 Open Access
Open Access
ARTICLE
Diagnostic and prognostic significance of the lymphocyte/C-reactive protein ratio, neutrophil/lymphocyte ratio, and D-dimer values in patients with COVID-19
1 Department of Medical Biochemistry, Amasya University Faculty of Medicine, Amasya, 05000, Turkey
2 Department of Infectious Diseases and Clinical Microbiology, Beylikduzu State Hospital, Istanbul, 34000, Turkey
* Corresponding Author: ALPASLAN OZTURK. Email:
(This article belongs to the Special Issue: Biochemical and Epigenetics Changes in Health and Disease)
BIOCELL 2022, 46(12), 2625-2635. https://doi.org/10.32604/biocell.2022.023124
Received 11 April 2022; Accepted 24 May 2022; Issue published 10 August 2022
Abstract
In this study, our aim was to examine the diagnostic and prognostic significance of lymphocyte/C-reactive protein ratio (LCR), neutrophil/lymphocyte ratio (NLR) and D-dimer parameters in COVID-19 infection. The LCR, NLR, neutrophil count, mean platelet volume (MPV), C-reactive protein (CRP), and D-dimer parameters were evaluated retrospectively. This was a retrospective cohort study with 1000 COVID-19 positive and 1000 healthy control groups, all over the age of 18 years. Odds ratio (OR) and 95% confidence interval (CI) values were calculated for each parameter found to be statistically significant in the univariate and multivariate logistic regression models. Herein, 127 (12.7%) of the COVID-19+ patients, whose data was included in this study, died. The neutrophil, MPV, CRP, D-dimer, and NLR values were higher in the COVID-19+/deceased group than in the COVID-19+/alive and control groups (p < 0.001, p < 0.001, p < 0.001, p < 0.001, p < 0.001). The lymphocyte and LCR values were lower in the COVID-19+/deceased group than in the COVID-19+/alive and control groups (p < 0.001, p < 0.001). Variables with statistically significance in predicting COVID-19 infection were lymphocyte, LCR, D-dimer, NLR, CRP, MPV, PLT, and neutrophil values. Statistically significant variables in predicting mortality due to COVID-19 were LCR, CRP, NLR, lymphocyte, D-dimer, neutrophil, and MPV values. A low LCR and high NLR are associated with the presence, prognosis, and mortality due to COVID-19. LCR and NLR parameters can thus be used in clinical monitoring to reduce morbidity and mortality rates.Keywords
China announced the outbreak of a new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December, 2019 (Guan et al., 2020), and the disease was declared a pandemic by the World Health Organization (WHO) in March, 2020 (Cucinotta and Vanelli, 2020). Globally, as of 7:03 pm CEST, September 20, 2021, there have been 228,394,572 confirmed cases of COVID-19, including 4,690,186 deaths, as reported by the WHO. As of September 19, 2021, a total of 5,771,619,897 vaccine doses have been administered (https://covid19.who.int/). Coronavirus 2019 (COVID-19) infection can initiate with flu-like common symptoms (Aktas, 2020); however, ∼10% of symptomatic patients develop a more severe disease (Lavezzo et al., 2020). Symptoms of COVID-19 infection, such as fever, weakness, loss of taste and smell, and cough, are observed in clinical practice. Although thorax computed tomography findings progress in the first days of this disease, the prognosis is often good (Chan et al., 2020). Acute respiratory distress syndrome and mortality are observed in patients with a more severe disease (Chen et al., 2020).
The most effective method for the diagnosis of COVID-19 infection is the detection of viral nucleic acid using reverse transcription-polymerase chain reaction (RT-PCR). Laboratory findings and radiology tests confirm the clinical diagnosis (Yang et al., 2020). As in all viral infections, changes in hematological laboratory parameters are observed in patients with COVID-19. A high neutrophil count and lymphopenia are among the common ones (Carpio-Orantes et al., 2020). the neutrophil/lymphocyte ratio (NLR), platelet (PLT)/lymphocyte ratio (PLR), and mean platelet volume (MPV)/PLT ratios in peripheral blood are reported to be the indicators of the infection type and prognosis (Ugurlu et al., 2021).
C-reactive protein (CRP) is a commonly used inflammatory marker. Moreover, CRP or its derivates have been suggested to be associated with chronic low inflammatory conditions (Aktas, 2021). CRP is present in very low levels or even absent in healthy individuals. Significantly elevated CRP levels are observed in conditions such as infection and trauma (Bal et al., 2021).
NLR, PLR, and high CRP are also reported as prognostic indicators in systemic inflammatory diseases. Elevated CRP level is also observed in patients having COVID-19 (Aktas, 2021). Studies have shown that the correlation of lymphocyte/CRP ratio with clinical severity is superior to NLR in COVID-19 patients (Bal et al., 2021; Lagunas-Rangel, 2020). Similar to CRP, elevated D-dimer value is a typical phenomenon in patients with COVID-19 (Wang et al., 2011).
The NLR is a novel inflammatory predictor derived from routine hemogram tests. It has been associated with various clinical inflammatory conditions, including irritable bowel disease (Aktas et al., 2020), thyroiditis (Aktas et al., 2017), type 2 diabetes (Duman et al., 2019), diabetic nephropathy (Bilgin et al., 2020), malignancy (Sit et al., 2019), and COVID-19 infection (Yildiz et al., 2021). Similarly, the PLR is a novel predictor of the inflammatory burden and has been reported to be associated with COVID-19 infection (Aktas, 2021), diabetes mellitus (Atak et al., 2019), and cancer (Atak et al., 2021). The MPV is the first studied hemogram-derived inflammatory marker and is reported to be associated with the inflammatory burden in functional bowel diseases (Aktas et al., 2014), type 2 diabetes mellitus (Cakir et al., 2014), infections (Aktas et al., 2013), rheumatoid arthritis (Cakir et al., 2020), vertebral disc hernia (Dagistan et al., 2016), dementia (Dagistan and Cosgun, 2019), cardiac conditions (Sincer et al., 2018), obesity (Aktas et al., 2018) and frailty (Bilgin et al., 2021).
The prognostic and diagnostic association of these parameters with COVID-19 remains unclear (Bal et al., 2021; ElshazliI et al., 2020). The present study compared the lymphocyte/CRP ratio (LCR), NLR, neutrophil count, MPV, PLT, neutrophil, CRP, and D-dimer values in patients with COVID-19 and a non-COVID-19 control group. Hence, the present study aimed to reveal their significance in patients with COVID-19.
The laboratory data of 1,000 patients diagnosed with COVID-19 and hospitalized at the Erbaa State Hospital (Erbaa, Turkey) between April 1, 2020, and April 01, 2021, were used. The LCR, NLR, neutrophil count, MPV, PLT, neutrophil, CRP, and D-dimer values of these patients were evaluated retrospectively. Laboratory data were obtained from the electronic information system of the hospital. Patients with a confirmed diagnosis of COVID-19 through RT-PCR were included in the present study. Complete blood count, biochemical tests, and D-dimer were determined with Mindray BC 6000 (Mindray Global, Shenzhen, China), Beckman Coulter AU680 (Beckman Coulter, California, USA) and Succeeder Sf 8100 (Beijing Succeeder Technology Inc., Beijing, China), respectively. Further, the differences in the values between the COVID-19+ patients as well as those of 1,000 healthy controls, whose parameters were previously measured at the hospital, were investigated. This was a retrospective cohort study with patients over 18 years of age (Fig. 1).

Figure 1: Recruitment and assessment flowchart for the patients and healthy controls.
STROBE, Strengthening the Reporting of Observational studies in Epidemiology.
An application was made to the Tokat Gaziosmanpaşa University Clinical Research Ethics Committee (as Erbaa State Hospital, in Erbaa, Tokat Province, Turkey), and the necessary approval was obtained from the ethics committee (approval date, August 17, 2021; decision number, HRU/20.14.13). The study was carried out in compliance with the ethical principles of the Declaration of Helsinki.
The data collected in the present study were statistically analyzed using IBM SPSS Statistics for Windows 22.0. Proportion comparisons between the research groups were made using the Chi-squared test. The normality distribution of the data was evaluated using the Kolmogorov-Smirnov test. Levene’s test was used to test the homogeneity of the variances. The Student’s t-test was used to compare the normally distributed data between two independent groups, and the Mann-Whitney U test was performed to compare the non-normally distributed data. To determine the differences between groups following ANOVA, Tukey’s post hoc test was used when the assumption of homogeneity of the variances was met, and the Games-Howell post hoc test was used when it was not. Receiver operating characteristic (ROC) analysis was used to determine whether age, sex, and certain laboratory parameter values could be prognostic indicators for the COVID-19 positivity and mortality of the COVID-19+ study participants. The ROC curves, area under the curve (AUC), and 95% confidence interval (CI) values were calculated. The AUC values obtained in the analyses were classified as follows: 0.9–1, excellent; 0.8–0.9, good; 0.7–0.8, fair; 0.6–0.7, poor; and 0.5–0.6, unsuccessful. Youden index (maximum sensitivity and specificity) was used to find the optimal cut-off point in the ROC analysis. According to the univariate analysis, all the variables related to COVID-19-associated mortality, at a significance level of <0.10, were included in the multivariate binary logistic regression model. The odds ratio (OR) and 95% CI values were calculated for each statistically significant parameter in the univariate and multivariate logistic regression models. A value with p < 0.05 was considered to indicate a statistically significant difference.
In the study, the data of 2,000 participants, 1,000 of whom were in the control group and 1,000 patients positive for COVID-19, were analyzed retrospectively. Of the COVID-19+ patients, 873 (87.3%) survived, and 127 (12.7%) succumbed to the disease. In addition, while a mutant variant (non-alpha) was detected in 55 (5.5%) patients, the mutant variant was not observed in 945 (94.5%) patients. The sex ratios were statistically similar between the study groups (p = 0.053): 52.9% of the participants in the control group were female (n = 529) and 47.1% were male (n = 471); 50.5% of the patients in the COVID-19+/alive group were female (n = 441) and 49.5% were male (n = 432); 41.7% of the patients in the COVID-19+/deceased group were female (n = 53) and 58.3% (n = 74) were male. The difference between the duration of hospitalization of the patients who survived and those who succumbed to COVID-19 was not statistically significant (p = 0.062). The mean length of hospital stay of the survivors was 7.72 ± 5.47 days [median (min-max), 7 (0–79) days], and the mean of the number of patients hospitalized among those who did not survive was 9.09 ± 6.23 [median (min-max), 8 (1–30)]. The difference in the mean age of the study participants was statistically significant (p < 0.001). According to the post hoc test results, the mean age of the deceased participants was significantly higher than that of the subjects in the control group and the survivors (p < 0.001 and p < 0.001) (Table 1). The mean age of the survivors was also significantly higher than that of the subjects in the control group (p < 0.001; Table 1).
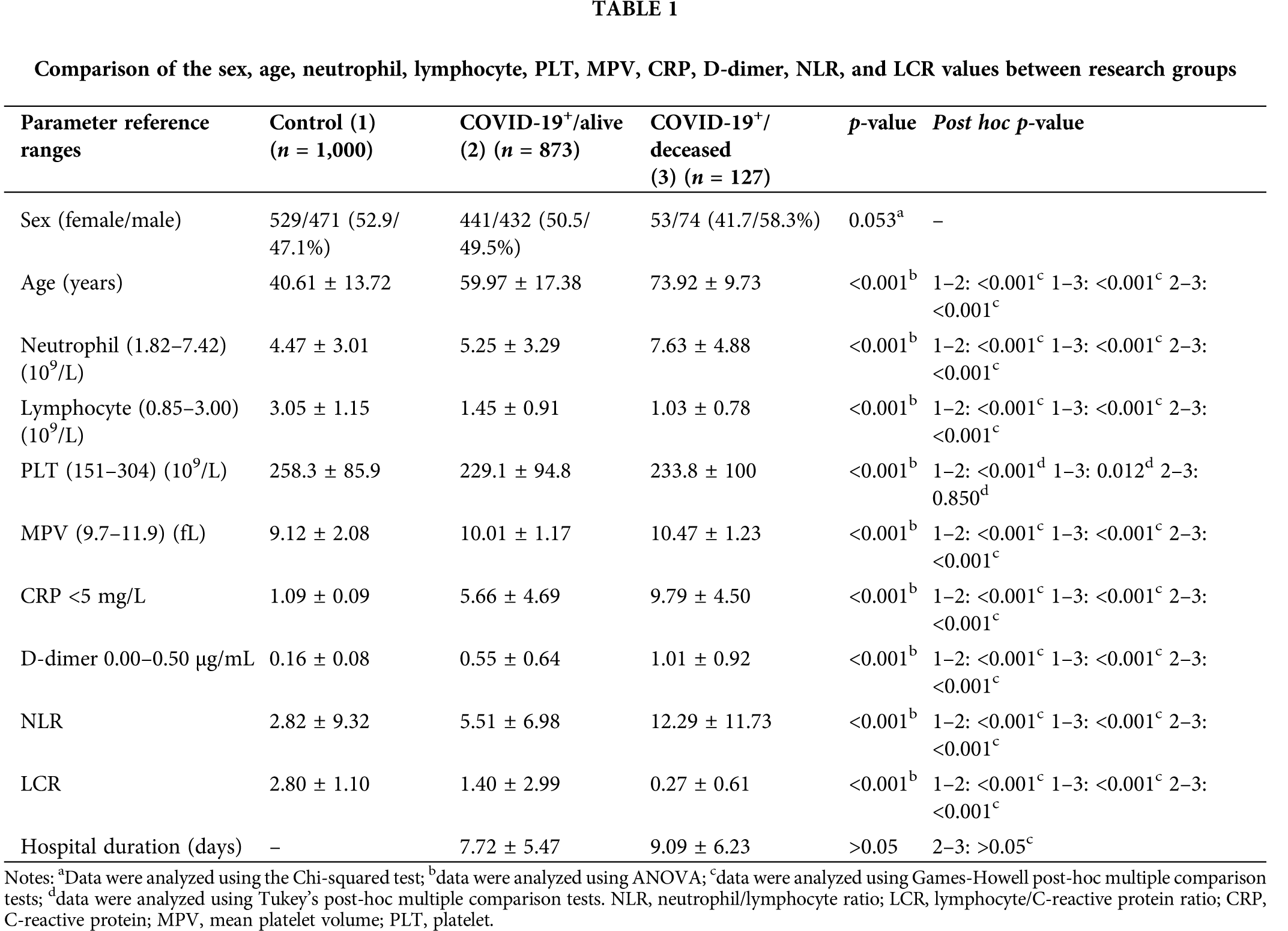
The results of the statistical analysis of the comparisons of the LCR, NLR, neutrophil, lymphocyte, PLT, MPV, CRP, and D-dimer values between the groups are presented in Table 1. Box plots of the distribution of the neutrophil, lymphocyte, LCR, CRP, MPV, PLT, and D-dimer values among the research groups are illustrated in Figs. 2 and 3. The differences in the LCR, NLR, neutrophil, lymphocyte, PLT, MPV, CRP, and D-dimer values were statistically significant between the study groups (p < 0.001). According to the post hoc test results, the neutrophil, MPV, CRP, D-dimer, and NLR values were significantly higher in the COVID-19+/deceased group than in the COVID-19+/alive and control groups (p < 0.001). These values were also significantly higher in the COVID-19+/alive group compared with the control group (p < 0.001). The lymphocyte and LCR values were significantly lower in the COVID-19+/deceased group than in the COVID-19+/alive and control groups (p < 0.001). These values were also significantly lower in the COVID-19+/alive group than in the control group (p < 0.001). The PLT values were significantly higher in the control group than in the COVID-19+/deceased and COVID-19+/alive groups (p-values were p = 0.012 and p < 0.001, respectively). No statistically significant differences were observed in the PLT values between the COVID-19+/deceased and COVID-19+/alive groups (p = 0.850).

Figure 2: Boxplot of the distribution of the (A) neutrophil, (B) lymphocyte, (C) NLR, and (D) LCR values among the research groups. NLR, neutrophil/lymphocyte ratio; LCR, lymphocyte/C-reactive protein ratio.

Figure 3: Boxplot of the distribution of the (A) CRP, (B) MPV, (C) PLT, and (D) D-dimer values among the research groups. CRP, C-reactive protein; MPV, mean platelet volume; PLT, platelet.
ROC analysis was then performed to determine the efficiency of using the LCR, NLR, neutrophil, lymphocyte, PLT, MPV, CRP, and D-dimer values in predicting COVID-19 prognosis and mortality. The sensitivity and selectivity values calculated according to the AUC values and cut-off points with 95% CI values are presented in Table 2. The ROC curve is illustrated in Fig. 4. The ROC analysis indicated that all parameters were statistically significant (p < 0.001, Table 2). According to the AUC values, statistically significant variables in predicting COVID-19 included lymphocyte, LCR, D-dimer, NLR, CRP, MPV, PLT, and neutrophil values. The success of using the lymphocyte, LCR, D-dimer, and NLR values in predicting COVID-19 positivity was good, while that of using the CRP value was fair. The success of using the MPV and PLT values was poor. The neutrophil value was found to be not of use. The sensitivity and selectivity levels calculated according to the cut-off points determined using the Youden index were calculated only for the parameters with good AUC values and are presented in Table 2.
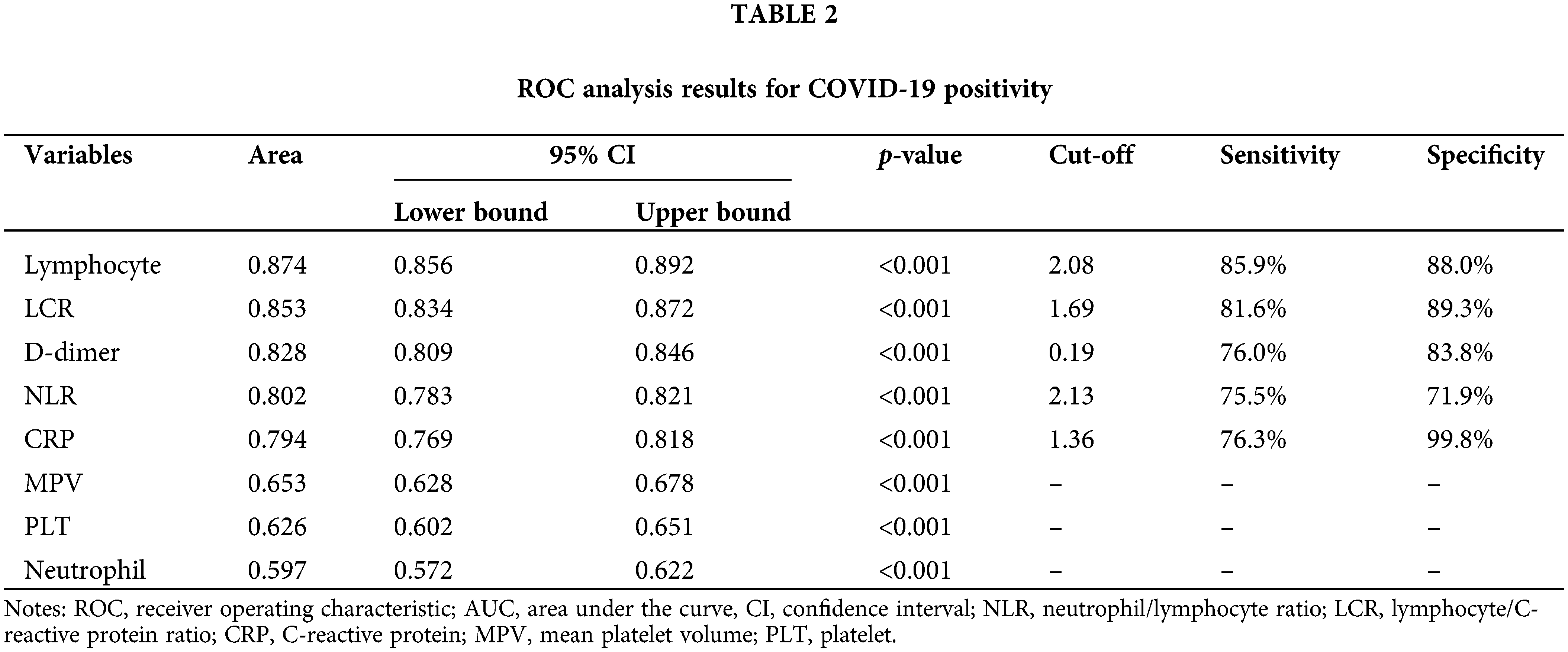

Figure 4: ROC curves for the (A) NLR, neutrophil, MPV, CRP, and D-dimer values, and (B) lymphocyte, PLT and LCR values in the prediction of COVID-19 positivity. NLR, neutrophil/lymphocyte ratio; LCR, lymphocyte/C-reactive protein ratio; CRP, C-reactive protein; MPV, mean platelet volume; PLT, platelet.
ROC analysis was also performed to determine the efficiency of using the LCR, NLR, neutrophil, lymphocyte, PLT, MPV, CRP, and D-dimer values in predicting mortality in patients with COVID-19. The sensitivity and selectivity values calculated according to the AUC values and cut-off points with 95% CIs are presented in Table 3. The ROC curve is illustrated in Fig. 5. As a result of the ROC analysis, all the parameters apart from the PLT count (p = 0.723) were found to be statistically significant (p < 0.001, Table 3). Statistically significant variables in predicting COVID-19-associated mortality based on the AUC value were LCR, CRP, NLR, lymphocyte, D-dimer, neutrophil, and MPV. The use of LCR, CRP, and NLR values was fairly successful. The success of using the lymphocyte, D-dimer, neutrophil, and MPV values was poor. The sensitivity and selectivity levels calculated according to the cut-off points determined using the Youden index were calculated only for parameters with fair AUC values and are presented in Table 3.
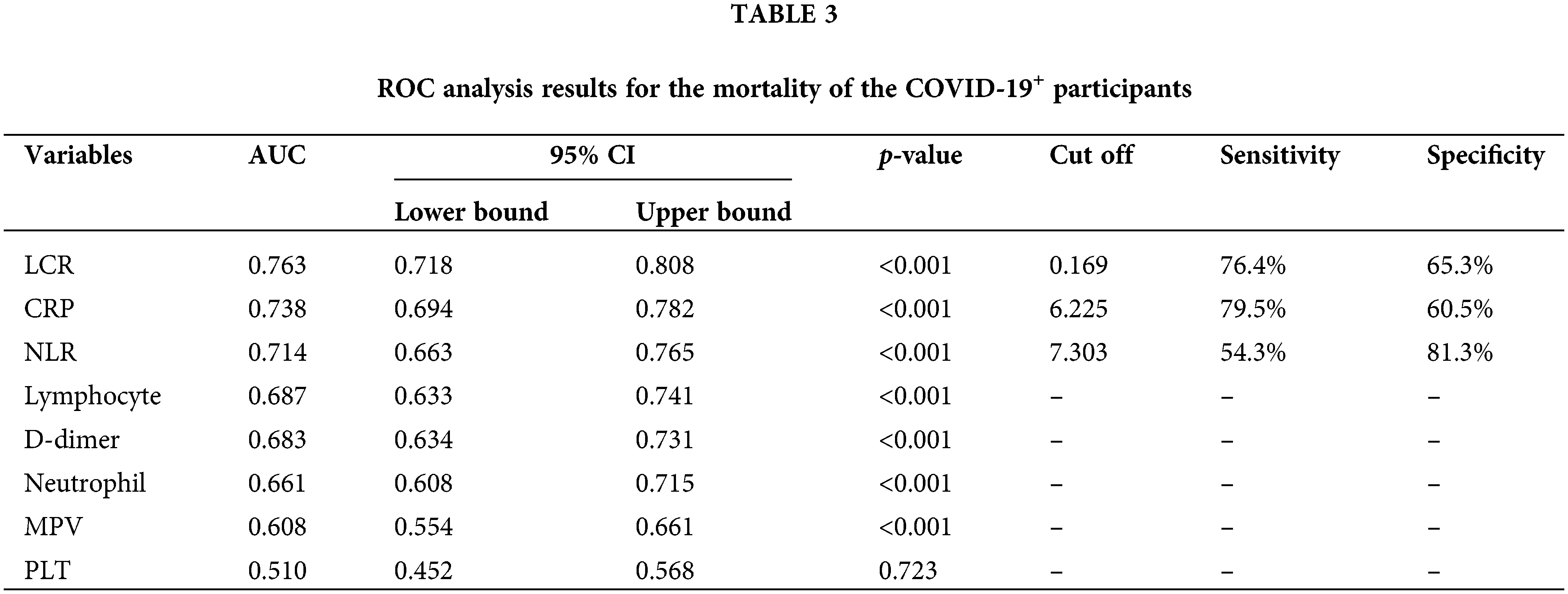

Figure 5: ROC curves for the (A) NLR, neutrophil, MPV, CRP, and D-dimer values, and (B) lymphocyte and LCR values in the mortality of the COVID-19+ participants. NLR, neutrophil/lymphocyte ratio; LCR, lymphocyte/C-reactive protein ratio; CRP, C-reactive protein; MPV, mean platelet volume; PLT, platelet.
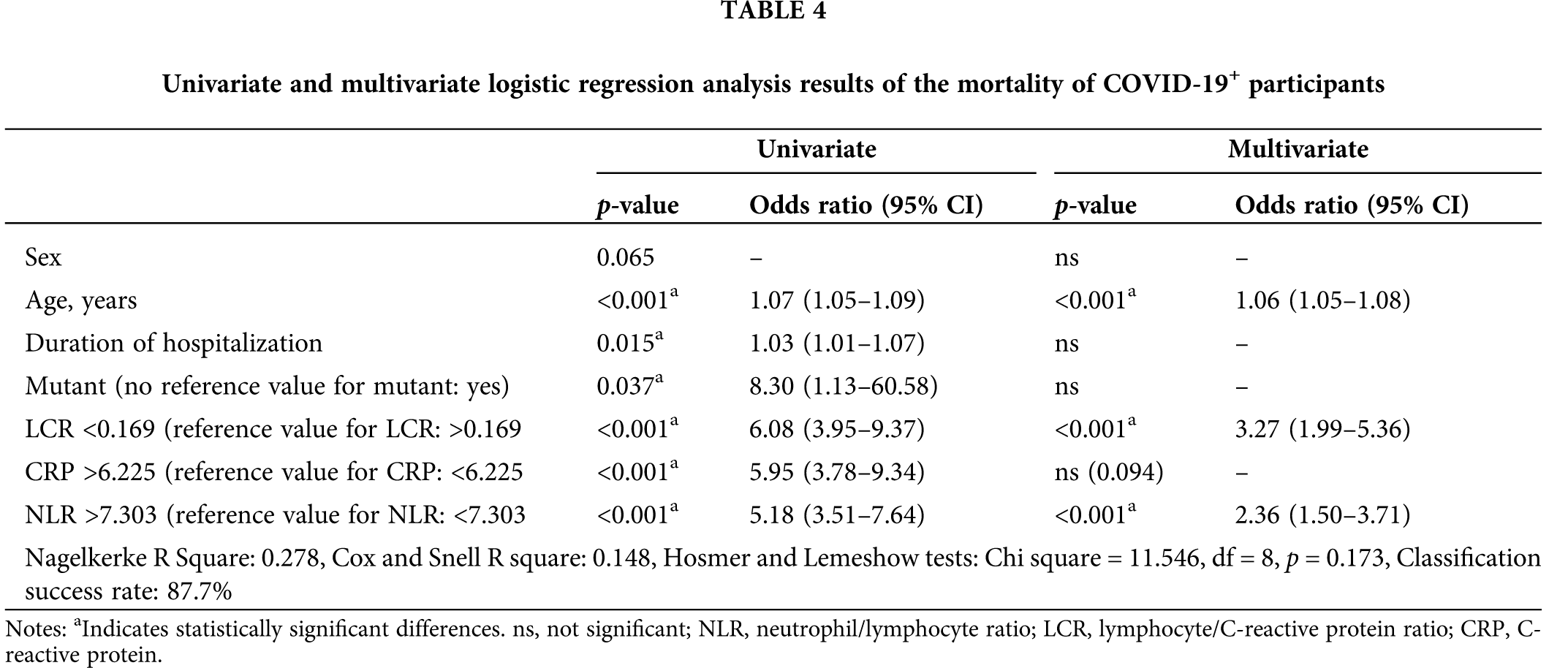
The results of the univariate and multivariate binary logistic regression analysis performed to determine the factors affecting mortality and the OR values in the COVID-19+ patients are presented in Table 4. Sex, age, the duration of hospitalization, the mutant variant, and the LCR, CRP, and NLR values, which were significant (p < 0.01) in the univariate model, were included in the multivariate model. The final model created by subtracting the sex, mutant variant, duration of hospitalization, and CRP value, which were insignificant in the multivariate model, is presented in Table 4. According to the model results, being 1 unit older in the COVID-19+ patients increased the probability of mortality (OR, 1.06; 95% CI, 1.05–1.08). A 1-unit lower LCR in the COVID-19+ patients increased the probability of mortality (OR, 3.27; 95% CI, 1.99–5.36), while an NLR > 1 unit in these patients increased the probability of mortality (OR, 2.36; 95% CI, 1.50–3.71) (Table 4).
The hematological laboratory parameters of COVID-19 patients also exhibit significant changes. The LCR and NLR ratios are parameters that have been reported to indicate the type of infection and prognosis (Carpio-Orantes et al., 2020; Ugurlu et al., 2021; Bal et al., 2021). The prognostic significance of LCR and NLR has also been reported in acute conditions other than COVID-19 (such as acute appendicitis and acute thyroiditis) (Ugurlu et al., 2021; Aktas et al., 2017) and in intensive care patients (Salciccioli et al., 2015; Wang et al., 2017).
In the present study, 2,000 participants, including 1,000 patients hospitalized due to COVID-19 and 1,000 healthy controls, were analyzed retrospectively. The neutrophil, MPV, CRP, D-dimer, and NLR values were significantly higher in the deceased patients than in the survivors and the control group. However, the LCR and lymphocyte values were significantly lower in this group when compared with the other two groups. The success of these parameters in predicting COVID-19 positivity, in descending order, were lymphocyte, LCR, D-dimer, NLR, CRP, MPV, PLT, and neutrophil values. The success of using the lymphocyte, LCR, D-dimer, and NLR values in predicting COVID-19 positivity was good. The success of these parameters in predicting COVID-19 mortality, in descending order, were LCR, CRP, NLR, lymphocyte, D-dimer, neutrophil, and MPV values.
In their study, Liu et al. (2020) reported hospital mortality at a rate of 13.47% for 245 patients with COVID-19, similar to the present study. Their study reported that a high NLR was significantly associated with hospital mortality, and each 1-unit increase in the NLR increased mortality by 1.1; in the present study, this rate was 2.36. Moreover, herein, we observed that the lymphocyte and PLT counts were not associated with hospital mortality. In addition, herein, the lymphocyte count was significantly lower in the patients who succumbed to COVID-19 when compared with the other groups. Likewise, no significant association was found between the PLT count and mortality rates. In the present study, it was observed that having a high NLR was a predictor of in-hospital mortality in patients diagnosed with COVID-19 and also determined high-risk patients.
Ye et al. (2020) reported a hospital mortality rate of 14.9% in their study of 349 hospitalized patients with COVID-19, which was similar to that determined herein. Their study also reported that the NLR and D-dimer parameters were highly associated with mortality (Yan et al., 2020). Herein, the D-dimer and NLR values were significantly higher in the COVID-19+/deceased group than in the COVID-19+/alive and control groups (p < 0.001); besides, the success of these parameters in predicting COVID-19 positivity was good.
In the study by Jimeno et al. (2021), comprising 119 patients with COVID-19, a high NLR was associated with clinical severity and mortality, consistent with those found herein.
In the study by Yan et al. (2020), which included 191 patients with COVID-19, the NLR, and CRP values were significantly higher, and the lymphocyte values were significantly lower in the patient group. In the study by Seyit et al. (2020) on 110 patients with COVID-19, the NLR, and CRP values were significantly higher in the patient group than in the healthy control group. The optimal cut-off values of these parameters were reported as 0.615 and 0.691, respectively (Seyit et al., 2020), while in the present study, these values were 2.13 and 1.36, respectively.
In a previous retrospective study conducted by Shang et al. (2020), which included 443 patients with COVID-19, the NLR, PLT and CRP values were found associated with disease severity, and the NLR was the most effective marker among them. In the present study, the NLR was also associated with mortality, although the LCR was more valuable in this regard.
In the study by Ding et al. (2020), comprising 72 patients with COVID-19, the leukocyte, neutrophil, and NLR values were significantly higher, and the lymphocyte counts were significantly lower in patients with the non-severe disease. In this respect, their results were compatible with those reported herein. Their study reported that the NLR was associated with the length of hospital stay and disease severity. The NLR and other parameters examined in the current study were found to not affect the duration of hospitalization.
In another study by Yang et al. (2020) on 69 patients with non-critical and 24 with critical COVID-19 infection, age, leukocyte count, NLR, the lymphocyte-monocyte ratio, PLR, and CRP were reported to be significantly higher than the other patients with non-critical COVID-19 infection. By contrast, the lymphocyte count was significantly lower. In addition, their study reported that the NLR was associated with clinical severity and disease prognosis and could be used as a marker in clinical applications. The most notable difference between their study and the current one was that the LCR was not examined. Apart from that, similar results were obtained.
Kong et al. (2020) also examined the NLRs of 210 patients with COVID-19 and reported that they were associated with disease severity following linear regression analysis (β = 0.056 and p = 0.000). They also demonstrated that the NLR was higher in the patient group with severe disease than in the group with mild disease (6.6 vs. 3.3, p < 0.001). Qin et al. (2020) reported that the NLR increased in patients with COVID-19 and could aid in diagnosis and treatment in the early period. These findings were consistent with those determined in the present study.
Bal et al. (2021) investigated 61 patients with COVID-19 and reported significantly decreased LCR in the patient group when compared with the healthy group and could be used to distinguish those with critical illness from the severely ill group (cut-off value, 43.21; sensitivity, 84%; specificity, 69%). The LCR (ROC AUC, 0.820) was observed to be more effective than the NLR (0.751) in distinguishing these patient groups. Similarly, in the present study, the LCR was more valuable than the NLR in predicting the disease and mortality.
The NLR is an easy-to-obtain marker and is elevated in a number of inflammatory conditions (Qin et al., 2020). The NLR increases following an increase in the neutrophil count and a decrease in the lymphocyte count. An increase in the number of neutrophils occurs due to systemic inflammation. The lymphocyte count may decrease due to bone marrow suppression, stimulation of apoptosis, increased lymphocyte destruction, and sequestration to the lungs. The mechanism of this association between the neutrophils and lymphocytes has not been precisely unraveled yet. While neutrophils play a crucial role in innate immunity, lymphocytes play an essential role in adaptive immunity (Liu et al., 2016). Lymphocytes are cells that regulate both cellular and humoral immunity and tend to decrease continuously in long-lasting inflammations. In addition, lymphocytes are responsible for destroying virus-infected cells. However, the role of neutrophils in defense against viral infections is not clear (Henry et al., 2020; Camp and Jonsson, 2017). The neutrophil infiltration seen in patients with acute respiratory distress syndrome supports the hypothesis of the possible role of neutrophils in anti-viral protection.
The LCR is one of the novel markers for systemic inflammation, similar to the NLR (Okugawa et al., 2021). It is calculated from the lymphocyte count and CRP value. The current study showed that the LCR was more significant than the NLR in predicting the presence of COVID-19 and mortality. The reason for the lower LCR in critically ill patients may be the decrease in the lymphocyte count following immune dysfunction and a higher CRP value in proportion to the severity of systemic inflammation.
D-dimer, which occurs after fibrin destruction, is significantly increased after microthrombotic events in peripheral blood vessels in patients with COVID-19 (Jin et al., 2020). In our study and other studies, D-dimer was found to be significant as a potential biomarker in predicting mortality and prognosis (Liu et al., 2020; Fu et al., 2020). The D-dimer elevation is likely to be associated with resistant hypoxemia, respiratory failure, disseminated intravascular coagulation, severe coagulation disorders, microthrombotic formation, pulmonary thromboembolism, and myocardial infarction.
The LCR, NLR, and D-dimer values can be used as markers to reduce COVID-19 morbidity and mortality. In addition, using these parameters may be necessary to monitor patients more closely, change the method of treatment, and move them to the intensive care unit. The most significant advantages of the present study were that, at least to the best of our knowledge, the number of study subjects (n = 2,000) was markedly higher than that in all previous studies (Yang et al., 2020; Bal et al., 2021; Salciccioli et al., 2015; Jimeno et al., 2021; Yan et al., 2020) conducted, and strong statistical analyses could be made.
However, certain limitations of the present study should be mentioned. The present study was a single-center retrospective study. Thus, the possibility of overlooked asymptomatic or mild patients, the inability to monitor changes in the parameters in these patients due to the single blood result of patients whose clinical exacerbation rapidly exacerbated, the uncertainty of the effect of patient comorbidity and treatment on the blood results, and the improvement in some patients at the time of hospitalization were limitations of the study. Another limitation was that the pediatric age group was not included in our study because the number of patients was not sufficient.
In conclusion, the low LCR and NLR are biomarkers associated with the presence, prognosis, and mortality of COVID-19. The low LCR is more useful in this regard. These parameters can be used in clinical monitoring to reduce morbidity and mortality rates. Patients with a low LCR or high NLR should be admitted to an isolation ward with respiratory monitoring and supportive care. Supportive treatment can be applied early using these markers for a better prognosis and reduced mortality and cost. The ease of measurement and low cost of these markers can increase their usability. Despite the large number of patients herein, prospective studies with more extensive patient series are required to more clearly reveal the association between the LCR, NLR, and D-dimer values with the presence of COVID-19 and disease severity.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contribution: AO conceptualized the main idea and hypothesis of the study, obtained permission from the ethics committee, developed the theory, arranged the material-method section, evaluated the data in the results section, wrote the discussion section, reviewed and made the necessary arrangements, and approved the manuscript. AO confirms the authenticity of all the raw data. The author has read and approved the final manuscript. MK conceptualized the main idea and hypothesis of the study, evaluated the data in the results section, wrote the discussion section, reviewed and made the necessary arrangements, and approved the manuscript. MK confirms the authenticity of all the raw data. The author has read and approved the final manuscript.
Transparency Statement: AO and MK affirm that this manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
Ethics Approval: An application was made to the Tokat Gaziosmanpaşa University Clinical Research Ethics Committee (as Erbaa State Hospital, Erbaa, Tokat Province), and the necessary approval was obtained (approval date, August 17, 2021; decision number, HRU/20.14.13). The study was carried out in compliance with the ethical principles of the Declaration of Helsinki. This was a retrospective study; therefore, informed consent was neither necessary nor obtained.
Patient Consent for Publication: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Aktas G, Cakiroglu B, Sit M, Uyeturk U, Alcelik A et al. (2013). Mean platelet volume: A simple indicator of chronic prostatitis. Acta Medica Mediterranea 29: 551–560. [Google Scholar]
Aktas G, Alcelik A, Tekce BK, Tekelioglu V, Sit M et al. (2014). Red cell distribution width and mean platelet volume in patients with irritable bowel syndrome. Przegląd Gastroenterologiczny 9: 160–163. DOI 10.5114/pg.2014.43578. [Google Scholar] [CrossRef]
Aktas G, Sit M, Dikbas O, Erkol H, Altinordu R et al. (2017). Elevated neutrophil-to-lymphocyte ratio in the diagnosis of hashimoto’s thyroiditis. Revista da Associação Médica Brasileira 63: 1065–1068. DOI 10.1590/1806-9282.63.12.1065. [Google Scholar] [CrossRef]
Aktas G, Kocak MZ, Duman TT, Erkus E, Atak BM et al. (2018). Mean platelet volume (MPV) as an inflammatory marker in type 2 diabetes mellitus and obesity. Bali Medical Journal 7: 650–653. DOI 10.15562/bmj.v7i3.806. [Google Scholar] [CrossRef]
Aktas G (2020). A comprehensive review on rational and effective treatment strategies against an invisible enemy; SARS Cov-2 infection. Experimental Biomedical Research 3: 293–311. DOI 10.30714/j-ebr.2020463629. [Google Scholar] [CrossRef]
Aktas G, Duman T, Atak B, Kurtkulagi O, Bilgin S et al. (2020). Irritable bowel syndrome is associated with novel inflammatory markers derived from hemogram parameters. Family Medicine and Primary Care Review 2: 107–110. DOI 10.5114/fmpcr.2020.95311. [Google Scholar] [CrossRef]
Aktas G (2021). Hematological predictors of novel coronavirus infection. Revista da Associação Médica Brasileira 67: 1–2. DOI 10.1590/1806-9282.67.suppl1.20200678. [Google Scholar] [CrossRef]
Atak B, Aktas G, Duman TT, Erkus E, Kocak MZ et al. (2019). Diabetes control could through platelet-to-lymphocyte ratio in hemograms. Revista da Associação Médica Brasileira 65: 38–42. DOI 10.1590/1806-9282.65.1.38. [Google Scholar] [CrossRef]
Atak MB, Bakir-Kahveci G, Bilgin S, Kurtkulagi O, Kosekli MA (2021). Platelet to lymphocyte ratio in differentiation of benign and malignant thyroid nodules. Experimental Biomedical Research 4: 148–153. DOI 10.30714/j-ebr.2021267978. [Google Scholar] [CrossRef]
Bal T, Dogan S, Cabalak M, Dirican E (2021). Lymphocyte-to-C-reactive protein ratio may serve as an effective biomarker to determine COVID-19 disease severity. Turkish Journal of Biochemistry 46: 23–28. DOI 10.1515/tjb-2020-0410. [Google Scholar] [CrossRef]
Bilgin S, Aktas G, Kocak MZ, Atak BM, Kurtkulagi O et al. (2020). Association between novel inflammatory markers derived from hemogram indices and metabolic parameters in type 2 diabetic men. The Aging Male 23: 923–927. DOI 10.1080/13685538.2019.1632283. [Google Scholar] [CrossRef]
Bilgin S, Aktas G, Kahveci G, Atak BM, Kurtkulagi O et al. (2021). Does mean platelet volume/lymphocyte count ratio associate with frailty in type 2 diabetes mellitus? Bratislavske Lekarske Listy 122: 116–119. DOI 10.4149/BLL_2021_017. [Google Scholar] [CrossRef]
Cakir L, Aktas G, Enginyurt O, Cakir SA (2014). Mean platelet volume increases in type 2 diabetes mellitus independent of HbA1c level. Acta Medica Mediterranea 30: 425–428. [Google Scholar]
Cakir L, Aktas G, Mercimek B, Enginyurt O, Kaya Y et al. (2020). Are red cell distribution width and mean platelet volume associated with rheumatoid arthritis? Biomedical Research 27: 292–294. [Google Scholar]
Camp JV, Jonsson CB (2017). A role for neutrophils in viral respiratory disease. Frontiers in Immunology 8: 550. DOI 10.3389/fimmu.2017.00550. [Google Scholar] [CrossRef]
Carpio-Orantes LD, García-Méndez S, Hernández-Hernández SN (2020). Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index in patients with COVID-19-associated pneumonia. Gaceta Medica de Mexico 156: 527–531. DOI 10.24875/GMM.M21000480. [Google Scholar] [CrossRef]
Chan JFW, Yuan S, Kok KH, Kelvin KWT, Hin C et al. (2020). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 395: 514–523. DOI 10.1016/S0140-6736(20)30154-9. [Google Scholar] [CrossRef]
Chen N, Zhou M, Dong X, Qu J, Gong F et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395: 507–513. DOI 10.1016/S0140-6736(20)30211-7. [Google Scholar] [CrossRef]
Cucinotta D, Vanelli M (2020). WHO declares COVID-19 a pandemic. Acta Biomedica 91: 157–160. DOI 10.23750/abm.v91i1.9397. [Google Scholar] [CrossRef]
Dagistan Y, Dagistan E, Gezici AR, Halicioglu S, Akar S et al. (2016). Could red cell distribution width and mean platelet volume be a predictor for lumbar disc hernias? Ideggyogyaszati Szemle 69: 411–414. DOI 10.18071/isz.69.0411. [Google Scholar] [CrossRef]
Dagistan E, Cosgun Z (2019). Could hemogram parameters be predictors of dementia in elderly patients? Aging Male 22: 192–197. DOI 10.1080/13685538.2019.1618821. [Google Scholar] [CrossRef]
Ding X, Yu Y, Lu B, Huo J, Chen M, Kang Y, Lou J, Liu Z (2020). Dynamic profile and clinical implications of hematological parameters in hospitalized patients with coronavirus disease 2019. Clinical Chemistry and Laboratory Medicine 58: 1365–1371. DOI 10.1515/cclm-2020-0411. [Google Scholar] [CrossRef]
Duman TT, Aktas G, Atak BM, Kocak MZ, Erkus E et al. (2019). Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. African Health Sciences 19: 1602–1606. DOI 10.4314/ahs.v19i1.35. [Google Scholar] [CrossRef]
ElshazliI RM, Toraih EA, Elgaml A, El-MowafyI M, El-MeseryI M et al. (2020). Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PLoS One 15: e0238160. DOI 10.1371/journal.pone.0238160. [Google Scholar] [CrossRef]
Fu J, Kong J, Wang W, Wu M, Yao L et al. (2020). The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: A retrospective study in Suzhou China. Thrombosis Research 192: 3–8. DOI 10.1016/j.thromres.2020.05.006. [Google Scholar] [CrossRef]
Guan W, Ni Z, Hu Y, Liang W, Ou C et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine 382: 1708–1720. DOI 10.1056/NEJMoa2002032. [Google Scholar] [CrossRef]
Henry MB, Santos de Oliveira HM, Benoit S, Plebani M, Lippi G (2020). Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19A meta-analysis. Clinical Chemistry and Laboratory Medicine 58: 1021–1028. DOI 10.1515/cclm-2020-0369. [Google Scholar] [CrossRef]
Jimeno S, Ventura PS, Castellano JM, Garcia-Adasme SI, Miranda M et al. (2021). Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. European Journal of Clinical Investigation 51: 1–9. DOI 10.1111/eci.13404. [Google Scholar] [CrossRef]
Jin YH, Cai L, Cheng ZS, Cheng ZS, Cheng H et al. (2020). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Military Medical Research 7: 4. DOI 10.1186/s40779-020-0233-6. [Google Scholar] [CrossRef]
Kong M, Zhang H, Cao X, Mao X, Lu Z (2020). Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiology and Infection 148: e139. DOI 10.1017/S0950268820001557. [Google Scholar] [CrossRef]
Lagunas-Rangel FA (2020). Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19A meta-analysis. Journal of Medical Virology 92: 1733–1734. DOI 10.1002/jmv.25819. [Google Scholar] [CrossRef]
Lavezzo E, Franchin E, Ciavarella C, Cuomo-Dannenburg G, Barzon L et al. (2020). Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 584: 425–429. DOI 10.1038/s41586-020-2488-1. [Google Scholar] [CrossRef]
Liu X, Shen Y, Wang H, Ge Q, Fei A et al. (2016). Prognostic significance of neutrophil-to-lymphocyte ratio in patients with sepsis: A prospective observational study. Mediators of Inflammation 2016: 8191254–8191258. DOI 10.1155/2016/8191254. [Google Scholar] [CrossRef]
Liu Y, Du X, Chen J, Jin Y, Peng L et al. (2020). Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. Journal of Infection 81: e6–e12. DOI 10.1016/j.jinf.2020.04.002. [Google Scholar] [CrossRef]
Okugawa Y, Toiyama Y, Fujikawa H, Ide S, Yamamoto A et al. (2021). Prognostic potential of lymphocyte–C-reactive protein ratio in patients with rectal cancer receiving preoperative chemoradiotherapy. The Journal of Gastrointestinal Surgery 25: 492–502. DOI 10.1007/s11605-019-04495-4. [Google Scholar] [CrossRef]
Qin C, Zhou L, Hu Z, Zhang S, Yang S et al. (2020). Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clinical Infectious Diseases 71: 762–768. DOI 10.1093/cid/ciaa248. [Google Scholar] [CrossRef]
Salciccioli JD, Marshall DC, Pimentel AF, Santos MD, Pollard T et al. (2015). The association between the neutrophil-tolymphocyte ratio and mortality in critical illness: An observational cohort study. Critical Care 19: 13. DOI 10.1186/s13054-014-0731-6. [Google Scholar] [CrossRef]
Seyit M, Avci E, Nar R, Senol H, Yilmaz A et al. (2020). Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. American Journal of Emergency Medicine 40: 110–114. DOI 10.1016/j.ajem.2020.11.058. [Google Scholar] [CrossRef]
Shang W, Dong J, Ren Y, Tian M, Li W et al. (2020). The value of clinical parameters in predicting the severity of COVID-19. Journal of Medical Virology 2020: 1–5. DOI 10.1002/jmv.26031. [Google Scholar] [CrossRef]
Sincer I, Gunes Y, Mansiroglu AK, Coskun M, Aktas G (2018). Association of mean platelet volume and red blood cell distribution width with coronary collateral development in stable coronary artery disease. Advances in Interventional Cardiology 14: 263–269. DOI 10.5114/aic.2018.78329. [Google Scholar] [CrossRef]
Sit M, Aktas G, Erkol H, Yaman S, Keyif F et al. (2019). Neutrophil to lymphocyte ratio is useful in differentiation of malign and benign thyroid nodules. Puerto Rico Health Sciences Journal 38: 60–63. [Google Scholar]
Ugurlu C, Yildirim M, Ozturk A, Ozcan O, Bostan MS et al. (2021). Lymphocyte-to-C-reactive protein ratio: A new biomarker to predict perforation in acute appendicitis. Indian Journal of Surgery 83: 8. DOI 10.1007/s12262-021-02937-5. [Google Scholar] [CrossRef]
Wang Y, Wang D, Fu J, Liu Y (2017). Predictive value of SOFA, qSOFA score and traditional evaluation index on sepsis prognosis. Chinese Critical Care Medicine 29: 700–704. DOI 10.3760/cma.j.issn.2095-4352.2017.08.006. [Google Scholar] [CrossRef]
Wang ZF, Su F, Lin XJ, Dai B, Kong LF et al. (2011). Serum D-dimer changes and prognostic implication in 2009 novel influenza A (H1N1). Thrombosis Research 127: 198–201. DOI 10.1016/j.thromres.2010.11.032. [Google Scholar] [CrossRef]
Yang AP, Liu JP, Tao WQ, Li HM (2020). The diagnostic and predictive role of NLR, d-NLR, and PLR in COVID-19 patients. International Immunopharmacology 84: 106504. DOI 10.1016/j.intimp.2020.106504. [Google Scholar] [CrossRef]
Ye W, Chen G, Li X, Lan X, Ji C et al. (2020). Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respiratory Research 21: 1–7. DOI 10.1186/s12931-020-01428-7. [Google Scholar] [CrossRef]
Yildiz E, Cigri E, Dincer Z, Narsat MA, Calisir B (2021). High neutrophil/lymphocyte ratios in symptomatic pediatric COVID-19 patients. Journal of College of Physicians and Surgeons Pakistan 31: 93–98. DOI 10.29271/jcpsp.2021. [Google Scholar] [CrossRef]
Yan TF, Liu ML, Li XJ, Deng XM, Jin YM et al. (2020). Utility of the neutrophil-to-lymphocyte ratio and C-reactive protein level for coronavirus disease 2019 (COVID-19). Scandinavian Journal of Clinical and Laboratory Investigation 80: 536–540. DOI 10.1080/00365513.2020.1803587. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools