DOI:10.32604/biocell.2021.016302

| BIOCELL DOI:10.32604/biocell.2021.016302 |  |
| Article |
Oxidative stress indicators in human and bottlenose dolphin leukocytes in response to a pro-inflammatory challenge
1Centro de Investigaciones Biológicas del Noroeste, La Paz, 23096, México
2Hospital General de Zona No. 1, Instituto Mexicano del Seguro Social, La Paz, 23000, México
3Centro Interdisciplinario de Ciencias Marinas, Instituto Politécnico Nacional, La Paz, 23096, México
*Address correspondence to: Tania Zenteno-Savín, tzenteno04@cibnor.mx
Received: 24 February 2021; Accepted: 16 April 2021
Abstract: Marine mammals undergo cycles of tissue ischemia and reperfusion during the dive response. Reperfusion injury can result in oxidative tissue damage and the activation of a pro-inflammatory immune response. The risk of oxidative damage is reduced by antioxidants. Our hypothesis is that the reported higher antioxidant defenses within marine mammal tissues provide additional protection in situations that produce oxidative stress, like inflammation, in comparison to terrestrial mammal tissues. Leukocytes were isolated from the whole blood of Pacific bottlenose dolphins (Tursiops truncatus gilli) and humans (Homo sapiens) and were exposed to lipopolysaccharides (LPS, 10 µg/mL) in vitro to simulate a pro-inflammatory challenge. Oxidative stress indicators, including superoxide radical (O2•−) production, activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione S-transferase (GST), as well as oxidative protein damage, were quantified by spectrophotometry. Following 48 h under experimental conditions, bottlenose dolphin leukocytes produced 1.9 times more O2•− but displayed 2.0 times lower protein carbonyl concentrations compared to human leukocytes. Following 48 h under experimental conditions, bottlenose dolphin leukocytes displayed 7.9, 2.0, 11.1, and 3.3 times more activities of CAT, GPx, GR, and GST, respectively, compared to human leukocytes. These results suggest that, under cell culture conditions, the antioxidant defenses in bottlenose dolphin leukocytes provide additional protection against pro-inflammatory challenges in comparison to human leukocytes, likely as an adaptive advantage.
Keywords: Antioxidant defenses; Marine mammals; Oxidative stress; Reactive oxygen species; White blood cells
In contrast to terrestrial mammals, cetaceans such as bottlenose dolphins (Tursiops truncatus gilli) frequently engage in the dive response as part of a completely aquatic lifestyle. The dive response of mammals is characterized by apnea, bradycardia, and peripheral vasoconstriction (Elsner et al., 1969). Therefore, during a dive, tissues, except for those within the central nervous system, experience ischemia (Elsner et al., 1969; Ridgway, 1986). Reactive oxygen species (ROS), such as superoxide radical (O2•−) and hydrogen peroxide (H2O2), are produced throughout the dive response, during reserve oxygen (O2) metabolism, adenosine triphosphate (ATP) catabolism, and tissue reperfusion (Kevin et al., 2005; Granger and Kvietys, 2015). Antioxidants reduce the likelihood of oxidative tissue damage resulting from ROS overproduction and accumulation (Fridovich and Freeman, 1986; Halliwell and Gutteridge, 1990). However, the accumulation of oxidative products in tissues can initiate processes of cellular apoptosis or necrosis, leading to the activation of the pro-inflammatory response as part of the innate immune system (Kevin et al., 2005; Hussain et al., 2016).
Activation of a pro-inflammatory response also occurs following exposure to pathogens, including bacterial products (Ulevitch and Tobias, 1999). For defense, all living organisms possess an innate immune system (Keogh et al., 2011; Miller et al., 2005). Lipopolysaccharides (LPS) are structural elements in the external membrane of gram-negative bacteria, including Escherichia coli (Guha and Mackman, 2001; Ohishi et al., 2011). Through interactions with host receptors, the innate immune system recognizes LPS as a foreign body and initiates a pro-inflammatory response within hours to days (Ulevitch and Tobias, 1999; Davis et al., 2008; de Vos et al., 2009; Keogh et al., 2011). The activation of innate immunity results in the recruitment of inflammatory cells, including phagocytic leukocytes, and the liberation of pro-inflammatory mediators, such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Repine et al., 1997; Miller et al., 2005). NADPH oxidase catalyzes the oxidative burst, the rapid production of ROS, as a mechanism to destroy foreign bodies and damaged tissues (Halliwell and Gutteridge, 1990; Kevin et al., 2005; Sokolova, 2005; Birben et al., 2012).
Endotoxin A, a constituent of LPS, is associated with illness, including sepsis and septic shock, in humans and other mammals (Ulevitch and Tobias, 1999; Guha and Mackman, 2001). Endotoxin sensitivity in mammals occurs over a wide spectrum (Lehmann et al., 1987). Basic investigations indicate that humans are highly sensitive models, as physiological alterations occur rapidly following endotoxin exposure (Wolff, 1973; Copeland et al., 2005; de Vos et al., 2009). Current knowledge of the immune system and immune response mechanisms in marine mammals, especially cetaceans, is limited (de Guise et al., 1995; Bossart et al., 2019). Previous studies have analyzed the immune response of marine mammals following in vitro exposure, including the response of leukocytes from bottlenose dolphins exposed to bacteria (Sokolova, 2005; Keogh et al., 2011) and bacterial products (Shiraishi et al., 2002; Ohishi et al., 2011) under laboratory conditions. Of these studies, Ohishi et al. (2011) report that the exposure of bottlenose dolphin leukocytes to LPS concentrations as low as 5 µg/mL LPS is sufficient to induce an immune response.
Few comparative immunotoxicity studies have been conducted analyzing the immune response between terrestrial and marine mammals (Shiraishi et al., 2002), largely in relation to legal, ethical, and financial constraints, as well as limitations in immune function assay availability (Copeland et al., 2005; Levin et al., 2007; Ruiz et al., 2009). Under physiological conditions, the tissues of marine mammals generally display greater antioxidant defenses, compared to terrestrial mammals, likely as an adaptive advantage to decrease oxidative damage resulting from the dive response (Wilhelm Filho et al., 2002; Vázquez-Medina et al., 2006; Vázquez-Medina et al., 2007). Few studies have analyzed the indicators of oxidative stress in the tissues of marine mammals following immune activation (Walsh et al., 2015; Del-Águila-Vargas et al., 2019).
The objective of this study was to compare the indicators of oxidative stress, including the production of superoxide radical (O2•−), antioxidant enzyme defenses, and oxidative damage to proteins in leukocytes isolated from marine and terrestrial mammals in response to a pro-inflammatory challenge. Our hypothesis is that the greater antioxidant defenses in the tissues of marine mammals provide additional protection against situations that induce oxidative stress, such as inflammation, compared to the tissues of terrestrial mammals.
Unless otherwise stated, all materials and solutions were obtained or prepared from reactants acquired from Sigma (St. Louis, MO, USA). All analyses were performed in triplicate.
Residual whole blood samples (20 mL) were obtained from 12 healthy human (Homo sapiens) volunteers of both genders between the ages of 18–65, from the Instituto Mexicano del Seguro Social (IMSS) blood bank in La Paz, Baja California Sur, Mexico. Human blood samples were collected in blood collection bags containing the anticoagulant citrate phosphate dextrose (CPD). All donors were deemed eligible to donate blood following the donor’s guidelines established by IMSS; donor identities, including name, age, and gender, were maintained anonymous. All blood samples were collected with permission from the Comisión Nacional de Bioética (CONBIOÉTICA-09-CEI-009-20160601; registration number 2018-785-010). Whole blood samples (8–15 mL) were collected from 12 healthy Pacific bottlenose dolphins of both sexes between the ages of 4 to 16 (average 9.3 years) maintained under human care by Cabo Dolphins, Cabo San Lucas, Baja California Sur, Mexico. Blood samples from bottlenose dolphins were collected from the ventral caudal fluke by veterinarians using established sanitary protocols. Bottlenose dolphin blood samples were collected in plastic tubes (BD Vacutainer, Franklin Lakes, NJ, USA) containing the anticoagulant dipotassium ethylenediaminetetraacetic acid (EDTA-K2). Samples from humans and bottlenose dolphins were maintained at 4°C during transport to the Oxidative Stress Laboratory within the Centro de Investigaciones Biológicas del Noroeste (CIBNOR), where they were maintained refrigerated at 4°C overnight.
Leukocytes were isolated from whole-blood samples following the methods of Corredor (1994). Briefly, whole blood samples obtained from humans and bottlenose dolphins were individually mixed with Hanks balanced saline solution (HBSS; Gibco, Paisley, RFW, UK) or Dulbecco phosphate buffer solution (PBS; Gibco, Paisley, RFW, UK) in a Nest centrifugation tube (Fisher Scientific, Hampton, NH, USA). Each mixture was transferred to a separate centrifugation tube containing Ficoll-Paque PLUS (GE Healthcare, Chicago, IL, USA) and centrifuged (Legend RT, Sorvall, Germany) at 650 × g and 25°C. The leukocyte layer was manually isolated, rinsed with PBS or HBSS, and centrifuged at 350 × g and 25°C. The supernatant was discarded, leukocyte pellets were rinsed a second time and centrifuged as previously described. Following supernatant removal, RPMI-1640 culture medium containing 1% streptomycin/penicillin (Gibco, Paisley, RFW, UK) and 10% fetal bovine serum (Gibco, Paisley, RFW, UK) was added to each leukocyte pellet.
Leukocyte viability and total count
Leukocyte viability was assessed using a Neubauer chamber (Hausser Scientific, Horsham, PA, USA) following the methods of Colotta et al. (1992). To be utilized in this investigation, aliquots of leukocytes isolated from whole blood (0 h) were required to present at least 90% viability. The average cell count of each aliquot was assessed, and the estimated total leukocyte count was determined.
Exposure of leukocytes to lipopolysaccharides (LPS)
A solution of LPS isolated from E. coli O55:B5 (028M4138V) was prepared following the manufacturer’s instructions. Approximately 1 × 106 leukocytes from each sample were separated into individual cell culture flasks. Human and bottlenose dolphin leukocytes were exposed to control (LPS, 0 µg/mL) or experimental (LPS, 10 µg/mL) conditions and incubated (Thermo Scientific, Waltham, MA, USA) at 37°C with 5% CO2 for 24 and 48 h, in duplicate. Following the designated incubation time, the cell culture flasks were gently mixed, and the samples were centrifuged at 360 × g at ambient temperature. The supernatant was eliminated, and PBS was added to the leukocyte packets. Samples were stored in labeled cryogenic vials (Corning Incorporated, Corning, NY, USA) and maintained at −80°C until analyzed.
Production of superoxide radical (O2•−)
The production of O2•− was determined in human and bottlenose dolphin leukocytes following methods adapted for microplate (Costar assay plate, Corning Incorporated, Corning, NY, USA), from Markert et al. (1984) and Drossos et al. (1995). Samples were thawed on ice, and aliquots were separated into individual 1.5 mL tubes (Neptune Scientific, San Diego, CA, USA). Samples were mixed with 18 mM Krebs buffer, and 15 µM cytochrome C was added. Samples were transferred to a 37°C water bath (Polyscience, Niles, IL, USA) for 15 min, then placed in ice water for 5 min while 3 mM N-ethylmaleimide was added. Samples were centrifuged at 500 × g and 4°C, and the absorbance of the supernatant was determined at 550 nm using a spectrophotometer (Multiskan FC, Thermo Scientific, Waltham, MA, USA). The production of O2•− was determined following 24 and 48 h of incubation. Results are expressed in nanomoles O2•− per milligram of protein per minute.
To quantify antioxidant enzyme activities, 50 mM homogenization solution (0.1 M potassium phosphate buffer and 60 mM EDTA (Fermont, Monterrey, SA, MX) and 1 mM phenylmethylsulfonyl fluoride (PMSF)) was added to each sample. The activity of superoxide dismutase (SOD; E.C. 1.15.1.1) was determined following methods, adapted for microplate, from Suzuki (2000). Samples were mixed with working solution (50 mM sodium carbonate buffer solution, 100 µM xanthine, 0.25 mM nitroblue tetrazolium (NBT), 1 mM EDTA, and xanthine oxidase (0.1 units)). The absorbance was determined at 240 nm every minute over 3–5 min. The activity of SOD was determined following 24 and 48 h of incubation. The activity of SOD is expressed as units (U) per milligram of protein. One unit of activity is defined as the quantity of SOD necessary to inhibit the reaction between O2•− and NBT by 50%.
The activity of catalase (CAT; E.C. 1.11.1.6) was determined following methods adapted from Aebi (1984). Each sample was placed into a crystal cuvette (VWR Spectrophotometry Cell, Radnor, PA, USA), and working solution (0.1 M phosphate buffer solution, 20 mM H2O2) was added. The absorbance was determined at 240 nm every 15 s over 120–180 s (DU 800, Beckman Coulter, Brea, CA, USA). The activity of CAT was determined following 24 and 48 h of incubation. The activity of CAT is expressed as U per milligram of protein. One unit of activity is defined as the quantity of CAT necessary to reduce 1 µmol of H2O2 per minute.
The activity of glutathione peroxidase (GPx; E.C. 1.11.1.9) was determined following methods, adapted for microplate, from Flohé and Günzler (1984). Samples were mixed with 500 mM potassium phosphate buffer solution, 50 mM EDTA, 20 mM sodium azide, 15 U/mL glutathione reductase (GR), 1.5 mM NADPH, 250 mM glutathione (GSH), and 10 mM H2O2. The absorbance was determined at 340 nm every minute over 3–5 min. The activity of GPx was determined following 24 and 48 h of incubation. The activity of GPx is expressed as U per milligram of protein. One unit of activity is defined as the quantity of GPx necessary to cause the oxidation of 1.0 µmol GSH to form glutathione disulfide (GSSG) per minute.
The activity of glutathione reductase (GR; E.C. 1.8.1.7) was determined following methods, adapted for microplate, from Goldberg and Spooner (1987). Samples were mixed with 500 mM potassium phosphate buffer solution, 50 mM EDTA, 2 mM NADPH, and 10 mM GSSG. The absorbance was determined at 340 nm every minute over 3–5 min. The activity of GR was determined following 24 and 48 h of incubation. The activity of GR is expressed as U per milligram of protein. One unit of activity is defined as the quantity of GR necessary to reduce 1.0 µmol GSSG to GSH per minute.
The activity of glutathione S-transferase (GST; E.C. 4.4.1.34) was determined following methods, adapted for microplate, from Habig and Jakoby (1981). Samples were mixed with 0.1 M phosphate buffer solution, 10 mM GSH, 60 mM EDTA, and 10 mM 1-chloro-2,4-dinitrobenzene (CDNB). The absorbance was determined at 340 nm every minute over 3–5 min. The activity of GST was determined following 24 and 48 h of incubation. The activity of GST is expressed as U per milligram of protein. One unit of activity is defined as the quantity of GST necessary to catalyze the conjugation of 1.0 µmol of CDNB per minute.
Oxidative damage to proteins was determined following methods, adapted for microplate, from Levine et al. (1990). Each sample was mixed with 5% sulfosalicylic acid. Following centrifugation at 350 × g and 4°C, the supernatant was removed. Dinitrophenyl hydrazine (10 mM) or hydrochloric acid (HCl, 2.5 M; Fermont, Monterrey, MX; blanks) was added. Vials were maintained in a dark area for 1 h, with mixing every 15 min. Following this period, 20% trichloroacetic acid was added, and the tubes were incubated in 4°C water for 10 min. Following centrifugation at 350 × g at ambient temperature, the supernatant was removed, and each tube was rinsed with 1:1 ethanol:ethyl acetate (J.T. Baker, Phillipsburg, NJ, USA). Following centrifugation using the aforementioned conditions, 6 M guanidine chlorate was added, and all tubes were incubated in a 37°C water bath for 15 min. Each tube was centrifuged, and the absorbance of the supernatant was analyzed in the range between 340 and 405 nm. The concentration of protein carbonyls was determined using the maximum absorbance registered for each sample. The concentration of protein carbonyls was determined following 24 and 48 h of incubation. The concentration of protein carbonyls is expressed as micromoles per milligram of protein.
The concentration of total soluble proteins in each sample was determined following methods, adapted for microplate, from Bradford (1976), to standardize the results for the oxidative stress indicators. A standard curve was prepared using albumin dilutions between 0.005 to 0.2 mg/mL. Each sample and the standard curve dilutions were mixed with homogenization solution, PMSF, distilled water, and Coomassie blue (Bio-Rad, Hercules, CA, USA). After 15 min incubation under ambient conditions, the absorbance of the supernatant was registered at 590 nm. The total concentration of soluble proteins in each sample was determined using the equation of the standard curve following 24 and 48 h of incubation. The concentration of total proteins is expressed in micrograms per milliliter.
Statistical analyses were conducted using IBM SPSS Statistics Viewer software. Normality and homogeneity were determined using Kolmogorov-Smirnov and Levene’s tests, respectively (Zar, 1999; Garson, 2012). Parametric results were analyzed with independent t-tests and dependent t-tests to determine if differences existed in the indicators of oxidative stress between species and between treatments within each species, respectively (Zar, 1999). Non-parametric results were analyzed with Mann–Whitney and Wilcoxon tests for two related samples to determine if differences existed in the indicators of oxidative stress between species and between treatments within each species, respectively (Zar, 1999). Statistical significance was assumed when P < 0.050 (α of 5%).
Production of superoxide radical (O2•−)
Results of the O2•− production in human (N = 12) and bottlenose dolphin (N = 12) leukocytes in response to LPS are shown in Fig. 1. Significant differences between species were observed. Under control conditions (LPS, 0 µg/mL) following 24 h, O2•− production in human leukocytes was 1.7 times higher than in bottlenose dolphin leukocytes (P = 0.001). Under experimental conditions (LPS, 10 µg/mL) following 48 h, O2•− production in bottlenose dolphin leukocytes was 1.9 times higher than in human leukocytes (P = 0.004).
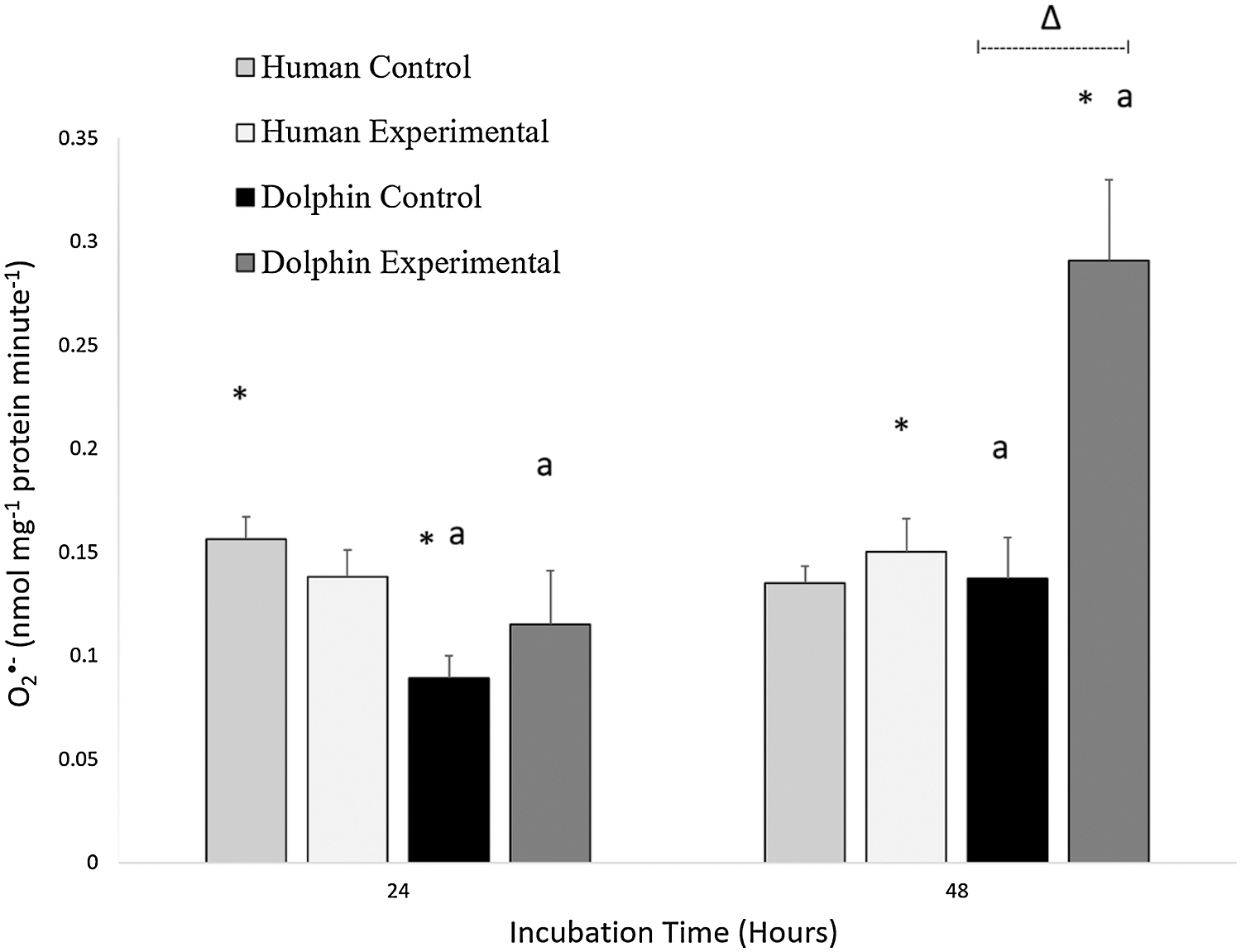
Figure 1: Production of superoxide radical (O2•−, nanomoles milligram−1 of protein minute−1) in human, Homo sapiens (N = 12), and bottlenose dolphin, Tursiops truncatus gilli (N = 12) leukocytes, maintained under primary culture in control (LPS, 0 µg/mL) and experimental (LPS, 10 µg/mL) conditions following 24 and 48 h incubation.
Data are presented as mean ± standard error. Significant differences (P < 0.050) *between species, Δwithin the same species and time of incubation, but different lipopolysaccharide concentrations, awithin the same species and lipopolysaccharide concentrations, but different incubation times.
An effect of exposure to LPS was observed in bottlenose dolphin leukocytes (Fig. 1). Under experimental conditions (LPS, 10 µg/mL) following 48 h, bottlenose dolphin leukocytes produced 2.1 times more O2•− compared to those cultured under control conditions (LPS, 0 µg/mL) for 48 h (P = 0.005).
An effect of incubation time was observed in bottlenose dolphin leukocytes (Fig. 1). Under control conditions (LPS, 0 µg/mL) following 48 h, bottlenose dolphin leukocytes produced 1.5 times more O2•− compared to leukocytes cultured for 24 h (P = 0.019). Under experimental conditions (LPS, 10 µg/mL) following 48 h, bottlenose dolphin leukocytes produced 2.5 times more O2•− compared to those exposed for 24 h (P = 0.010).
The results for the activities of SOD, CAT, GPx, GR, and GST in human (N = 12) and bottlenose dolphin (N = 12) leukocytes in response to LPS are shown in Fig. 2. Between species, significant differences in antioxidant enzyme activities were found. Following 24 h under control conditions (LPS, 0 µg/mL), bottlenose dolphin leukocytes displayed 2.4 times less SOD activity (P = 0.002), 7.6 times more CAT activity (P = 0.038), 6.1 times more GR activity (P = 0.001), and 2.2 times more GST activity (P = 0.002) compared to human leukocytes. Following 24 h under experimental conditions (LPS, 10 µg/mL), bottlenose dolphin leukocytes displayed 2.3 times less SOD activity (P = 0.015), 12.9 times more GR activity (P = 0.000), and 2.4 times more GST activity (P = 0.005) compared to human leukocytes. Following 48 h under control conditions, bottlenose dolphin leukocytes displayed 4.4 times more CAT activity (P = 0.050), 7.2 times more GR activity (P = 0.000), and 1.9 times more GST activity (P = 0.007) compared to human leukocytes. Following 48 h under experimental conditions, bottlenose dolphin leukocytes displayed 7.9 times more CAT activity (P = 0.001), 2.0 times more GPx activity (P = 0.038), 11.1 times more GR activity (P = 0.000), and 3.3 times more GST activity (P = 0.000) compared to human leukocytes.
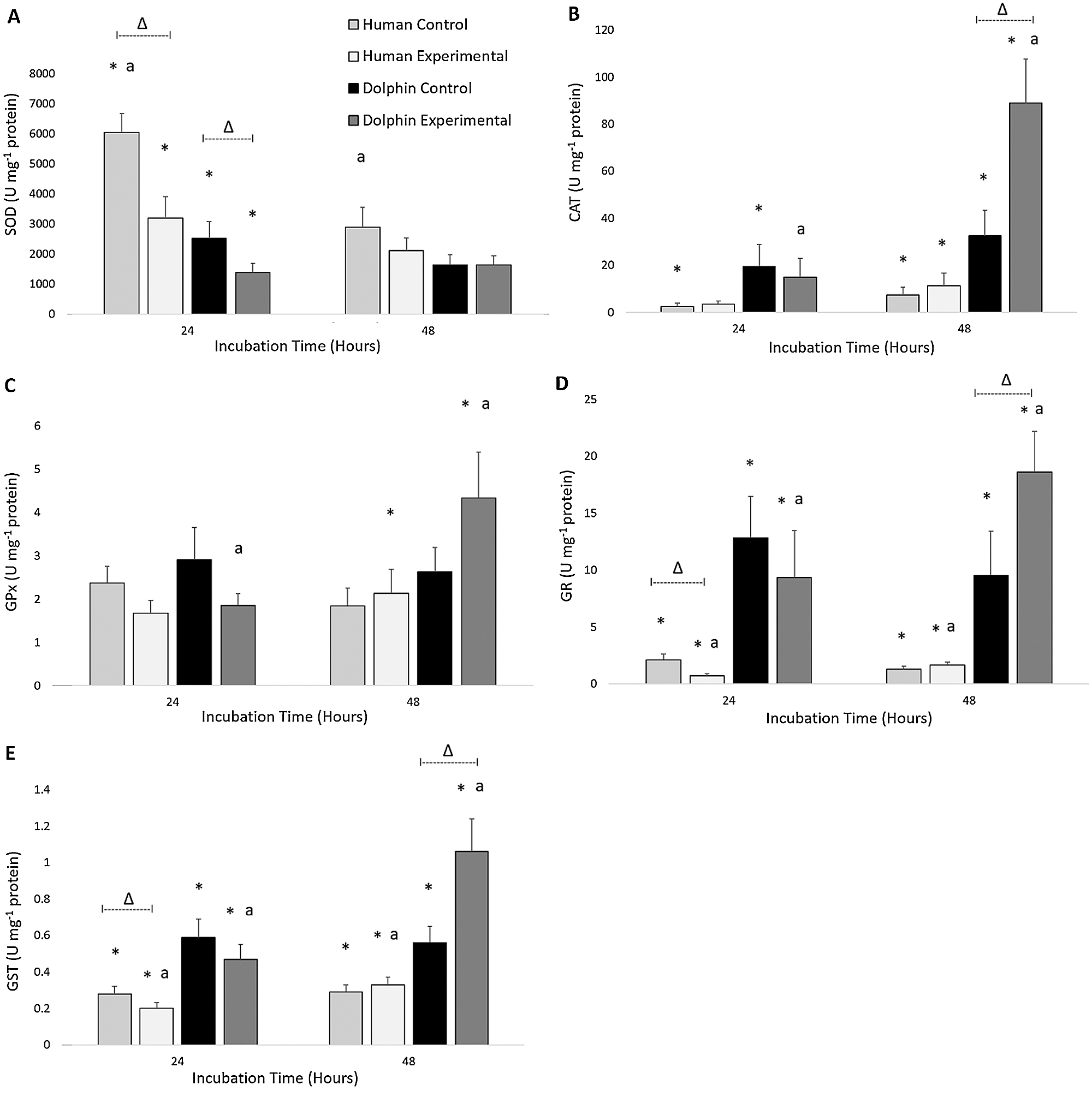
Figure 2: Activities of (A) superoxide dismutase (SOD; units (U) milligram−1 of protein), (B) catalase (CAT; units milligram−1 of protein), (C) glutathione peroxidase (GPx; units milligram−1 of protein), (D) glutathione reductase (GR; units milligram−1 of protein), and (E) glutathione S-transferase (GST; units milligram−1 of protein) in human, Homo sapiens (N = 12), and bottlenose dolphin, Tursiops truncatus gilli (N = 12) leukocytes, maintained under primary culture in control (LPS, 0 µg/mL) and experimental (LPS, 10 µg/mL) conditions following 24 and 48 h incubation.
Data are presented as mean ± standard error. Significant differences (P < 0.050) *between species, Δwithin the same species and time of incubation, but different lipopolysaccharide concentrations, awithin the same species and lipopolysaccharide concentrations, but different incubation times.
Within each species, effects of LPS exposure were found in relation to antioxidant enzyme activities (Fig. 2). Following 24 h under control conditions (LPS, 0 µg/mL), human leukocytes displayed 1.9 times more SOD activity (P = 0.008), 2.9 times more GR activity (P = 0.028), and 1.4 times more GST activity (P = 0.034) compared to those exposed to experimental conditions (LPS, 10 µg/mL) for 24 h. Following 24 h under control conditions, bottlenose dolphin leukocytes displayed 1.8 times more SOD activity (P = 0.019) compared to those exposed to experimental conditions for 24 h. Following 48 h under experimental conditions, bottlenose dolphin leukocytes displayed 2.7 times more CAT activity (P = 0.015), 2.0 times more GR activity (P = 0.041), and 1.9 times more GST activity (P = 0.012) compared to those cultured under control conditions for 48 h.
Significant differences in antioxidant enzyme activities between incubation times were observed within each species (Fig. 2). Under control conditions (LPS, 0 µg/mL) following 48 h, human leukocytes displayed 2.1 times less SOD activity compared to those cultured for 24 h (P = 0.004). Under experimental conditions (LPS, 10 µg/mL) following 48 h, human leukocytes displayed 2.3 times more GR activity (P = 0.010) and 1.7 times more GST activity (P = 0.003) compared to those incubated for 24 h. Under experimental conditions following 48 h, bottlenose dolphin leukocytes displayed 5.9 times more CAT activity (P = 0.006), 2.3 times more GPx activity (P = 0.050), 2.0 times more GR activity (P = 0.034), and 2.3 times more GST activity (P = 0.004) compared to those incubated for 24 h.
The results for the concentration of protein carbonyls in human (N = 12) and bottlenose dolphin (N = 12) leukocytes in response to LPS are shown in Fig. 3. Significant differences in oxidative damage to proteins between species were observed. Under control conditions (LPS, 0 µg/mL) following 48 h, human leukocytes displayed a protein carbonyl concentration 2.0 times greater than that in bottlenose dolphin leukocytes (P = 0.029). Under experimental conditions (LPS, 10 µg/mL) following 24 and 48 h, human leukocytes displayed protein carbonyl concentrations 3.4 and 2.0 times greater, respectively, than bottlenose dolphin leukocytes (P = 0.002, P = 0.023, respectively).
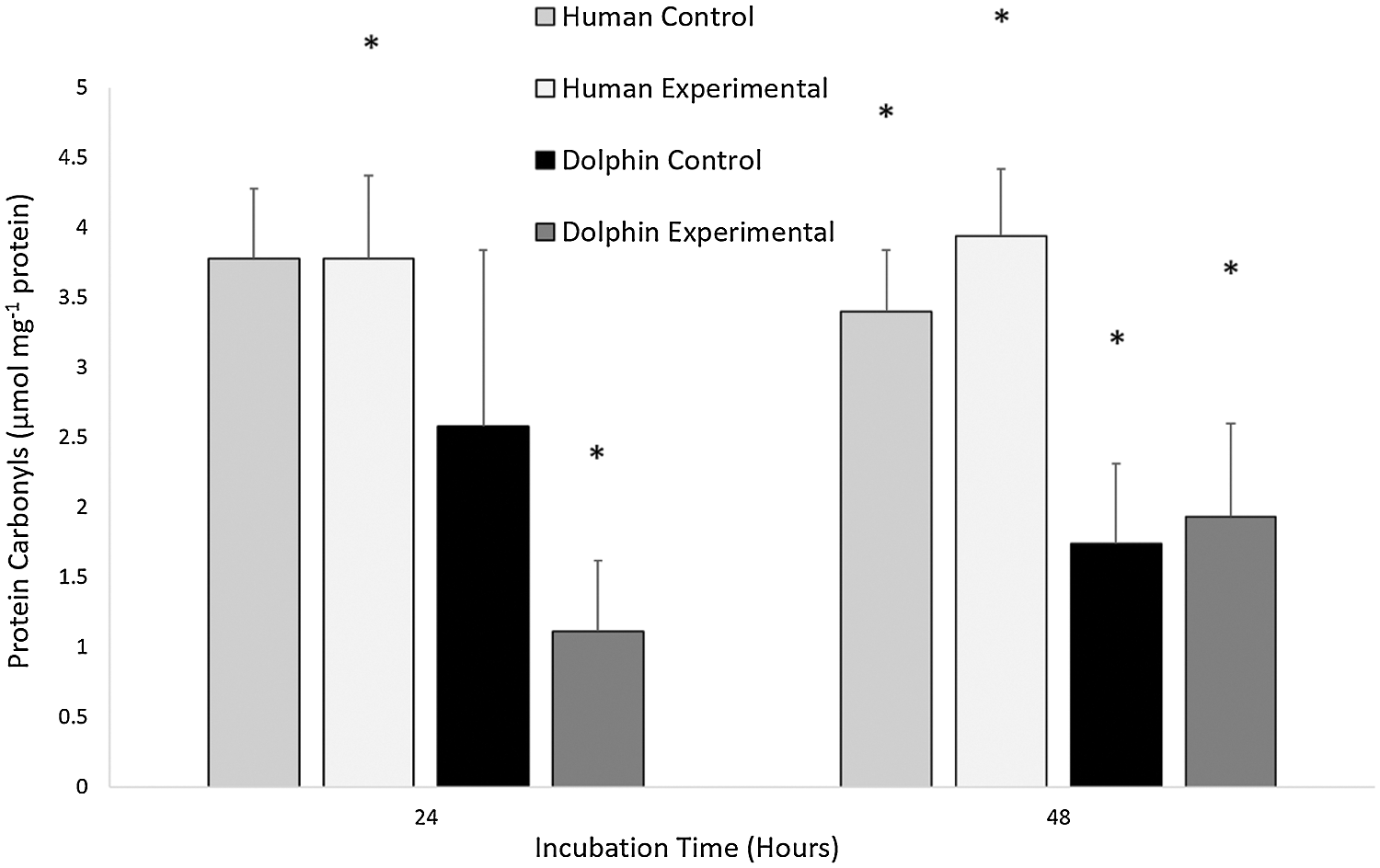
Figure 3: The concentration of protein carbonyls (μmol milligram−1 of protein) in human, Homo sapiens (N = 12), and bottlenose dolphin, Tursiops truncatus gilli (N = 12) leukocytes, maintained under primary culture in control (LPS, 0 µg/mL) and experimental (LPS, 10 µg/mL) conditions following 24 and 48 h incubation.
Data are presented as mean ± standard error. Significant differences (P < 0.050) *between species, Δwithin the same species and time of incubation, but different lipopolysaccharide concentrations, awithin the same species and lipopolysaccharide concentrations, but different incubation times.
Reactive oxygen species, including O2•−, are produced during normal O2 metabolism, phagocytosis, and reperfusion (Fridovich and Freeman, 1986; Halliwell and Gutteridge, 1990; Kevin et al., 2005; Birben et al., 2012). Approximately 1–4% of O2 is reduced to ROS during mitochondrial O2 metabolism (Kevin et al., 2005). Around one dozen mitochondria are distributed throughout the cytoplasm in human leukocytes (Anderson, 1966). ROS production, observed in this study, in human and bottlenose dolphin leukocytes under control conditions, at both 24 and 48 h, was likely associated with normal cellular processes, including ATP metabolism and apoptosis (Geering et al., 2013; Granger and Kvietys, 2015).
In this study, differences in oxidative stress indicators in bottlenose dolphin and human leukocytes were found at different incubation times. Differences in phagocyte quantity and phagocytic capacity influence ROS production and, thus, oxidative stress indicators (Sokolova, 2005; Keogh et al., 2011; Geering et al., 2013). Reports indicate that bottlenose dolphins have 42 to 75% neutrophils, 15 to 34% lymphocytes, 2 to 40% eosinophils, 0 to 4% monocytes, and <1% basophils in circulation, depending on the pathophysiological conditions (Medway and Geraci, 1964; Ridgway et al., 1970; Engelhardt, 1979; Shirai and Sakai, 1997). A similar leukogram has been reported in humans, except for a lower eosinophil count ranging from 1 to 4 % (Shirai and Sakai, 1997; Francischetti et al., 2010; Kobayashi et al., 2017; Nouri-Shirazi et al., 2017). Neutrophils act as the first line of defense against bacteria and bacterial products (Kobayashi et al., 2017) and have been reported to demonstrate greater phagocytic capacity compared to eosinophils and basophils (Geering et al., 2013). Differences in half-life between leukocyte cell types, related to function, may also contribute to explain the observed differences, even in the absence of LPS. In humans, unstimulated neutrophils have a half-life of approximately 7.6 to 90 h in vivo (Dancey et al., 1976; Pillay et al., 2010) and approximately 5 to 10 h in vitro (Dancey et al., 1976; Lee et al., 1993; Francischetti et al., 2010). In vivo studies in humans indicate that unstimulated T-lymphocytes have an approximate half-life of 77 to 87 days (Hellerstein et al., 1999), basophils have a half-life of up to a few days, eosinophils have a half-life of approximately 8 to 18 h (Stone et al., 2010), and monocytes have a half-life of 3 days (Whitelaw, 1972) within the general circulation. Stimulation with LPS delays apoptosis in polymorphonuclear leukocytes as a means to prolong their functional life (Colotta et al., 1992; Lee et al., 1993). At the end of their lifecycle, leukocytes enter apoptosis, contributing to ROS production (Kevin et al., 2005).
Differences in oxidative stress indicators in bottlenose dolphin and human leukocytes between treatments (control and experimental groups) were found. During the respiratory burst, phagocytes increase their O2 metabolism, resulting in ROS production from both NADPH oxidase and mitochondrial metabolism (Babior, 1999; Shiraishi et al., 2002; Keogh et al., 2011). In humans, rapid immune responses are initiated by small LPS concentrations (Guha and Mackman, 2001; de Vos et al., 2009). Modifications in antioxidant enzyme expression occur during periods of increased ROS production, including during immune responses (Davies, 2000; Marikovsky et al., 2003), contributing to decrease the likelihood of oxidative damage (Fridovich and Freeman, 1986; Halliwell and Gutteridge, 1990; Kevin et al., 2005). Following exposure to LPS isolated from E. coli, SOD, GR, and GPx activities significantly increased in the livers of Balb/c mice (Mus musculus) in comparison with controls (Kurhaluk et al., 2018). In this study, the significant increases observed in GPx, CAT, GST, and GR activities in bottlenose dolphin leukocytes and of GST and GR in human leukocytes, under experimental conditions between 24 and 48 h, likely relate to immune and antioxidant responses resulting from altered leukocyte metabolism in the presence of LPS.
Differences in oxidative stress indicators between bottlenose dolphin and human leukocytes were found in this study; greater ROS production was observed in bottlenose dolphin leukocytes following 48 h under experimental conditions as compared to human leukocytes. In this study, bottlenose dolphin leukocytes generally presented greater antioxidant defenses, which likely contributed to the lower oxidative damage observed as compared to human leukocytes. Previous reports suggest that H. sapiens are highly sensitive endotoxin models, compared to other mammals (Wolff, 1973; Guha and Mackman, 2001; Shiraishi et al., 2002; Copeland et al., 2005; de Vos et al., 2009). Marine mammals experience drastic fluctuations in O2 availability during the dive response. These species generally have greater concentrations of O2-binding proteins (hemoglobin, myoglobin) and greater antioxidant defenses in comparison with terrestrial mammals (Kooyman and Ponganis, 1998; Wilhelm Filho et al., 2002; Ponganis et al., 2003). Wilhelm Filho et al. (2002) reported that under physiological conditions, in the erythrocytes of various marine mammal species, GPx, CAT, GST, and GR activities were significantly greater, as compared to the erythrocytes of various terrestrial mammal species. Vázquez-Medina et al. (2007) reported significantly greater GR activity and GSH concentrations in the tissues of ringed seals (Phoca hispida) in comparison with the tissues of domestic pigs (Sus scrofa domesticus), indicating a greater capacity to recycle GSH in marine mammals. Both GPx and CAT act to reduce H2O2 produced during phagocytosis (Fridovich and Freeman, 1986). CAT primarily acts during periods of escalated H2O2 production, such as during inflammation (Fridovich and Freeman, 1986; Burdon, 1995). GR catalyzes the formation of GSH from GSSG (Sies, 1997), while GST acts to eliminate reactive electrophiles, such as carbonyl groups, through their conjugation with GSH (Sies, 1997). Based on the results obtained in this study, it is likely that bottlenose dolphin leukocytes are better equipped at clearing H2O2 during a pro-inflammatory response, have a greater capacity to eliminate reactive groups, and demonstrate a greater capacity to recycle GSH, in comparison with the leukocytes of humans.
Leukocytes and other immune cells incorporate free unsaturated fatty acids into their cellular membranes, increasing their susceptibility to oxidative damage (Rustan and Drevon, 2005; Poggi et al., 2015). Aldehyde formation following lipid peroxidation can damage membrane-bound proteins, leading to the loss of membrane integrity, structure, and function (Birben et al., 2012). In this study, following 24 and 48 h under both control and experimental conditions, greater protein carbonyl concentrations were observed in human leukocytes compared to bottlenose dolphin leukocytes. These results were similar to those reported by Del-Águila-Vargas et al. (2019) in that the skeletal muscle cells from northern elephant seals (Mirounga angustirostris) incubated under control conditions demonstrated lower (non-significant) protein carbonyl concentrations compared to the muscles of humans.
In summary, the results from this study suggest that in response to LPS and compared to human leukocytes, bottlenose dolphin leukocytes may increase their O2 metabolism to a higher degree as a means to quickly and efficiently terminate the pro-inflammatory response, resulting in increased ROS production. Greater antioxidant defenses were found in bottlenose dolphin leukocytes compared to human leukocytes. This likely contributed to the reduced oxidative damage observed in bottlenose dolphin leukocytes. Host immune responses can vary between individuals, between species in relation to habitat, diet, metabolic rate, sex, age, and in relation to the presenting pathogen, dose, and administration method (Lehmann et al., 1987; Shiraishi et al., 2002; Copeland et al., 2005; Miller et al., 2005; Venn-Watson et al., 2011; Kurhaluk et al., 2018). Thus, discrepancies with other reports likely relate to the tissue analyzed, the phagocytic capacity of each leukocyte group, and species-specific evolutionary histories. How diet, sex, age, and habitat affect the indicators of oxidative stress during a pro-inflammatory response in bottlenose dolphins leukocytes are not known. Further studies should aim to compare immune sensitivity to LPS and the production of immune factors, such as cytokines, between marine and terrestrial mammals. Studies addressing the effects of pro-inflammatory factors in other marine mammal species, including comparisons between cetaceans and pinnipeds, are needed.
Our knowledge of the immune response in marine mammals remains fragmentary. In this study, exposure to LPS induced an immune response in the leukocytes of both H. sapiens and T. truncatus gilli. Following 48 h under experimental conditions, greater ROS production and lower oxidative damage were observed in bottlenose dolphin leukocytes, likely as a result of greater antioxidant defenses compared to human leukocytes. These results provide support to the hypothesis that greater antioxidant defenses in the tissues of marine mammals provide additional protection against situations that induce oxidative stress, such as inflammation, compared to the tissues of terrestrial mammals. The results of this study suggest that the immune system of marine mammals is better equipped for the efficient termination of pro-inflammatory responses, likely as an evolutionary advantage, in comparison with their terrestrial counterparts.
Acknowledgement: The authors wish to thank O. Lugo Lugo, P. Hernández Almaraz, and C. Reyes Ramos for their assistance in the laboratory. The authors thank the management, training, and veterinary teams (especially L. Ibarra Vargas, M. C. Lopez, M. F. Ramírez, and R. Santos Camacho) from Cabo Dolphins for generously providing blood samples (Tursiops truncatus gilli) for this study. Finally, the authors thank the staff from IMSS for the generous donation of residual blood (Homo sapiens) for this investigation.
Availability of Data and Materials: Requests for the datasets analyzed during the current study can be requested from the corresponding author.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: T. Zenteno-Savín; data collection: T. E. Symon; analysis and interpretation of results: T. E. Symon, R. Gaxiola-Robles, T. Zenteno-Savín; draft manuscript and preparation: T. E. Symon, T. Zenteno-Savín, R Gaxiola-Robles, C. J. Hernández-Camacho. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: All blood samples were collected with permission from the Comisión Nacional de Bioética (CONBIOÉTICA-09-CEI-009-20160601; registration No. 2018-785-010). Bottlenose dolphin blood samples were obtained with informed consent from Cabo Dolphins.
Funding Statement: Funding for this project was received from CONACyT [Project CB-2016-01-283669] and CIBNOR (Línea Estratégica II. Estrés Oxidativo). TES received a CONACYT graduate studies scholarship (CVU No. 922584).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Aebi H (1984). Catalase in vitro. In: Packer (ed.Methods in Enzymology, vol. 105, pp. 121–126. San Diego: Academic Press. [Google Scholar]
Anderson DR (1966). Ultrastructure of normal and leukemic leukocytes in human peripheral blood. Journal of Ultrastructure Research 1: 5–42. [Google Scholar]
Babior BM (1999). NADPH oxidase: an update. Blood 93: 1464–1476. [Google Scholar]
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012). Oxidative stress and antioxidant defense. WAO Journal 5: 9–19. [Google Scholar]
Bossart GD, Romano TA, Peden-Adams MM, Schaefer AM, Rice CD, Fair PA, Reif JS (2019). Comparative innate and adaptive immune responses in Atlantic bottlenose dolphins (Tursiops truncatus) with viral, bacterial, and fungal infections. Frontiers in Immunology 10: 1125. [Google Scholar]
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254. [Google Scholar]
Burdon RH (1995). Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radical Biology and Medicine 18: 775–794. [Google Scholar]
Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A (1992). Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80: 2012–2020. [Google Scholar]
Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D, the Inflammation and the Host Response to Injury Investigators (2005). Acute inflammatory response to endotoxin in mice and humans. Clinical and Diagnostic Laboratory Immunology 12: 60–67. [Google Scholar]
Corredor RC (1994). Separación rápida de leucocitos de sangre periférica. Revista de la Facultad de Medicina 42: 5–8. [Google Scholar]
Dancey JT, Deubelbeiss KA, Harker LA, Finch CA (1976). Neutrophil kinetics in man. Journal of Clinical Investigation 58: 705–715. [Google Scholar]
Davies KJA (2000). Oxidative stress, antioxidant defenses, and damage removal, repair and replacement systems. Life 50: 279–289. [Google Scholar]
Davis AK, Maney DL, Maerz JC (2008). The use of leukocyte profiles to measure stress in vertebrates: a review for Ecologists. Functional Ecology 22: 760–772. [Google Scholar]
de Guise S, Flipo D, Boehm JR, Martineau D, Béland P, Fournier M (1995). Immune functions in beluga whales (Delphinapterus leucasEvaluation of phagocytosis and respiratory burst with peripheral blood leukocytes using flow cytometry. Veterinary Immunology and Immunopathology 47: 351–362. [Google Scholar]
de Vos AF, Pater JM, van den Pangaart PS, de Kruif MD, van’t Veer C, van der Poll T (2009). In vivo lipopolysaccharide exposure of human blood leukocytes induces cross-tolerance to multiple TLR ligands. Journal of Immunology 183: 533–542. [Google Scholar]
Del-Águila-Vargas AC, Vázquez-Medina JP, Crocker DE, Méndez-Rodriguez LC, Gaxiola-Robles R, de Anda-Montañez JA, Ramirez-Jirano LJ, Lugo-Lugo O, Zenteno-Savín T (2019). Antioxidant response to cadmium exposure in the primary skeletal muscle cells isolated from humans and elephant seals. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology 227: 108641. [Google Scholar]
Drossos G, Lazou A, Panagopoulos P, Westaby S (1995). Deferoxamine cardioplegia reduces superoxide radical production in human myocardium. Annals of Thoracic Surgery 59: 169–172. [Google Scholar]
Elsner R, Hammond DD, Parker HR (1969). Circulatory responses to asphyxia in pregnant and fetal animals: a comparative study of Weddell seals and sheep. Yale Journal of Biology and Medicine 42: 202–217. [Google Scholar]
Engelhardt FR (1979). Haematology and plasma chemistry of captive pinnipeds and cetaceans. Aquatic Mammals 7: 11–20. [Google Scholar]
Flohé L, Günzler WA (1984). Assays for glutathione peroxidase. In: Packer (ed.Methods in Enzymology: Oxygen Radicals in Biological Systems, vol. 105, pp. 114–120. San Diego: Academic Press. [Google Scholar]
Francischetti I, Moreno JB, Scholz M, Yoshida WB (2010). Leukocytes and the inflammatory response in ischemia-reperfusion injury. Brazilian Journal of Cardiovascular Surgery 25: 575–584. [Google Scholar]
Fridovich I, Freeman B (1986). Antioxidant defenses in the lung. Annual Review of Physiology 48: 693–702. [Google Scholar]
Garson GD (2012). Testing Statistical Assumptions. Asheboro: Statistical Associates Publishing. [Google Scholar]
Geering B, Stoeckle C, Conus S, Simon H (2013). Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends in Immunology 34: 398–409. [Google Scholar]
Goldberg DM, Spooner RJ (1987). Glutathione reductase. In: Bermeyer-Ulrich (ed.Methods of Enzymatic Analysis, pp. 258–265, Germany: Verlog Chemie. [Google Scholar]
Granger DN, Kvietys PR (2015). Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biology 6: 524–551. [Google Scholar]
Guha M, Mackman N (2001). LPS induction of gene expression in human monocytes. Cellular Signalling 13: 85–94. [Google Scholar]
Habig WH, Jakoby HB (1981). Glutathione S-transferases (Rat and human). In: Jakoby (ed.Methods in Enzymology, vol. 77, pp. 218–235. San Diego: Academic Press. [Google Scholar]
Halliwell B, Gutteridge JMC (1990). Role of free radicals and catalytic metal ions in human disease: an overview. Methods in Enzymology 186: 1–85. [Google Scholar]
Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, Hoh R, Neese R, Macallan D, Deeks S, McCune JM (1999). Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nature Medicine 5: 83–89. [Google Scholar]
Hussain T, Tan B, Yin Y, Blachier F, Tossou MCB, Rahu N (2016). Oxidative stress and inflammation; what polyphenols can do for us? Oxidative Medicine and Cellular Longevity 2016: 7432797. [Google Scholar]
Keogh MJ, Spoon T, Ridgway SH, Jensen E, van Bonn W, Romano TA (2011). Simultaneous measurement of phagocytosis and respiratory burst of leukocytes in whole blood from bottlenose dolphins (Tursiops truncatus) utilizing flow cytometry. Veterinary Immunology and Immunopathology 144: 468–475. [Google Scholar]
Kevin LG, Novalija E, Stowe DF (2005). Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesthesia and Analgesia 101: 1275–1287. [Google Scholar]
Kobayashi SD, Malachowa N, DeLeo FR (2017). Influence of microbes on neutrophil life and death. Frontiers in Cellular and Infection Microbiology 7: 159. [Google Scholar]
Kooyman GL, Ponganis PJ (1998). The physiological basis of diving to depth: birds and mammals. Annual Review of Physiology 60: 19–32. [Google Scholar]
Kurhaluk N, Szarmach A, Zaitseva OV, Sliuta A, Kyriienko S, Winklewski PJ (2018). Effects of melatonin on low-dose lipopolysaccharide-induced oxidative stress in mouse, liver, muscle, and kidney. Canadian Journal of Physiology and Pharmacology 96: 1153–1160. [Google Scholar]
Lee A, Whyte MKB, Haslett C (1993). Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. Journal of Leukocyte Biology 54: 282–288. [Google Scholar]
Lehmann V, Freudenberg MA, Galanos C (1987). Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. Journal of Experimental Medicine 165: 657–663. [Google Scholar]
Levin M, Morsey B, de Guise S (2007). Non-coplanar PCBs induce calcium mobilization in bottlenose dolphin and beluga whale, but not in mouse leukocytes. Journal of Toxicology and Environmental Health, Part A: Current Issues 70: 1220–1231. [Google Scholar]
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A, Ahn B, Shaltiel S, Stadtman ER (1990). Determination of carbonyl content in oxidatively modified proteins. Methods in Enzymology 186: 464–478. [Google Scholar]
Marikovsky M, Ziv V, Nevo N, Harris-Cerruti C, Mahler O (2003). Cu/Zn superoxide dismutase plays important role in immune response. Journal of Immunology 170: 2993–3001. [Google Scholar]
Markert M, Andrews PC, Babior BM (1984). Measurement of O2− production by human neutrophils. In: Packer (ed.Methods in Enzymology, Oxygen Radicals in Biological Systems, vol. 105, pp. 358–365. Orlando: Academic Press. [Google Scholar]
Medway W, Geraci JR (1964). Hematology of the bottlenose dolphin (Tursiops truncatus). American Journal of Physiology 207: 1367–1370. [Google Scholar]
Miller SI, Ernst RK, Bader MW (2005). LPS, TLR4 and infectious disease diversity. Nature Reviews Microbiology 3: 36–46. [Google Scholar]
Nouri-Shirazi M, Bible BF, Zeng M, Tamjidi S, Bossart GD (2017). Phenotyping and comparing the immune cell populations of free-ranging Atlantic bottlenose dolphins (Tursiops truncatus) and dolphins under human care. BMC Veterinary Research 13: 78–91. [Google Scholar]
Ohishi K, Shishido R, Iwata Y, Saitoh M, Takenaka R, Ohtsu D, Okutsu K, Maruyama T (2011). Lipopolysaccharide-induced innate immune factors in the bottlenose dolphin (Tursiops truncatus) detected in expression sequence tag analysis. Microbiology and Immunology 55: 790–797. [Google Scholar]
Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JAM, Tesselaar K, Koenderman L (2010). In vivo labeling with H2O2 reveals a human neutrophil lifespan of 5.4 days. Blood 116: 625–627. [Google Scholar]
Poggi P, Mirabella R, Neri S, Assirelli E, Dolzani P, Mariani E, Calder PC, Chatgilialoglu A (2015). Membrane fatty acid heterogeneity of leukocyte classes is altered during in vitro cultivation but can be restored with ad-hoc lipid supplementation. Lipids in Health and Disease 14: 165–178. [Google Scholar]
Ponganis PJ, Kooyman GL, Ridgway SH (2003). Comparative diving physiology. In: Brubakk, Neuman (eds.Bennett and Elliott’s Physiology and Medicine of Diving, vol. 5, pp. 211–226. New York: Saunders. [Google Scholar]
Repine JE, Bast A, Lankhorst I, The Oxidative Stress Study Group (1997). Oxidative stress in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 156: 341–357. [Google Scholar]
Ridgway SH (1986). Diving by cetaceans. Royal Norwegian Society of Science and Letters 1: 33–62. [Google Scholar]
Ridgway SH, Simpson JG, Patton GS, Gilmartin WG (1970). Hematologic findings in certain small cetaceans. Journal of the American Veterinary Medical Association 157: 566–575. [Google Scholar]
Ruiz CL, Nollens HH, Venn-Watson S, Green LG, Wells RS, Walsh MT, Nolan EC, McBain JF, Jacobson ER (2009). Baseline circulating immunoglobulin G levels in managed collection and free-ranging bottlenose dolphins (Tursiops truncatus). Developmental and Comparative Immunology 33: 449–455. [Google Scholar]
Rustan AC, Drevon CA (2005). Fatty acids: structures and properties. Encyclopedia of Life Sciences 1: 1–7. [Google Scholar]
Shirai K, Sakai T (1997). Haematological findings in captive dolphins and whales. Australian Veterinary Journal 175: 512–514. [Google Scholar]
Shiraishi R, Itou T, Sugisawa H, Shoji Y, Endo T, Sakai T (2002). The respiratory burst activity of bottlenose dolphin neutrophils elicited by several stimulants. Journal of Veterinary Medical Science 64: 711–714. [Google Scholar]
Sies H (1997). Oxidative stress: oxidants and antioxidants. Experimental Physiology 82: 291–295. [Google Scholar]
Sokolova OV (2005). Peculiarities of phagocytosis in bottlenose dolphin (Tursiops truncatus) during the period of adaptation to the captivity conditions. Doklady Biological Sciences 403: 263–266. [Google Scholar]
Stone KD, Prussin C, Metcalfe DD (2010). IgE, mast cells, basophils, and eosinophils. Journal of Allergy and Clinical Immunology 125: S73–80. [Google Scholar]
Suzuki K (2000). Measurement of Mn-SOD and Cu, Zn-SOD. In: Taniguchi, Gutteridge (eds.Experimental Protocols for Reactive Oxygen and Nitrogen Species, vol. 1, pp. 91–95. Oxford: Oxford University Press. [Google Scholar]
Ulevitch RJ, Tobias PS (1999). Recognition of gram-negative bacteria and endotoxin by the innate immune system. Immunology 11: 19–22. [Google Scholar]
Vázquez-Medina JP, Zenteno-Savín T, Elsner R (2006). Antioxidant enzymes in ringed seal tissues: potential protection against dive-associated ischemia/reperfusion. Comparative Biochemistry and Physiology Part C 142: 198–204. [Google Scholar]
Vázquez-Medina JP, Zenteno-Savín T, Elsner R (2007). Glutathione protection against dive-associated ischemia/reperfusion in ringed seal tissues. Journal of Experimental Marine Biology and Ecology 345: 110–118. [Google Scholar]
Venn-Watson S, Smith CR, Gomez F, Jensen ED (2011). Physiology of aging among healthy, older bottlenose dolphins (Tursiops truncatuscomparisons with aging humans. Journal of Comparative Physiology B 181: 667–680. [Google Scholar]
Walsh CJ, Butawan M, Yordy J, Ball R, Flewelling L, de Wit M, Bonde RK (2015). Sublethal red tide toxin exposure in free-ranging manatees (Trichechus manatus) affects the immune system through reduced lymphocyte proliferation responses, inflammation, and oxidative stress. Aquatic Toxicology 161: 73–84. [Google Scholar]
Whitelaw DM (1972). Observations on human monocyte kinetics after pulse labeling. Cell and Tissue Kinetics 5: 311–317. [Google Scholar]
Wilhelm Filho D, Sell F, Ribeiro L, Ghislandi M, Carrasquedo F, Fraga CG, Wallauer JP, Simões-Lopes PC, Uhart MM (2002). Comparison between the antioxidant status of terrestrial and diving mammals. Comparative Biochemistry and Physiology Part A 133: 885–892. [Google Scholar]
Wolff SM (1973). Biological effects of bacterial endotoxins in man. Journal of Infectious Diseases 128: 259–264. [Google Scholar]
Zar JH (1999). Biostatistical Analysis, vol. 4, pp. 1–663. Upper Saddle River: Prentice Hall. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |