DOI:10.32604/biocell.2022.022015

| BIOCELL DOI:10.32604/biocell.2022.022015 |  |
| Article |
Es-SOX8 regulates the morphological changes of the sperm nucleus of Eriocheir sinensis by activating Es-BMP2 transcription
1The Sperm Laboratory, College of Life Sciences, Zhejiang University, Hangzhou, 310058, China
2The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, 310003, China
*Address correspondence to: Shuang-Li Hao, haosli0620761@zju.edu.cn; Wan-Xi Yang, wxyang@zju.edu.cn
Received: 18 February 2022; Accepted: 06 April 2022
Abstract: The SRY-related high mobility group (HMG) box (SOX) transcription factors participate in many physiological processes of animal growth, development, and reproduction and are related to spermatogenesis in many species. However, the relationship between SOX and spermatogenesis in Eriocheir sinensis is rarely reported. Here, we studied the role of Es-SOX8 in the spermatogenesis of E. sinensis and its possible regulation mechanism. Immunofluorescence results demonstrated Es-SOX8 signal in both cytoplasm and nucleus of spermatogonia, spermatocytes as well as spermatids, but not in mature spermatozoa. Hematoxylin and eosin staining showed a significant increase in the number of spermatozoa with abnormal nuclear morphology in vivo, described as prominent edges and corners, after the knockdown of Es-SOX8 through RNA interference. This indicated a possible role of Es-SOX8 in the nuclear deformation process in the spermatogenesis of E. sinensis. Analysis of the mRNA levels of Es-bone morphogenetic protein 2(BMP2) in the Es-Sox8 knocked-down testis tissue revealed significantly decreased transcription of Es-BMP2. Chromatin immunoprecipitation results showed the binding of Es-SOX8 protein to the promoter region of Es-BMP2. Thus, Es-SOX8 can directly regulate the transcription of Es-BMP2 by activating the promoter of Es-BMP2 and thus affects the sperm nucleus deformation of E. sinensis.
Keywords: SOX; Transcription factor; Spermatogenesis; BMP2
Spermatogenesis is the key to the normal maintenance of male reproductive ability (Satouh and Ikawa, 2018). This process includes mitosis, meiosis, and sperm deformation (Clermont, 1962; de Kretser et al., 1998; Staub and Johnson, 2018). Abnormality in any of these stages results in the obstruction of spermatogenesis or the production of abnormal sperm (Hann et al., 2011).
The process of spermatogenesis is highly complex and requires strict transcriptional regulation. During meiosis, the transcription rate reaches its peak in the late period of the pachytene stage (Eddy and O’Brien, 1998; Margolin et al., 2014; Cruz et al., 2016). Spermatogonia differentiation is regulated by several transcription factors. Stimulated by retinoic acid gene 8 is a transcription factor and a marker for meiotic initiation and is related to the stability of meiosis (Koubova et al., 2006; Bolcun-Filas et al., 2011). Yin Yang 1 works synergistically with polycomb repressive complex to maintain meiosis, which also involves other transcriptional activators and regulatory factors (Cheon et al., 2020).
The transcription factors, SRY-related high mobility group (HMG) box (SOX) proteins, are members of the SOX family and have HMG, a conserved region. SOX proteins act as key regulators that determine cell fate and participate in several life activities (Wegner, 1999; Sarkar and Hochedlinger, 2013; Grimm et al., 2020). These proteins perform various functions in processes such as gender differentiation, neural regulation, cartilage formation, and the development of different organs (Laudet et al., 1993; Whittington et al., 2015; Pennisi et al., 2000; Kamachi and Kondoh, 2013). The SOX family currently has more than 20 members. These 20 members are categorized into 10 subfamilies, many of which have been found to participate in spermatogenesis (Schepers et al., 2000; Bowles et al., 2000). For example, knockout of Sox3 causes the loss of differentiation ability of the spermatogonia, and stalls spermatogenesis (Laronda and Jameson, 2011; Raverot et al., 2005; McAninch et al., 2020). Testis-specific protein SOX30 is a key factor for gene expression after meiosis (Bai et al., 2019). SOX30 also cooperates with histone deacetylase 3 to regulate the acetylation of proteins in the later stages of spermatogenesis (Yin et al., 2021).
Bone morphogenetic protein 2 (BMP2) is a member of the transforming growth factorβ superfamily and it dominates bone morphogenesis. BMP8A regulates the differentiation of spermatogonia in mice through Suppressor of Mothers against Decapentaplegic (SMAD)1/5/8, and its loss can lead to increased germ cell apoptosis in adult mice (Zhao et al., 1998; Wu et al., 2017). Likewise, the lack of BMP8B results in the loss of germ cells in mice after birth (Zhao and Hogan, 1996). Sperm counts are severely reduced in heterozygous mice with BMP4 mutations (Hu et al., 2004). In E riocheir sinensis (Chinese mitten crab), BMP2 is involved in maintaining the normal progression of spermatogenesis and the morphology of sperm nuclei (Yang et al., 2020).
The SOX family has a certain regulatory relationship with the BMP family. In the mouse fibroblast C3H10T1/2, BMP2 is the upstream regulatory factor of SOX9 (Zehentner et al., 1999). BMP7 up-regulates the expression of SOX8, 9, and 10 in chicken embryo finger cartilage (Chimal-Monroy et al., 2003). While in the chicken pyloric sphincter, Sox9 expression is regulated by BMP signaling (Theodosiou and Tabin, 2005). However, in human nasopharyngeal carcinoma cell lines, SOX9 acts as the upstream transcription factor of BMP2 to directly activate its transcription (Xiao et al., 2019).
E. sinensis is a commonly used species in molecular studies of crustaceans and an economically important aquaculture item in China. Studying its gametes is highly important to understand its reproductive characteristics and population control. Our understanding of SOX8 comes from its role in cancer and its relationship with gender differentiation (Koopman, 2005; Kennedy et al., 2007; Turnescu et al., 2018; Xie et al., 2018; Portnoi et al., 2018; Tang et al., 2019). Some studies have shown that SOX8 also plays an indispensable role in spermatogenesis in mice. For example, Sox8-knockout mice have reduced sperm counts and disordered germ cell positioning (O’Bryan et al., 2008; Kataruka et al., 2017).
At present, our knowledge about the role of SOX proteins in the spermatogenesis of E. sinensis is very limited. We speculated a possible regulatory relationship between Es-SOX8 and Es-BMP2. Therefore, this study aimed to explore the function of Es-Sox8 in the testis of E. sinensis and study the possible mechanism of its spermatogenesis regulation.
The experimental animals were Institute of Cancer Research (ICR) mice and mature male E. sinensis. The mice were used to prepare antibodies. Three-week-old female ICR mice were purchased from Shanghai Slack Company provided through the Experimental Animal Center of Zhejiang University and raised in the Experimental Animal Center of Zhejiang University. E. sinensis were used for RNA and protein extraction as well as knockdown experiments in vivo. E. sinensis were purchased from Luojiazhuang Farmers Market in Hangzhou City, Zhejiang Province, and Chongming Breeding Base of Shanghai Ocean University. One group included six animals. All the experimental groups were kept in an aquarium at a constant temperature. Air, through pumps, and food were provided to ensure proper living conditions.
Total RNA extraction and reverse transcription
E. sinensis tissue was mixed with 1 mL RNAisoPlus (Takara, Dalian, China), homogenized in an ice bath for 2 min, and then kept in the ice bath for 10 min to lyse the tissue fully. Two hundred microliters of chloroform was added to the centrifuge tube of the previous step and vigorously shaken until it was milky white. These samples were kept at room temperature for 5 min to achieve full precipitation. The centrifuge tube was centrifuged at 12000 g under 4°C for 10 min; the solution in the centrifuge tubes displayed three layers. The top layer of colorless and transparent solution was then transferred to a new 1.5 mL centrifuge tube. An equal volume of isopropanol was added to the supernatant of the previous step, and the sample was gently mixed and allowed to settle at room temperature for 15 min. The tube was centrifuged at 12000 g and 4°C for 15 min. At this time, a white RNA precipitate could be seen at the bottom of the centrifuge tube. The supernatant was cautiously discarded to avoid touching the precipitate. Then, 1 mL of 75% ethanol was added to wash the precipitate twice. The sample was centrifuged at 5000 g under 4°C for 5 min. The pellet was dried at room temperature for 2 min. An appropriate amount of RNase-free ddH2O was added to the tube to dissolve the precipitate. For reverse transcription, PrimeScript™ RTMasterMix kit (Takara, Dalian, China) was used to obtain cDNA.
Cloning of Es-Sox8 in Eriocheir sinensis
We used the Primer-Blast tool in NCBI (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) to design the primers based on the predicted sequence in the transcriptome database. The CDS region was then cloned in two stages. The primers and their sequences are listed in Table 1.
For CDS cloning, 2 × Flash Hot Start MasterMix (CoWin Biosciences, Jiangsu, China) was used. The operation is carried out according to the manual. For the polymerase chain reaction (PCR) template, reversed transcribed E. sinesis testis RNA was used. The amplification procedure was as follows: 34 cycles of 98°C for 5 s, 55°C for 10 s and 72°C for 15 s. The PCR products were sent to the Hzykang company (Hangzhou, China) for sequencing. The sequencing results were compared with the transcriptome data in the Vector NT1.
Semi-quantitative real-time PCR (RT-PCR)
Tissue mRNA was extracted from the testis, seminal vesicle, epididymis, heart, muscle, intestine, hepatopancreas, and gills of E. sinensis. RNA from the testis tissue of E. sinensis was extracted from June to October. The extracted RNA was used to prepare cDNA.
Es-Sox8 and β-actin primers were designed based on sequences available in NCBI (Table 1). To the PCR tubes, 2 × Flash Hot Start Master Mix (CoWin Biosciences, Jiangsu, China), cDNA templates of different tissues, and testis (sampled at different periods) were added. From the final PCR product, 5 μL was resolved on 1% agarose gel. Grayscale analysis was conducted by ImageJ. Plotting and t-test analysis were conducted by GraphPad Prism 8. The housekeeping gene β-actin was used for internal reference.
We submitted the protein sequence of Es-SOX8 to the website (http://crdd.osdd.Net/raghava/bcepred/bcepred_submission.html) to determine the antigen-binding site, selected fragments containing the maximum antigen-binding sites, and designed primers based on the Primer-Blast tool on NCBI (Table 1). Then, BamH I and EcoR I restriction sites and protective bases were added at 5’end of the primers. For PCR, 2 × Flash Hot Start Master Mix (CoWin Biosciences, Jiangsu, China) was used. The PCR products were purified using a gel cutting recovery kit (Sangon, Shanghai, China). Then the purified product was ligated to the pMD™ 19-T Vector (Takara, Dalian, China), and the final plasmid was transfected into Top10 E. coli. After colony PCR, the confirmed sample was sent to the Hzykang Company (Hangzhou, China) for sequencing. Then, the sequence in Vector NT1 was analyzed, and the extracted plasmid was double digested with BamH I and EcoR I enzymes at 37°C for 4 h. Simultaneously, the pET-28a plasmid was digested with the same enzymes under the same conditions. The desired bands of the digestion product were sliced from the gel and purified. The purified target fragment and pET-28a vector were ligated using the T4 ligase. The ligated plasmid was transformed into E. coli BL21 to express polypeptide fragments carrying the 6 × His tag. When the OD 600 value of the bacterial solution reached 0.4~0.6, 0.5 mM IPTG was added to induce protein expression. A single target peptide was obtained after purification on a nickel column and injected into mice once a week for four consecutive weeks to produce antigens. Blood from the mouse eyeball was withdrawn on the 7th day after the last injection. This blood was centrifuged, and the serum was used as an antibody.
For protein extraction, different tissues of E. sinensis and testis at different developmental stages were used. The tissues were transferred to 1 mL RIPA (Beyotime, Shanghai, China) containing 10 μL phenylmethylsulfonyl fluoride (Sangon, Shanghai, China). Samples were placed on ice and ground with a homogenizer for 2 min. After grinding, the sample was centrifuged at 12000 g, 4°C for 10 min. The supernatant contained the protein solution. The entire supernatant was frozen at –80°C.
For polyacrylamide gel electrophoresis, a gel containing 12% acrylamide in the lower layer and 5% in the upper layer was prepared. Ten microliters of the protein was loaded per well and resolved for 40 min at 80 V and 100 min at 100 V. The protein on the gel was transferred to a polyvinylidene fluoride (PVDF) membrane using a transfer instrument. The PVDF membrane was exposed to a 200 mA constant current for 80 min. During the entire transfer process, the device was placed in an ice bath. The final PVDF membranes were blocked with 5% skimmed milk for 1 h. The primary antibody was incubated overnight at 4°C. The PVDF membranes were washed thrice with phosphate-buffered saline with Tween (PBST), incubated with a secondary antibody for 1 h at room temperature, and washed thrice. The gel was developed on a chemiluminescence instrument using the developer from Beyotime (Shanghai, China).
The testis tissue blocks embedded with OCT (SAKURA, Torrance, California, USA) were sliced as frozen sections (8 μm thickness). The slices were placed at room temperature for 10 min to thaw and then washed twice with 1×PBS, 2 min each time, followed by permeabilization with PBST for 15 min. The slices were washed again with 1×PBS thrice, 5 min each time. Then 100 μL of blocking solution was added to the surface of the slice, placed in a wet box, and sealed at room temperature for 1 h. The primary antibody, diluted in the blocking solution, was added to the surface of the slice and incubated overnight at 4°C. The slices were then washed thrice with PBST, and the secondary antibody was added and kept for 1 h incubation at room temperature. From this step onwards, the samples were handled in the dark to prevent fluorescence quenching. The samples were washed thrice with PBST, and 100 μL 4’,6-diamidino-2-phenylindole (DAPI) was added to the surface of the slice to stain the nuclei and kept for 5 min at room temperature. Later 10 μL anti-fluorescence quencher was added, slides were covered with a cover glass and nail polish and stored at 4°C. The processed slices were scanned using a confocal microscope (FV3000; Olympus).
In vivo RNA interference (RNAi) assay
Es-Sox8 and GFP primers were designed based on sequences available on NCBI (Table 1), followed by the addition of XbaI and KpnI restriction sites and protective bases to the 5’end of the primers. For the PCR reaction, 2×Flash Hot Start Master Mix (CoWin Biosciences, Jiangsu, China) was used. The products were purified using a gel recovery kit (Sangon, Shanghai, China) and ligated to pMD™19-T Vector (Takara, Dalian, China) overnight. This ligated plasmid was transformed into Top10 E. coli the next day. After colony PCR the samples were sent to Hzykang company (Hangzhou, China) for sequencing. The plasmid was extracted and double digested with Xba I and Kpn I enzymes at 37°C for 4 h. Simultaneously, the L4440 plasmid was digested with the same set of enzymes under the same conditions. The desired bands of the digestion product were resolved on a gel, selected, sliced, and purified. The target fragment and L4440 vector were ligated with the T4 enzyme and then transformed into HT115 competent cells. The dsRNA expression was induced by adding 0.5 mM IPTG when the OD 600 value of the bacterial culture reached 0.4~0.6.
We categorized E. sinensis into two groups, six in each group. One group was injected with Es-Sox8-dsRNA for the knockdown of Es-Sox8. The other group was injected with GFP-dsRNA as a control. Each crab was injected with 200 μg of dsRNA. The samples were injected into the body cavity from the base of the fourth foot. The injections were conducted every three days for a total of five injections. Tissue samples were taken on the third day after the last injection. The testis of each E. sinensis was divided into three parts for RNA extraction, protein extraction, and tissue embedding.
Hematoxylin-eosin (H&E) staining
Frozen sections were processed in sequence according to the following incubation steps: 100% alcohol (5 min), 100% alcohol (5 min), 95% alcohol (2 min), 90% alcohol (2 min), 80% alcohol (2 min), 70% alcohol (2 min), distilled water (2 min), hematoxylin (5 min), tap water rinse (3 times), hydrochloric acid (2 mL hydrochloric acid + 300 mL distilled water) (15 s), tap water rinse once, ammonia water (1.2 mL ammonia water + 300 mL distilled water) (10 s), tap water rinse (15 min), distilled water (2 min), 70% alcohol (2 min), 80% alcohol (2 min), 90% alcohol (2 min), eosin (5 min), 95% alcohol (2 min), 95% alcohol (2 min), 100% alcohol (5 min), 100% alcohol (5 min), xylene (5 min), xylene (5 min), quickly seal with neutral resin. The morphology of the nucleus and cytoplasm was observed under an optical microscope (Olympus).
Luciferase reporter gene assay
Primers were designed based on the sequences available on the NCBI website (Table 1), and the predicted Es-BMP2 promoter fragment was cloned using genomic DNA as a template. The predicted promoter sequence of Es-BMP2 was inserted in the PGL4.23 plasmid by restriction enzyme digestion. The EndoFree plasmids were extracted using the EndoFree Plasmid Midi Kit (CoWin Biosciences, Jiangsu, China). The plasmid was transferred into HEK 293 T cells using lipo8000 (Beyotime, Shanghai, China). The lysate was collected after 48 h, and the fluorescence intensity was detected by Multimode Plate Reader (Thermo Fisher, America).
Chromatin immunoprecipitation (ChIP) assay
The ChIP Assay Kit from Beyotime (Shanghai, China) was used according to the provided instructions. Testis of E. sinensis was ground in 1×PBS to obtain testis tissue cells. Formaldehyde was added to fix the tissue at 37°C for 10 min. Glycine Solution (1.1 mL; 10×) was added to stop the reaction. The collected cells were washed with PBS. Then, SDS Lysis buffer was added to lyse the cells. DNA was sheared by ultrasonic treatment (3 s each of on and off frequency processing) for 2 min.
After centrifugation, the supernatant was collected and ChIP Dilution buffer (1.8 mL) was added. From the centrifuge tube, 20 μL inputs were taken. Protein A + G Agarose/Salmon Sperm DNA (70 μL) was added, and the tube was rotated slowly at 4°C for 30 min to reduce non-specific binding. The supernatant was obtained after centrifugation. Serum antibody (10 μL) was added to the tube containing the supernatant and rotated slowly overnight at 4°C. Then, 60 μL Protein A + G agarose/salmon sperm DNA was added the next day, rotated at 4°C for 1 h, and centrifuged at 1000 g for 1 min.
After centrifugation, the precipitate was washed according to the instructions. The solutions at each step were rotated at 4°C for 5 min and finally centrifuged at 1000 g at 4°C for 1 min.
Then 250 μL of Elution buffer (containing 1% SDS and 0.1 M NaHCO3) was added to the precipitate and the tube was rotated for 5 min. Finally, this was centrifuged at 1000 g for 1 min. The supernatant was transferred to a 2 mL centrifuge tube. Then, the precipitate was washed with 250 μL Elution buffer. Twenty microliters of 5 M NaCl was added to about 500 μL of supernatant and the samples were heated at 65°C for 4 h. Finally, EDTA (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), Tris (Sangon, Shanghai, China), and proteinase K (Sangon, Shanghai, China) were added. After mixing, the samples were incubated at 45°C for 60 min. The purified products were obtained using a DNA purification kit (Sangon, Shanghai, China).
We designed the primers for the Es-BMP2 promotor based on sequences available on NCBI (Table 1) and 2 × Flash Hot Start Master Mix (CoWin Biosciences, Jiangsu, China) was used for PCR. ChIP product was used as a template.
Western blot and RT-PCR results were analyzed under grayscale by ImageJ software. At least three experimental data were analyzed by Graphpad Prism 8 software. Unpaired two-tailed Student’s t-test was used to perform statistical analysis on the means of biological replicates between two groups. One-way ANOVA was used to analyze the difference among multiple groups. Results were considered statistically significant if p-value < 0.05 (*p < 0.05, ** for p < 0.01, *** for p < 0.001).
Identification and characterization of Es-Sox8 from E. sinensis
The full-length CDS sequence of Es-Sox8 was estimated to be 1506 bp, with a predicted molecular weight of the Es-SOX8 protein being 53.51 kDa, containing 501 amino acid residues and a theoretical isoelectric point of 8.62 (Fig. S1). The sequence has been uploaded to GenBank (sequence number MZ542346).
Similar to SOX8 of other species, Es-SOX8 contained an HMG domain (161AA-231AA) and no other apparent domains. The three-stage structure was predicted using ZHANG-Lab (https://zhanggroup.org//I-TASSER/), and the Es-SOX8 protein was found to possess 5 α spirals, with curled structures in the remaining protein (Fig. S1).
The Es-SOX8 protein sequences of E. sinensis were compared with the SOXE protein sequences of other species; SOX8, SOX9, and SOX10 of the SOXE subfamily showed a high degree of homology (Figs. S1 and S2). We selected 18 species of the SOXE family for sequence alignment, homology analysis, and construction of a phylogenetic tree (Figs. S1 and S2). Consistent with the characteristics of the SOX family, the Es-SOX8 protein of the E. sinensis was highly conserved in the HMG domain segment compared to other species.
The results showed that the Es-SOX8 of E. sinensis has the same evolutionary status as the SOX10 of Chinopecetes opilio and Portunus trituberculatus (80% and 78%, respectively), and the evolutionary status of SOX8-like protein of Penaeus vannamei and SOXE of Macrobrachium nipponense was similar (80% and 57%, respectively). The sequence numbers of SOX protein in GenBank used for sequence analysis were as follows: Portunus trituberculatus (XP_045138684.1), Chionoecetes opilio (KAG0714866.1), Penaeus vannamei (XP_027226033.1), Macrobrachium nipponense (QPC96191.1), Leptinotarsa decemlineata (XP_023023564.1), Diabrotica virgifera virgifera (XP_028134523.1), Tribolium castaneum (XP_015833813.1), Sitophilus oryzae (XP_030765604.1), Dendroctonus ponderosae (XP_019755831.1), Aethina tumida (XP_019876895.1), Nicrophorus vespilloides (XP_017780683.1), Microplitis demolitor (XP_008559245.1), Bemisia tabaci (XP_018898911.1), Danio rerio (AY883015.1), Homo sapiens (NP_055402.2), and Mus musculus (NP_035577.1).
Distribution of Es-Sox8 and the protein in the testis of E. sinensis
We detected the expression level of Es-Sox8 in the testis, seminal vesicles, epididymis, liver, heart, and gills of E. sinensis. Es-Sox8 was the highest in the gills, and the levels in the reproductive system, such as testis, seminal vesicles, and the epididymis were relatively low (Figs. 1A and 1B).
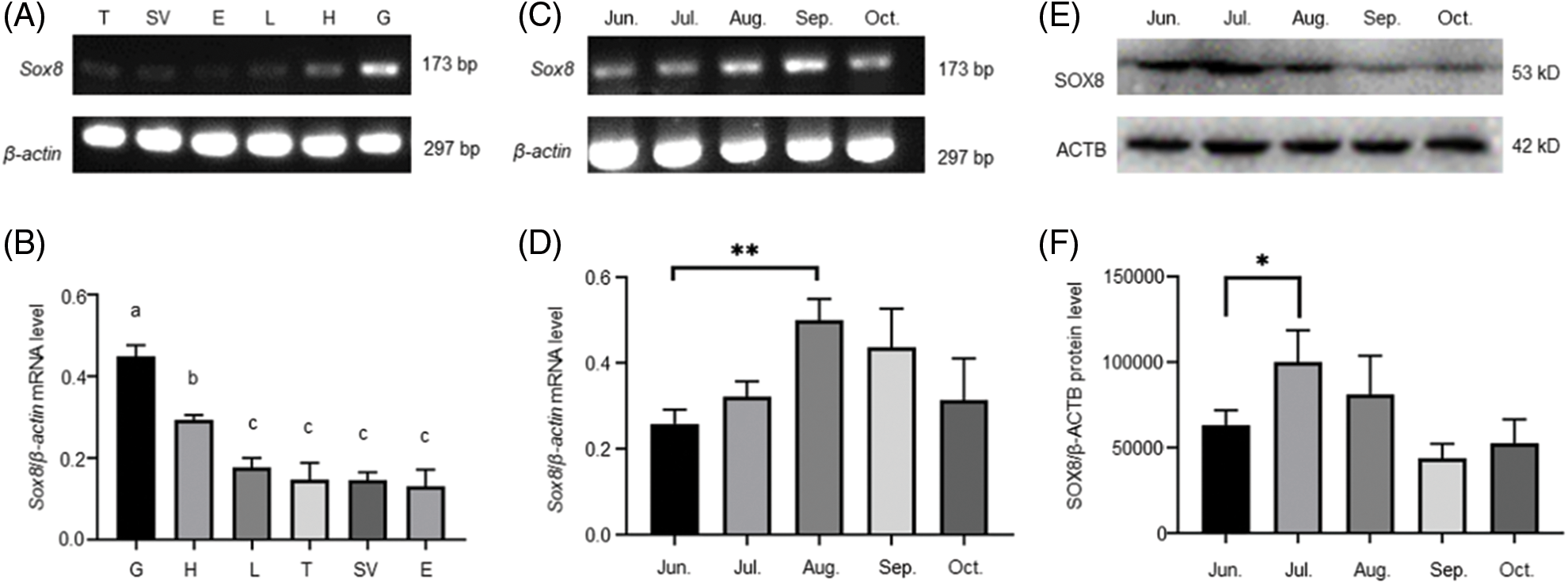
Figure 1: Transcription levels of Es-Sox8 in different tissues and the different stages of testis development in E. sinensis. (A) and (B) Semi-quantitative PCR of Es-Sox8 in different tissues, with β-actin as the internal control. Six types of tissues from E. sinensis were selected. T: testis; SV: seminal vesicles; E: epididymis; L: liver, H: heart; G: gills. (C) and (D) Semi-quantitative PCR of Es-Sox8 with β-actin as the internal control. (E) and (F) Western blot result of Es-SOX8, with ACTB as the internal control. Different letters (a, b, c) on the histogram mean significant differences between groups. * p < 0.05, ** p < 0.01, *** p < 0.001.
The development of E. sinensis testis follows an annual seasonal cycle, from May to December each year. Spermatogonia develop from May to June. In this period, spermatogonia dominate in the testis. The spermatocytes predominate from July to August, when there are a high number of spermatocytes in the seminiferous tubules. The sperm cell stage occurs from September to October, when the number of spermatocytes in the germinal area is only a few, with a high number of sperm cells. The sperm period is from November to December when the germinal areas begin to shrink, and the seminiferous tubules are filled with mature sperm.
The transcription level of Es-Sox8 in the testis of E. sinensis from June to October was determined by RT-PCR. The Es-Sox8 mRNA was expressed during all of this months. The expression level of Es-Sox8 increased from July onwards and decreased from September, but overall, the expression became stable (Figs. 1C and 1D).
Western blotting results showed that the protein level of Es-SOX8 peaked in July, which was significantly higher than that in June (Figs. 1E and 1F). The protein expression was lower in the following months, but the difference was not significant, consistent with the trend of mRNA expression. In July, the spermatocyte stage prevails and Es-SOX8 may begin to participate in the differentiation of spermatogonia into spermatocytes. Low protein expression in September and October suggests that Es-SOX8 may have a limited role in the mature sperm of E. sinensis.
Localization of Es-SOX8 in the testis of E. sinensis at different stages
The localization of Es-SOX8 in the testis tissue of E. sinensis was detected by immunofluorescence (IF) to understand the function of Es-SOX8 in different stages of spermatogenesis.
Signals of Es-SOX8 in E. sinensis were detected in the cytoplasm and the nucleus of immature germ cells, such as spermatogonia, spermatocytes, and round sperm cells (Fig. 2A). However, the fluorescent signal of Es-SOX8 was not detected in mature sperms (Fig. 2A), indicating that Es-SOX8 was likely to play an important role in the process of meiosis and sperm maturation (Fig. 2). This was consistent with the expression results of Es-SOX8 in the testis at different developmental stages. A negative control group without a primary antibody was also set up (Fig. 2D).
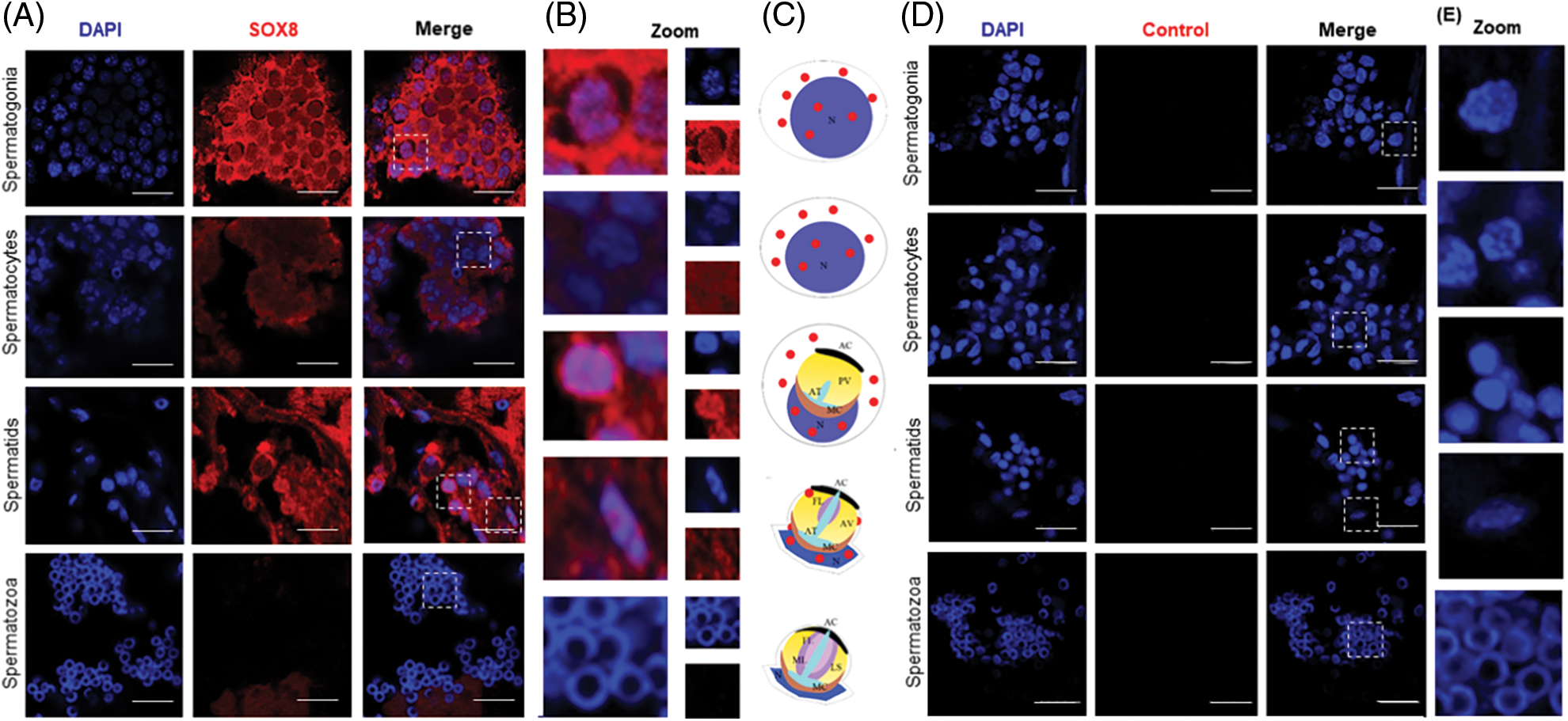
Figure 2: Localization of Es-SOX8 protein in the testis of E. sinensis. (A) Es-SOX8 localization in testis detected by immunofluorescence. The red signal represents the Es-SOX8 protein, and the blue signal represents the nucleus (DAPI-stained). The pictures from top to bottom are spermatogonia, spermatocytes, sperm cells, and mature spermatozoa. (B) Partially enlarged view of (A). (C) Schematic diagram of the location of Es-SOX8 protein in the spermatogenesis of E. sinensis. (D) Negative control. Es-SOX8 is localized in the cytoplasm and nucleus in spermatogonia, spermatocytes, round sperm, and elongated sperm except mature sperm; however, no Es-SOX8 signal was detected in mature spermatozoa. N: nucleus, AC: acrosome cap, ML: middle layer, FL: fibrous layer, AT: acrosome tube, PV: proacrosomal vesicle, AV: acrosome vesicle, LS; lamellar structure, MC membrane complex. Bars = 20 μm.
To further understand the role of Es-Sox8 in E. sinensis, Es-Sox8-dsRNA was injected into adult living male E. sinensis, and knockdowns were established. RT-PCR results confirmed notably reduced Es-Sox8 mRNA expression and a significant decrease in protein levels (Fig. 3), indicating a successful knockdown of Es-Sox8.
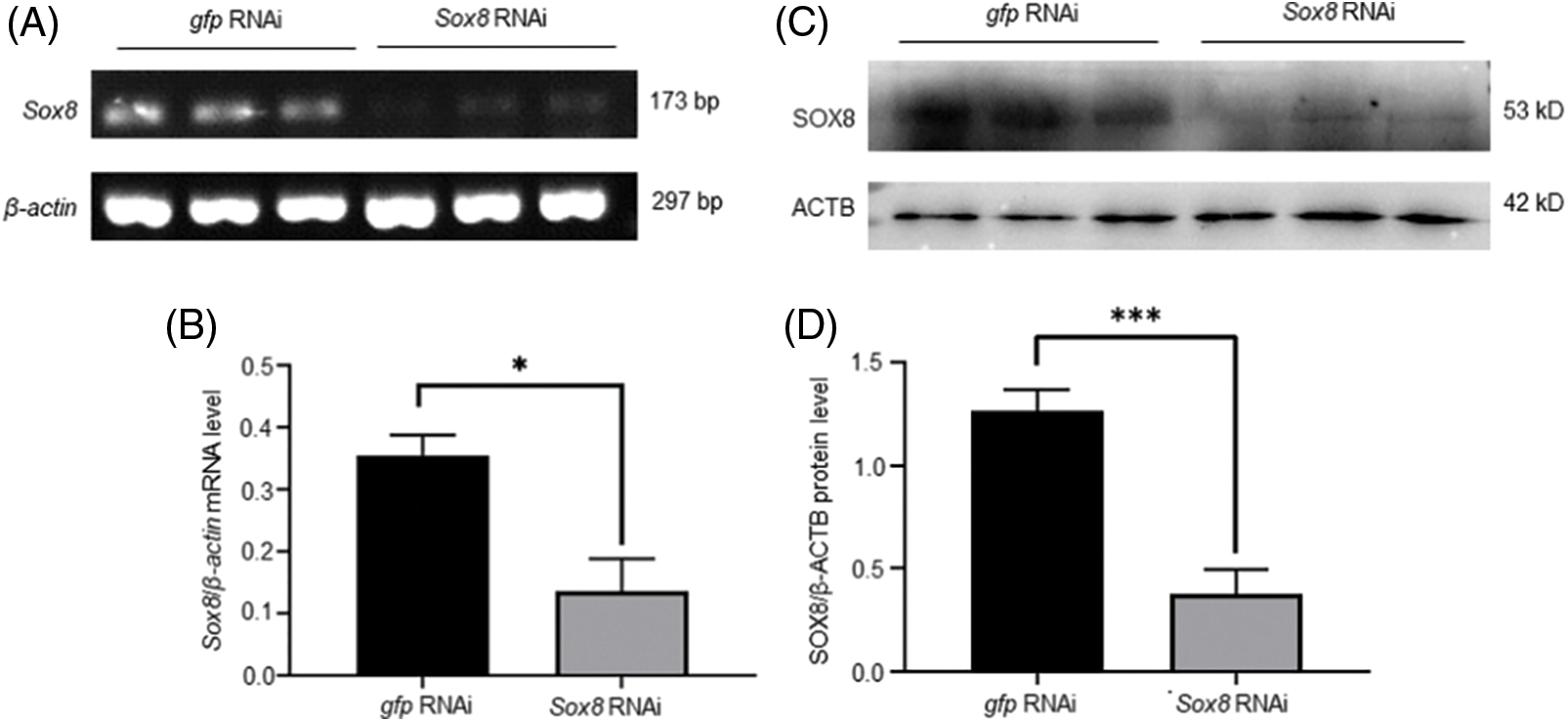
Figure 3: The transcript and protein levels of Es-Sox8 were significantly reduced in the testis of E. sinensis after Es-Sox8 knockdown treatment. (A) and (B) Semi-quantitative PCR of Es-Sox8, control is the sample injected with GFP-dsRNA, and the treated group is the sample injected with Es-Sox8-dsRNA. (C) and (D) Western blot analyses of Es-SOX8 in Es-Sox8 RNAi-treated E. sinensis. An obvious decrease in the expression of Es-Sox8 and Es-SOX8 in the knockdown group indicated a successful knockdown operation. β-actin and ACTB were used as internal controls for mRNA and protein levels, respectively. * p < 0.05, ** p < 0.01, *** p < 0.001.
H&E staining of the sections of E. sinensis testis showed that after Es-Sox8 silencing, there were some morphological changes in germ cells (Fig. 4). In the control group, the sperm nucleus cup was round at the later stage of deformation, without obvious edges and protrusions, and the ring-shaped nucleus cup in the outer layer of the cell was observed in mature sperms (Figs. 4A–4C). These results indicate that the nuclei of the sperm cells in the treatment group were primarily abnormal (Figs. 4D–4F).
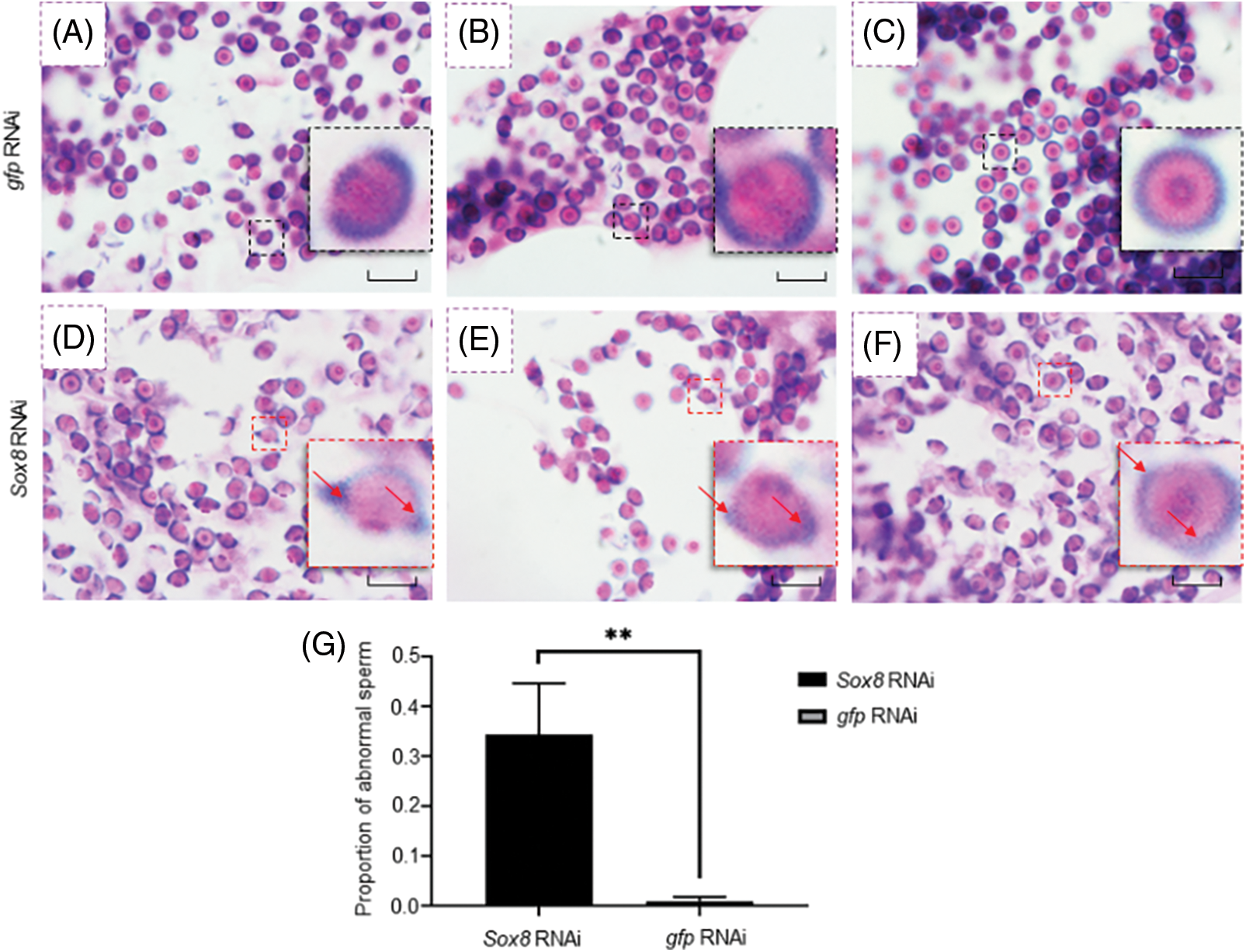
Figure 4: Es-Sox8 is involved in sperm nucleus deformation during spermatogenesis of E. sinensis. (A), (B), and (C) Negative control group treated with GFP-dsRNA. The inset images (black boxes) show a normal sperm shape, with a round and cup-shaped sperm nucleus. (D), (E), and (F) Experimental group treated with Es-Sox8-dsRNA. The inset images (red boxes) show abnormal sperm cells, with abnormal morphology of the nucleus and prominent edges and corners (pointed by the red arrow). (G) Analysis of the proportion of abnormal sperm. We used GraphPad Prism8 software to perform t detection analysis. * p < 0.05, ** p < 0.01, *** p < 0.001. Bars = 20 μm.
Statistical analysis showed a significant increase in the percentage of abnormal spermatids in the Es-Sox8 knockdown group (Fig. 4G). This indicated that Es-Sox8 likely assists the process of sperm nucleus deformation in E. sinensis.
Knockdown of Es-Sox8 in vivo changes the distribution of lamin
To further confirm that nuclear deformation was hindered, we assessed the distribution of lamin protein in the testis of E. sinensis after Es-Sox8 knockdown. Lamin is an important component of the nuclear skeleton (de Leeuw et al., 2018). Immunofluorescence results showed a tight distribution of the lamin proteins near the nuclear membrane in the normal E. sinensis testis cells. Lamin could be found around the nucleus of spermatogonia, spermatocytes, and round spermatids. The signal of lamin diminished when the sperm nucleus began to deform and disappeared when the sperm matured (Fig. 5A). In the Es-Sox8-RNAi group, lamin distribution was not orderly around the nucleus; instead, it spread throughout the cells in an unpredictable, stochastic manner. This phenomenon was especially severe during the spermatocyte and round sperm stages. Within the sperm cells of the metamorphic stage, a high deposition of lamin residues was observed, which still existed in a loose state. Weak lamin signals could still be detected even in the mature spermatozoa (Fig. 5B). Thus, the nuclear deformation was kinetically blocked after disturbing Es-Sox8 expression.
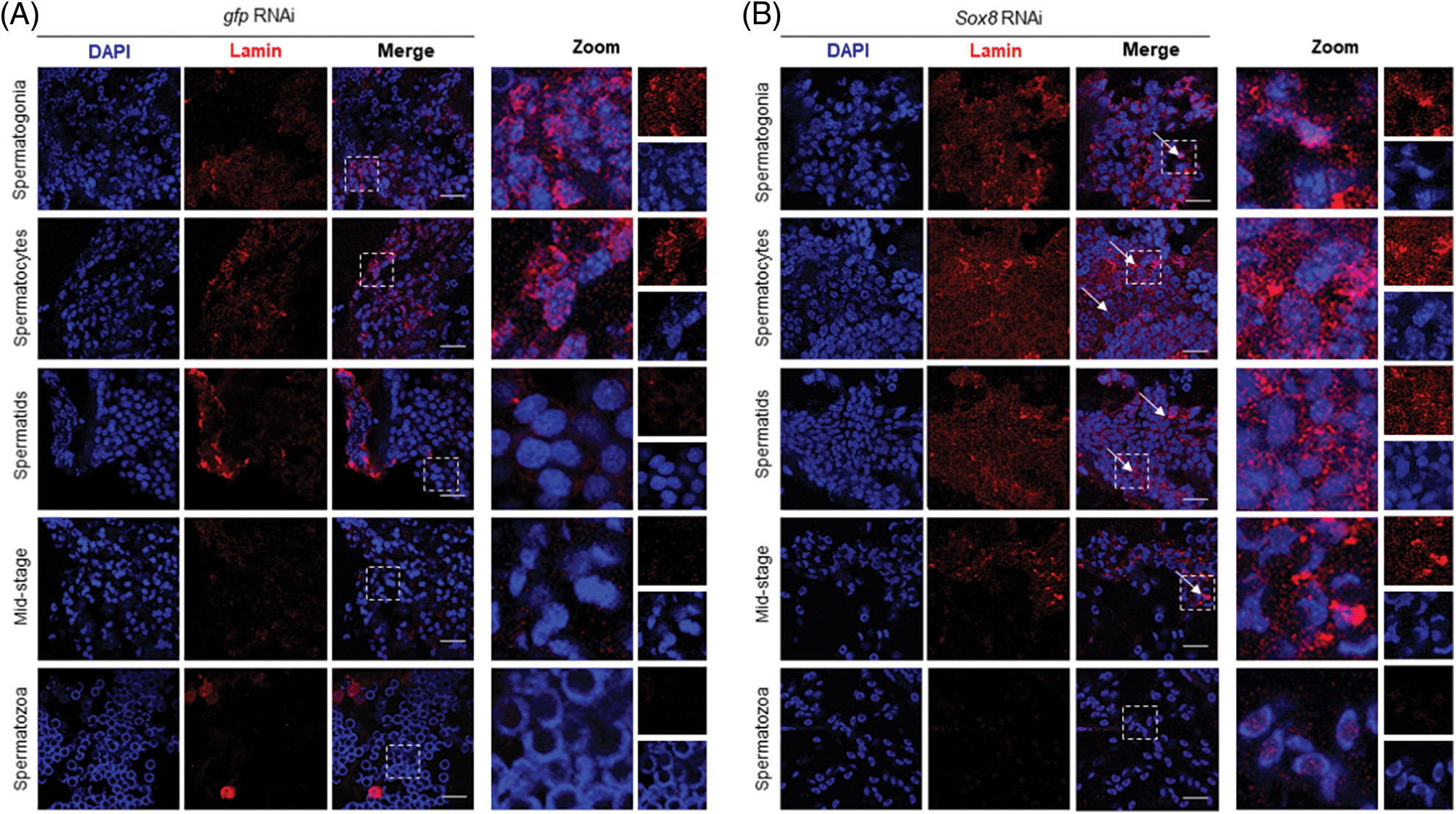
Figure 5: Lamin immunofluorescence in the Es-Sox8 knockdown group and GFP-dsRNA treated Eriocheir sinensi. (A) Control group. (B) Es-Sox8 knockdown group. The red signal represents lamin. The blue signal (DAPI) represents the nucleus. White arrows indicate abnormally localized lamin. Lamin localization was chaotic and irregular in Es-Sox8-dsRNA-treated tissues than in controls. Bars = 20 μm.
Es-SOX8 may regulate spermatogenesis by regulating Es-BMP2
Es-BMP2 has a role in the nuclear morphogenesis of E. sinensis (Yang et al., 2020). SOX can regulate the transcription of Es-BMP2; therefore, we presumed that Es-BMP2 was also a downstream target gene of Es-SOX8. RT-PCR results revealed that the transcription level of Es-BMP2 in the knockdown group was apparently lower than in the GFP-dsRNA group, showing that knockdown of Es-Sox8 indeed decreased Es-BMP2 transcription (Figs. 6A and 6B).
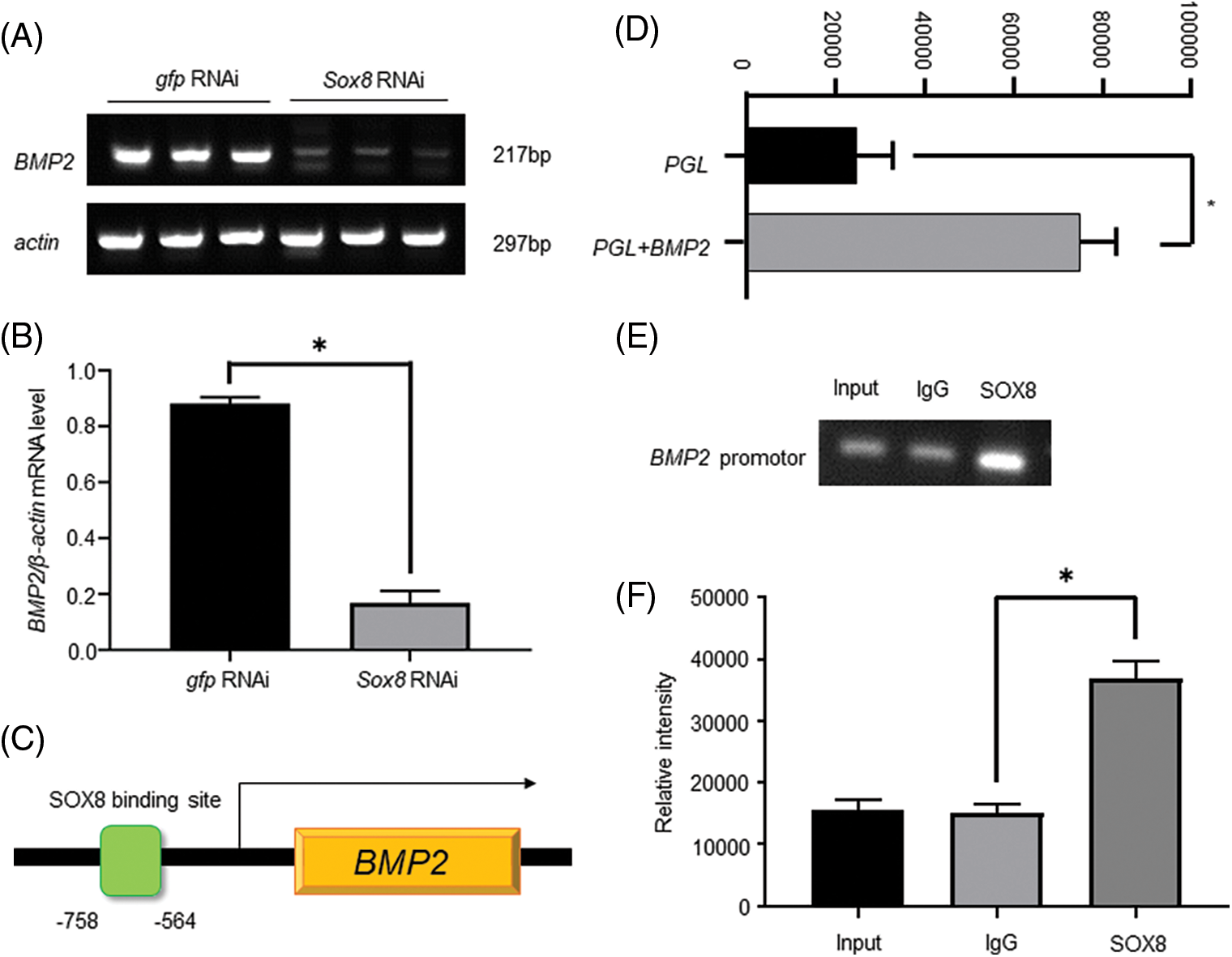
Figure 6: Es-SOX8 directly regulates Es-BMP2 transcription. (A) and (B) Es-BMP2 RT-PCR after knocking down Es-Sox8. The control group was injected with GFP-dsRNA, and the knockdown group was injected with Es-Sox8-dsRNA. (C) A model showing the binding of Es-SOX8 to the Es-BMP2 promoter. The green box represents the binding of Es-SOX8 to the Es-BMP2 promoter site. The orange box represents the coding region of Es-BMP2. (D) Es-BMP2 promoter activity. (E) and (F) Es-BMP2 promoter PCR after chromatin immunoprecipitation experiment. GraphPad Prism8 software was used for t detection analysis. * p < 0.05, ** p < 0.01, *** p < 0.001.
We determined the SOX binding site in the Es-BMP2 promoter segment based on the genome sequence of Es-BMP2 available in the NCBI database and the online prediction results. The reporter assay using the Es-SOX8-binding fragment in Es-BMP2 in a luciferase reporter plasmid demonstrated promoter activity of this fragmen (Fig. 6D).
ChIP analysis using the Es-SOX8 antibody explored whether there was an Es-BMP2 promoter in the nucleic acid sequence bound by the Es-SOX8 protein. For the PCR, a pull-down DNA purification product was used as a template, and the primers of the Es-SOX8 binding segment on the Es-BMP2 promoter are listed in Table 1. In the Es-SOX8 antibody-treated group, a clearly clustered Es-BMP2 promoter was observed (Figs. 6E and 6F). The results reveal that the control group had certain false positives indicating non-specific binding, whereas the Es-SOX8 antibody-treated group produced a far greater number of products than the control group. These findings indicate that the nucleic acid bound by Es-SOX8 contained the Es-BMP2 promoter segment (Fig. 6).
Es-Sox8 regulates sperm nuclear deformation of E. sinensis
The normal progression of spermatogenesis is critical for male reproductive functioning. Several transcription factors, including members of the SOX family, are also key factors that regulate spermatogenesis. Different SOX proteins may be involved in different stages of spermatogenesis. SOX5 functions in the post-meiotic stage of male germ cells in mice (Connor et al., 1994; Zheng et al., 2021). The Sox18 mRNA is mainly expressed in spermatogonia of mice (Roumaud et al., 2018). SOX30 regulates the development of round sperm cells (Bai et al., 2019). Knock-out of some Sox genes hinders spermatogenesis. Particularly, spermatogonia lacking Sox3 lose their differentiation ability (Raverot et al., 2005; Laronda and Jameson, 2011; McAninch et al., 2020). The testis of severely damaged mice reveal the presence of only spermatogonia and supporting cells (Raverot et al., 2005). After Sox4 is knocked out, spermatogenesis in mice is also impaired (Zhao et al., 2017). Loss of Sox8 can cause infertility and spermatogenesis disorders in male mice (O’Bryan et al., 2008). In E. sinensis, SOXB2-1 expression in the testis is related to the formation of nuclear arms of spermatozoa (Liu et al., 2016). Based on these findings, we wanted to explore the relationship between Es-SOX8 and spermatogenesis in E. sinensis.
SOX8 has certain homology with other members in the SOXE family from other species. The evolutionary status of Es-SOX8 in E. sinensis is similar to that in other crustaceans. We found that Es-Sox8 may have a more important role in spermatogonia to spermatocytes differentiation.
In this study, we observed that Es-SOX8 protein was expressed in spermatogonia, spermatocytes, and spermatids. It can be detected in the whole cell, including the nucleus and the cytoplasm. However, no positive signal of Es-SOX8 was detected in mature sperm, indicating the role of Es-SOX8 in spermatogenesis rather than in subsequent steps such as acrosome reaction and fertilization. Based on the above observations, we concluded that Es-SOX8 participates in many life processes of mammals and is also indispensable in the spermatogenesis of E. sinensis.
The spermatogenesis of E. sinensis undergoes a process of sperm nucleus deformation in the later stage, wherein the shape of the nucleus turns from spherical to cup shape (Du et al., 1987; Du et al., 1988; Du, 1998). The process of sperm deformation is regulated by transcription factors, such as cyclic AMP element modulator and spermatogenesis and oogenesis bHLH transcription factor 1 (Martianov et al., 2010; Liu et al., 2021). Here, we found the role of the Es-SOX8 transcription factor in sperm nucleus formation in E. sinensis spermatogenesis. A large number of abnormal sperm were observed in the Es-Sox8 knockdown crab. Unlike the rounded nuclear cup of mature sperm in the control group, the sperm nuclear cup in the treatment group was severely deformed, with sharp corners or protrusions (Fig. 7).
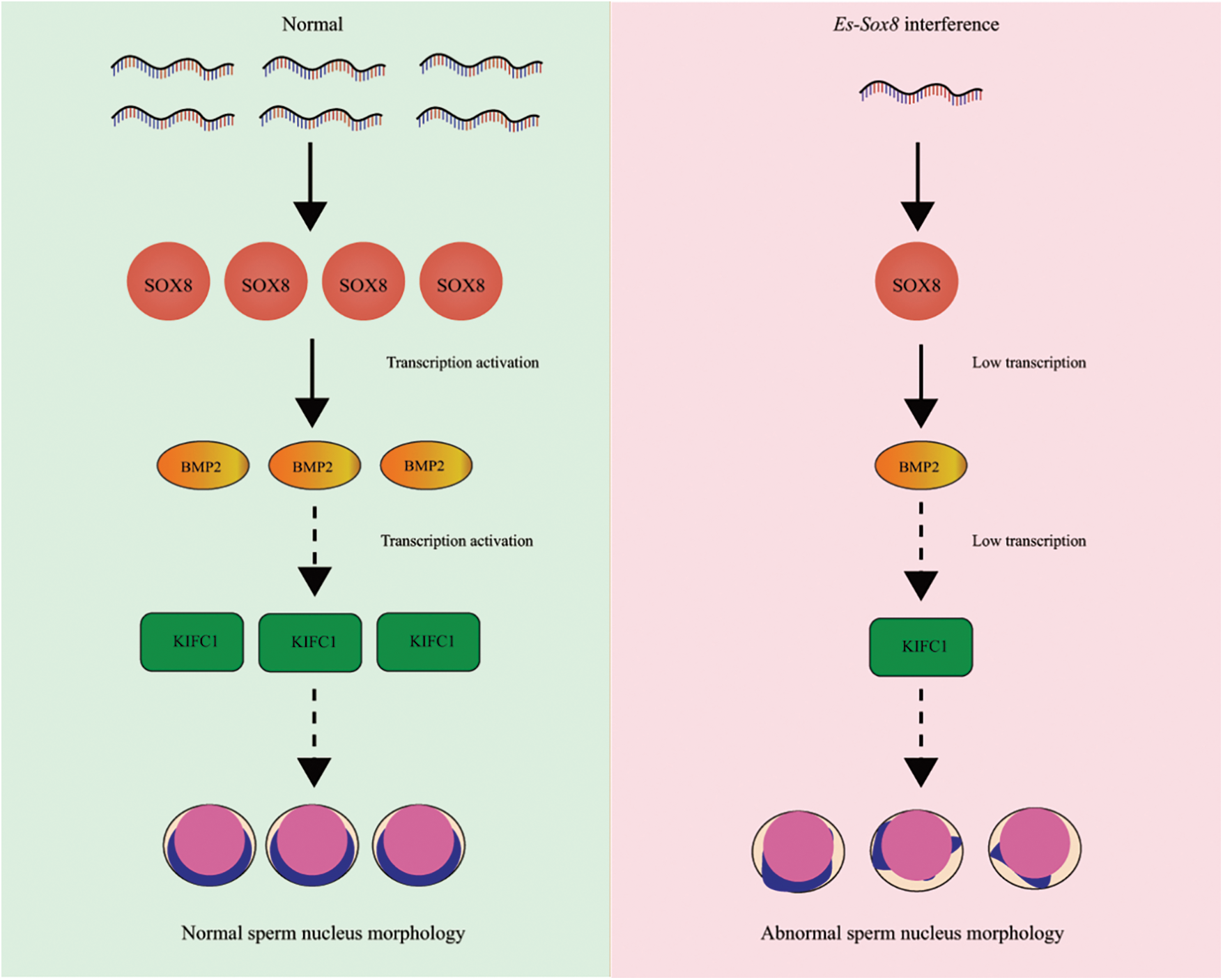
Figure 7: In normal E. sinensis, Es-SOX8 activates the transcription of Es-BMP2, leads to normal expression of Es-kifc1, and maintains normal sperm nucleus deformation. RNA silencing resulted in a decrease in Es-Sox8 mRNA and protein levels. When Es-SOX8-mediated transcription of Es-BMP2 is not successfully activated, the level of downstream Es-kifc1 decreases, and the normal deformation of sperm nucleus morphology is not maintained. As a result, this leads to abnormal morphology of mature sperm cells. The abnormal nucleus of the sperm is angular and has a raised shape.
Es-Sox8 regulates Es-kifc1 by activating Es-BMP2
Es-BMP2 also plays a key role in sperm nucleus development (Yang et al., 2020), and SOX can regulate the expression of BMP2 (Xiao et al., 2019). Accordingly, we observed a significantly repressed transcription level of Es-BMP2 in the Es-Sox8 knockdown group. ChIP results showed a direct binding of Es-SOX8 to the promoter of Es-BMP2; thus, confirming that Es-SOX8 can directly activate the transcription of Es-BMP2 while a knockdown of Es-SOX8 reduces the level of Es-BMP2 transcription.
Microtubules and motor proteins play several roles in acrosome formation and other processes during spermatogenesis (Clubb and Locke, 1996; Kierszenbaum, 2002; Weber et al., 2004; Cheng et al., 2005; Sun et al., 2010; Wang et al., 2012). Kinesin can be transported along microtubules of the cytoskeleton. During spermatogenesis, kinesin proteins, KIFs can help acrosome genesis and coordinate nuclear deformation (Yang and Sperry, 2003). KIFC1 is co-located with some organelles on one side of the nucleus, such as Golgi and mitochondria. The transport of KIFC1 to these organelles can assist in nuclear deformation (Sun et al., 2010). During the sperm deformation process of E. sinensis, Es-KIFC1 maintains the nucleus morphology (Wei et al., 2019), and Es-BMP2 adjusts the expression level of Es-KIFC1 (Yang et al., 2020). Therefore, we can conclude that Es-SOX8, Es-BMP2, and Es-KIFC1 can jointly constitute a regulatory pathway for the morphological changes in the sperm nucleus of E. sinensis (as shown in Fig. 8).
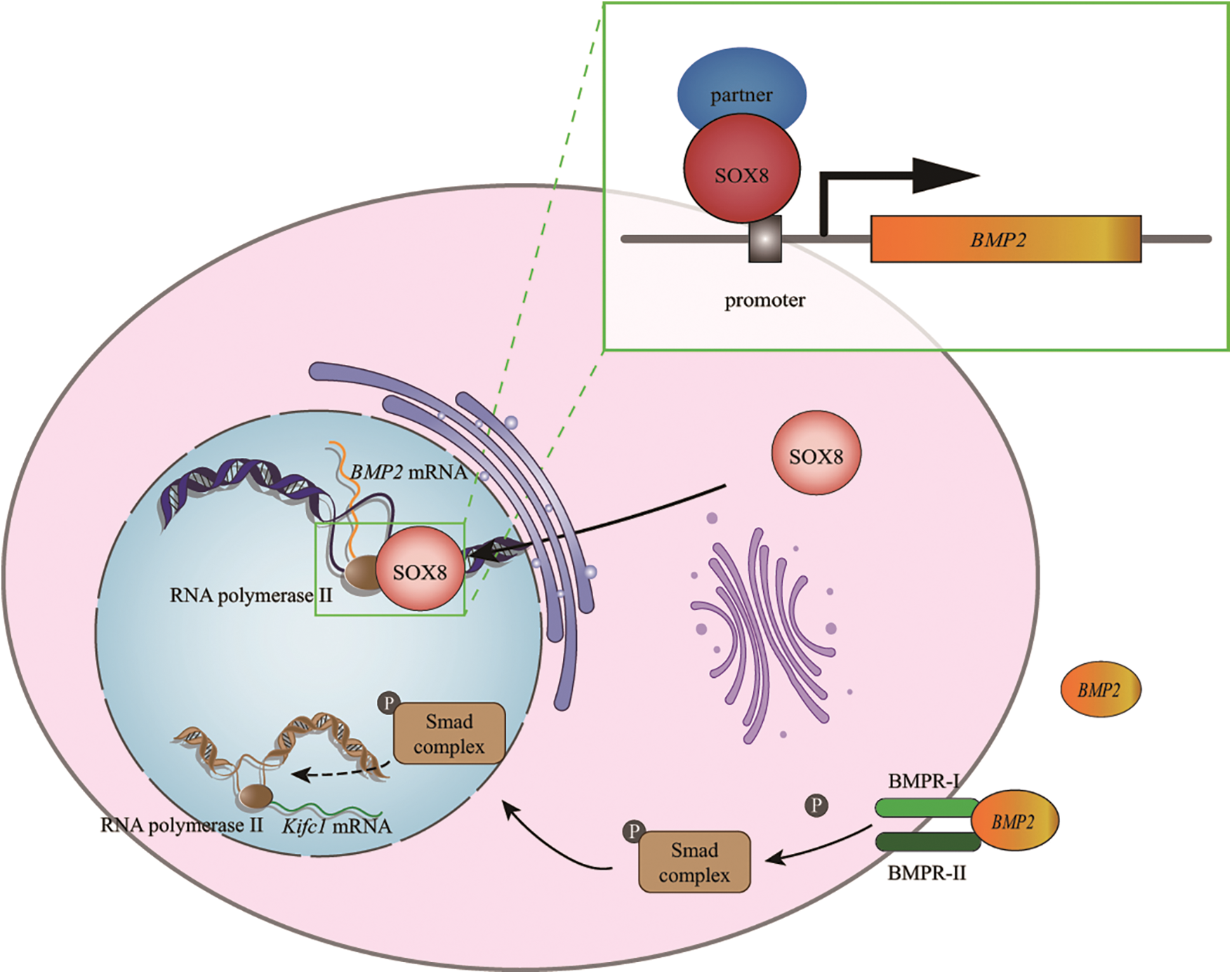
Figure 8: A schematic diagram showing that Es-SOX8 directly regulates Es-BMP2 transcription. Es-SOX8 and the transcription partner combine to form a transcription complex, which binds to the promoter of Es-BMP2, opens the DNA strands and initiates the transcription of Es-BMP2, which is then transported to the cytoplasm to be translated into Es-BMP2 protein. The Es-BMP2 protein is secreted extracellularly; it binds to membrane receptors and activates the phosphorylation of the intracellular SMAD complex. The phosphorylated complex functions to regulate the transcription of Es-kifc1. As a kinesin, Es-KIFC1 regulates the normal morphology of spermatozoa.
However, SOX transcription factors usually cannot work independently and often exist in the form of transcription complexes. In this study, we could not determine the chaperone factor of Es-SOX8 when it functions in spermatogenesis. We also do not know which motor proteins are responsible for the shuttle of Es-SOX8 between the nucleus and the cytoplasm, as well as other members in the SOX8-BMP2-KIFC1 pathway, and these need to be explored further.
Based on the distribution characteristics of Es-SOX8 protein in the germ cells of male E. sinensis and its knockdown effect on sperm nucleus morphology, along with the inhibition of Es-BMP2 transcription, we can conclude that Es-SOX8 has an important function in the spermatogenesis of E. sinensis. Knockdown of Es-Sox8 inhibits the transcription of Es-BMP2, and Es-SOX8 binds to the promoter region of Es-BMP2, indicating that Es-SOX8 directly affects nuclear morphogenesis by regulating Es-BMP2. The mechanism by which Es-BMP2 maintains the normal morphology of the sperm nucleus through Es-kifc1 has been confirmed before. Therefore, to summarize, Es-SOX8 up-regulates Es-kifc1 by activating the transcription of Es-BMP2, thereby regulating the normal deformation of the sperm nucleus.
Acknowledgement: The authors thank all members of the Sperm Laboratory of Zhejiang University for their help. Thanks for the experiment platform provided by Zhejiang University. We also appreciate the College Students Creative Research Training Center, National Biological Demonstration Experimental Center, Zhejiang University.
Statement of Ethics: The study was approved by Animal Experimental Ethical Inspection of the First Affiliated Hospital, College of Medicine, Zhejiang University (Reference No. 2021-557).
Author Contribution Statement: K. Jia designed and performed the experiments, collected and analyzed the data, wrote and edited the manuscript. S. L. Hao participated in data analysis and manuscript revision, provided funding, W. X. Yang guided experiments, provided funding, and approved the final manuscript.
Funding Statement: This work was supported by the National Natural Science Foundation of China (Nos. 32072954 and 32102786).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Bai S, Fu K, Yin H, Cui Y, Yue Q et al. (2019). Sox30 initiates transcription of haploid genes during late meiosis and spermiogenesis in mouse testes. Development 145: dev164855. [Google Scholar]
Bolcun-Filas E, Bannister LA, Barash A, Schimenti KJ, Hartford SA et al. (2011). A-MYB (MYBL1) transcription factor is a master regulator of male meiosis. Development 138: 3319–3330. DOI 10.1242/dev.067645. [Google Scholar] [CrossRef]
Bowles J, Schepers G, Koopman P (2000). Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Developmental Biology 227: 239–255. DOI 10.1006/dbio.2000.9883. [Google Scholar] [CrossRef]
Cheng YM, Shi XQ, Yu H, Wu Y, Jia MC (2005). Specific expression of β-actin during spermatogenesis in rats. National Journal of Andrology 11: 755–760. [Google Scholar]
Cheon YP, Choi D, Lee SH, Kim CG (2020). YY1 and CP2c in unidirectional spermatogenesis and stemness. Development and Reproduction 24: 249–262. DOI 10.12717/DR.2020.24.4.249. [Google Scholar] [CrossRef]
Chimal-Monroy J, Rodriguez-Leon J, Montero JA, Gañan Y, Macias D et al. (2003). Analysis of the molecular cascade responsible for mesodermal limb chondrogenesis: Sox genes and BMP signaling. Developmental Biology 257: 292–301. [Google Scholar]
Clermont Y (1962). Quantitative analysis of spermatogenesis of the rat: A revised model for the renewal of spermatogonia. American Journal of Anatomy 111: 111–129. [Google Scholar]
Clubb BH, Locke M (1996). F-actin forms transient perinuclear shells at the mitosis-interphase transition. Cell Motility and the Cytoskeleton 33: 151–162. [Google Scholar]
Connor F, Cary PD, Read CM, Preston NS, Driscoll PC et al. (1994). DNA binding and bending properties of the post-meiotically expressed Sry-related protein Sox-5. Nucleic Acids Research 22: 3339–3346.da. [Google Scholar]
Cruz I, Rodríguez-Casuriaga R, Santiñaque FF, Farías J, Curti G et al. (2016). Transcriptome analysis of highly purified mouse spermatogenic cell populations: Gene expression signatures switch from meiotic-to postmeiotic-related processes at pachytene stage. BMC Genomics 17: 294. [Google Scholar]
de Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N (1998). Spermatogenesis. Human Reproduction 13 Suppl 1: 1–8. [Google Scholar]
de Leeuw R, Gruenbaum Y, Medalia O (2018). Nuclear lamins: Thin filaments with major functions. Trends in Cell Biology 28: 34–45. [Google Scholar]
Du NS (1998). Fertilization of chinese mitten crab. Fisheries Science and Technology Information 25: 9–13. [Google Scholar]
Du NS, Lai W, Xue LZ (1987). Studies on the sperm of Chinese mitten-handed crab, Eriocheir sinensis (Crustacea, Decapoda). I. The morphology and ultrastructure of mature sperm. Oceanologia et Limnologia Sinica 18: 119–125. [Google Scholar]
Du NS, Xue LZ, Lai W (1988). Studies on the sperm of Chinese mitten-handed crab, Eriocheir sinensis (Crustacea, Decapoda). II. Spermatogenesis. Oceanologia et Limnologia Sinica 19: 71–75. [Google Scholar]
Eddy EM, O’Brien DA (1998). Gene expression during mammalian meiosis. Current Topics in Developmental Biology 37: 141–200. [Google Scholar]
Grimm D, Bauer J, Wise P, Krüger M, Simonsen U et al. (2020). The role of SOX family members in solid tumours and metastasis. Seminars in Cancer Biology 67: 122–153. [Google Scholar]
Hann MC, Lau PE, Tempest HG (2011). Meiotic recombination and male infertility: From basic science to clinical reality? Asian Journal of Andrology 13: 212–218. [Google Scholar]
Hu J, Chen YX, Wang D, Qi X, Li TG (2004). Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Developmental Biology 276: 158–171. [Google Scholar]
Kamachi Y, Kondoh H (2013). Sox proteins: Regulators of cell fate specification and differentiation. Development 140: 4129–4144. [Google Scholar]
Kataruka S, Akhade VS, Kayyar B, Rao MRS (2017). Mrhl long noncoding RNA mediates meiotic commitment of mouse spermatogonial cells by regulating Sox8 expression. Molecular and Cellular Biology 37: e00632–16. [Google Scholar]
Kennedy CL, Koopman P, Mishina Y, O’Bryan MK (2007). Sox8 and Sertoli-cell function. Annals of the New York Academy of Sciences 1120: 104–113. [Google Scholar]
Kierszenbaum AL (2002). Intramanchette transport (IMTManaging the making of the spermatid head, centrosome, and tail. Molecular Reproduction and Development 63: 1–4. [Google Scholar]
Koopman P (2005). Sex determination: A tale of two Sox genes. Trends in Genetics 21: 367–370. [Google Scholar]
Koubova J, Menke DB, Zhou Q, Page DC (2006). Retinoic acid regulates sex-specific timing of meiotic initiation in mice. PNAS 103: 2474–2479. [Google Scholar]
Laronda MM, Jameson JL (2011). Sox3 functions in a cell-autonomous manner to regulate spermatogonial differentiation in mice. Endocrinology 152: 1606–1615. [Google Scholar]
Laudet V, Stehelin D, Clevers H (1993). Ancestry and diversity of the HMG box superfamily. Nucleic Acids Research 21: 2493–2501. [Google Scholar]
Liu M, Yang Y, Wang Y, Chen S, Shen Y (2021). The mutation c.346-1G > a in SOHLH1 impairs sperm production in the homozygous but not in the heterozygous condition. Human Molecular Genetics 27: 242. [Google Scholar]
Liu ZQ, Jiang XH, Qi HY, Xiong LW, Qiu GF (2016). A novel SoxB2 gene is required for maturation of sperm nucleus during spermiogenesis in the Chinese mitten crab, Eriocheir sinensis. Scientific Reports 6: 32139. [Google Scholar]
Margolin G, Khil PP, Kim J, Bellani MA, Camerini-Otero RD (2014). Integrated transcriptome analysis of mouse spermatogenesis. BMC Genomics 15: 39. [Google Scholar]
Martianov I, Choukrallah MA, Krebs A, Ye T, Legras S et al. (2010). Cell-specific occupancy of an extended repertoire of CREM and CREB binding loci in male germ cells. BMC Genomics 11: 530. [Google Scholar]
McAninch D, Mäkelä JA, La HM, Hughes JN, Lovell-Badge R et al. (2020). SOX3 promotes generation of committed spermatogonia in postnatal mouse testes. Scientific Reports 10: 6751. [Google Scholar]
O’Bryan MK, Takada S, Kennedy CL, Scott G, Harada S et al. (2008). Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Developmental Biology 316: 359–370. [Google Scholar]
Pennisi D, Gardner J, Chambers D, Hosking B, Peters J et al. (2000). Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nature Genetics 24: 434–437. [Google Scholar]
Portnoi MF, Dumargne MC, Rojo S, Witchel SF, Duncan AJ et al. (2018). Mutations involving the SRY-related gene SOX8 are associated with a spectrum of human reproductive anomalies. Human Molecular Genetics 27: 1228–1240. [Google Scholar]
Raverot G, Weiss J, Park SY, Hurley L, Jameson JL (2005). Sox3 expression in undifferentiated spermatogonia is required for the progression of spermatogenesis. Developmental Biology 283: 215–225. [Google Scholar]
Roumaud P, Haché J, Martin LJ (2018). Expression profiles of Sox transcription factors within the postnatal rodent testes. Molecular and Cellular Biochemistry 447: 175–187. [Google Scholar]
Sarkar A, Hochedlinger K (2013). The sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell 12: 15–30. [Google Scholar]
Satouh Y, Ikawa M (2018). New insights into the molecular events of mammalian fertilization. Trends in Biochemical Sciences 43: 818–828. [Google Scholar]
Schepers GE, Bullejos M, Hosking BM, Koopman P (2000). Cloning and characterisation of the Sry-related transcription factor gene Sox8. Nucleic Acids Research 28: 1473–1480. [Google Scholar]
Staub C, Johnson L (2018). Review: Spermatogenesis in the bull. Animal 12: s27–s35. [Google Scholar]
Sun X, He Y, Hou L, Yang WX (2010). Myosin Va participates in acrosomal formation and nuclear morphogenesis during spermatogenesis of Chinese mitten crab Eriocheir sinensis. PLoS One 5: e12738. [Google Scholar]
Tang H, Chen B, Liu P, Xie X, He R et al. (2019). SOX8 acts as a prognostic factor and mediator to regulate the progression of triple-negative breast cancer. Carcinogenesis 40: 1278–1287. [Google Scholar]
Theodosiou NA, Tabin CJ (2005). Sox9 and Nkx2.5 determine the pyloric sphincter epithelium under the control of BMP signaling. Developmental Biology 279: 481–490. [Google Scholar]
Turnescu T, Arter J, Reiprich S, Tamm ER, Waisman A et al. (2018). Sox8 and Sox10 jointly maintain myelin gene expression in oligodendrocytes. Glia 66: 279–294. [Google Scholar]
Wang YT, Mao H, Hou CC, Sun X, Wang DH et al. (2012). Characterization and expression pattern of KIFC1-like kinesin gene in the testis of the Macrobrachium nipponense with discussion of its relationship with structure lamellar complex (LCx) and acroframosome (AFS). Molecular Biology Reports 39: 7591–7598. [Google Scholar]
Weber KL, Sokac AM, Berg JS, Cheney RE, Bement WM (2004). A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature 431: 325–329. [Google Scholar]
Wegner M (1999). From head to toes: The multiple facets of Sox proteins. Nucleic Acids Research 27: 1409–1420. [Google Scholar]
Wei YL, Yang T, Kovacs T, Yang WX (2019). C-terminal kinesin motor es-KIFC1 regulates nuclear formation during spermiogenesis in Chinese mitten crab Eriocheir sinensis. Gene 719: 144074. [Google Scholar]
Whittington N, Cunningham D, Le TK, De Maria D, Silva EM (2015). Sox21 regulates the progression of neuronal differentiation in a dose-dependent manner. Developmental Biology 397: 237–247. [Google Scholar]
Wu FJ, Lin TY, Sung LY, Chang WF, Wu PC et al. (2017). BMP8A sustains spermatogenesis by activating both SMAD1/5/8 and SMAD2/3 in spermatogonia. Science Signaling 10: eaal1910. [Google Scholar]
Xiao B, Zhang W, Kuang Z, Lu J, Li W et al. (2019). SOX9 promotes nasopharyngeal carcinoma cell proliferation, migration and invasion through BMP2 and mTOR signaling. Gene 715: 144017. [Google Scholar]
Xie SL, Fan S, Zhang SY, Chen WX, Li QX et al. (2018). SOX8 regulates cancer stem-like properties and cisplatin-induced EMT in tongue squamous cell carcinoma by acting on the Wnt/β-catenin pathway. International Journal of Cancer 142: 1252–1265. [Google Scholar]
Yang T, Wei BH, Hao SL, Wei YL, Yang WX (2020). Bone morphogenetic protein 2 (BMP2) mediates spermatogenesis in Chinese mitten crab Eriocheir sinensis by regulating kinesin motor KIFC1 expression. Gene 754: 144848. [Google Scholar]
Yang WX, Sperry AO (2003). C-terminal kinesin motor KIFC1 participates in acrosome biogenesis and vesicle transport. Biology of Reproduction 69: 1719–1729. [Google Scholar]
Yin H, Kang Z, Zhang Y, Gong Y, Liu M et al. (2021). HDAC3 controls male fertility through enzyme-independent transcriptional regulation at the meiotic exit of spermatogenesis. Nucleic Acids Research 49: 5106–5123. [Google Scholar]
Zehentner BK, Dony C, Burtscher H (1999). The transcription factor Sox9 is involved in BMP-2 signaling. Journal of Bone and Mineral Research 14: 1734–1741. [Google Scholar]
Zhao GQ, Hogan BL (1996). Evidence that mouse Bmp8a (Op2) and Bmp8b are duplicated genes that play a role in spermatogenesis and placental development. Mechanisms of Development 57: 159–168. [Google Scholar]
Zhao GQ, Liaw L, Hogan BL (1998). Bone morphogenetic protein 8A plays a role in the maintenance of spermatogenesis and the integrity of the epididymis. Development 125: 1103–1112. [Google Scholar]
Zhao L, Arsenault M, Ng ET, Longmuss E, Chau TC et al. (2017). SOX4 regulates gonad morphogenesis and promotes male germ cell differentiation in mice. Developmental Biology 423: 46–56. [Google Scholar]
Zheng B, Huang C, Zhou J, Ye L (2021). Identification of a novel Sox5 transcript in mouse testis. Gene Expression Patterns 41: 119197. [Google Scholar]
Supplementary Information
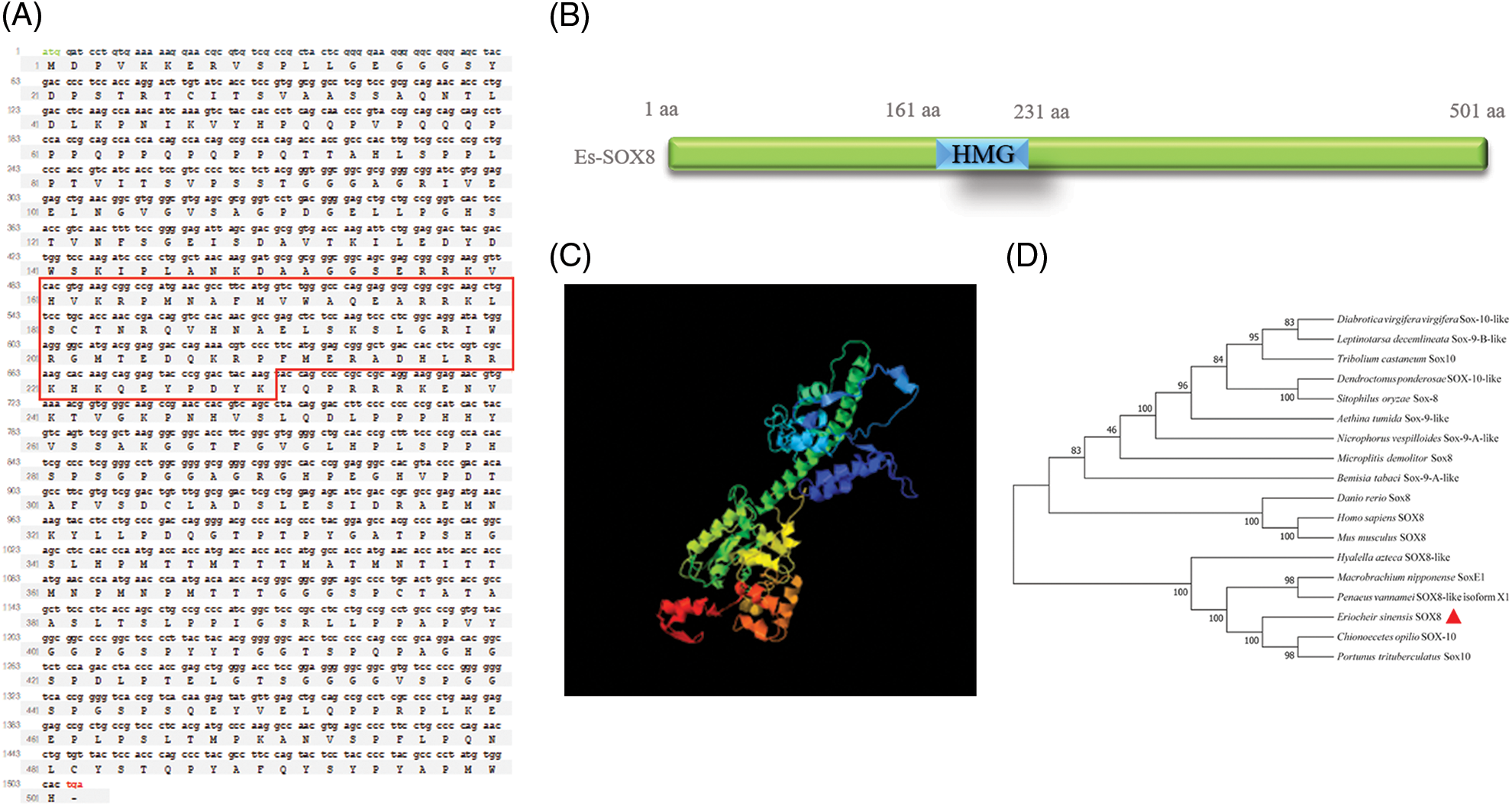
Supplemental Figure 1: Es-Sox8 CDS sequence and the predicted protein. (A) Es-Sox8 CDS equence and predicted amino acid sequence. CDS: 1506 bp; amino acid: 501 aa; mass: 53.51 kDa; the red box is the position of the high mobility group (HMG) domain. (B) Predicted structure of the domain. (C) Predicted three-level structure. (D) Phylogenetic analysis of Es-Sox8.
Supplemental Figure 2: Multiple sequence alignment of Es-SOX8. The red box indicates the position of the HMG domain. 1: Aethina tumida Sox-9-like, 2: Bemisia tabaci Sox-9-A-like, 3: Chionoecetes opilio SOX-10, 4: Danio rerio SOX8, 5: Dendroctonus ponderosa SOX-10-like, 6: Diabrotica virgifera virgifera Sox-10-like, 7: Eriocheir sinensis SOX8, 8: Homo sapiens SOX8, 9: Leptinotarsa decemlineata Sox-9-B-like,10: Macrobrachium nipponense SoxE1, 11: Microplitis demolitor Sox8, 12: Mus musculus SOX8, 13: Nicrophorus vespilloides Sox-9-A-like, 14:Penaeus vannamei SOX8-like isoform X1, 15: Sitophilus oryzae Sox-8, 16: Tribo lium castaneum Sox10.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |