DOI:10.32604/biocell.2022.020697

| BIOCELL DOI:10.32604/biocell.2022.020697 |  |
| Article |
BET protein inhibitor apabetalone represses Porphyromonas gingivalis LPS-induced macrophage M1 polarization via regulating miR-130a/STAT3 axis
1Department of Periodontics, Shanghai Stomatological Hospital & School of Stomatology, Fudan University, Shanghai, 200001, China
2Shanghai Key Laboratory of Craniomaxillofacial Development and Diseases, Fudan University, Shanghai, 200001, China
3Department of Stomatology, Shanghai Fifth People’s Hospital, Fudan University, Shanghai, 200240, China
*Address correspondence to: Tianying Bian, tianying_bian@fudan.edu.cn
Received: 07 December 2021; Accepted: 16 February 2022
Abstract: Periodontitis is a frequent chronic inflammatory disorder destroying periodontium. Recent studies have revealed the role of bromodomain and extraterminal domain inhibitor (BETi) and microRNA (miR)-130a in regulating macrophage polarization and pro-inflammatory response. However, little is known about whether apabetalone (a novel BETi) and miR-130a are correlated with chronic inflammatory state in periodontitis by regulating macrophage polarization. Here murine RAW264.7 macrophages were applied as an in vitro inflammatory model. After treatment with Porphyromonas gingivalis-derived lipopolysaccharide (Pg LPS) and apabetalone, the expression of macrophage M1 polarization markers and inflammatory cytokines was assessed using real-time PCR, western blot, and enzyme-linked immuno sorbent assay (ELISA). MiR-130a level was assessed using real-time PCR, and the target gene was identified using dual luciferase reporter assay. We demonstrated that apabetalone repressed Pg LPS-induced macrophage M1 polarization in a dose-dependent manner, as evidenced by decreased expression of inducible nitric oxide synthase (iNOS), CD86, and pro-inflammatory cytokines, and increased expression of Arg-1 and CD206. Mechanistically, Pg LPS increased miR-130a expression in macrophages, whereas apabetalone treatment repressed the effect. Functionally, forced expression of miR-130a promoted macrophage M1 polarization, and signal transducer and activator of transcription (STAT)-3 was the direct target gene of miR-130a in the process. Taken together, apabetalone decreases Pg LPS-induced macrophage M1 polarization via regulating miR-130a-3p/STAT3 axis, and may be a promising target for the clinical management of periodontitis.
Keywords: Periodontitis; Macrophage polarization; Porphyromonas gingivalis; BET inhibitor; miRNA
Periodontitis is a highly prevalent oral disease characterized by low-grade inflammation (Nazir, 2017). Periodontitis is caused by constant periodontopathic bacterial (e.g., Porphyromonas gingivalis) infection, and eventually leads to chronic inflammation-induced tissue destruction and bone loss (Chen et al., 2021; Papapanou et al., 2018). The local inflammation in oral mucosa is epidemiologically correlated with other inflammation-triggering diseases such as neurodegenerative, autoimmune and cardio-metabolic diseases (Hamza et al., 2021; Makkar et al., 2018).
Macrophages are activated by recognizing pathogen (or damage)-associated molecular patterns through pattern-recognition receptors (Luan et al., 2021a; Takeuchi and Akira, 2010). Once activation, macrophage can trigger appropriate or pathogenic inflammatory response (Chen et al., 2021). Macrophages display phenotypic plasticity and functional heterogeneity in response to different environmental signals (Hussell and Bell, 2014). This capacity enables macrophages to orchestrate specific immune response to distinct pathogens. Macrophages are often divided into two subpopulations: classically activated macrophages (M1) and alternatively activated macrophages (M2). M1 macrophages secrete pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF)-α and IL-12, etc. (Arora et al., 2018). Macrophages are polarized to M2 phenotype after stimulation with IL-4, IL-10 and IL-13, and generate anti-inflammatory factors including IL-10, IL-4, etc. (Kopf et al., 2015; Murray et al., 2014). In periodontitis, Pg LPS converts macrophages to M1 phenotype to trigger inflammatory response, thus driving these macrophages to M2 macrophages is an effective treatment to inhibit inflammation-induced tissue destruction and bone loss.
MicroRNAs (miRNAs) are a class of approximately 21-nucleotide non-coding RNAs and involve in multiple physiological and pathological processes (Miranda et al., 2006; Wu et al., 2021). Given that miRNAs are able to regulate multiple target genes in different biological processes (Selbach et al., 2008), the involvement of miRNAs in macrophage-polarization is interesting. Recent studies also showed that a great deal of miRNAs participate in the fine-tuning regulation of immune responses (Li et al., 2021; Wang et al., 2021). For example, miR-223 is necessary for macrophage M2 polarization and downregulated miR-223 in septic patients converts macrophages to M1 phenotype (Ying et al., 2015). Periodontal miR-125a-5p level is increased and the miRNA contributes to bone healing through facilitating macrophage M2 polarization (He et al., 2021).
The bromodomain and extra-terminal (BET) proteins are a kind of epigenetic adaptors and exert an important role in cell proliferation, neurological disorders, and inflammation (Cochran et al., 2019). Previous studies showed that BET inhibitor (BETi), I-BET151 and JQ1, represses excessive secretion of pro-inflammatory cytokines in gingival fibroblasts and epithelial cells from periodontitis patients (Maksylewicz et al., 2019). Apabetalone, a novel BETi, is undergoing phase III clinical trials in patients with high-risk cardiovascular disease (CVD). Apabetalone also exhibits an anti-inflammatory effect in CVD patients (Jahagirdar et al., 2014; Tsujikawa et al., 2019). Based on the above findings, we investigated the role of apabetalone in controlling Pg LPS-induced excessive inflammatory cytokines production in macrophages. The current study demonstrated that apabetalone inhibits Pg LPS-induced macrophage M1 polarization via decreasing miR-130a-3p and thus de-repressing STAT3 expression.
A murine macrophage-like cell line (RAW264.7) was obtained from the American Type Culture Collection (ATCC, MD, USA), and cultivated in DMEM containing 10% FBS (Tianhang, Hangzhou, China). RAW264.7 cells were maintained in a humidified incubator (Yiheng Instruments, Shanghai, China) containing 5% CO2 at 37°C. Ultrapure Pg LPS (5 µg/mL), purchased from InvivoGen (San Diego, CA, USA), was used to treat RAW264.7 cells.
Quantitative real-time PCR (qRT-PCR)
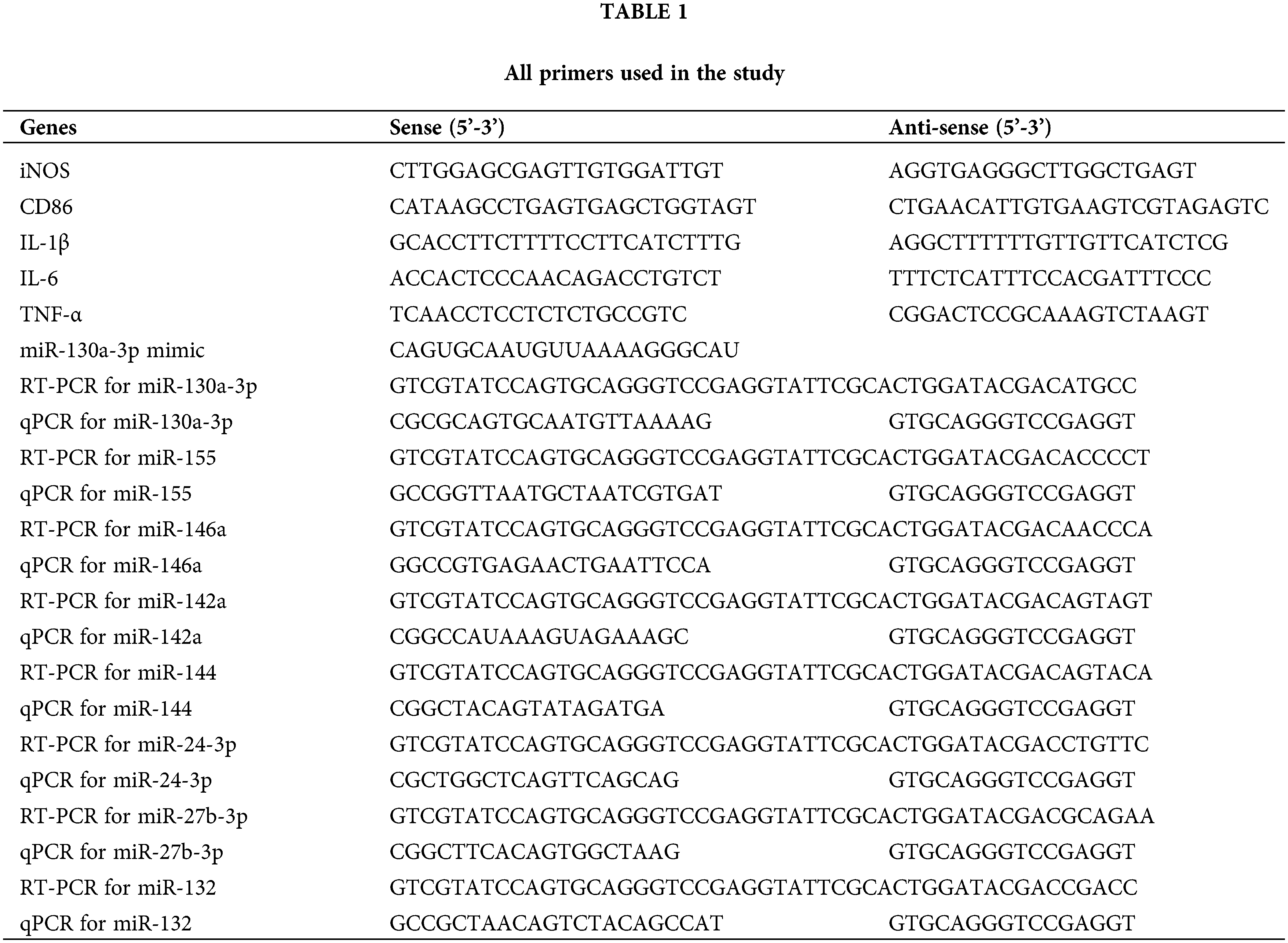
Total RNA was extracted from RAW264.7 cells with TRIzol™ reagent (15596-026; ThermoFisher, MA, USA) according to the manufacturer’s instructions. Total RNAs were quantified using NanodropND-2000 spectrophotometer (ThermoFisher). cDNAs were synthesized using Moloney’s murine leukemiavirus reverse transcriptase (Thermo Fisher Scientific) and Oligo (dT) primers in accordance with manufacturer’s instructions (70°C for 12 min, ice bath for 2 min, and then 42°C for 60 min). Reverse transcription PCR (RT-PCR) for miR-130a was performed with special stem-loop RT primers. qRT-PCR was carried out using cDNA template and Light Cycler 480 SYBR Green I Master (Roche, Basel, Switzerland) on ABI 7500 qRT-PCR System (Thermo Fisher Scientific). The temperature protocol for qRT-PCR was 95°C for 20 min, followed by 45 cycles of 95°C for 10 s and 58°C for 15 s. β-actin was applied as internal control for mRNAs. U6 was applied as internal control for miRNAs. Relative expression level of mRNAs or miRNAs was calculated using 2(-ΔΔCT) method, as previously described (Livak and Schmittgen, 2001). All primers used in the study were listed in Table 1.
RAW264.7 cells were treated with Pg LPS (5 µg/mL) for 24 h in the presence or absence of apabetalone (2 µM, 10 µM, or 30 µM) and lysed with RIPA buffer (Beyotime, Shanghai, China). After measuring total protein concentration with a BCA protein assay kit (Beyotime), approximate 80 µg of protein were segregated through SDS-PAGE gels (12%) and then electro-transferred onto PVDF membranes (Thermo Fisher Scientific). The membranes were blocked with 3% bovine serum albumin and then incubated with the indicated primary antibodies (iNOS: ab178945, 1:1000, Abcam; CD86: ab112490, 1:1000, Abcam; Arg-1: ab239731, 1:2000, Abcam; CD206: ab64693, 1:1500, Abcam; STAT3: ab68153, 1:1500, Abcam; beta-actin: ab8226, 1:2000, Abcam, MA, USA) for 1 h at room temperature (RT). Then the membranes were incubated with HRP-labelled anti-rabbit secondary antibodies (1:6000) for 1 h at RT. Immunoblots were visualized using a chemiluminescence assay system (Thermo Fisher Scientific).
Enzyme linked immunosorbent assay (ELISA)
RAW264.7 cells were treated with Pg LPS (5 µg/mL) for 24 h in the presence or absence of apabetalone (10 µM), and then the supernatant was collected. The IL-6 and IL-1β levels in supernatant were assessed using the indicated ELISA kits (Mouse IL-1β ELISA Kit: MLB00C, R&D; IL-6 ELISA Kit, M6000B, R&D). The absorbance at 450 nm was assessed on a Varioskan LUX Multimode Microplate Reader.
Dual-luciferase reporter assay
The recombinant plasmids of pGL3-STAT3-3’UTR or pGL3-STAT3-3’UTR-mut was constructed by inserting STAT3-3’UTR containing predictive binding site or its mutant (STAT3-3’UTR-mut) into pGL3 vector. RAW264.7 cells were plated into 96-well plates. After 24 h, pGL3-STAT3-3’UTR or pGL3-STAT3-3’UTR-mut plasmids were co-transfected with miR-130a mimics (GenePharma, Shanghai, China) using Advanced DNA/RNA Transfection Reagent™ (ZETA LIFE, CA, USA) in accordance with manufacturer’s instructions. Forty-eight hours later, luciferase activity was assessed using Dual Luciferase Reporter Assay Kit (Beyotime). pRL-TK was applied as the internal control to normalize luciferase activity.
Data were presented as mean ± SD from three independent experiments. Comparison between different treatments was performed with GraphPad Prism (GraphPad Software, CA, USA). The significance of differences between groups was assessed by one-way analysis of variance followed by the Scheffé test or Student’s t test. p < 0.05 was considered as significant.
Apabetalone repressed Pg LPS-induced macrophage M1 polarization
Although apabetalone is well known to exert a critical role in vascular inflammation (Jahagirdar et al., 2014; Tsujikawa et al., 2019), the role of apabetalone in regulating macrophage polarization remains unclear. To investigate the potential correlation between apabetalone and periodontitis, Pg LPS-treated RAW264.7 macrophages were applied as the in vitro inflammatory model. As shown in Fig. 1A, Pg LPS treatment enhanced the expression of macrophage M1 polarization markers (iNOS and CD86), whereas apabetalone repressed Pg LPS-induced up-regulation of iNOS and CD86 in a dose-dependent manner. Western blot analysis further showed that apabetalone inhibited Pg LPS-induced upregulation of iNOS and CD86 protein level (Figs. 1B and 1C). As expected, Pg LPS increased pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) production, whereas apabetalone treatment repressed the effect (Figs. 1D–1F). Consistent with the above results, Pg LPS decreased M2 polarization markers (Arg-1 and CD206) expression, whereas apabetalone treatment restored Arg-1 and CD206 expression in Pg LPS-treated RAW264.7 cells (Figs. 2A and 2B). The results from ELISA also showed that apabetalone repressed Pg LPS-induced increase of pro-inflammatory cytokines (Figs. 2C and 2D). These results demonstrate that apabetalone suppresses Pg LPS-induced macrophage M1 polarization.

Figure 1: Apabetalone repressed Pg LPS-triggered macrophage M1 polarization. (A) RAW264.7 cells were treated with Pg LPS (5 µg/mL) and apabetalone (2 µM, 10 µM, and 30 µM), and qRT-PCR analysis was carried out to assess iNOS and CD86 mRNA level. (B and C) RAW264.7 cells were treated with Pg LPS (5 µg/mL) and apabetalone (2 µM, 10 µM, and 30 µM), and western blot analysis was carried out to assess iNOS and CD86 protein level. (D–F) RAW264.7 cells were treated with Pg LPS (5 µg/mL) and apabetalone (2 µM, 10 µM, and 30 µM), and qRT-PCR analysis was carried out to assess IL-1β (D), IL-6 (E), and TNF-α (F) mRNA level. **p < 0.01.

Figure 2: Apabetalone increased the expression of macrophage M2 polarization markers. (A and B) RAW264.7 cells were treated with Pg LPS (5 µg/mL) and apabetalone (10 µM), and western blot analysis was performed to assess Arg-1 and CD206 protein level. RAW264.7 cells were treated with Pg LPS (5 µg/mL) and apabetalone (10 µM), and ELISA was carried out to assess IL-1β (C) and IL-6 (D) level in supernatant. **p < 0.01.
Apabetalone repressed Pg LPS-induced miR-130a expression
Given the important role of miRNAs in periodontitis (Luan et al., 2021b; Santonocito et al., 2021) and macrophage polarization (Nakao et al., 2021; Tao et al., 2021), we next investigated whether miRNAs were involved in the regulation of macrophage polarization after Pg LPS and apabetalone treatment. To this end, several dys-regulated miRNAs in periodontitis (miR-155, miR-146a, miR-130a-3p, miR-142a-3p, miR-144, miR-24-3p, miR-27b-3p, and miR-132) (Asa’ad et al., 2020) were analyzed in Pg LPS-treated RAW264.7 in the presence or absence of apabetalone. Among these miRNAs, only miR-130a-3p (hereafter named as miR-130a) was increased in RAW264.7 cells following Pg LPS treatment, whereas apabetalone repressed the increase (Figs. 3A and 3B). Functionally, miR-130a overexpression increased iNOS and CD86 expression (Fig. 3C), and promoted pro-inflammatory cytokines production (Fig. 3D), indicating the effect of miR-130a on facilitating macrophage M1 polarization. More important, miR-130a inhibition decreased Pg LPS-induced macrophage M1 polarization (Fig. 3E). These results showed that apabetalone represses Pg LPS-triggered macrophage M1 polarization, at least in part by repressing miR-130a.

Figure 3: Apabetalone repressed Pg LPS-induced miR-130a expression. (A) RAW264.7 cells were treated with Pg LPS (5 µg/mL) and apabetalone (10 µM), and qRT-PCR analysis was carried out to assess the level of miR-155, miR-146a, miR-130a, miR-142a-3p, miR-144, miR-24-3p, miR-27b-3p, and miR-132. (B) qRT-PCR analysis of miR-130a in RAW64.7 cells after treatment with Pg LPS (5 µg/mL) in the presence or absence of apabetalone (10 µM). (C) qRT-PCR analysis of miR-130a, iNOS, and CD86 level in RAW64.7 cells after treatment with miR-130a mimics (40 nM). (D) qRT-PCR analysis of IL-1β, IL-6, and TNF-α level in RAW64.7 cells after treatment with miR-130a mimics (40 nM). (E) qRT-PCR analysis of miR-130a, iNOS, CD86, IL-1β, IL-6, and TNF-α level in RAW64.7 cells after treatment with miR-130a inhibitor (40 nM). *p < 0.05. **p < 0.01.
miR-130a facilitated macrophage M1 polarization by targeting STAT3
To identify the potential targets of miR-130a, bioinformatical analysis was performed with TargetScan7.1 (http://www.targetscan.org/vert_71/). There are 1029 transcripts possibly targeted by miR-130a. Among these transcripts, STAT3 was selected for further validation because STAT3 is a critical factor in regulating macrophage polarization (Ren et al., 2021; Tian et al., 2021). To prove the prediction, recombinant plasmids of pGL3-STAT3-3’UTR and its mutant (pGL3-STAT3-3’UTR-mut) were constructed by inserting STAT3-3’UTR into pGL3 vector (Fig. 4A), and co-transfected with miR-130a into RAW264.7 cells. It has revealed that the luciferase activity of pGL3-STAT3-3’UTR was significantly reduced following transfection with miR-130a, but four nucleotides mutation in STAT3-3’UTR led to complete loss of the suppressive effect (Fig. 4B). Furthermore, forced expression of miR-130a significantly decreased STAT3 protein level in RAW264.7 cells (Figs. 4C and 4D), whereas miR-130a inhibition up-regulated STAT3 expression (Figs. 4E and 4F).

Figure 4: MiR-130a facilitated macrophage M1 polarization by targeting STAT3. (A) Schematic representation of the miR-130a site in STAT3-3’UTR. (B) Luciferase activity was assessed in RAW264.7 cells after co-transfection with miR-130a mimics (40 nM) and 20 ng of STAT3-3’UTR luciferase reporters (or its mutant). (C and D) Western blot analysis of STAT3 protein level in RAW264.7 cells after transfection with miR-130a mimics (40 nM). (E and F) Western blot analysis of STAT3 protein level in RAW264.7 cells after treatment with miR-130a inhibitor (40 nM). **p < 0.01.
Macrophages can polarize into different phenotypes in response to different environmental signals. In periodontitis, M1 macrophages initiate pro-inflammatory response and contribute to maintain inflammatory state (Lam et al., 2016). Therefore, driving these macrophages to M2 macrophages may be an effective treatment for inhibiting inflammation-induced tissue destruction and bone loss. In the current study, we demonstrated that, I) apabetalone restrains Pg LPS-induced macrophage M1 polarization, II) apabetalone decreases Pg LPS-triggered miR-130a expression, III) miR-130a promotes macrophage M1 polarization by targeting STAT3, IV) apabetalone represses Pg LPS-induced macrophage M1 polarization via regulating miR-130a-3p/STAT3 axis. These results reveal the important role of apabetalone in inhibiting Pg LPS-induced macrophage M1 polarization, indicating that apabetalone has the potential to treat periodontitis.
There are more than 700 kinds of bacteria in mouth cavity, and Gram-negative bacteria are the major pathogens in chronic periodontitis. Among these, Pg is regarded as the keystone pathogen. Mounting evidence has demonstrated that LPS derived from Porphyromonas gingivalis (Pg LPS) is a major virulence factor in the pathology of periodontitis through triggering periodontal inflammation (Yao et al., 2021). Macrophage is a key regulator of innate and adaptive immune response, and exerts a critical role in regulating periodontal homeostasis and pathogenesis (Nędzi-Góra et al., 2017). Dysregulation of macrophage function leads to the breakdown of periodontal homeostasis. M1 macrophage, induced by periodontopathic bacteria or bacteria-derived LPS, produces a large amount of inflammatory factors, including IL-1β, IL-6, TNF-α, etc., and thus acts as an antimicrobial function. Nevertheless, chronic and excess inflammatory factors derived from over-activated M1 macrophage exacerbate inflammation and periodontal tissue injury. Lam et al. (2014) has demonstrated that macrophages depletion using clodronate-liposomes alleviates alveolar bone loss in a murine model of periodontitis. Viniegra et al. (2018) revealed that macrophages depletion during onset of experimental periodontitis prevents bone resorption.
Epigenetic mechanisms, including DNA and histone modifications, contribute to the onset and progression of periodontitis. In vitro studies have demonstrated the ability of Pg LPS to stimulate epigenetic modifications associated with DNA methylation and histone acetylation in human periodontal ligament stem cells (Diomede et al., 2017). Furthermore, Pg LPS can also trigger abrupt but short-lived acetylation of histone H3 in oral epithelial cells (Martins et al., 2016). Epigenetic therapeutics have been identified as a promising therapeutic target for chronic periodontitis. Effects of some small-molecular inhibitors of epigenetic regulators in periodontitis models in vitro and in vivo are reviewed and discussed (Jurdziński et al., 2020).
MiRNAs are able to regulate multiple genes involved in different biological processes (Selbach et al., 2008). For example, miR-146a can repress IFN-γ-triggered macrophage M1 polarization via directly targeting signal transducer and activator of transcription 1 (STAT1) (He et al., 2016). The miR-146a level is increased in patients with periodontitis, and miR-146a overexpression in gingival fibroblasts or macrophages decreases pro-inflammatory cytokines production by targeting tumor necrosis factor receptor-associated factor (TRAF)-6 (Motedayyen et al., 2015; Tang et al., 2019). These results suggest that miR-146a might protect against periodontitis through regulating macrophage polarization. MiR-130a is also increased in periodontitis (Perri et al., 2012), and the role of miR-130a in macrophage polarization has been demonstrated (Lin et al., 2015; Shi et al., 2020). However, little is known about whether miR-130a is correlated with chronic inflammatory state in periodontitis by regulating macrophage polarization. In the study, we demonstrated that miR-130a level is increased in RAW264.7 cells after Pg LPS treatment. Forced expression of miR-130a up-regulates iNOS and CD86 expression and promotes pro-inflammatory cytokines production, suggesting the role of miR-130a in facilitating macrophage M1 polarization. More important, miR-130a inhibition represses Pg LPS-induced macrophage M1 polarization.
BET protein has two tandem bromodomains, BD1 and BD2. BET inhibitors are small molecules which can bind to BET bromodomains, inhibiting the interactions of BET proteins with acetylated lysines on histone tails, thus modulating downstream gene transcription. BET inhibitors are demonstrated to have beneficial roles in diabetes, cardiovascular diseases, and cancer (Kalantar-Zadeh et al., 2021; Kulikowski et al., 2018). The pan-BETi JQ1 was demonstrated to resolve inflammation in vitro and bone destruction in a murine periodontitis model (Meng et al., 2014). However, compared with selective BET inhibitors, pan-BETi with equal affinity towards BD1 and BD2 leads to larger side effects and toxicity. Apabetalone is a selective BD2 bromodomain inhibitor with 20 to 30-fold selectivity over BD1 (Ray et al., 2019), which is different from JQ1, a pan-BET inhibitor, thus resulting in different biological processes. As a novel BETi, apabetalone is being developed as the potential treatment of acute coronary syndrome, diabetes and chronic kidney failure. Here we assessed whether apabetalone regulates macrophage polarization and function. We found that apabetalone represses Pg LPS-triggered macrophage M1 polarization in a dose-dependent manner. Mechanistically, apabetalone inhibits Pg LPS-induced miR-130a expression in macrophages. STAT3 is identified as a novel target gene of miR-130a in the regulation of macrophage polarization.
The current study demonstrated that apabetalone suppresses Pg LPS-induced macrophage M1 polarization via decreasing miR-130a-3p and de-repressing STAT3 expression.
Authors’ Contributions: T.B. generated research ideas and acquired fundings. M.C., H.W., X.C. performed the research. M.C., T.B. wrote the first draft. Y.C. analyzed the data. H.W. edited the manuscript for scientific accuracy. T.B. supervised the project and revised the manuscript. All authors have read and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Ethics Approval: Not applicable.
Funding Statement: This work was supported by the National Natural Science Foundation for Young Scientists of China (Grant No. 81901004), the Youth Program Shanghai Municipal Health and Family Planning Commission grant (Grant No. 20194Y0227) and Shanghai Stomatological Hospital Science Foundation (Grant No. SSDC-2018-02).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Arora S, Dev K, Agarwal B, Das P, Syed MA (2018). Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 223: 383–396. DOI 10.1016/j.imbio.2017.11.001. [Google Scholar] [CrossRef]
Asa’ad F, Garaicoa-Pazmino C, Dahlin C, Larsson L (2020). Expression of MicroRNAs in periodontal and peri-implant diseases: A systematic review and meta-analysis. International Journal of Molecular Sciences 21: 4147. DOI 10.3390/ijms21114147. [Google Scholar] [CrossRef]
Chen M, Wang Y, Sun B, Yu L, Chen Q et al. (2021). HIF-1alpha activator DMOG inhibits alveolar bone resorption in murine periodontitis by regulating macrophage polarization. International Immunopharmacology 99: 107901. DOI 10.1016/j.intimp.2021.107901. [Google Scholar] [CrossRef]
Cochran AG, Conery AR, Sims RJ (2019). Bromodomains: A new target class for drug development. Nature Reviews Drug Discovery 18: 609–628. DOI 10.1038/s41573-019-0030-7. [Google Scholar] [CrossRef]
Diomede F, Thangavelu SR, Merciaro I, D’Orazio M, Bramanti P et al. (2017). Porphyromonas gingivalis lipopolysaccharide stimulation in human periodontal ligament stem cells: Role of epigenetic modifications to the inflammation. European Journal of Histochemistry 61: 2826. DOI 10.4081/ejh.2017.2826. [Google Scholar] [CrossRef]
Hamza SA, Asif S, Khurshid Z, Zafar MS, Bokhari SAH (2021). Emerging role of epigenetics in explaining relationship of periodontitis and cardiovascular diseases. Diseases 9: 48. DOI 10.3390/diseases9030048. [Google Scholar] [CrossRef]
He W, Zhang N, Lin Z (2021). MicroRNA-125a-5p modulates macrophage polarization by targeting E26 transformation-specific variant 6 gene during orthodontic tooth movement. Archives of Oral Biology 124: 105060. DOI 10.1016/j.archoralbio.2021.105060. [Google Scholar] [CrossRef]
He X, Tang R, Sun Y, Wang Y, Zhen K et al. (2016). MicroR-146 blocks the activation of M1 macrophage by targeting signal transducer and activator of transcription 1 in hepatic schistosomiasis. EBioMedicine 13: 339–347. DOI 10.1016/j.ebiom.2016.10.024. [Google Scholar] [CrossRef]
Hussell T, Bell TJ (2014). Alveolar macrophages: Plasticity in a tissue-specific context. Nature Reviews Immunology 14: 81–93. DOI 10.1038/nri3600. [Google Scholar] [CrossRef]
Jahagirdar R, Zhang H, Azhar S, Tobin J, Attwell S et al. (2014). A novel BET bromodomain inhibitor, RVX-208, shows reduction of atherosclerosis in hyperlipidemic ApoE deficient mice. Atherosclerosis 236: 91–100. DOI 10.1016/j.atherosclerosis.2014.06.008. [Google Scholar] [CrossRef]
Jurdziński KT, Potempa J, Grabiec AM (2020). Epigenetic regulation of inflammation in periodontitis: Cellular mechanisms and therapeutic potential. Clinical Epigenetics 12: 186. DOI 10.1186/s13148-020-00982-7. [Google Scholar] [CrossRef]
Kalantar-Zadeh K, Schwartz GG, Nicholls SJ, Buhr KA, Ginsberg HN et al. (2021). Effect of apabetalone on cardiovascular events in diabetes, CKD, and recent acute coronary syndrome: Results from the BETonMACE randomized controlled trial. Clinical Journal of the American Society of Nephrology 16: 705–716. DOI 10.2215/CJN.16751020. [Google Scholar] [CrossRef]
Kopf M, Schneider C, Nobs SP (2015). The development and function of lung-resident macrophages and dendritic cells. Nature Immunology 16: 36–44. DOI 10.1038/ni.3052. [Google Scholar] [CrossRef]
Kulikowski E, Halliday C, Johansson J, Sweeney M, Lebioda K et al. (2018). Apabetalone mediated epigenetic modulation is associated with favorable kidney function and alkaline phosphatase profile in patients with chronic kidney disease. Kidney and Blood Pressure Research 43: 449–457. DOI 10.1159/000488257. [Google Scholar] [CrossRef]
Lam RS, O’Brien-Simpson NM, Lenzo JC, Holden JA, Brammar GC et al. (2014). Macrophage depletion abates Porphyromonas gingivalis-induced alveolar bone resorption in mice. Journal of Immunology 193: 2349–2362. DOI 10.4049/jimmunol.1400853. [Google Scholar] [CrossRef]
Lam RS, O’Brien-Simpson NM, Holden JA, Lenzo JC, Fong SB et al. (2016). Unprimed, M1 and M2 macrophages differentially interact with Porphyromonas gingivalis. PLoS One 11: e0158629. DOI 10.1371/journal.pone.0158629. [Google Scholar] [CrossRef]
Li W, Wang J, Hao W, Yu C (2021). MicroRNA-543-3p down-regulates inflammation and inhibits periodontitis through KLF6. Bioscience Reports 41: 1–10. DOI 10.1042/BSR20210138. [Google Scholar] [CrossRef]
Lin L, Lin H, Wang L, Wang B, Hao X et al. (2015). miR-130a regulates macrophage polarization and is associated with non-small cell lung cancer. Oncology Reports 34: 3088–3096. DOI 10.3892/or.2015.4301. [Google Scholar] [CrossRef]
Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. DOI 10.1006/meth.2001.1262. [Google Scholar] [CrossRef]
Luan G, Pan F, Bu L, Wu K, Wang A et al. (2021a). Butorphanol promotes macrophage phenotypic transition to inhibit inflammatory lung injury via kappa receptors. Frontiers in Immunology 12: 692286. DOI 10.3389/fimmu.2021.692286. [Google Scholar] [CrossRef]
Luan X, Zhou X, Fallah P, Pandya M, Lyu H et al. (2021b). MicroRNAs: Harbingers and shapers of periodontal inflammation. Seminars in Cell and Developmental Biology 124: 85–98. DOI 10.1016/j.semcdb.2021.05.030. [Google Scholar] [CrossRef]
Makkar H, Reynolds MA, Wadhawan A, Dagdag A, Merchant AT et al. (2018). Periodontal, metabolic, and cardiovascular disease: Exploring the role of inflammation and mental health. Pteridines 29: 124–163. DOI 10.1515/pteridines-2018-0013. [Google Scholar] [CrossRef]
Maksylewicz A, Bysiek A, Lagosz KB, Macina JM, Kantorowicz M et al. (2019). BET bromodomain inhibitors suppress inflammatory activation of gingival fibroblasts and epithelial cells from periodontitis patients. Frontiers in Immunology 10: 933. DOI 10.3389/fimmu.2019.00933. [Google Scholar] [CrossRef]
Martins MD, Jiao Y, Larsson L, Almeida LO, Garaicoa-Pazmino C et al. (2016). Epigenetic modifications of histones in periodontal disease. Journal of Dental Research 95: 215–222. DOI 10.1177/0022034515611876. [Google Scholar] [CrossRef]
Meng S, Zhang L, Tang Y, Tu Q, Zheng L et al. (2014). BET inhibitor JQ1 blocks inflammation and bone destruction. Journal of Dental Research 93: 657–662. DOI 10.1177/0022034514534261. [Google Scholar] [CrossRef]
Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL et al. (2006). A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 126: 1203–1217. DOI 10.1016/j.cell.2006.07.031. [Google Scholar] [CrossRef]
Motedayyen H, Ghotloo S, Saffari M, Sattari M, Amid R (2015). Evaluation of MicroRNA-146a and its targets in gingival tissues of patients with chronic periodontitis. Journal of Periodontology 86: 1380–1385. DOI 10.1902/jop.2015.150319. [Google Scholar] [CrossRef]
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW et al. (2014). Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 41: 14–20. DOI 10.1016/j.immuni.2014.06.008. [Google Scholar] [CrossRef]
Nakao Y, Fukuda T, Zhang Q, Sanui T, Shinjo T et al. (2021). Exosomes from TNF-alpha-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomaterialia 122: 306–324. DOI 10.1016/j.actbio.2020.12.046. [Google Scholar] [CrossRef]
Nazir MA (2017). Prevalence of periodontal disease, its association with systemic diseases and prevention. International Journal of Health Sciences 11: 72–80. [Google Scholar]
Nędzi-Góra M, Kowalski J, Górska R (2017). The immune response in periodontal tissues. Archivum Immunologiae et Therapiae Experimentalis 65: 421–429. DOI 10.1007/s00005-017-0472-8. [Google Scholar] [CrossRef]
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M et al. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. Journal of Periodontology 89 Suppl 1: S173–S182. DOI 10.1002/JPER.17-0721. [Google Scholar] [CrossRef]
Perri R, Nares S, Zhang S, Barros SP, Offenbacher S (2012). MicroRNA modulation in obesity and periodontitis. Journal of Dental Research 91: 33–38. DOI 10.1177/0022034511425045. [Google Scholar] [CrossRef]
Ray KK, Nicholls SJ, Ginsberg HD, Johansson JO, Kalantar-Zadeh K et al. (2019). Effect of selective BET protein inhibitor apabetalone on cardiovascular outcomes in patients with acute coronary syndrome and diabetes: Rationale, design, and baseline characteristics of the BETonMACE trial. American Heart Journal 217: 72–83. DOI 10.1016/j.ahj.2019.08.001. [Google Scholar] [CrossRef]
Ren J, Han X, Lohner H, Liang R, Liang S et al. (2021). Serum- and glucocorticoid-inducible kinase 1 promotes alternative macrophage polarization and restrains inflammation through FoxO1 and STAT3 signaling. Journal of Immunology 207: 268–280. DOI 10.4049/jimmunol.2001455. [Google Scholar] [CrossRef]
Santonocito S, Polizzi A, Palazzo G, Isola G (2021). The emerging role of microRNA in periodontitis: Pathophysiology, clinical potential and future molecular perspectives. International Journal of Molecular Sciences 22: 5456. DOI 10.3390/ijms22115456. [Google Scholar] [CrossRef]
Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R et al. (2008). Widespread changes in protein synthesis induced by microRNAs. Nature 455: 58–63. DOI 10.1038/nature07228. [Google Scholar] [CrossRef]
Shi J, Chen M, Ouyang L, Wang Q, Guo Y et al. (2020). miR-142-5p and miR-130a-3p regulate pulmonary macrophage polarization and asthma airway remodeling. Immunology and Cell Biology 98: 715–725. DOI 10.1111/imcb.12369. [Google Scholar] [CrossRef]
Takeuchi O, Akira S (2010). Pattern recognition receptors and inflammation. Cell 140: 805–820. DOI 10.1016/j.cell.2010.01.022. [Google Scholar] [CrossRef]
Tang L, Li X, Bai Y, Wang P, Zhao Y (2019). MicroRNA-146a negatively regulates the inflammatory response to Porphyromonas gingivalis in human periodontal ligament fibroblasts via TRAF6/p38 pathway. Journal of Periodontology 90: 391–399. DOI 10.1002/JPER.18-0190. [Google Scholar] [CrossRef]
Tao L, Pang Y, Wang A, Li L, Shen Y et al. (2021). Functional miR-142a-3p induces apoptosis and macrophage polarization by targeting tnfaip2 and glut3 in grass carp (Ctenopharyngodon idella). Frontiers in Immunology 12: 633324. DOI 10.3389/fimmu.2021.633324. [Google Scholar] [CrossRef]
Tian Z, Zeng F, Zhao C, Dong S (2021). Angelicin alleviates post-trauma osteoarthritis progression by regulating macrophage polarization via STAT3 signaling pathway. Frontiers in Pharmacology 12: 669213. DOI 10.3389/fphar.2021.669213. [Google Scholar] [CrossRef]
Tsujikawa LM, Fu L, Das S, Halliday C, Rakai BD et al. (2019). Apabetalone (RVX-208) reduces vascular inflammation in vitro and in CVD patients by a BET-dependent epigenetic mechanism. Clinical Epigenetics 11: 102. DOI 10.1186/s13148-019-0696-z. [Google Scholar] [CrossRef]
Viniegra A, Goldberg H, Çil Ç., Fine N, Sheikh Z et al. (2018). Resolving macrophages counter osteolysis by anabolic actions on bone cells. Journal of Dental Research 97: 1160–1169. DOI 10.1177/0022034518777973. [Google Scholar] [CrossRef]
Wang L, Liu T, Chen G, Li Y, Zhang S et al. (2021). Exosomal microRNA let-7-5p from taenia pisiformis cysticercus prompted macrophage to M2 polarization through inhibiting the expression of C/EBP delta. Microorganisms 9: 1403. DOI 10.3390/microorganisms9071403. [Google Scholar] [CrossRef]
Wu P, Feng J, Wang W (2021). Expression of miR-155 and miR-146a in the saliva of patients with periodontitis and its clinical value. American Journal of Translational Research 13: 6670–6677. [Google Scholar]
Yao S, Jiang C, Zhang H, Gao X, Guo Y et al. (2021). Visfatin regulates Pg LPS-induced proinflammatory/prodegradative effects in healthy and inflammatory periodontal cells partially via NF-kappaB pathway. Biochimica et Biophysica Acta-Molecular Cell Research 1868: 119042. DOI 10.1016/j.bbamcr.2021.119042. [Google Scholar] [CrossRef]
Ying W, Tseng A, Chang RC, Morin A, Brehm T et al. (2015). MicroRNA-223 is a crucial mediator of PPARgamma-regulated alternative macrophage activation. Journal of Clinical Investigation 125: 4149–4159. DOI 10.1172/JCI81656. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |