 Open Access
Open Access
VIEWPOINT
Long term immunological perturbations post DAA therapy in chronic HCV/HIV co-infected patients
National HIV/AIDS Research Center, Istituto Superiore di Sanità, Rome, 00161, Italy
* Corresponding Author: ALESSANDRA BORSETTI. Email:
BIOCELL 2022, 46(12), 2695-2699. https://doi.org/10.32604/biocell.2022.022257
Received 01 March 2022; Accepted 22 June 2022; Issue published 10 August 2022
Abstract
Direct-acting antiviral (DAA) therapies are efficacious for the achievement of sustained virologic response (SVR) in almost all treated hepatitis C virus (HCV)-infected patients. However, the impacts of HCV eradication on immune function and chronic immune activation in the long-term remain controversial and limited, especially in patients co-infected with human immunodeficiency virus (HIV). Indeed, although restoration of many immune responses clearly can be observed, several features of immune perturbations persist over time after HCV clearance. Understanding the degree and reasons of the partial recovery of the immune system in chronic HCV/HIV co-infection after HCV elimination is pivotal to avoid disease progression and possible long-term clinical outcomes in cured patients, as well as contributing to the development of immunotherapy drug design.Keywords
Hepatitis C virus (HCV) infection among HIV infected people is a pivotal cause of comorbidities and overall mortality. In the combined antiretroviral therapy (cART) era, liver disease is the main cause of non-AIDS death in HIV infected individuals and HCV co-infection is responsible for 90% of deaths in HIV positive patients with end stage liver disease. The clinical course of HCV disease is accelerated during co-infection with HIV, with higher rates of progression to liver disease in terms of fibrosis progression, risk of decompensation, cirrhosis and liver cancer development. Both HCV and HIV infection lead to increased production of inflammatory cytokines and chemokines, giving rise to a state of chronic inflammation, with consequent progressive T cell exhaustion and immune dysregulation in HCV/HIV co-infected individuals (Kim and Chung, 2009; Chen et al., 2014; Mastroianni et al., 2014; Gonzalez et al., 2010). Suppression of HCV replication by DAA regimens, greatly improves rates of cure in HCV/HIV co-infected patients on cART, probably by decreasing immune activation and ameliorating efficacy of cART in these individuals. However, it is not completely acknowledged whether, following DAA treatment, HCV eradication brings to a recovery of both innate and adaptive immune responses, and of lymphocyte homeostasis previously dysregulated by the virus (Rial-Crestelo et al., 2020).
What are the effects of DAA therapy on immune reconstitution in HCV/HIV co-infected patients?
HCV/HIV co-infected patients suffer from higher inflammation than HCV mono-infected patients, and despite cART, residual immune activation remains also in HIV mono-infected patients. Furthermore, recovery of immune response to HCV is important in cases of re-exposure, or for the resolution of extrahepatic manifestations. It has been well accepted that achievement of sustained virologic response (SVR) is associated with at least partial restoration of T cell immune function, downregulation of hepatic inflammation and reduction in liver-related mortality in cART treated HCV/HIV co-infected individuals (Parisi et al., 2018; Emmanuel et al., 2019; Shrivastava et al., 2018; López-Cortés et al., 2018). DAA therapy is expected to ameliorate symptoms and mortality from severe extrahepatic manifestations, as well as improve quality of life in HCV/HIV co-infected individuals. However, it does not completely restore immune and liver functions (Brochado-Kith et al., 2021). Indeed, although a reduction of global T cell immune response occurs after DAA therapy, higher immune activation may remain for a longtime after the end of treatment. This suggests that a complete restoration of the immune response, similar to that observed after spontaneous viral clearance, may not be obtained, remarking that long-term antigenic stimulation gives an irreversible modification on the T cell compartment. Different authors showed conflicting data in HCV/HIV co-infected patients on cART. In fact, while some studies displayed a functional improvement of immune system by a significant decrease of plasma biomarkers and a decline in the activation of immune cells, others suggested a long-term imprint of HCV on immune cells, that remained largely unchanged following HCV clearance. In particular, studies focused on total CD4+ and CD8+ T cell activation, provided evidences on whether T cell activation was reduced following DAA treatment in HCV/HIV co-infected patients on cART (Parisi et al., 2018; Emmanuel et al., 2019; Shrivastava et al., 2018; López-Cortés et al., 2018; Brochado-Kith et al., 2021; Najafi Fard et al., 2018). Emmanuel and co-workers reported a decrease in total activated (HLA-DR+ and CD38+) CD4+ and CD8+ T cells up to one year post-SVR in HCV mono-infected and more evident in HCV/HIV co-infected patients (Emmanuel et al., 2019). In another study, T cell activation was found unvaried both in HCV mono- and HCV/HIV co-infected patients 12 weeks after DAA treatment, with only a reduction of total CD4+ and CD8+ T cells producing IFN-gamma, IL-17, and IL-22 (Najafi Fard et al., 2018). Recently, Brochado-Kith and collaborators showed a significant reduction in plasma biomarkers and gene expression related to antiviral/inflammatory response in HCV/HIV co-infected patients, especially in levels of chemokines and interferon-stimulated genes (ISGs). However, a complete recovery of the immune function was not achieved, when compared with HCV and HIV mono-infected patients (Brochado-Kith et al., 2021). All the studies described above considered the effects of DAA therapy on immune system at SVR, or for short time follow-up. Conversely, a recent study by our group aimed at evaluating the impact of HCV eradication following DAA therapy on early and late longitudinal phenotypic changes in activated, differentiated and exhausted T cells, and also in inflammatory biomarkers, indoleamine 2–3 dioxygenase (IDO) activity, and liver stiffness scores in HCV mono-infected and HCV/HIV co-infected patient on cART. We showed that, especially in the HCV/HIV co-infected patients, immune activation may persist for a long time after the end of treatment despite an improvement of liver-specific outcomes, thus confirming the previous reported conclusions (Farcomeni et al., 2021). Whether these effects were sustained and/or were extended to the memory T cell subsets or innate cell compartment was unclear.
The frequency of T cells with phenotype Foxp3+ CD25+ CD4+, known as Tregs, is often increased in chronic HCV infection and is responsible of the immune suppressive response in the liver. However, after HCV eradication by DAA therapy, frequency of Tregs did not completely normalize in co-infected subjects on successful cART (Tumino et al., 2017). Similarly, the frequency of HLA-DR-CD33+CD11b+ myeloid-derived suppressor cells (MDSC) were expanded in co-infected subjects (Tumino et al., 2017). The frequency of MDSC increased post-DAA therapy in HCV/HIV co-infected individuals, probably participating to the overall immune suppression (Tumino et al., 2017). In summary, studies that describe DAA-mediated clearance of HCV, suggested only limited recovery of the immune system and an immune suppressive environment with persistence of cells like Tregs and MDSC. These immune suppressive conditions might decrease immune response to vaccinations, reduce immune surveillance against cancer, and increase re-activation of latent infections of herpesviruses and hepatitis B virus, respectively (Macias et al., 2019a; Fabbri et al., 2017). Finally, an incomplete reconstitution of HCV-specific CD4+ and CD8+ T cells might increase the risk of HCV persistence upon re-exposure and re-infection in high-risk populations like HCV/HIV co-infected patients.
Clinical benefits on disease progression in HCV/HIV co-infected subjects were also observed in terms of liver disease and fibrosis scores, after HCV eradication with DAA therapy (Macias et al., 2019b; Schwabl et al., 2017; Navarro et al., 2017; Lledo et al., 2018).
In conclusion, additional evaluations examining immune reconstitution after treatment with DAA drugs in HCV/HIV co-infected subjects are warranted, to more fully appreciate the long-term implications and health outcomes of HCV clearance.
Can DAA treatment restore the metabolic disturbances in HCV/HIV co-infection?
Both HCV and HIV infections have been related with metabolic alterations affecting lipid and glucose metabolism. In particular, in chronic HCV/HIV co-infection on cART a higher prevalence of dyslipidemia and dysglycemia due to the host’s inflammatory and immune activated state was observed (Collins et al., 2019). Because of the important metabolic disorder and related diseases due to HCV infection, the question of whether the benefit of DAA treatment can restore the metabolic impairment is of pivotal importance. Recent studies reported that DAA therapy may provide beneficial effects on both glucose and lipid metabolism early after treatment in HCV/HIV co-infected individuals on cART. However, these effects gradually decline with the extension of follow-up (Zeng et al., 2020). In contrast, another study did not find the same effect on glucose metabolism post DAA treatment in HCV/HIV co-infected patients (Chaudhury et al., 2017). The contradictory of these results may be explained by the duration of follow-up after the end of treatment and by the different characteristics of infected individuals (Zeng et al., 2020). Another recent study described that in cART treated HCV/HIV co-infected patients with advanced HCV-related cirrhosis, plasma metabolomic profile changed after HCV clearance with DAA therapy. Besides, plasma level changes were related to improvements in cirrhosis scores and inflammatory status of patients (Virseda-Berdices et al., 2022). Therefore, the question of whether DAA therapy can bring to a positive impact on the metabolic impairment is still on debate and needs further studies with longer duration of follow-up, particularly in the HCV/HIV co-infected population.
What is the residual risk of disease progression in HCV/HIV co-infection after sustained virologic response?
It is plausible that HCV eradication by DAA therapy helps to replacing an antiviral immune function that may hinder some features of HIV-1 replication in HCV/HIV co-infection (Sikavi et al., 2018). Recovery of immune homeostasis may involve many clinical benefits, ranging from protection of reinfection or reactivation of HCV and of other viruses, to protection from hepatocellular carcinoma (HCC) development, and conceivably to regression of liver fibrosis. In fact, data from many studies have shown a clear advantage of HCV cure in reducing liver damage progression and also in reducing the probability of HCC in people living with HIV, especially in those treated at an initial stage of fibrosis (Salmon-Ceron et al., 2019). On the contrary, evidence that DAA treatment did not completely hamper progressive inflammation and liver damage in co-infected people (Ganesan et al., 2019), gave rise to post-SVR outcomes, such as liver fibrosis’ advancement, risk of reinfection, and HCC development. In fact, the risk of developing HCC remained elevated in co-infected people, and was not completely abolished upon HCV clearance, in those subjects with advanced fibrosis or cirrhosis (Gobran et al., 2021). Also, molecular studies on HCV-infected hepatocytes or on liver biopsy samples from infected patients, told us that chronic HCV infection causes epigenetic and gene expression alterations. These abnormalities, that persisted after HCV eradication in HCV/HIV co-infected subjects on cART, may be associated with risk for HCC development (Rial-Crestelo et al., 2020). Other factors may be implicated in liver disease progression and HCC development in HCV/HIV co-infected subjects. First, metabolic abnormalities including insulin resistance that may result in accumulation of triglycerides in hepatocytes, with consequent hepatic steatosis. This can accelerate liver damage and increase risk of developing HCC regardless HCV status (Jeyarajan and Chung, 2020). Second, gut dysbiosis and intestinal impairment are characteristics of HIV infection that are not restored by ART (Gobran et al., 2021). Considering that DAA mediated HCV eradication does not influence gut dysbiosis in cirrhotic HCV mono-infected subjects, dysbiosis may persist as an important risk of liver disease progression in HCV/HIV co-infection after DAA therapy. DAA treatment might not be sufficient to stop liver disease’ progression regarding inflammation, oxidative stress, liver damage and metabolic dysregulation, especially in HCV/HIV co-infection. Therefore, defining how inflammation and chronic immune activation are interconnected during DAA therapy, understanding the mechanisms of HCV related immune dysfunction and barriers to immune reconstitution after viral clearance is of pivotal importance to diminish the long-term effects and progression of chronic HCV infection in HCV/HIV co-infection (Fig. 1).
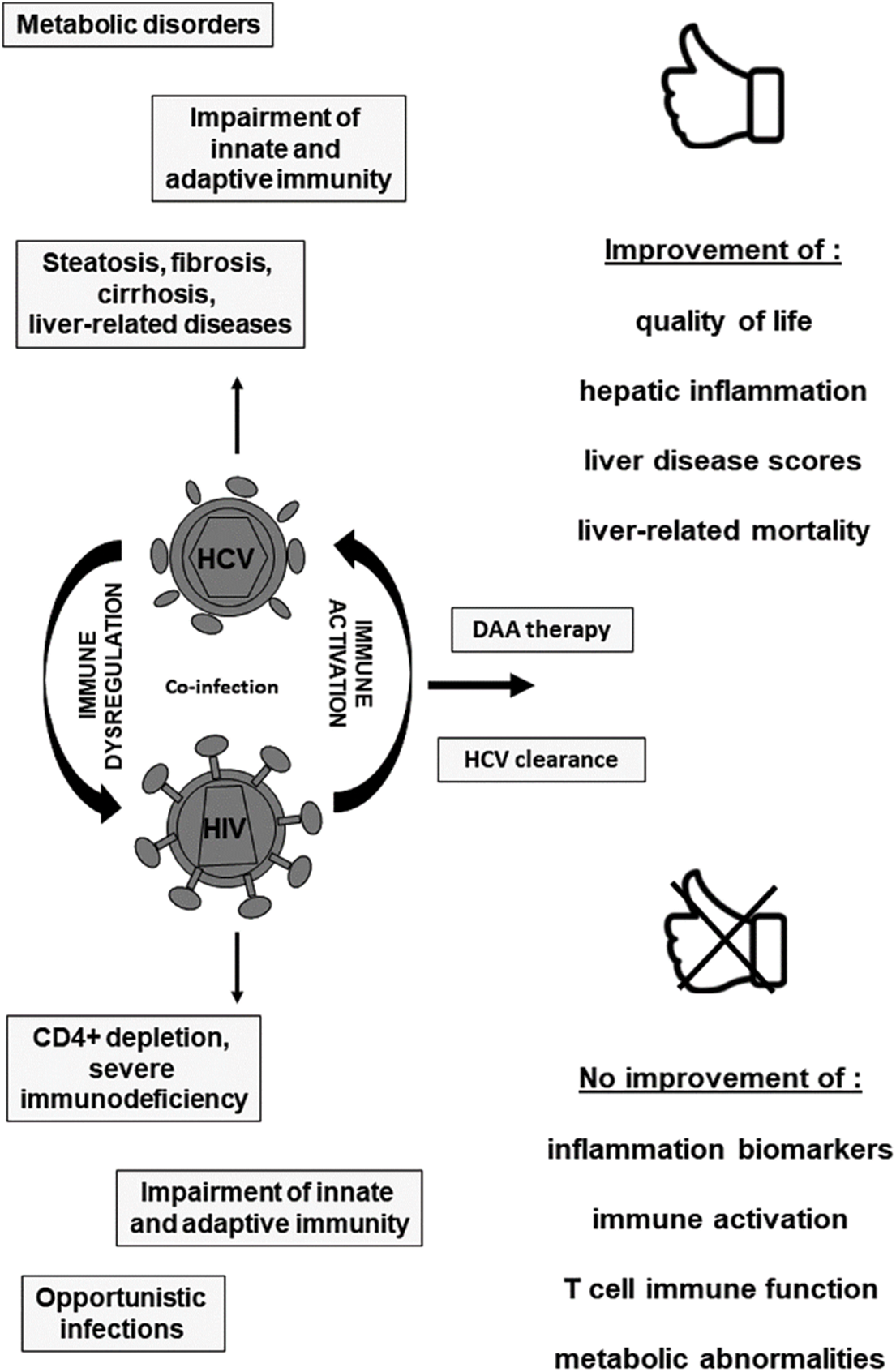
Figure 1: Outcomes of DAA therapy in chronic HCV/HIV co-infection.
Several important questions remain to be investigated, as many effects of chronic HCV infection on different compartments of the immune system which may persist for years despite antigen elimination. Longer follow-up studies are needed to verify whether there is a decline of inflammatory parameters over time in successfully DAA treated HCV/HIV co-infected patients, and whether following HCV clearance, long-term recovery of the interferon system is achieved. Identifying differences in immune responses between HCV mono- and HCV/HIV co-infected subjects might help to understand the relationship between HCV/HIV co-infection and progression of HIV disease. More comprehensive studies on exhausted HCV-specific CD4+ and CD8+ T cells are necessary since there might be certain levels of heterogeneity regarding subpopulations of exhausted HCV-specific cells becoming restored after DAA mediated clearance of the virus. Moreover, clearance of HCV by DAA therapy may cause a higher efficacy to cART therapy by reducing immune activation in co-infected individuals and a better clinical management of co-infected patients under suppressive cART. Furthermore, the effect of DAA treatment on HIV reservoir persistence in ART-treated HCV/HIV patients, should be analyzed in new longitudinal studies, and additional therapeutic interventions can be required to monitor residual HIV transcription in ART-treated patients and restore immune competence following DAA therapy. Another aspect may be defining the risk of disease progression and HCC development after HCV clearance by DAA treatment which is an important issue that could be relatively easy to solve, utilizing large number of patients in long-term follow-up studies. Association of specific clinical characteristics such as diabetes, alcohol intake, advanced age, coexistent nonalcoholic fatty liver disease, with a higher risk of HCC development among post-SVR patients may impact surveillance recommendations and increase cost-effectiveness.
In most cases, HCV elimination by DAA treatment in HCV/HIV co-infected individuals on cART improves liver inflammation and HCV-related extrahepatic diseases. However, while some aspects of the immune response are restored, others remain altered, indicating an active role of immunity besides DAA mediated viral clearance. Long-term chronic HCV infection might irreversibly damage the immune system, perhaps by inducing epigenetic changes that persist after virus eradication (Perez et al., 2019; Polyak et al., 2021).
• a long-term antigenic stimulation may imprint an irreversible change on the T cell compartment in HCV/HIV co-infected individuals.
Clarifying the extent and levels of the deficient recovery of the immune system in ART treated HIV patients after HCV eradication by DAA therapy might be relevant:
• to understand the susceptibility to the development of immune-mediated diseases, cancer and co-infections, also contributing to drug design and vaccine.
• to appropriately manage and improve clinical outcomes for HCV/HIV co-infected patients.
• to clarify how HCV and HIV reciprocally influence hepatic pathophysiology.
Until more definitive knowledge can be developed, continued assessment of immune activation and inflammation biomarkers as well as clinical follow-up of HCV/HIV patients treated with DAA therapy, including assessment of fibrosis progression and HCC surveillance, would appear to be warranted.
Acknowledgement: All contributors who do not meet the criteria for authorship should be listed in this section.
Author Contribution: SM, FM and AB contributed to drafting the work, revised the final manuscript, and approved submission.
Availability of Data and Materials: No data or materials were generated from this study.
Ethics Approval: There were no experiments involving humans, animals, or plants in this study.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: No competing financial interests exist.
References
Brochado-Kith Ó, Martínez I, Berenguer J, González-García J, Salgüero S et al. (2021). HCV cure with direct-acting antivirals improves liver and immunological markers in HIV/HCV-coinfected patients. Frontiers in Immunology 12: 723196. DOI 10.3389/fimmu.2021.723196. [Google Scholar] [CrossRef]
Chaudhury CS, Sheehan J, Chairez C, Akoth E, Gross C et al. (2017). No improvement in hemoglobin A1c following hepatitis C viral clearance in patients with and without HIV. The Journal of Infectious Diseases 217: 47–50. DOI 10.1093/infdis/jix517. [Google Scholar] [CrossRef]
Chen JY, Feeney ER, Chung RT (2014). HCV and HIV co-infection: Mechanisms and management. Nature Reviews Gastroenterology and Hepatology 11: 362–371. DOI 10.1038/nrgastro.2014.17. [Google Scholar] [CrossRef]
Collins LF, Adekunle RO, Cartwright EJ (2019). Metabolic syndrome in HIV/HCV co-infected patients. Current Treatment Options in Infectious Diseases 11: 351–371. DOI 10.1007/s40506-019-00207-3. [Google Scholar] [CrossRef]
Emmanuel B, El-Kamary SS, Magder LS, Stafford KA, Charurat ME et al. (2019). Immunological recovery in T-cell activation after sustained virologic response among HIV positive and HIV negative chronic Hepatitis C patients. Hepatology International 13: 270–276. DOI 10.1007/s12072-019-09941-8. [Google Scholar] [CrossRef]
Fabbri G, Mastrorosa I, Vergori A, Mazzotta V, Pinnetti C, Grisetti S, Zaccarelli M, Ammassari A, Antinori A (2017). Reactivation of occult HBV infection in an HIV/HCV co-infected patient successfully treated with Sofosbuvir/Ledipasvir: A case report and review of the Literature. BMC Infectious Diseases 17: 182. DOI 10.1186/s12879-017-2287-y. [Google Scholar] [CrossRef]
Farcomeni S, Moretti S, Fimiani C, Sulekova LF, Vescio F et al. (2021). Short- and long-term immunological responses in chronic HCV/HIV co-infected compared to HCV mono-infected patients after DAA therapy. Pathogens 10: 1488. DOI 10.3390/pathogens10111488. [Google Scholar] [CrossRef]
Ganesan M, Poluektova LY, Kharbanda KK, Osna NA (2019). Human immunodeficiency virus and hepatotropic virus co-morbidities as the inducers of liver injury progression. World Journal of Gastroenterology 25: 398–410. DOI 10.3748/wjg.v25.i4.398. [Google Scholar] [CrossRef]
Gobran ST, Ancuta P, Shoukry NH (2021). A tale of two viruses: Immunological insights into HCV/HIV coinfection. Frontiers in Immunology 12: 26419. DOI 10.3389/fimmu.2021.726419. [Google Scholar] [CrossRef]
Gonzalez VD, Landay AL, Sandberg JK (2010). Innate immunity and chronic immune activation in HCV/HIV-1 co-infection. Clinical Immunology 135: 12–25. DOI 10.1016/j.clim.2009.12.005. [Google Scholar] [CrossRef]
Kim AY, Chung RT (2009). Coinfection with HIV-1 and HCV—A one-two punch. Gastroenterology 137: 795–814. DOI 10.1053/j.gastro.2009.06.040. [Google Scholar] [CrossRef]
Jeyarajan AJ, Chung RT (2020). Insights into the pathophysiology of liver disease in HCV/HIV: Does it end with HCV cure? Journal of Infectious Diseases 222: 802–813. DOI 10.1093/infdis/jiaa279. [Google Scholar] [CrossRef]
Lledo GM, Carrasco I, Benitez-Gutierrez LM, Arias A, Royuela A, Requena S, Cuervas-Mons V, de Mendoza C (2018). Regression of liver fibrosis after curing chronic hepatitis C with oral antivirals in patients with and without HIV coinfection. AIDS 32: 2347–2352. DOI 10.1097/QAD.0000000000001966. [Google Scholar] [CrossRef]
López-Cortés LF, Trujillo-Rodríguez M, Báez-Palomo A, Benmarzouk-Hidalgo OJ, Dominguez-Molina B, Milanés-Guisado Y, Espinosa N, Viciana P, Gutiérrez-Valencia A (2018). Eradication of hepatitis C virus (HCV) reduces immune activation, microbial translocation, and the HIV DNA level in HIV/HCV-coinfected patients. Journal of Infectious Diseases 218: 624–632. DOI 10.1093/infdis/jiy136. [Google Scholar] [CrossRef]
Macias J, Tellez F, Rivero-Juarez A, Palacios R, Morano LE et al. (2019a). Early emergence of opportunistic infections after starting direct-acting antiviral drugs in HIV/HCV-coinfected patients. Journal of Viral Hepatitis 26: 48–54. DOI 10.1111/jvh.13003. [Google Scholar] [CrossRef]
Macias J, Granados R, Tellez F, Merino D, Perez M et al. (2019b). Similar recovery of liver function after response to all-oral HCV therapy in patients with cirrhosis with and without HIV coinfection. Journal of Viral Hepatitis 26: 16–24. DOI 10.1111/jvh.12990. [Google Scholar] [CrossRef]
Mastroianni CM, Lichtner M, Mascia C, Zuccalà P, Vullo V (2014). Molecular mechanisms of liver fibrosis in HIV/HCV coinfection. International Journal of Molecular Sciences 15: 9184–9208. DOI 10.3390/ijms15069184. [Google Scholar] [CrossRef]
Najafi Fard S, Schietroma I, Corano Scheri G, Giustini N, Serafino S et al. (2018). Direct-acting antiviral therapy enhances total CD4+ and CD8+ T-cells responses, but does not alter T-cells activation among HCV mono-infected, and HCV/HIV-1 co-infected patients. Clinics and Research in Hepatology and Gastroenterology 42: 319–329. DOI 10.1016/j.clinre.2017.11.006. [Google Scholar] [CrossRef]
Navarro J, Laguno M, Vilchez HH, Guardiola JM, Carrion JA et al. (2017). Efficacy and safety of direct antiviral agents in a cohort of cirrhotic HCV/HIV-coinfected patients. Journal of Antimicrobial Chemotherapy 72: 2850–2856. DOI 10.1093/jac/dkx223. [Google Scholar] [CrossRef]
Parisi SG, Andreis S, Mengoli C, Menegotto N, Cavinato S, Scaggiante R, Andreoni M, Palù G, Basso M, Cattelan AM (2018). Soluble CD163 and soluble CD14 plasma levels but not cellular HIV-DNA decrease during successful interferon-free anti-HCV therapy in HIV-1-HCV co-infected patients on effective combined anti-HIV treatment. Medical Microbiology and Immunology 207: 183–194. DOI 10.1007/s00430-018-0538-1. [Google Scholar] [CrossRef]
Perez S, Kaspi A, Domovitz T, Davidovich A, Lavi-Itzkovitz A et al. (2019). Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PLoS Genetics 15: e1008181. DOI 10.1371/journal.pgen.1008181. [Google Scholar] [CrossRef]
Polyak SJ, Crispe IN, Baumert TF (2021). Liver abnormalities after elimination of HCV infection: Persistent epigenetic and immunological perturbations post-cure. Pathogens 10: 44. DOI 10.3390/pathogens10010044. [Google Scholar] [CrossRef]
Rial-Crestelo D, Sepúlveda MA, González-Gasca FJ, Geijo-Martínez P, Martínez-Alfaro E et al. (2020). Does fibrosis really regress in HIV/hepatitis C virus co-infected patients after treatment with direct antiviral agents? AIDS 34: 427–432. [Google Scholar]
Salmon-Ceron D, Nahon P, Layese R, Bourcier V, Sogni P et al. (2019). Human immunodeficiency Virus/Hepatitis C virus (HCV) co-infected patients with cirrhosis are no longer at higher risk for hepatocellular carcinoma or end-stage liver disease as compared to HCV mono-infected patients. Hepatology 70: 939–954. DOI 10.1002/hep.30400. [Google Scholar] [CrossRef]
Schwabl P, Mandorfer M, Steiner S, Scheiner B, Chromy D et al. (2017). Interferon-free regimens improve portal hypertension and histological necroinflammation in HIV/HCV patients with advanced liver disease. Alimentary Pharmacology & Therapeutics 45: 139–149. DOI 10.1111/apt.13844. [Google Scholar] [CrossRef]
Shrivastava S, Bhatta M, Ward H, Romani S, Lee R et al. (2018). Multitarget direct-acting antiviral therapy is associated with superior immunologic recovery in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology Communications 2: 1451–1466. DOI 10.1002/hep4.1258. [Google Scholar] [CrossRef]
Sikavi C, Chen PH, Lee AD, Saab EG, Choi G, Saab S (2018). Hepatitis C and human immunodeficiency virus coinfection in the era of direct-acting antiviral agents: No longer a difficult-to-treat population. Hepatology 67: 847–857. DOI 10.1002/hep.29642. [Google Scholar] [CrossRef]
Tumino N, Casetti R, Fabbri G, Cimini E, Romanelli A et al. (2017). In HIV/HCV co-infected patients T regulatory and myeloid-derived suppressor cells persist after successful treatment with directly acting antivirals. Journal of Hepatology 67: 422–424. DOI 10.1016/j.jhep.2017.03.036. [Google Scholar] [CrossRef]
Virseda-Berdices A, Rojo D, Martínez I, Berenguer J, González-García J et al. (2022). Metabolomic changes after DAAs therapy are related to the improvement of cirrhosis and inflammation in HIV/HCV-coinfected patients. Biomedicine & Pharmacotherapy 147: 112623. DOI 10.1016/j.biopha.2022.112623. [Google Scholar] [CrossRef]
Zeng H, Li L, Hou Z, Zhang Y, Tang Z, Liu S (2020). Direct-acting antiviral in the treatment of chronic hepatitis C: Bonuses and challenges. International Journal of Medical Sciences 17: 892–902. DOI 10.7150/ijms.43079. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools