DOI:10.32604/biocell.2022.021399

| BIOCELL DOI:10.32604/biocell.2022.021399 |  |
| Viewpoint |
Mesenchymal stem cells and cell-free preparations for treating atopic dermatitis
1Dermatology Department, Virgen de las Nieves University Hospital, Granada, Spain
2Instituto de Investigación Biosanitaria Granada, Granada, Spain
3Pharmacy Department, Virgen de las Nieves University Hospital, Granada, Spain
4Dermatology Department, Faculty of Medicine, University of Granada, Granada, Spain
*Address correspondence to: Trinidad Montero-Vilchez, tmonterov@gmail.com
Received: 12 January 2022; Accepted: 11 April 2022
Abstract: Atopic dermatitis (AD) is a chronic cutaneous inflammatory disease caused by an interaction between genetic, immune and epidermal barrier factors. Several treatments can be used to treat this disease but there are patients that do not respond to actual drugs. So, there is a need to develop effective therapies for AD. Mesenchymal stem cells (MSCs) are non-hematopoietic multipotent adult progenitor cells with immunomodulatory power and self-regenerating capacity to repair tissue damage, so they could be a potential effective treatment for AD. MSCs-Conditioned Medium (CM) and MSCs-exosomes are cell-free preparation with molecules secreted by stem cells that could be also beneficial for AD. This viewpoint reviews the actual development of MSCs, MSCs-CM and MSCs-exosomes for treating patients with AD.
Keywords: Atopic dermatitis; Conditioned medium; Dermatology; Mesenchymal stem cells; Stem cells
There is a need to develop effective drugs for treating atopic dermatitis (AD). Mesenchymal stem cells (MSCs) are non-hematopoietic multipotent adult progenitor cells with immunomodulatory potential and self-regenerating capacity to restore tissue damage. MSCs-Conditioned Medium (CM) and MSCs-exosomes are cell-free preparation that contains molecules secreted by stem cells and have similar therapeutic potential than MSCs. This viewpoint reviews the actual development of MSCs, MSCs-CM and MSCs-exosomes for treating patients with AD in clinical settings.
Human mesenchymal stem cells (MSCs) are non-hematopoietic multipotent adult progenitor cells that can be isolated from several sources, including adipose tissue (AT-MSCs), umbilical cord blood (UC-MSCs), bone marrow (BM-MSCs) and skin (Sierra-Sanchez et al., 2021). The beneficial effect of MSCs is not only in the cell component but also in its secretome, due to its abundant resource of paracrine factors. The secretome include the molecules secreted by stem cells, containing proteins, microRNA, growth factors, antioxidants, proteasomes and exosomes (Montero-Vilchez et al., 2021). MSCs secretome has one free fraction- called Conditioned Media (CM), and other encapsulated into extracellular vesicles–called exosomes (Quinones-Vico et al., 2021). It has been described that cell to cell interaction or a long-term remaining in the body can be the strong point in efficacy or safety, but cell-free preparations could have several advantages over cell therapy, as their manufacturing process is easier and more economic and there is no risk of adverse events associated with cell, including rejection, tumors, thrombosis, ossification or calcification (Bogatcheva and Coleman, 2019). Nevertheless, it is still difficult to define the composition of the secretome, there are inconsistencies about cell-free preparation, the quantities needed are about 10–25 times higher than directly administered live cells and there could be instability and short half-life of some proteins (Ahangar et al., 2020). Despite all these facts, MSCs, CM-MSCs and MSCs-exosomes are a promising therapeutic option for several skin conditions due to their potential to proliferate and repair tissue damage and to modulate immune responses (Kim et al., 2017). They have been mainly used in ulcers, hair restoration, skin rejuvenation and inflammatory skin diseases (Montero-Vilchez et al., 2021; Quinones-Vico et al., 2021; Sierra-Sanchez et al., 2021).
Atopic dermatitis (AD) is a chronic cutaneous inflammatory disease characterized by pruritic and recurrent eczematous lesions (Wollenberg et al., 2020). It is one of the most prevalent (Bylund et al., 2020) and most expensive cutaneous disease (Sacotte and Silverberg, 2018). Its etiopathogenesis is multifactorial and includes genetic factors, immune dysregulation, epidermal barrier dysfunction, and gut dysbiosis (Luger et al., 2021). Topical corticosteroids are the basic treatment for AD, but patients with severe disease need systemic immunosuppressants, including cyclosporin or methotrexate, or biologic therapies, such as dupilumab, tralokinumab and JAK inhibitors (Wollenberg et al., 2020). Long-term use of immunosuppressants may cause side-effects, including nephrotoxicity, hematological alterations or gastrointestinal problems, and multiple administrations of these drugs are needed to achieve effectiveness. Moreover, there are patients that do not reach a clinical response with these treatments (Simpson et al., 2016). Therefore, there is still a need to develop safe and effective therapies for AD.
MSCs, CM-MSCs and MSCs-exosomes could be a promising treatment for AD patients. Their main advantages compared to traditional treatment could be their longer-term effect with a capacity to restore the immune system and the possibility to avoid traditional drugs side events (Shin et al., 2020). MSCs and cell free preparations could be effective for AD due to their ability to regulate innate and acquired immune responses and their capacity of homing and repairing areas of inflammation and tissue damage (Balato and Caiazzo, 2017; Fig. 1). The imbalance between type 2 helper T cell (Th2) and type 1 helper T cell (Th1) plays a key role in the pathogenesis of AD. This disease is mainly characterized by excessive Th2 mediated inflammatory responses, but other subsets of helper T cells, including Th1, Th17, and Th22 might also be involved in its pathogenesis. Preclinical studies have shown that different types of MSCs have immunomodulatory effects on lymphocyte function, suppress T-cell proliferation, reduce the number of CD4+ and CD8+ T cells in the spleen, lymph and skin, and reduce cytokine production, including Th2 cytokines (IL-4, IL-5 and IL-13) and other proinflammatory cytokines such as TNF-α, TGF-β or IFN-γ (Sierra-Sanchez et al., 2021). MSCs derived from skin of AD patients also overexpress Th1/Th17 cytokines, emphasizing the role of the Th17 pathway in the chronic phase of AD (Orciani et al., 2017). Moreover, MSCs inhibit B cell proliferation and maturation, decrease Ig E levels and inhibit mast cell degranulation and histamine and prostaglandin E2 level (Daltro et al., 2020). Moreover, the impact of MSCs on epidermal barrier function in animal models have been shown by a reduced transepidermal water loss (TEWL) and epidermal thickness in the areas treated with MSCs (Sierra-Sanchez et al., 2021). It should be also considered that MSCs therapeutic effects could be disturb by conventional AD treatments, such as calcineurin inhibitors that decrease the production of prostaglandin E2, one of the most critical immunomodulatory factors for MSCs treatment (Shin et al., 2021).
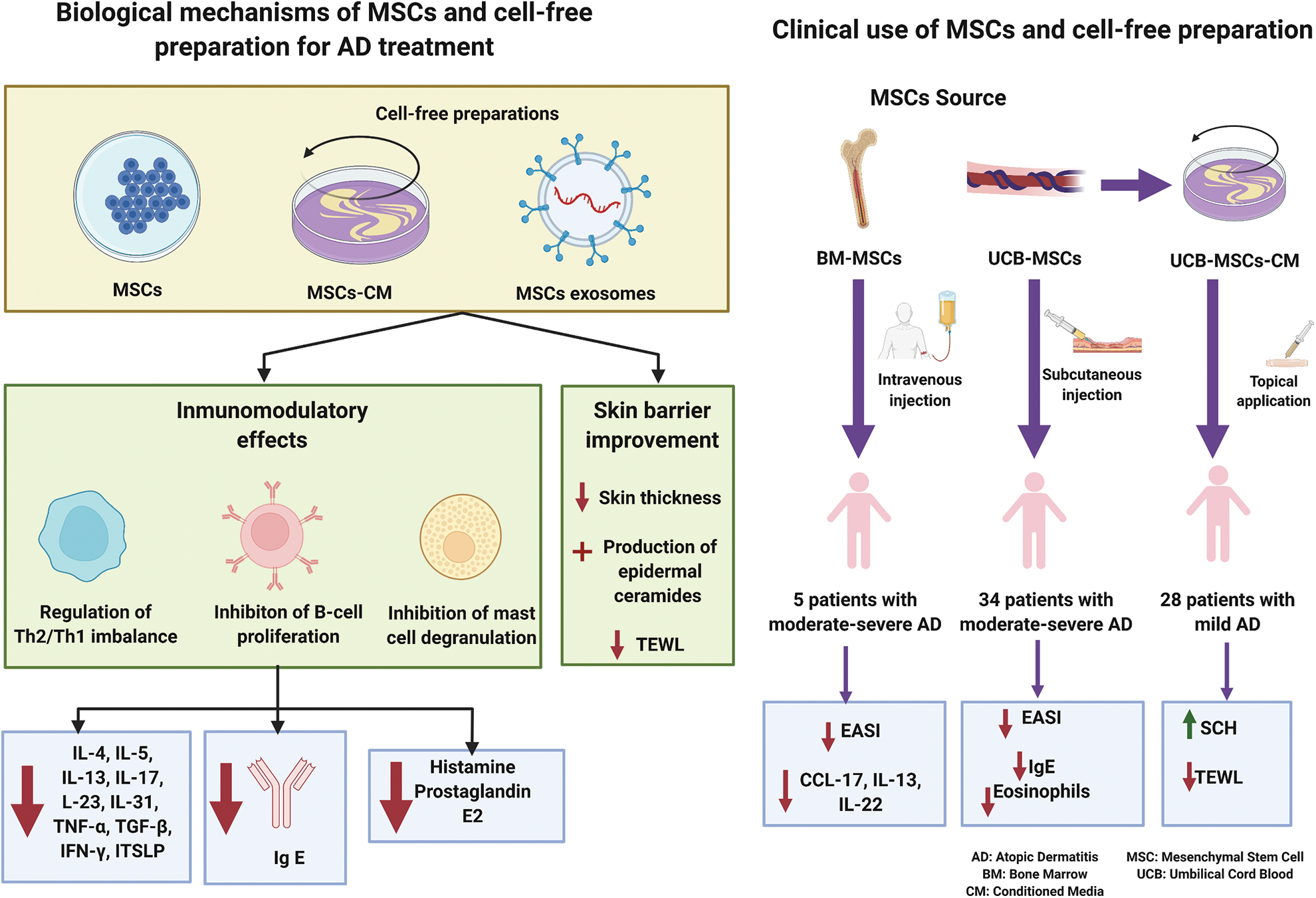
Figure 1: Summarize of biological mechanism and clinical use of mesenchymal stem cell and their cell-free preparations for treating atopic dermatitis.
MSCs-CM derived from murine adipose tissue and injected subcutaneously decrease AD severity in murine model, skin thickness, mast cell infiltration, Th2 expression, Ig E levels and IL-4 (Park et al., 2019). Furthermore, MSCs-exosomes decrease the production of Th2 cytokines (including IL-4, IL-5, IL-13, IL-23 and IL-31) and others pro-inflammatory cytokines (such as IL-17, TNF-α, IFN-γ and TSLP) in a dose-dependent manner, stimulate the production of epidermal ceramides and activate genes associated with keratinocyte differentiation, improving epidermal barrier function (Quinones-Vico et al., 2021).
The clinical use of MSC and MSCs-CM has been reported in few studies (Table 1). Two studies have evaluated the effect of MSCs derived from human umbilical cord blood (Kim et al., 2017) and human bone marrow (Shin et al., 2021) on patients with moderate to severe AD with persistent symptoms that had not previously responded to conventional therapies. Thirty-four patients with moderate to severe AD received two doses of UC-MSCs subcutaneously injected. Patients were assigned to receive a low dose of MSCs (2.5 × 107 cells) or a high dose (5.0 × 107 cells). After 12-weeks follow-up, 36% of the patients treated with a low dose of UC-MSCs and 55% of the patients treated with a high dose reached a clinical respond, assessed by a decreased higher than 50% in the Eczema Area and Severity Index (EASI)-50 (Kim et al., 2017). Ig E and eosinophil counts also decreased after the treatment (Kim et al., 2017). Moreover, BM-MSCs injected intravenously three times every 2 weeks were tested in five patients with moderate to severe AD. 80% of the participants reached EASI-50 after one or two cycles of treatment and was maintained. CCL-17, IL-13, and IL-22 decreased in these patients, but Ig E was not reduced after 16 weeks follow-up (Shin et al., 2021). In this study two patients maintained long-term efficacy (84 weeks follow-up) without systemic steroids and immunomodulators, and increased levels of IL-17, so the authors suggested that MSCs therapy could be a therapeutic option for moderate to severe AD with high IL-17 levels (Shin et al., 2021). No serious adverse events were reported in these studies (Kim et al., 2017; Shin et al., 2021). The adverse events were transient and mild and mainly related to injection site responses (Kim et al., 2017). Another clinical trial has been completed evaluating the effect of one intravenous injection of AT-MSCs (NCT02888704), but the results have not been posted. There are also ongoing clinical trials testing UC-MSCs subcutaneously injected (NCT05004324), BM-MSCs intravenously injected (NCT04179760) and AT-MSCs intravenously injected (NCT04137562). The routes of administration evaluated are subcutaneous and intravenous injections. Subcutaneous injections could be safer, due to a lower rate of side events such as pulmonary embolism (Kim et al., 2021), and more effective to reduce gross and histological signatures of AD (Kim et al., 2017).
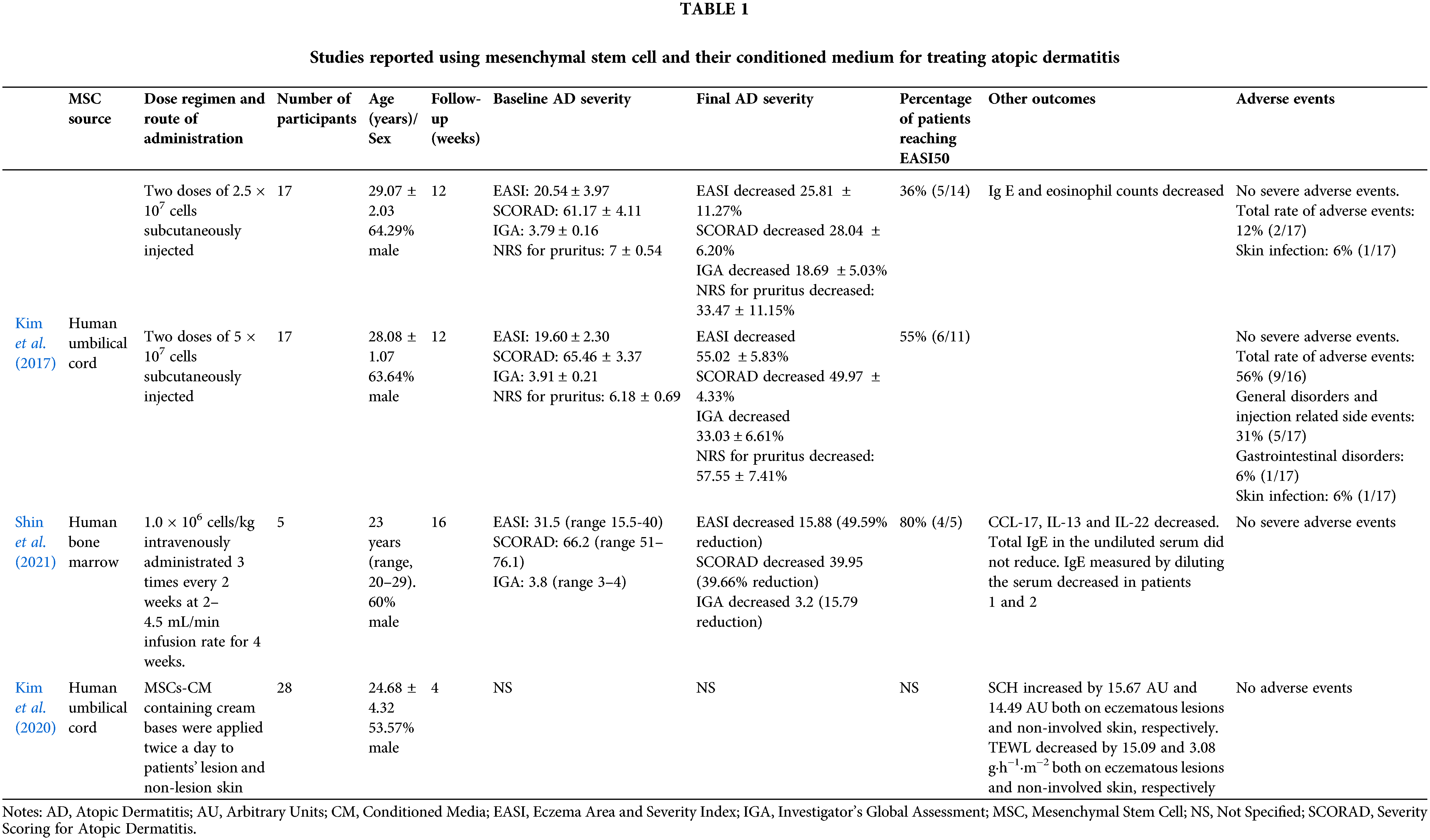
Regarding cell-free preparations, one study evaluated the effects of MSCs-CM, derived from human umbilical cord blood, for treating patients with mild AD. Twenty-eight patients with AD were treated with MSCs-CM in cream base for 4 weeks. MSCs-CM was applied on both eczematous lesions and non-involved skin twice a day. After treatment, stratum corneum hydration increased while transepidermal water loss decreased both on eczematous lesions and non-involved skin, reflecting skin barrier improvement (Kim et al., 2020). No study about the use of MSCs-exosomes in human has been reported yet.
In conclusion, MSCs, MSCs-CM and exosomes seem promising therapy for AD. It is difficult to know what therapy could be more effective at such early stages. Cell-free preparations could have advantages over cell therapy as risks related to cell therapies may be avoided. Nevertheless, there are no clinical trials about CM and exosomes use in AD. Cell free preparations should be further investigated for treating AD and clinical trials comparing MSCs, MSCs-CM and exosomes should be conducted. More studies regarding the ideal source of MSCs and the route of administration should also be carried out.
Availability of Data and Materials: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contribution: This statement should make clear the contribution of all authors. An author name can appear multiple times, and each author name must appear at least once. It can be described in the following manner: The authors confirm contribution to the paper as follows: study conception and design: TMV; data collection: TMV, MSD, CMV, ASS; analysis and interpretation of results: TMV and SAS; draft manuscript preparation: TMV and SAS. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ahangar P, Mills SJ, Cowin AJ (2020). Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. International Journal of Molecular Sciences 21: 7038. DOI 10.3390/ijms21197038. [Google Scholar] [CrossRef]
Balato A, Caiazzo G (2017). Mesenchymal stem cells participate to inflammatory skin process of atopic dermatitis. British Journal of Dermatoly 176: 1437–1438. DOI 10.1111/bjd.15412. [Google Scholar] [CrossRef]
Bogatcheva NV, Coleman ME (2019). Conditioned medium of mesenchymal stromal cells: A new class of therapeutics. Biochemistry 84: 1375–1389. DOI 10.1134/S0006297919110129. [Google Scholar] [CrossRef]
Bylund S, Kobyletzki LB, Svalstedt M, Svensson A (2020). Prevalence and incidence of atopic dermatitis: A systematic review. Acta Dermato-Venereologica 100: adv00160. DOI 10.2340/00015555-3510. [Google Scholar] [CrossRef]
Daltro SRT, Meira CS, Santos IP, Ribeiro Dos Santos R, Soares MBP (2020). Mesenchymal stem cells and atopic dermatitis: A review. Frontiers in Cell and Developmental Biology 8: 326. DOI 10.3389/fcell.2020.00326. [Google Scholar] [CrossRef]
Kim EY, Kim HS, Hong KS, Chung HM, Park SP, Noh G (2021). Mesenchymal stem/stromal cell therapy in atopic dermatitis and chronic urticaria: Immunological and clinical viewpoints. Stem Cell Research & Therapy 12: 539. DOI 10.1186/s13287-021-02583-4. [Google Scholar] [CrossRef]
Kim HS, Lee JH, Roh KH, Jun HJ, Kang KS, Kim TY (2017). Clinical trial of human umbilical cord blood-derived stem cells for the treatment of moderate-to-severe atopic dermatitis: Phase I/IIa studies. Stem Cells 35: 248–255. DOI 10.1002/stem.2401. [Google Scholar] [CrossRef]
Kim KH, Blasco-Morente G, Cuende N, Arias-Santiago S (2017). Mesenchymal stromal cells: Properties and role in management of cutaneous diseases. Journal of the European Academy of Dermatology and Venereology 31: 414–423. DOI 10.1111/jdv.13934. [Google Scholar] [CrossRef]
Kim YJ, Ahn HJ, Lee SH, Lee MH, Kang KS (2020). Effects of conditioned media from human umbilical cord blood-derived mesenchymal stem cells in the skin immune response. Biomedicine & Pharmacotherapy 131: 110789. DOI 10.1016/j.biopha.2020.110789. [Google Scholar] [CrossRef]
Luger T, Amagai M, Dreno B, Dagnelie MA, Liao W et al. (2021). Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. Journal of Dermatological Science 102: 142–157. DOI 10.1016/j.jdermsci.2021.04.007. [Google Scholar] [CrossRef]
Montero-Vilchez T, Sierra-Sanchez A, Sanchez-Diaz M, Quinones-Vico MI, Sanabria-de-la-Torre R, Martinez-Lopez A, Arias-Santiago S (2021). Mesenchymal stromal cell-conditioned medium for skin diseases: A systematic review. Frontiers in Cell and Developmental Biology 9: 654210. DOI 10.3389/fcell.2021.654210. [Google Scholar] [CrossRef]
Orciani M, Campanati A, Caffarini M, Ganzetti G, Consales V, Lucarini G, Offidani A, Di Primio R (2017). T helper (Th)1, Th17 and Th2 imbalance in mesenchymal stem cells of adult patients with atopic dermatitis: At the origin of the problem. British Journal of Dermatoly 176: 1569–1576. DOI 10.1111/bjd.15078. [Google Scholar] [CrossRef]
Park HS, Son HY, Choi MH, Son Y, Kim S, Hong HS, Park JU (2019). Adipose-derived stem cells attenuate atopic dermatitis-like skin lesions in NC/Nga mice. Experimenta Dermatoly 28: 300–307. DOI 10.1111/exd.13895. [Google Scholar] [CrossRef]
Quinones-Vico MI, Sanabria-de la Torre R, Sanchez-Diaz M, Sierra-Sanchez A, Montero-Vilchez T, Fernandez-Gonzalez A, Arias-Santiago S (2021). The role of exosomes derived from mesenchymal stromal cells in dermatology. Frontiers in Cell and Developmental Biology 9: 647012. DOI 10.3389/fcell.2021.647012. [Google Scholar] [CrossRef]
Sacotte R, Silverberg JI (2018). Epidemiology of adult atopic dermatitis. Clinics in Dermatology 36: 595–605. DOI 10.1016/j.clindermatol.2018.05.007. [Google Scholar] [CrossRef]
Shin HT, Lee SH, Yoon HS, Heo JH, Lee SB et al. (2021). Long-term efficacy and safety of intravenous injection of clonal mesenchymal stem cells derived from bone marrow in five adults with moderate to severe atopic dermatitis. The Journal of Dermatology 48: 1236–1242. DOI 10.1111/1346-8138.15928. [Google Scholar] [CrossRef]
Shin KO, Ha DH, Kim JO, Crumrine DA, Meyer JM et al. (2020). Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells 9: 680. DOI 10.3390/cells9030680. [Google Scholar] [CrossRef]
Shin N, Jung N, Lee SE, Kong D, Kim NG, Kook MG, Park H, Choi SW, Lee S, Kang KS (2021). Pimecrolimus interferes the therapeutic efficacy of human mesenchymal stem cells in atopic dermatitis by regulating NFAT-COX2 signaling. Stem Cell Research & Therapy 12: 482. DOI 10.1186/s13287-021-02547-8. [Google Scholar] [CrossRef]
Sierra-Sanchez A, Montero-Vilchez T, Quinones-Vico MI, Sanchez-Diaz M, Arias-Santiago S (2021). Current advanced therapies based on human mesenchymal stem cells for skin diseases. Frontiers in Cell and Developmental Biology 9: 643125. DOI 10.3389/fcell.2021.643125. [Google Scholar] [CrossRef]
Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A et al. (2016). Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. New England Journal of Medicine 375: 2335–2348. DOI 10.1056/NEJMoa1610020. [Google Scholar] [CrossRef]
Wollenberg A, Christen-Zach S, Taieb A, Paul C, Thyssen JP et al. (2020). ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. Journal of the European Academy of Dermatology and Venereology 34: 2717–2744. DOI 10.1111/jdv.16892. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |