DOI:10.32604/biocell.2022.021161

| BIOCELL DOI:10.32604/biocell.2022.021161 |  |
| Article |
Induction of adaptive response in utero by ionizing radiation: A radiation quality dependent phenomenon
National Institutes for Quantum Science and Technology, Chiba, 263-8555, Japan
*Address correspondence to: Bing Wang, wang.bing@qst.go.jp; Mitsuru Nenoi, nenoi.mitsuru@qst.go.jp
Received: 30 December 2021; Accepted: 14 February 2022
Abstract: Investigation on possible induction of adaptive response (AR) by high-liner energy transfer (LET) particle radiation for protection against low-LET photon radiation-induced detrimental effects has not yet been performed in utero. This study verified if an AR could be induced by high-LET particle radiation from accelerated heavy ions against low-LET X-ray radiation-induced detrimental effects on fetal mice. Total body irradiation of pregnant C57BL/6J mice were performed by delivering a priming dose ranging from 10 mGy to 320 mGy of particle radiation on gestation day 11 followed one day later by a challenge dose at 3500 mGy from X-ray radiation. The monoenergetic beams of carbon, silicon and iron with the LET values of about 15, 55, and 200 KeV/μm, respectively, were examined. Significant suppression by the priming radiation of the detrimental effects (fetal death, malformation, or low body weight) was used as the endpoints for judgment of a successful AR induction on gestation day 18. Existence of AR was not observed. On the other hand, the priming dose of high-LET particle radiation, in some cases, even increased the detrimental effects induced by the challenge dose from low-LET X-ray radiation. Although existence of AR induced by high-LET radiation in cultured mammalian cells in vitro and in certain tissues of laboratory mice in vivo was demonstrated, the present study did not suggest that low dose of high-LET particle radiation could induce an AR in fetal mice in utero under the setup of our experimental system.
Keywords: Heavy particle radiation; Adaptive response; High liner energy transfer; Teratogenesis; Fetal mice
Abbreviations
| AR: | adaptive response |
| DSB: | double-strand break |
| E: | embryonic day |
| HIMAC: | Heavy Ion Medical Accelerator in Chiba |
| IR: | ionizing radiation |
| LET: | liner energy transfer |
| RBE: | relative biological effectiveness |
| SD: | standard deviation |
| TBI: | total body irradiation |
Exposure to teratogens is one of the main concerns on health risk for embryogenesis and fetal development in humans. As a teratogen, carcinogen and mutagen, in utero exposure to ionizing radiation (IR) could cause teratogenic, carcinogenic and mutagenic effects on the fetus (Williams and Fletcher, 2010). At a moderate to high dose IR could result in varied detrimental effects. For examples, exposure of the embryo or fetus to a dose higher than 500 mGy (0.5 Gy) at a high dose rate could increase the risk of cancer in epidemiological studies in humans and experimental studies in mammalian animals (Brent, 2014; Brent, 2015). During organogenesis in laboratory murine, the detrimental effects manifest such as elevated occurrence rate of prenatal death, malformation and intrauterine growth retardation. These effects depend on the quality and quantity of radiation (such as types of radiation (i.e., electromagnetic photon and particle), linear energy transfer (LET), dose, and dose rate) and characteristics of the fetuses (such as developmental stage and genetic susceptibility) (ICRP, 1986; NCRP, 1994; NCRP, 2013; Wang et al., 1999a; Wang et al., 2000b; Wang, 2001; De Santis et al., 2007; Wang et al., 2007; Wang et al., 2008; Williams and Fletcher, 2010). Meanwhile, studies also show that IR at low doses is capable of inducing an adaptive response (AR) which could protect against the detrimental effects resulting from a subsequent challenge IR at higher doses (Wang et al., 1998; Wang et al., 2000a; Mitchel et al., 2002; Okazaki et al., 2005; Boreham et al., 2006; Wang et al., 2012; Howell et al., 2013).
AR, as one of the specific phenomena induced by low doses of IR, has been studied for several decades (UNSCEAR, 1994). Studies on AR provide important scientific basis for radiation risk estimates and offer significant insight into the novel biological defense mechanisms regarding protection against radiation (Mortazavi et al., 2003; Streffer, 2004; Mitchel, 2006; Varès et al., 2006; Tapio and Jacob, 2007; Takahashi and Ohnishi, 2009; Nenoi et al., 2015). However, most of the investigations on AR at the whole-body level have been performed using low-LET photon radiation (i.e., X-ray and gamma-ray radiation) for both the priming radiation and the challenge radiation (UNSCEAR, 1994; Tapio and Jacob, 2007; Takahashi and Ohnishi, 2009; Nenoi et al., 2015). Due to the difference of radiation quality, difference occurs in induction of DNA damage by low- and high-LET radiations. Low-LET IR damages the structure of DNA directly via introducing base lesions and DNA strand breaks, and indirectly via generating reactive oxygen species (Goodhead, 1994). On the other hand, high-LET particle radiation causes damage mainly through a particle track and by energy deposited radial to this track leading to clustered DNA damage and complex DNA lesions (Prise et al., 1994). High-LET particle radiation-induced clustered DNA damage is less easily repaired, subsequently resulting in efficient cell killing than low-LET photon radiation (Goodhead, 1994; Suzuki et al., 2000; Nikjoo et al., 2001). Study on gene expression also shows that a variety of DNA repair mechanisms were downregulated after exposure to high-LET particle radiation, correlating with complex DNA damage formation. However, DNA repair mechanisms continue to take place after exposure to low-LET X-ray radiation (Michalettou et al., 2021). As most of the damage induced by high-LET particle radiation is hard to be repaired or non-repairable (Hada and Georgakilas, 2008), high-LET particle radiation usually shows larger relative biological effectiveness (RBE) on various endpoints, such as cell killing, animal development and lethality, and their RBE values depend mainly on the LET and the type of the particle (Aoki et al., 2000; Rydberg et al., 2005; Wang et al., 2007; Wang et al., 2008; Hunter and Muirhead, 2009; Sørensen et al., 2011; Okayasu, 2012; Wang and Yasuda, 2020).
Medical application of IR in diagnosis and treatment, and manned exploration activities in space are associated with concerns on exposure to both low- and high-LET radiations (Furukawa et al., 2020). These concerns necessitate the study on radiation quality-dependent biological effects in both basic and translational fields, and the study must be performed in the context of the mixed low- and high-LET radiations that humans encounter. Recent advances in generation of accelerated high-LET particle radiation provide a useful tool and a valuable opportunity for more in-depth investigations on the biological effects from exposure to high-LET particle radiation. In our previous work at whole-body level, AR was induced both in the fetal mice in utero and in the adult mice in vivo by delivery of the priming low doses of low-LET X-ray radiation in advance against the detrimental effects from subsequent challenge high doses from high-LET particle radiation (Wang et al., 2010; Wang et al., 2012). As the current knowledge of the effects and basic mechanisms specific to low doses of high-LET particle radiation is still poor, the present study is aimed to verify possible existence of high-LET particle radiation-induced AR against the detrimental effects of subsequent challenge radiation from low-LET X-ray radiation in fetal mice and to provide possible insight to explore its underlying mechanisms. Being different from some studies reporting successful induction of AR by high-LET particle radiation in vitro in cultured cell lines and in vivo in certain organs in laboratory animals, results obtained under the experimental setup in the present work did not show a successful AR induction by high-LET particle radiation against low-LET X-ray radiation-induced detriment in utero in fetal mice. Together, our studies suggested induction of AR in utero by IR possible a radiation quality dependent phenomenon in the context of exposure to both the mixed low- and high-LET radiations.
Eight-week-old C57BL/6J Jms strain mice of both sexes were purchased from SLC, Inc. (Hamamatsu, Japan). All animals were maintained in a conventional animal facility under a 12 h light-12 h dark photoperiod, controlled temperature (23 ± 2°C) and humidity (50 ± 10%). They were housed in autoclaved cages with sterilized wood chips and allowed free access to standard laboratory chow (MB-1, Funabashi Farm Co., Japan) and acidified water ad libitum. The pregnant mice were timely prepared accordingly (Wang et al., 2000a; Wang et al., 2012). In brief, after being acclimatized to the laboratory conditions for 2 weeks, animals to be used were first selected by body weight. To avoid possible effects from the developmental condition of the mice, any mouse of 10 weeks old with a significantly higher or lower body weight (more or less than the mean ± 2 standard deviation (SD)) was omitted from this study. Using the selected mice, the female mice in estrus were mated with male mice (two females housed with one male in the same cage) overnight (from 11:30 p.m. to 5:30 a.m.). The following day when a copulatory plug was found the day was designated as gestation day 1 (embryonic day 1, E1), and then each of pregnant animals was housed in one cage. To avoid possible effects from the developmental condition of the fetuses and the litter size, the pregnant mice were selected by body weight on E11. Any pregnant mouse with a significantly higher or lower body weight was omitted from this investigation. In this work at least 5 pregnant mice were used for each experimental point and all the experiment was repeated once. There were totally 317 pregnant mice used in this study.
For high-LET particle radiation, carbon, silicon and iron particles were generated and accelerated by a synchrotron, the Heavy Ion Medical Accelerator in Chiba (HIMAC), National Institutes for Quantum Science and Technology, Japan. The monoenergetic carbon, silicon and iron beams having 290, 490 and 500 MeV/nucleon of initial energy respectively were expanded by wobbler magnets to a 10 cm irradiation field with homogeneous irradiation dose. Animals were irradiated at the entrance (plateau) region of the beams. The dose-averaged LET value for carbon, silicon and iron particle radiation was at about 15, 55 and 200 KeV/μm, respectively. Total body irradiation (TBI) of the fetal mice in utero in the pregnant mice with a priming dose ranging from 10 mGy to 320 mGy was delivered on E11 at a dose rate ranging from 10 to 100 mGy/min (Fig. 1). The latitude of the priming dose was chosen to be comparable to the efficient doses of X-ray radiation by taking into account the RBE value (Wang and Yasuda, 2020) and/or the average number of particle hits to the cell (Katsube et al., 2021) for each type of particle radiation under HIMAC setup conditions. For receiving TBI with particle radiation, the pregnant mice were held in a Lucite columnar container, which was with an outer diameter of 10 cm and 3 individual cells of the same size (each mouse in each cell). The mice were in an air-breathing condition (there were six holes 5 mm in diameter in the wall of each cell). The containers were set on the beam track and the focused 10 cm diameter ion beam was delivered to the animals at room temperature without anesthesia.
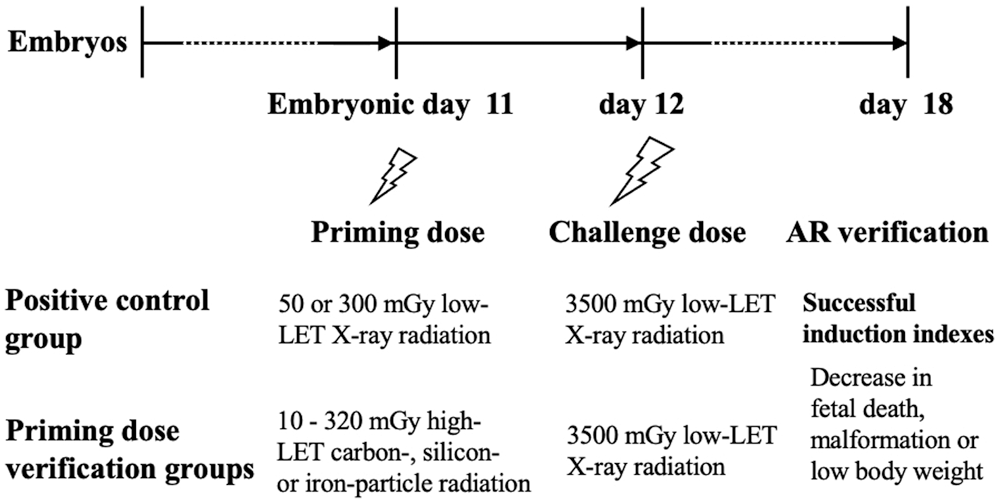
Figure 1: Experimental schedule.
For low-LET X-ray radiation, X-rays were generated with an X-ray machine (Pantak-320S, Shimadzu, Japan) operated at 200 kVp and 20 mA, using a 0.50 mm Al + 0.50 mm Cu filter. An exposure-rate meter (AE-1321M, Applied Engineering Inc., Japan) with an ionization chamber (C-110, 0.6 ml, JARP, Applied Engineering Inc., Japan) was used for the dosimetry. TBI of the fetal mice in utero in the pregnant mice with an efficient priming dose at 50 mGy or 300 mGy was delivered on E11 at a dose rate of about 300 mGy/min, and a challenge dose of 3500 mGy was delivered on E12 at a dose rate of about 0.55 Gy/min. The latitude of these two efficient priming doses used in the present work was according to our previous studies (Wang et al., 2000a; Wang et al., 2012) (Fig. 1). For receiving TBI with X-ray radiation, the pregnant mice were held in a Lucite columnar container, which was with an outer diameter of 23.5 cm and 12 individual cells of the same size (each mouse in each cell and one cell for the dosimetry meter). The mice were in an air-breathing condition (there were 11 holes 5 mm in diameter in the wall of each cell). The container was set on the radiation target platform and BTI were delivered to the animals at room temperature without anesthesia.
Fetal mouse model for induction of AR
The efficient and essential conditions for successful induction of AR in fetal mice established in our previous studies were applied to the present work (Wang et al., 2000a; Wang et al., 2012). In brief, the timing and interval for delivery of the priming dose and challenge dose was on E11 and E12, respectively. Compared to the detrimental effects induced by the challenge radiation alone, significant decrease in fetal death, malformation in live fetuses, or low body weight induced by the priming radiation when examined on E18 was used as the criteria for phenotypic verification of a successful induction of AR. Serving as a positive control, the phenotypic verification work using X-ray radiation for delivery of both the priming dose and the challenge dose was always performed in parallel to the investigations with priming dose from high-LET particle radiation on E11 in combination of the challenge dose from low-LET X-ray radiation on E12 (Fig. 1).
Phenotypic verification of AR induction
The procedures and criteria for verification of AR induction in our previous work (Wang et al., 2012) were adopted and used. In brief, the pregnant mice on gestation day 18 (E18) were sacrificed by CO2 asphyxiation followed by cervical dislocation. The fetuses were obtained by cesarean section after euthanasia of the pregnant animals. The numbers of live fetuses, dead fetuses and live fetuses with external gross malformations were examined and scored. The body weight of live fetuses was weighed. In the present study, the external gross malformations induced by radiations were mainly limb defects (missing or shortness of digits) and malformed tails (short, kinked or bent tails). For fetal death, it was defined as those without a heartbeat, having signs of autolysis (i.e., dark red body discoloration), and those showing fetal residues. In this work, as radiations delivered to the fetus were in the period of late organogenesis, thus those with only placenta residues were defined as early death and they were excluded when evaluating the radiation effects. A successful induction of AR was defined as a marked suppression which was induced by the priming radiation at low doses against either the incidence of fetal death, the incidence of live malformed fetuses or low body weight that was induced by the challenge radiation at higher doses (Fig. 1).
As data obtained in each experiment did not show statistical differences across the replicates, thus data were pooled. Results were presented as absolute value (mean ± SD) for fetal death, live malformed fetuses, and fetal body weight using little as the unit. The litter was used as the unit of statistical analysis. Statistical evaluation of the data was carried out using Student’s t-test. Statistical significance was assigned to p < 0.05.
Reproducibility of AR induction in fetal mouse model using low-LET X-ray radiation
The fetal mouse model for successful induction of AR in utero established in our previous study (Wang et al., 2012) was verified under the experiment setup in the present study. Both the priming dose at 50 mGy and 300 mGy on E11 and challenge dose at 3500 mGy on E12 were delivered by using low-LET X-ray radiation. Results showed that the priming dose (50 mGy and 300 mGy) could significantly reduce the percentage of fetal death (Fig. 2a), percentage of live malformed fetuses (Fig. 2b) and decrease in body weight (Fig. 2c). For examples, the percentage of fetal death was 4.1 ± 1.0% in the control group receiving neither the priming dose on E11 nor the challenge dose on E12, while the percentage of fetal death was 12.9 ± 1.9% in the group receiving only the challenge dose on E12. The challenge dose could markedly cause increased fetal death (p < 0.01). On the other hand, the percentage of fetal death was respectively 6.8 ± 2.1% and 7.1 ± 1.7% in the group receiving a priming dose at 50 mGy on E11 followed by the challenge dose on E12 and the group receiving a priming dose at 300 mGy on E11 followed by the challenge dose on E12. These results indicated that successful induction of AR was confirmed under the experiment setup in the present study.
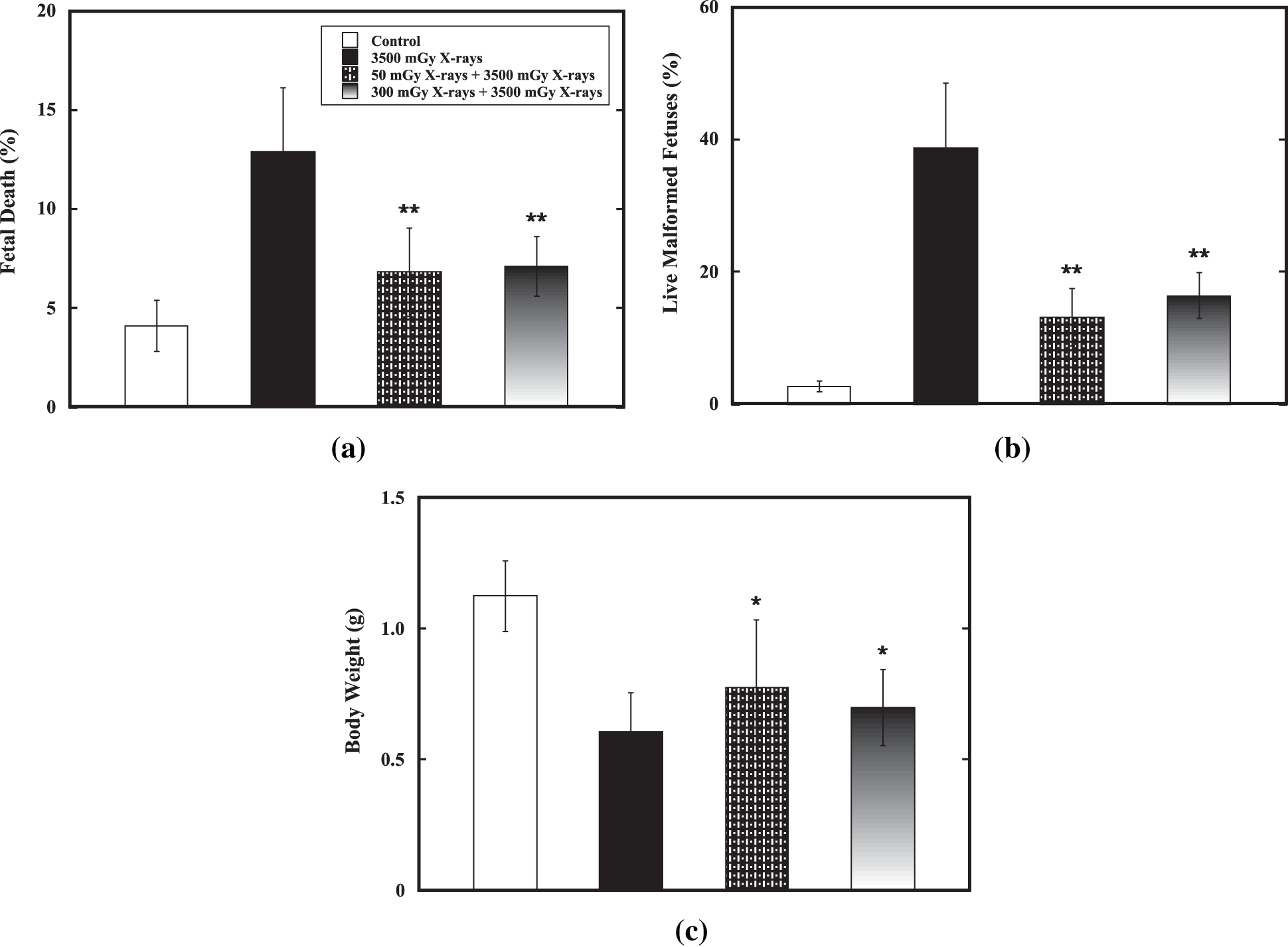
Figure 2: Reproducibility of AR induction in fetal mouse model. Reproducibility of AR induction by the priming doses (50 mGy and 300 mGy) from low-LET X-ray radiation on E11 against the challenge dose (3500 mGy) from low-LET X-ray radiation on E12 in the fetal mouse model was confirmed. Datum is presented as mean ± SD. (a) Percentage of fetal death. (b) Percentage of live malformed fetuses. (c) Body weight (in gram). One asterisk (*) and two asterisks (**) indicate statistically marked difference between the group receiving only the challenge dose (3500 mGy on E12) and the irradiated group receiving both the priming dose on E11 and the challenge dose on E12 at p < 0.05 and p < 0.01, respectively. Abbreviation: AR, adaptive response; E11, embryonic day 11; E12, embryonic day 12; SD, standard deviation.
No AR induction by high-LET carbon-particle radiation
Effect of a priming dose ranging from 10 mGy to 250 mGy on E11 on reduction of the detrimental effect of the challenge dose of 3500 mGy from low-LET X-ray radiation on E12 was verified. Results showed that no priming dose could significantly reduce the percentage of fetal death (Fig. 3a), percentage of live malformed fetuses (Fig. 3b) and decrease in body weight (Fig. 3c). On the contrary, a priming dose at 23 mGy Gy could significantly increase fetal death and a priming dose ranging from 10 mGy to 250 mGy could cause markedly increase in induction of malformation in fetal mice. For examples, for the the percentage of fetal death, it was 3.3 ± 0.9% in the control group while it was 15.5 ± 4.2% in the group receiving only the challenge dose. The challenge dose could markedly cause increased fetal death (p < 0.01). However, the percentage of fetal death was 20.1 ± 4.2% in the group receiving a priming dose at 230 mGy followed by the challenge dose. The percentage of live malformed fetuses, it was 2.3 ± 0.2% in the control group while it was 39.6 ± 10.5% in the group receiving only the challenge dose. The challenge dose could markedly cause increased malformation in fetal mice (p < 0.01). A priming dose ranging from 10 mGy to 250 mGy significantly increase the percentage of live malformed fetuses to 58.3 ± 15.7% to 72.2 ± 25.3%. On the other hand, the priming dose did not show any marked effect on reduction of body weight induced by the challenge dose. These results indicated that no induction of AR by carbon-particle radiation was demonstrated.
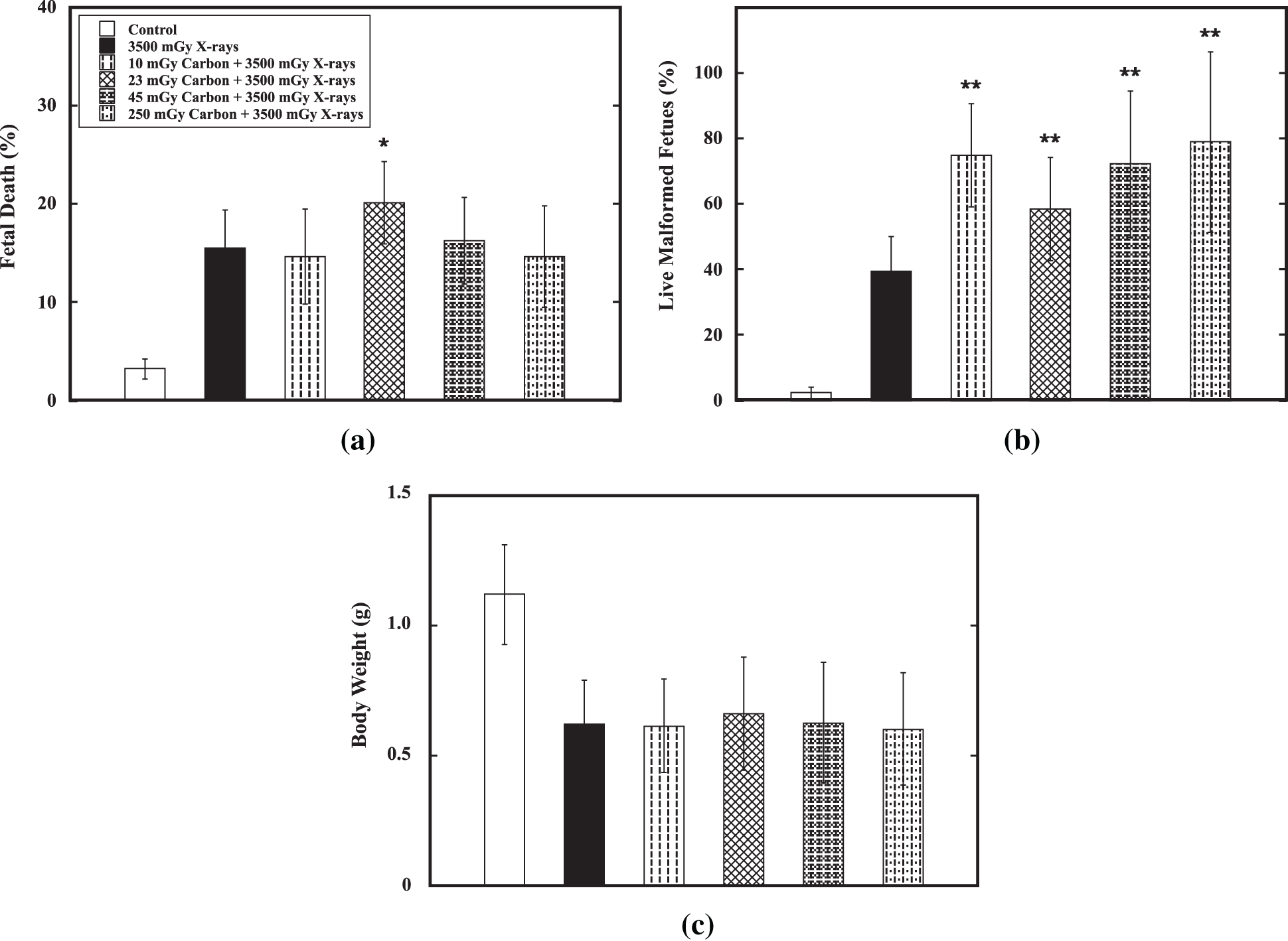
Figure 3: Verification of AR induction by high-LET carbon-particle radiation. Verification of AR induction by a priming dose ranging from 10 mGy to 250 mGy of high-LET carbon-particle radiation on E11 against the challenge dose (3500 mGy) from low-LET X-ray radiation on E12 was carried out in the fetal mouse model. Datum is presented as mean ± SD. (a) Percentage of fetal death. (b) Percentage of live malformed fetuses. (c) Body weight (in gram). One asterisk (*) and two asterisks (**) indicate statistically marked difference between the group receiving only the challenge dose (3500 mGy on E12) and the irradiated group receiving both the priming dose on E11 and the challenge dose on E12 at p < 0.05 and p < 0.01, respectively.
No AR induction by high-LET silicon-particle radiation
Effect of a priming dose ranging from 35 mGy to 93 mGy on E11 on reduction of the detrimental effect of the challenge dose of 3500 mGy from low-LET X-ray radiation on E12 was verified. Results showed that no priming dose could significantly reduce the percentage of fetal death (Fig. 4a), percentage of live malformed fetuses (Fig. 4b) and decrease in body weight (Fig. 4c). On the contrary, a priming dose at 35 mGy or 45 mGy could significantly increase fetal death and a priming dose ranging from 35 mGy to 93 mGy could cause markedly increase in induction of malformation in fetal mice. For examples, for the the percentage of fetal death, it was 3.9 ± 0.9% in the control group while it was 12.5 ± 3.3% in the group receiving only the challenge dose. The challenge dose could markedly cause increased fetal death (p < 0.01). However, the percentage of fetal death was 20.7 ± 8.8% and 26.8 ± 9.2%, respectively, in the group receiving a priming dose at 35 mGy followed by the challenge dose and in the group receiving a priming dose at 45 mGy followed by the challenge dose. The percentage of live malformed fetuses, it was 2.3 ± 0.6% in the control group while it was 37.8 ± 12.8% in the group receiving only the challenge dose. The challenge dose could markedly cause increased malformation in fetal mice (p < 0.01). A priming dose ranging from 10 mGy to 250 mGy significantly increase the percentage of live malformed fetuses to 80.6 ± 14.8% to 96.6 ± 16.4%. On the other hand, the priming dose did not show any marked effect on reduction of body weight induced by the challenge dose. These results indicated that no induction of AR by silicon-particle radiation was demonstrated.
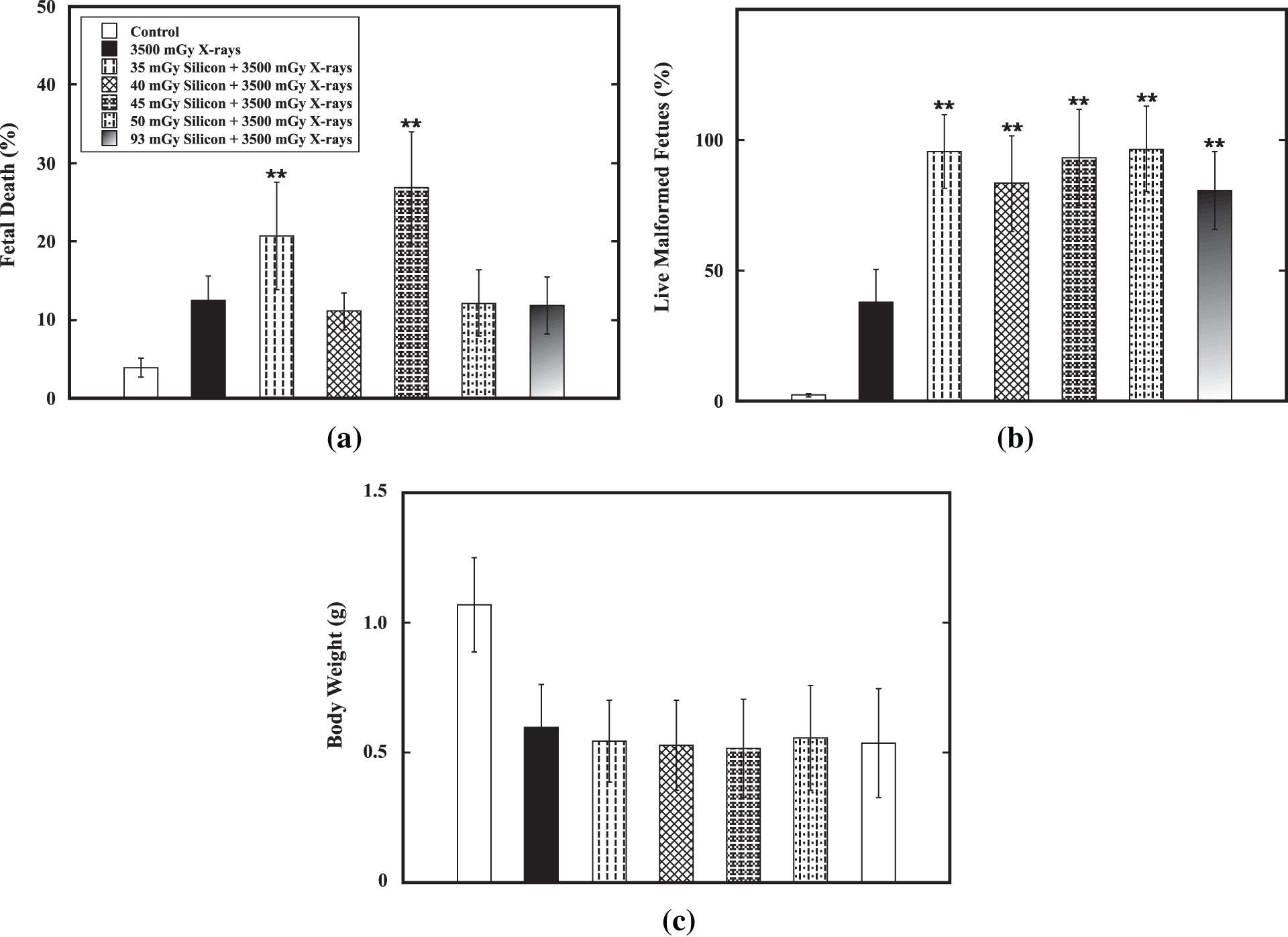
Figure 4: Verification of AR induction by high-LET silicon-particle radiation. Verification of AR induction by a priming dose ranging from 35 mGy to 93 mGy of high-LET silicon-particle radiation on E11 against the challenge dose (3500 mGy) from low-LET X-ray radiation on E12 was performed in the fetal mouse model. Datum is presented as mean ± SD. (a) Percentage of fetal death. (b) Percentage of live malformed fetuses. (c) Body weight (in gram). Two asterisks (**) indicate statistically marked difference between the group receiving only the challenge dose (3500 mGy on E12) and the irradiated group receiving both the priming dose on E11 and the challenge dose on E12 at p < 0.01.
No AR induction by high-LET iron-particle radiation
Effect of a priming dose ranging from 35 mGy to 320 mGy on E11 on reduction of the detrimental effect of the challenge dose of 3500 mGy from low-LET X-ray radiation on E12 was verified. Results showed that no priming dose could significantly reduce the percentage of fetal death (Fig. 5a), percentage of live malformed fetuses (Fig. 5b) and decrease in body weight (Fig. 5c). On the contrary, a priming dose ranging from 40 mGy to 320 mGy could significantly increase fetal death and each of the priming doses used could cause markedly increase in induction of malformation in fetal mice. For examples, for the the percentage of fetal death, it was 3.9 ± 1.0% in the control group while it was 12.4 ± 3.0% in the group receiving only the challenge dose. The challenge dose could markedly cause increased fetal death (p < 0.01). However, the percentage of fetal death was in the range from 15.5 ± 4.1% to 24.4 ± 7.5% in the group receiving a priming dose ranging from 40 mGy to 320 mGy followed by the challenge dose. The percentage of live malformed fetuses, it was 2.4 ± 0.7% in the control group while it was 38.1 ± 12.6% in the group receiving only the challenge dose. The challenge dose could markedly cause increased malformation in fetal mice (p < 0.01). A priming dose ranging from 35 mGy to 320 mGy significantly increase the percentage of live malformed fetuses to 93.1 ± 24.2% to 100.0 ± 24.0%. On the other hand, the priming dose did not show any marked effect on reduction of body weight induced by the challenge dose. These results indicated that no induction of AR by iron-particle radiation was demonstrated.
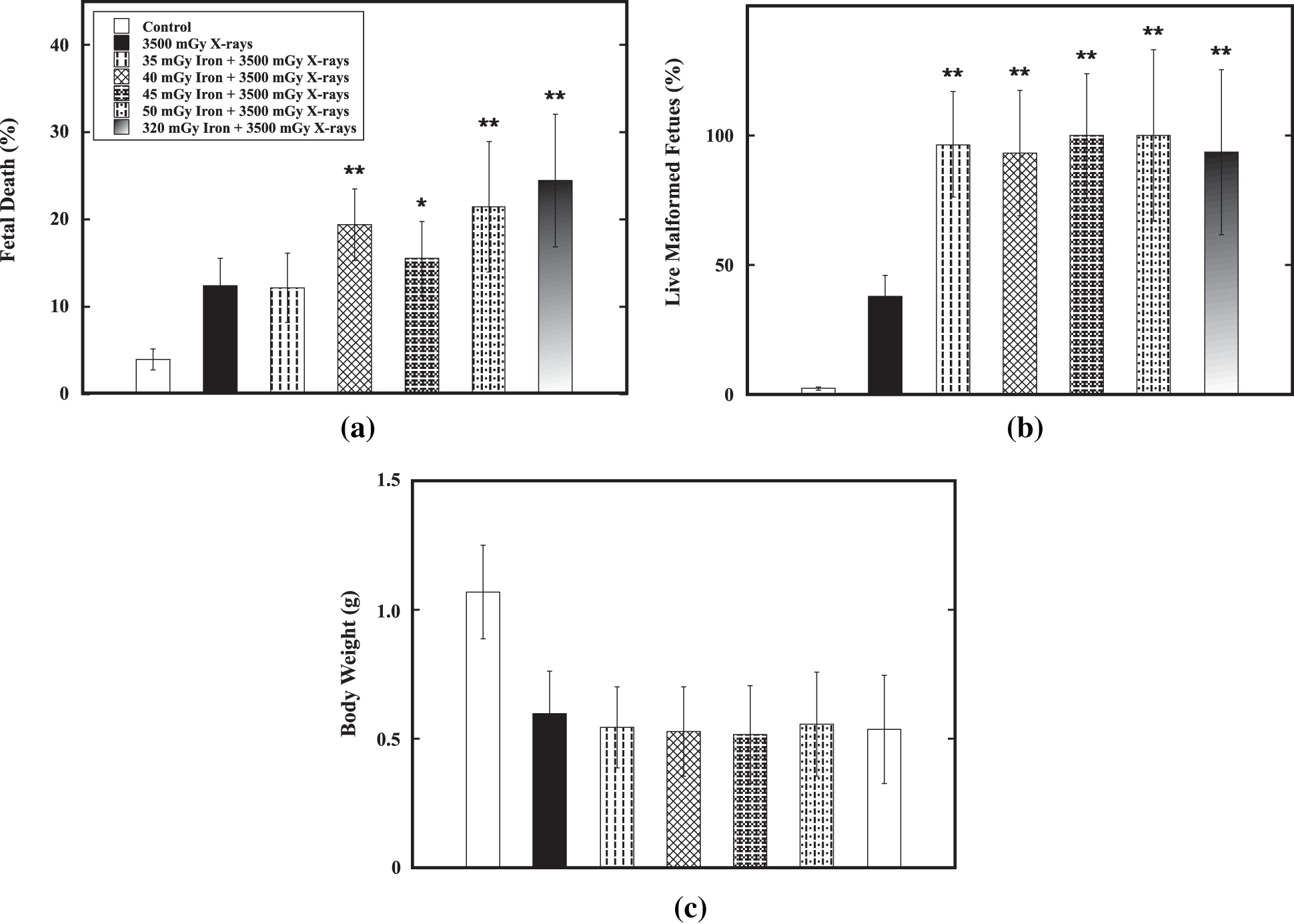
Figure 5: Verification of AR induction by high-LET iron-particle radiation. Verification of AR induction by a priming dose ranging from 35 mGy to 320 mGy of high-LET iron-particle radiation on E11 against the challenge dose (3500 mGy) from low-LET X-ray radiation on E12 was done in the fetal mouse model. Datum is presented as mean ± SD. (a) Percentage of fetal death. (b) Percentage of live malformed fetuses. (c) Body weight (in gram). One asterisk (*) and two asterisks (**) indicate statistically marked difference between the group receiving only the challenge dose (3500 mGy on E12) and the irradiated group receiving both the priming dose on E11 and the challenge dose on E12 at p < 0.05 and p < 0.01, respectively.
These data indicated none of the conditions tested the induction of an AR, namely, a priming dose ranging from 10 mGy to 320 mGy of high-LET carbon-, silicon or iron-particle radiation on E11 did not induce an AR against the detrimental effects of the challenge dose at 3500 mGy from low-LET X-ray radiation on E12 measured as reduction of fetal death, gross malformation and low body weight on E18 in fetal mice.
Since AR was first reported in cultured cells in 1984 (Olivieri et al., 1984), it has been demonstrated in diverse biological systems in vitro and in vivo (UNSCEAR, 1994; Yonezawa et al., 1990; Yonezawa et al., 1996; Yonezawa, 2006). For induction of AR in developing birds and mammals, pioneer verification work in chicken embryos, preimplantation mouse embryos and fetal rats was done nearly 30 years ago (Müller et al., 1992; Wojcik et al., 1992; Hays et al., 1993; Tempel and Schleifer, 1995). Successful AR induction was demonstrated by priming radiation of chicken embryo in ovo followed by challenge radiation of brain- and liver-cell suspensions in vitro, showing a significant reduction of the initial radiation damage. Both the priming dose and the challenge dose were from low-LET X-ray radiation (Tempel and Schleifer, 1995). However, AR was not observed neither in preimplantation mouse embryos in vitro and in vivo, nor in fetal rats in utero using chromosome aberrations, cell proliferation, cell differentiation and cell death as the endpoints (Müller et al., 1992; Wojcik et al., 1992; Hays et al., 1993). Although AR was demonstrated in vivo at whole body level in young adult mice in early 1990 (Yonezawa et al., 1990), it was in 1998 when AR was first demonstrated in utero in fetal mice (Wang et al., 1998). After then AR in utero in fetal mice was verified and confirmed under similar experimental setup, and further demonstrated in different experimental conditions (Wang et al., 2000a; Mitchel et al., 2002; Okazaki et al., 2005; Boreham et al., 2006; Wang et al., 2012; Howell et al., 2013). In our laboratory, a series of investigations on characterization of our AR fetal mouse model were performed. In these investigations, timing and interval (i.e., embryonic developmental stage) for delivery of the priming dose and the challenge dose, radiation quantity (dose and dose rate) of priming radiation, radiation quality (low-LET X-rays and high-LET particle radiation) of challenge radiation, mouse strain and AR mechanisms (Trp53 protein phosphorylation, Trp53-dependent apoptosis, and Trp53 related signal transduction pathways) were extensively studied in utero and ex vivo (Wang et al., 1998; Wang et al., 1999b; Wang et al., 2000a; Wang et al., 2000b; Wang et al., 2004a; Wang et al., 2004b; Varès et al., 2009; Varès et al., 2011a; Varès et al., 2011b; Wang et al., 2012). These investigations indicate that successful AR induction in fetal mice is a complicated phenomenon resulted from the interplay among the quantity of priming radiation, the quality of challenge radiations, the timing and interval for delivery of radiations and the animal characteristics (i.e., mouse strain). As there is still a lack of information on induction and characterization of AR in the period of fetal development (Streffer, 2004; Varès et al., 2006; Nenoi et al., 2015), in the present study, we further verified if the priming dose from high-LET particle radiation could induce AR in fetal mice. Results showed that no AR was induced using multiple combinations of the latitude of priming dose and the type of particle radiation.
To date, some studies have been successfully demonstrated that AR could be induced by a priming dose from high-LET particle radiation in vitro and in vivo in the experimental systems using either cultured mammalian cells or laboratory mice. For examples, it is reported that priming low doses of high-LET particle radiation could reduce subsequent high doses of low-LET X- or gamma-ray radiation-induced detrimental effects such as mutation in lymphoblastoid cells in vitro (10 mGy at 20 keV/μm of carbon particles, and 10 mGy at 150 keV/μm of neon particles) (Varès et al., 2011c), and chromosomal aberrations in spermatogonia and spermatocytes in mice when the priming doses were delivered in vivo and the challenge doses ex vivo (50 mGy at 70 keV/μm of oxygen particles, and 50 mGy at 45.2 keV/μm of carbon particles) (Zhang et al., 1998; Zhang et al., 2008). Of note, as space radiation consists of both low- and high-LET radiations causing various biological effects on humans (Nelson, 2003; Held, 2009; Furukawa et al., 2020), a study on chromosomal aberrations in lymphocytes from cosmonauts involved in two or more space flights suggested possible existence of AR induced by space radiation in vivo (Durante et al., 2003). For induction of AR by high-LET radiation in embryo and fetus, successful demonstration came from only quite a few of work on investigations using vertebrates. For examples, it is reported that a priming dose (ranging from 110 mGy to 650 mGy) of high-LET particle radiation from proton microbeam with an energy of 3.4 MeV could induce AR against a challenge dose from low-LET X-ray radiation-induced apoptosis in zebrafish embryos in vitro (Choi et al., 2010; Choi et al., 2013). However, when the priming doses (ranging from 6 mGy to 100 mGy) were from neutron microbeam with an energy of 2 MeV, no AR was inducible (Ng et al., 2016). These studies also suggested that successful AR induction by high-LET radiation depends on the quality of priming radiation including the LET value and/or the particle type in vertebrate embryos. Interestingly, our previous work on verification of the protective efficacy from priming low-LET X-ray radiation against high-LET particle radiation in the same AR model in fetal mice also showed dependence of challenge radiation quality for successful AR induction, namely, the priming low-LET X-ray radiation could protect detrimental effect of challenge radiation from carbon-, neon- and silicon-particle radiation but not from iron-particle radiation (Wang et al., 2012). In addition, under the similar experimental setup, it was reported that the priming low-LET X-ray radiation did not protect neutron-particle radiation-induced teratogenesis (Lee et al., 2008).
Why was no AR induced in the present work? The reasons are still unknown. Exposure to low doses of IR induces profound effects on embryonic and fetal development (ICRP, 1986; NCRP, 1994; NCRP, 2013). AR is amongst these effects to be further studied in radiation biology and toxicology. It is known that mechanisms underlying AR generally include such as the enhanced antioxidative capacities and increased repair capacities of DNA double-strand breaks (DSBs), leading to the reduction of initial DNA damage, cell death, chromosomal aberrations, mutations and malignant transformation (UNSCEAR, 1994; Otsuka et al., 2006; Varès et al., 2006; Tapio and Jacob, 2007; Takahashi and Ohnishi, 2009; Nenoi et al., 2015; Liu et al., 2021). In fact, these induced defensive mechanisms are tightly conserved throughout evolution, being the basic responses critical to life (Mitchel, 2006). On the other hand, as radiation effects depend on the quality of IR (ICRP, 1989; NCRP, 1990), due to the result of the condensed energy deposition pattern, high-LET particle radiation is more lethal than the same doses of low-LET radiation by inducing hardly repairable clustered DNA damage which is considered a unique feature of high-LET IR (Suzuki et al., 2000; Shikazono et al., 2009; Eccles et al., 2011; Wada et al., 2013; Roobol et al., 2020). The consequences of DNA repair processes reflect LET-dependent particle track structure in the biological effects (Tsuruoka et al., 2005; Tsuruoka et al., 2008). Evidence from gene expression analysis showing downregulation of a variety of DNA repair mechanisms after high-LET particle radiation well correlated with the complex DNA damage formation induced by high-LET particle radiation while DNA repair mechanisms continue to function after low-LET X-ray radiation (Michalettou et al., 2021). As a fact, combination of closely interspaced DNA DSBs and perturbed DNA damage processing cause the increased RBE for high-LET radiation (Mehnati et al., 2005; Roobol et al., 2020; Wang and Yasuda, 2020). As IR-induced detrimental effect in fetal mice are closely linked to IR-induced Trp53-dependent apoptosis and one of the mechanisms underlying induction of AR is via inhibition of Trp53-dependent apoptosis (Wang, 2001; Boreham et al., 2002) while induction of apoptosis by high-LET radiation is independent of Trp53 (Mori et al., 2009), perturbed DNA repair mechanisms induced by the priming dose of high-LET particle radiation leading to failure of enhanced DNA repair is probably responsible for the unsuccessful induction of AR in fetal mice. An interesting study by Boreham and colleagues indirectly provided supporting evidence to this hypothesis. In their study, fetal mice were pretreated on E11 with heat stress (40.5°C, 60 min) followed by exposure to IR on E12. As the heat stress could inhibit the active DNA repair which occurs in the developing fetus under normal physiological conditions, the pretreatment significantly increased the detrimental effects of IR on E12 (Boreham et al., 2002). As a fact, in the present work enhanced detrimental effects including death and malformation in fetal mice were observed in the combination of a priming dose from high-LET particle radiation followed by the challenge dose. Another possible reason for the successful AR induction in fish embryos at early developmental stage but not in mice at late organogenesis may exist in the huge difference in species and complexity (i.e., developmental stage and cell types) between fish embryo and mouse fetus used in the studies, as the huge difference could lead different susceptibility and responses to the high-LET particle radiation. It should be noticed that as AR could also be induced in fetal mice using conditions different from ours including such as the timing and interval for delivery of the priming dose and the challenge dose, the latitude of the dose and the endpoints to evaluate AR induction (Okazaki et al., 2005; Howell et al., 2013), and animal characteristics (i.e., mouse strain and age), we could not exclude the possibility that AR could be induced by high-LET particle radiation under different experimental setup.
Taken together, using our fetal mouse AR model the present study showed no AR could be induced by using low doses of high-LET carbon-, silicon- or iron-particle radiation as the priming radiation against the detrimental effects induced by subsequent high dose of challenge radiation from low-LET X-ray radiation. Although it is demonstrated that priming low doses of high-LET particle radiation could induce AR in cultured cells and fish embryos in vitro and certain cells in mice in vivo, it seems that priming low doses of high-LET particle radiation could be hardly capable of inducing AR in fetal mice in utero. These findings suggest that the efficacy of the biological defense mechanisms induced by low doses in fetal mice depends largely on the quality of the priming radiation. A better understanding of AR is needed to understand what extent low dose radiation could be beneficially functional in humans, and it makes the studies in the context of the mixed low- and high-LET radiations as humans encounter. New knowledge to AR induction should be obtained in further investigations to provide a new insight into the mechanistic study.
Acknowledgement: The authors would like to thank Ms. Hiromi Arai, Ms. Mikiko Nakajima, Ms. Nobuko Tsutsumi, Ms. Satoko Idohara, Ms. Ichika Kishi, Ms. Kaori Tateno, Mr. Tatsuo Hayao, Ms. Maki Asano and Ms. Yasuko Morimoto for their expert technical assistance and administrative support. We are also deeply grateful to Dr. Takeshi Murakami, Dr. Guillaume Varès and Dr. Kiyomi Eguchi-Kasai for their continual support that made this study possible. Great appreciation is especially given to late Dr. Takeo Ohnishi, professor emeritus, Nara Medical University School of Medicine, Japan for his constructive comments and continual encouragement throughout the study.
Availability of Data and Materials: Data supporting this article are details in this manuscript.
Author Contribution: BW, MN conceptualized, conceived and designed the study. All authors conducted the experiment. BW and MN supervised the experiment. BW analyzed the data. BW prepared the first draft of the manuscript. BW, TK and MN reviewed, edited and finalized the manuscript. All authors have read and approved the final manuscript.
Ethics Approval: The experimental protocol involving the caring, handling, and treatment of mice for the experiment was reviewed and approved by The Institutional Animal Care and Use Committee of the National Institute of Radiological Sciences, National Institutes for Quantum Science and Technology (ethical approval code was 07-2023 and date of approval was 30 August 2007; ethical approval code was 07-2023-1 and date of approval was 01 April 2011). The experiments were performed in strict accordance with the Institutional Guidelines for the Care and Use of Laboratory Animals.
Funding Statement: This research was financially supported in part by the Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI 21510060 and JSPS KAKENHI 25340041) and Research Project Grants with Heavy Ions at HIMAC, QST, Japan (19B-258 and 22B-258).
Conflicts of Interest: The authors declare that they have no conflicts of interest. The authors declare no conflict of interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Aoki M, Furusawa Y, Yamada T (2000). LET dependency of heavy-ion induced apoptosis in V79 cells. Journal of Radiation Research 41: 163–175. DOI 10.1269/jrr.41.163. [Google Scholar] [CrossRef]
Boreham DR, Dolling JA, Misonoh J, Mitchel RE (2002). Radiation-induced teratogenic effects in fetal mice with varying Trp53 function: Influence of prior heat stress. Radiation Research 158: 449–457. DOI 10.1667/0033-7587(2002)158[0449:RITEIF]2.0.CO;2. [Google Scholar] [CrossRef]
Boreham DR, Dolling JA, Somers C, Quinn J, Mitchel RE (2006). The adaptive response and protection against heritable mutations and fetal malformation. Dose Response 4: 317–326. DOI 10.2203/dose-response.06-104.Boreham. [Google Scholar] [CrossRef]
Brent RL (2014). Carcinogenic risks of prenatal ionizing radiation. Seminars in Fetal and Neonatal Medicine 19: 203–213. DOI 10.1016/j.siny.2013.11.009. [Google Scholar] [CrossRef]
Brent RL (2015). Protection of the gametes embryo/fetus from prenatal radiation exposure. Health Physics 108: 242–274. DOI 10.1097/HP.0000000000000235. [Google Scholar] [CrossRef]
Choi VW, Konishi T, Oikawa M, Cheng SH, Yu KN (2013). The threshold number of protons to induce an adaptive response in zebrafish embryos. Journal of Radiological Protection 33: 91–100. DOI 10.1088/0952-4746/33/1/91. [Google Scholar] [CrossRef]
Choi VW, Konishi T, Oikawa M, Iso H, Cheng SH et al. (2010). Adaptive response in zebrafish embryos induced using microbeam protons as priming dose and X-ray photons as challenging dose. Journal of Radiation Research 51: 657–664. DOI 10.1269/jrr.10054. [Google Scholar] [CrossRef]
de Santis M, Cesari E, Nobili E, Straface G, Cavaliere AF et al. (2007). Radiation effects on development. Birth Defects Research, Part C 81: 177–182. DOI 10.1002/(ISSN)1542-9768. [Google Scholar] [CrossRef]
Durante M, Snigiryova G, Akaeva E, Bogomazova A, Druzhinin S et al. (2003). Chromosome aberration dosimetry in cosmonauts after single or multiple space flights. Cytogenetic and Genome Research 103: 40–46. DOI 10.1159/000076288. [Google Scholar] [CrossRef]
Eccles LJ, O’Neill P, Lomax ME (2011). Delayed repair of radiation induced clustered DNA damage: friend or foe? Mutation Research 711: 134–141. DOI 10.1016/j.mrfmmm.2010.11.003. [Google Scholar] [CrossRef]
Furukawa S, Nagamatsu A, Nenoi M, Fujimori A, Kakinuma S et al. (2020). Space radiation biology for living in space. Biomed Research International 2020: 4703286. DOI 10.1155/2020/4703286. [Google Scholar] [CrossRef]
Goodhead DT (1994). Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA. International Journal of Radiation Biology 65: 7–17. DOI 10.1080/09553009414550021. [Google Scholar] [CrossRef]
Hada M, Georgakilas AG (2008). Formation of clustered DNA damage after high-LET irradiation: A review. Journal of Radiation Research 49: 203–210. DOI 10.1269/jrr.07123. [Google Scholar] [CrossRef]
Hays SR, Li X, Kimler BF (1993). Is there an adaptive response to radiation in the developing brain of the fetal rat? Radiation Research 136: 293–296. DOI 10.2307/3578624. [Google Scholar] [CrossRef]
Held KD (2009). Effects of low fluences of radiations found in space on cellular systems. International Journal of Radiation Biology 85: 379–390. DOI 10.1080/09553000902838558. [Google Scholar] [CrossRef]
Howell EK, Gaschak SP, Griffith KD, Rodgers BE (2013). Radioadaptive response following in utero low-dose irradiation. Radiation Research 179: 29–37. DOI 10.1667/RR3029.1. [Google Scholar] [CrossRef]
Hunter N, Muirhead CR (2009). Review of relative biological effectiveness dependence on linear energy transfer for low-LET radiations. Journal of Radiological Protection 29: 5–21. DOI 10.1088/0952-4746/29/1/R01. [Google Scholar] [CrossRef]
ICRP (International Commission on Radiological Protection) (1986). Developmental effects of radiation on the brain of the embryo and fetus, In: Annals of the ICRP. ICRP Publication, vol. 49, pp. 2–30. New York: Pergamon Press. [Google Scholar]
ICRP (International Commission on Radiological Protection) (1989). RBE for deterministic effects. ICRP Publication, vol. 58, pp. 10–35. Oxford, UK: Pergamon Press. [Google Scholar]
Katsube T, Wang B, Tanaka K, Ninomiya Y, Hirakawa H et al. (2021). Synergistic effects of chronic restraint-induced stress and low-dose 56Fe-particle irradiation on induction of chromosomal aberrations in Trp53-heterozygous mice. Radiation Research 196: 100–112. DOI 10.1667/RADE-20-00218.1. [Google Scholar] [CrossRef]
Lee HJ, Kim JS, Song MS, Seo HS, Moon C et al. (2008). Lack of adaptive response of gamma radiation for protection against neutron-induced teratogenesis. Birth Defects Research Part B: Developmental and Reproductive Toxicology 83: 502–506. DOI 10.1002/bdrb.20171. [Google Scholar] [CrossRef]
Liu C, Hirakawa H, Katsube T, Fang Y, Tanaka K et al. (2021). Altered induction of reactive oxygen species by X-rays in hematopoietic cells of C57BL/6-Tg (CAG-EGFP) mice. International Journal of Molecular Sciences 22: 6929. DOI 10.3390/ijms22136929. [Google Scholar] [CrossRef]
Mehnati P, Morimoto S, Yatagai F, Furusawa Y, Kobayashi Y et al. (2005). Exploration of “over kill effect” of high-LET Ar- and Fe-ions by evaluating the fraction of non-hit cell and interphase death. Journal of Radiation Research 46: 343–350. DOI 10.1269/jrr.46.343. [Google Scholar] [CrossRef]
Michalettou TD, Michalopoulos I, Costes SV, Hellweg CE, Hada M et al. (2021). A meta-analysis of the effects of high-LET ionizing radiations in human gene expression. Life 11: 115. DOI 10.3390/life11020115. [Google Scholar] [CrossRef]
Mitchel RE (2006). Low doses of radiation are protective in vitro and in vivo: Evolutionary origins. Dose Response 4: 75–90. DOI 10.2203/dose-response.04-002.Mitchel. [Google Scholar] [CrossRef]
Mitchel RE, Dolling JA, Misonoh J, Boreham DR (2002). Influence of prior exposure to low-dose adapting radiation on radiation-induced teratogenic effects in fetal mice with varying Trp53 function. Radiation Research 158: 458–463. DOI 10.1667/0033-7587(2002)158[0458:IOPETL]2.0.CO;2. [Google Scholar] [CrossRef]
Mori E, Takahashi A, Yamakawa N, Kirita T, Ohnishi T (2009). High LET heavy ion radiation induces p53-independent apoptosis. Journal of Radiation Research 50: 37–42. DOI 10.1269/jrr.08075. [Google Scholar] [CrossRef]
Mortazavi SM, Cameron JR, Niroomand-rad A (2003). Adaptive response studies may help choose astronauts for long-term space travel. Advances in Space Research 31: 1543–1551. DOI 10.1016/S0273-1177(03)00089-9. [Google Scholar] [CrossRef]
Müller WU, Streffer C, Niedereichholz F (1992). Adaptive response in mouse embryos? International Journal of Radiation Biology 62: 169–175. DOI 10.1080/09553009214551981. [Google Scholar] [CrossRef]
NCRP (National Council of Radiation Protection and Measurements) (1990). The Relative Biological Effectiveness of Radiations of Different Quality. NCRP Report No. 104. Bethesda, MD, USA: NCRP. [Google Scholar]
NCRP (National Council of Radiation Protection and Measurements) (1994). Considerations regarding the unintended radiation exposure of the embryo, fetus or nursing child, In: NCRP Publications Office, NCRP Commentary, No. 9, pp. 3–14. Bethesda. [Google Scholar]
NCRP (National Council of Radiation Protection and Measurements) (2013). Preconception & prenatal radiation exposure: Health Effects & Protective Guidance. NCRP Report, No. 174, pp. 1–150. Bethesda: NCRP. [Google Scholar]
Nelson GA (2003). Fundamental space radiobiology. Gravitational and Space Biology Bulletin 16: 29–36. [Google Scholar]
Nenoi M, Wang B, Varès G (2015). In vivo radioadaptive response: A review of studies relevant to radiation-induced cancer risk. Human and Experimental Toxicology 34: 272–283. DOI 10.1177/0960327114537537. [Google Scholar] [CrossRef]
Ng CY, Kong EY, Kobayashi A, Suya N, Uchihori Y et al. (2016). Non-induction of radioadaptive response in zebrafish embryos by neutrons. Journal of Radiation Research 57: 210–219. DOI 10.1093/jrr/rrv089. [Google Scholar] [CrossRef]
Nikjoo H, O’Neill P, Wilson WE, Goodhead DT (2001). Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiation Research 156: 577–583. DOI 10.1667/0033-7587(2001)156[0577:CAFDTS]2.0.CO;2. [Google Scholar] [CrossRef]
Okayasu R (2012). Repair of DNA damage induced by accelerated heavy ions-A mini review. International Journal of Cancer 130: 991–1000. DOI 10.1002/ijc.26445. [Google Scholar] [CrossRef]
Okazaki R, Ootsuyama A, Norimura T (2005). Radioadaptive response for protection against radiation-induced teratogenesis. Radiation Research 163: 266–270. DOI 10.1667/RR3315. [Google Scholar] [CrossRef]
Olivieri G, Bodycote J, Wolff S (1984). Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 223: 594–597. DOI 10.1126/science.6695170. [Google Scholar] [CrossRef]
Otsuka K, Koana T, Tauchi H, Sakai K (2006). Activation of antioxidative enzymes induced by low-dose-rate whole-body gamma irradiation: Adaptive response in terms of initial DNA damage. Radiation Research 166: 474–478. DOI 10.1667/RR0561.1. [Google Scholar] [CrossRef]
Prise KM, Folkard M, Newman HC, Michael BD (1994). Effect of radiation quality on lesion complexity in cellular DNA. International Journal of Radiation Biology 66: 537–542. DOI 10.1080/09553009414551581. [Google Scholar] [CrossRef]
Roobol SJ, van den Bent I, van Cappellen WA, Abraham TE, Paul MW et al. (2020). Comparison of high- and low-LET radiation-induced DNA double-strand break processing in living cells. International Journal of Molecular Sciences 21: 6602. DOI 10.3390/ijms21186602. [Google Scholar] [CrossRef]
Rydberg B, Cooper B, Cooper PK, Holley WR, Chatterjee A (2005). Dose-dependent misrejoining of radiation-induced DNA double-strand breaks in human fibroblasts: experimental and theoretical study for low and high-LET radiation. Radiation Research 163: 526–534. DOI 10.1667/RR3346. [Google Scholar] [CrossRef]
Shikazono N, Noguchi M, Fujii K, Urushibara A, Yokoya A (2009). The yield, processing, and biological consequences of clustered DNA damage induced by ionizing radiation. Journal of Radiation Research 50: 27–36. DOI 10.1269/jrr.08086. [Google Scholar] [CrossRef]
Sørensen BS, Overgaard J, Bassler N (2011). In vitro RBE-LET dependence for multiple particle types. Acta Oncologica 50: 757–762. DOI 10.3109/0284186X.2011.582518. [Google Scholar] [CrossRef]
Streffer C (2004). Bystander effects, adaptive response and genomic instability induced by prenatal irradiation. Mutation Research 568: 79–87. DOI 10.1016/j.mrfmmm.2004.07.014. [Google Scholar] [CrossRef]
Suzuki M, Kase Y, Kanai T, Ando K (2000). Correlation between cell killing and residual chromatin breaks measured by PCC in six human cell lines irradiated with different radiation types. International Journal of Radiation Biology 76: 1189–1196. DOI 10.1080/09553000050134429. [Google Scholar] [CrossRef]
Takahashi A, Ohnishi T (2009). Molecular mechanisms involved in adaptive responses to radiation, UV light, and heat. Journal of Radiation Research 50: 385–393. DOI 10.1269/jrr.09048S. [Google Scholar] [CrossRef]
Tapio S, Jacob V (2007). Radioadaptive response revisited. Radiation and Environmental Biophysics 46: 1–12. DOI 10.1007/s00411-006-0078-8. [Google Scholar] [CrossRef]
Tempel K, Schleifer S (1995). Adaptive response of the chicken embryo to low doses of x-irradiation. Radiation and Environmental Biophysics 34: 177–183. DOI 10.1007/BF01211545. [Google Scholar] [CrossRef]
Tsuruoka C, Suzuki M, Hande MP, Furusawa Y, Anzai K et al. (2008). The difference in LET and ion species dependence for induction of initially measured and non-rejoined chromatin breaks in normal human fibroblasts. Radiation Research 170: 163–171. DOI 10.1667/RR1279.1. [Google Scholar] [CrossRef]
Tsuruoka C, Suzuki M, Kanai T, Fujitaka K (2005). LET and ion species dependence for cell killing in normal human skin fibroblasts. Radiation Research 163: 494–500. DOI 10.1667/RR3360. [Google Scholar] [CrossRef]
UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) (1994). Adaptive responses to radiation in cells and organisms, UNSCEAR 1994 Report: Sources and Effects of Ionizing Radiation, pp. 185–272. New York: United Nations. [Google Scholar]
Varès G, Wang B, Shang Y, Ohyama H, Tanaka K et al. (2009). Adaptive response in embryogenesis: VI. Comparative microarray analysis of gene expressions in mouse fetuses. International Journal of Radiation Biology 85: 70–86. DOI 10.1080/09553000802635039. [Google Scholar] [CrossRef]
Varès G, Wang B, Tanaka K, Kakimoto A, Eguchi-Kasai K et al. (2011c). Mutagenic adaptive response to high-LET radiation in human lymphoblastoid cells exposed to low doses of heavy-ion radiation. Mutation Research 712: 49–54. DOI 10.1016/j.mrfmmm.2011.04.004. [Google Scholar] [CrossRef]
Varès G, Wang B, Tanaka K, Nakajima T, Nenoi M et al. (2006). Radiation-induced adaptive response with reference to evidence and significance: A review. Indian Journal of Radiation Research 3: 16–34. [Google Scholar]
Varès G, Wang B, Tanaka K, Shang Y, Fujita K et al. (2011a). Trp53 activity is repressed in radio-adapted cultured murine limb bud cells. Journal of Radiation Research 52: 727–734. DOI 10.1269/jrr.10092. [Google Scholar] [CrossRef]
Varès G, Wang B, Tanaka K, Shang Y, Taki K et al. (2011b). Gene silencing of Tead3 abrogates radiation-induced adaptive response in cultured mouse limb bud cells. Journal of Radiation Research 52: 39–46. DOI 10.1269/jrr.10101. [Google Scholar] [CrossRef]
Wada M, Suzuki M, Liu C, Kaneko Y, Fukuda S et al. (2013). Modeling the biological response of normal human cells, including repair processes, to fractionated carbon beam irradiation. Journal of Radiation Research 54: 798–807. DOI 10.1093/jrr/rrt012. [Google Scholar] [CrossRef]
Wang B (2001). Involvement of p53-dependent apoptosis in radiation teratogenesis and in the radioadaptive response in the late organogenesis of mice. Journal of Radiation Research 42: 1–10. DOI 10.1269/jrr.42.1. [Google Scholar] [CrossRef]
Wang B, Fujita K, Watanabe K, Odaka T, Mitani H et al. (1999a). Radiation-induced apoptosis and limb bud teratogenesis in embryonic mice. Radiation Research 151: 63–68. DOI 10.2307/3579748. [Google Scholar] [CrossRef]
Wang B, Haginoya K, Ohyama H, Nose M, Itsukaichi H et al. (1999b). Adaptive response in embryogenesis: II. Postnatal developmental retardation in the prenatally irradiated mice. Radiation Research 152: 119–123. DOI 10.2307/3580084. [Google Scholar] [CrossRef]
Wang B, Murakami M, Eguchi-Kasai K, Nojima K, Shang Y et al. (2008). Prenatal irradiation with accelerated-heavy-ion beams induced LET-dependent detrimental effects on prenatal development and postnatal neurophysiologic accomplishment in rats. Indian Journal of Radiation Research 5: 15–23. [Google Scholar]
Wang B, Murakami M, Eguchi-Kasai K, Nojima K, Shang Y et al. (2007). Effects of prenatal irradiation with an accelerated heavy-ion beam on postnatal development in rats: II. Further study on neurophysiologic alterations. Advances in Space Research 39: 994–1003. DOI 10.1016/j.asr.2006.11.011. [Google Scholar] [CrossRef]
Wang B, Ninomiya Y, Tanaka K, Maruyama K, Varès G et al. (2012). Adaptive response of low linear energy transfer X-rays for protection against high linear energy transfer accelerated heavy ion-induced teratogenesis. Birth Defects Research Part B: Developmental and Reproductive Toxicology 95: 379–385. DOI 10.1002/bdrb.21027. [Google Scholar] [CrossRef]
Wang B, Ohyama H, Haginoya K, Odaka T, Itsukaichi H et al. (2000a). Adaptive response in embryogenesis: III. Relationship to radiation-induced apoptosis and Trp53 gene status. Radiation Research 154: 277–282. DOI 10.1667/0033-7587(2000)154[0277:arieir]2.0.co;2. [Google Scholar] [CrossRef]
Wang B, Ohyama H, Haginoya K, Odaka T, Yamada T et al. (2000b). Prenatal radiation-induced limb defects mediated by Trp53-dependent apoptosis in mice. Radiation Research 154: 673–679. DOI 10.1667/0033-7587(2000)154[0673:prildm]2.0.co;2. [Google Scholar] [CrossRef]
Wang B, Ohyama H, Nose T, Itsukaichi H, Nakajima T et al. (1998). Adaptive response in embryogenesis: I. Dose and timing of radiation for reduction of prenatal death and congenital malformation during the late period of organogenesis. Radiation Research 150: 120–122. [Google Scholar]
Wang B, Ohyama H, Shang Y, Fujita K, Tanaka K et al. (2004b). Adaptive response in embryogenesis: IV. Protective and detrimental bystander effects induced by X radiation in cultured limb bud cells of fetal mice. Radiation Research 161: 9–16. DOI 10.1667/RR3106. [Google Scholar] [CrossRef]
Wang B, Ohyama H, Shang Y, Tanaka K, Aizawa S et al. (2004a). Adaptive response in embryogenesis: V. Existence of two efficient dose-rate ranges for 0.3 Gy of priming irradiation to adapt mouse fetuses. Radiation Research 161: 264–272. DOI 10.1667/RR3141. [Google Scholar] [CrossRef]
Wang B, Tanaka K, Varès G, Shang Y, Fujita K et al. (2010). X-ray-induced radioresistance against high-LET radiations from accelerated heavy ions in mice. Radiation Research 174: 532–536. DOI 10.1667/RR2133.1. [Google Scholar] [CrossRef]
Wang B, Yasuda H (2020). Relative biological effectiveness of high LET particles on the reproductive system and fetal development. Life 10: 298. DOI 10.3390/life10110298. [Google Scholar] [CrossRef]
Williams PM, Fletcher S (2010). Health effects of prenatal radiation exposure. American Family Physician 82: 488–493. [Google Scholar]
Wojcik A, Bonk K, Müller WU, Streffer C, Weissenborn U et al. (1992). Absence of adaptive response to low doses of X-rays in preimplantation embryos and spleen lymphocytes of an inbred mouse strain as compared to human peripheral lymphocytes: A cytogenetic study. International Journal of Radiation Biology 62: 177–186. DOI 10.1080/09553009214551991. [Google Scholar] [CrossRef]
Yonezawa M (2006). Induction of radio-resistance by low dose X-irradiation. Yakugaku Zasshi (Journal of the Pharmaceutical Society of Japan) 126: 833–840. DOI 10.1248/yakushi.126.833. [Google Scholar] [CrossRef]
Yonezawa M, Misonoh J, Hosokawa Y (1990). Acquired radioresistance after small dose X-irradiation in mice. Journal of Radiation Research 31: 256–262. DOI 10.1269/jrr.31.256. [Google Scholar] [CrossRef]
Yonezawa M, Misonoh M, Hosokawa Y (1996). Two types of X-ray-induced radioresistance in mice: Presence of 4 dose ranges with distinct biological effects. Mutation Research 358: 237–243. DOI 10.1016/s0027-5107(96)00126-1. [Google Scholar] [CrossRef]
Zhang H, Zhao W, Wang Y, Li N, Wu Z et al. (2008). Induction of cytogenetic adaptive response in spermatogonia and spermatocytes by pre-exposure of mouse testis to low-dose 12C6+ ions. Mutation Research 653: 109–112. DOI 10.1016/j.mrgentox.2008.04.001. [Google Scholar] [CrossRef]
Zhang H, Zheng RL, Wei ZQ, Li WJ, Gao QX et al. (1998). Effects of pre-exposure of mouse testis with low-dose 16O8+ ions or 60Co gamma-rays on sperm shape abnormalities, lipid peroxidation and superoxide dismutase (SOD) activity induced by subsequent high-dose irradiation. International Journal of Radiation Biology 73: 163–167. DOI 10.1080/095530098142545. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |