DOI:10.32604/biocell.2022.020325

| BIOCELL DOI:10.32604/biocell.2022.020325 |  |
| Article |
Berberine inhibits the proliferation of pancreatic cancer cells by targeting pancreatic cancer stem cells through regulating EMT signaling pathway
1State Key Laboratory of Fine Chemicals, Department of Pharmaceutical Sciences, School of Chemical Engineering, Dalian University of Technology, Dalian, 116023, China
2Ningbo Institute of Dalian University of Technology, Ningbo, 315000, China
3Department of Pharmacy, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450000, China
4Henan Key Laboratory of Precision Clinical Pharmacy, Zhengzhou University, Zhengzhou, 450000, China
*Address correspondence to: Yongjie Yang, fccyangyj@zzu.edu.cn; Gary Guishan Xiao, gxiao@dlut.edu.cn
#These authors have contributed equally to this work and share first authorship
Received: 17 November 2021; Accepted: 07 February 2022
Abstract: Pancreatic ductal adenocarcinoma (PDAC) is universally acknowledged as the cancer with the highest mortality rate. Berberine has high medicinal value and has been used as an anti-cancer agent. Hence the purpose of this study was to investigate the anti-cancer effect of berberine in PDAC. Berberine was shown to have a selective anti-cancer effect on PDAC by MTT assay in vitro. Pancreatic cancer stem cells (PCSCs), regulated by epithelial–mesenchymal transition (EMT), could promote the proliferation of PDAC cells. However, berberine suppressed the proliferation and stemness of PCSCs through immunofluorescence staining, stem cell sphere assays and so forth in vitro. In vivo, berberine reduced tumor size and decreased the expression levels of Ki67, a marker of cellular proliferation, in orthotopic pancreatic tumors. In addition, berberine inhibited the EMT signaling pathway by RT-PCR and Western blotting methods both in vitro and in vivo. Our study indicates that berberine inhibits the proliferation of PDAC cells both in vivo and in vitro. The mechanism of the anti-cancer effect of berberine likely involves the inhibition of EMT. Therefore, berberine may be a novel antineoplastic drug with clinical efficacy in PDAC.
Keywords: Berberine; PDAC; PCSCs; EMT; Antineoplasic
Pancreatic ductal adenocarcinoma (PDAC), is universally acknowledged to have one of the highest mortality rates of all cancer types (Cronin et al., 2018; Nimmakayala et al., 2018; Olivares et al., 2017; Takahashi et al., 2021). Although there have been advances in therapeutic methods and treatment technologies for PDAC, surgery combined with chemotherapy is the classical clinical treatment (Gao et al., 2019; He et al., 2018). Cancer stem cells (CSCs) play a critical role in cancer due to their self-renewal potential. It is now widely accepted that pancreatic CSCs (PCSCs) facilitate the proliferation, invasion, and metastasis of PDAC (Nimmakayala et al., 2018; Subramaniam et al., 2018; Wang et al., 2019a). PCSCs may survive after gemcitabine treatment, leading to the failure of chemotherapy (de Jesus-Acosta et al., 2020; Kaushik et al., 2021). Overall, drug resistance and other side effects may be induced by long-term chemotherapy (Gout et al., 2021). Therefore, there is an urgent need to develop new therapies targeting PCSCs with improved safety.
Epithelial–mesenchymal transition (EMT) is characterized by a morphological change from an epithelial to a mesenchymal phenotype. When the EMT process is activated, E-cadherin is replaced by vimentin and N-cadherin. Noteworthy, the first molecular alteration for inducing EMT is reducing E-cadherin levels (Ashrafizadeh et al., 2021). The disappearance of E-cadherin is accompanied by Snail and Twist (Sun et al., 2019a). It is now widely accepted that the EMT process is activated in a variety of cancer types, such as breast carcinoma, glioblastoma, colorectal cancer and pancreatic cancer. In addition, the activation of EMT diminishes intercellular adhesion and enhances migration of cells that are necessary for carcinogenesis (Mirzaei et al., 2021). EMT is associated with the proliferation and stemness of CSCs (Nomura et al., 2015). For instance, Luo et al. (2017) showed that NR5A2 inhibits CSCs by negatively modulating EMT. Consequently, targeting EMT inhibits the PCSCs that are associated with chemotherapy resistance in PDAC (Zhou et al., 2017).
Berberine (Zhao et al., 2021) is a naturally occurring quaternary ammonium alkaloid that has effective anti-cancer activity. Our previous study showed that berberine enhances the chemosensitivity of breast cancer (Pan et al., 2017a). In 2016, Naveen et al. (2016) demonstrated that berberine inhibits the stemness of CSCs. Coincidentally, Lin et al. (2017) also reported an anti-CSC effect of berberine in oral cancer. Furthermore, Kim et al. (2018) reported that berberine inhibits CSCs by downregulating the expression levels of EMT signaling pathway components in breast cancer. These data imply that berberine may be a candidate for cancer therapy by targeting CSCs. Nevertheless, the effectiveness of berberine against PDAC and PCSCs is unknown.
In this study, we investigated whether berberine could inhibit the proliferation of PDAC cells in vivo and in vitro. We then determined the mechanism whereby berberine regulates PCSCs and EMT. Data from this study indicate that berberine may be a candidate for clinical PDAC therapy.
Berberine hemisulfate salt was purchased from Sigma-Aldrich (Cat. #B3412; St Louis, MO, USA). The anti-mouse Twist antibody (Cat. #ab5088) and anti-rabbit Ki67 (Cat. #ab15580) were purchased from Abcam (Cambridge, UK). The anti-rabbit OCT-4 (Cat. #2750), anti-rabbit Nanog (Cat. #8822), anti-rabbit SOX2 (Cat. #3579), anti-rabbit CD133 (Cat. #64326), anti-rabbit Snail (Cat. #3879), anti-rabbit vimentin (Cat. #5741), anti-rabbit N-cadherin (Cat. #13116), anti-rabbit E-cadherin (Cat. #14472), anti-rabbit β-actin (Cat. #4970), anti-mouse CD44 (Cat. #3570), anti-rabbit IgG (Cat. #7074), and anti-mouse IgG (Cat. #7076) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
Human pancreatic cancer cell lines (PANC-1, BXPC-3, ASPC-1, SW1990), mouse pancreatic cancer cell line PANC-02 and human normal pancreatic cell line H6C7 were purchased from the Peking Union Medical College Cell Bank (Beijing, China). All these cells were treated with berberine at final concentrations of 0, 1.25, 2.5, 5, 10, 20, 40, 80 and 160 μM for 48 or 72 h. MTT assays were performed as previously described (Pan et al., 2017b).
Sphere forming capacity of bulk tumor cells were determined by seeding 5 × 103 PANC-1 or PANC 02 cells/well in ultra-low attachment 6-well plate with stem cell growth media containing DMEM-F12 medium, 0.4% BSA, EGF 20 ng/mL, insulin 5 μg/mL, 2% B27, penicillin 100 U/mL and streptomycin 100 μg/mL. Meanwhile, 10 μM berberine were added in berberine group. Then cells were incubated for a week. After treatment, number of spheres were counted and their size was measured by taking images using a light microscope (Olympus DP73; Tokyo, Japan) inverted microscope.
Wound-healing assay was performed as previously described (Sun et al., 2019a). PANC-1 and PANC-02 cells were treated with 10 μM berberine for 24 h. Firstly, PANC-1 and PANC-02 cells were seeded into a 6-well plate and was allowed to grow to 80%–90% confluence. Subsequently, the cell was wounded by a 20 μL pipette tip and washed three times with 1 × phosphate buffer saline (PBS) to remove the to clear cell debris and suspension cells. Cells were then incubated in DMEM with 10 μM berberine for 24 h. Eventually, cell migration was observed by an inverted microscopy (BioTeK; Vermont, USA) and the migration distance was calculated by the change in wound size during the 24 h period using adobe Photoshop cS6 software (Adobe Systems, Inc., headquartered in San Jose, California, USA). The experiment was performed in triplicate. The specific statistic method is as followed. △width = 0 h width–24 h width The wound healing = △width in BBR group /△width in CON group × 100%. The results were analyzed using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Transwell assay was performed as previously described (Sun et al., 2019a). PANC-1 and PANC-02 cells were treated with 10 μM berberine for 24 h. A 8 μm pore-size of Transwell chamber (Corning Costar; Cambridge, USA) was obtained and coated with a thin layer of 0.25 mg/ml Matrigel Basement Membrane Matrix (BD Biosciences) at 37°C for 30 min. Briefly, PANC-1 and PANC-02 cells were incubated in 100 µl of serum-free medium and berberine (Cells: 10 μM) for 24 h respectively. Complete medium (600 μl) containing 10% FBS was added to the lower chamber. Triplicate wells were used for each group. The cells were allowed to migrate through the filters for 24 h in a humidified incubator at 37°C with 5% CO2. Cells attached to the lower surface of the membrane were fixed in 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet for 20 min at room temperature. A cotton swab was used for wiping off the cells on the upper surface of the filters. The number of stained cells on the lower surface of the filters was counted under a fluorescence microscope at 20 × magnification. A total of five fields of view were counted for each Transwell filter. The results were analyzed using ImageJ (National Institutes of Health, Bethesda, MD, USA).
RNA isolation and quantitative reverse transcription PCR
PANC-1 and PANC-02 cells were treated with 10 μM berberine for 48 h. Cells were washed and resuspended in ice-cold Trizol LS Reagent (Invitrogen; Carlsbad, USA) and then total mRNA was extracted by an RNA Easy Kit (Invitrogen) according to the manufacturer’s instructions and prepared for subsequent experiments. Total mRNA was extracted from orthotopic pancreatic tumors from C57BL/6 mice treated with berberine. The concentration of mRNA was measured by a microspectrophotometer, with the ratio of OD260/OD280 > 1.8. Furthermore, reverse transcription was performed using the Prime Script TM RT Reagent Kit (TaKaRa; Dalian, China) and all reverse-transcription of total RNA into cDNA was performed using the SYBR® Premix Ex TaqTM (Roche Diagnostics, Basel, Switzerland) system on a Light Cycler 480 II machine (Roche; Basel, Switzerland). Specific forward and reverse PCR primers were designed using a PRIMER5/NCBI system. β-actin served as an internal control in all reactions. Quantitative reverse transcription PCR was then performed as previously described (Sun et al., 2019b).
PANC-1 and PANC-02 cells were treated with 10 μM berberine for 48 h. Total protein was extracted and prepared for subsequent experiments. Total protein was extracted from orthotopic pancreatic tumors from C57BL/6 mice treated with berberine. Western blotting was then performed as previously described (Domenichini et al., 2019).
Twenty C57BL/6 male mice were purchased from Liaoning Changsheng Bioteconology Co., Ltd. (Benxi, China). All experiments were performed in accordance with the China Public Health Service Guide for the Care and Use of Laboratory Animals. Experiments involving mice and study protocols were approved by Dalian University of Technology. To generate the PANC-02 orthotopic pancreatic cancer mouse model, 2 × 106 PANC-02 cells were injected into the pancreas tissue of male C57BL/6 mice. The animals were divided into two groups: control group (CON) and berberine group. Mice in the control group were treated with saline daily by intragastric (i.g.) administration, whereas mice in the berberine group were treated with berberine (200 mg·kg−1) daily by i.g. administration. Body weight was measured every 2 days. The mice were administered the drugs for 28 days, after which they were sacrificed and pancreas tumors were collected for mechanism research.
The collected pancreas tumors were dipped in 4% paraformaldehyde, embedded in paraffin and cut into small pieces. Then, according to the manufacturer’s instructions, sections were performed with hemayoxylin-eosin (H&E) staining. Besides, to implement immunohistochemical (IHC) assay, pancreatic sections were also probed with primary antibody against Ki67. Simple images were derived by using a light microscope with 200 × magnification (Tao et al., 2020). The IHC images were quantified by ImageJ.
Data were analyzed using Prism 8.0 statistical analysis software (GraphPad, San Diego, CA, USA). All the fluorescence and the flow cytometry were quantified by ImageJ. Data are expressed as the mean ± standard deviation (SD) of at least three independent experiments. Differences between two groups were performed by using an unpaired Student’s t-test, and the comparisons among multi-groups were detected through using the one-way analysis of variance (ANOVA). P values < 0.05 were deemed statistically significant.
Berberine inhibits the viability of PDAC cells
Berberine is a naturally occurring compound that has anti-tumor effects against a variety of tumor types, such as hepatoma, breast cancer, and colon cancer. To investigate the effect of berberine on PDAC, the PDAC cell lines PANC-1, ASPC-1, SW1990, BXPC-3, and PANC-02 were treated with various concentrations of berberine (0.125–160 μM) for 48 and 72 h. Cell viability was then measured by MTT assay. As shown in Figs. 1A and 1B, berberine markedly reduced cell viability in a time- and dose-dependent manner. We then tested the normal pancreatic cell line H6C7. As shown in Figs. 1C and 1D, the viability of H6C7 cells was significantly higher than that of the PDAC cells BXPC-3 after treatment with the same concentration of berberine. These results showed that berberine inhibited PDAC cell viability and had a selective killing effect on PDAC cells.
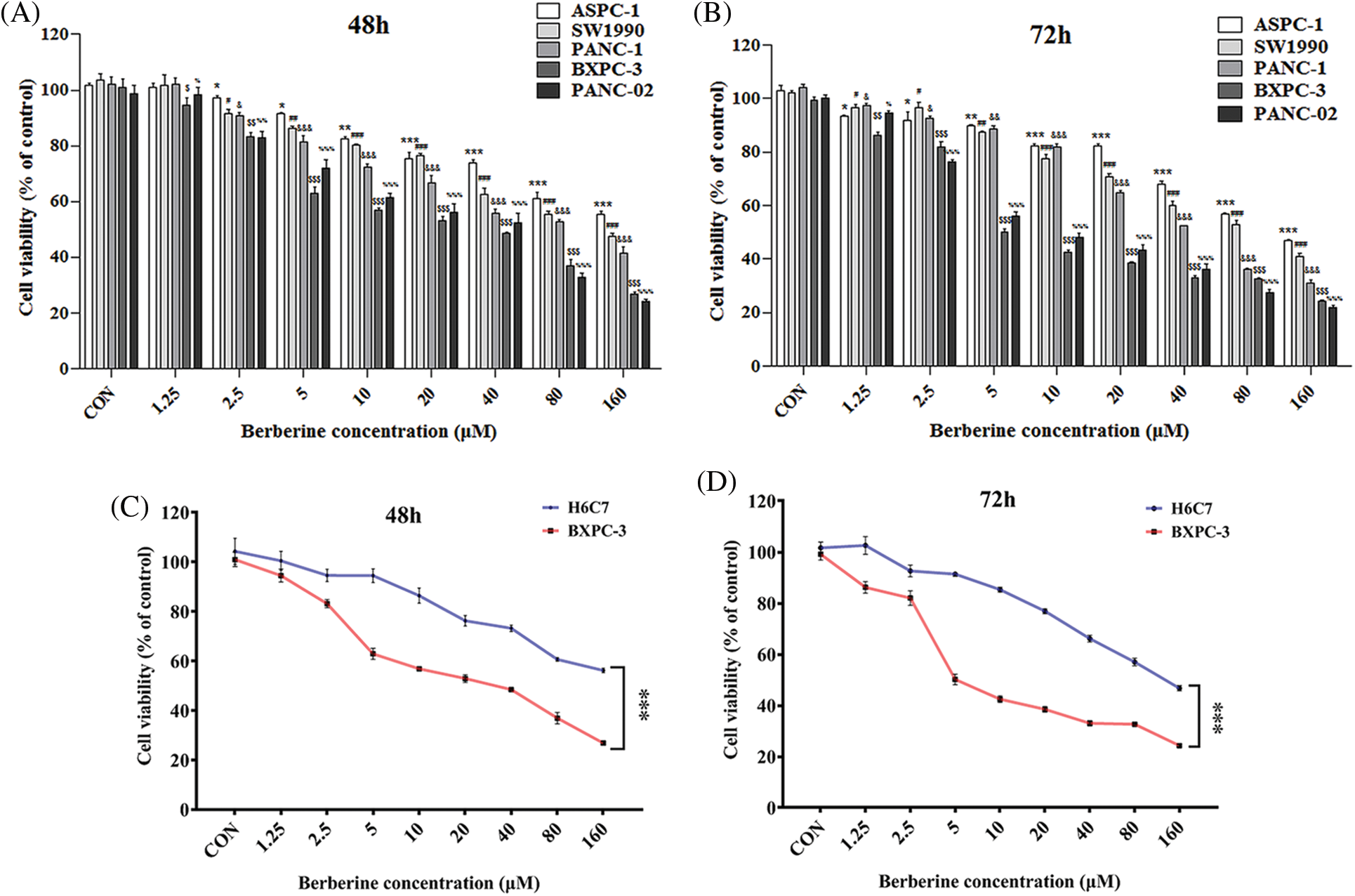
Figure 1: Effect of berberine on PDAC and normal pancreatic cells. (A–B) Viability of PANC-1, ASPC-1, SW1990, BXPC-3, and PANC-02 cells treated with berberine. (C–D) Viability of BXPC-3 and H6C7 cells treated with berberine. Data are expressed as the mean ± SD. (A–B) Statistical differences were analyzed using the one-way analysis. *P < 0.05, #P < 0.05, &P < 0.05, $P < 0.05, and %P < 0.05 vs. control cells; (C–D) Statistical differences were analyzed using the unpaired Student’s t-test. ***P < 0.001 vs. H6C7 cells.
Berberine inhibits PCSCs in PDAC
The acquisition of stem-like characteristics by PDAC cells serves as a critical driver of cancer proliferation. Sphere-formation ability is one of the main characteristics of PCSCs. Compared with its effect on control cells, the BBR group which berberine was treated with 10 μM or 40 μM for one week decreased the sphere-formation ability of PANC-1 and PANC-02 cells in a dose-dependent manner (Figs. 2A and 2B). We then evaluated the PCSC markers CD44 and CD133 by flow cytometry and found that the proportion of CD44+CD133+ cells treated with 10 μM berberine for 48 h was decreased (Figs. 2C and 2D). Furthermore, immunofluorescence analysis confirmed that the proportion of CD44+CD133+ cells incubated with 10 μM berberine for 48 h was decreased similarly (Figs. 2E and 2F). To further investigate the inhibitory effect of berberine on PCSCs, we measured the expression levels of the PCSC-related genes encoding OCT4, Nanog, SOX2, CD44, and CD133. As shown in Figs. 2G and 2H, berberine suppressed the mRNA and protein expression levels of these genes. In conclusion, these findings confirmed that berberine inhibits PCSCs proliferation in vitro.
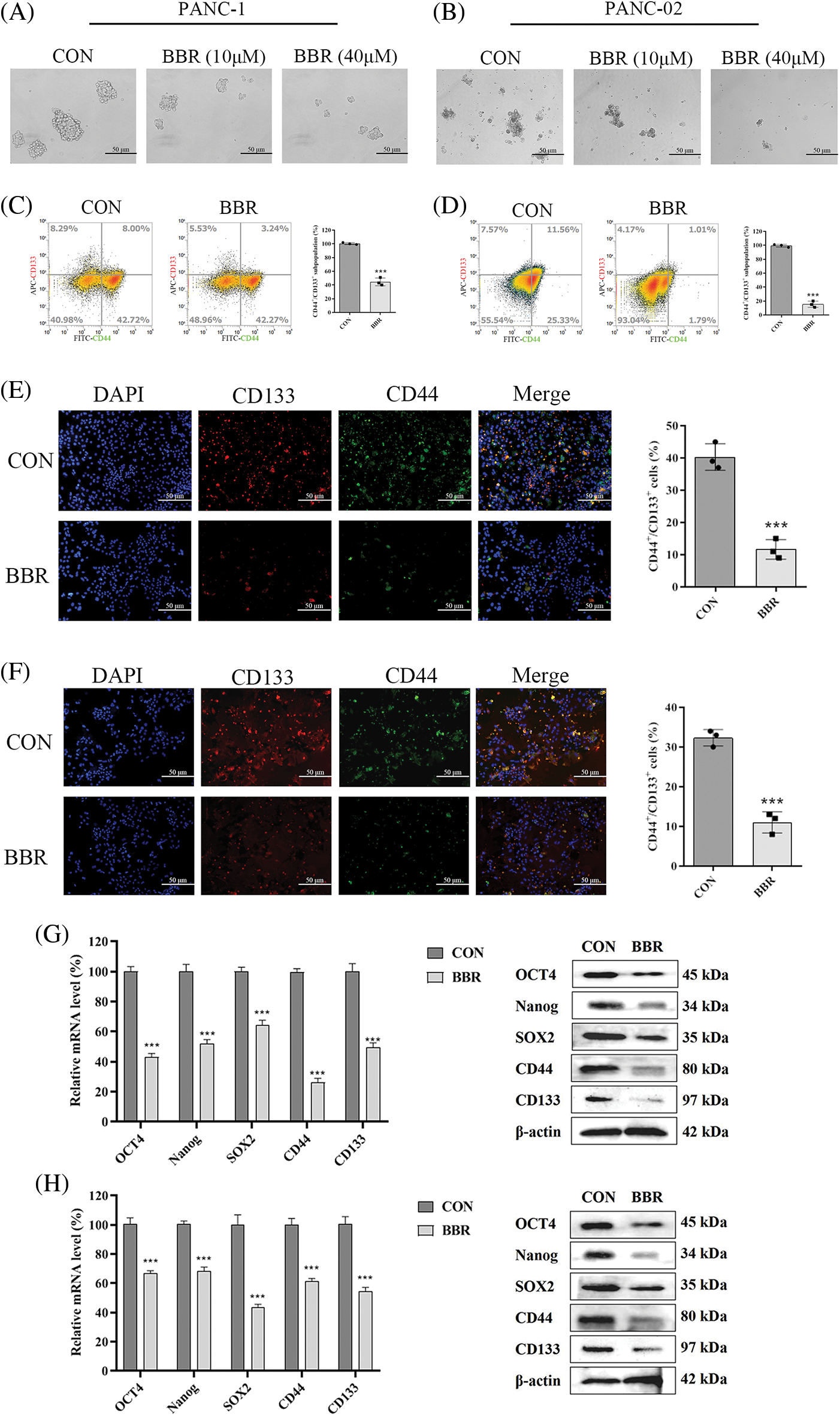
Figure 2: Effect of berberine on PCSCs. (A–B) Sphere-formation ability of PANC-1 and PANC-02 cells after berberine treatment. (C–D) Proportion of CD133+CD44+ PANC-1 and PANC-02 cells determined by flow cytometry after treatment with 10 μM berberine for 48 h. (E–F) CD44 and CD133 expression in PANC-1 and PANC-02 cells determined by immunofluorescence after treatment with 10 μM berberine for 48 h. (G–H) PCSC-related gene expression levels in PANC-1 and PANC-02 cells after treatment with 10 μM berberine for 48 h. Data are expressed as the mean ± SD. Statistical differences were analyzed using the unpaired Student’s t-test. ***P < 0.001 vs. control cells.
Berberine inactivates the EMT signaling pathway
Migration ability is involved in the stemness of PCSCs. We assessed the migration capacity of PANC-1 and PANC-02 cells using wound-healing assays. The results showed that compared with cells in the control group, PANC-1 cells and PANC-02 cells in the berberine group spread slower to the wound area after 24 h of incubation (Figs. 3A and 3B). In addition, transwell migration assay results demonstrated that berberine decreased the number of PANC-1 and PANC-02 cells passing through the polyester fiber membrane (Figs. 3C and 3D).
Activation of the EMT signaling pathway is a characteristic of CSCs. Snail, Twist, vimentin, N-cadherin, and E-cadherin are the main biomarkers of EMT. We measured the mRNA and protein levels of genes involved in the EMT of PANC-1 and PANC-02 cells. The results showed that berberine downregulated the levels of N-cadherin, vimentin, Snail, and Twist and upregulated the levels of E-cadherin expression at both the mRNA and protein levels (Figs. 3E and 3F).
Thus, our results showed that berberine decreased the stemness of PCSCs by regulating the EMT signaling pathway.
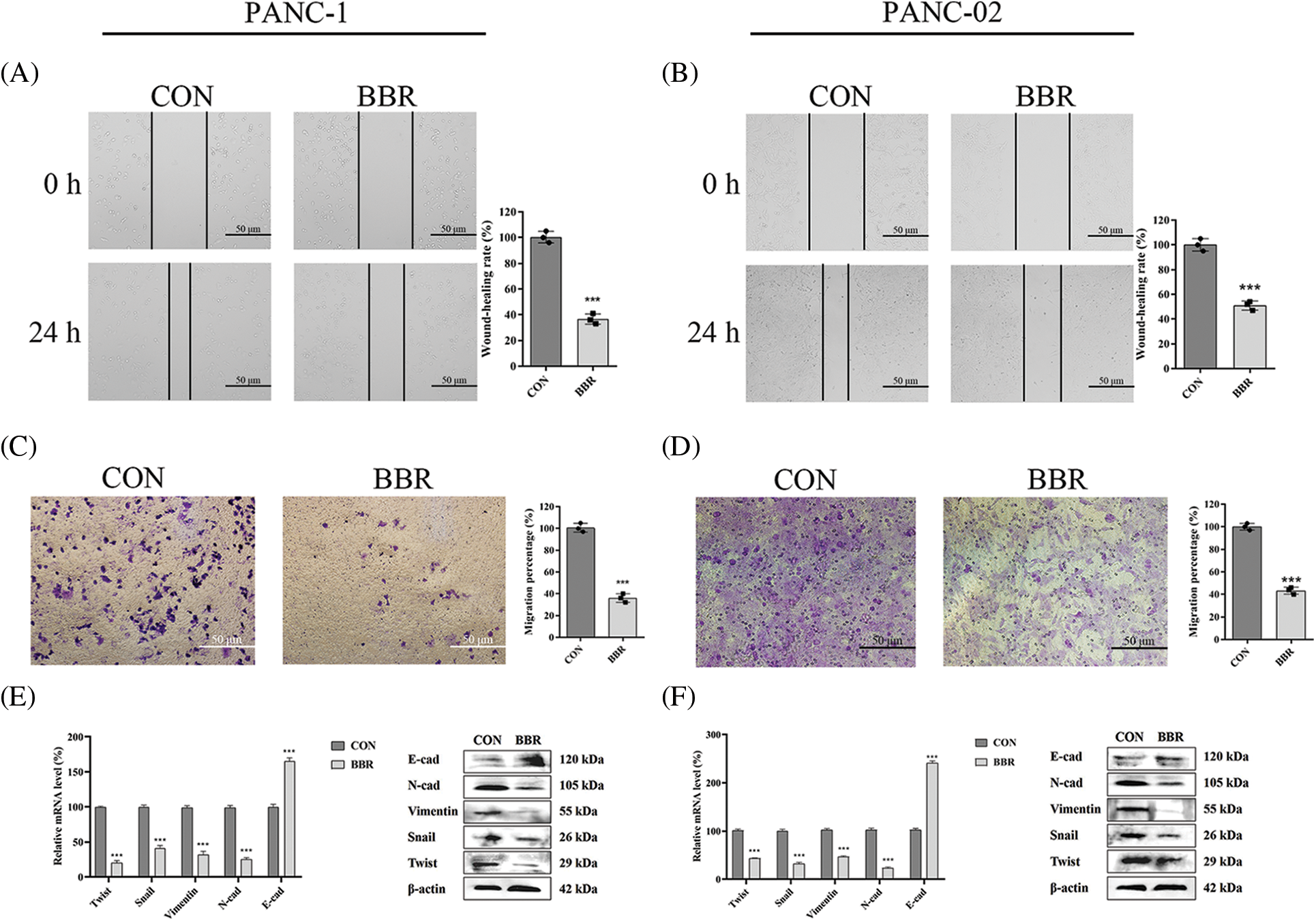
Figure 3: Effect of berberine on the stemness of PCSCs and EMT. (A–B) Migration ability of PANC-1 and PANC-02 cells in wound-healing assays after treatment with 10 μM berberine for 24 h. (C–D) Invasion ability of PANC-1 and PANC-02 cells in transwell assays after treatment with 10 μM berberine for 24 h. (E–F) EMT-related gene expression levels in PANC-1 and PANC-02 cells after treatment with 10 μM berberine for 48 h. Data are expressed as the mean ± SD. Statistical differences were analyzed using the unpaired Student’s t-test. ***P < 0.001 vs. control cells.
Berberine inhibits PDAC proliferation and PCSCs proliferation in vivo
The above experiments showed that berberine inhibited PDAC proliferation in vitro. To determine the effect of berberine on PDAC cell proliferation in vivo, we established a PANC-02 orthotopic pancreatic cancer model in C57BL/6 mice. As shown in Fig. 4A, berberine reduced the size of orthotopic pancreatic tumors. Hematoxylin and eosin (H&E) staining showed that the mucus surrounding the PDAC lesions clearly decreased after berberine treatment (Fig. 4B). Ki67 marker was then assessed to evaluate cell proliferation. The expression levels of Ki67 decreased significantly after treatment with berberine (Fig. 4C). Thus, these results indicated that berberine markedly inhibited the proliferation of PDAC cells in vivo. Consistent with these results, CD133 and CD44 immunofluorescence showed that berberine decreased the number of PCSCs (CD44+CD133+) in vivo (Fig. 4D). As shown in Fig. 4E, berberine significantly reduced the expression levels of OCT4, Nanog, SOX2, CD44, and CD133. In addition, berberine clearly inhibited EMT, which was consistent with the in vitro data (Fig. 4F). In agreement with the in vitro results, these data further confirmed that berberine inhibited PCSCs and the EMT signaling pathway in PDAC in vivo.
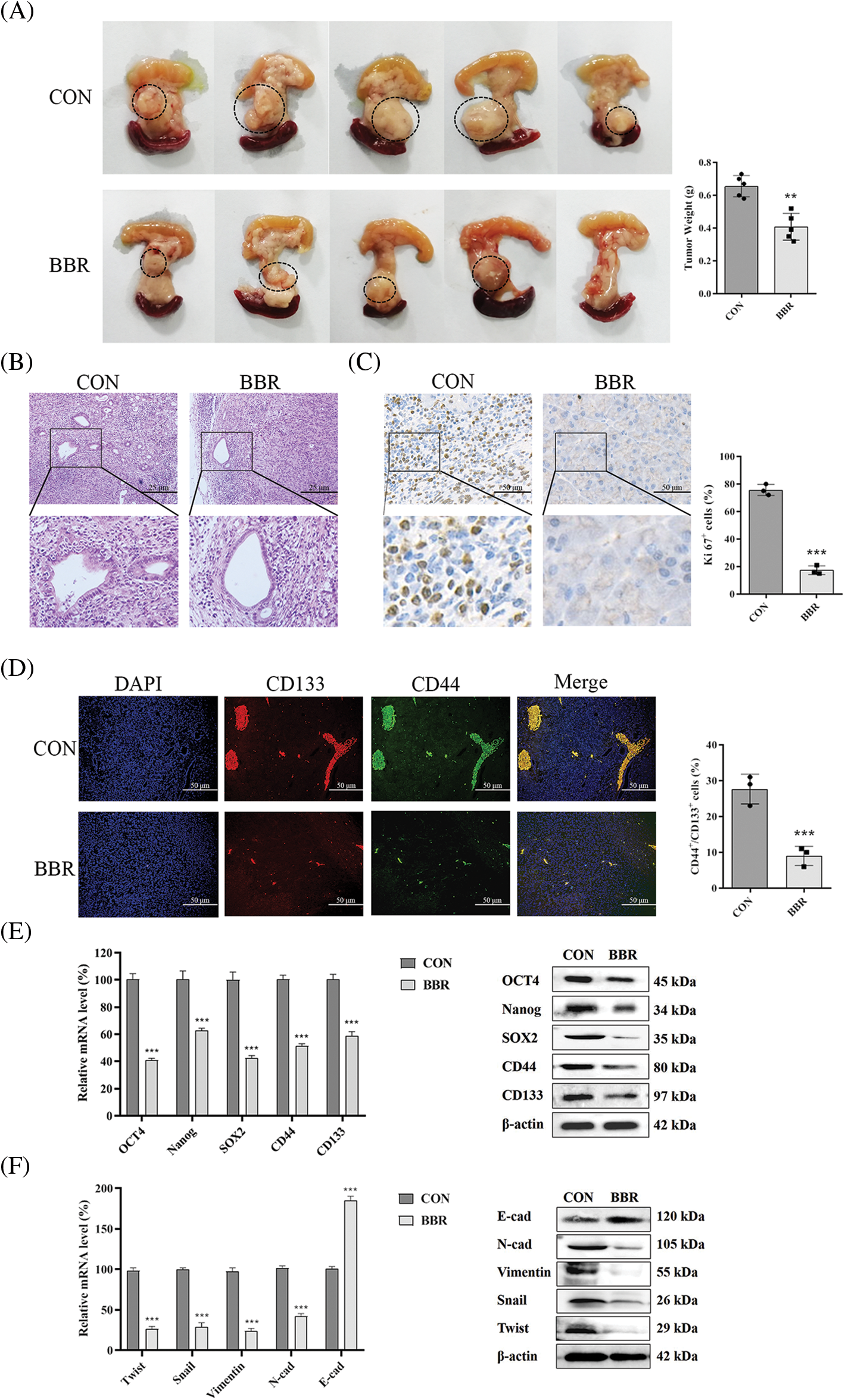
Figure 4: Effect of berberine on PDAC in vivo. (A) Photographs of pancreatic tumors treated with berberine. (B) H&E staining of pancreatic tissue after treatment with berberine. (C) Immunohistochemical staining of Ki67 in pancreatic tissue after treatment with berberine. (D) CD44 and CD133 expression in pancreatic tissue determined by immunofluorescence after treatment with berberine. (E) PCSC-related gene expression levels in pancreatic tissue after treatment with berberine. (F) EMT-related gene expression levels in pancreatic tissue after treatment with berberine. Data are expressed as the mean ± SD. Statistical differences were analyzed using the unpaired Student’s t-test. ***P < 0.001 vs. controls.
PCSCs are associated with PDAC proliferation, metastasis, and apoptosis (Domenichini et al., 2019; Subramaniam et al., 2018; Zheng et al., 2015). Previous studies have shown that PCSCs are important mediators of therapy resistance and cancer relapse in PDAC (Domenichini et al., 2019; He et al., 2018; Stoica et al., 2020). Our results demonstrated that berberine inhibited the proliferation and stemness of PCSCs (Figs. 1 and 2). Furthermore, we showed that berberine decreased the number of PCSCs by inhibiting EMT. In addition to its effect on EMT, berberine inhibits CSC stemness by downregulating the PI3K/Akt and Ras–Raf–ERK signaling pathways in neuroblastoma (Naveen et al., 2016). In addition, berberine reduces the stemness of CSCs through the SDF-1/CXCR4 signaling pathway in acute myeloid leukemia (Li et al., 2008). These results indicate that berberine may be a candidate for PDAC therapy by targeting PCSCs.
Abnormal energy metabolism is one of the main features of tumors. It causes the proliferation of CSCs, which is a critical factor in oncogenesis and tumor progression (Kim, 2019). AMP-activated protein kinase (AMPK), a sensor of cellular energy, is involved in cancer development by regulating energy metabolism (Pan et al., 2017b). Cheng et al. (2016) showed that methylisoindigo preferentially reduces the number of PCSCs by activating AMPK in PDAC. Furthermore, Wang et al. (2019b) showed that the activation of AMPK decreases CSC stemness in prostate cancer. Moreover, our previous study showed that AMPK is one of the main targets of berberine. Berberine enhances the chemosensitivity of breast cancer to doxorubicin in vivo and in vitro by regulating the AMPK–HIF-1α–P-gp pathway (Pan et al., 2017a). Furthermore, Xu et al. (2017) reported that AMPK activation inhibits EMT in cervical cancer. Importantly, in PDAC, EMT can also be negatively regulated via the activation of AMPK (Sun et al., 2019a). Hence, we speculated that AMPK may be one of the targets of berberine in the regulation of EMT in PDAC.
As a naturally occurring compound, berberine has fewer side effects and better safety than some chemotherapy drugs. Our results also showed that berberine did not affect safety-related serological indicators or animal body weight (Supplemental Fig. 1) (Pan et al., 2017b; Wu et al., 2021). Furthermore, berberine did not show toxicity or side effects after long-term treatment (Supplemental Fig. 1). In addition to its anti-cancer effects, Gu et al. (2021) demonstrated that berberine suppresses bone loss and inflammation in ligature-induced periodontitis. Moreover, recent study have declared the pharmacokinetics and pharmacological activities of berberine in diabetes mellitus treatment (Han et al., 2021). Similarly, berberine has excellent therapeutic effect on cardiovascular disease (Feng et al., 2019), obesity (Ilyas et al., 2020), and neurodegenerative disorders (Fan et al., 2019). Therefore, berberine, as a classical ingredient of Chinese medicine, has therapeutic effects on a variety of diseases.
Berberine was shown to have selective anti-cancer effects on PDAC cell lines (Fig. 1). This study also showed that berberine inhibited the PCSC phenotype and downregulated the EMT signaling pathway in vitro (Figs. 2 and 3). We compared tumor weight and other indicators between CON group and BBR group and the results implied significantly berberine has an effective treatment (Fig. 4). The limitations of this study were that, recent study have declared berberine has amazing therapeutic effects on cancer cells invasion and metastasis on other tumors like breast cancer. In addition, berberine has the ability to increase the sensitivity of miR-34a curing pancreatic cancer. Therefore, we will probe the effect of berberine in pancreatic cancer drug resistant, invasion and metastasis in the future. Furthermore, bererine affects other tumors through AMPK pathway and the AMPK is the upstream of EMT signal pathway. Therefore, AMPK may be the key target of berberine influencing PCSCs and we will further explore relative questions in the next research.
In conclusion, our study demonstrates that berberine is a novel antineoplastic agent that targets PCSCs by inhibiting EMT signaling in PDAC. These findings indicate that berberine is a potential candidate for PDAC therapy and are expected to promote the further application of berberine.
Our study demonstrates that berberine inhibits the proliferation of PDAC cells both in vivo and in vitro. The mechanism of the anti-cancer effect of berberine likely involves the suppression of PCSCs through the inhibition of EMT. Therefore, berberine may be a novel antineoplastic drug for the treatment of PDAC.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: Gary Guishan Xiao and Yongjie Yang; data collection: Yue Pan; analysis and interpretation of results: Xufeng Tao and Wenli Kang; draft manuscript preparation: Mengmeng Liu, Yingjie Liu and Yue Pan. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: The animal study was reviewed and approved by the Animal Care and Use Committee of Dalian University of Technology in 4th January, 2022. The ethical approval code is DUTSCE20220223 01.
Funding Statement: This research was supported by 81803024 (National Natural Science Foundation of China) and DUT21LK23 (Fundamental Research Funds for the Central Universities).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Ashrafizadeh M, Mirzaei S, Hashemi F, Zarrabi A, Zabolian A et al. (2021). New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomedicine & Pharmacotherapy 141: 111824. DOI 10.1016/j.biopha.2021.111824. [Google Scholar] [CrossRef]
Cheng X, Kim JY, Ghafoory S, Duvaci T, Rafiee R et al. (2016). Methylisoindigo preferentially kills cancer stem cells by interfering cell metabolism via inhibition of LKB1 and activation of AMPK in PDACs. Molecular Oncology 10: 806–824. DOI 10.1016/j.molonc.2016.01.008. [Google Scholar] [CrossRef]
Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM et al. (2018). Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 124: 2785–2800. DOI 10.1002/cncr.31551. [Google Scholar] [CrossRef]
de Jesus-Acosta A, Sugar EA, O’Dwyer PJ, Ramanathan RK, Von Hoff DD et al. (2020). Phase 2 study of vismodegib, a hedgehog inhibitor, combined with gemcitabine and nab-paclitaxel in patients with untreated metastatic pancreatic adenocarcinoma. British Journal of Cancer 122: 498–505. DOI 10.1038/s41416-019-0683-3. [Google Scholar] [CrossRef]
Domenichini A, Edmands JS, Adamska A, Begicevic RR, Paternoster S et al. (2019). Pancreatic cancer tumorspheres are cancer stem-like cells with increased chemoresistance and reduced metabolic potential. Advances in Biological Regulation 72: 63–77. DOI 10.1016/j.jbior.2019.02.001. [Google Scholar] [CrossRef]
Fan D, Liu L, Wu Z, Cao M (2019). Combating neurodegenerative diseases with the plant alkaloid berberine: Molecular mechanisms and therapeutic potential. Current Neuropharmacology 17: 563–579. DOI 10.2174/1570159X16666180419141613. [Google Scholar] [CrossRef]
Feng X, Sureda A, Jafari S, Memariani Z, Tewari D et al. (2019). Berberine in cardiovascular and metabolic diseases: From mechanisms to therapeutics. Theranostics 9: 1923–1951. DOI 10.7150/thno.30787. [Google Scholar] [CrossRef]
Gao C, Chen G, Zhang DH, Zhang J, Kuan SF et al. (2019). PYK2 is involved in premalignant acinar cell reprogramming and pancreatic ductal adenocarcinoma maintenance by phosphorylating β-Catenin (Y654). Cellular and Molecular Gastroenterology and Hepatology 8: 561–578. DOI 10.1016/j.jcmgh.2019.07.004. [Google Scholar] [CrossRef]
Gout J, Perkhofer L, Morawe M, Arnold F, Ihle M et al. (2021). Synergistic targeting and resistance to PARP inhibition in DNA damage repair-deficient pancreatic cancer. Gut 70: 743–760. DOI 10.1136/gutjnl-2019-319970. [Google Scholar] [CrossRef]
Gu L, Ke Y, Gan J, Li X (2021). Berberine suppresses bone loss and inflammation in ligature-induced periodontitis through promotion of the G protein-coupled estrogen receptor-mediated inactivation of the p38 MAPK/NF-κB pathway. Archives of Oral Biology 122: 104992. DOI 10.1016/j.archoralbio.2020.104992. [Google Scholar] [CrossRef]
Han Y, Xiang Y, Shi Y, Tang X, Pan L et al. (2021). Pharmacokinetics and pharmacological activities of berberine in diabetes mellitus treatment. Evidence-Based Complementary and Alternative Medicine 2021: 9987097. DOI 10.1155/2021/9987097. [Google Scholar] [CrossRef]
He P, Yang JW, Yang VW, Bialkowska AB (2018). Krüppel-like factor 5, increased in pancreatic ductal adenocarcinoma, promotes proliferation, acinar-to-ductal metaplasia, pancreatic intraepithelial neoplasia, and tumor growth in mice. Gastroenterology 154: 1494–1508. DOI 10.1053/j.gastro.2017.12.005. [Google Scholar] [CrossRef]
Ilyas Z, Perna S, Al-Thawadi S, Alalwan TA, Riva A et al. (2020). The effect of berberine on weight loss in order to prevent obesity: A systematic review. Biomedicine & Pharmacotherapy 127: 110137. DOI 10.1016/j.biopha.2020.110137. [Google Scholar] [CrossRef]
Kaushik G, Seshacharyulu P, Rauth S, Nallasamy P, Rachagani S et al. (2021). Selective inhibition of stemness through EGFR/FOXA2/SOX9 axis reduces pancreatic cancer metastasis. Oncogene 40: 848–862. DOI 10.1038/s41388-020-01564-w. [Google Scholar] [CrossRef]
Kim SY (2019). Targeting cancer energy metabolism: A potential systemic cure for cancer. Archives of Pharmacal Research 42: 140–149. DOI 10.1007/s12272-019-01115-2. [Google Scholar] [CrossRef]
Kim S, Lee J, You D, Jeong Y, Jeon M et al. (2018). Berberine suppresses cell motility through downregulation of TGF-β1 in triple negative breast cancer cells. Cellular Physiology Biochemistry 45: 795–807. DOI 10.1159/000487171. [Google Scholar] [CrossRef]
Li H, Guo L, Jie S, Liu W, Zhu J et al. (2008). Berberine inhibits SDF-1-induced AML cells and leukemic stem cells migration via regulation of SDF-1 level in bone marrow stromal cells. Biomedicine & Pharmacotherapy 62: 573–578. DOI 10.1016/j.biopha.2008.08.003. [Google Scholar] [CrossRef]
Lin CY, Hsieh PL, Liao YW, Peng CY, Lu MY et al. (2017). Berberine-targeted miR-21 chemosensitizes oral carcinomas stem cells. Oncotarget 8: 80900–80908. DOI 10.18632/oncotarget.20723. [Google Scholar] [CrossRef]
Luo Z, Li Y, Zuo M, Liu C, Tian W et al. (2017). Effect of NR5A2 inhibition on pancreatic cancer stem cell (CSC) properties and epithelial-mesenchymal transition (EMT) markers. Molecular Carcinogenesis 56: 1438–1448. DOI 10.1002/mc.22604. [Google Scholar] [CrossRef]
Mirzaei S, Abadi AJ, Gholami MH, Hashemi F, Zabolian A et al. (2021). The involvement of epithelial-to-mesenchymal transition in doxorubicin resistance: Possible molecular targets. European Journal of Pharmacology 908: 174344. DOI 10.1016/j.ejphar.2021.174344. [Google Scholar] [CrossRef]
Naveen CR, Gaikwad S, Agrawal-Rajput R (2016). Berberine induces neuronal differentiation through inhibition of cancer stemness and epithelial-mesenchymal transition in neuroblastoma cells. Phytomedicine 23: 736–744. DOI 10.1016/j.phymed.2016.03.013. [Google Scholar] [CrossRef]
Nimmakayala RK, Seshacharyulu P, Lakshmanan I, Rachagani S, Chugh S et al. (2018). Cigarette smoke induces stem cell features of pancreatic cancer cells via PAF1. Gastroenterology 155: 892–908. DOI 10.1053/j.gastro.2018.05.041. [Google Scholar] [CrossRef]
Nomura A, Banerjee S, Chugh R, Dudeja V, Yamamoto M et al. (2015). CD133 initiates tumors, induces epithelial-mesenchymal transition and increases metastasis in pancreatic cancer. Oncotarget 6: 8313–8322. DOI 10.18632/oncotarget.3228. [Google Scholar] [CrossRef]
Olivares O, Mayers JR, Gouirand V, Torrence ME, Gicquel T et al. (2017). Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nature Communications 8: 16031. DOI 10.1038/ncomms16031. [Google Scholar] [CrossRef]
Pan Y, Shao D, Zhao Y, Zhang F, Zheng X et al. (2017a). Berberine reverses hypoxia-induced chemoresistance in breast cancer through the inhibition of AMPK-HIF-1α. International Journal of Biological Sciences 13: 794–803. DOI 10.7150/ijbs.18969. [Google Scholar] [CrossRef]
Pan Y, Zhang F, Zhao Y, Shao D, Zheng X et al. (2017b). Berberine enhances chemosensitivity and induces apoptosis through dose-orchestrated AMPK signaling in breast cancer. Journal of Cancer 8: 1679–1689. DOI 10.7150/jca.19106. [Google Scholar] [CrossRef]
Stoica AF, Chang CH, Pauklin S (2020). Molecular therapeutics of pancreatic ductal adenocarcinoma: Targeted pathways and the role of cancer stem cells. Trends in Pharmacological Sciences 41: 977–993. DOI 10.1016/j.tips.2020.09.008. [Google Scholar] [CrossRef]
Subramaniam D, Kaushik G, Dandawate P, Anant S (2018). Targeting cancer stem cells for chemoprevention of pancreatic cancer. Current Medicinal Chemistry 25: 2585–2594. DOI 10.2174/0929867324666170127095832. [Google Scholar] [CrossRef]
Sun L, Cao J, Chen K, Cheng L, Zhou C et al. (2019a). Betulinic acid inhibits stemness and EMT of pancreatic cancer cells via activation of AMPK signaling. International Journal of Oncology 54: 98–110. DOI 10.3892/ijo.2018.4604. [Google Scholar] [CrossRef]
Sun S, Zhang X, Xu M, Zhang F, Tian F et al. (2019b). Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death & Disease 10: 274. DOI 10.1038/s41419-019-1510-8. [Google Scholar] [CrossRef]
Takahashi R, Macchini M, Sunagawa M, Jiang Z, Tanaka T et al. (2021). Interleukin-1β-induced pancreatitis promotes pancreatic ductal adenocarcinoma via B lymphocyte-mediated immune suppression. Gut 70: 330–341. DOI 10.1136/gutjnl-2019-319912. [Google Scholar] [CrossRef]
Wang VM, Ferreira R, Almagro J, Evan T, Legrave N et al. (2019a). CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nature Cell Biology 21: 1425–1435. DOI 10.1038/s41556-019-0407-1. [Google Scholar] [CrossRef]
Wang X, Jin J, Wan F, Zhao L, Chu H et al. (2019b). AMPK promotes SPOP-mediated NANOG degradation to regulate prostate cancer cell stemness. Developmental Cell 48: 345–360. DOI 10.1016/j.devcel.2018.11.033. [Google Scholar] [CrossRef]
Wu P, Gao M, Dong J, Xu C, Li B et al. (2021). The role of mTOR signaling pathway in regulating autophagy in liver injury of TX mice with Wilson’s disease. BIOCELL 45: 109–117. DOI 10.32604/biocell.2021.012048. [Google Scholar] [CrossRef]
Tao X, Chen Q, Li N, Xiang H, Pan Y et al. (2020). Serotonin-RhoA/ROCK axis promotes acinar-to-ductal metaplasia in caerulein-induced chronic pancreatitis. Biomedicine & Pharmacotherapy 125: 109999. DOI 10.1016/j.biopha.2020.109999. [Google Scholar] [CrossRef]
Xu G, Ge Y, Tao X, Gao Q, Liang X (2017). MARK2 inhibits the growth of HeLa cells through AMPK and reverses epithelial-mesenchymal transition. Oncology Reports 38: 237–244. DOI 10.3892/or.2017.5686. [Google Scholar] [CrossRef]
Zhao Y, Yang X, Zhao J, Gao M, Zhang M et al. (2021). Berberine inhibits chemotherapy-exacerbated ovarian cancer stem cell-like characteristics and metastasis through GLI1. European Journal of Pharmacology 895: 173887. DOI 10.1016/j.ejphar.2021.173887. [Google Scholar] [CrossRef]
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J et al. (2015). Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527: 525–530. DOI 10.1038/nature16064. [Google Scholar] [CrossRef]
Zhou P, Li B, Liu F, Zhang MC, Wang Q et al. (2017). The epithelial to mesenchymal transition (EMT) and cancer stem cells: Implication for treatment resistance in pancreatic cancer. Molecular Cancer 16: 52. DOI 10.1186/s12943-017-0624-9. [Google Scholar] [CrossRef]
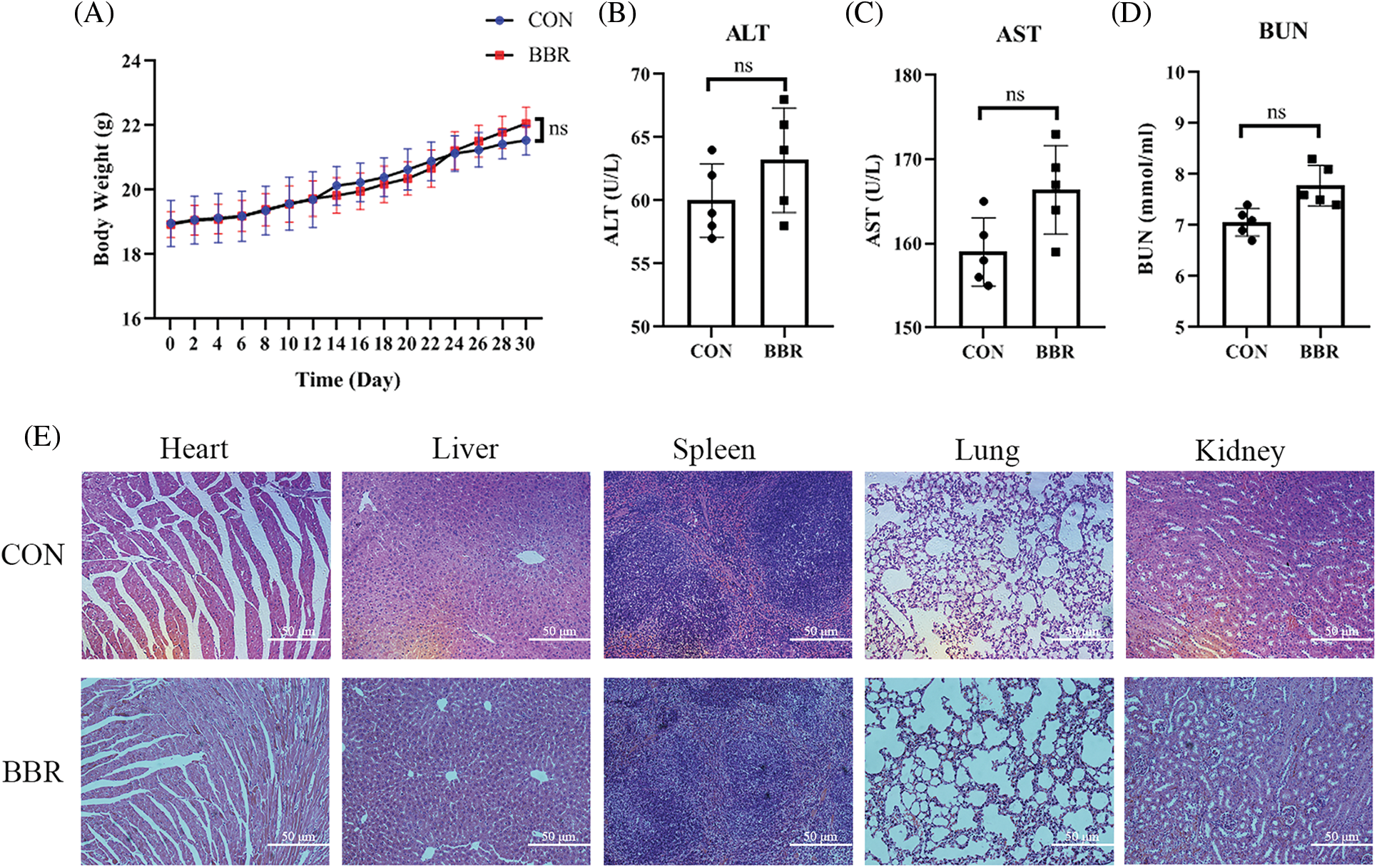
Supplemental Figure 1: Safety evaluation of berberine in PDAC. (A) Body weight of mice treated with berberine. (B) Serum alanine transaminase levels after treatment with berberine. (C) Serum aspartate aminotransferase levels after treatment with berberine. (D) Blood urea nitrogen levels after treatment with berberine. (E) H&E staining treated with berberine in heart, liver, spleen, lung, kidney tissues. Data are expressed as the mean ± SD. Statistical differences were analyzed using the unpaired Student’s t-test. No significant difference when compared with controls.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |