DOI:10.32604/biocell.2022.021510

| BIOCELL DOI:10.32604/biocell.2022.021510 |  |
| Article |
Exosomal miR-218 regulates the development of endometritis in dairy cows by targeting TGIF2/TGF-β pathway
1Animal Science and Technology College, Beijing University of Agriculture, Beijing, 102206, China
2Department of Animal Husbandry and Veterinary, Beijing Vocational College of Agriculture, Beijing, 102442, China
3Beijing Changping Animal Disease Prevention and Control Center, Beijing, 102200, China
4China Institute of Veterinary Drugs Control, Beijing, 100000, China
*Address correspondence to: Longfei Xiao, xiaolf1989@bua.edu.cn; Xiangguo Wang, xiangguo731@163.com
#These authors contributed equally to this work
Received: 18 January 2022; Accepted: 30 March 2022
Abstract: Endometritis affects the reproductive capacity of dairy cows and leads to serious economic losses in dairy farming. Clarification of the pathogenesis of endometritis is necessary to improve the reproductive efficiency of dairy cows. Exosomes and their miRNAs have been proven to play an important role in inflammatory regulation. Exosomal miR-218 is a differentially expressed miRNA found in endometrial epithelial cells (EECs) under endometrial inflammation. Therefore, we investigated the expression of miR-218 in the uterine tissue of dairy cows, lipopolysaccharide (LPS) treated EECs, exosomal vesicles, and regulation of exosomal miR-218 by targeting TGIF-2 inducible factor homology frame 2 (TGIF2)/transforming growth factor-beta (TGF-β). The expression of miR-218 was suppressed in inflammatory uterine tissues and LPS treated EECs. The expression of TGIF2 and TGF-β in inflammatory uterine tissues and LPS treated EECs was significantly higher than those in healthy uterine tissues and EECs (p < 0.01). Interestingly, miR-218 derived from donor cells was found to regulate the expression of the target gene TGIF2 in recipient cells through the fusion of exosomes. Concurrently, the expression of its target gene TGIF2 was also suppressed by miR-218 in donor cells resulting in fewer TGIF2 being transported into recipient cells with exosomal fusion. This may be a novel mechanism of miRNAs-mediated regulation and provides a new reference for analyzing the pathogenesis of endometritis in dairy cows.
Keywords: Dairy cows; Endometritis; Exosomes; miR-218; TGIF2/TGF-β
Endometritis, a common disease of the reproductive system, mostly occurs in dairy cows after delivery or childbirth and causes infertility in dairy cows (Wang et al., 2021a). The causes of cow endometritis are relatively complex, and bacterial infection is one the most important etiological agents linked with this disease (Li et al., 2021a). Besides infection by pathogenic microorganisms, the autoimmune status of dairy cows determines the occurrence and development of endometrial inflammation (Siqueira et al., 2020). Clinical diagnosis of endometritis in cows typically includes rectal, vaginal speculum, routine blood, and biochemical indexes examinations, bacterial culture, and endometrial biopsy. The main treatment methods include antibiotics (Dubuc et al., 2011), traditional Chinese medicine (Jinliang et al., 2011), microbial preparation (Osawa, 2021), and ozone therapy treatments (Escandon et al., 2020).
Bovine endometrial epithelial cells (EECs) are the first line of defense against infections caused by various invasive factors (Piras et al., 2017). The lipopolysaccharide (LPS) released from the bacterial surface mainly binds to the toll-like receptor 4 (TLR4) and myeloid differentiation protein-2 (MD2) to form protein complexes (Kim et al., 2007). The binding of TLR4-LPS leads to the activation of the nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK), which then activates downstream inflammatory mediators, including the cytokines IL-1β and IL-6, tumor necrosis factor (TNF)-α, cyclooxygenase-2, inducible nitric oxide synthase, and chemokine IL-8 (Regueiro et al., 2009). The number of studies on the immune response to endometritis in dairy cows has greatly increased over the past 20 years.
Currently, extracellular vesicles are considered an effective vehicle for intercellular communication because of their ability to transfer proteins, lipids, and nucleic acids (O’Brien et al., 2020). Exosomes are a subset of extracellular vesicles released by almost all cell types and contain several biological components, including membrane proteins, lipids, RNA, and DNA (Yáñez-Mó et al., 2015). After being released from donor cells, exosomes are distributed in biological fluids and taken up by the same or different types of cells to exert biological regulatory function (Sahoo and Losordo, 2014). Exosome microRNAs (miRNAs), the main molecules playing a regulatory role in exosomes, primarily cause gene silencing in receptor cells (Sato et al., 2017a; Liu et al., 2021). The transfer of exosome miRNAs between DCs is considered a means of communication and post-transcriptional regulation (Montecalvo et al., 2012). The exosome miR-150 released by B lymphocytes is internalized by CD8 + T lymphocytes, leading to significant downregulation of endogenous miR-150 (Almanza et al., 2013). B lymphocyte-derived exosomes are effective vectors for delivering anti-miR-155 to macrophages, thereby down-regulating endogenous miRNA in recipient cells (Momen-Heravi et al., 2014). Exosome-mediated transfer of Let-7d from Treg cells to Th1 cells helps suppress and prevent systemic disease (Okoye et al., 2014). MiR-218, a pre-miRNA sequence, is located in the noncoding region of the host genes of the Slit guidance ligands 2 and 3 (SLIT2 and SLIT3). Mature miR-218 affects cell function by interacting with transcription targets such as roundabout guidance receptor (ROBO)-1 and modulating cell migration (Wang et al., 2020). MiR-218 plays a functional role in human endothelial cells, and its inhibition confers a migratory phenotype (Pang et al., 2017). MicroRNA-218-5p promotes embryonic trophoblast differentiation and is involved in the pathogenesis of pre-eclampsia (Brkić et al., 2018). We previously found that exosomal-derived uterine miRNAs isolated 6from dairy cows with endometritis impede blastocyst development (Wang et al., 2019b). We also found that endometrial inflammation decreases the expression of miR-218 in the uterus of dairy cows, and exosome-derived uterine miR-218 isolated from dairy cows with endometritis regulates the release of cytokines and chemokines (Wang et al., 2020). TGIF-2 inducible factor homology frame 2 (TGIF2) belongs to the TALE homologous structural domain protein family, including TGIF, TGIF1, and TGIF2LX/Y (Imoto et al., 2000). We identified TGIF2 as a target gene for miR-218 bio-prediction using a bioinformatics approach. TGIF2 interacts with the suppressor of mothers against decapentaplegic (SMAD) protein to recruit histone deacetylase (HDAC)1 to inhibit transcription of related genes and participate in the regulation of various physiological activities (Du et al., 2019). Transforming growth factor β (TGF-β) is a group of regulatory and fibrotic proteins that affect cell growth and differentiation, apoptosis, cell motility, extracellular matrix production, angiogenesis, and immune response (Shi et al., 2021).
Therefore, in this study, we investigated the expression of miR-218 in uterine tissue of dairy cows and LPS treated EECs and exosomal vesicles, and the regulation of exosomal miR-218 by targeting TGIF2/TGF-β. By exploring the association of exosome miR-218 with TGIF2 and its downstream TGF-β in the mechanism of pathogenesis of endometritis in dairy cows, we tentatively provide a new reference for analysis of the pathogenesis of endometritis in dairy cows.
Diagnosis of endometritis and uterus tissue collection in dairy cows
This study was performed in accordance with the guidelines of the Animal Ethics Committee of the Beijing University of Agriculture. Bovine uteri were obtained from a slaughterhouse. Before slaughter, the postpartum Holstein cows from the Beijing Shun Sunshine Farm were monitored by rectal temperature measurement and rectal examination for uterine rejuvenation on days 1, 7, 14, 21, and 30 after delivery, combined with the daily disease and medication treatments of dairy cows used in this study. The bovine (parity 2–4, body condition score 3.25–4.0) uterus without other diseases such as mastitis, hoof disease, dermatitis, and postpartum paralysis, body temperature lower than 39.5°C and mucopurulent or purulent secretions secreted through vagina reaching the level 3 of vaginal mucus secretion in bovine endometritis were collected at 21–30 days postpartum as the clinical endometritis group. The control group included healthy bovine uteri with normal body temperature and without disease, determined by vaginal endoscopy to be without mucopurulent discharge 21–30 days postpartum. The cows in all groups had no prominently protruding estrous or corpus luteum from the surface of the ovary within 21–30 days after delivery, as determined by rectal examination. Five uteri from healthy cows and five from cows with endometritis were dissected to observe inflammatory changes on the surface of the uterus, and then diff rapid cell staining (Solarbio, G1540, Beijing, China) was used to count the proportion of polymorphonuclear leukocytes in the uterine cavity fluid (Wang et al., 2020). After confirming uterine samples from healthy bovine uteruses and the cows with endometritis, the uterus tissue was collected and stored at –80°C for immunohistochemistry, quantitative reverse transcription-polymerase chain reaction (qRT-PCR), and western blot analyses.
Isolation and identification of exosomes derived from endometrial epithelial cells (EECs)
Cell models were established by stimulating EECs (EEC, A commercial cell line purchased from Tongpai Biotechnology Co., Ltd., GDC-9643015, Shanghai, China) with 50 μg/mL LPS. After incubation for 24 h, exosomes from normal cultured EECs and those from LPS treated EECs were extracted according to the instructions of the Exosomes Isolation Kit (Shanghai Bestbio Biotechnology Co., Ltd., Shanghai, China). Extracted exosomes were identified by electron microscopy, and the expression of CD63 marker protein and Calnexin was detected by western blotting.
Examination of exosome morphology by electron microscopy
The exosomes were re-suspended in 30 μL PBS, then 10 μL samples were poured on the copper net to precipitate for 1 min, and the floating liquid was absorbed with filter paper. After that, uranyl acetate (phosphotungstic acid) 10 μL was added to the copper net for precipitation for 1 min. The floating liquid was absorbed using a filter paper and was dried for a few minutes at 25°C. Electron microscopy imaging was performed at 80 KV.
Uterine tissues were fixed in 4% paraformaldehyde, and tissue sections were baked at 60°C, dewaxed and rehydrated, microwave repaired, washed thrice with PBS containing 0.1% Triton X100 (Solarbio, Beijing, China), and then sealed with 5% BSA (Sigma Aldrich, Missouri, USA). After that, the sections were incubated and washed with primary antibody (1:100 dilution, Abcam, Cambridge, UK), secondary antibody (1:100 dilution, Abcam, Cambridge, UK), and DAPI (Solarbio, Beijing, China) in turn, following instructions of the immunohistochemistry kit. Finally, after washing thrice with PBS, cells were observed with a laser scanning confocal microscope (FV10i, Olympus, Tokyo, Japan).
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) detection
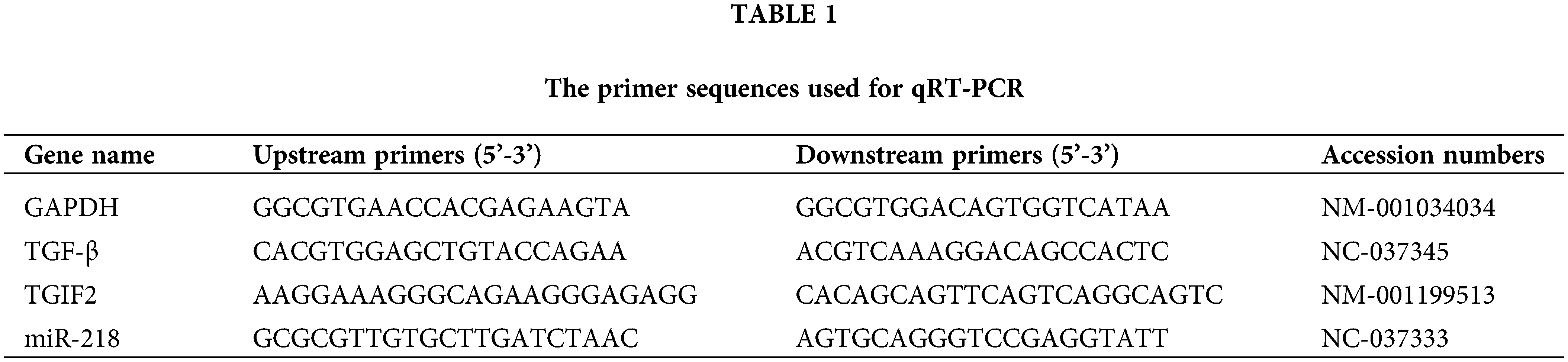
Total RNA of the exosomes, EECs, and uterine tissues were extracted using Trizol (Invitrogen Inc., California, USA), and cDNA was synthesized with the Prime Script RT Reagent Kit (TaKaRa Bio Inc., Dalian, China) according to the manufacturer’s protocols. MiR-218, TGIF2, and TGF-β expressions in this study were detected as described previously (Wang et al., 2019a). The GenBank accession numbers and primer sequences of miR-218, TGIF2, TGF-β, and GADPH are summarized in Table 1.
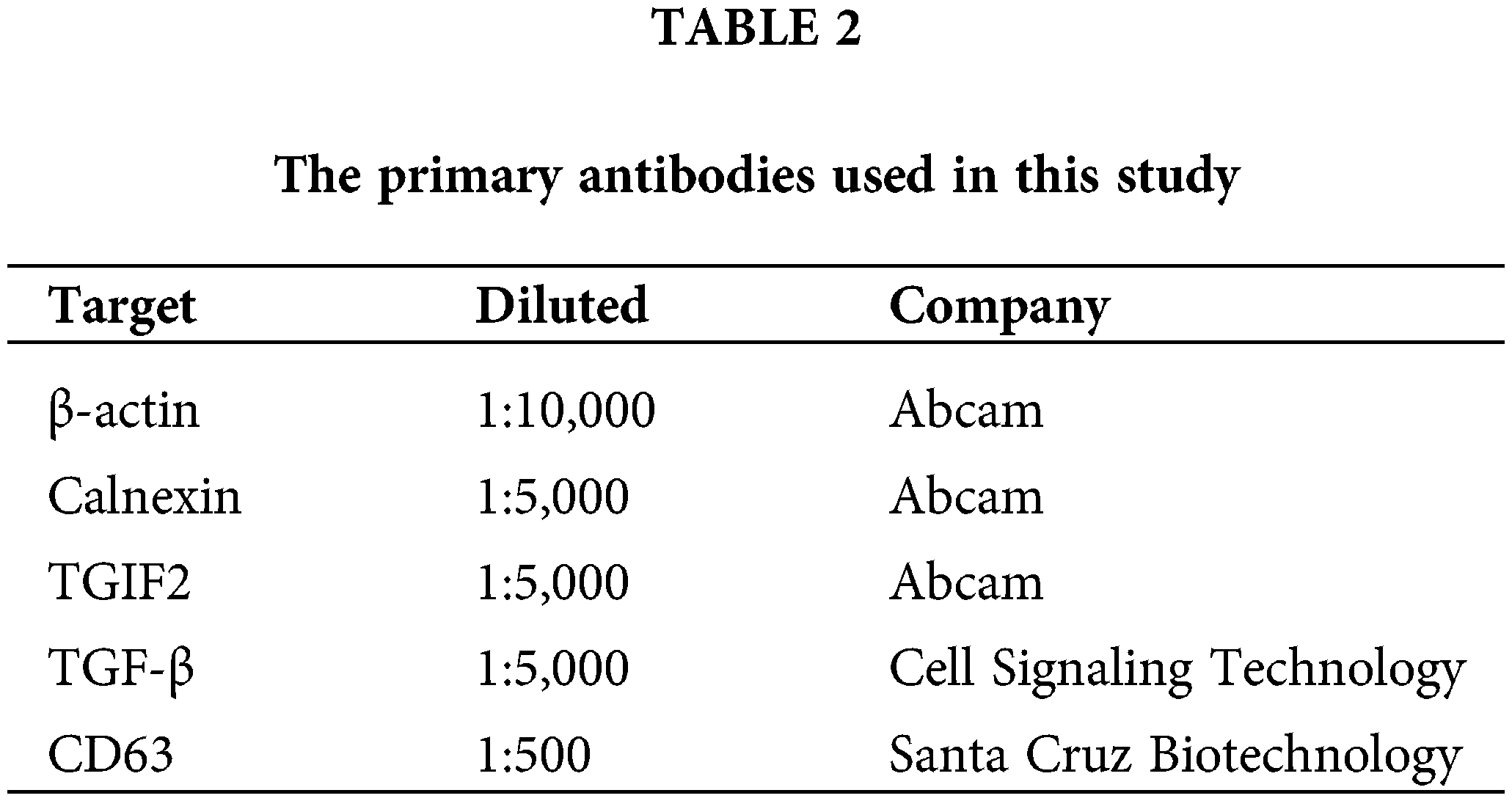
Exosomes, EECs, and uterine tissues were lysed with RIPA buffer (Solarbio, Beijing, China) on ice. Protein concentrations were measured by the BCA assay (Beyotime, Beijing, China). Forty micrograms of protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12% SDS/PAGE gel then electro-transferred onto polyvinylidene fluoride membranes (Millipore, Massachusetts, USA). The membranes were blocked with 5% dry nonfat milk for 1 h at 25°C, and incubated with a primary antibody (Table 2) at 4°C overnight. The next day, membranes were washed and incubated with the corresponding secondary antibody conjugated to horseradish peroxidase (1:2,000; Zhongshan Golden Bridge Biotechnology, Beijing, China) for 30 min at 25°C. Finally, immunoreactive bands were visualized on a gel imaging system (Shanghai Tianeng Technology Co., Ltd., Shanghai, China).
The targets of differentially expressed miRNAs were predicted using miRanda software (Enright et al., 2003) using the following parameters: S ≥ 150, ΔG ≤ –30 kcal/mol and searched for strict 5’ seed pairing. TargetFinder software (Fahlgren and Carrington, 2010) was also used for miRNA target prediction.
The reporter plasmid p-MIR-TGIF2 was designed by GenScript (Nanjing, China). For the luciferase reporter assays, 2 mg of firefly luciferase reporter plasmid, 2 mg of β-galactosidase vector, and 200 pmol of mimic or scrambled NC RNA were transfected into EECs. The β-galactosidase vector was used as a transfection control. At 24 h after transfection, cells were analyzed using a Dual-Luciferase Assay Kit (Promega, Madison, Wisconsin, USA). The miR-218 mimic and NC sequences designed and made by Shanghai Gemar Co., Ltd., were as follows: 5’-UUGUGCUUGAUCUAACCAUGUG-3’(miR-218 mimic, sense), 5’-CAUGGUUAGAUCAAGCACAAUU-3’ (miR-218 mimic, antisense), 5’-UUCUCCGAACGUGUCACGUTT-3’ (NC, sense), and 5’-ACGUGACACGUUCGGAGAATT-3’ (NC, antisense).
Data were analyzed by one-way analysis of variance, followed by the Fisher’s least significant different test or independent-samples t-test using the Statistical Package for the Social Sciences software (Version 16.0; SPSS, Inc.). Differences were considered significant when p < 0.05.
Anatomical and cytological identification of bovine uteri with endometritis
Results from the healthy dairy cows’ uterus revealed a flesh-colored, smooth inner wall and a small amount of clear fluid inside. Inside the uterus of dairy cows with endometritis, inflamed swelling and ulcerations were found on the inner wall along with dark red fluid (Fig. 1A). Exfoliated epithelial cells presented only in healthy uterine luminal fluid in comparison to a large number of neutrophils in the uterine luminal fluid with endometritis (Fig. 1B).
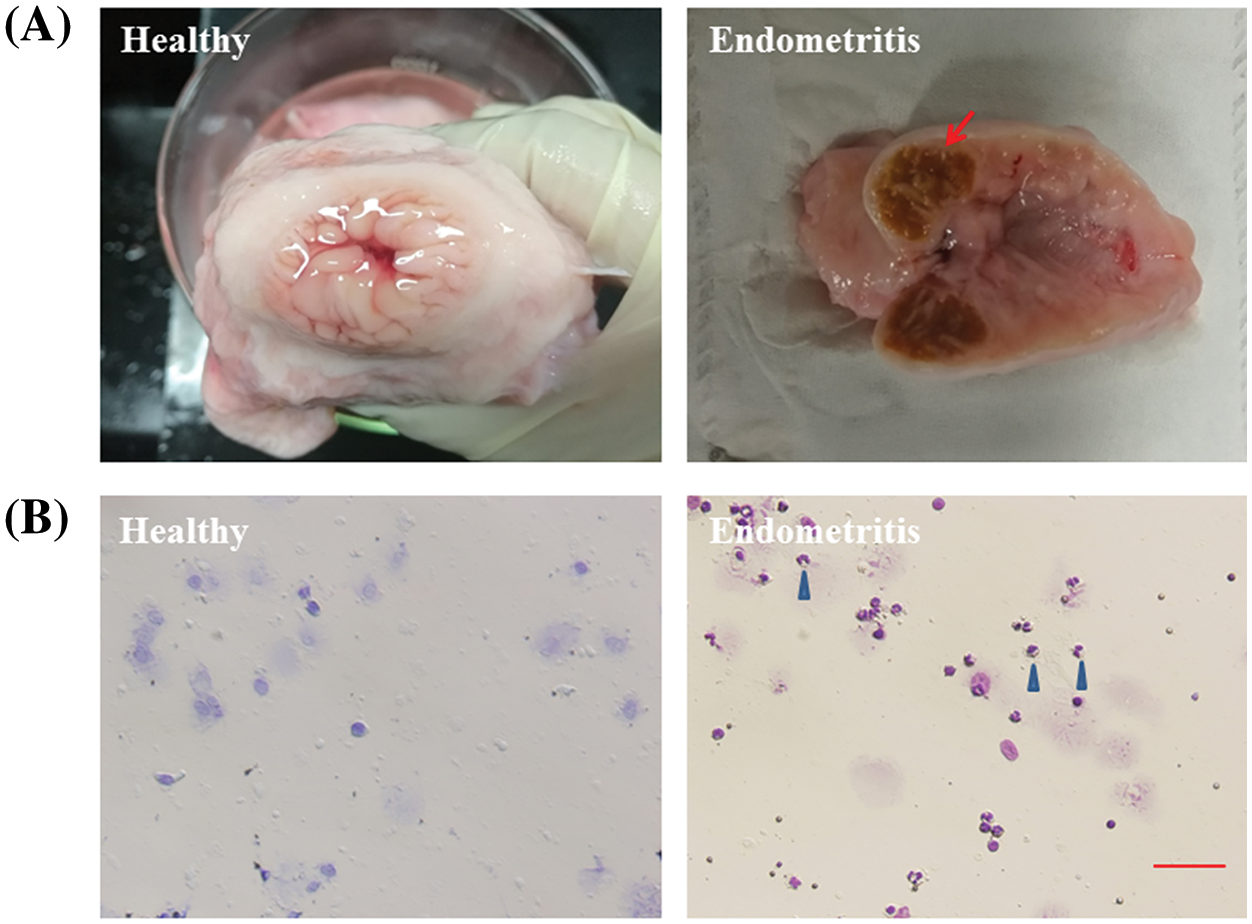
Figure 1: Anatomical and cytological identification of cow uterus. (A) Endometrial cavity of healthy cows and dairy cows with endometritis. (B) Uterine lavage fluid cytology of healthy cows and dairy cows with endometritis. The arrow points to the inflamed uterus. The blue triangle points to a neutrophil.
Localization of TGF-β and TGIF2 in uterine tissues of healthy cows and dairy cows with endometritis
Immunohistochemical analysis showed the localization of TGIF2 mainly in the uterine glandular epithelium of healthy dairy cows. However, TGIF2 was mainly localized in the uterine stroma and endometrial epithelium of dairy cows with endometritis (Fig. 2A). As a TGIF2-induced transcription product, TGF-β was also mainly localized in the uterine glandular epithelium of healthy dairy cows. TGF-β was mainly localized in the uterine stroma and endometrial epithelium of dairy cows with endometritis (Fig. 2B).
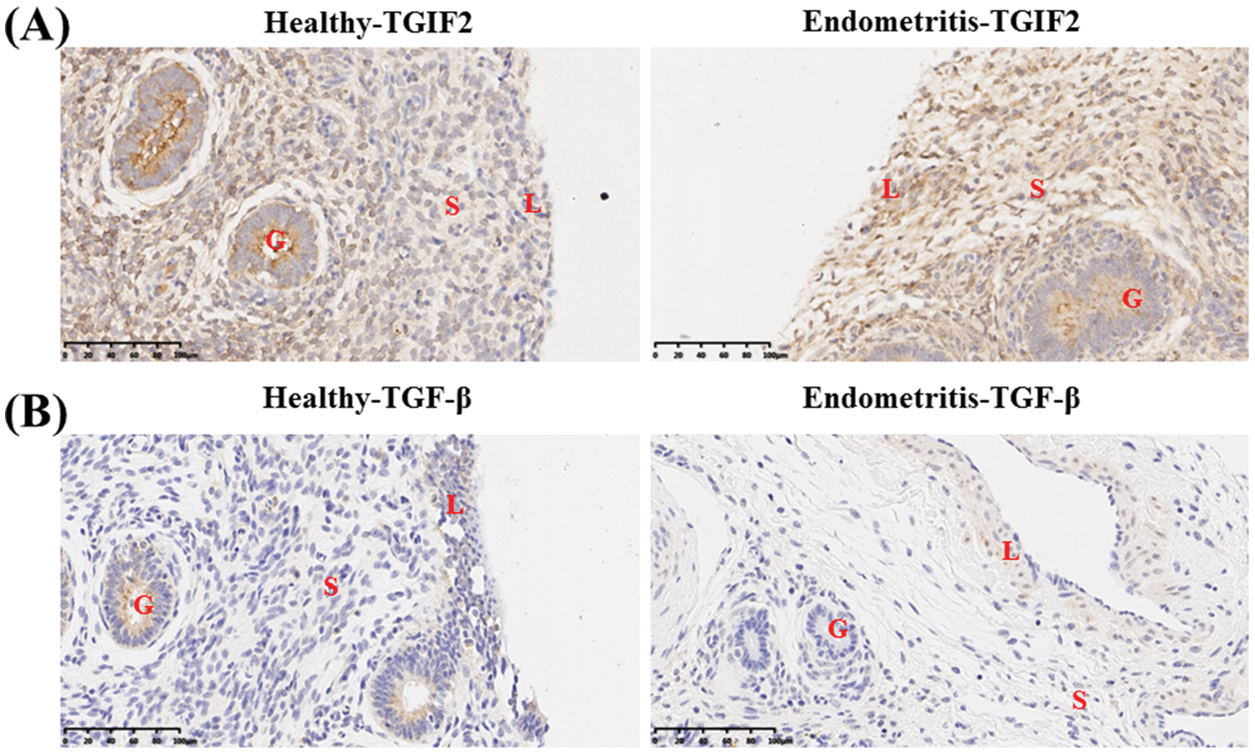
Figure 2: Localization of TGIF2/TGF-β in uterine tissue of healthy cows and dairy cows with endometritis. (A) Localization of TGIF2 in uterine tissue of healthy cows and dairy cows with endometritis. (B) Localization of TGF-β in uterine tissue of healthy cows and dairy cows with endometritis. Data are representative of three independent experiments. S: stroma, G: glandular epithelium, L: endometrial epithelium.
Expression of miR-218, TGF-β, and TGIF2 in uterine tissues of healthy cows and those with endometritis
The expression of TGF-β and TGIF2 mRNAs in uterine tissue of dairy cows with endometritis was significantly higher than those in uterine tissue of healthy dairy cows (Figs. 3A and 3B, p < 0.01). Consistent with our previous report, miR-218 expression in the uterine tissues of dairy cows with endometritis was significantly lower than that of healthy dairy cows (Fig. 3C, p < 0.01). The expression of TGF-β and TGIF2 proteins were consistent with mRNA expression in uterine tissues of healthy dairy cows and dairy cows with endometritis (Fig. 3D).
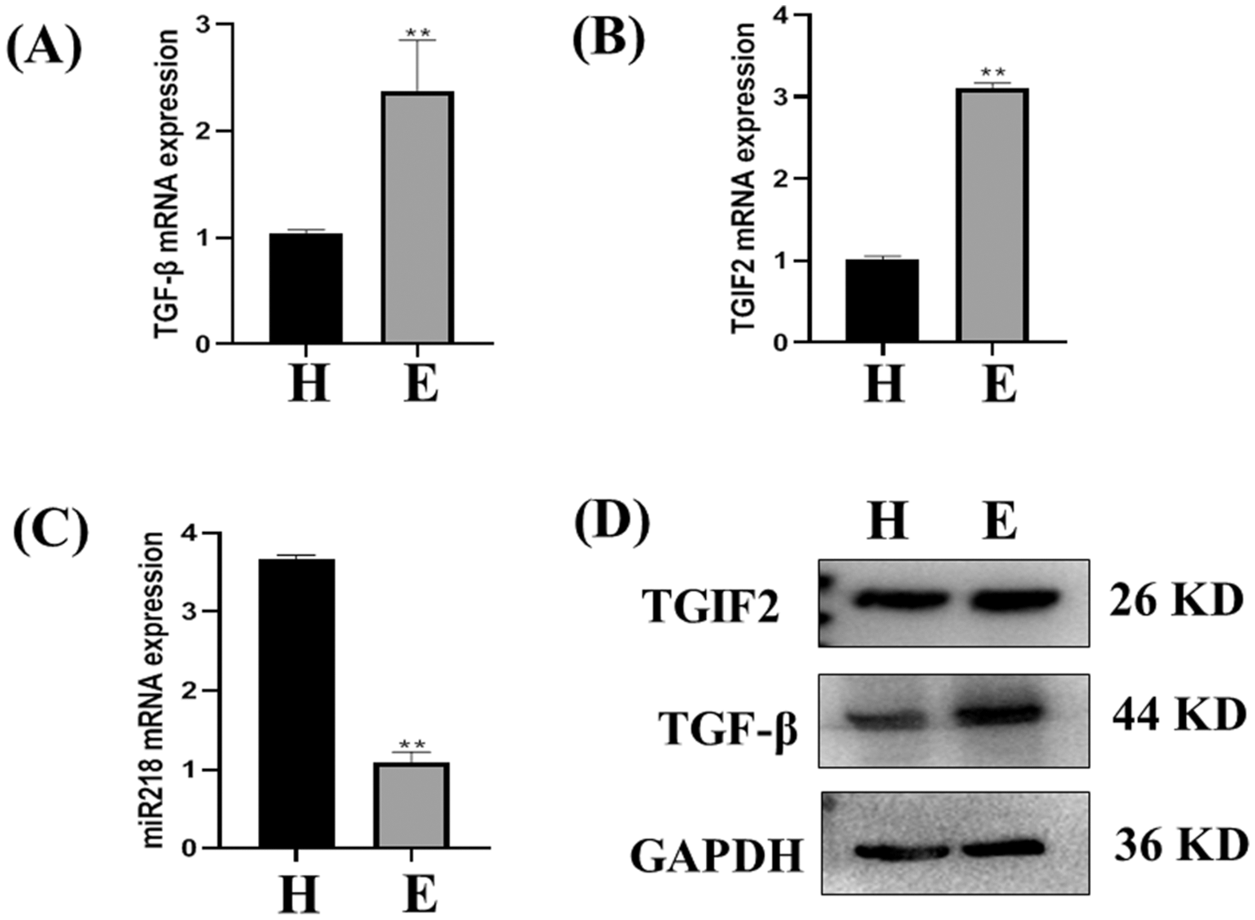
Figure 3: The expression of miR-218/TGF-β/TGIF2 in the uterus of healthy cows and dairy cows with endometritis. Expression of TGF-β (A), TGIF2 (B), miR-218 (C) in uterine tissue of healthy cows and those with endometritis as detected with qRT-PCR. Data are represented with mean + standard deviation from three independent experiments, ** p < 0.01. (D) Protein expression of the TGF-β and TGIF2 as detected by western blotting. Data are representative of three independent experiments.
Expression changes of miR-218, TGF-β, and TGIF2 in EECs and LPS-treated EECs
The expression of TGF-β and TGIF2 mRNAs in LPS treated EECs was significantly higher than in normal cultured EECs (Figs. 4A and 4B, p < 0.01). Consistent with our previous report, miR-218 expression in LPS treated EECs was significantly lower than in normal cultured EECs (Fig. 4C, p < 0.01). TGF-β and TGIF2 expressions in normal cultured EECs and LPS treated EECs were consistent with mRNA expression (Fig. 4D).
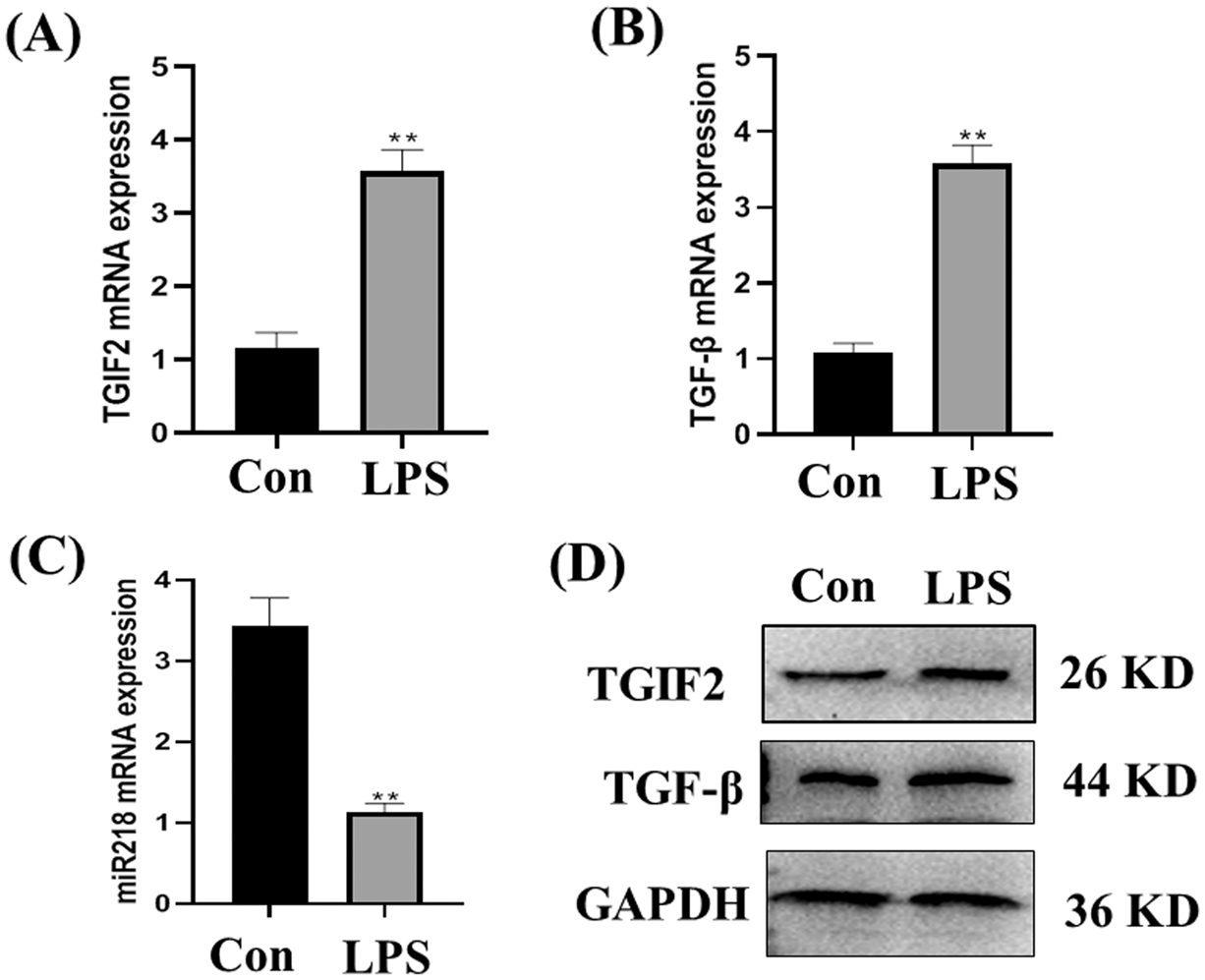
Figure 4: Expression of miR-218/TGF-β/TGIF2 in EECs and LPS treated EECs. Expression of TGF-β (A), TGIF2 (B), miR-218 (C) in EECs and LPS treated EECs (50 μg/mL) as detected with qRT-PCR. Data are represented with mean + standard deviation from three independent experiments, ** p < 0.01. (D) Protein expression of the TGF-β and TGIF2 as detected by western blotting. Data are representative of three independent experiments. Con: control group, LPS: lipopolysaccharide.
Isolation and identification of exosomes derived from EECs and LPS-treated EECs
Electron microscopy results showed that both normal cultured EECs-derived exosomes and LPS treated EECs-derived exosomes had a 30–150 nm particle size and cystic structure (Fig. 5A). Exosome marker protein CD63 was normally expressed (Fig. 5B), while the expression of calnexin protein was not detected (Figs. 5C and 5D).
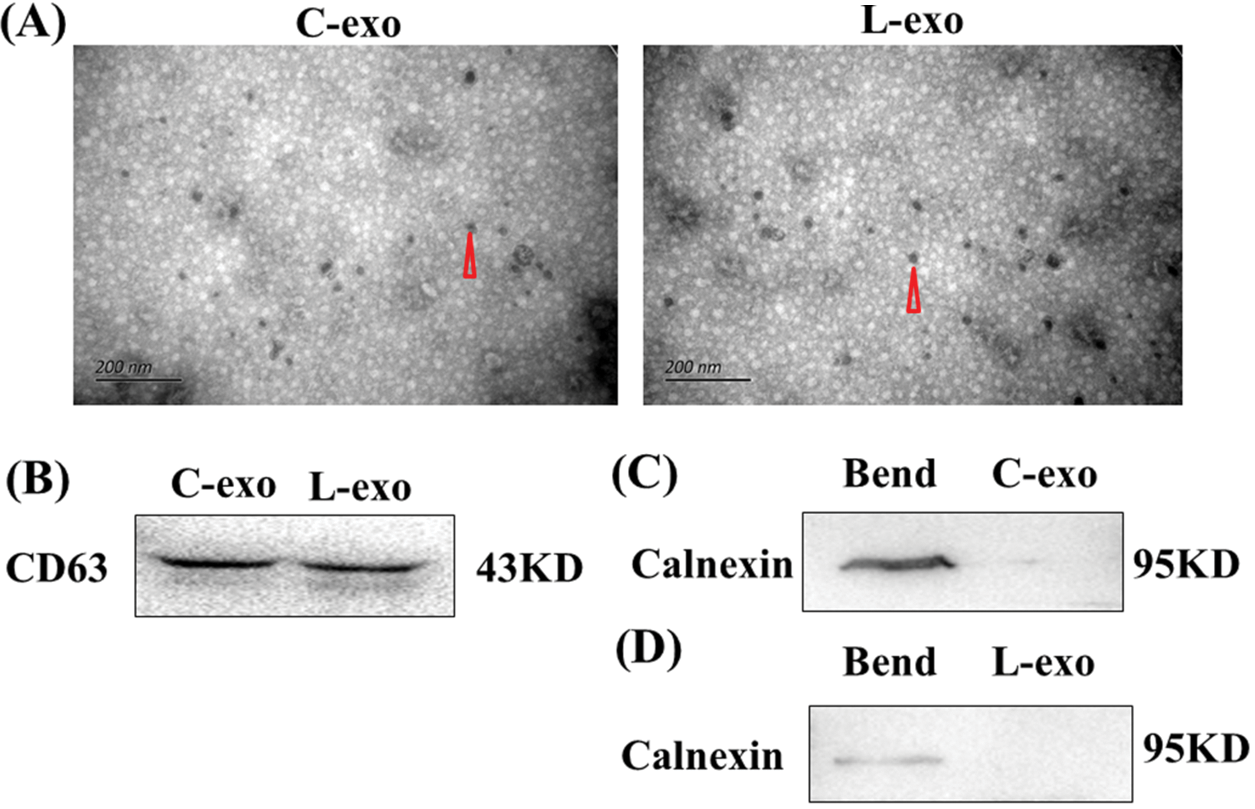
Figure 5: Isolation and identification of EECs and LPS treated EECs derived exosomes. (A) Exosomal vesicle detection with electron microscopy. Scale: 200 nm. Data are representative of three independent experiments. The triangular points for an exosome. (B&C&D) Protein expression of the exosome marker CD63 and non-exosome labeled protein calnexin as detected by western blot. Data are representative of three independent experiments. C-exo: exosome derived from EECs, L-exo: exosome derived from LPS treated EECs.
Exosomal miR-218 targets the TGIF2/TGF-β pathway
MiR-218 expression in LPS treated EECs-derived exosomes was significantly lower than in normal cultured EECs-derived exosomes (Fig. 6A, p < 0.01). In contrast, TGIF2 mRNA expression in LPS treated EECs-derived exosomes was significantly higher than in normal cultured EECs-derived exosomes (Fig. 6B, p < 0.01). Concomitantly, TGIF2 mRNA protein expression in LPS treated EECs-derived exosomes was also significantly higher than that in normal cultured EECs-derived exosomes (Fig. 6C). Bioinformatic analysis suggested that miR-218 can directly target the 3’-untranslated region (3’-UTR) of TGIF2 mRNA (Fig. 6D). EECs transfected with miR-218-mimic had increased miR-218 expression (Fig. 6E, p < 0.01). The relative luciferase activity was inhibited when miR-218-mimic was co-transfected with the luciferase reporter (Fig. 6F, p < 0.05).
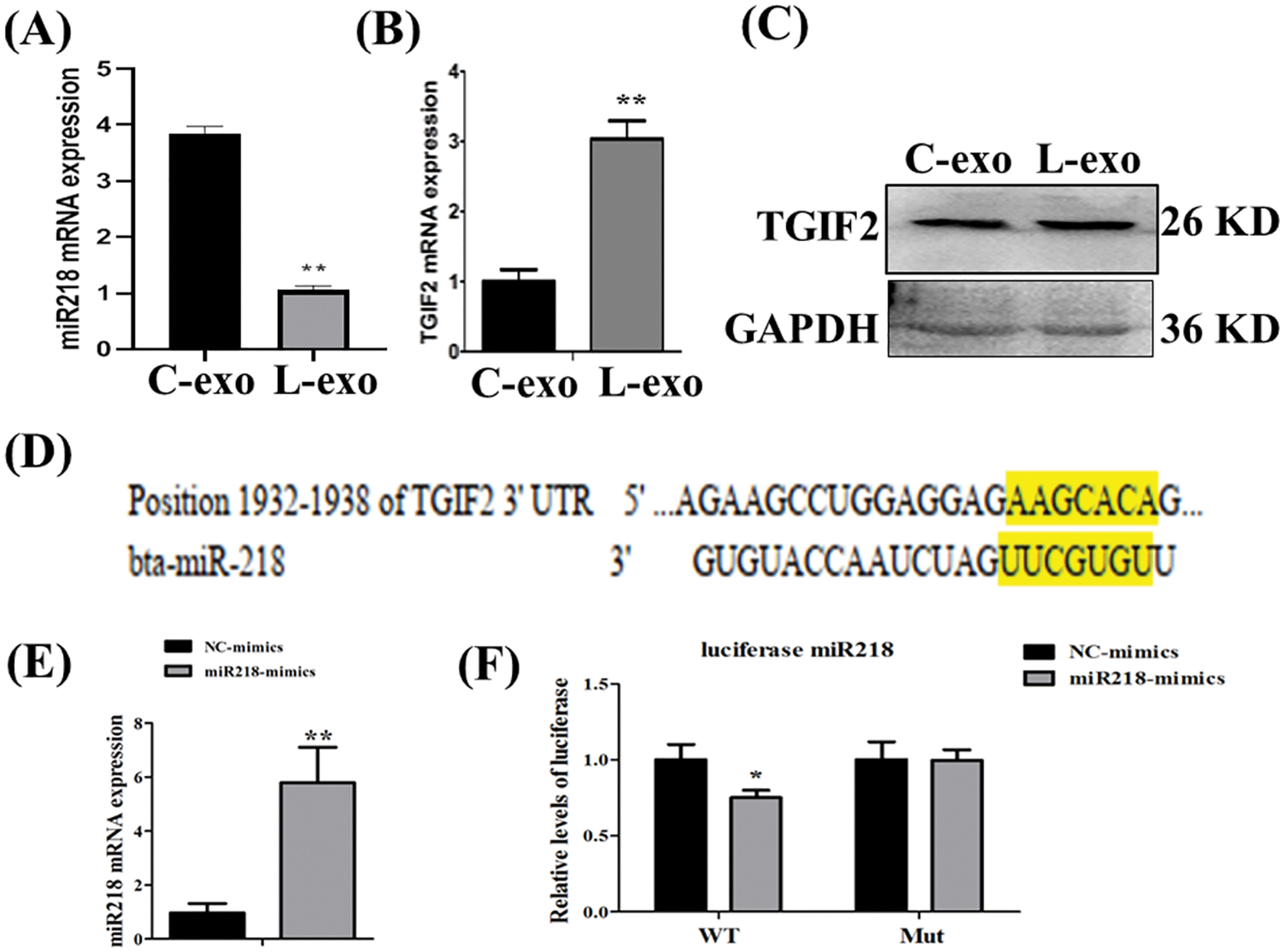
Figure 6: Exosomal miR-218 targets the TGIF2/TGF-β pathway. Expression of miR-218 (A) and TGIF2 (B) in LPS treated EECs exosomes (50 μg/mL) as detected with qRT-PCR. Data are represented with mean + standard deviation (SD) from three independent experiments, ** p < 0.01. (C) Protein expression of the TGIF2 as detected by western blotting. Data are representative of three independent experiments. C-exo: exosome derived from EECs, L-exo: exosome derived from LPS treated EECs. (D) Predicted binding sites of miR-218 within the 3′-UTR of TGIF2 mRNA. (E) qRT-PCR analysis of relative miR-218 levels in EECs transfected with miR-218-mimic. Data shown are the mean±SD from three independent experiments; ** p < 0.01. (F) Direct recognition of the TGIF2 3′-UTR by miR-218. Data are shown as the mean±SD from three independent experiments. 3′-UTR: 3′-untranslated region.
In our previous study, we demonstrated the decrease in miR-218 expression in the uterus of dairy cows with endometritis (Wang et al., 2020). Bioinformatics analysis in this study suggested that miR-218 can directly target the 3’-UTR of TGIF2 mRNA. Immunohistochemical analyses confirmed that the occurrence of endometritis changed the localization and expression of TGIF2 and TGF-β in the endometrial tissues of dairy cows, in contrast to the expression pattern of miR-218 (Wang et al., 2020). TGIF2 is highly expressed in cervical cancer tissues, and inhibition of TGIF2 expression inhibits the proliferation, migration, and invasion of cervical cancer cells (Jiang et al., 2020). In addition, the expression of TGIF2 is upregulated in skin cancer (Tian et al., 2015), ovarian cancer (Ebrahimi and Reiisi, 2019), adult neural tube cell tumors (Taniguchi et al., 2017), multiple myeloma (Wu et al., 2016), and rectal cancer (Liu et al., 2017). Thus, inflammation reduces miR-218 expression, increases TGIF2 expression, and promotes abnormal proliferation and injury of uterine epithelial cells, which may be an important mechanism for the occurrence and development of endometritis in dairy cows. Related studies have confirmed that TGIF2 interacts with SMAD proteins and recruits the histone deacetylase HDAC1 to repress the transcription of related genes in the TGF-β signaling pathway (Glenisson et al., 2007; Tao et al., 2021).
A variety of life processes are affected by TGF-β, including cell growth and differentiation, apoptosis, cell motility, extracellular matrix production, angiogenesis, and immune response (Kondo et al., 2021; Lu et al., 2004). Meanwhile, the TGF-β signaling pathway is closely related to immune regulation as well as angiogenesis (Ali et al., 2013). The expression of TGF-β and IL-10 decreased in chronic endometrial inflammation (Wang et al., 2019a). TGF-β is highly expressed in the endometrium during the secretory period of the menstrual cycle and implantation window period, which can improve endometrial receptivity and participate in the formation of endometrium decidualization, thus facilitating the implantation of embryos (Li et al., 2021b).
With the occurrence of endometrial inflammation, there is an increase in the localization and expression of TGIF2 and TGF-β in endometrial epithelial cells. EECs are the first line of defense against infections caused by various invasive factors (Piras et al., 2017). LPS, as a component of the outer membrane of the cell wall of gram-negative bacteria, has been widely used in inflammatory models such as acute lung injury, mastitis, and endometritis (Wang et al., 2020). In this study, an in vitro model of endometritis was constructed using LPS-treated EECs. The expression of miR-218 decreased, and the mRNA and protein expression of TGF-β and TGIF-2 increased in this in vitro model, consistent with the results obtained in vivo. As a target gene of miR-218 predicted by bioinformatics, TGIF2 and its downstream TGF-β showed increased expression as miR-218 level decreased in uterine tissues of dairy cows with endometritis and LPS-treated EECs. The luciferase reporter assay confirmed the miR-218 targets the TGIF2/TGF-β pathway. In our previous study, we found a significantly lower expression of exosomal miR-218 derived from the uterine fluid of dairy cows than that derived from the uterine fluid of healthy cows (Wang et al., 2021a).
Exosomes, as a secretory mediator carrying components of their miRNAs in the regulation of a variety of physiological activities, have been reported frequently (Sharma, Gupta & Mohanty, 2021). Exosomal miRNAs, the molecules mainly playing a regulatory role in exosomes, have a primarily gene-silencing function in receptor cells (Sato et al., 2017a). In one study, miR-218 could enhance chemosensitivity to oxaliplatin-resistant colorectal cancer through exosomal delivery (Liu et al., 2020). MiR-218 also plays an important role in the regulation of osteoclast differentiation and inflammatory response in rats with periodontitis and the development of human natural killer cells (Guo et al., 2019; Victor et al., 2018). In this study, we observed that the expression of miR-218 decreased under uterine inflammatory conditions, which relieved its inhibitory response to the inhibition of TGIF2/TGF-β and regulated the development of endometritis in dairy cows. MiR-218 has often been reported to play a role in cancer progression by significantly downregulating its expression in cancer tissues (Li et al., 2015). Most interestingly, when miR-218 derived from donor cells fuses into recipient cells with exosomes to regulate target gene TGIF2 expression in recipient cells, miR-218 simultaneously inhibits TGIF2 expression in donor cells, resulting in a decrease in TGIF2 with the fusion of exosomes into recipient cells. Our study may have found a new regulatory mechanism of miRNA, and further studies are needed to consolidate these findings (Fig. 7).
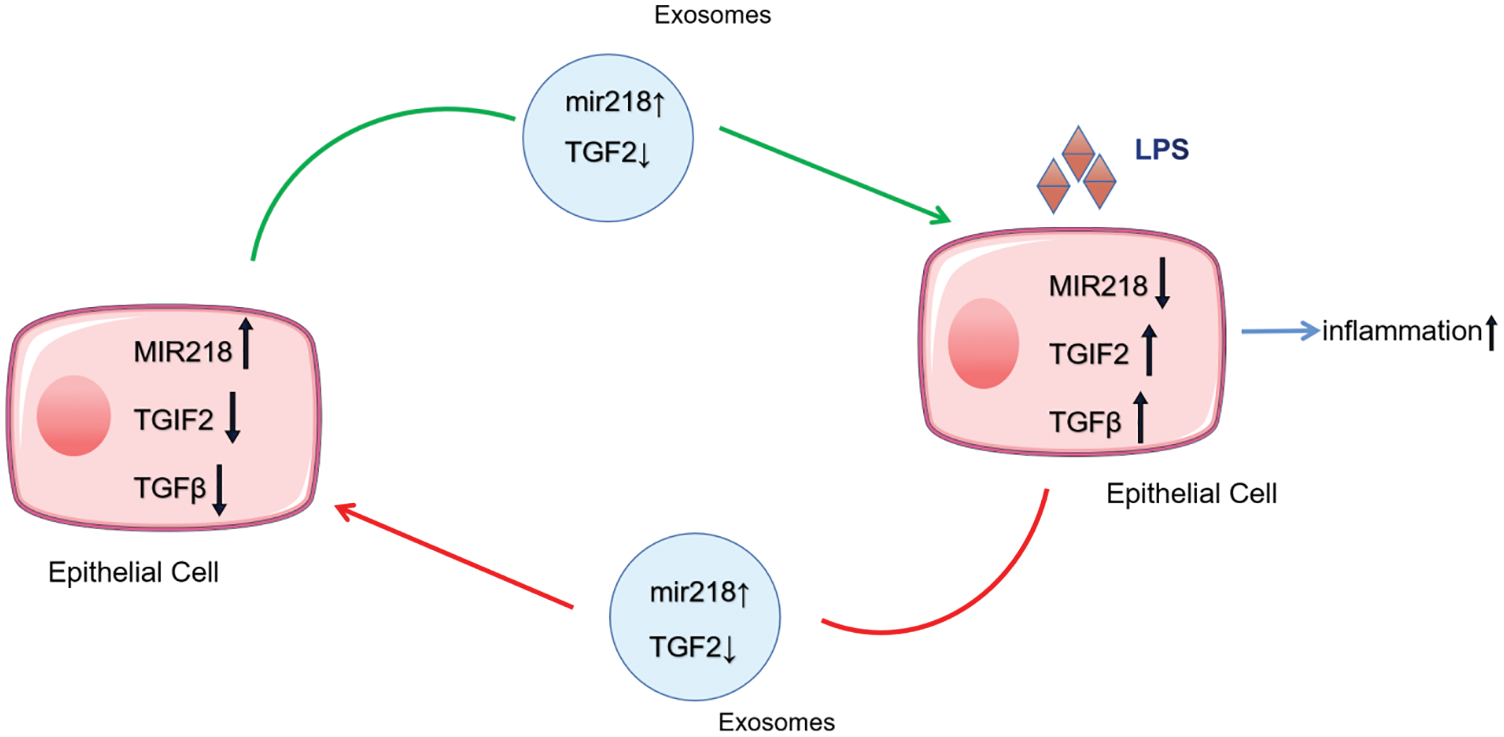
Figure 7: The delivery pattern of miR-218, TGIF2, and TGF-β with exosomes between EECs and LPS-treated EECs.
In summary, our study elucidates a new mechanism for the pathogenesis of endometritis in dairy cows and provides a new potential biomarker for the diagnosis of endometritis.
Acknowledgement: This research was supported by the National Natural Science Foundation of China (No. 31802263).
Availability of Data and Materials: All data generated or analyzed in this study are available from the corresponding author on reasonable request.
Authors’ Contributions: CC, LMQ, and XGW performed the experiments and collected and interpreted the data. KJG, XBG, and HMN provided initial help with analysis. MYY, BFF, and YQW collected the samples. XGW and CC conceptualized and wrote the manuscript. LFX reviewed the manuscript prior to publication.
Ethics Approval and Consent to Participate: All experimental and surgical procedures involving animals were approved by the Beijing Laboratory Animal Management Committee (Permit No. SYXK (JING) 2015-0004).
Funding Statement: This research was supported by the National Natural Science Foundation of China (No. 31802263) and Outstanding Young Talent Project of the Beijing Municipal Party Committee Organization Department (No. 2018000020124G081).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ali A, Wang Z, Fu J, Ji L, Liu J et al. (2013). Differential regulation of the REGγ-proteasome pathway by p53/TGF-β signalling and mutant p53 in cancer cells. S8/ncomms3667. [Google Scholar]
Almanza G, Anufreichik V, Rodvold JJ, Chiu KT, DeLaney A et al. (2013). Synthesis and delivery of short, noncoding RNA by B lymphocytes. PNAS 110: 20182–20187. DOI 10.1073/pnas.1311145110. [Google Scholar] [CrossRef]
Brkić J, Dunk C, Brien O, Fu J, Nadeem G et al. (2018). MicroRNA-218-5p promotes endovascular trophoblast differentiation and spiral artery remodeling. Molecular Therapy 26: 2189–2205. DOI 10.1016/j.ymthe.2018.07.009. [Google Scholar] [CrossRef]
Du R, Shen W, Liu Y, Gao W, Zhou W et al. (2019). TGIF2 promotes the progression of lung adenocarcinoma by bridging EGFR/RAS/ERK signaling to cancer cell stemness. Signal Transduction and Targeted Therapy 4: 60. DOI 10.1038/s41392-019-0098-x. [Google Scholar] [CrossRef]
Dubuc J, Duffield TF, Leslie KE, Walton JS, Leblanc SJ (2011). Randomized clinical trial of antibiotic and prostaglandin treatments for uterine health and reproductive performance in dairy cows. Journal of Dairy Science 94: 1325–1338. DOI 10.3168/jds.2010-3757. [Google Scholar] [CrossRef]
Ebrahimi SO, Reiisi S (2019). Downregulation of miR-4443 and miR-5195-3p in ovarian cancer tissue contributes to metastasis and tumorigenesis. Archives of Gynecology and Obstetrics 299: 1453–1458. DOI 10.1007/s00404-019-05107-x. [Google Scholar] [CrossRef]
Enright AJ, John B, Gaul U, Tuschl T, Sander C et al. (2003). MicroRNA targets in Drosophila. Genome Biology 5: R1. DOI 10.1186/gb-2003-5-1-r1. [Google Scholar] [CrossRef]
Escandon BM, Espinoza JS, Perea FP, Quito F, Ochoa R et al. (2020). Intrauterine therapy with ozone reduces subclinical endometritis and improves reproductive performance in postpartum dairy cows managed in pasture-based systems. Tropical Animal Health and Production 52: 2523–2528. DOI 10.1007/s11250-020-02298-3. [Google Scholar] [CrossRef]
Fahlgren N, Carrington JC (2010). miRNA target prediction in plants. Methods in Molecular Biology 592: 51–57. DOI 10.1007/978-1-60327-005-2. [Google Scholar] [CrossRef]
Glenisson W, Castronovo V, Waltregny D (2007). Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochimica et Biophysica Acta 1773: 1572–1582. DOI 10.1016/j.bbamcr.2007.05.016. [Google Scholar] [CrossRef]
Guo J, Zeng X, Miao J, Liu C, Wei F et al. (2019). MiRNA-218 regulates osteoclast differentiation and inflammation response in periodontitis rats through Mmp9. Cellular Microbiology 21: e12979. DOI 10.1111/cmi.12979. [Google Scholar] [CrossRef]
Imoto I, Pimkhaokham A, Watanabe T, Saito-Ohara F, Soeda E, Inazawa J (2000). Amplification and overexpression of TGIF2, a novel homeobox gene of the TALE superclass, in ovarian cancer cell lines. Biochemical and Biophysical Research Communications 276: 264–270. DOI 10.1006/bbrc.2000.3449. [Google Scholar] [CrossRef]
Jiang J, Wu RH, Zhou HL, Li ZM, Kou D et al. (2020). TGIF2 promotes cervical cancer metastasis by negatively regulating FCMR. European Review for Medical and Pharmacological Sciences 24: 5953–5962. DOI 10.26355/eurrev_202006_21488. [Google Scholar] [CrossRef]
Jinliang D, Jianhua Q, Jingsheng C, Lina X, Yuzhong M (2011). Effects of traditional Chinese medicine Yimu Shenghuatang on cytochrome P450 in cow inflammatory endometrial cells. Frontiers of Agriculture in China 5: 102–105. [Google Scholar]
Kim HM, Park BS, Kim JI, Kim SE, Lee J et al. (2007). Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130: 906–917. DOI 10.1016/j.cell.2007.08.002. [Google Scholar] [CrossRef]
Kondo Y, Suzuki S, Takahara T, Ono S, Goto M et al. (2021). Improving function of cytotoxic T-lymphocytes by transforming growth factor-beta inhibitor in oral squamous cell carcinoma. Cancer Science 112: 4037–4049. DOI 10.1111/cas.15081. [Google Scholar] [CrossRef]
Li PL, Zhang X, Wang LL, Du LT, Yang YM et al. (2015). MicroRNA-218 is a prognostic indicator in colorectal cancer and enhances 5-fluorouracil-induced apoptosis by targeting BIRC5. Carcinogenesis 36: 1484–1493. DOI 10.1093/carcin/bgv145. [Google Scholar] [CrossRef]
Li Y, Ma X, Yang J, Wu X, Yan Z, He B (2021a). Expression pattern of cathelicidins in dairy cows during endometritis and role of bovine endometrial epithelial cells in production of cathelicidins. Frontiers in Veterinary Science 8: 675669. DOI 10.3389/fvets.2021.675669. [Google Scholar] [CrossRef]
Li Y, Yan J, Chang HM, Chen ZJ, Leung P (2021b). Roles of TGF-beta superfamily proteins in extravillous trophoblast invasion. Trends in Endocrinology and Metabolism 32: 170–189. DOI 10.1016/j.tem.2020.12.005. [Google Scholar] [CrossRef]
Liu J, Cheng Y, Wang X, Zhang L, Liu H (2017). An optimal mean based block robust feature extraction method to identify colorectal cancer genes with integrated data. Scientific Reports 7: 8584. DOI 10.1038/s41598-017-08881-3. [Google Scholar] [CrossRef]
LiuP, ZouA, ChenQ, ChengB, LiQ (2021). Basing on microRNA-mRNA analysis identifies microRNA in exosomes associated with wound repair of diabetic ulcers. Biocell 45: 27–39. [Google Scholar]
Liu T, Zhang X, Du L, Wang Y, Liu X et al. (2020). Correction to: Exosome-transmitted miR-128-3p increase chemosensitivity of oxaliplatin-resistant colorectal cancer. Molecular Cancer 19: 89. DOI 10.1186/s12943-020-01211-8. [Google Scholar] [CrossRef]
Lu SL, Reh D, Li AG, Woods J, Corless CL et al. (2004). Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Research 64: 4405–4410. DOI 10.1158/0008-5472.CAN-04-1032. [Google Scholar] [CrossRef]
Momen-Heravi F, Bala S, Bukong T, Szabo G (2014). Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine: Nanotechnology, Biology, and Medicine 10: 1517–1527. DOI 10.1016/j.nano.2014.03.014. [Google Scholar] [CrossRef]
Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML et al. (2012). Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 119: 756–766. DOI 10.1182/blood-2011-02-338004. [Google Scholar] [CrossRef]
O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO (2020). RNA delivery by extracellular vesicles in mammalian cells and its applications. Nature Reviews. Molecular Cell Biology 21: 585–606. DOI 10.1038/s41580-020-0251-y. [Google Scholar] [CrossRef]
Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V et al. (2014). MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 41: 89–103. DOI 10.1016/j.immuni.2014.05.019. [Google Scholar] [CrossRef]
Osawa T (2021). Predisposing factors, diagnostic and therapeutic aspects of persistent endometritis in postpartum cows. Journal of Reproduction and Development 67: 291–299. DOI 10.1262/jrd.2021-052. [Google Scholar] [CrossRef]
Pang P, Abbott M, Chang SL, Abdi M, Chauhan N et al. (2017). Human vascular progenitor cells derived from renal arteries are endothelial-like and assist in the repair of injured renal capillary networks. Kidney International 91: 129–143. DOI 10.1016/j.kint.2016.07.037. [Google Scholar] [CrossRef]
Piras C, Guo Y, Soggiu A, Chanrot M, Greco V et al. (2017). Changes in protein expression profiles in bovine endometrial epithelial cells exposed to E. coli LPS challenge. Molecular BioSystems 13: 392–405. DOI 10.1039/C6MB00723F. [Google Scholar] [CrossRef]
Regueiro V, Moranta D, Campos MA, Margareto J, Garmendia J et al. (2009). Klebsiella pneumoniae increases the levels of Toll-like receptors 2 and 4 in human airway epithelial cells. Infection and Immunity 77: 714–724. DOI 10.1128/IAI.00852-08. [Google Scholar] [CrossRef]
Sahoo S, Losordo DW (2014). Exosomes and cardiac repair after myocardial infarction. Circulation Research 114: 333–344. DOI 10.1161/CIRCRESAHA.114.300639. [Google Scholar] [CrossRef]
Sato M, Suzuki T, Kawano M, Tamura M (2017a). Circulating osteocyte-derived exosomes contain miRNAs which are enriched in exosomes from MLO-Y4 cells. Biomedical Reports 6: 223–231. DOI 10.3892/br.2016.824. [Google Scholar] [CrossRef]
SharmaY, GuptaS, MohantyS (2021). Mesenchymal stem cell-derived exosome as a nano weapon to target the COVID-19 pandemic. Biocell 45: 517–520. [Google Scholar]
Shi A, Li J, Qiu X, Sabbah M, Boroumand S et al. (2021). TGF-β loaded exosome enhances ischemic wound healing in vitro and in vivo. Theranostics 11: 6616–6631. DOI 10.7150/thno.57701. [Google Scholar] [CrossRef]
Siqueira LC, Favaretto B, Moraes BT, de Freitas VO, Bicalho RC et al. (2020). Bovine endometritis and the inflammatory peripheral cholinergic system. Applied Biochemistry and Biotechnology 190: 1242–1256. DOI 10.1007/s12010-019-03157-0. [Google Scholar] [CrossRef]
Taniguchi K, Anderson AE, Melhuish TA, Carlton AL, Manukyan A et al. (2017). Genetic and molecular analyses indicate independent effects of TGIFs on Nodal and Gli3 in neural tube patterning. European Journal of Human Genetics 25: 208–215. DOI 10.1038/ejhg.2016.164. [Google Scholar] [CrossRef]
Tao W, Cao C, Ren G, Zhou D (2021). CircularRNA circCPA4 promotes tumorigenesis by regulatingmiR-214-3p/TGIF2 in lung cancer. Thoracic Cancer 12: 3356–3369. DOI 10.1111/1759-7714.14210. [Google Scholar] [CrossRef]
Tian Y, Wei W, Li L, Yang R (2015). Down-Regulation of miR-148a promotes metastasis by DNA methylation and is associated with prognosis of skin cancer by targeting TGIF2. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 21: 3798–3805. DOI 10.12659/MSM.894826. [Google Scholar] [CrossRef]
Victor AR, Weigel C, Scoville SD, Chan WK, Chatman K et al. (2018). Epigenetic and posttranscriptional regulation of CD16 expression during human NK cell development. Journal of Immunology 200: 565–572. DOI 10.4049/jimmunol.1701128. [Google Scholar] [CrossRef]
Wang W, Zhang H, Chen Z, Zhang W, Liu X et al. (2019a). Endometrial TGF-β, IL-10, IL-17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reproductive Biology and Endocrinology 17: 14. DOI 10.1186/s12958-018-0444-9. [Google Scholar] [CrossRef]
Wang X, Li Q, Xie T, Yuan M, Sheng X et al. (2021a). Exosomes from bovine endometrial epithelial cells ensure trophoblast cell development by miR-218 targeting secreted frizzled related protein 2. Journal of Cellular Physiology 236: 4565–4579. DOI 10.1002/jcp.30180. [Google Scholar] [CrossRef]
Wang X, Tian F, Chen C, Feng Y, Sheng X et al. (2019b). Exosome-derived uterine microRNAs isolated from cows with endometritis impede blastocyst development. Reproductive Biology 19: 204–209. DOI 10.1016/j.repbio.2019.06.003. [Google Scholar] [CrossRef]
Wang X, Yao X, Xie T, Chang Z, Guo Y et al. (2020). Exosome-derived uterine miR-218 isolated from cows with endometritis regulates the release of cytokines and chemokines. Microbial Biotechnology 13: 1103–1117. DOI 10.1111/1751-7915.13565. [Google Scholar] [CrossRef]
Wang Y, Chen T, Gan Z, Li H, Li Y et al. (2021b). Metabolomic analysis of untargeted bovine uterine secretions in dairy cows with endometritis using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Research in Veterinary Science 139: 51–58. DOI 10.1016/j.rvsc.2021.07.006. [Google Scholar] [CrossRef]
Wu S, He X, Li M, Shi F, Wu D et al. (2016). MiRNA-34a overexpression inhibits multiple myeloma cancer stem cell growth in mice by suppressing TGIF2. American Journal of Translational Research 8: 5433–5443. [Google Scholar]
Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE et al. (2015). Biological properties of extracellular vesicles and their physiological functions. Journal of Extracellular Vesicles 4: 27066. DOI 10.3402/jev.v4.27066. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |