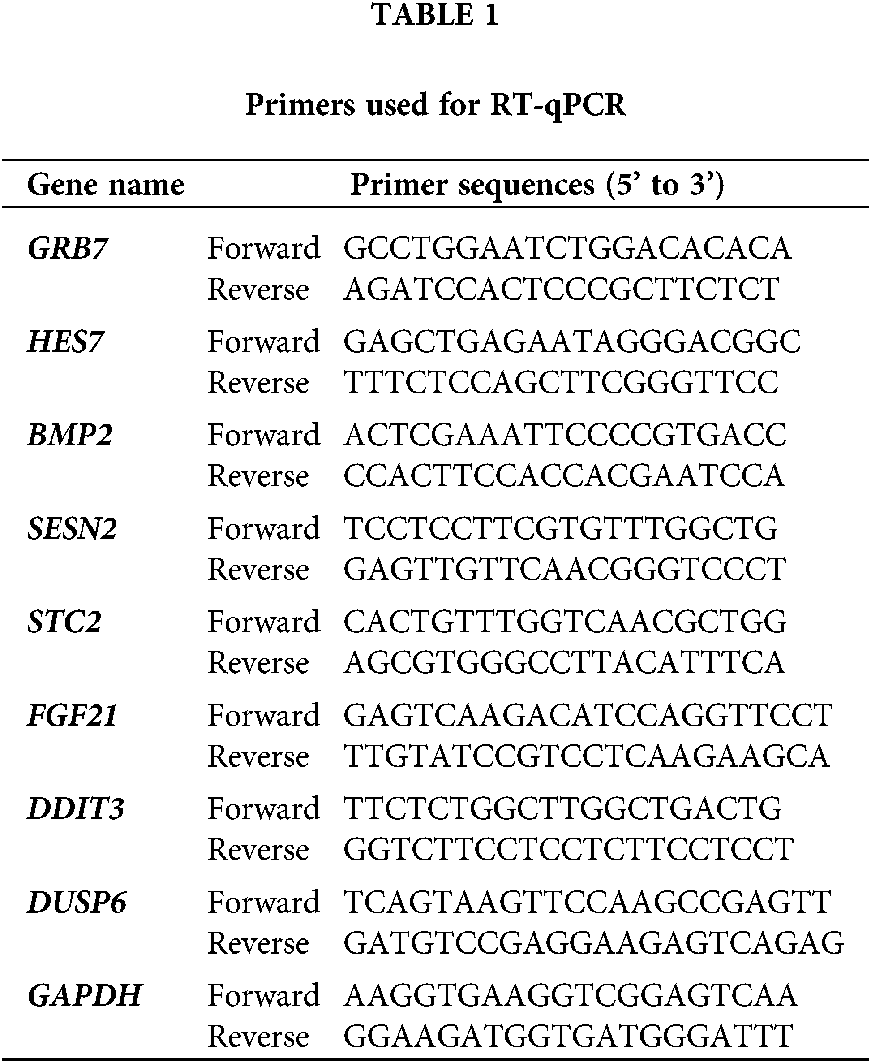
Total RNA (1 µg) was first removed from the genomic DNA with gDNA Eraser (Takara) and then reverse-transcribed into complementary DNA (cDNA, 100 ng) at 37°C for 15 min using the PrimeScript RT Reagent Kit (Takara). A CFX Connect™ Real-time PCR detection system (Bio-Rad) was used for quantitative PCR (qPCR) to verify the results of the gene chip. The amplification conditions were 95°C for 30 s, 95°C for 5 s, 60°C for 34 s, and 40 cycles. Gene-specific primers and SYBR Green Master Mix (Takara) were used in the experiment, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) RNA levels were used as internal controls. The primers (shown in Table 1) were synthesized by the GENEWIZ Company.
The RNA-seq data were analyzed by BGI Technology Co., Ltd. (Beijing, China) using professional bioinformatics statistical software. Reverse transcription and quantitative PCR (RT qPCR) data were performed with Statistical Package for the Social Sciences (version 16.0) software. Statistical significance was assessed using a two-tailed unpaired t-test with a P-value threshold of <0.05.
Transcriptome alterations after metformin treatment
In this study, 153 differentially expressed genes (FC > 2, q-values < 0.001) were observed in HepG2 cells treated with metformin. Seventy-seven upregulated genes and 76 downregulated genes in HepG2 cells treated with metformin were detected (Fig. 1). Some of the upregulated genes, DUSP6, FGF21, GRB7, HES7, HSPA6, NGFR, and RASGRF2, and some of the downregulated genes, DDTI3, BMP2, SESN2, STC2, TARP, and TNFAIP3, exhibited significant changes following metformin treatment, and most of them were involved in the antitumor effects of metformin.
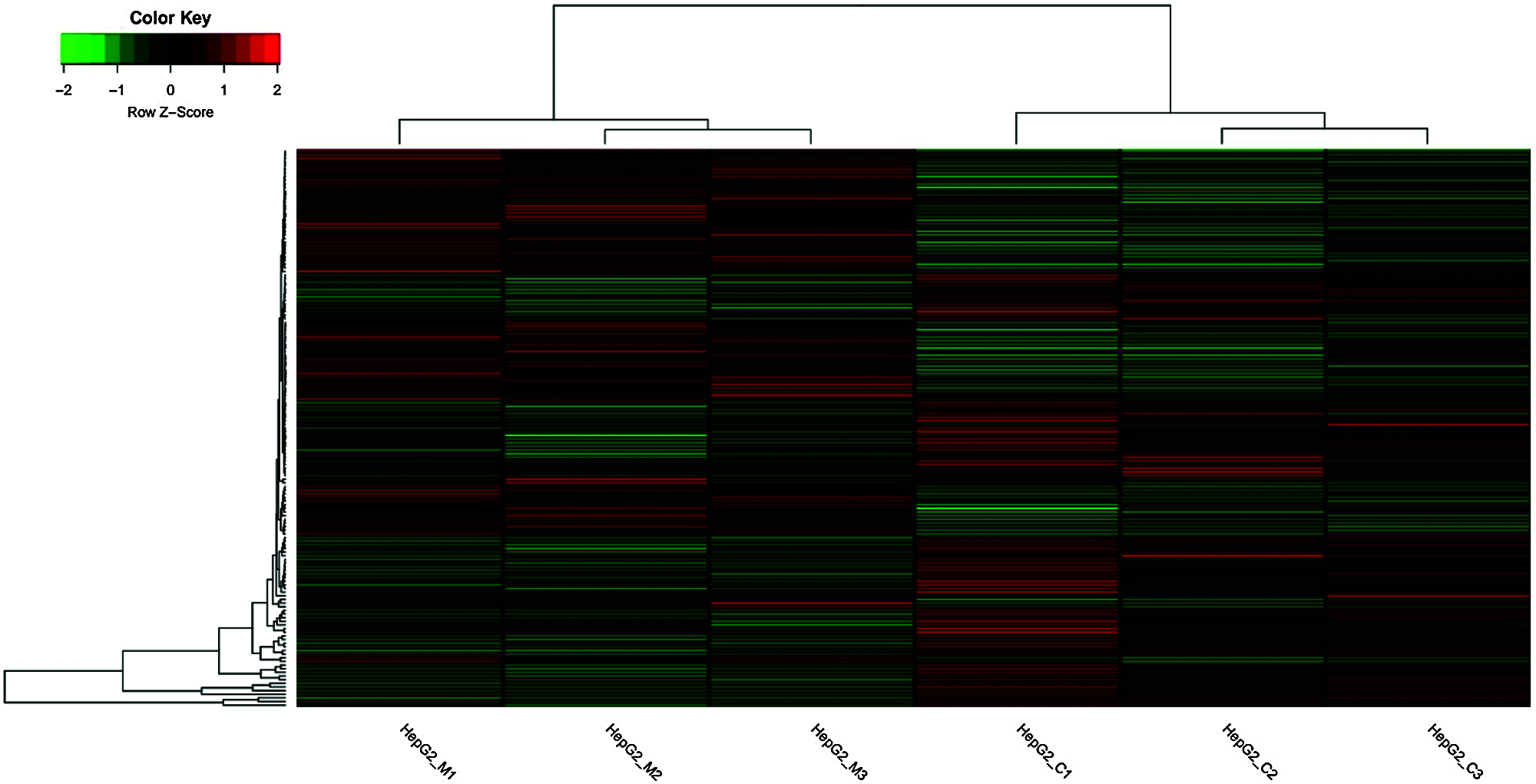
Figure 1: Heat map of the expression profiles of 153 significantly dysregulated genes with or without metformin treatment (HepG2_M1,2.3: Metformin Treatment Groups 1, 2, 3; HepG2_C1, 2, 3: Control Groups 1, 2, 3).
Gene ontology analysis of the differentially expressed genes
The Gene Ontology functions of 153 differentially expressed genes after metformin treatment were classified and analyzed. With R software, enrichment analysis was carried out using the Phyper function. The results of the gene ontology enrichment of the DEGs are shown in Fig. 2. The GO analysis results showed that these DEGs were mainly enriched in cell migration, localization of cells, cell motility, regulation of the Wnt signaling pathway and extracellular matrix.
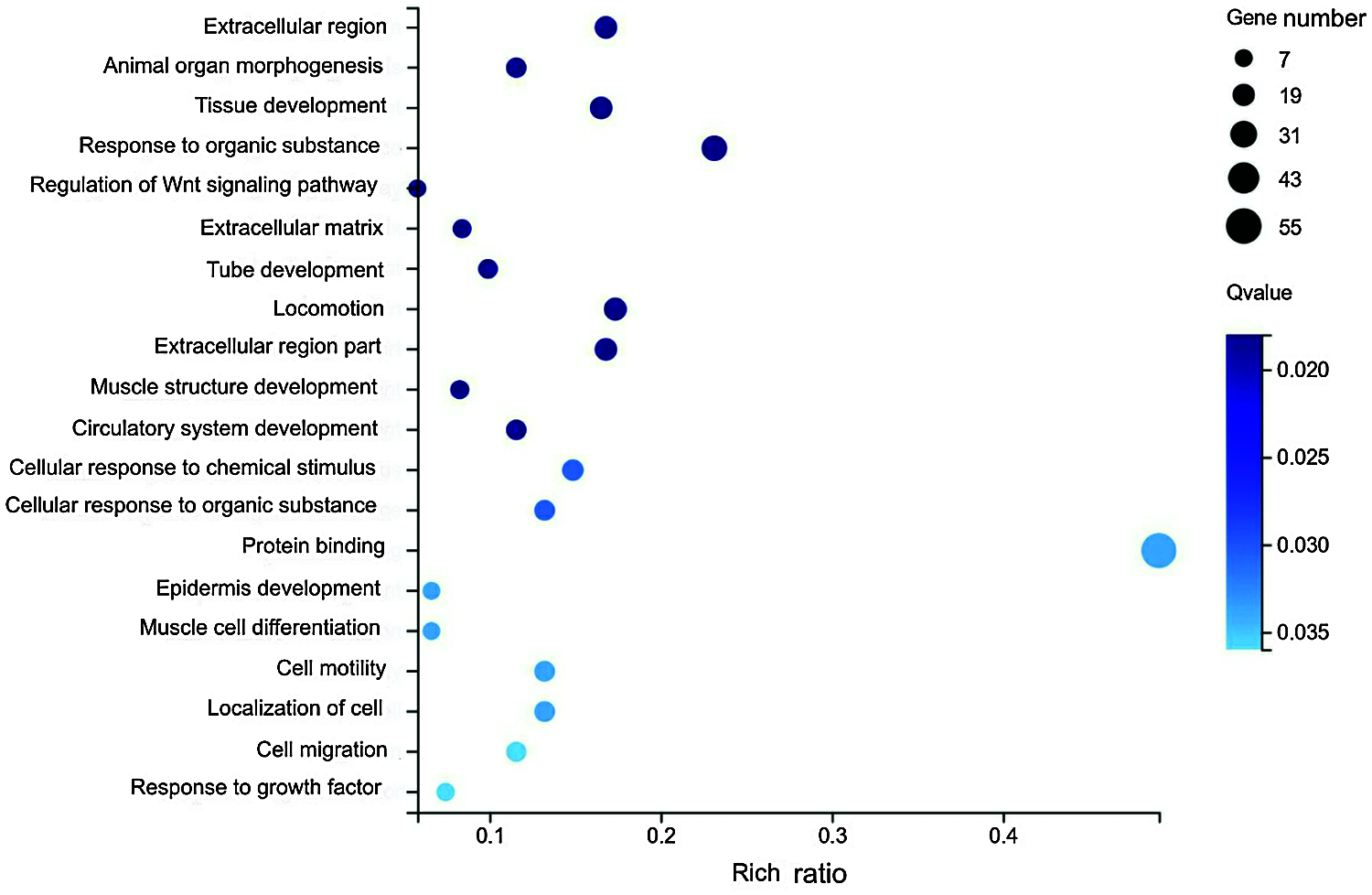
Figure 2: Gene Ontology analysis results of differentially expressed genes with or without metformin treatment.
KEGG signaling pathway analysis of differentially expressed genes
In this study, KOBAS software was used to detect the enrichment of DEGs in the KEGG pathways. The results showed that the DEGs were involved in MAPK, NF-kappa B, cell adhesion molecules (CAMs), and leukocyte transendothelial migration signaling pathways (Fig. 3). In particular, 8 differentially expressed genes (DDIT3, DUSP6, FGFR2, MAPK8IP2, FGF21, HSPA6, NGFR, and RASGRF2) were involved in the MAPK signaling pathway.
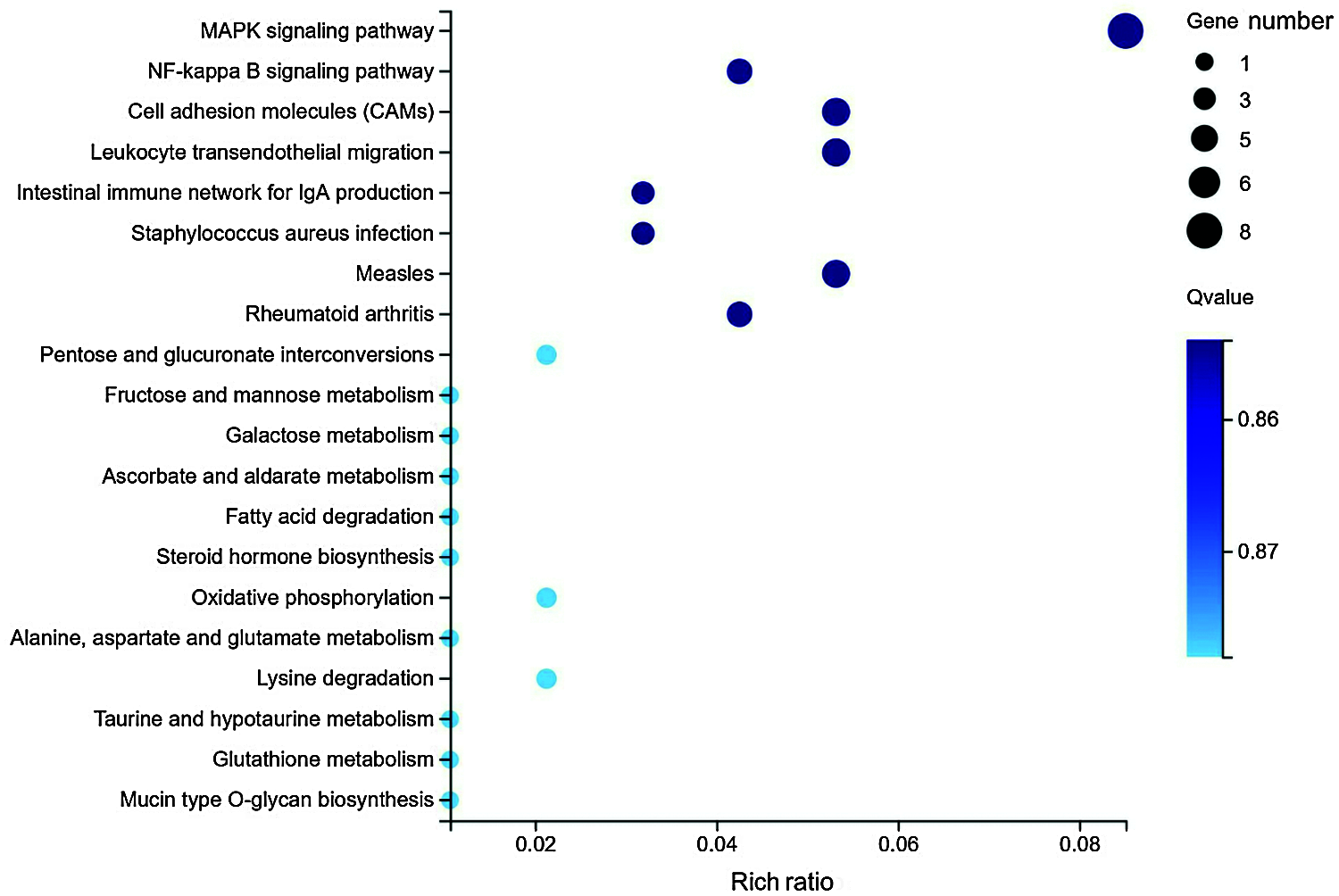
Figure 3: Statistical analysis of pathway enrichment for the differentially expressed genes in HepG2 cells with or without metformin treatment.
RT-qPCR validation of the differentially expressed genes
To verify the RNA-Seq data, RT-qPCR was used to analyze the relative expression levels of eight selected genes (including FGF21, DDIT3, DUSP6, BMP2, GRB7, HES7, SESN2, and STC2). The 8 genes we chose were mainly related to antitumor effects, and 3 genes were related to the MAPK pathway and were used to explore the relationship between the preventive effects of metformin on liver cancer and the MAPK pathway. We used the 2–ΔΔCt method to calculate the fold change and normalized the expression value to GAPDH expression. We found that the results of the RT-qPCR data were similar to those of RNA-Seq (Fig. 4), although the two techniques differed due to the inherent characteristics of the different procedures. Therefore, the RT-qPCR analysis confirmed the results of RNA-Seq. The RT-qPCR results of genes related to the MAPK pathway were consistent with the RNA-Seq results, further confirming our new discovery that the effects of metformin on HCC cells are related to the MAPK pathway.

Figure 4: Validation of RNA-Seq results. (a) The results from the RNA-Seq. (b) Validation of RNA-Seq results by RT-qPCR (mean ± SEM, **P < 0.01, *P < 0.05).
Metformin has a certain role in cancer prevention and treatment, but its mechanism is still unclear. To date, few researchers have discovered DEGs in HepG2 cells treated with or without metformin. In this study, we used RNA-Seq to investigate the gene expression pattern in metformin-treated HepG2 cells.
The results showed that metformin could significantly affect the expression of 153 genes (FC > 2, q-values < 0.001) in metformin-treated HepG2 cells. Notably, some of these genes are involved in MAPK, NF-kappa B, cell adhesion molecules (CAMs), and leukocyte transendothelial migration signaling pathways. This study provides a new approach to unveiling the molecular mechanism of metformin in oncology prevention and treatment.
The NF-kappa B and CAMs pathways are related to the antitumor effects of metformin. Metformin can reduce the number of epithelial cell adhesion molecule (EpCAM) (+) liver cancer stem cells in rats and reduce their self-renewal ability, thus inhibiting the growth and recurrence of tumors (Saito et al., 2013).
According to previous results, Luizon et al. (2016) used primary human hepatocytes to observe the effects of metformin on gluconeogenesis and found that it is related to its upregulation of AMP-activated protein kinase (AMPK)-dependent genes. Qu et al. found that metformin (5 and 10 mM) can increase the level of AMPK protein in hepatoma cells in a concentration-dependent manner (Qu et al., 2012). However, according to our studies, metformin had little effect on AMPK-dependent gene expression. The possible reason is that the metformin used in our experiment was a low dose (1 mM), while the metformin used by other researchers were medium and high doses (5 and 10 mM), and these results suggest that the effects of metformin on the AMPK pathway is dose-dependent (Qu et al., 2012).
Tseng, (2018) fully considered the influence of various interference factors when analyzing whether metformin can reduce the risk of PHC in patients with type 2 diabetes, analyzing the potential role of aspirin and statins, and finally found that metformin reduced the risk of PHC in diabetic patients by 76% (95%CI: 0.67–0.85). Luizon et al. (2016) suggested that in in vitro experiments conducted on primary human liver cells rather than tissues, the concentration of cells treated with metformin—2.5 mM—was higher than the physiological condition for patients taking metformin. Therefore, the 1 mM metformin we chose more clearly investigate its role in cancer prevention in patients with diabetes treated with metformin.
KEGG analysis revealed that the DEGs in metformin-treated HepG2 cells were involved in the MAPK signaling pathway. Moreover, some of these DEGs were further confirmed by RT-qPCR. To date, a few studies have reported that the anti-liver cancer effects of metformin are related to the MAPK pathway. MAPK is a serine threonine (Ser/Thr) protein kinase activated by various stimuli (such as growth factors, osmotic pressure, toxins, cytokines, ionizing radiation and chemotherapeutic drugs) both inside and outside the cell (Munshi and Ramesh, 2013). The MAPK signaling pathway is first activated by activated MAPK kinase, which then activates MAPK. Phosphorylated MAPK activates transcription factors in the cytoplasm and nucleus, and they affect their corresponding target genes to regulate and participate in biological processes such as cell growth, survival, differentiation, division, apoptosis, and autophagy. MAPK can also activate protein kinases in downstream pathways and participate in corresponding biological processes (Li et al., 2011). Alcohol, chemical carcinogens, and other causes of intrahepatic inflammation can upregulate the expression of inflammatory-related factors in liver tissues and cells and cause the abnormal activation of signaling pathways in hepatocytes. Abnormal activation of MAPK signaling pathways plays an important role in the occurrence, development, metastasis, and angiogenesis of hepatocellular carcinoma. The RAS/MAPK signaling pathway is abnormally activated in 50%–100% of hepatocellular carcinomas and is correlated with a poor prognosis in HCC (Delire and Stärkel, 2015). The MAPK pathway is closely related to the occurrence and development of liver cancer, and we found that inhibition of the MAPK signaling pathway was associated with phosphorylation-related genes. Therefore, we speculate that cancer prevention in patients with diabetes treated with metformin may be due to its inhibition of phosphorylation of the MAPK signaling pathway. In the future, we will use western blot technology to verify this mechanism.
It is worth noting that there are several limitations to our findings. This is an in vitro study on one hepatoma cell line, and there are certain differences in the environment of the cells between in vitro and in vivo. Therefore, in the future, we can further study the role of metformin in vivo or use another liver cancer cell line to study the role of metformin. In this study, we observed the results of metformin gene expression at a concentration of 1 mM to try to explain the reason for the reduction of liver cancer caused by long-term metformin administration at the genetic level. In the future, we can observe the results of metformin on gene expression using multiple concentrations in an attempt to comprehensively understand the role of metformin, such as whether the effect of metformin on the MAPK pathway is dose-dependent.
Overall, this study revealed the differences in gene expression profiles in liver cancer cells treated with or without metformin. The results of this study provide a new approach to the treatment and prevention of liver cancer with metformin. In addition, we observed a relationship between the effects of metformin on HepG2 cells and the MAPK signaling pathway. These findings may be significant for the clinical application of metformin. In the future, more studies are needed to elucidate the functional mechanism of different genes involved in the antitumor effects of metformin in other HCC lines.
Acknowledgement: The authors extend their appreciation to the institute of Epigenetics & Epigenomics of Yangzhou University for their kind support.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Author Contribution: Study conception and design: YH, HC; experiment and data collection: YH, CD; analysis and interpretation of results: YH, XH; draft manuscript preparation: YH, XH, HC. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This research was supported by the National Natural Science Foundation of China (81773013), the National Key Research and Development Program in China (2016YFC1303604) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Animal Science and Veterinary Medicine) and Special Subject of Youth Science and Technology of Suzhou Vocational Health College (szwzy201910).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG et al. (2012). Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prevention Research 5: 544–552. DOI 10.1158/1940-6207.CAPR-11-0228. [Google Scholar] [CrossRef]
Chen TM, Lin CC, Huang PT, Wen CF (2011). Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. Journal of Gastroenterology and Hepatology 26: 858–865. DOI 10.1111/j.1440-1746.2011.06664.x. [Google Scholar] [CrossRef]
Cheng J, Huang T, Li Y, Guo Y, Zhu Y et al. (2014). AMP-activated protein kinase suppresses the in vitro and in vivo proliferation of hepatocellular carcinoma. PLoS One 9: e93256. DOI 10.1371/journal.pone.0093256. [Google Scholar] [CrossRef]
Delire B, Stärkel P (2015). The Ras/MAPK pathway and hepatocarcinoma: Pathogenesis and therapeutic implications. European Journal of Clinical Investigation 45: 609–623. DOI 10.1111/eci.12441. [Google Scholar] [CrossRef]
Gerbes A, Zoulim F, Tilg H, Dufour JF, Bruix J et al. (2018). Gut roundtable meeting paper: Selected recent advances in hepatocellular carcinoma. Gut 67: 380–388. DOI 10.1136/gutjnl-2017-315068. [Google Scholar] [CrossRef]
Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J (2018). Chemotherapy for hepatocellular carcinoma: Current status and future perspectives. Japanese Journal of Clinical Oncology 48: 103–114. [Google Scholar]
Li J, Deng Y, Zhang L, Chen H (2011). The role of Ras/ERK signal transduction pathway in the occurrence and development of hepatic fibrosis. Journal of Clinical Hepatology 27: 325–327. [Google Scholar]
Lin X-L, Liu M, Liu Y, Hu H, Pan Y et al. (2018). Transforming growth factor β1 promotes migration and invasion in HepG2 cells: Epithelial-to-mesenchymal transition via JAK/STAT3 signaling. International Journal of Molecular Medicine 41: 129–136. [Google Scholar]
Luizon MR, Eckalbar WL, Wang Y, Jones SL, Smith RP et al. (2016). Genomic characterization of metformin hepatic response. PLoS Genetics 12: e1006449. [Google Scholar]
Miyoshi H, Kato K, Iwama H, Maeda E, Masaki T (2014). Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. International Journal of Oncology 45: 322–332. [Google Scholar]
Munshi A, Ramesh R (2013). Mitogen-activated protein kinases and their role in radiation response. Genes & Cancer 4: 401–408. [Google Scholar]
Qu Z, Zhang Y, Liao M, Chen Y, Zhao J et al. (2012). In vitro and in vivo antitumoral action of metformin on hepatocellular carcinoma. Hepatology Research 42: 922–933. [Google Scholar]
Raoul JL, Kudo M, Finn RS, Edeline J, Reig M et al. (2018). Systemic therapy for intermediate and advanced hepatocellular carcinoma: Sorafenib and beyond. Cancer Treatment Reviews 68: 16–24. [Google Scholar]
Saito T, Chiba T, Yuki K, Zen Y, Oshima M et al. (2013). Metformin, a diabetes drug, eliminates tumor-initiating hepatocellular carcinoma cells. PLoS One 8: e70010. [Google Scholar]
Singh S, Singh PP, Singh AG, Murad MH, Sanchez W (2013). Anti-diabetic medications and the risk of hepatocellular cancer: A systematic review and meta-analysis. Official Journal of the American College of Gastroenterology 108: 881–891. [Google Scholar]
Sun Y, Tao C, Huang X, He H, Shi H et al. (2016). Metformin induces apoptosis of human hepatocellular carcinoma HepG2 cells by activating an AMPK/p53/miR-23a/FOXA1 pathway. OncoTargets and Therapy 9: 2845. [Google Scholar]
Tseng CH (2018). Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver International 38: 2018–2027. DOI 10.1111/liv.13872. [Google Scholar] [CrossRef]
Zhang H, Gao C, Fang L, Zhao HC, Yao SK (2012). Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: A meta-analysis. Scandinavian Journal of Gastroenterology 48: 78–87. DOI 10.3109/00365521.2012.719926. [Google Scholar] [CrossRef]
Zhang Q, Kong J, Dong S, Xu W, Sun W (2017). Metformin exhibits the anti-proliferation and anti-invasion effects in hepatocellular carcinoma cells after insufficient radiofrequency ablation. Cancer Cell International 17: E359. DOI 10.1186/s12935-017-0418-6. [Google Scholar] [CrossRef]
Zhou YY, Zhu GQ, Liu T, Zheng JN, Cheng Z et al. (2016). Systematic review with network meta-analysis: Antidiabetic medication and risk of hepatocellular carcinoma. Scientific Reports 6: 1118. DOI 10.1038/srep33743. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |