DOI:10.32604/biocell.2022.020865

| BIOCELL DOI:10.32604/biocell.2022.020865 |  |
| Article |
Metformin alleviates LTA-induced inflammatory response through PPARγ/MAPK/NF-κB signaling pathway in bovine mammary epithelial cells
1College of Animal Science and Technology, Yangzhou University, Yangzhou, 225009, China
2Joint International Research Laboratory of Agriculture and Agri-Product Safety of Ministry of Education of China, Yangzhou University, Yangzhou, 225009, China
3Biomedical Research Institute, Darfur College, Nyala, 63313, Sudan
4Shandong Haiding husbandry Co., Ltd., Jinan, 250100, China
*Address correspondence to: Zhangping Yang, yzp@yzu.edu.cn
#These authors contributed equally for this work
Received: 16 December 2021; Accepted: 11 February 2022
Abstract: Mastitis is a common inflammatory cow mammary infection; that causes significant economic loss in dairy industry. Given the interesting connection between metformin’s anti-inflammatory function and mastitis model induced by LTA in pbMECs, our objective was to prove that metformin was beneficial in suppressing proinflammatory response induced by LTA through modulation of mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) signaling pathways and activation of peroxisome proliferator-activated receptor-γ (PPARγ) in pbMECs. The proliferation of cells and mRNA expression were measured using EdU assay and quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). Immunoblotting and immunofluorescence analysis were conducted to evaluate the expression of target proteins in inflammatory and anti-inflammatory responses to metformin and LTA. Finally, pbMECs were allowed to treat with the PPAR antagonist GW9662, and inflammatory markers were detected in the cells. Our results showed that LTA concentration at 100 µg/mL significantly stimulated the MAPK14, IL-6 and IL-1β mRNA expressions compared to the control cells (P < 0.05) in dose-dependent tests for LTA. Metformin suppressed the phosphorylation expressions of MAPK (ERK1/2, p38, and JNK) in LTA-stimulated pbMECs. Metformin also reduced the protein expression of NF-κB, interleukin-8 (IL-8), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in pbMECs pretreated with LTA. Metformin administration activated PPARγ phosphorylation by up-regulating the expression of PPARγ in LTA-stimulated pbMECs. Treatment with GW9662 resulted in increased IL-6 expression, which was reversed by metformin. These findings collectively indicated that metformin act to attenuate LTA-stimulated inflammatory response in pbMECs by suppressing MAPK and NF-κB activation via a mechanism partially dependent on PPARγ activation. These results suggested that metformin could function as an anti-inflammatory drug in the treatment of mastitis.
Keywords: Metformin; LTA; Primary bovine mammary epithelial cell; Anti-inflammatory effect; PPARγ; Nuclear factor-κB; Mitogen-activated protein kinase
Mastitis is a common and serious infectious disease in the dairy business caused by many distinct kinds of contagious pathogens spread among cows, resulting in mastitis infection; these pathogens include Staphylococcus aureus, Streptococcus agalactiae, Mycoplasma spp. and Corynebacterium bovis (Patterson, 2017). Mastitis is a common, inflammatory and costly bovine mammary illness that affects the milk production sectors (Günther et al., 2010); and significantly affects animal health, production ability and may eventually cause a major economic loss to the dairy farm business (Halasa et al., 2007; Petrovski et al., 2006). Staphylococcus aureus infection causes almost one-third of dairy cattle’s clinical and subclinical mastitis infections. Among the numerous bacterial and fungal pathogenic factors, S. aureus is one of the key pathogens that cause mastitis (Pereira et al., 2011). Lipoteichoic acid (LTA), as a cell wall component of gram-positive bacteria (S. aureus), is a key element used to trigger inflammatory reactions (Wall et al., 2016) and influences lactation in dairy cows’ mammary glands (Mount et al., 2009; Wu et al., 2020).
Nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways helped in the regulation of cytokines and chemokines, which are important immune mediators during inflammation (Calzado et al., 2007). Furthermore, the interaction of TLR2-S. aureus resulted in the rapid activation and coordination of different intracellular NF-κB and MAPK signaling pathways (Hines et al., 2013).
Selenium inhibits the production of TNF-α, IL-1β and IL-6 inflammatory biomarkers in S. aureus-stimulated pbMECs, via TLR2, NF-κB and MAPK signaling pathways, which may be attributed to the Selenium anti-inflammatory role (Wang et al., 2018). Morin’s (flavono pigment) ability to suppress the NF-B and MAPK signaling may also represent its protective effects against LPS-induced inflammatory responses in pbMECs; as a result, Morin may be used as an anti-inflammatory medicine for mastitis (Wang et al., 2016). Furthermore, in an LPS-induced mastitis model using isolated pbMECs, tea tree oil decreased cellular death while decreasing protein quantities of NF-κB, mitogen-activated protein kinase 4 (MAPK4), and caspase-3 (Chen et al., 2020b). Docosahexaenoic acid (DHA) reduced the inflammatory response to LPS in pbMECs by decreasing NF-B activation via a mechanism that was partly reliant on PPAR activity (He et al., 2017). In addition, cis-9, trans-11-conjugated linoleic acid (CLAs) can reduce Escherichia coli-induced inflammation in pbMECs, and this effect is mediated through the TLR4-NF-B pathway and PPAR participation, as cells pretreated with CLA expressed significantly lower levels of p-p65, p-IκB, TLR4, and higher PPAR gene and protein expressions level following E. coli challenge (Ma et al., 2019).
Currently, antibiotics are used to treat mastitis, which has resulted in an antibiotic resistance problem in dairy production (Guérin-Faublée et al., 2003; Constable and Morin, 2002). Therefore, it is critical and urgent to identify and develop an alternative safe and effective medication to replace antibiotics in the treatment of cow mastitis, which is a growing area of interest in veterinary research.
Metformin (1, 1-dimethylbiguanide hydrochloride), an oral anti-diabetic medication derived from natural constituents present in plant Galega officinalis, often known as French lilac or goat’s rue, and has been used for treating type 2 diabetes for decades (Saisho, 2015). Metformin has many biological functions that have been studied, including anti-inflammatory and anti-carcinogenic properties (Hyun et al., 2013; Liu et al., 2020). Alternatives promising antibiotics have been suggested as an effective strategic means to circumvent drug resistance and counteract the negative effects of pathogenic bacterial infection during inflammation (Liu et al., 2020). Many studies have been done on the animal model in different conditions. A study in Colitic mice reported that metformin is an important candidate for suppressing gut pathological inflammation (di Fusco et al., 2018). Moreover, Metformin injection in the Parkinson’s animal model reduced inflammatory markers such as IL-1β, iNOS, and TNF-α, and reduced the number of microglia cells, therefore suppressed the inflammatory response (Ashabi et al., 2015). Moreover, metformin has been found to enhance p-ERK1/2 expression and reduce cell proliferation in human immortalized keratinocytes; this process may be connected to the activation of the MAPK signaling system (Li et al., 2014). Recently, we discovered that metformin treatment assists in the decrease of inflammatory process in LPS-challenged bMECs via AMPK/NF-κB signaling (Xu et al., 2021b). In addition, metformin inhibits inflammation and oxidative stress by modulating the AMPK/Nrf2/NF-κB signaling pathway in LTA-stimulated pbMECs, highlighting the importance of AMPK as a potential therapeutic approach for the treatment of bovine mastitis (Arbab et al., 2021). However, the actual mechanisms underlying metformin’s anti-inflammatory action on LTA-stimulated pbMECs have not been widely researched. The main objective of this work was to prove that metformin was beneficial in suppressing proinflammatory response induced by LTA through modulation of mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB) signaling pathways and activation of peroxisome proliferator-activated receptor-γ (PPARγ) in pbMECs.
Metformin (Met) with a purity of higher than 97 percent was bought from Sigma (D150959, Sigma-Aldrich, St. Louis, USA). Sigma sold the LTA (derived from Staphylococcus aureus) used in this research (L2515, Sigma-Aldrich, St. Louis, USA).
All experimental procedures were certified by Yangzhou University’s Animal Experiment Committee (YZUDWLL-202003-209) in accordance with the Regulations concerning the Administration of Affairs Concerning Experimental Animals (The State Science and Technology Commission of China, 1988), which were published in 2004 by the Ministry of Science and Technology of China.
Primary bovine mammary epithelial cells isolation and culture
Biopsy mammary gland was performed on three dairy cows at peak lactation (Chen et al., 2019a). Fat and connective tissue was removed after PBS washing. After subculturing, bovine mammary epithelial cells were isolated using the tissue block technique, purified by differential digestion, and cryopreserved. Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12) supplemented with 10% (vol/vol) fetal bovine serum, 5 g/mL bovine insulin, and 10 Ku/L cyan/streptomycin was used to culture the cells. The resuscitated mammary epithelial cells were grown in an incubator at 37°C, 5% CO2, and appropriate humidity. The medium was changed every 48 h. The pbMECs were digested with 0.25% trypsin for passaging, and the growth of cells was observed using an inverted microscope (Chen et al., 2020b).
As previously reported, the culturing of pbMECs was carried out (Chen et al., 2019a; Ma et al., 2019). Cell isolation and purification were performed on mammary tissue collected from three dairy cattle with no historical clinical symptoms. The basal media used to grow the pbMECs included DMEM/F12 (Catalogue No. 11320082, American Thermo Fisher, Waltham, MA, USA) and 10% fetal bovine serum, and various cytokines (e.g., 5 μg/mL bovine insulin, 10 kU/L cyan/streptomycin) (Catalog No. 7120-30, Invitrogen, Carls-bad, CA, USA). The resuscitated pbMECs were cultured in a humidifier at 37°C, 5% CO2. When the cell confluence attained 80%, after infection with small RNA chemical synthesis reagents, cells were harvested 48 h later for the following analyses. Each treatment was performed in triplicate. All experiments were performed on cells at passages 4 to 6. Cells (2 × 105 cells/well) were seeded in 6-well plates and incubated overnight in complete medium (90% RPMI 1640, 8119417 Gibco, CA, 10% fetal bovine serum and antibiotics (penicillin 100 IU/mL; streptomycin 100 μg/mL). All supplemented mediums were from Gibco (Thermo Fisher Scientific, CA). The cells were preserved in a humidified incubator at 37°C and 5% CO2 until they reached confluence.
This study evaluated and optimized the inflammation model regarding LTA and metformin concentrations. To find an appropriate metformin treatment for the subsequent experimental work, we used the EdU assay to identify metformin dose-dependent effect on cell viability for the pbMECs pretreated with metformin for 12 hours at graded concentrations of (0, 3, and 10 mM) (Fig. 1). The qRT-PCR analysis was used to measure LTA dose-dependent in pbMECs stimulated by LTA at (0, 50, and 100 µg/mL) for 6 h (Figs. 2A–2C).
LTA was used to stimulate the mastitis setting in vitro. To make a 1 µg/mL stock solution, a total of 5 µg of LTA was dissolved in 5 mL of RPMI 1640 (CCM). As study design, four experimental trials set as follow: a control group (Control) cells supplied only with PBS; LTA treatment refers (LTA) cells administrated with LTA at a concentration of 100 μg /mL for 6 h; combined metformin with LTA group (MET + LTA) for cells pretreated with metformin and subsequently primed with LTA and metformin group (MET) for cells only pretreated with metformin at a dose of 3 mM according to the dose-dependent assay for 12 h (Fig. 6). In addition, to clarify that metformin’s effect on the inflammatory process is PPARγ activation-dependent, GW9662 (1 mM), a PPARγ inhibitor, was used at a dosage of 1 mM.
5-Ethynyl-2’-deoxyuridine (EdU) detection
The BeyoClick EdU cell proliferation kit with Alexa Fluor 555 (Beyotime, Shanghai, China) was used in accordance with the manufacturer’s protocols to measure the ability of pbMECs to proliferate in response to different treatments. Cells were incubated with 1X EdU (10 μM) solution for 2 h, subsequently fixed with 4% paraformaldehyde at room temperature (RT) for 20 min and permeabilized with 0.3% Triton X-100 in PBS for 15 min at RT. For nuclear staining, cells were incubated with 1X Hoechst for 10 min at RT in the absence of light. Finally, cells were imaged at 200× magnification using a DMi8 Microsystems GmbH (Leica, Wetzlar, Germany) fluorescence microscope.
RNA isolation, cDNA and gene expression by using quantitative real-time PCR analysis
After discarding the cell culture, PBS was used to wash the cells two times before using the TRIzol reagent (Catalog No. 9108, Takara, Dalian, China) to isolate the Total RNA in cells and a RNA concentration was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). The purity of RNA (A260/A280) for all samples was above 1.9. RNA quality was assessed using a 2100 Bioanalyzer (Agilent Technologies). Samples had a median RNA integrity value of 6.7 ± 1.2. 1 µg Total RNA was added to the reaction system and reverse transcribed into cDNA using a Prime Script RT Master Mix Kit (Catalog No. RR036A, Takara), as described in the manufacturer. Premier 6.0 software (Premier Biosoft International, Palo Alto, CA, USA) was used to create related primers, and the primer sequences are shown in Table 1. Before using primers, their efficacy was evaluated. Each cDNA sample was amplified through qRT-PCR using the SYBR Premix Ex Taq Kit (Catalog No. DRR420A, Takara) on an ABI 7300 Fast Real-time PCR system (Applied Biosystems, Foster City, CA, USA). In our research, we used Glyceraldehyde phosphate dehydrogenase (GAPDH), RPS9, and UXT as internal controls for RNA expression in our experiments. Fold changes of the related mRNAs were quantified by the 2−ΔΔCt method (Pfaffl, 2001).
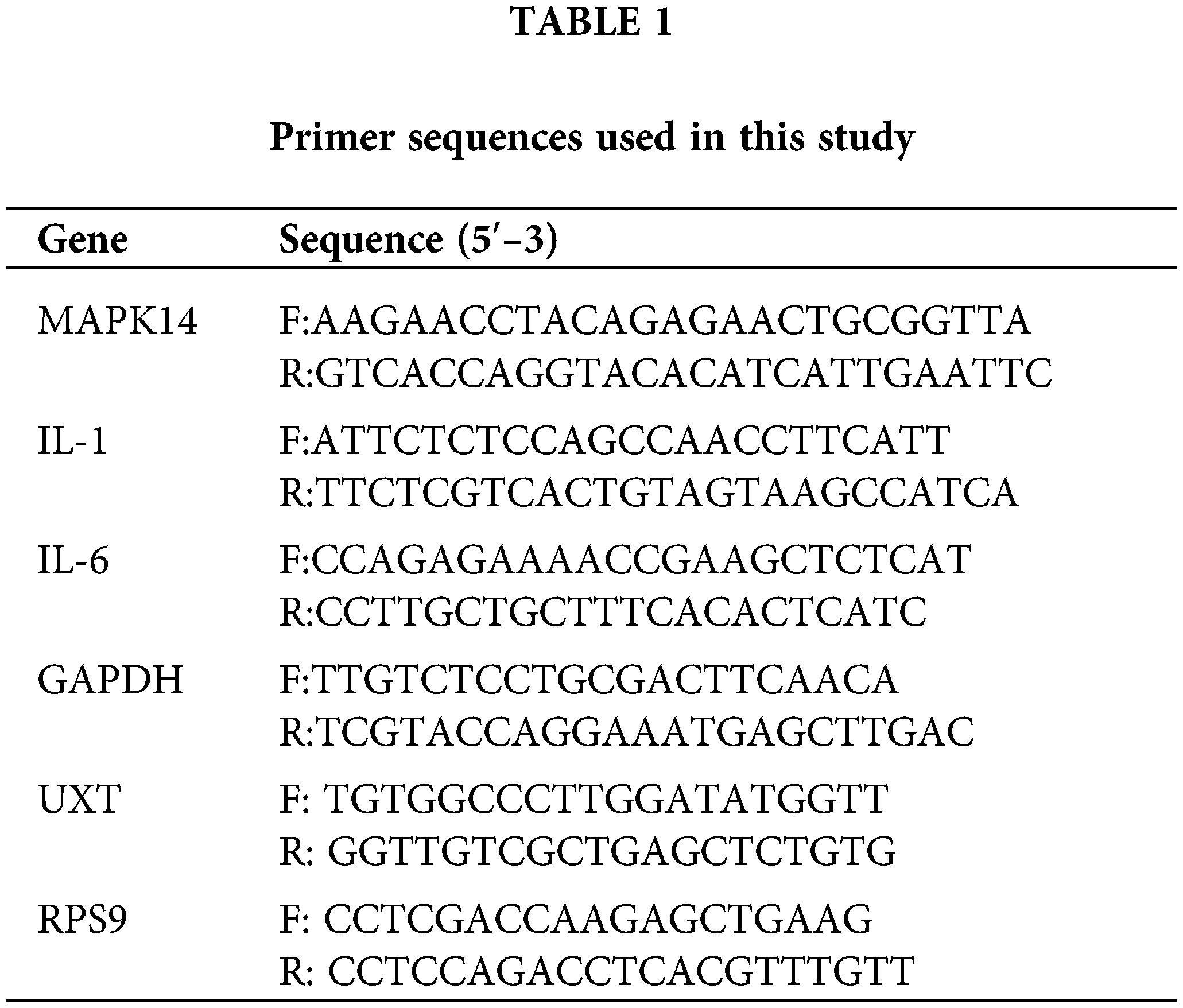
The reverse transcription–generated cDNA encoding, Mitogen-activated protein kinase 14 (MAPK14), interleukin-1β (IL-1β), interleukin 6 (IL-6), GAPDH (glyceraldehyde-3 phosphate dehydrogenase), UXT (Ubiquitously-expressed transcript), RPS9 (Ribosomal Protein S9) were amplified by RT-PCR using primers. F, forward primer (5′-3′); and R, reverse primer (3′-5′).
Western blot was carried out in accordance with the previously published procedures (Chen et al., 2019a). In brief, pbMECs were lysed in RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China), then electrophoresed in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS−PAGE) gels and then were transferred onto nitrocellulose membranes (Bio Trace, Pall Corp, Port Washington, NY, USA). After blocking for 2 h at room temperature with 7% skim milk or 5% bovine serum albumin (BSA) (used for phosphorylated protein) in Tris-buffered saline with Tween (TBST), the nitrocellulose membranes were incubated overnight at 4°C with different anti-p65 (#AF1234), p-IKβα (#AF1870) IL-1β (#A108133611), IL-6, IL-8 (#AH09037217), MAPK Family Antibody (JNK (#AF1048), p-JNK (#AF1767), ERK1/2 (# AF1051), p-ERK1/2 (#AF1891) and p-p38 (#AF5884), PPARγ (#AF7797) Family Antibody overnight, and GAPDH (#AF1186)), Rabbit antibodies were purchased from Beyotime; IL-8 (#AH09037217), and IL-1β were obtained from Bioss ANTIBODIES (AL08133511/bs-6319R). P-p65 (#3033) was derived from Cell Signaling Technology (Danvers, MA). After washing with TBST, 6 times 5 min each time, the blots were incubated at room temperature for 2 h with a diluted 1:5,000 goat anti-rabbit secondary antibody obtained from Abcam (ab6712). After being washed with TBST 6 times 5 min each and chemiluminescence, bands were detected and analyzed with Bio-Rad Gel Doc 2000 system analysis software (Bio-Rad, Hercules, CA). GAPDH was used as a reference in our present study. A quantitative analysis of protein expression was performed using Image J software.
Ten thousand (2 × 104 cells/well) pbMECs were seed per well in 12-well plates. The cells were cultured overnight before being treated according to the experimental protocol. For the immunofluorescence technique, PBS (200 L/well) was used to rinse the coverslips bounded by cells triple before adding 4 percent paraformaldehyde (500 L/well) to fix the cells structure for 15 minutes. Cells were washed three times with PBS before perforating with 0.3% Triton X-100 (T9284, Sigma-Aldrich) at room temperature for 15 minutes to increase permeability. After three times washed with PBS, the cells’ surfaces were blocked using 1 × PBS/5% BSA/0.3% Triton-100 blocking buffer at room temperature for 1 h. The resultant cells were then incubated against p38, p65, IL-6, and PPARγ primary antibodies (same as those used in the Western blot analysis) in antibody buffer (1 × PBS/1% BSA/0.3% Triton X-100) at 4°C overnight, followed by washing triple with PBS, the cells were then stained for 1 h in the dark at 37°C with the FITC-conjugated goat anti-rabbit antibody (A0562, Beyotime). PBS was used to wash the cells three times gently. The cell nucleus was stained by DAPI (1 μg/mL) (D8417, Sigma-Aldrich) for 5 min before being rinsed triple with PBS. Using a DMi8 Microsystems GmbH (Leica, Wetzlar, Germany), the expression of p38, p65, IL-6, and PPARγ was visualized.
The means ± standard error (mean ± SEM) was used to report the data. Significant differences between treatments groups were determined by one-way ANOVA with Duncan’s multiple range-tests by IBM SPSS 20.0 Statistics for Windows (IBM Inc., New York, NY, United States). P-values less than 0.05 were considered statistically significant. Experiments were conducted in triplicate.
Determination of the metformin effect on pbMECs proliferation
Metformin concentration was carried out using Flow Cytometry, western blot analysis and CCK-8 assay, and determined the dose of metformin 3 mM was suitable in accordance with our previous published work (Arbab et al., 2021; Xu et al., 2021b). This study used EdU assay to confirm the proper dose of metformin and investigate pbMECs proliferation following metformin treatment. Compared to the control group, metformin at dose 10 mM affected the cell proliferation due to the inhibited EdU staining. Cells pretreated with metformin at a concentration of 3 mM blocked the effect of LTA-mediated suppression of cells proliferation (Fig. 1). This metformin (3 mM) dose was considered suitable and used in this experiment as no cytotoxic effect was observed.

Figure 1: EdU assay for determination of cell proliferation. Cells were pretreated with or without metformin at doses of 3 mM and 10 mM for 12 h, followed by the induction of 100 µg/ml LTA for 6 h.
Optimizing LTA concentration by using qRT-PCR technique in pbMECs
In our previous study, the qRT-PCR technique was used to measure LTA dose-dependency (Arbab et al., 2021). In this experiment, the mRNA expressions of Mitogen-Activated Protein Kinase 14 (MAPK14), interleukin 6 (IL-6), and interleukin-1β (IL-1β) in LTA-induced pbMECs by was measured at doses (0, 50, and 100 μg/mL) doses for 6 h, as shown in Fig. 2. The results revealed that pbMECs stimulated with LTA at dosages of 0, 50, and 100 μg/mL for 6 h; the MAPK14 gene expression showed difference between the control and 100 μg/mL groups (P > 0.05) (Fig. 2A). Compared to the control group, the IL-6 gene expression was different at 50 and 100 μg/mL of LTA groups (P < 0.05) (Fig. 2B). Compared to the control group, the mRNA levels of IL-1β increased in LTA treatments at 50 and 100 μg/mL (P < 0.05) (Fig. 2C). Therefore, we determined from these results that LTA at a concentration of 100 μg/mL was suitable for the next experiments of this study.

Figure 2: Dose-dependent effect of LTA in PBMECs. Effect of LTA on MAPK14, IL-6 and IL-1β gene expressions measured in pbMECs by qRT-PCR (A–C). Glyceraldehyde-3 phosphate dehydrogenase (GAPDH) is used as a reference control. Values were represented as means ± SEM. LTA, lipoteichoic acid, NC, control. Different uppercase (A and B) letters on the top of bars indicate significant differences (P < 0.05).
Metformin inhibits the LTA-induced activation of the MAPK pathway in pbMECs
In response to bacterial toxins, MAPKs play a key role in activating the gene expression encoding a wide range of cytokines and chemokines. To investigate the inhibitory effect of metformin supplementation on the key proteins of the MAPK signaling pathways in pbMECs that LTA indeed activated. Our Western blotting results revealed that the levels of phosphorylated (p-ERK1/2, p-p38 and p-JNK) were increased following LTA stimulation in the context of pbMECs mastitis model. While, metformin decreased the expression of MAPK kinase components (p-ERK1/2, p-p38, and p-JNK) in pbMECs treated with metformin 3 mM dose for 12 h followed by incubation with 100 µl/ml LTA for 6 h (Figs. 3A and 3B). These data indicate that metformin inhibits MAPK signaling, suggesting that metformin can exert an anti-inflammatory effect by preventing the MAPK signaling pathway from being activated.
Furthermore, we used immunofluorescence to validate p-p38 activation in the current study. Metformin enhanced staining of phosphorylated p-p38, as seen in Fig. 3C. In comparison, the LTA treatment resulted in a light staining level. Thus, the LTA challenge does not affect the staining level of p-p38 phosphorylation in metformin-treated cells.
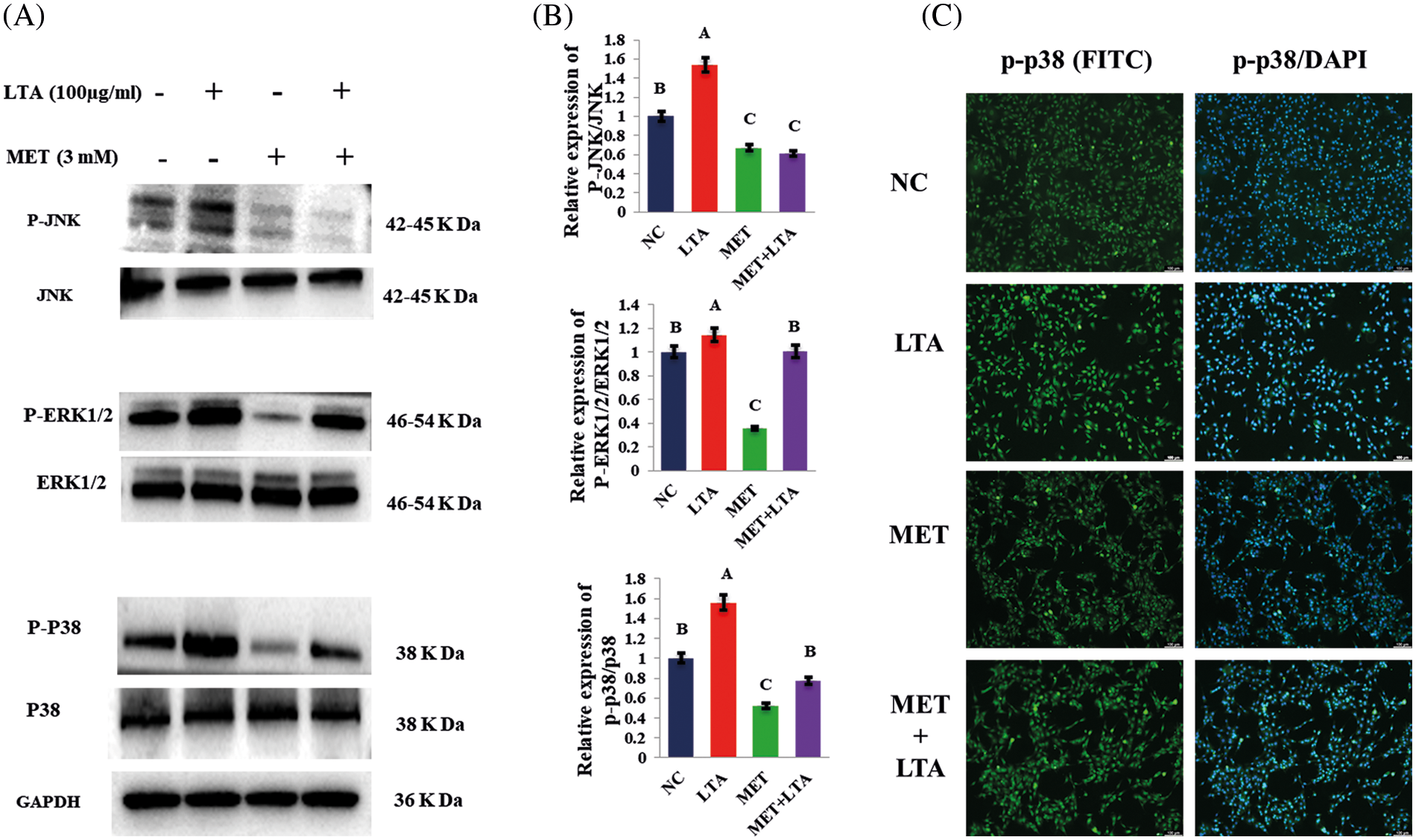
Figure 3: Inhibitory effect of metformin on the MAPKs protein levels in LTA-induced pbMECs. Cells were stimulated by LTA (100 µg/mL) for 6 h in the presence of metformin (3 mM). Western blotting was used to identify the protein expression of MAPK subfamilies (p-ERK1/2, p-p38 and p-JNK). Representative examples of western blots are shown on the left (A), and results from densitometry analysis of the western blots are shown in the middle panel (B). GAPDH was used as a reference control. Relative protein expression levels of p-p38/p38, p-ERK/ERK and p-JNK/JNK were quantified by Image J software. While, the p-p38 protein immunofluorescence (FITC) was performed, and the nuclear was stained with dye DAPI (blue) (C). These values are presented as the means ± SEM of three independent experiments with similar results. Different uppercase letters (A, B and C) on the top of the bars consider significant differences at (P < 0.05).
Metformin inhibits LTA-induced activation of NF-κB pathway in pbMECs
To understand whether metformin functioned in suppressing the NF-κB pathway and alleviated the inflammation induced by LTA in pbMECs. The effects of metformin on the inactivation of NF-κB and IκBα in LTA-stimulated pbMECs were examined using western blotting. Compared to the control group, the protein level of phosphorylated NF-κB (p-p65) and IκBα were upregulated in LTA-stimulated pbMECs (P < 0.05). However, compared to the LTA group, a suppression of p-p65 and IκBα was observed in pbMECs pretreated with metformin (P < 0.05), as shown in Fig. 4.
Moreover, metformin reduced the protein expression levels of inflammatory cytokines (IL-8 and IL-6) besides IL-1β proteins expression in pbMECs challenged with LTA, respectively (Fig. 4B). As shown by Western blotting, metformin pretreatment decreased the protein expression levels of inflammatory cytokines in pbMECs. Thus, our findings suggest that metformin can suppress the production of inflammatory cytokines, thereby alleviating the LTA-induced inflammatory response in pbMECs.
This study also used immunofluorescence to demonstrate the p-p65 translocation and subsequently the induction of IL-6 in nuclei after LTA-induced inflammatory response in pbMECs or pretreatment with metformin to reverse the process by visualizing the fluorescence signal using immunofluorescence microscopy. NF-κB and IL-6 were visualized using FITC fluorescence (green), and the nuclei were visualized using DAPI (blue). When pbMECs were stimulated with LTA, the strongest signal was p-p65, and the green fluorescence was predominantly located in the nuclei. However, there was less p-p65 signal in cells pretreated with 3 mM metformin, and it was metformin mainly in the cytoplasm. These results indicated that metformin acted as an anti-inflammatory agent by preventing p-p65 translocation into the nucleus, and consequently showed a decrease in IL-6 expression level (Fig. 4C).

Figure 4: Inhibitory effect of metformin on LTA-induced NF-κB pathway activation in pbMECs. The cells were pre-incubated with metformin (3 μM) for 12 h and then treated with 100 μg/ml LTA for 6 h. Analysis of NF-κB, IκBα and the downstream (IL-8, IL-6 and IL-1β) protein expressions in pbMECs was determined using western blotting. Representative examples of western blots are shown on the left in (A), and results from densitometry analysis of the western blots are shown in the middle in (B). While the immunofluorescence was used to detect p-p65 (FITC) and IL-6 (FITC) activity and nuclear was stained with DAPI dyne (blue) (C). These values are presented as the means ± SEM of three independent experiments. Different uppercase letters (A, B, C, and D) on the top of the bars considered significant differences at (P < 0.05).
Metformin activated PPARγ signaling pathway in LTA-induced pbMECs
Metformin was used to alleviate the inflammatory response of LTA-induced pbMECs. The activity of PPARγ was detected with western blotting to investigate the possible molecular mechanism of metformin’s action against the LTA-induced inflammatory response on pbMECs. In comparison to the control group, metformin pretreatment and the combined (metformin plus LTA) group activated PPARγ phosphorylation, as metformin up-regulated the expression of PPARγ in LTA-stimulated pbMECs (P < 0.05, Fig. 5A). To further investigate metformin’s anti-inflammatory effect, pbMECs were treated with the PPARγ antagonist GW9662, which resulted in increased IL-6 expression. Conversely, GW9662 and metformin combination treatments significantly reduced the expression of IL-6 inflammatory factors (P < 0.05, Fig. 5B).
The activity of PPARγ in pbMECs was determined using immunofluorescence. The PPARγ (green) was seen using FITC fluorescence, while DAPI was used to show the nuclei (blue) (Fig. 5C). These findings suggest that metformin supplementation exerts anti-inflammatory effects during LTA-induced inflammatory response on pbMECs.

Figure 5: Effects of metformin on PPARγ activation in LTA-stimulated pbMECs. The cells were pre-incubated with metformin at 3 mM for 12 h before being treated with 100 μg/ml LTA for 6 h. Representative western blots and results from densitometry analysis of the western blots are shown in the left panels (A, B). GAPDH was used as a loading control. Metformin pretreatment effect on the location of PPARγ protein in bovine pbMECs stimulated with LTA was performed using immunofluorescence for PPARγ (FITC), and the nuclear was stained with dye DAPI (blue) was shown the right panels (C). The PPARγ protein expression in pbMECs was determined by Western-blot. For the PPARγ inhibitory activity, GW9662 was used. These values are presented as the means ± SEM of three independent experiments, and different uppercase letters (A, B and C) on the top of the bars considered significant differences at (P < 0.05).
The main purpose of this study was to investigate if the activation of PPARγ via metformin could help in inhibiting inflammatory responses in an LTA-induced mastitis model using isolated pbMECs. The current study demonstrated a protective action of metformin on LTA-induced inflammation bovine mastitis by notably activating anti-inflammatory PPARγ signaling and inhibition of MAPK and NF-κB signaling pathways, which play a central role during the inflammatory process. Uncovering the functional relationship between (MAPK and NF-κB) inflammatory axis and PPARγ pathways is significant because it reveals a promising anti-inflammatory mechanism for inflammation suppression (Fig. 6). This discovery may greatly contribute to the development of new therapeutic approaches for inflammatory illnesses like mastitis.

Figure 6: Possible Schematic representation underlying the protective effects of metformin against S. aureus LTA-induced cellular inflammatory responses in pbMECs. S aureus -LTA interaction leads to the rapid and coordinated activation of NF-κB and MAPK signaling pathways. Metformin activated the anti-inflammatory PPARγ and reduced LTA-induced inflammatory damage to pbMECs by inhibiting the MAPK and NF-κB signaling pathways.
Mastitis is a frequent, inflammatory bovine mammary disease; this condition is one of the major causes of economic losses in dairy industries worldwide (Halasa et al., 2007; Gomes and Henriques, 2016). Another option to antibiotics have been suggested as one of the best solutions to reduce the emergence of drug resistance and mitigate the impact of inflammation caused by bacterial invasion (Liu et al., 2020). Therefore, veterinary science is becoming increasingly interested in the safe and effective treatment of bovine mastitis. Metformin, a commonly used anti-diabetic medication, has been shown to have various biological activities such as anti-inflammatory and benefits animals suffering from inflammatory diseases such as mastitis. The current study aimed to investigate metformin’s anti-inflammatory properties and the action mechanism against LTA-induced inflammation in pbMECs.
To comprehend the critical role of metformin as an anti-inflammatory drug in such situations, as well as the cross association between PPARγ activation and suppression of MAPK and NF-κB signaling axis; the pbMECs were exposed to metformin (3 mM) for 12 h followed by the addition of 100 μg/mL LTA for 6 h. We used qRT-PCR to determine the mRNA expression. We used Western blot analysis and immunofluorescence to evaluate the target proteins in inflammatory and anti-inflammatory responses metformin and LTA.
Metformin has been shown in previous in vitro and in vivo studies to inhibit the growth of human prostate cancer (Sahara et al., 2008). Another research reported that pretreatment with metformin increased the intensity of β-hydroxybutyric acid-inhibited cell staining in the EdU assay in bovine hepatocytes (Xu et al., 2021a). The metformin (3 mM) pretreated pbMECs blocked the effect of LTA-mediated cell proliferation suppression. Pretreatment with metformin increased the intensity of LTA-inhibited cells staining in the EdU test. Our data indicated that metformin (3 mM) had potential activity as it has a similar effect on pbMECs proliferation in vitro compared to the control group. This dose of metformin was consistent with what was reported in our previous study (Arbab et al., 2021); therefore, it might be used as a novel therapeutic agent.
MAPKs are families of closely related kinases of those three major subfamilies: ERK, JNK, and p38. MAPK and PPAR pathways were highly linked to the inflammatory response. MAPK pathway plays an important in the onset and development of inflammation. As it is known that, the NF-κB and MAPK signalling pathways are shown to contribute to control cytokines and chemokine production, which are essential immune mediators during inflammation (Calzado et al., 2007). Furthermore, the TLR2-S. aureus reaction involves the rapid and coordinated activation of various intracellular signaling pathways, including NF-κB and MAPK (Hines et al., 2013).
Many studies have demonstrated that different inducers other than metformin can inhibit the inflammation process in bMECs by blocking NF-κB and MAPK signaling pathways. For example, Selenium inhibited the gene expressions of inflammatory mediators TNF-α, IL-1β and IL-6 in S. aureus-stimulated pbMECs via TLR2, NF-κB and MAPK signaling pathway, implying an anti-inflammatory effect of Se (Wang et al., 2018). Morin also showed the ability to suppress NF-κB and MAPK signaling pathways in pbMECs induced inflammatory response by LPS, suggesting that it could be used as an anti-inflammatory therapy for mastitis (Wang et al., 2016). Moreover, in the lipopolysaccharide (LPS)-induced mastitis model using isolated bMECs, tea tree oil decreased cellular death along with downregulated protein concentrations of NF-κB, MAPK4, and caspase-3 (Chen et al., 2020b).
To the best of our understanding, the promising metformin mode of action as an anti-inflammatory agent against LTA induced mastitis models in pbMECs, as well as the precise role and connection of activating PPAR and inhibiting the MAPK and NF-κB pathways on inflammatory suppression, has not been clearly reported; hence, from a mechanism perspective, this results indicated that MAPK and NF-κB axis is considered to be involved in the metformin-mediated suppression of inflammatory process in this settings. Herein, we tested the effects of metformin on MAPK activation, which plays a central role during the inflammatory response (Herlaar and Brown, 1999). The levels of p38, JNK, and ERK MAPKs were identified using Western blot to determine whether the MAPK pathway is implicated in metformin’s anti-inflammatory actions. The results demonstrated that the expression of phosphorylated ERK1/2 (p-ERK1/2), phosphorylated p38 (p-p38) and phosphorylated JNK (p-JNK) proteins were increased upon LTA stimulation in pbMECs; while metformin pretreatment obviously reduced the phosphorylated proteins of p-p38, p-JNK, and p-ERK1/2. According to this evidence, this study suggested that metformin functions as an anti-inflammatory agent in mastitis.
LTA, an endotoxin of S. aureus, is released during the cellular proliferation process and after death and has been shown to stimulate inflammatory responses (Bougarn et al., 2010). Interestingly, S. aureus and its related endotoxins internalization into pbMECs were critical factors connected with the NF-κB activation and induction of inflammatory reaction in cows’ mammary glands, which affected lactation (Oviedo-Boyso et al., 2008; Mount et al., 2009; Wu et al., 2020).
NF-κB, a pivotal transcription factor, is a key regulator of inflammation, and immunological responses (Lawrence, 2009) is normally localized in the cytoplasm as a complex with IKB in normal cells, in the presence of inducer, IκBα if degraded and NF-κB is phosphorylated and translocate into the nucleus and promote the transcription of inflammation cytokines(Mardirossian et al., 2018). TNF-α, IL-1β and IL-6 cytokines are released in response to endotoxins and mastitis pathogens (Blum et al., 2017; Nakajima et al., 1997). Thus, the downregulation of proteins IL-8, IL6 and IL1B in response to metformin after LTA stimulation indicate metformin’s anti-inflammatory action in bovine cells. Metformin inhibited NF-κB activation and proinflammatory cytokine secretion (Lei et al., 2017). Recent research found that metformin pretreatment reduced NF-κB, COX2, and TNF-α levels in pbMECs stimulated by LTA, all of which are considered essential proteins implicated in the inflammation process. Metformin inhibited p-p65 expression in the pbMECs exposed to LTA. Metformin’s anti-inflammatory effect was also reported; interestingly, it has significantly reduced the expression of NF-κB downstream protein expressions COX2, IL-1β, and TNF-α, which were upregulated in response to LTA stimulation (Arbab et al., 2021). In the current study, we further characterize the nature of the metformin mechanism; we assumed that IL-8, IL-6, IL-1β cytokines and NF-κB and IKBα kinases are possibly involved in the anti-inflammatory effect of metformin supplementation on pbMECs. This study’s findings were in part replicated in the current study because compared to control cells, LTA (100 µg/ml) activated NF-κB signaling by upregulating the expression of phosphorylated NF-κB and (IL-8, IL-6 and IL-1β) pro-inflammatory cytokines in the pbMECs, respectively (P < 0.05). However, by adding metformin, the protein expression of these inflammatory cytokines was reduced. These results might point to the remarkable protective role of metformin in the regulation of the NF-κB pathway as a potential therapeutic method for treating bovine inflammation.
The peroxisome proliferator-activated receptor-γ (PPARγ) is a ligand-inducible transcription factor involved in adipogenesis, glucose metabolism, angiogenesis and inflammation (Sarafidis and Bakris, 2006). Several studies have proven that some herbal products have anti-inflammatory properties via PPARγ activation (Lim et al., 2012). Emodin activates PPARγ, thereby attenuating LPS-induced inflammatory response in mouse mammary epithelial cells (Yang et al., 2014). Moreover, accumulating data have shown that PPARγ might negatively modulate LPS-induced inflammatory responses. On the other hand, DHA has attenuated LPS-stimulated inflammatory response in pbMECs by suppressing NF-κB activation via a partially reliant mechanism on PPARγ activation (He et al., 2017).
Moreover, CLA can reduce inflammation caused by E. coli in pbMECs, and this process is mediated via the TLR4-NF-κB pathway and PPARγ participation. These results showed that the cells that were pretreated with CLA expressed significantly lower p-p65, p-IκB, TLR4 and a higher level of PPARγ after the E. coli challenge at the gene and protein levels (Ma et al., 2019). Research with various cell types has clarified that PPARγ signaling is important for anti-inflammatory function (Ul Hasan et al., 2019). However, the mechanisms underlying metformin protective effects on the pro-inflammatory reactions in bMEC have not been clearly understood. This study investigated the effects of metformin on PPARγ activation. Compared to the control group, the metformin pretreatment and the combined (metformin plus LTA) group activated PPARγ phosphorylation, as metformin up-regulated the expression of PPARγ in LTA-stimulated pbMECs. Thereby metformin could be used as an anti-inflammatory compound to attenuate LTA-induced inflammatory response in pbMECs.
In summary, these results demonstrated that metformin could suppress proinflammatory cytokine production in LTA-stimulated pbMECs, by suppressing MAPK and NF-κB activation, and this mechanism is probably dependent on PPARγ activation (Fig. 6). Therefore, these results suggested that metformin could be an anti-inflammatory medication for mastitis in future studies.
Authors’ Contribution: The authors confirm contribution to the paper as follows: study conception and design: Abdelaziz Adam Idriss Arbab and Tianle Xu; data collection: Abdelaziz Adam Idriss Arbab, Chunqing Yin, Xubin Lu and Yan Liang; analysis and interpretation of results: Abdelaziz Adam Idriss Arbab, Chunqing Yin, Tianle Xu; Amer Adam Idris; and Ismail Mohamed Abdalla; draft manuscript preparation: Abdelaziz Adam Idriss Arbab; Chunqing Yin, Yongjiang Mao and Xubin Lu. Zhangping Yang support to the financial cost throughout the experiment. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated during or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: All experimental procedures were certified by Yangzhou University’s Animal Experiment Committee (YZUDWLL-202003-209) in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (The State Science and Technology Commission of China, 1988), which were published in 2004 by the Ministry of Science and Technology of China.
Funding Statement: This study was supported by the National Natural Science Foundation of China (Grant Nos. 32102731; 31872324).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Arbab AAI, Lu X, Abdalla IM, Idris AA, Chen Z, Li M, Mao Y, Xu T, Yang Z (2021). Metformin inhibits lipoteichoic acid-induced oxidative stress and inflammation through AMPK/NRF2/NF-κB signaling pathway in bovine mammary epithelial cells. Frontiers in Veterinary Science 8: 661380. DOI 10.3389/fvets.2021.661380. [Google Scholar] [CrossRef]
Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A (2015). Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metabolic Brain Disease 30: 747–754. DOI 10.1007/s11011-014-9632-2. [Google Scholar] [CrossRef]
Blum SE, Heller ED, Jacoby S, Krifucks O, Leitner G (2017). Comparison of the immune responses associated with experimental bovine mastitis caused by different strains of Escherichia coli. Journal of Dairy Research 84: 190–197. DOI 10.1017/S0022029917000206. [Google Scholar] [CrossRef]
Bougarn S, Cunha P, Harmache A, Fromageau A, Gilbert FB, Rainard P (2010). Muramyl dipeptide synergizes with Staphylococcus aureus lipoteichoic acid to recruit neutrophils in the mammary gland and to stimulate mammary epithelial cells. Clinical and Vaccine Immunology 17: 1797–1809. DOI 10.1128/CVI.00268-10. [Google Scholar] [CrossRef]
Calzado M, Bacher S, Schmitz ML (2007). NF-κB inhibitors for the treatment of inflammatory diseases and cancer. Current Medicinal Chemistry 14: 367–376. DOI 10.2174/092986707779941113. [Google Scholar] [CrossRef]
Chen Z, Chu S, Wang X, Sun Y, Xu T, Mao Y, Loor JJ, Yang Z (2019a). MiR-16a regulates milk fat metabolism by targeting large tumor suppressor kinase 1 (LATS1) in bovine mammary epithelial cells. Journal of Agricultural and Food Chemistry 67: 11167–11178. DOI 10.1021/acs.jafc.9b04883. [Google Scholar] [CrossRef]
Chen Z, Zhang Y, Zhou J, Lu L, Wang X, Liang Y, Loor JJ, Gou D, Xu H, Yang Z (2020b). Tea tree oil prevents mastitis-associated inflammation in lipopolysaccharide-stimulated bovine mammary epithelial cells. Frontiers in Veterinary Science 7: 496. DOI 10.3389/fvets.2020.00496. [Google Scholar] [CrossRef]
Constable PD, Morin DE (2002). Use of antimicrobial susceptibility testing of bacterial pathogens isolated from the milk of dairy cows with clinical mastitis to predict response to treatment with cephapirin and oxytetracycline. Journal of the American Veterinary Medical Association 221: 103–108. DOI 10.2460/javma.2002.221.103. [Google Scholar] [CrossRef]
Di Fusco D, Dinallo V, Monteleone I, Laudisi F, Marafini I et al. (2018). Metformin inhibits inflammatory signals in the gut by controlling AMPK and p38 MAP kinase activation. Clinical Science 132: 1155–1168. DOI 10.1042/CS20180167. [Google Scholar] [CrossRef]
Gomes F, Henriques M (2016). Control of bovine mastitis: Old and recent therapeutic approaches. Current Microbiology 72: 377–382. DOI 10.1007/s00284-015-0958-8. [Google Scholar] [CrossRef]
Guérin-Faublée V, Carret G, Houffschmitt P (2003). In vitro activity of 10 antimicrobial agents against bacteria isolated from cows with clinical mastitis. Veterinary Record 152: 466–471. DOI 10.1136/vr.152.15.466. [Google Scholar] [CrossRef]
Günther J, Liu S, Esch K, Schuberth HJ, Seyfert HM (2010). Stimulated expression of TNF-α and IL-8, but not of lingual antimicrobial peptide reflects the concentration of pathogens contacting bovine mammary epithelial cells. Veterinary Immunology and Immunopathology 135: 152–157. DOI 10.1016/j.vetimm.2009.11.004. [Google Scholar] [CrossRef]
Halasa T, Huijps K, Østerås O, Hogeveen H (2007). Economic effects of bovine mastitis and mastitis management: A review. Veterinary Quarterly 29: 18–31. DOI 10.1080/01652176.2007.9695224. [Google Scholar] [CrossRef]
He X, Liu W, Shi M, Yang Z, Zhang X, Gong P (2017). Docosahexaenoic acid attenuates LPS-stimulated inflammatory response by regulating the PPARγ/NF-κB pathways in primary bovine mammary epithelial cells. Research in Veterinary Science 112: 7–12. DOI 10.1016/j.rvsc.2016.12.011. [Google Scholar] [CrossRef]
Herlaar E, Brown Z (1999). p38 MAPK signalling cascades in inflammatory disease. Molecular Medicine Today 5: 439–447. DOI 10.1016/S1357-4310(99)01544-0. [Google Scholar] [CrossRef]
Hines DJ, Choi HB, Hines RM, Phillips AG, MacVicar BA (2013). Prevention of LPS-induced microglia activation, cytokine production and sickness behavior with TLR4 receptor interfering peptides. PLoS One 8: e60388. DOI 10.1371/journal.pone.0060388. [Google Scholar] [CrossRef]
Hyun B, Shin S, Lee A, Lee S, Song Y, Ha NJ, Cho KH, Kim K (2013). Metformin down-regulates TNF-α secretion via suppression of scavenger receptors in macrophages. Immune Network 13: 123. DOI 10.4110/in.2013.13.4.123. [Google Scholar] [CrossRef]
Lawrence T (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology 1: a001651. DOI 10.1101/cshperspect.a001651. [Google Scholar] [CrossRef]
Lei Y, Yi Y, Liu Y, Liu X, Keller ET, Qian CN, Zhang J, Lu Y (2017). Metformin targets multiple signaling pathways in cancer. Chinese Journal of Cancer 36: 1–9. DOI 10.1186/s40880-017-0184-9. [Google Scholar] [CrossRef]
Li W, Ma W, Zhong H, Liu W, Sun Q (2014). Metformin inhibits proliferation of human keratinocytes through a mechanism associated with activation of the MAPK signaling pathway. Experimental and Therapeutic Medicine 7: 389–392. DOI 10.3892/etm.2013.1416. [Google Scholar] [CrossRef]
Lim HA, Lee EK, Kim JM, Park MH, Kim DH et al. (2012). PPARγ activation by baicalin suppresses NF-κB-mediated inflammation in aged rat kidney. Biogerontology 13: 133–145. DOI 10.1007/s10522-011-9361-4. [Google Scholar] [CrossRef]
Liu Y, Jia Y, Yang K, Li R, Xiao X, Zhu K, Wang Z (2020). Metformin restores tetracyclines susceptibility against multidrug resistant bacteria. Advanced Science 7: 1902227. DOI 10.1002/advs.201902227. [Google Scholar] [CrossRef]
Ma N, Chang G, Huang J, Wang Y, Gao Q, Cheng X, Liu J, Shen X (2019). Cis-9, trans-11-conjugated linoleic acid exerts an anti-inflammatory effect in bovine mammary epithelial cells after Escherichia coli stimulation through NF-κB signaling pathway. Journal of Agricultural and Food Chemistry 67: 193–200. DOI 10.1021/acs.jafc.8b05500. [Google Scholar] [CrossRef]
Mardirossian M, Barrière Q, Timchenko T, Müller C, Pacor S, Mergaert P, Scocchi M, Wilsona DN (2018). Fragments of the nonlytic proline-rich antimicrobial peptide Bac5 kill Escherichia coli cells by inhibiting protein synthesis. Antimicrobial Agents and Chemotherapy 62: e00534-18. DOI 10.1128/AAC.00534-18. [Google Scholar] [CrossRef]
Mount JA, Karrow NA, Caswell JL, Boermans HJ, Leslie KE (2009). Assessment of bovine mammary chemokine gene expression in response to lipopolysaccharide, lipotechoic acid + peptidoglycan, and CpG oligodeoxynucleotide 2135. Canadian Journal of Veterinary Research 73: 49–57. [Google Scholar]
Nakajima Y, Mikami O, Yoshioka M, Motoi Y, Ito T, Ishikawa Y, Fuse M, Nakano K, Yasukawa K (1997). Elevated levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) activities in the sera and milk of cows with naturally occurring coliform mastitis. Research in Veterinary Science 62: 297–298. DOI 10.1016/S0034-5288(97)90209-5. [Google Scholar] [CrossRef]
Oviedo-Boyso J, Barriga-Rivera JG, Valdez-Alarcón JJ, Bravo-Patiño A, Cárabez-Trejo A, Cajero-Juárez M, Baizabal-Aguirre VM (2008). Internalization of Staphylococcus aureus by bovine endothelial cells is associated with the activity state of NF-κB and modulated by the pro-inflammatory cytokines TNF-α and IL-1β. Scandinavian Journal of Immunology 67: 169–176. DOI 10.1111/j.1365-3083.2007.02056.x. [Google Scholar] [CrossRef]
Patterson C (2017). Veterinary medicine: A textbook of the diseases of cattle, horses, sheep, pigs, and goats, 11th edition, Volumes 1 and 2. The Canadian Veterinary Journal 10: 2045–2050. [Google Scholar]
Pereira UP, Oliveira DGS, Mesquita LR, Costa GM, Pereira LJ (2011). Efficacy of Staphylococcus aureus vaccines for bovine mastitis: A systematic review. Veterinary Microbiology 148: 117–124. DOI 10.1016/j.vetmic.2010.10.003. [Google Scholar] [CrossRef]
Petrovski KR, Trajcev M, Buneski G (2006). A review of the factors affecting the costs of bovine mastitis: Review article. Journal of the South African Veterinary Association 77: 52–60. DOI 10.4102/jsava.v77i2.344. [Google Scholar] [CrossRef]
Pfaffl MW (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29: 45e–45. DOI 10.1093/nar/29.9.e45. [Google Scholar] [CrossRef]
Sahra IB, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, Auberger P, Tanti JF, Le Marchand-Brustel Y, Bost F (2008). The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 27: 3576–3586. DOI 10.1038/sj.onc.1211024. [Google Scholar] [CrossRef]
Saisho Y (2015). Metformin and inflammation: Its potential beyond glucose-lowering effect. Endocrine, Metabolic & Immune Disorders-Drug Targets 15: 196–205. DOI 10.2174/1871530315666150316124019. [Google Scholar] [CrossRef]
Sarafidis PA, Bakris GL (2006). Protection of the kidney by thiazolidinediones: An assessment from bench to bedside. Kidney International 70: 1223–1233. DOI 10.1038/sj.ki.5001620. [Google Scholar] [CrossRef]
Ul Hasan A, Rahman A, Kobori H (2019). Interactions between host PPARs and gut microbiota in health and disease. International Journal of Molecular Sciences 20: 387. DOI 10.3390/ijms20020387. [Google Scholar] [CrossRef]
Wall SK, Hernández-Castellano LE, Ahmadpour A, Bruckmaier RM, Wellnitz O (2016). Differential glucocorticoid-induced closure of the blood-milk barrier during lipopolysaccharide- and lipoteichoic acid-induced mastitis in dairy cows. Journal of Dairy Science 99: 7544–7553. DOI 10.3168/jds.2016-11093. [Google Scholar] [CrossRef]
Wang H, Bi C, Wang Y, Sun J, Meng X, Li J (2018). Selenium ameliorates Staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-κB and MAPK signaling pathways. BMC Veterinary Research 14: 197. DOI 10.1186/s12917-018-1508-y. [Google Scholar] [CrossRef]
Wang J, Guo C, Wei Z, He X, Kou J, Zhou E, Yang Z, Fu Y (2016). Morin suppresses inflammatory cytokine expression by downregulation of nuclear factor-κB and mitogen-activated protein kinase (MAPK) signaling pathways in lipopolysaccharide-stimulated primary bovine mammary epithelial cells. Journal of Dairy Science 99: 3016–3022. DOI 10.3168/jds.2015-10330. [Google Scholar] [CrossRef]
Wu Y, Chen J, Sun Y, Dong X, Wang Z, Chen J, Dong G (2020). PGN and LTA from Staphylococcus aureus induced inflammation and decreased lactation through regulating DNA methylation and histone H3 acetylation in bovine mammary epithelial cells. Toxins 12: 238. DOI 10.3390/toxins12040238. [Google Scholar] [CrossRef]
Xu T, Lu X, Idriss Arbab AA, Wu X, Mao Y, Loor JJ, Yang Z (2021a). Metformin acts to suppress β-hydroxybutyric acid-mediated inflammatory responses through activation of AMPK signalling in bovine hepatocytes. Journal of Animal Science 99: skab153. DOI 10.1093/jas/skab153. [Google Scholar] [CrossRef]
Xu T, Wu X, Lu X, Liang Y, Mao Y, Loor JJ, Yang Z (2021b). Metformin activated AMPK signaling contributes to the alleviation of LPS-induced inflammatory responses in bovine mammary epithelial cells. BMC Veterinary Research 17: 97. DOI 10.1186/s12917-021-02797-x. [Google Scholar] [CrossRef]
Yang Z, Zhou E, Wei D, Li D, Wei Z, Zhang W, Zhang X (2014). Emodin inhibits LPS-induced inflammatory response by activating PPAR-γ in mouse mammary epithelial cells. International Immunopharmacology 21: 354–360. DOI 10.1016/j.intimp.2014.05.019. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |