 Open Access
Open Access
VIEWPOINT
Exosomes from adipose tissue-derived stem/stromal cells: A key to future regenerative medicine
1 University of Limoges, Neuropathies and Therapeutic Innovations (UR 20218), Faculties of Medicine and Pharmacy, Limoges, F-87000, France
2 Department of Maxillo-Facial and Reconstructive Surgery and Stomatology, University Hospital Dupuytren, Limoges, F-87000, France
* Corresponding Author: ALEXIS DESMOULIÈRE. Email:
(This article belongs to the Special Issue: Mesenchymal Stem Cells, Secretome and Biomaterials: Regenerative Medicine Application)
BIOCELL 2022, 46(12), 2701-2704. https://doi.org/10.32604/biocell.2022.022229
Received 28 February 2022; Accepted 20 May 2022; Issue published 10 August 2022
Abstract
Advances in regenerative medicine correlate strongly with progress in the use of adipose tissue-derived mesenchymal stem/stromal cells. The range of therapeutic indications has also expanded over recent years. Numerous recent studies have highlighted the primary importance of paracrine secretion by these cells. Though it is interesting to compare the different types of such secretions, we believe that exosomes (extra-cellular vesicles possessing the same properties as their source cells) will likely be the main key in tomorrow’s cell therapy. Exosomes also have many advantages compared to the direct use of cells, making these particles a major target in fundamental and translational research.Keywords
Mesenchymal stem/stromal cells (MSCs) have an extremely broad therapeutic potential and numerous applications have already emerged over the last decade. Correlated with improvements in extraction and isolation techniques for these cells, the clinical indications for their use have become more precise and more numerous. As adipose tissue constitutes a very important reservoir for MSCs (Zuk et al., 2002) and allows an easy extraction of MSCs, adipose tissue-derived (AD)-MSCs are considered as the cell of choice for regenerative medicine (Laloze et al., 2021). Indeed, they have similar anti-inflammatory properties than their counterparts from other tissues (such as bone marrow-derived- or umbilical cord-derived-MSCs) (Puissant et al., 2005; Yoo et al., 2009) making them the best candidate to treat diseases such as rheumatoid arthritis or systemic lupus erythematosus (Lipsky, 2001). They are also known for their high angiogenic potential which allows their use in various ischemic diseases such as diabetic foot (da Silva et al., 2017; da Silva et al., 2019).
In addition to their capacity to differentiate into mesodermal cells and their self-renewal, which are the main characteristics of these cells, they have paracrine properties which would be beneficial in wound healing, for example, but also in tissue bioengineering. Studies in the 2000s already showed the paracrine action of AD-MSCs which by secreting in particular interleukin-10 (IL-10), hepatocyte growth factor (HGF) and transforming growth factor (TGF)-beta inhibit the proliferation and secretion of pro-inflammatory cytokines by T lymphocytes (Puissant et al., 2005; Wolf and Wolf, 2008; Yañez et al., 2010) and induce a transition from the pro-inflammatory macrophage phenotype to an anti-inflammatory macrophage phenotype (Kim and Hematti, 2009; Manning et al., 2015).
The paracrine functions of AD-MSCs are exerted in a variety of ways: cell to cell contact, secretion of soluble factors into the interstitium or secretion of extracellular vesicles. This last mechanism includes microvesicles (>150 nm) and exosomes (<150 nm) (Galipeau and Sensébé, 2018). The latter particles have gained more and more attention in translational research over recent years as they have many advantages for clinical use (Hu et al., 2016).
Exosomes are ubiquitous structures that reproduce most of the functional effects of the cells from which they are derived (Le Lay et al., 2018) (Fig. 1). The main superiority of these particles is delivering higher levels of biomolecules leading to prolonged desired effect. In addition, exosomes show better homing to target tissue compared with parental cells. However, for optimal mass production of exosomes, it is necessary to have a reliable cell source (Mendt et al., 2019). Due to the advantages described above, AD-MSCs currently represent the most promising cell source (Yeo et al., 2013). Moreover, their small size avoids the problems of venous or arterial thrombosis (Cho et al., 2018).

Figure 1: Exosomes obtained from adipose tissue-derived mesenchymal stem/stromal cells (AD-MSCs) for cutaneous wound treatment. AD-MSCs are easy to isolate and they produce significant quantities of exosomes, permitting their allogeneic use and the creation of exosome banks (realized by using in part Servier Medical ART, https://smart.servier.com/).
Vizoso et al. (2017) has also demonstrated the possibility of modifying exosomes before their use in order to obtain specific desired effects on targeted cells according to the required effect or therapy (Vizoso et al., 2017). Additionally, it has been shown that the use of stem-cell-cultured conditioned media or exosomes was more economical and convenient than using cells since it avoids invasive cell transplantation procedures (Osugi et al., 2012; Bian et al., 2022). Thus, for all of the above-mentioned reasons, exosomes from AD-MSCs deserve the attention of researchers.
Moreover, numerous studies have already shown the efficacy of exosomes in pre-clinical models. Exosomes from AD-MSCs can stimulate cell migration, proliferation and collagen synthesis in fibroblasts, leading to accelerated wound healing in vivo (Hu et al., 2016). Thus, there seem to be several mechanisms through which they could potentially enhance wound repair. They can also improve lipofilling by increasing angiogenesis and the rate of fat resorption. Indeed, they are similar to AD-MSCs in terms of fat resorption, up-regulating early inflammation and angiogenesis (Chen et al., 2019). Recently, exosomes derived from hypoxia-treated AD-MSCs have shown great capacity to promote angiogenesis in lipofilling (Han et al., 2019). Li et al. (2018) recently showed in vitro that exosomes secreted by AD-MSCs induced endothelial progenitor cell proliferation and the overexpression of nuclear factor erythroid 2 related factor (Nrf2), showing protective effects in a rat model of diabetic foot ulcer (Li et al., 2018). They also promote increased collagen deposition in the late stage of wound healing in diabetic mice (Wang et al., 2020).
Lastly, they have been shown to be therapeutically effective in animal disease models, for example by exhibiting immunosuppressive activity against atopic dermatitis (Cho et al., 2018).
Exosomes derived from AD-MSCs possess all the characteristics to be one of the main therapeutic tools in regenerative medicine in the coming years. We therefore encourage the scientific community to concentrate their efforts on this subject. Indeed, some important aspects remain to be investigated.
One of the advantages of AD-MSCs is their heterogeneity, coming from multiple sources. They thus have different characteristics depending on their region of origin or even depending on the tissue layer from which they are collected (Vijay et al., 2020; Raajendiran et al., 2019). It therefore seems obvious that exosomes will not all possess the same properties and advantages. Comparative studies would therefore allow targeting their use according to the pathology.
It will also be important to compare the efficacy of exosomes to soluble secreted factors. It is known that trophic factors produced by these stem cells can promote growth and viability of adjacent cells. Exosomes are also able to support angiogenesis by secreting factors such as vascular endothelial growth factor (VEGF) and angiopoietin 1 (Sacchetti et al., 2007; Lin et al., 2012; Watt et al., 2013; Bortolotti et al., 2015). C-X-C motif chemokine ligand 12 (CXCL12) also named stromal cell-derived factor 1 (SDF-1) is also secreted by MSCs and allows the recruitment of other immune cells and progenitors to the site of injury (Oswald et al., 2004). The use of these soluble factors would be faster, easier and more cost effective; however, we believe that exosomes contain all the necessary materials to reproduce the same effects as the source cell.
The concept of tissue engineering in regenerative medicine integrates all the technologies using living cells or biomaterials (synthetic or natural), in order to reconstruct or regenerate human tissues and organs. MSC-based therapy combined with artificial scaffolds offers a promising strategy to promote wound healing or complete reconstruction of full-thickness skin. In this context, exosomes can be considered as a combined carrier and scaffold. Their natural biocompatibility and cell-targeting characteristics allow exosomes to transport drugs (Taverna et al., 2017). As Vizoso and his colleagues have previously shown (Vizoso et al., 2017), many recent studies have also revealed that some characteristics and contents of exosomes can be modified by other substances. Under particular culture conditions, exosomes can serve as stable and efficient vehicles to be loaded with specific proteins, lipids, and genetic material, including mRNAs, miRNAs, other small non-coding RNAs, and DNA (Bunggulawa et al., 2018). Recently, Shafei et al. (2020) used exosomes loaded in alginate gel as a bioactive scaffold in an in vivo study. They showed that this active dressing technique could significantly promote wound healing, collagen synthesis and local angiogenesis (Shafei et al., 2020).
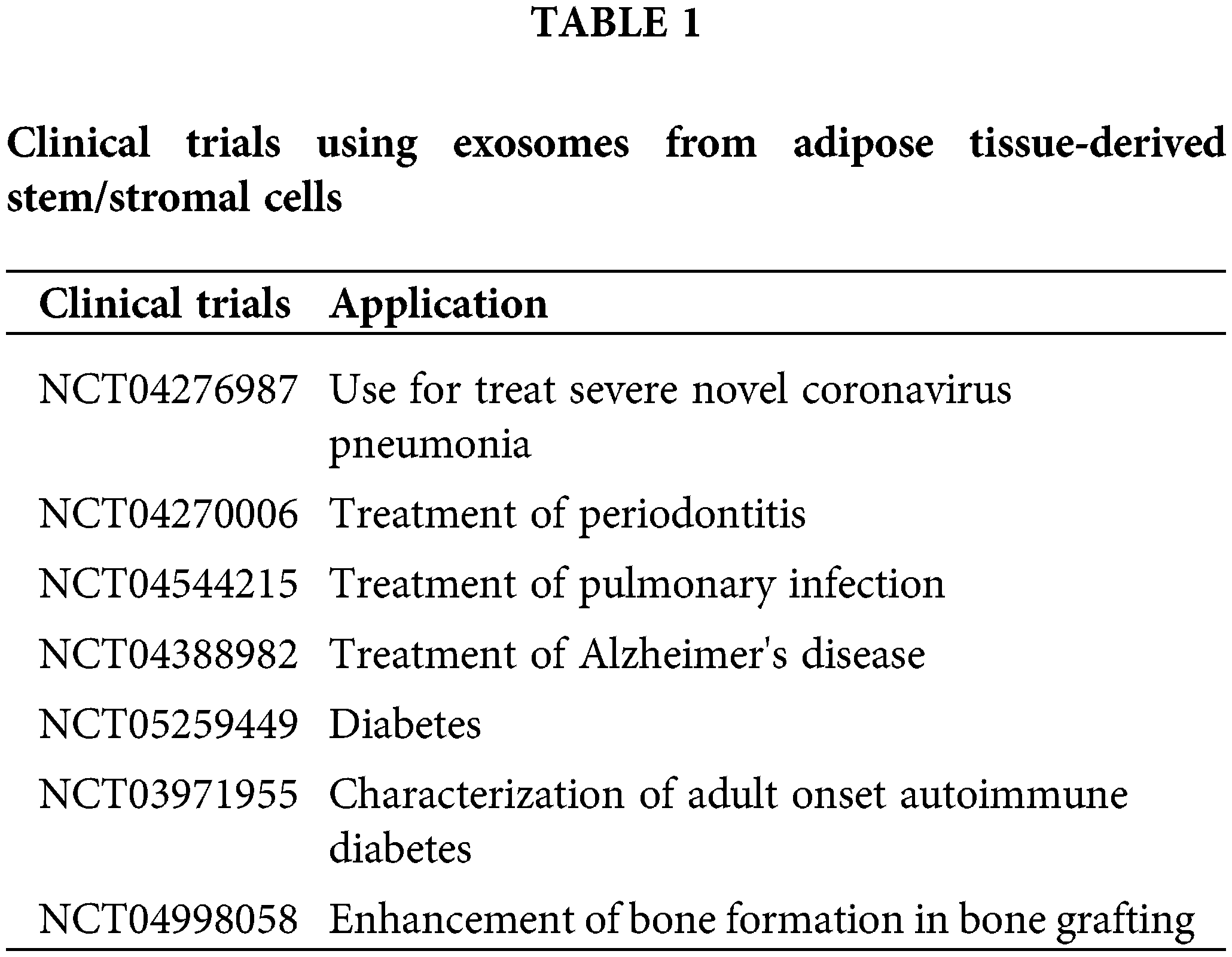
Finally, despite the reservations expressed by some (Rezabakhsh et al., 2021), the increasing number of clinical trials using exosomes (https://ClinicalTrials.gov) illustrates the safety and the potential efficacy of this type of treatment for treating diseases (Table 1).
Thus, we can conclude that exosomes represent a real challenge in basic research in order to benefit from their full potential. Numerous studies are still necessary to compare them to other actors but we see that exosomes are now a major key in regenerative medicine future.
Availability of Data and Materials: No data are included within this viewpoint.
Author Contribution: Both authors contributed equally for this viewpoint.
Ethics Approval: No ethics approval was required for this study.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Bian D, Wu Y, Song G, Azizi R, Zamani A (2022). The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: A comprehensive review. Stem Cell Research & Therapy 13: 24. DOI 10.1186/s13287-021-02697-9. [Google Scholar] [CrossRef]
Bortolotti F, Ukovich L, Razban V, Martinelli V, Ruozi G, Pelos B, Dore F, Giacca M, Zacchigna S (2015). In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure. Stem Cell Reports 4: 332–339. DOI 10.1016/j.stemcr.2015.01.001. [Google Scholar] [CrossRef]
Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, Wang G (2018). Recent advancements in the use of exosomes as drug delivery dystems. Journal of Nanobiotechnology 16: 81. DOI 10.1186/s12951-018-0403-9. [Google Scholar] [CrossRef]
Chen B, Cai J, Wei Y, Jiang Z, Desjardins HE et al. (2019). Exosomes are comparable to source adipose stem cells in fat graft retention with up-regulating early inflammation and angiogenesis. Plastic and Reconstructive Surgery 144: 816e–827e. DOI 10.1097/PRS.0000000000006175. [Google Scholar] [CrossRef]
Cho BS, Kim JO, Ha DH, Yi YW (2018). Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Research & Therapy 9: 187. DOI 10.1186/s13287-018-0939-5. [Google Scholar] [CrossRef]
da Silva LP, Reis RL, Correlo VM, Marques AP (2019). Hydrogel-based strategies to advance therapies for chronic skin wounds. Annual Review of Biomedical Engineering 21: 145–169. DOI 10.1146/annurev-bioeng-060418-052422. [Google Scholar] [CrossRef]
da Silva LP, Santos TC, Rodrigues DB, Pirraco RP, Cerqueira MT, Reis RL, Correlo VM, Marques AP (2017). Stem cell-containing hyaluronic acid-based spongy hydrogels for integrated diabetic wound healing. Journal of Investigative Dermatology 137: 1541–1551. DOI 10.1016/j.jid.2017.02.976. [Google Scholar] [CrossRef]
Galipeau J, Sensébé L (2018). Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22: 824–833. DOI 10.1016/j.stem.2018.05.004. [Google Scholar] [CrossRef]
Han Y, Ren J, Bai Y, Pei X, Han Y (2019). Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. The International Journal of Biochemistry & Cell Biology 109: 59–68. DOI 10.1016/j.biocel.2019.01.017. [Google Scholar] [CrossRef]
Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L (2016). Exosomes derived from human adipose mesenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Scientific Reports 6: 32993. DOI 10.1038/srep32993. [Google Scholar] [CrossRef]
Kim J, Hematti P (2009). Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Experimental Hematology 37: 1445–1453. DOI 10.1016/j.exphem.2009.09.004. [Google Scholar] [CrossRef]
Laloze J, Fiévet L, Desmoulière A (2021). Adipose-derived mesenchymal stromal cells in regenerative medicine: state of play, current clinical trials, and future prospects. Advances in Wound Care 10: 24–48. DOI 10.1089/wound.2020.1175. [Google Scholar] [CrossRef]
Le Lay S, Martinez MC, Andriantsitohaina R (2018). Extracellular vesicles as biomarkers and bioeffectors of metabolic syndrome. Médecine/Sciences 34: 936–943. DOI 10.1051/medsci/2018239. [Google Scholar] [CrossRef]
Li X, Xie X, Lian W, Shi R, Han S, Zhang H, Lu L, Li M (2018). Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Experimental & Molecular Medicine 50: 1–14. DOI 10.1038/s12276-018-0058-5. [Google Scholar] [CrossRef]
Lin RZ, Moreno-Luna R, Zhou B, Pu WT, Melero-Martin JM (2012). Equal modulation of endothelial cell function by four distinct tissue-specific mesenchymal stem cells. Angiogenesis 15: 443–455. DOI 10.1007/s10456-012-9272-2. [Google Scholar] [CrossRef]
Lipsky PE (2001). Systemic lupus erythematosus: An autoimmune disease of B cell hyperactivity. Nature Immunology 2: 764–766. DOI 10.1038/ni0901-764. [Google Scholar] [CrossRef]
Manning CN, Martel C, Sakiyama-Elbert SE, Silva MJ, Shah S, Gelberman RH, Thomopoulos S (2015). Adipose-derived mesenchymal stromal cells modulate tendon fibroblast responses to macrophage-induced inflammation in vitro. Stem Cell Research & Therapy 6: 74. DOI 10.1186/s13287-015-0059-4. [Google Scholar] [CrossRef]
Mendt M, Rezvani K, Shpall E (2019). Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplantation 54: 789–792. DOI 10.1038/s41409-019-0616-z. [Google Scholar] [CrossRef]
Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M (2012). Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Engineering Part A 18: 1479–1489. DOI 10.1089/ten.tea.2011.0325. [Google Scholar] [CrossRef]
Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C (2004). Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22: 377–384. DOI 10.1634/stemcells.22-3-377. [Google Scholar] [CrossRef]
Puissant B, Barreau C, Bourin P, Clavel C, Corre J et al. (2005). Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. British Journal of Haematology 129: 118–129. DOI 10.1111/j.1365-2141.2005.05409.x. [Google Scholar] [CrossRef]
Raajendiran A, Ooi G, Bayliss J, O’Brien PE, Schittenhelm RB, Clark AK, Taylor RE, Rodeheffer MS, Burton PR, Watt MJ (2019). Identification of metabolically distinct adipocyte progenitor cells in human adipose tissues. Cell Reports 27: 1528–1540.e7. DOI 10.1016/j.celrep.2019.04.010. [Google Scholar] [CrossRef]
Rezabakhsh A, Sokullu E, Rahbarghazi R (2021). Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Research & Therapy 12: 521. DOI 10.1186/s13287-021-02596-z. [Google Scholar] [CrossRef]
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S et al. (2007). Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131: 324–336. DOI 10.1016/j.cell.2007.08.025. [Google Scholar] [CrossRef]
Shafei S, Khanmohammadi M, Heidari R, Ghanbari H, Nooshabadi VT, Farzamfar S, Akbariqomi M, Sanikhani NS, Absalan M, Tavoosidana G (2020). Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: an in vivo study. Journal of Biomedical Materials Research Part A 108: 545–556. DOI 10.1002/jbm.a.36835. [Google Scholar] [CrossRef]
Taverna S, Pucci M, Alessandro R (2017). Extracellular vesicles: small bricks for tissue repair/regeneration. Annals of Translational Medicine 5: 83. DOI 10.21037/atm.2017.01.53. [Google Scholar] [CrossRef]
Vijay J, Gauthier MF, Biswell RL, Louiselle DA, Johnston JJ et al. (2020). Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nature Metabolism 2: 97–109. DOI 10.1038/s42255-019-0152-6. [Google Scholar] [CrossRef]
Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R (2017). Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. International Journal of Molecular Sciences 18: 1852. DOI 10.3390/ijms18091852. [Google Scholar] [CrossRef]
Wang J, Yi Y, Zhu Y, Wang Z, Wu S, Zhang J, Hu X, Nie J (2020). Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice. Chinese Journal of Reparative and Reconstructive Surgery 34: 124–131. [Google Scholar]
Watt SM, Gullo F, van der Garde M, Markeson D, Camicia R, Khoo CP, Zwaginga JJ (2013). The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. British Medical Bulletin 108: 25–53. DOI 10.1093/bmb/ldt031. [Google Scholar] [CrossRef]
Wolf D, Wolf AM (2008). Mesenchymal stem cells as cellular immunosuppressants. Lancet 371: 1553–1554. DOI 10.1016/S0140-6736(08)60666-2. [Google Scholar] [CrossRef]
Yañez R, Oviedo A, Aldea M, Bueren JA, Lamana ML (2010). Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Experimental Cell Research 316: 3109–3123. DOI 10.1016/j.yexcr.2010.08.008. [Google Scholar] [CrossRef]
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK (2013). Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Advanced Drug Delivery Reviews 65: 336–341. DOI 10.1016/j.addr.2012.07.001. [Google Scholar] [CrossRef]
Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS et al. (2009). Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunology 259: 150–156. DOI 10.1016/j.cellimm.2009.06.010. [Google Scholar] [CrossRef]
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002). Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell 13: 4279–4295. DOI 10.1091/mbc.e02-02-0105. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools