DOI:10.32604/biocell.2022.021050

| BIOCELL DOI:10.32604/biocell.2022.021050 |  |
| Viewpoint |
Possible mechanisms of bidirectional nuclear transport during neuronal migration
1Graduate School of Biostudies, Kyoto University, Kyoto, 606-8501, Japan
2Institute for Integrated Cell-Material Sciences (WPI-iCeMS), Kyoto University, Kyoto, 606-8501, Japan
*Address correspondence to: Mineko Kengaku, kengaku@icems.kyoto-u.ac.jp
Received: 05 January 2022; Accepted: 18 April 2022
Abstract: Neuronal migration is a fundamental process of mammalian brain development. In migrating neurons, the nuclear membrane protein Nesprin-2 has been shown to serve as an adaptor to pull the nucleus along microtubule tracks. Current evidence has shown that Nesprin-2 binds to both the minus-end-directed motor dynein as well as the plus-end-directed motor kinesin. However, translocation of neuronal nucleus has long been thought to be primarily driven by dynein motors. Intriguing questions could be raised about the role of kinesin in nuclear transport and how the activities of opposing motors are coordinated through interactions with Nesprin. Combining evidence from recent studies, we propose that Nesprin-2 serves as a switchboard in mediating bidirectional neuronal nuclear movements.
Keywords: Nesprin-2; Nucleus; Microtubules; Kinesin; Dynein
The positioning of cell nucleus is essential in many developmental events, including the multinucleated arrangement in myoblast syncytium, apicobasal polarization of epithelial cells in the cochlea, and pronuclear migration in fertilized zygotes (Bone and Starr, 2016; Gundersen and Worman, 2013). Especially in highly polarized cells like neurons, nuclear movements and positioning are tightly aligned with developmental stages and cellular functions. One important example is the interkinetic nuclear migration of neuroepithelial progenitor cells, where the apicobasal movements of the nucleus is coupled with the cell division cycle to produce neurons and glia in the brain (Bertipaglia et al., 2018; Taverna and Huttner, 2010). Following neurogenesis, the migration of post-mitotic neurons during the formation of the laminated cortex also requires active nuclear movements (Nakazawa and Kengaku, 2020; Tsai et al., 2007). Here we focus our discussion on how nuclear movements are regulated in mammalian neuronal migration, which would hopefully provide new insights into nucleocytoskeletal interactions under normal and pathological conditions in different tissue types.
The forward translocation of the cell nucleus is a critical step of neuronal migration, which was first described in detail by Rakic (1972) in the developing cerebral cortex. Pioneering studies using the cerebellar granule cells identified perinuclear microtubule network which connects to microtubule bundles in the leading process, and proposed the preliminary hypothesis that the polarized microtubules may create forces for nuclear displacement (Fig. 1) (Rivas and Hatten, 1995; Rakic et al., 1996). Breakthrough was made by the discovery of the causal genes for type I lissencephaly, a heterogeneous group of disorders of cortical formation caused by abnormal neuronal migration. LIS1 (official symbol PAFAH1B1, for platelet-activating factor acetylhydrolase isoform 1b regulatory subunit 1) was identified as a causal gene product, which binds to the motor domain of cytoplasmic dynein and regulates dynein-dependent transport of the nucleus along intracellular microtubule tracks (Hirotsune et al., 1998; Tanaka et al., 2004; Shu et al., 2004). Later, more dynein-related mutations that lead to neuronal migration defects were identified, contributing to the common view that the cytoplasmic dynein complex (dynein hereafter) drives forward nuclear translocation in neurons (Ayala et al., 2007; Tsai and Gleeson, 2005). Since dynein transports cargoes towards microtubule minus ends, it is in line with the observation that most peri-nuclear microtubules have their minus ends pointing forward (Fig. 1) (Tsai et al., 2007). Nonetheless, recent studies further revealed that perinuclear microtubules are of mixed polarity and that KIF5, the microtubule plus-end-driven kinesin-1 motor, is also involved in facilitating neuronal nuclear translocation during cerebellar granule cell migration (Umeshima et al., 2007; Wu et al., 2018). Due to the opposite nature of dynein and kinesin motilities on microtubules, a consensus has not been reached on the role of kinesin in driving nuclear movements (Kengaku, 2018). In the case of bidirectional interkinetic nuclear migration of neuroepithelial cells, kinesin-3 motor KIF1A and dynein are responsible for basal (away from centrosome) and apical (towards centrosome) nuclear movements, respectively, at distinct cell cycle stages (Tsai et al., 2010). However, in the case of one-way nuclear translocation of migrating neurons, novel mechanisms need to be proposed to explain the biological significance of kinesin involvement.
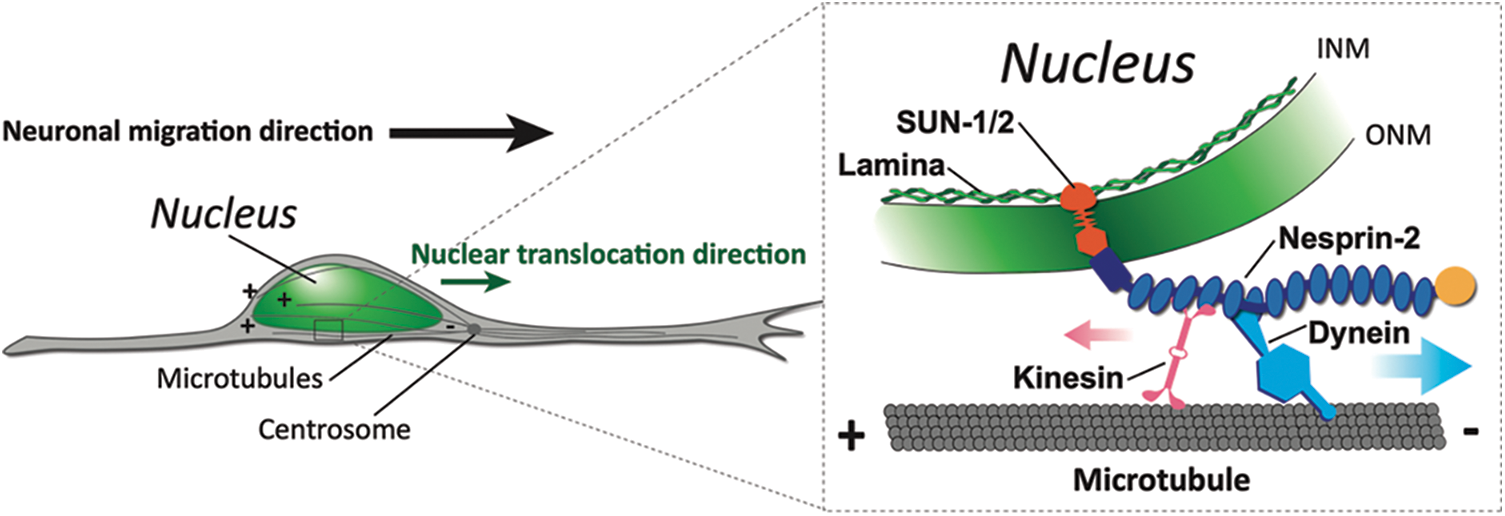
Figure 1: The forward translocation of nucleus during neuronal migration is driven by microtubule motors via LINC complex. In migrating neurons, the cell nucleus is transported along migration direction towards the minus ends of perinuclear microtubules which are embedded in the centrosome in front (left). The LINC complex of INM (inner nuclear membrane)-locating SUN proteins and ONM (outer nuclear membrane)-locating Nesprin-2 mediates recruitment of kinesin and dynein motors onto nuclear envelope (right).
Meanwhile, increasing evidence suggests that the LINC complex (Linker of Nucleoskeleton and Cytoskeleton) acts as a key mediator in nuclear transport driven by microtubule motors. The LINC complex is composed of SUN proteins located on the inner nuclear membrane which interact with Lamin A/C of the nuclear lamina and KASH (Klarsicht/ANC-1/Syne Homology) family proteins traversing the outer nuclear membrane (Fig. 1). The binding between SUN and KASH domains anchors the C-terminus of KASH protein to the nuclear envelope while its gigantic N-terminus extends out to the cytoplasm, providing a scaffold for interactions with cytoskeletons (Starr and Fridolfsson, 2010; Friedl et al., 2011; Rajgor and Shanahan, 2013). KASH proteins in vertebrates are known as nesprins, for nuclear envelope spectrin repeat protein (Zhang et al., 2001). Human nesprin mutations defective in nucleus-cytoskeleton coupling are associated with muscular, neurological, pre-mature aging diseases and cancer in human (Zhang et al., 2007; Attali et al., 2009; Young et al., 2021; Gros-Louis et al., 2007; Kandert et al., 2007; Dawe et al., 2009; Doherty et al., 2010; Östlund et al., 2019; Bone and Starr, 2016). Among the nesprin family, Nesprin-2 has been shown to recruit both dynein and KIF5 motors onto the nucleus during neuronal migration in the developing mouse brain (Zhang et al., 2009). While the kinesin-binding motif has been identified to be the LEWD sequence near the C-terminus of the cytoplasmic stretch, the dynein-binding regions are still unclear (Wilson and Holzbaur, 2015). Zhu et al. (2017) have presented the initial evidence that the dynein and/or dynactin-binding sites are within a region in close proximity to the LEWD motif, followed by another study reporting that the same region also recruits BICD2, a key component of dynein/dynactin complex (Gonçalves et al., 2020).
Although the recruitment of both dynein and kinesin by Nesprin-2 is evident, the mechanism of motor activation remains unknown. In addition to serving merely as a docking site for motor recruitment, spectrin repeats in the cytoplasmic stretch of Nesprin-2 might also play regulatory roles by adopting conformational transformations or post-translational modifications upon interactions with cytoskeletal or signaling molecules (Djinovic-Carugo et al., 2002). One possibility is that Nesprin-2 acts as a molecular switch to selectively turn on/off the activities of dynein and kinesin in response to spatiotemporal cues. Another possibility is that Nesprin-2 mediates new modes of cooperation between dynein and kinesin while both motors are simultaneously attached and active. In fact, continuous progress has been made to characterize the coordinating roles of adaptor or scaffolding proteins which link dynein and kinesin simultaneously to specific intracellular organelles or vesicles (Olenick and Holzbaur, 2019; Fu and Holzbaur, 2014). For instance, phosphorylation/dephosphorylation of HAP-1 (Huntingtin-associated protein 1, a motor-adaptor protein for neuronal intracellular vesicles) can enhance or lessen recruitment of the kinesin-1 light chain in competition with dynein, which determines the direction of cargo transport (Colin et al., 2008). Likewise, TRAK1/2 proteins (the adaptors responsible for mitochondria trafficking) are capable of recruiting both dynactin p150 and KIF5 to generate bidirectional cargo movements along microtubules, but their association with KIF5 can be downregulated when TRAK proteins adopt a head-to-tail folded structure (van Spronsen et al., 2013). Another bidirectional adaptor protein HOOK3 forms a complex with dynein/dynactin and KIF1C, which can adjust the frequency of plus-end-directed runs depending on the local concentration of KIF1C motors (Kendrick et al., 2019). Although the large size and complexity of Nesprin-2 make it challenging to fully understand its functional interactions with motors, Nesprin-2 shares some similarities with those characterized bidirectional adaptors, including the extended coiled-coil structures and physical proximity between kinesin and dynein binding motifs. Therefore, it is tempting to speculate that Nesprin-2 might function not only as a physical linker, but also a coordinating moderator between opposing microtubule motors.
By forming an integrated complex with motors and motor accessory proteins, cargo adaptors may also acquire new motility properties rather than a simple stochastic combination of motor activities (Elshenawy et al., 2019; Mckenney et al., 2014). It is particularly interesting to find out whether kinesin plays an inhibitory, subsidiary, or assistive role with dynein in neuronal nuclear translocation. There are different theories about the function of kinesin in dynein-dominating cargo transport (Fig. 2):
1. Brake control: by interfering kinesin function in migrating cerebral neurons, Gonçalves et al. (2020) showed that nuclear translocation and neuronal migration were accelerated, implying that kinesin restrains forward nuclear movements while dynein moves it forward.
2. Increase flexibility: studies in myotubes demonstrated that kinesin generates dynamic nuclear rotation and backward stepping of the nucleus, which may help to untangle the nucleus from roadblock and to enable smooth transport through crowded cytoplasm and to finetune its correct positioning (Wilson and Holzbaur, 2012). In migrating cerebellar granule cells, downregulation of kinesin activities has been shown to decrease nuclear rotation and impede cell migration (Wu et al., 2018).
3. Microtubule tethering: kinesin may also help with cargo attachment onto microtubules to facilitate dynein-mediated transport. The mitochondria adaptor protein TRAK2 has been shown to have a higher affinity to microtubule tracks when kinesin is also present, which, in turn, enables a higher frequency of active dynein-driven movements (Fenton et al., 2021).
4. Mechanical activation: some evidence from in vitro studies suggested that the presence of opposite pulling force by kinesin enhances dynein stalling force, which resembles a catch-bond mechanism (reviwed in Hancock, 2014).
5. Steric disinhibition or hinderance: conformational changes could occur when kinesin and dynein bind to cargo adaptors (Hancock, 2014). It should be noted that these hypothesized models are not mutually exclusive and multiple mechanisms might be applied to dictate how nuclear transport is achieved coordinately by opposing motors.
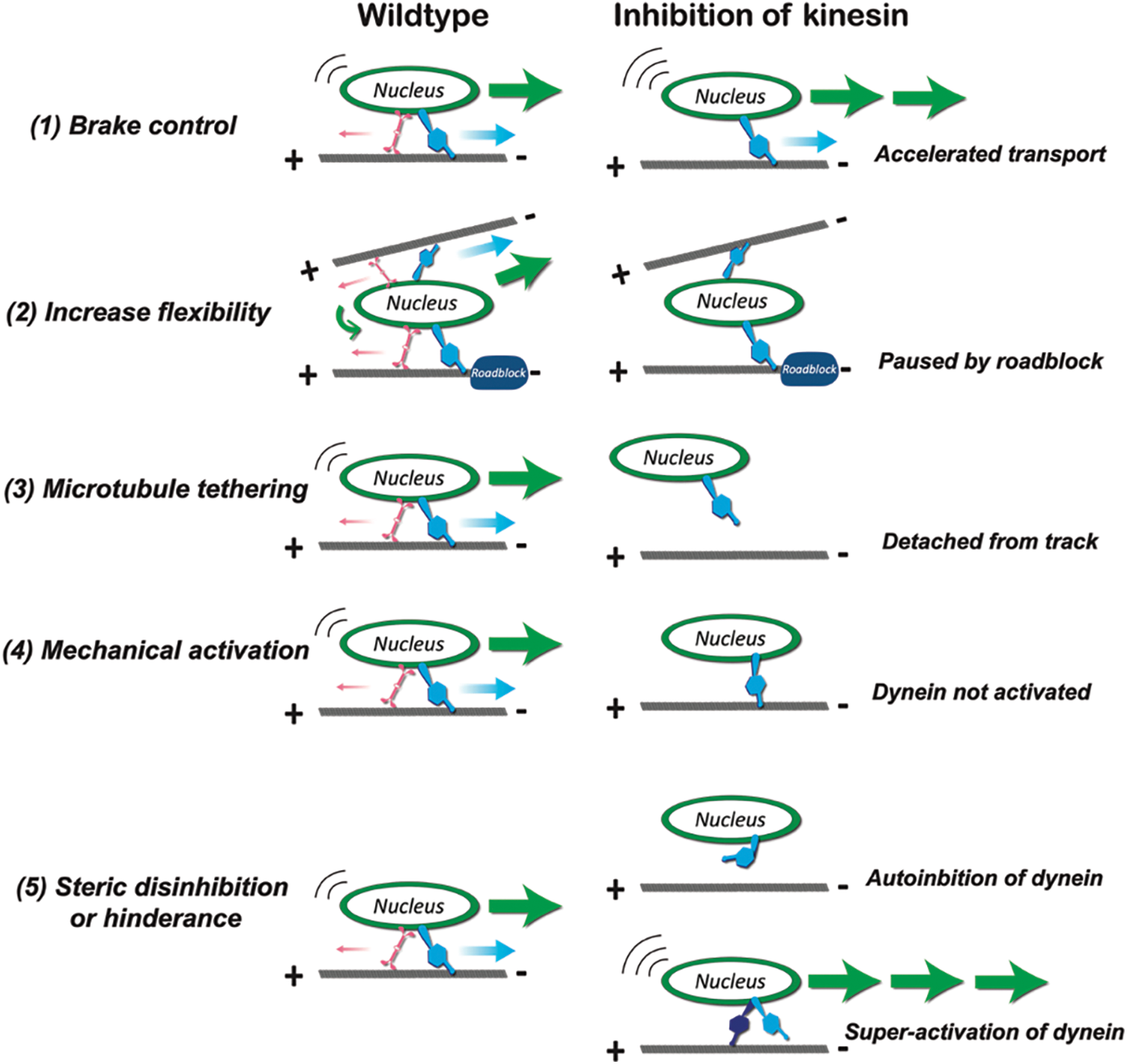
Figure 2: The hypothesized roles of kinesin in dynein-dominating nuclear transport. (1) When functioning as brake control, kinesin competes with dynein. Under kinesin inhibition, nucleus moves faster towards microtubule minus ends due to the absence of opposite forces. (2) When functioning to increase flexibility of transport, kinesin generates nuclear rotation and backward stepping to overcome roadblocks or to switch to another microtubule track. Under kinesin inhibition, nuclear movement is impeded by roadblocks or crowded intracellular environment. (3) When functioning to tether nucleus to microtubule tracks, kinesin enhances attachment between dynein-bound nucleus to microtubules. Under kinesin inhibition, nucleus detaches from tracks and fails to be transported. (4) When functioning as mechanical activator, the opposite stalling forces generated by kinesin activates dynein activities. Under kinesin inhibition, dynein motor is not activated, and nuclear transport is suppressed. (5) When functioning as steric disinhibition or hinderance effector, the presence of kinesin either relieves auto-inhibition or suppress super-activation of dynein. Under kinesin inhibition, dynein remains at auto-inhibited state or resumes to super-activated conformation (Adapted from Hancock, 2014).
To test the hypothesized mechanisms of nesprin-mediated nuclear transport, complex intracellular environment and multiple players should be considered. Kinetics of neuronal migration show great diversity depending on neuronal types and trajectories. This may be caused by diverse roles of kinesin in different cell types and stages with different microtubule arrangement, types of microtubule-associated proteins, and post-translational modifications of tubulin. In addition, depending on the distinct properties of dynein and kinesin, including processivity, detachment rate and stalling force, they probably behave differently under intracellular environment with highly polarized parallel microtubule assemblies, or a more mixed-oriented microtubule tracks with frequent intersections. Moreover, the availability of dynein regulators, including LIS1, NDE1/NDEL1 and BICD2 also affects transport. Combinatorial approach with molecular biophysics, high temporospatial imaging, and structural analysis of macromolecular protein complex will be required to reveal detailed motor dynamics regulated by nesprins in various neuronal migration, including radial migration of excitatory neurons and tangential migration of interneurons in the telencephalon.
In summary, we think that Nesprin-2 acts as the core adaptor protein of a complex with kinesin and dynein motors to facilitate nuclear translocation during neuronal migration. Understanding the mediator function of Nesprin-2 could be a promising direction leading to mechanistic understanding of neuronal migration.
Author Contribution: Both authors contributed to conceptualizing, researching, writing, and editing the manuscript.
Funding Statement: This work was supported by KAKENHI grant from the Japan Society for the Promotion of Science (JSPS) to MK (20H00483). CZ was supported by the Otsuka Toshimi Scholarship Foundation.
Conflicts of Interest: The authors declare no conflicts of interest.
Attali R, Warwar N, Israel A, Gurt I, McNally E, Puckelwartz M, Glick B, Nevo Y, Ben-Neriah Z, Melki J (2009). Mutation of SYNE-1, encoding an essential component of the nuclear lamina, is responsible for autosomal recessive arthrogryposis. Human Molecular Genetics 18: 3462–3469. DOI 10.1093/hmg/ddp290. [Google Scholar] [CrossRef]
Ayala R, Shu T, Tsai LH (2007). Trekking across the Brain: The journey of neuronal migration. Cell 128: 29–43. DOI 10.1016/j.cell.2006.12.021. [Google Scholar] [CrossRef]
Bertipaglia C, Gonçalves JC, Vallee RB (2018). Nuclear migration in mammalian brain development. Seminars in Cell & Developmental Biology 82: 57–66. DOI 10.1016/j.semcdb.2017.11.033. [Google Scholar] [CrossRef]
Bone CR, Starr DA (2016). Nuclear migration events throughout development. Journal of Cell Science 129: 1951–1961. DOI 10.1242/jcs.179788. [Google Scholar] [CrossRef]
Colin E, Zala D, Liot G, Rangone H, Borrell-Pagè M, Li XJ, Saudou F, Humbert S (2008). Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. The EMBO Journal 27: 2124–2134. DOI 10.1038/emboj.2008.133. [Google Scholar] [CrossRef]
Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, Gull K, Johnson CA (2009). Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. Journal of Cell Science 122: 2716–2726. DOI 10.1242/jcs.043794. [Google Scholar] [CrossRef]
Djinovic-Carugo K, Gautel M, Ylänne J, Young P (2002). The spectrin repeat: A structural platform for cytoskeletal protein assemblies. FEBS Letters 513: 119–123. DOI 10.1016/S0014-5793(01)03304-X. [Google Scholar] [CrossRef]
Doherty JA, Rossing MA, Cushing-Haugen KL, Chen C, Van Den Berg DJ et al. (2010). ESR1/SYNE1 polymorphism and invasive epithelial ovarian cancer risk: An ovarian cancer association consortium study. Cancer Epidemiology, Biomarkers & Prevention 19: 245–250. DOI 10.1158/1055-9965.EPI-09-0729. [Google Scholar] [CrossRef]
Elshenawy MM, Canty JT, Oster L, Ferro LS, Zhou Z, Blanchard SC, Yildiz A (2019). Cargo adaptors regulate stepping and force generation of mammalian dynein-dynactin. Nature Chemical Biology 15: 1093–1101. DOI 10.1038/s41589-019-0352-0. [Google Scholar] [CrossRef]
Fenton AR, Jongens TA, Holzbaur ELF (2021). Mitochondrial adaptor TRAK2 activates and functionally links opposing kinesin and dynein motors. Nature Communications 12: 4578. DOI 10.1038/s41467-021-24862-7. [Google Scholar] [CrossRef]
Friedl P, Wolf K, Lammerding J (2011). Nuclear mechanics during cell migration. Current Opinion in Cell Biology 23: 55–64. DOI 10.1016/j.ceb.2010.10.015. [Google Scholar] [CrossRef]
Fu MM, Holzbaur ELF (2014). Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends in Cell Biology 24: 564–574. DOI 10.1016/j.tcb.2014.05.002. [Google Scholar] [CrossRef]
Gonçalves JC, Quintremil S, Yi J, Vallee RB (2020). Nesprin-2 recruitment of BicD2 to the nuclear envelope controls dynein/kinesin-mediated neuronal migration in vivo. Current Biology 30: 3116–3129.e4. DOI 10.1016/j.cub.2020.05.091. [Google Scholar] [CrossRef]
Gros-Louis F, Dupré N, Dion P, Fox MA, Laurent S, Verreault S, Sanes JR, Bouchard JP, Rouleau GA (2007). Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nature Genetics 39: 80–85. DOI 10.1038/ng1927. [Google Scholar] [CrossRef]
Gundersen GG, Worman HJ (2013). Nuclear positioning. Cell 152: 1376–1389. DOI 10.1016/j.cell.2013.02.031. [Google Scholar] [CrossRef]
Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ, Wynshaw-Boris A (1998). Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nature Genetics 19: 333–339. DOI 10.1038/1221. [Google Scholar] [CrossRef]
Hancock WO (2014). Bidirectional cargo transport: Moving beyond tug of war. Nature Reviews. Molecular Cell Biology 15: 615–628. DOI 10.1038/nrm3853. [Google Scholar] [CrossRef]
Kandert S, Lüke Y, Kleinhenz T, Neumann S, Lu W et al. (2007). Nesprin-2 giant safeguards nuclear envelope architecture in LMNA S143F progeria cells. Human Molecular Genetics 16: 2944–2959. DOI 10.1093/hmg/ddm255. [Google Scholar] [CrossRef]
Kendrick AA, Dickey AM, Redwine WB, Tran PT, Vaites LP, Dzieciatkowska M, Harper JW, Reck-Peterson SL (2019). Hook3 is a scaffold for the opposite-polarity microtubule-based motors cytoplasmic dynein-1 and KIF1C. The Journal of Cell Biology 218: 2982–3001. DOI 10.1083/jcb.201812170. [Google Scholar] [CrossRef]
Kengaku M (2018). Cytoskeletal control of nuclear migration in neurons and non-neuronal cells. Proceedings of the Japan Academy, Series B 94: 337–349. DOI 10.2183/pjab.94.022. [Google Scholar] [CrossRef]
Mckenney RJ, Huynh W, Tanenbaum ME, Bhabha G, Vale RD (2014). Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science 345: 337–341. DOI 10.1126/science.1254198. [Google Scholar] [CrossRef]
Nakazawa N, Kengaku M (2020). Mechanical regulation of nuclear translocation in migratory neurons. Frontiers in Cell and Developmental Biology 8: 150. DOI 10.3389/fcell.2020.00150. [Google Scholar] [CrossRef]
Olenick MA, Holzbaur ELF (2019). Dynein activators and adaptors at a glance. Journal of Cell Science 132: jcs227132. DOI 10.1242/jcs.227132. [Google Scholar] [CrossRef]
Östlund C, Chang W, Gundersen GG, Worman HJ (2019). Pathogenic mutations in genes encoding nuclear envelope proteins and defective nucleocytoplasmic connections. Experimental Biology and Medicine 244: 1333–1344. DOI 10.1177/1535370219862243. [Google Scholar] [CrossRef]
Rajgor D, Shanahan CM (2013). Nesprins: From the nuclear envelope and beyond. Expert Reviews in Molecular Medicine 15: e5. DOI 10.1017/erm.2013.6. [Google Scholar] [CrossRef]
Rakic P (1972). Mode of cell migration to the superficial layers of fetal monkey neocortex. The Journal of Comparative Neurology 145: 61–83. DOI 10.1002/(ISSN)1096-9861. [Google Scholar] [CrossRef]
Rakic P, Knyihar-Csillik E, Csillil B (1996). Polarity of microtubule assemblies during neuronal cell migration. PNAS 93: 9218–9222. DOI 10.1073/pnas.93.17.9218. [Google Scholar] [CrossRef]
Rivas RJ, Hatten ME (1995). Motility and cytoskeletal organization of migrating cerebellar granule neurons. Journal of Neuroscience 15: 981–989. DOI 10.1523/JNEUROSCI.15-02-00981.1995. [Google Scholar] [CrossRef]
Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH (2004). Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron 44: 263–277. DOI 10.1016/j.neuron.2004.09.030. [Google Scholar] [CrossRef]
Starr DA, Fridolfsson HN (2010). Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annual Review of Cell and Developmental Biology 26: 421–444. DOI 10.1146/annurev-cellbio-100109-104037. [Google Scholar] [CrossRef]
Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG (2004). Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. The Journal of Cell Biology 165: 709–721. DOI 10.1083/jcb.200309025. [Google Scholar] [CrossRef]
Taverna E, Huttner WB (2010). Neural progenitor nuclei IN motion. Neuron 67: 906–914. DOI 10.1016/j.neuron.2010.08.027. [Google Scholar] [CrossRef]
Tsai LH, Gleeson JG (2005). Nucleokinesis in neuronal migration. Neuron 46: 383–388. DOI 10.1016/j.neuron.2005.04.013. [Google Scholar] [CrossRef]
Tsai JW, Bremner H, Vallee RB (2007). Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nature Neuroscience 10: 970–979. DOI 10.1038/nn1934. [Google Scholar] [CrossRef]
Tsai JW, Lian WN, Kemal S, Kriegstein AR, Vallee RB (2010). Kinesin 3 and cytoplasmic dynein mediate interkinetic nuclear migration in neural stem cells. Nature Neuroscience 13: 3–6. DOI 10.1038/nn.2665. [Google Scholar] [CrossRef]
Umeshima H, Hirano T, Kengaku M (2007). Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. PNAS 104: 16182–16187. DOI 10.1073/pnas.0708047104. [Google Scholar] [CrossRef]
van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ et al. (2013). TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 77: 485–502. DOI 10.1016/j.neuron.2012.11.027. [Google Scholar] [CrossRef]
Wilson MH, Holzbaur ELF (2015). Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development 142: 218–228. DOI 10.1242/dev.114769. [Google Scholar] [CrossRef]
Wilson MH, Holzbaur ELF (2012). Opposing microtubule motors drive robust nuclear dynamics in developing muscle cells. Journal of Cell Science 125: 4158–4169. DOI 10.1242/jcs.108688. [Google Scholar] [CrossRef]
Wu YK, Umeshima H, Kurisu J, Kengaku M (2018). Nesprins and opposing microtubule motors generate a point force that drives directional nuclear motion in migrating neurons. Development 145: dev158782. DOI 10.1242/dev.158782. [Google Scholar] [CrossRef]
Young N, Asif M, Jackson M, Fernández-Mayoralas DM, de la Peña MJ et al. (2021). Biallelic SYNE2 missense mutations leading to nesprin-2 giant hypo-expression are associated with intellectual disability and autism. Genes 12: 1294. DOI 10.3390/genes12091294. [Google Scholar] [CrossRef]
Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM (2001). Nesprins: A novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. Journal of Cell Science 114: 4485–4498. DOI 10.1242/jcs.114.24.4485. [Google Scholar] [CrossRef]
Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C et al. (2007). Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Human Molecular Genetics 16: 2816–2833. DOI 10.1093/hmg/ddm238. [Google Scholar] [CrossRef]
Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M (2009). SUN1/2 and syne/nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 64: 173–187. DOI 10.1016/j.neuron.2009.08.018. [Google Scholar] [CrossRef]
Zhu R, Antoku S, Gundersen GG (2017). Centrifugal displacement of nuclei reveals multiple LINC complex mechanisms for homeostatic nuclear positioning. Current Biology 27: 3097–3110.e5. DOI 10.1016/j.cub.2017.08.073. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |