DOI:10.32604/biocell.2022.021661

| BIOCELL DOI:10.32604/biocell.2022.021661 |  |
| Article |
Dexmedetomidine alleviates oxygen and glucose deprivation-induced apoptosis in mesenchymal stem cell via downregulation of MKP-1
1Department of Cardiovascular Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
2Department of Cardiothoracic Surgery, University Hospital, Linköping University, Linköping, Sweden
3Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
*Address correspondence to: Yanqi Yang, yangyq@mail.sysu.edu.cn
#The authors contributed equally to this work
Received: 26 January 2022; Accepted: 18 April 2022
Abstract: Bone marrow mesenchymal stem cell (MSC)-based therapy is a novel candidate for heart repair. But ischemia-reperfusion injury leads to low viability of MSC. Dexmedetomidine (Dex) has been found to protect neurons against ischemia-reperfusion injury. It remains unknown if Dex could increase the viability of MSCs under ischemia. The present study is to observe the potential protective effect of Dex on MSCs under ischemia and its underlying mechanisms. Specific mRNAs related to myocardial ischemia in the GEO database were selected from the mRNA profiles assessed in a previous study using microarray. The most dysregulated mRNAs of the specific ones from the above study were subject to bioinformatics analysis at our laboratory. These dysregulated mRNAs possibly regulated apoptosis of cardiomyocytes and were validated in vitro for their protective effect on MSCs under ischemia. MSCs were pre-treated with Dex at 10 μM concentration for 24 h under oxygen-glucose deprivation (OGD). Flow cytometry and TUNEL assay were carried out to detect apoptosis in Dex-pretreated MSCs under OGD. The relative expressions of mitogen-activated protein kinase phosphatase 1 (MKP-1) and related genes were detected by quantitative polymerase chain reaction and western blotting. Microarray data analysis revealed that Dex regulates MAPK phosphatase activity. Dex significantly reduced in vitro apoptosis of MSCs under OGD, which suppressed the synthesis level of Beclin1 and light chain 3 proteins. Dex down-regulated MKP-1 expression and attenuated an OGD-induced change in the mitogen activated protein kinase 3 (MAPK3) signaling pathway. Dex increases the viability of MSC and improves its tolerance to OGD in association with the MKP-1 signaling pathway, thus suggesting the potential of Dex as a novel strategy for promoting MSCs efficacy under ischemia.
Keywords: Dexmedetomidine; HIF-1α; MKP-1; Oxygen and glucose deprivation
Clinical trials have shown that bone marrow mesenchymal stem cell (MSC) transplantation via intracoronary or intramyocardial injection may improve cardiac function in patients with ischemic heart disease (IHD) (Choudhury et al., 2017; Miao et al., 2017). This approach opens up a fascinating area in the treatment of IHD. However, transplanted MSCs linked to poor viability and be inflammatory in the hypoxic micro-environment in the infarct area because of hypoperfusion (Yu et al., 2017; Van Nguyen et al., 2021; Ferro et al., 2019). After 4 days of MSCs transplantation, only 0.5% of MSCs could survive in hypoxic myocardium (Mihai et al., 2019). Several pathological changes occur in the hypoxic microenvironment, such as a reduction in ATP, dysfunction of sodium-potassium channels, retention of lactic acid, and inhibition of protein synthesis on the ribosomes (Wu et al., 2018). Glucose- and oxygen-deprivation is used as an in vitro model for studying cell biology in the hypoxic microenvironment (Ryou and Mallet, 2018). Apoptosis is one of the critical cell death mechanisms in glucose- and oxygen-deprived conditions (Bachmann et al., 2020). Karpov et al. (2017) highlighted that inhibition of apoptosis in ischemic MSCs in a hypoxic microenvironment could improve the efficacy of the therapy with transplanted MSCs in patients with IHD (Karpov et al., 2017).
Mitogen-activated protein kinase (MAPK) is evolutionally conserved from yeast to mammals and is one of the critical regulators of the cell cycle and apoptosis (Whitaker and Cook, 2021). Levels of Beclin1, which was first discovered in the yeast vacuolar system, can dictate autophagy and endocytosis (Tran et al., 2021). Nuclear LC3 is a critical initiator of the autophagic process in response to external nutrient deficiencies (Huang et al., 2015). Dual-specificity phosphatase (DUSP) or nuclear-localized phosphatase, also referred to as MAPK phosphatase 1 (MKP-1), plays a key role in cell survival (Kim and Asmis, 2017). Upregulation of MKP-1 using the gain of function method could augment MSC apoptotic rate in vitro (Yang et al., 2019).
Dexmedetomidine (Dex), a highly selective alpha-2 adrenergic receptor agonist, has been widely applied in sedation and analgesia (Keating, 2015). Yang et al. (2022) reported that Dex protects cardiomyocytes against ischemia injury by inhibiting the apoptosis signaling pathway (Yang et al., 2022). In another study, Zhai et al. (2019) highlighted that Dex attenuates ischemia-induced neuronal apoptosis (Zhai et al., 2019). Dex can protect both cardiomyocytes (Chang et al., 2020) and neurons (Zheng et al., 2020) against ischemia-reperfusion injury through the inhibition of apoptosis. Based on the above findings, we hypothesize that Dex might protect MSCs against ischemia-induced cell death. Hence, the present study aimed to observe the potential of Dex as a drug and the underlying mechanisms in mitigating MSCs apoptosis during ischemia injury.
We screened and downloaded public microarray data from the Gene Expression Omnibus (GEO) database by the National Center for Biotechnology Information for both the Dex and control groups under the following criteria: male rats weighing between 250 g to 350 g, ischemic rat models established by transiently ligating the left anterior descending coronary artery, and Dex administrated at 60 mL/kg/h. Our search narrowed down to the GEO series with the accession number of GSE126104, wherein differentially expressed miRNAs and mRNAs were identified using GEO2R. Five control samples and five Dex samples selected from Series GSE126104 were analyzed based on Platform GPL22440.
Data preprocessing and screening strategy
We used GEO2R to detect downregulated genes by comparing the Dex and control groups in Series GSE126104. As the cut-off criteria, parameters of p < 0.05 and |fold change | > 0 were used. Dysregulated genes were classified by the Database for Annotation, Visualization, and Integrated Discovery v6.8 according to shared data from Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). The MAPK-related mRNAs were screened based on enriched GO terms and significant KEGG pathways.
Rat bone marrow mesenchymal stem cell (MSC) line was purchased from Ginkgo Biotechnology Co., Ltd. (Guangzhou, China), and the cells were seeded in 75 cm2 flasks (Corning, USA) at a density of 1 − 2 × 106 cells/mL in a medium containing 88% DMEM(1×) supplemented with GlutaMAXTM-I (Gibco, Thermo Fisher Scientific Inc., USA), 10% newborn calf serum (Gibco Thermo Fisher Scientific Inc., USA), and 1% penicillin/streptomycin (GibcoTM; Thermo Fisher Scientific Inc., USA). The cells were then incubated in an incubator (Galaxy 48R) at 37°C with 5% CO2. The flasks were washed with PBS, and the media was changed every 2–3 days.
Dex treatment and the oxygen and glucose deprivation (OGD) model
The Dex group was pre-treated with Dex (Yangtze River Pharmaceutical Group, China) at a 10 μM concentration for 24 h, as previously reported (Yu et al., 2019). Then the OGD and OGD+Dex groups were transferred into a 75 cm2 culture flask containing Roswell Park Memorial Institute (RPMI) 1640 media devoid of any sugar (Procell Life Science & Technology Co., Ltd., China) and incubated in an oxygen-free environment with 95% N2 and 5% CO2 at 37°C for 24 h as previously described (Liu et al., 2020).
MSCs were collected and suspended twice with PBS before being divided into a blank group as the negative control and a positive group which was stained with Annexin V/ propidium iodide (Invitrogen eBioscienceTM Annexin V-FITC Apop Kit #BMS500FI-300). The cells were subsequently analyzed by a FACS flow cytometer (BD Biosciences).
Apoptosis detection by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Apoptosis in MSC cells was detected by TUNEL assay (#G1501-50 Wuhan Servicebio Technology Co., Ltd.). Media from different groups were removed, and the cells were washed with PBS. MSCs were fixed with 1% paraformaldehyde for 30 min before 50 μL of the permeabilizing working solution was added. The mixture was then left to incubate at room temperature for 20 min. An appropriate amount of terminal deoxynucleotidyl transferase enzyme, dUTP, and buffer were mixed at a 1:5:50 ratio according to the TUNEL kit protocol. Sections were observed using an OLYMPUS TH4-200 fluorescence microscope. Images were analyzed with Image-Pro Plus 6.0 software (USA).
Total RNA extraction and quantitative polymerase chain reaction (qPCR) assays
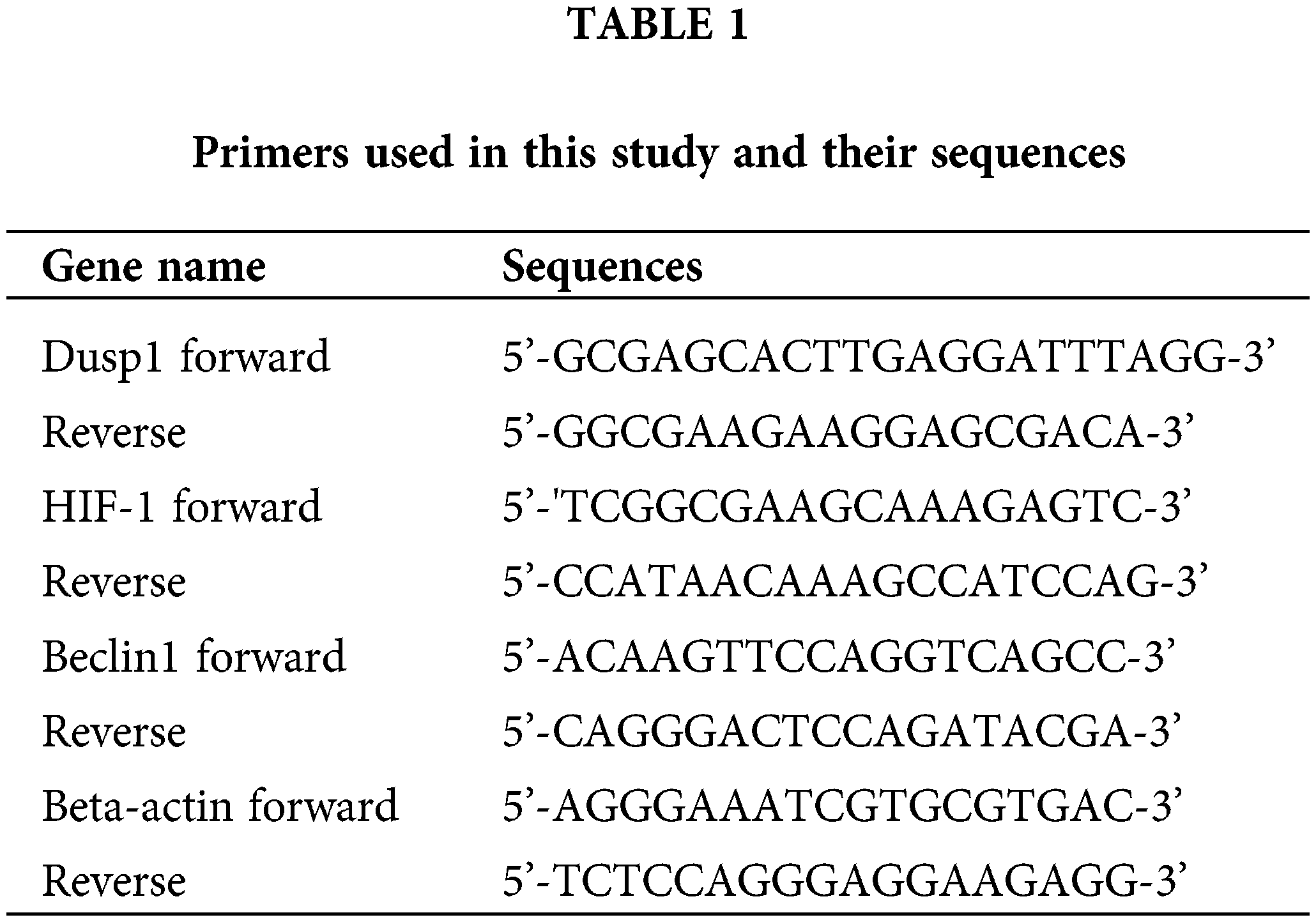
The MSCs were seeded into 24 wells at a density of 1 × 105/mL. Then the cells were preconditioned with Dex for 24 h, washed with PBS thrice and cultured in a serum- and sugar-free RPMI 1640 medium for an additional 24 h under OGD condition. Total RNA was extracted from the MSCs by spin column technology using a MolPure Cell RNA Kit (#M2010751, Yeasen Biotechnology, China) and was reverse-transcribed into cDNA using the RT First Strand cDNA Synthesis Kit for RT PCR (#LT202501, Servicebio, China). Primer sequences are shown in Table 1. The cDNA was then analyzed using 2× SYBR Green qPCR Master Mix (# MPC2011003, Servicebio, China) on a LightCycler® 96 SW 1.1 (Roche). Dusp1, hypoxia-inducible factor 1-alpha (HIF-1α), and Beclin-1 expression levels were analyzed using the 2-(ΔΔCT) method and were normalized to the beta-actin expression.
The expressions of MKP-1, HIF-1α, p38, pErk1/2, Erk1/2, Beclin1, and LC3 in different groups were detected using a standard western blot assay. An equivalent amount of proteins (25 μg/μL) measured by a BCA protein kit were boiled with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer at 95°C and then subjected to 8% SDS-PAGE before being transferred onto cropped 0.22 μm PVDF membranes (Servicebio, Wuhan, China) at 200 mA for 1.5 h. After that, the membranes were incubated at 4°C for 24 h with primary antibodies against MKP-1 (1:1000, Santa Cruz Biotechnology, #sc-373841), p-ERK1/2 (1:1000, CST, #4370S), ERK1/2 (1:1000, Servicebio, #GB11560), Beclin1 (1:1000, Santa Cruz Biotechnology, #sc-48341), p38 MAPK rabbit monoclonal antibody (1:1000, Beyotime, #AF1111), HIF-1α (1:1000, Wanleibio, #L09011607), LC3 (1:1000, Servicebio, #GB11124), GAPDH (1:1000, Servicebio, #GB11002), and beta-actin (1:2000, Servicebio, #GB11001). Next, the membranes were incubated with HRP-conjugated goat anti-rabbit secondary antibody (1:2000, Servicebio, #GB23303) or anti-mouse IgG (1:2000, Servicebio, #GB23301) at room temperature for 1 h. An Enhanced Chemiluminescence Reagent Kit was used to detect proteins on the blotting membranes, and the outcomes were subsequently quantified with ImageJ (1.52 h). The expressions of MKP-1, HIF-1α, p38, p-ERK1/2, ERK1/2, Beclin1, and LC3 proteins were normalized to those of beta-actin or GAPDH.
The experiments were repeated five times. The data were analyzed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) or the GraphPad Prism 7 software (GraphPad Software Inc., LaJolla, CA, USA) and presented as mean ± standard deviation (SD). Multiple groups were compared using the one-way analysis of variance (ANOVA) with Tukey’s post-hoc test for pairwise comparison of data. Measurement data with normal distribution were compared between two groups using the Student’s t-test. p < 0.05 was considered statistically significant.
Downregulation of MKP-1 gene expression in rat ischemic heart tissue
The mRNAs of 1833 differentially expressed genes (DEGs) were selected and marked with red and blue, of which Sufu, Nr1d1, Dusp1 (MKP-1), and Tspan1 were the most significantly downregulated according to the cut-off criteria (Fig. 1A). A GO enrichment analysis showed that the most significantly downregulated genes were related to biological processes, molecular functions, and cellular components (Figs. 1B–1D). The top five results of KEGG pathways included hypertrophic cardiomyopathy, pathways in cancer, MAPK signaling pathway, arrhythmogenic right ventricular cardiomyopathy, and protein processing in the endoplasmic reticulum (Fig. 1E). Of note, GO and KEGG analyses predicted that MAPK phosphatase activity and the inhibition of MKP-1 mRNA expression might exert a cardioprotective effect in the ischemic rat model (Webster et al., 2017; Yoshikawa et al., 2019).
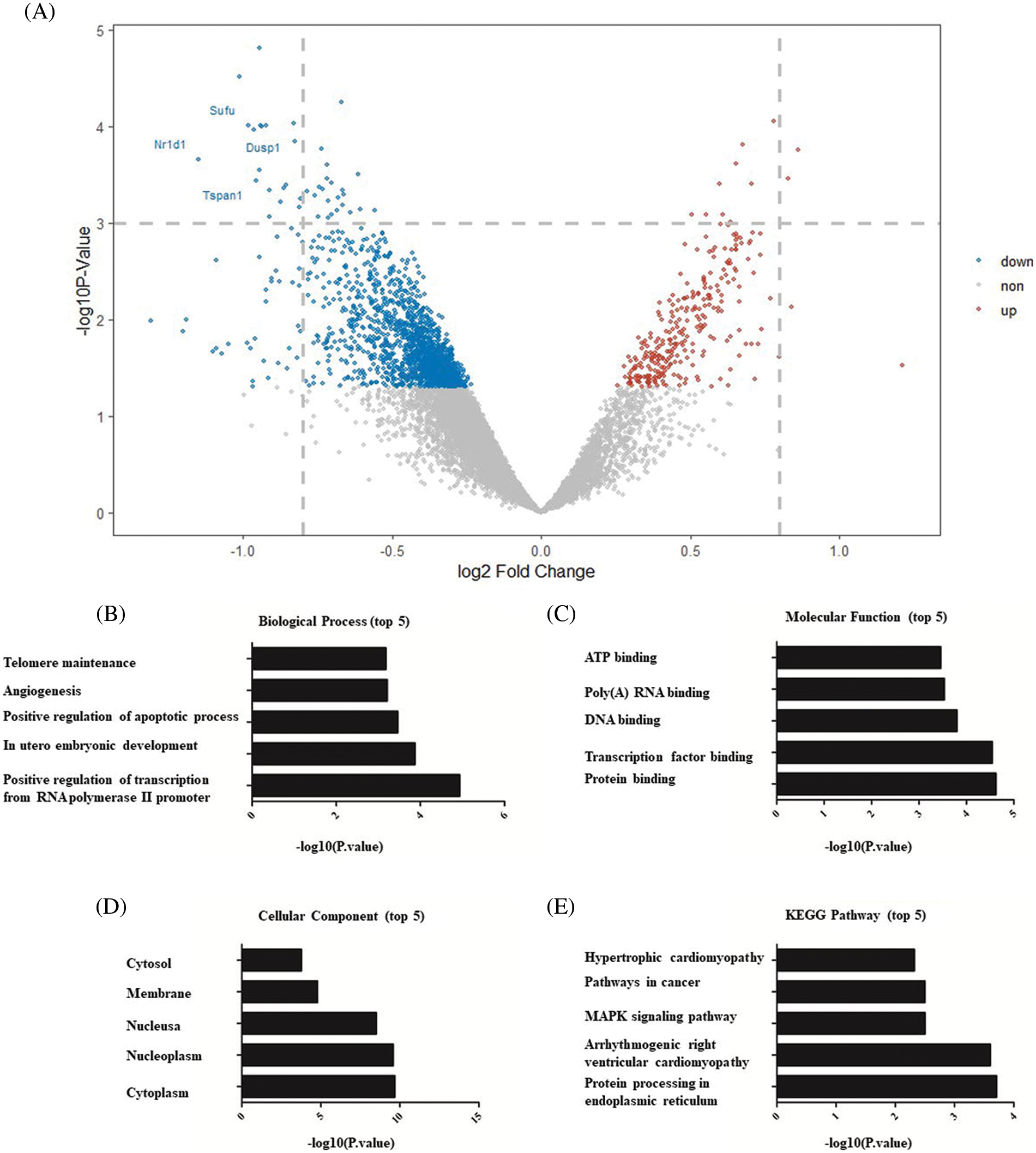
Figure 1: Bioinformatic analysis of GSE126104 related to Dex treatment in ischemic heart (A) Volcano plot generated from gene expression profile of Series GSE126104 DEG screening using the R studio software version 1.3.1093. The blue color indicates the downregulation of MKP-1 by Dex when compared to ischemic heart tissue; meanwhile red color indicates the upregulation of the genes. DUSP1 (MKP-1) is a candidate gene enriched in the cellular MAPK pathway. (B) Top five biological process enrichment of mRNAs downregulated by Dex. (C) Top five molecular function enrichment of mRNAs downregulated by Dex. (D) Top five cellular component enrichment of mRNAs downregulated by Dex. (E) Top 5 KEGG pathway enrichment of mRNAs that downregulated by Dex. Dex: dexmedetomidine, DUSP1: dual-specificity phosphatase, MAPK: mitogen-activated protein kinase, MKP-1: MAPK phosphatase-1, KEGG: Kyoto Encyclopedia of Genes and Genomes.
Attenuation of apoptosis in OGD-induced MSCs
The effect of Dex on OGD-induced MSCs apoptosis was assessed using flow cytometry analysis. As presented in Figs. 2A–2C, no significant difference was observed in apoptosis rate between the control and Dex groups. The apoptosis rate was 5% in the OGD+Dex group, significantly lower than 8% in the OGD group (Fig. 2D). The significantly lower apoptosis rate in the OGD+Dex group than in the OGD group was observed by the TUNEL assay as well (p < 0.01, Figs. 2E–2G).
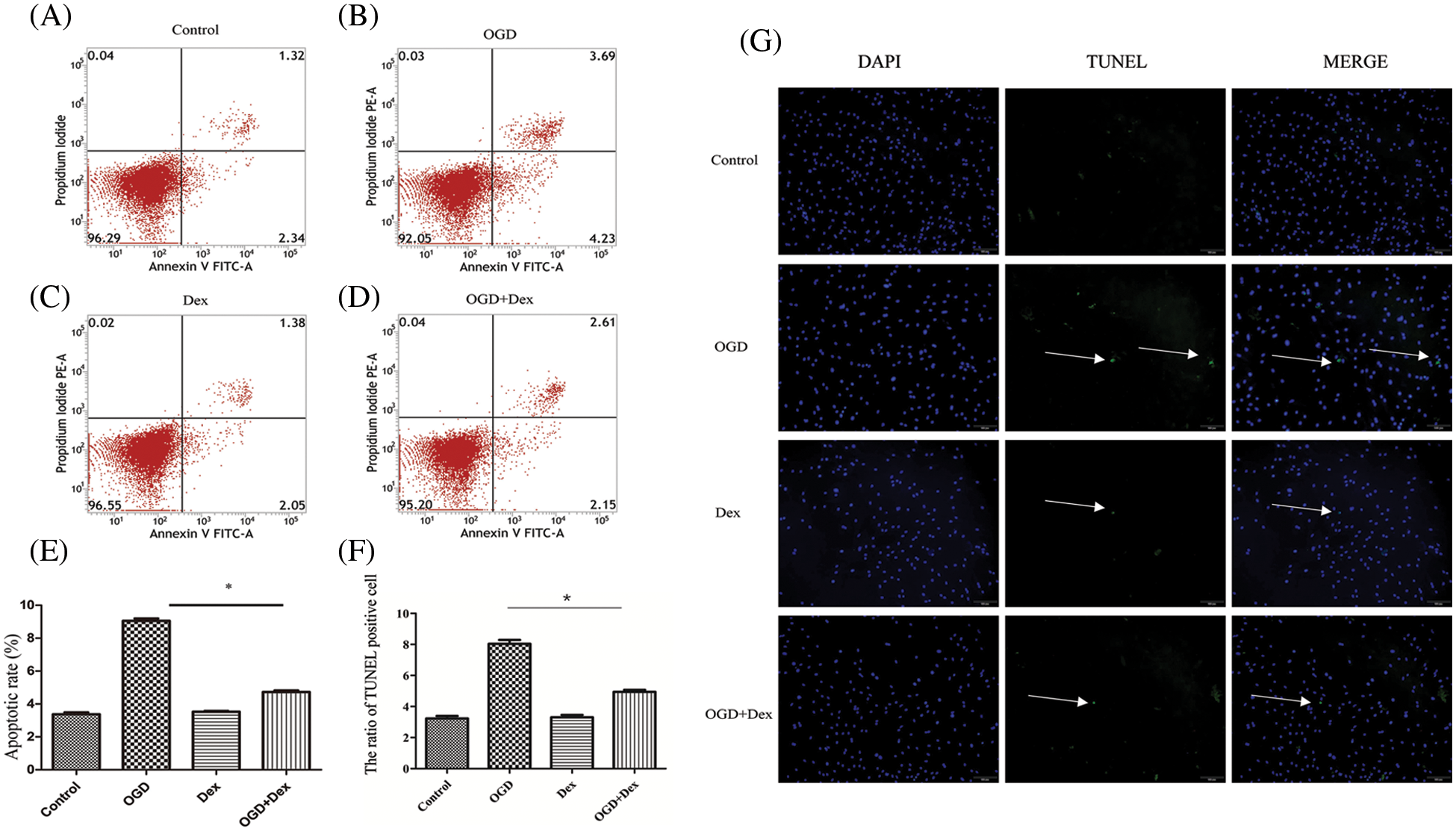
Figure 2: Flow cytometry and TUNEL-based analysis of apoptosis (n = 5 in each group). (A) The apoptosis rate of MSCs in the control group was less than 4%. (B) The apoptosis rate of MSCs after 24 h of OGD accounted to 8%, indicating incidences of cell death and apoptosis. (C) The apoptosis rate of MSCs after Dex pretreatment at 10 μM for 24 h was also less than 4%. (D) The apoptosis rate of the OGD+Dex group was less than 5%. (E) Both Annexin V and PI-positive cell populations were gated for analysis. Quantitative analysis of apoptotic cells was done by one-way ANOVA, and the results are expressed as the mean ± SD. F = 20. *p < 0.05 OGD vs. OGD+Dex. (F–G) Representative TUNEL images of MSCs (n = 5 in each group, 100×, scale bar = 100 μM). MSCs in the control group were cultured normally; MSCs in the OGD group were subjected to OGD for 24 h; MSCs in the Dex group were pretreated with Dex at a concentration of 10 μM for 1 h followed by normal culture, and MSCs in the OGD+Dex group were subjected to OGD for 24 h after pretreatment with Dex. White arrows point to apoptotic cells, while blue stains indicate the nuclei of MSCs. Dex: dexmedetomidine, DUSP1: dual-specificity phosphatase, OGD: oxygen and glucose deprivation, MAPK: mitogen-activated protein kinase, MKP-1: MAPK phosphatase-1, MSC: mesenchymal stem cells, PI: propidium iodide, TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
Suppression of protein synthesis upon oxygen and glucose deprivation
Results from the qPCR analysis suggested that compared with the control group, OGD significantly increased the expression of MKP-1 (Fig. 3A), while the expression of Beclin1 was markedly decreased (Fig. 3B) after MSCs were deprived of oxygen and glucose for 24 h.
Inhibition of Beclin1 protein synthesis by OGD in MSC might lead to downregulation of autophagy, and upregulation of MKP-1 expression by OGD might aggravate cell injury.
Figure 3: Levels of Dusp1, HIF, Beclin1, and beta-actin mRNAs in MSCs under OGD. (A) Under the OGD condition, the expression of the Dusp1 mRNA was significantly increased compared with both the control and OGD+Dex groups, while the gene expression level of MKP-1 was downregulated by Dex. (B) Dex upregulated the expression level of Beclin1 under OGD conditions. (C) Dex had no influence on HIF-1α changes induced by OGD (One-way ANOVA. Results are expressed as the mean ± SD. *p < 0.05 OGD vs. OGD+Dex). Dex: dexmedetomidine, Dusp1: dual-specificity phosphatase, HIF: hypoxia-inducible factor, MSC: mesenchymal stem cells, OGD: oxygen and glucose deprivation.
Downregulation of MKP-1 and attenuation of OGD-induced changes
Western blot results revealed that when MSCs were pretreated with 10 μM of Dex for 24 h before OGD, Dex markedly increased the levels of Beclin1 and LC3 proteins, whereas Dex had no effect on HIF-1α protein level, which may be an OGD-induced change in p38 (Fig. 4). The results suggest that Dex markedly increased protein levels of Beclin1 and LC3 related to autophagy and downregulated MKP-1 related to the MAPK signaling pathway, indicating that Dex induces autophagy in MSCs under OGD to produce energy for the metabolic need of other cells. Downregulation of MKP-1 by Dex had no effect on HIF-1α and p38 MAPK activity in response to OGD-induced cell death.
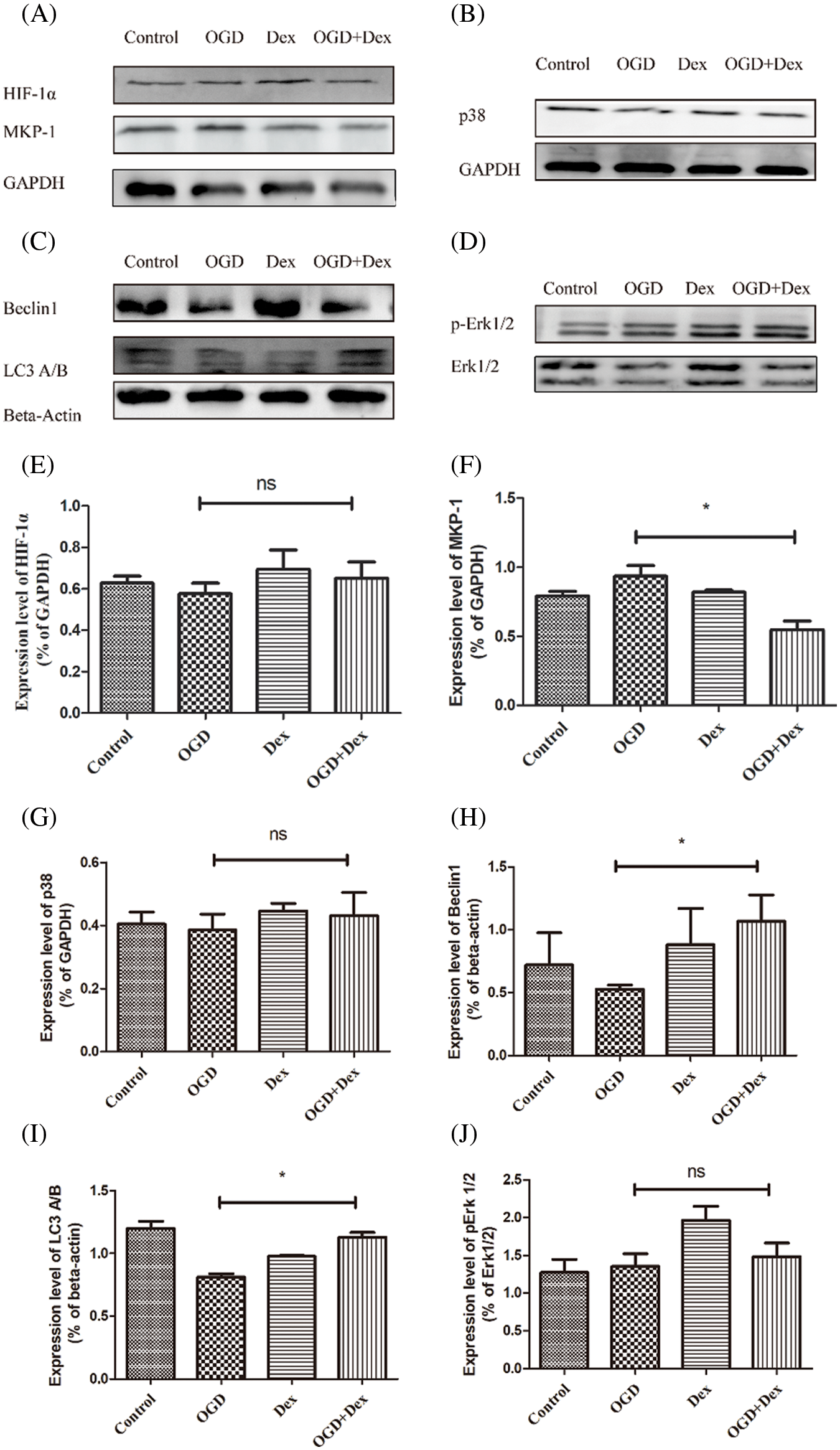
Figure 4: Upregulation of autophagy by Dex via the downregulation of MKP-1 in OGD-induced injury in MSCs. (A) Western blot images of MKP-1, p38, HIF-1α, p-ERK1/2, ERK1/2, Beclin1, LC3 A/B, GAPDH, and beta-actin in MSCs under different conditions. (B) Quantitative analysis of the ratio of MKP-1 or HIF-1α to GAPDH. The expression of MKP-1 was significantly downregulated in the Dex group compared with the control group. (C) The treatment of Dex had no effect on total ERK1/2, with focus given only to the effect of Dex on the ratio of pERK1/2 to ERK1/2. Quantitative analysis of the ratio of ERK1/2 phosphorylation to total ERK1/2 showed markedly increased phosphorylation of ERK1/2 in the Dex group, showing a significant difference compared to the OGD and the OGD+Dex groups. (D) Quantitative analysis of the ratio of Beclin1 phosphorylation to beta-actin. The Beclin1 level in MSCs was upregulated by Dex in OGD-induced apoptosis. (E) Quantitative analysis of the ratio of LC3 to beta-actin. LC3 level in MSCs was downregulated by Dex in OGD-induced apoptosis. (F) Quantitative analysis of the ratio of HIF-1α to beta-actin. The level of HIF-1α expression did not change significantly compared to that in the OGD and OGD+Dex groups. The results are expressed as the mean ± SD. *p < 0.05, *p < 0.01 Control vs. Dex, OGD vs. OGD+Dex. Dex: dexmedetomidine, Dusp1: dual-specificity phosphatase, GAPDH: glyceraldehyde 3-phosphate dehydrogenase, HIF: hypoxia-inducible factor, MAPK: mitogen-activated protein kinase, MKP-1: MAPK phosphatase-1, LC3: light chain 3, MSC: mesenchymal stem cells, OGD: oxygen and glucose deprivation.
The focus of this study was to elucidate the potential of Dex as a drug to mitigate MSC death during stem cell transplantation. This was done by identifying dysregulated mRNAs from microarray data downloaded from GEO and determining the expression levels of MKP-1 and MAPK in rat MSCs pre-treated with Dex and kept under an OGD condition. Oxygen and glucose deprivation stimulated an ischemic insult to the in vitro transplanted MSCs. Although stem cell transplant serves as a novel therapeutic approach, the survival rate of transplanted stem cells within the ischemic myocardium was unsatisfactory, hindering its clinical application (Kim et al., 2020).
Dex has been widely used in general anesthesia and intensive care for medications targeting the α2 receptors (Lankadeva et al., 2021). The results of GEO profiling show that Dex downregulates the gene expression of MKP-1 in the ischemia-reperfusion rat heart model, which is associated with enhanced MAPK phosphatase activity (Yoshikawa et al., 2019).
Initially, Yang et al. (2019) reported that MKP-1 pathways were considered apoptosis mediators modulating cell death (Yang et al., 2019). Chang et al. (2020) revealed that Dex pre-treatment can protect the ischemic heart via the inhibition of apoptosis signaling (Chang et al., 2020). Interestingly, MKP-1 may show similar effects to a switch that controls monocyte proliferation and activation (Comalada et al., 2012). Our major finding is the downregulation of MKP-1 along with the increase in autophagy-related Beclin1 and LC3 expression due to Dex treatment. The interplay between Beclin1, LC3, and MKP-1 phosphatase may allow them to work together as agonists and antagonists in regulating cell apoptosis.
Our study revealed that OGD for 24 h suppresses protein synthesis, consistent with previous studies. One earlier study reported that Dex reduces the infarct size/volume in mouse brain and downregulates the expression of autophagy-related proteins such as LC3 and Beclin1 in the OGD model (Luo et al., 2017).
In our study, the expression of HIF-1α and p38 MAPK remained unchanged in the OGD+Dex group than in the OGD group within 24 h of OGD. The timepoint of assessment of HIF expression may have been behind the peak activity of HIF and failed to catch the HIF expression, which should be considered while planning the future study. Limitations of the present study include that autophagy flux associated with MKP-1 expression levels and function of MSCs in vivo have not been observed.
We observed that OGD-induced changes in terms of HIF-2α expression were time-dependent. Studies have earlier shown that a period of OGD within 20 h induces elevated HIF-2α expression levels, whereas an extended period of OGD for over 60 h leads to the markedly decreased expression (Andreev et al., 2015). Interestingly, the results from this study suggested otherwise: the expression of HIF-1α in MSCs was not markedly suppressed after undergoing 24 h of OGD. Understanding this molecular mechanism may pave a novel way for stem cell treatment in IHD.
Pretreatment of MSCs with Dex demonstrated a protective effect against OGD-induced apoptosis along with the inactivation of the MKP-1 signaling pathway in vitro and may not be associated with HIF.
Author Contribution: Yanqi Y conceived and designed the experiments. Ruicong G and Kuan Z performed the experiments. Minnan G downloaded and analyzed the GEO data. Jianfen L, Huiqi J, Lu Z, Jingwen L, Bin Z, Yuqiang L, Zhu XL, and Dian W repeated the experiments and verified the statistical findings. All authors reviewed and approved the final version of the paper.
Availability of Data and Materials: All data used in this study would be made available through a request from the corresponding author.
Ethics Approval: Not applicable.
Funding Statement: This work was supported by grants from the 3 × 3 Clinical Scientist Fund of Sun Yat-sen Memorial Hospital (1320900026) and the National Natural Science Foundation for Young Scientists of China (81600245) from the Guangdong Science and Technology Department (2020B1212060018).
Conflicts of Interest: The authors declare no conflicts of interest associated with this study.
Andreev DE, O’Connor P, Zhdanov A, Dmitriev R, Shatsky I, Papkovsky D, Baranov P (2015). Oxygen and glucose deprivation induces widespread alterations in mRNA translation within 20 minutes. Genome Biology 16: 90. DOI 10.1186/s13059-015-0651-z. [Google Scholar] [CrossRef]
Bachmann J, Ehlert E, Becker M, Otto C, Radeloff K et al. (2020). Ischemia-like stress conditions stimulate trophic activities of adipose-derived stromal/stem cells. Cells 9: 1935. DOI 10.3390/cells9091935. [Google Scholar] [CrossRef]
Chang JH, Jin MM, Liu JT (2020). Dexmedetomidine pretreatment protects the heart against apoptosis in ischemia/reperfusion injury in diabetic rats by activating PI3K/Akt signaling in vivo and in vitro. Biomedicine & Pharmacotherapy 127: 110188. DOI 10.1016/j.biopha.2020.110188. [Google Scholar] [CrossRef]
Choudhury T, Mozid A, Hamshere S, Yeo C, Pellaton C et al. (2017). An exploratory randomized control study of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with ischaemic cardiomyopathy: The REGENERATE-IHD clinical trial. European Journal of Heart Failure 19: 138–147. DOI 10.1002/ejhf.676. [Google Scholar] [CrossRef]
Comalada M, Lloberas J, Celada A (2012). MKP-1: A critical phosphatase in the biology of macrophages controlling the switch between proliferation and activation. European Journal of Immunology 42: 1938–1948. DOI 10.1002/eji.201242441. [Google Scholar] [CrossRef]
Ferro F, Spelat R, Shaw G, Duffy N, Islam MN, O’Shea PM, O’Toole D, Howard L, Murphy JM (2019). Survival/Adaptation of bone marrow-derived mesenchymal stem cells after long-term starvation through selective processes. Stem Cells 37: 813–827. DOI 10.1002/stem.2998. [Google Scholar] [CrossRef]
Huang R, Xu Y, Wan W, Shou X, Qian J et al. (2015). Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Molecular Cell 57: 456–466. DOI 10.1016/j.molcel.2014.12.013. [Google Scholar] [CrossRef]
Karpov AA, Udalova DV, Pliss MG, Galagudza MM (2017). Can the outcomes of mesenchymal stem cell-based therapy for myocardial infarction be improved? Providing weapons and armour to cells. Cell Proliferation 50: e12316. DOI 10.1111/cpr.12316. [Google Scholar] [CrossRef]
Keating GM (2015). Dexmedetomidine: A review of its use for sedation in the intensive care setting. Drugs 75: 1119–1130. DOI 10.1007/s40265-015-0419-5. [Google Scholar] [CrossRef]
Kim CW, Kim CJ, Park EH, Lee E, Seong E et al. (2020). Intramyocardial transplantation of MSC-loading injectable hydrogels after myocardial infarction in a murine model. Journal of Visualized Experiments 163. DOI 10.3791/61752. [Google Scholar] [CrossRef]
Kim HS, Asmis R (2017). Mitogen-activated protein kinase phosphatase 1 (MKP-1) in macrophage biology and cardiovascular disease. A redox-regulated master controller of monocyte function and macrophage phenotype. Free Radical Biology and Medicine 109: 75–83. DOI 10.1016/j.freeradbiomed.2017.03.020. [Google Scholar] [CrossRef]
Lankadeva YR, Yahya S, Adam MD, Mark PP, Rinaldo B, Clive N (2021). Emerging benefits and drawbacks of α(2)-adrenoceptor agonists in the management of sepsis and critical illness. British Journal of Pharmacology 178: 1407–1425. DOI 10.1111/bph.15363. [Google Scholar] [CrossRef]
Liu D, Tang W, Zhang H, Huang H, Zhang Z, Tang D, Jiao F (2020). Icariin protects rabbit BMSCs against OGD-induced apoptosis by inhibiting ERs-mediated autophagy via MAPK signaling pathway. Life Science 253: 117730. DOI 10.1016/j.lfs.2020.117730. [Google Scholar] [CrossRef]
Luo C, Ouyang MW, Fang YY, Li SJ, Zhou Q, Fan J, Qin ZS, Tao T (2017). Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating HIF-1α. Frontiers in Cellular Neuroscience 11: 197. DOI 10.3389/fncel.2017.00197. [Google Scholar] [CrossRef]
Miao C, Lei M, Hu W, Han S, Wang Q (2017). A brief review: The therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Research and Therapy 8: 242. DOI 10.1186/s13287-017-0697-9. [Google Scholar] [CrossRef]
Mihai MC, Popa MA, Suica VI, Antohe F, Jackson EK et al. (2019). Mechanism of 17β-estradiol stimulated integration of human mesenchymal stem cells in heart tissue. Journal of Molecular and Cellular Cardiology 133: 115–124. DOI 10.1016/j.yjmcc.2019.06.007. [Google Scholar] [CrossRef]
Ryou MG, Mallet RT (2018). An in vitro oxygen-glucose deprivation model for studying ischemia-reperfusion injury of neuronal cells. Methods in Molecular Biology 1717: 229–235. DOI 10.1007/978-1-4939-7526-6. [Google Scholar] [CrossRef]
Tran S, Fairlie WD, Lee EF (2021). BECLIN1: Protein structure, function and regulation. Cells 10: 1522. DOI 10.3390/cells10061522. [Google Scholar] [CrossRef]
van Nguyen TT, Vu NB, van Pham P (2021). Mesenchymal stem cell transplantation for ischemic diseases: Mechanisms and challenges. Tissue Engineering and Regenerative Medicine 18: 587–611. DOI 10.1007/s13770-021-00334-3. [Google Scholar] [CrossRef]
Webster I, Smith A, Lochner A, Huisamen B (2017). The role of MKP-1 in insulin-induced cardioprotection. Cardiovascular Drugs and Therapy 31: 247–254. DOI 10.1007/s10557-017-6731-4. [Google Scholar] [CrossRef]
Whitaker RH, Cook JG (2021). Stress relief techniques: P38 MAPK determines the balance of cell cycle and apoptosis pathways. Biomolecules 11: 1444. DOI 10.3390/biom11101444. [Google Scholar] [CrossRef]
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, Li CY, Li CJ (2018). Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiology and Biochemistry 46: 1650–1667. DOI 10.1159/000489241. [Google Scholar] [CrossRef]
Yang J, Sun L, Han J, Zheng W, Peng W (2019). DUSP1/MKP-1 regulates proliferation and apoptosis in keratinocytes through the ERK/Elk-1/Egr-1 signaling pathway. Life Science 223: 47–53. DOI 10.1016/j.lfs.2019.03.018. [Google Scholar] [CrossRef]
Yang FY, Zhang L, Zheng Y, Dong H (2022). Dexmedetomidine attenuates ischemia and reperfusion-induced cardiomyocyte injury through p53 and forkhead box O3a (FOXO3a)/p53-upregulated modulator of apoptosis (PUMA) signaling signaling. Bioengineered 13: 1377–1387. DOI 10.1080/21655979.2021.2017611. [Google Scholar] [CrossRef]
Yoshikawa Y, Hirata N, Terada H, Sawashita Y, Yamakage M (2019). Identification of candidate genes and pathways in dexmedetomidine-induced cardioprotection in the rat heart by bioinformatics analysis. International Journal of Molecular Sciences 20: 1614. DOI 10.3390/ijms20071614. [Google Scholar] [CrossRef]
Yu H, Lu K, Zhu J, Wang J (2017). Stem cell therapy for ischemic heart diseases. British Medical Bulletin 121: 135–154. DOI 10.1093/bmb/ldw059. [Google Scholar] [CrossRef]
Yu T, Liu D, Gao M, Yang P, Zhang M, Song F, Zhang X, Liu Y (2019). Dexmedetomidine prevents septic myocardial dysfunction in rats via activation of α7nAChR and PI3K/Akt-mediated autophagy. Biomedicine & Pharmacotherapy 120: 109231. DOI 10.1016/j.biopha.2019.109231. [Google Scholar] [CrossRef]
Zhai M, Liu C, Li Y, Zhang P, Yu Z, Zhu H, Zhang L, Zhang Q, Wang J, Wang J (2019). Dexmedetomidine inhibits neuronal apoptosis by inducing Sigma-1 receptor signaling in cerebral ischemia-reperfusion injury. Sedentary Life and Nutrition 11: 9556–9568. DOI 10.18632/aging.102404. [Google Scholar] [CrossRef]
Zheng X, Cai X, Ye F, Li Y, Wang Q, Zuo Z, Huang W, Wang Z (2020). Perioperative dexmedetomidine attenuates brain ischemia reperfusion injury possibly via up-regulation of astrocyte Connexin 43. BMC Anesthesiology 20: 299. DOI 10.1186/s12871-020-01211-7. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |