DOI:10.32604/biocell.2021.015060

| BIOCELL DOI:10.32604/biocell.2021.015060 |  |
| Article |
Basal cell carcinoma stem cells exhibit osteogenic and chondrogenic differentiation potential
1Department of Human Genetics, School of Dental Medicine, University of Belgrade, Belgrade, 11000, Serbia
2Clinic for Maxillofacial Surgery, School of Dental Medicine, University of Belgrade, Belgrade, 11000, Serbia
3Department of Public Health, School of Dental Medicine, University of Belgrade, Belgrade, 11000, Serbia
*Address correspondence to: Jelena Milasin, jelena.milasin@stomf.bg.ac.rs
Received: 19 November 2020; Accepted: 11 May 2021
Abstract: Specific cell subpopulations identified as cancer stem cells (CSCs) can be found in basal cell carcinoma (BCC). Generally, CSCs have a marked trans-differentiation potential that could potentially be used in differentiation therapies. However, there are no studies regarding BCC CSCs multipotency. The aim of the study was to analyze the characteristic of CSCs of BCC with emphasis on their differentiation potential upon specific induction. Specific staining and cell morphology were used for differentiation confirmation, along with the expression analysis of osteogenic (ALP, BSP, Runx2, OCN, BMP2), chondrogenic (COL1 and COL2A1), adipogenic (PPAR-γ) and neurogenic (Nestin and MAP2) markers. BCC CSCs differentiated into osteogenic and chondrogenic lineages, as judged by staining and high expression of specific markers (from 2-to 92-fold higher upon induction). Concomitantly with differentiation, the levels of cancer stem cell markers decreased in the cultures. Adipo-differentiation and neuro-differentiation were unsuccessful. In conclusion, BCC CSCs exhibit the capacity to trans-differentiate, a characteristic that may potentially be useful in the development of new strategies for the treatment of aggressive BCCs.
Keywords: Basal cell carcinoma; Cancer stem cells; Differentiation potential; Osteo-differentiation; Chondro-differentiation
The renewal of the entire epidermis under normal conditions has been attributed to a single type of self-renewing progenitor cell (Clayton et al., 2007), and it is known that disruption of its renewal mechanisms can lead to various skin diseases, including cancer (Colmont et al., 2012). Basal cell carcinoma (BCC), a slowly progressive skin cancer with propensity to be locally destructive, accounts for almost 80% of all non-melanoma skin cancers worldwide (Alter et al., 2015; Raasch et al., 2006). Early treatment of BCC is curative in the vast majority of cases; after 5-year follow up, the total recurrence rate is around 4–5% (Kyrgidis et al., 2010). Based on recurrence after treatment, BCC can be classified as low or high risk. For most patients with high-risk lesions surgery and radiotherapy are the treatment of choice (Bath-Hextall et al., 2004).
Studies show that cells at the origin of BCC are long-term resident progenitor cells from the hair follicle, interfollicular epidermis and the upper infundibulum of the skin (Peterson et al., 2015). When the normal stem cells are hit by oncogenic mutations at an immature or less-differentiated stadium, they could transform into cancer cells (Sancho et al., 2004).
Cancer stem cells (CSCs) exhibit characteristics similar to those of normal stem cells, i.e., they have high proliferative and self-renewing capacity. That specific cell subpopulation, responsible for tumor initiation, development, progression, metastasis and recurrence, has been described in many solid tumors such as breast (Al-Hajj et al., 2003), pancreas (Bailey et al., 2014; Mohammed et al., 2013), brain (Singh et al., 2004), colon cancer (O’Brien et al., 2007; Ricci-Vitiani et al., 2007) and also BCC (Gailani and Bale, 1997; Milosevic et al., 2018). Studies suggest that CSCs have also a marked trans-differentiation potential and can differentiate into multiple cell types (Reya et al., 2001; Singh et al., 2004). Although this feature could potentially be exploited for novel therapeutic modalities in the treatment of aggressive BCC, there are no studies confirming the multipotency of BCC stem cells.
In a previous study of BCC by our group, a thorough characterization of BCC CSCs was performed and a high expression of embryonic (Oct4, Sox2 and Nanog) and tumor (CD44 and CD73) stem cell markers were showed. Also, the propensity to form tumorispheres–A feature of CSCs, which increased during passages, was demonstrated along with their migration and clonogenic capacity and resistance to anti-neoplastic agents (Milosevic et al., 2018). However, no differentiation experiments have been carried out at that time. Hence, the aims of the present study were (a) to analyze BCCs cells in terms of their multipotency, i.e., capacity of osteogenic, chondrogenic, adipogenic and neurogenic differentiation, upon induction; and (b) to analyze the status of Sonic Hedgehog (SHH) signaling, a master regulator of embryonic cell differentiation and crucial factor in the pathogenesis of BCC.
Five tumor specimens and their corresponding distant resection margins (>5 mm from the edge of the tumor), used as controls, were obtained from patients treated at the Clinic for Maxillofacial Surgery of the School of Dental Medicine, University of Belgrade. All samples were examined by a pathologist and the diagnosis of BCC was confirmed as well as the status of the margins (labeled as “clear”). The age of the patients was 73 ± 17 years, three were males and two were females. The localization of BCCs was as follows: two in the temporal region, two on the nose and one was periauricular. Histologically, three were nodular, one was superficial and one infiltrative, with average size 18 ± 1.2 mm. Patients were informed of the study and signed informed consent. The study was approved by the institutional Ethical Committee (No. 36/30) University of Belgrade, Republic of Serbia, in accordance with the Declaration of Helsinki. All the experiments were performed in accordance with relevant guidelines and regulations.
Tissue samples from tumors and healthy distant margins used as controls were cut with blades into small pieces and seeded onto T75 cell culture flasks in complete medium (DMEM supplemented with 10% FBS and 100 U/mL penicillin-streptomycin solution), as previously described (Milosevic et al., 2018). In brief, cells were cultivated under standard conditions in humidified atmosphere with 5% CO2 at 37°C. Contaminating fibroblasts were removed using 0.125% trypsin, 0.02% edetic acid (Grando et al., 1996). The medium was changed every 2–3 days, and after cells reached 80% of confluence, passage was done, and 5 × 105 cells were plated for the next passage. The number and viability of cells were recorded using Countess™ Automated Cell Counter (Thermo Fisher Scientific, Waltham, USA).
The experiments on differentiation into four different lineages and relative gene expression analyses were done using the 5th passage tumor cells (TU P5). All experiments were done in triplicate, repeated two times. Distant margin cells were used as control.
Differentiation of primary BCC CSCs
Tumor and control (distant margin) cells of fifth passage were seeded onto 24-well culture plates (8 × 104 per well for osteogenesis and chondrogenesis and 1 × 105 cells per well for adipogenesis experiments) and cultured in complete medium (DMEM supplemented with 10% FBS and 100 U/mL penicillin-streptomycin solution). After reaching approximately 80% culture confluence (for adipogenic) or 100% (for osteogenic and chondrogenic differentiation) cells were cultivated in appropriate medium for 14 days (adipogenic differentiation) and 21 days (chondrogenic and osteogenic differentiation); commercially available osteogenic, chondrogenic, and adipogenic differentiation medium were used (Life Technologies, USA).
Separate cultures were prepared for cell staining and for RNA extraction. Cells were incubated under standard conditions and the medium was changed every third day. After being fixed in paraformaldehyde, cells were stained using Alizarin Red for osteogenic differentiation (Lin et al., 2001; Yunze et al., 2020), Safranin for chondrogenic (Engle et al., 2012), and Oil Red for adipogenic differentiation (Hausman, 1981). The quantification of staining was done as described previously (Carrino et al., 1991; Saeed et al., 2017; Sekiya et al., 2002). For neurodiferentiation, cells were seeded in 6 well plates at the density of 2 × 104 per well and incubated for 24 h under standard conditions. After that period medium was discarded and cells were incubated in neural preinduction medium- DMEM with 100 mM betamercaptoethanol which was, after 4 h of incubation, replaced with neural induction medium DMEM/F12 supplemented with 100 U/mL streptomycin and penicillin, 20 ng/mL human epidermal growth factor (EGF, Thermo Fisher Scientific, USA), 20 ng/mL basic fibroblast growth factor (bFGF Thermo Fisher Scientific, USA), 20 ng/mL neural growth factor (NGF, Thermo Fisher Scientific, USA) and 2% B27 (Thermo Fisher Scientific, USA). Medium was changed every 3–4 days. After 7 days the morphology of cells was analyzed and photographed under inverted microscope (BIB-100/T, BOECO, Germany).
In parallel, for all four experiments, tumor cells were cultured with standard growth medium (DMEM, 10% FBS and 100 U/mL penicillin-streptomycin solution), without the addition of any differentiation agent. The same procedure of staining and RNA isolation was carried out.
Total RNA was extracted from the cultured cells with TRIzol Reagent (Invitrogen, Thermo Fisher Scientific, Waltham, Massachusetts, USA) and first-strand cDNA was synthesized from 2 µg of total RNA using Oligo d (T) primer (Invitrogen, USA) and Revert aid (Thermo Fisher Scientific, Waltham, Massachusetts, USA).
Gene expression analysis of markers of differentiation
For PCR analysis, cDNA was amplified by Taq DNA polymerase. Subsequent Real-time PCR analysis was performed in Line Gene-K Fluorescence Real-time PCR Detection System (Bioer, China) using Maxima™ SYBR Green/ROX qPCR Master Mix (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The expression of markers of osteogenic (ALP, BSP, Runx2, OCN, BMP2), chondrogenic (COL1, COL2A1), adipogenic (PPAR-γ) and neurogenic (Nestin, MAP2) differentiation and tumor markers (CD44, CD73) were analyzed under the same conditions. The housekeeping gene GAPDH was used as reference. Fold-induction values were calculated using the 2−ΔCt method.
Gene expression analysis of CSC markers
After differentiation, in tumors and controls, the levels of major cancer stem cell markers CD44 and CD73 were analyzed by the same qPCR protocol as mentioned above. In order to check the status of Sonic Hedgehog (SHH) signaling cascade, mRNA expression of two members of this pathway (PTCH1 and GLI1 genes), was also examined, before and after induction of differentiation.
The sequences of all primers used in the experiments are given in Tab. 1.

Student’s t-test was performed in the study. Statistical significance was set at P < 0.05. Software package SPSS ver. 20 was used for the analyses (SPSS Inc., Chicago, USA).
One of the CSCs characteristics is their ability to undergo multilineage differentiation. Tumor and control cells were induced by appropriate osteo-, chondro-, adipo-, neuro-medium to differentiate into the four lineages. Osteogenic, chondrogenic and adipogenic induction were confirmed by specific staining, followed by products’ quantification, while neurogenic differentiation was assessed by morphological analysis, using light microscopy.
Osteocyte formation was confirmed by the detection of extracellular calcium deposits using Alizarin red staining (Fig. 1A). Chondrogenic differentiation, i.e., cartilage formation was detected by staining cell cultures with Safranin (Fig. 1D). Intracellular lipid vesicles that characterize adipocytes could not be detected by standard Oil Red O staining (Fig. 1G). Also, no characteristic morphological changes typical of neuro-differentiation could be observed in cell culture after 7 days of neuroinduction (Fig. 1J). Quantifications of osteogenic, chondrogenic and adipogenic staining are given in Figs. 1M–1O, respectively.
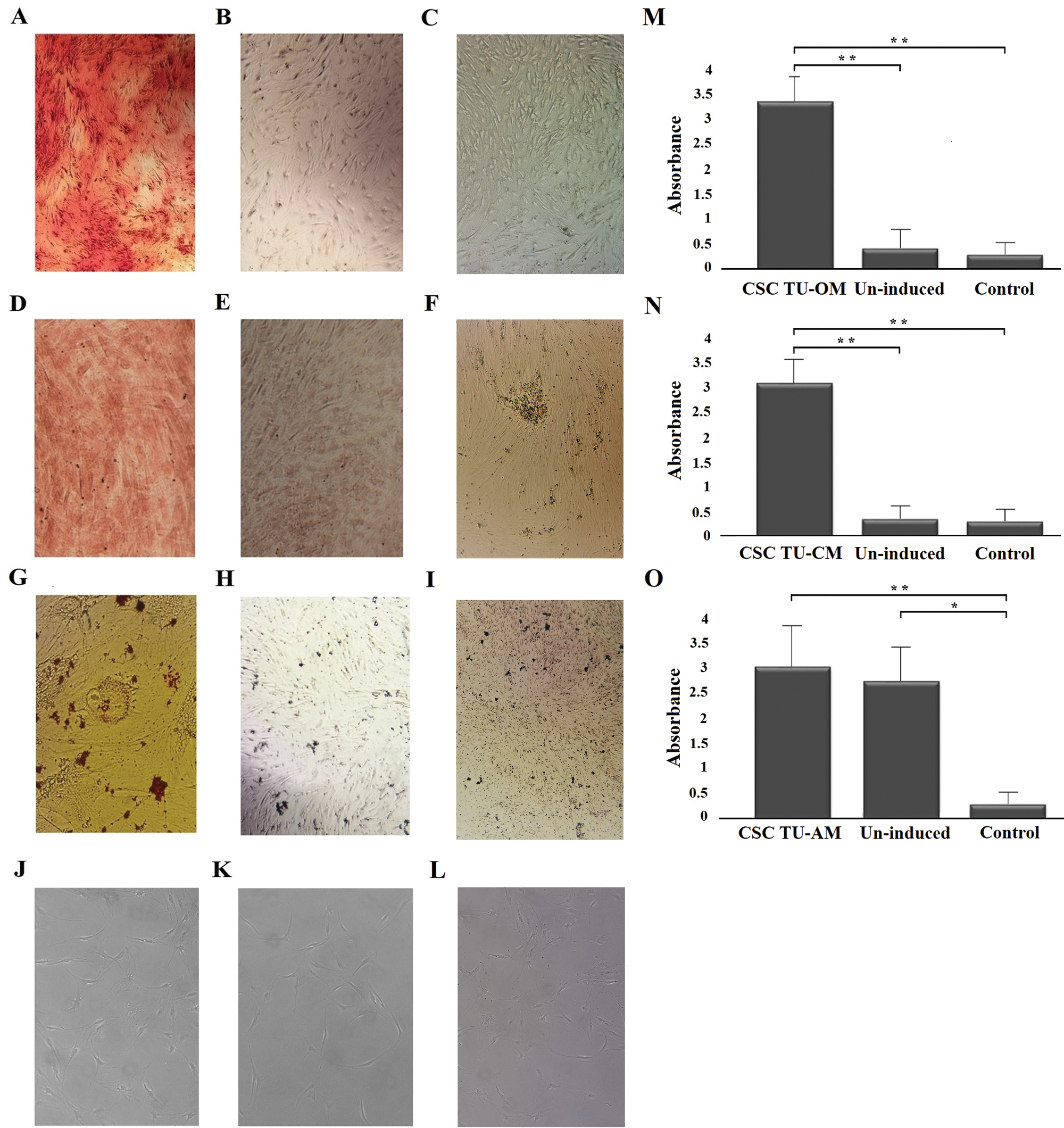
Figure 1: Osteogenic, chondrogenic, adipogenic and neurogenic differentiation potential of BCC CSCs. Induced (A) and un-induced (B) tumor cells and control cells (C) in successful osteogenic induction experiments; induced (D) and un-induced (E) tumor cells and control cells (F) in successful chondrogenic induction experiments; induced (G) an un-induced (H) tumor cells and control cells (I) in unsuccessful adipogenic induction experiments; induced (J), un-induced (K) and control cells (L) in unsuccessful neuro-induction experiments. Quantitatification of staining showed statistically significant differences between osteogenically (M) and chondrogenically (N) induced cells compared to un-induced and control cells. Quantification did not show differences between adipo-induced and un-induced tumor cells (O). Asterisks * and ** designate P-values lower than 0.05 and 0.01, respectively.
Gene expression of differentiation markers
Lineage specific gene expression was analyzed by qPCR. The following genes were used to demonstrate osteo-differentiation: ALP, BSP, Runx2, OCN and BMP2; chondro-differentiation: COL1 and COL2A1; adipo-differentiation: PPAR-γ; neuro-differentiation: Nestin and MAP2 (Fig. 2).
The expression of all genes related to osteogenesis was significantly higher in induced cells compared to un-induced and control cells and showed the following increase compared to un-induced cells: ALP 5.5-, BSP 92.0-, Runx2 6.7-, OCN 30-, and BMP2 3.3-fold (P < 0.05) (Figs. 2A–2E). Similarly, levels of COL1 and COL2A1 mRNA were higher in induced than in un-induced (2.0- and 10.0-fold, respectively) and control cells (Figs. 2G and 2H). However, PPAR-γ expression did not show a statistically significant difference between cells grown in adipogenic medium, un-induced and control cells (Fig. 2F). Similarly, after neuro-induction there was no statistically significant difference of expression of neural markers Nestin and MAP2 between induced, un-induced and control cells (Figs. 2I and 2J).
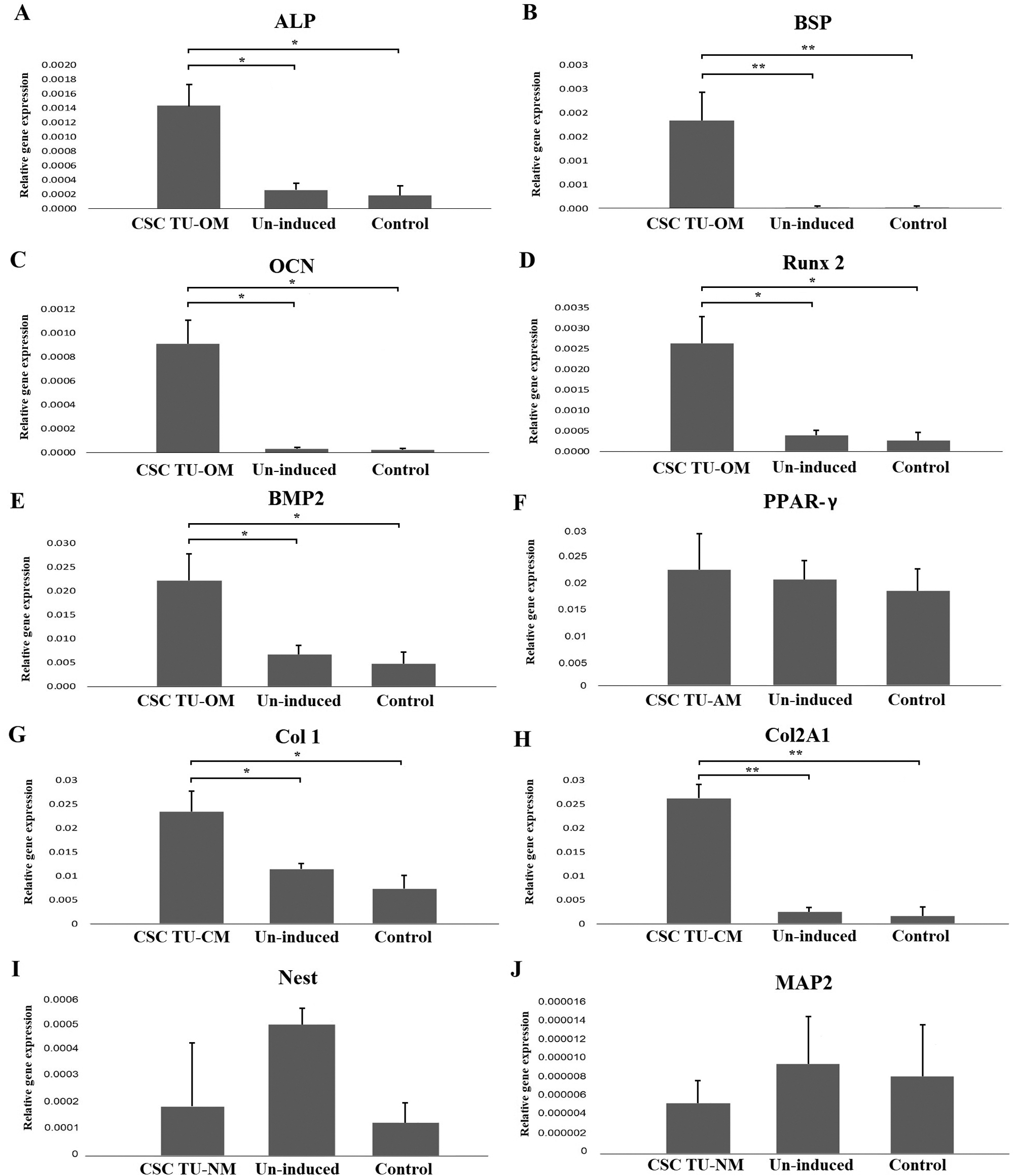
Figure 2: Gene expression analysis of differentiation markers. The mRNA levels of osteogenic markers ALP, BSP, OCN, Runx2 and BMP2 were significantly higher in induced tumor cells compared to un-induced and control cells (A, B, C, D, E); adipogenic marker PPARγ had similar expression levels in induced and un-induced tumor cells and control cells (F); the expression of COL1 and COL2A1 mRNAs was significantly higher in induced tumor cells than in un-induced and control cells (G, H); the neurogenic markers Nestin and MAP2 levels of expression (I, J) were lower in induced cells when compared to un-induced and control cells but without statistical difference. Error bars represent standard error calculated from experiments. Asterisks * and ** designate P-values lower than 0.05 and 0.01, respectively.
Gene expression of CSC markers
In order to verify whether differentiation led to the decrease of CSC markers, we selected one successfully (chondrogenic) and one unsuccessfully (adipogenic) induced cell lineage. It appeared that the differentiation into chondrogenic lineage was accompanied by the decrease of tumor markers expression. Namely, both CD44 and CD73 levels were significantly lower compared to un-induced cells (P < 0.05) and similar to the control. After unsuccessful adipo-induction the cells continued to express higher level of both markers (CD44 and CD73) than controls (P < 0.05) and when compared to un-induced cells, markers’ levels remained unchanged (Figs. 3A and 3B).
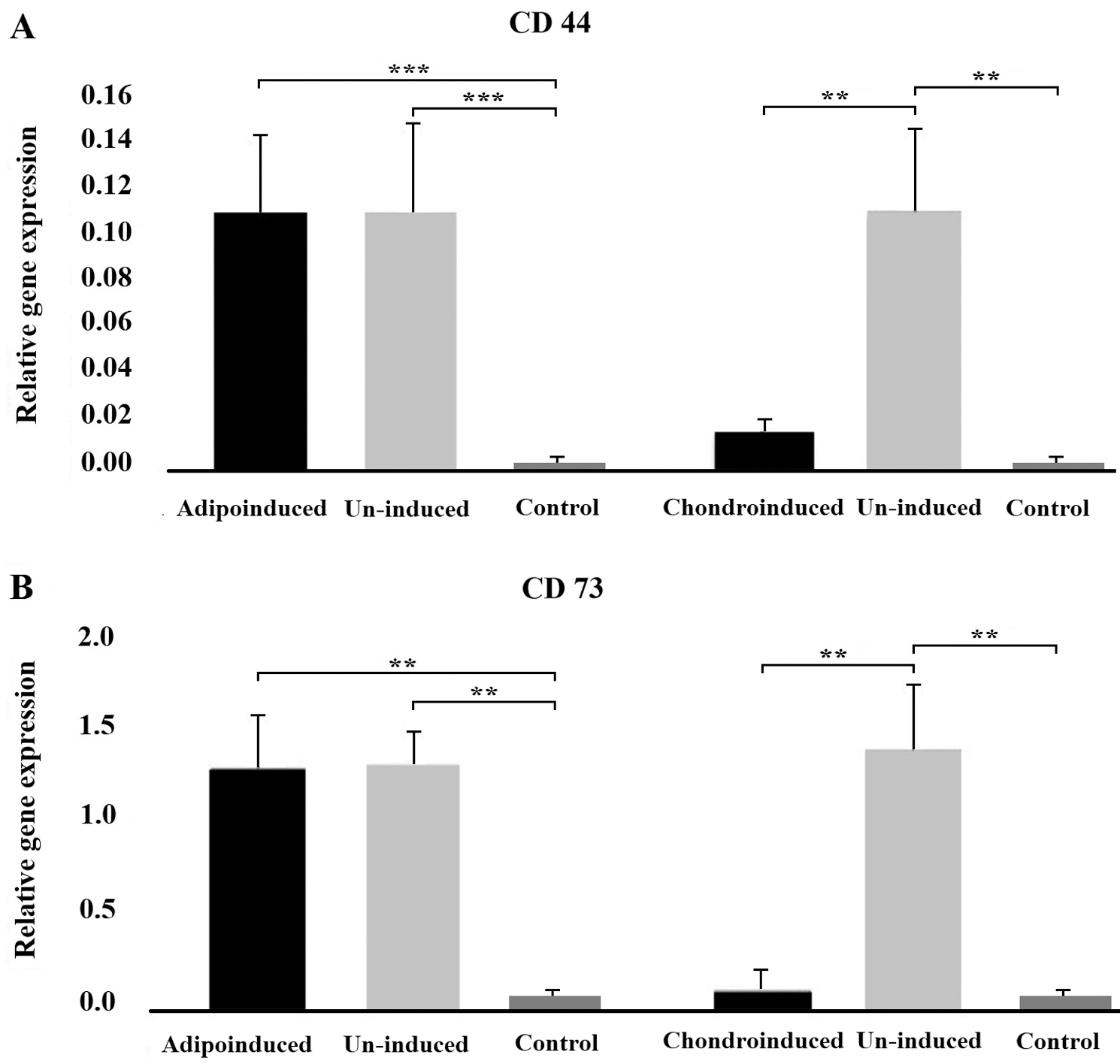
Figure 3: The effect of differentiation on tumor markers expression. The level of expression of CD44 (A) and CD73 (B) markers in tumor cells decreased after successful chondrogenic differentiation, unlike in cells exposed to adipogenic medium where there was no differentiation. Asterisks ** and *** designate P-values lower than 0.01 and 0.001, respectively.
Finally, given the importance of Sonic Hedgehog (SHH) signaling cascade in the pathogenesis of BCC, the mRNA expression of two members of this pathway (PTCH1 gene and GLI1 gene), was also examined in tumor cells of the fifth passage, before and after induction of differentiation as an additional test of phenotype reversion. The levels of PTCH1, lower in BCC cultures compared to control (P < 0.05), started to increase during transdiffentiation into chondrogenic lineage (P < 0.05). At the same time, levels of GLI1 which were higher before induction compared to control cells (P < 0.05), decreased during chondrogenic differentiation (P < 0.05), showing that normal SHH signaling was being restored (Fig. 4).
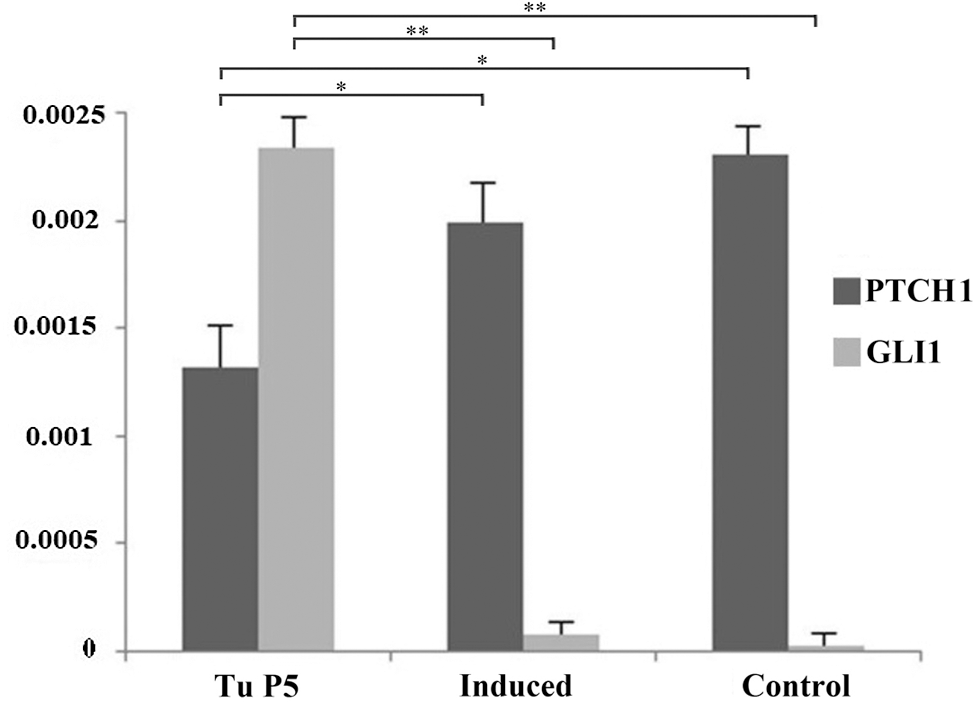
Figure 4: The effect of differentiation on Sonic hedgehog marker expression. The level of expression of PTCH1 in tumor cells increased while Gli1 marker decreased after successful chondrogenic differentiation. Asterisks * and ** designate P-values lower than 0.05 and 0.01, respectively.
The aggressive behavior of some cancers is attributed to the existence of specific cell subpopulations called cancer stem cells. Our previous investigation has already shown the existence of such a population in BCCs (Milosevic et al., 2018). Extensive proliferative and self-regeneration potential are fundamental characteristics of CSCs (Gailani and Bale, 1997). In vitro, CSCs grow faster than normal cells and have high colony formation ability (Faustino et al., 2013). As reported, CSCs markers such as Oct4, Sox2, CD44, with an essential role in stemness maintenance and cell migratory capacity, are expressed in BCC (Milosevic et al., 2018).
This is the very first study dealing with the differentiation capacity of BCCs stem cells, and, importantly, it shows that the process of differentiation was accompanied by substantial decrease of cancer stem cell markers and restoration of SHH signaling.
We demonstrated the potential of these cells to differentiate into osteogenic and chondrogenic but not adipogenic and neurogenic lineages. Zakaria et al. (2015) showed that lung CSCs were capable of differentiation into three lineages. Similar results were also obtained with ovarian CSCs while Ding et al. (2016) and Zhau et al. (2011) observed osteogenic and adipogenic differentiation of prostate cancer cells. The expression pattern of osteogenic markers (alkaline phosphatase, Runx 2 and osteocalcin) in this study was in agreement with the results of Zhang et al. (2013) although in their study protein expression, not mRNA, was analyzed. The expression pattern of type 1 (COL1) and type 2 collagen (COL2A1) was also in concordance with the reports of Ding et al. (2016) and Zhau et al. (2011) who demonstrated increased levels of COL1 in lung and ovarian cancer after CSC differentiation induction.
PPARγ, the master transcription regulator for adipogenic differentiation was similarly expressed in treated cells compared to un-treated and control cells, indicating the lack of adipo-differentiation potential of BCC CSCs. This in contrast with some other studies that showed the ability of ovarian and lung cancer stem cells, for instance, to differentiate into adipocytes (Ding et al., 2016; Zakaria et al., 2015). Lipid drops were also detected in prostate cancer stem cells after 7 days of induction (Ding et al., 2016). In our study, 21 days after seeding, the Oil Red color did not show the presence of lipid vesicles in the cells.
Negative results were also obtained when attempting to induce BCC CSCs into neuro-lineage. Namely, cells did not show typical neuron-like morphology with long, slender projection, nor did they express typical markers of neuro-differentiation (Nestin and MAP2). However, in the study of Zhang et al. (2013) neuro-differentiation could be observed in the case of small cell lung cancer stem cells induced with neurogenic medium.
As already stated, the process of differentiation was accompanied by the loss of cancer stemness characteristics. Namely, in the case of successful chondrogenic differentiation, two tested cancer stem cell markers, CD44 and CD73, considered as crucial CSC markers in several solid tumors (Spychala, 2000; Thapa and Wilson, 2016), showed markedly lower levels.
Bearing in mind the importance of SHH signaling cascade in the pathogenesis of BCC and its deregulation in this tumor, as an additional test of CSC phenotype reversion, mRNA levels of two members of this pathway (PTCH1 gene and GLI1 gene), were also examined in cells of the fifth passage, before and after induction of differentiation. The tumor suppressor gene PTCH1, normally downregulated in BCC cells, increased during trans-differentiation. At the same time GLI1, a transcription factor upregulated in BCC and related to poor prognosis in solid malignancies, decreased after differentiation, pointing to a possible mechanism of normalization of SHH signaling (Cheng et al., 2016).
A limitation of the present study is that the differentiation capacity was tested on the entire tumor population. In the future, experiments should be performed only after separating stem cells from non-stem cancer cells. However, the capacity of differentiation is a characteristic of stem cells, hence, most probably, the obtained results are basically due to changes occurring in BCC CSCs. In addition, it must be emphasized that in the course of cell passaging, cultures are enriched with CSCs. In our previous study we demonstrated that the proportion of CSCs (CD44 positive) increased from 5% in the first passage, to 26% in the fifth passage, making CSCs a substantial proportion of the whole culture (Milosevic et al., 2018).
Therapies based on the inhibition of various pathways involved in tumorigenesis are constantly emerging. However, these therapies are moderately efficient and are often accompanied with adverse effects (Bliss et al., 2018; Silapunt et al., 2016). Instead, future therapies should inhibit CSCs proliferation and induce terminal differentiation and/or apoptosis (Meng et al., 2012; Seigel, 2011). Differentiation therapy approach implies agents or methods that are able to induce terminal differentiation of CSCs (Sell, 2006). This therapeutic modality was successfully applied in chronic myeloid leukemia using imitinab, and acute promyelocytic leukemia using retinoids (Song et al., 2014). To date, no differentiation-inducing agents have been reported to have a comparable effect in solid tumors.
The present study suggests that cancer stem cells in primary BCC cultures exhibit the capacity to differentiate into osteoblast and chondroblast-like cells. Although BCC CSC capacity was limited, the finding may however be valuable for future strategies in the treatment of aggressive forms of this malignancy.
Availability of Data and Materials: Data and materials are available upon request to the corresponding author.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: M.M., M.L., J.M.; data collection: M.M., M.L., B.T., M.P., M.V., Z.J., B.A., D.J.; analysis and interpretation of results: M.M., M.L., B.T., S.J., J.M., D.J.; draft manuscript preparation: M.M., M.L., B.T., M.P., M.V., Z.J., B.A., D.J., S.J., and J.M. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (Grant No. 451-03-9/2021-14/200129).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America 100: 3983–3988. [Google Scholar]
Alter M, Hillen U, Leiter U, Sachse M, Gutzmer R (2015). Current diagnosis and treatment of basal cell carcinoma. Journal der Deutschen Dermatologischen Gesellschaft 13: 863–874. [Google Scholar]
Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY et al. (2014). DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology 146: 245–256. [Google Scholar]
Bath-Hextall F, Bong J, Perkins W, Williams H (2004). Interventions for basal cell carcinoma of the skin: Systematic review. British Medical Journal 329: 705–710. [Google Scholar]
Bliss SA, Paul S, Pobiarzyn PW, Ayer S, Sinha G et al. (2018). Evaluation of a developmental hierarchy for breast cancer cells to assess risk-based patient selection for targeted treatment. Scientific Reports 8: 367–381. [Google Scholar]
Carrino DA, Arias JL, Caplan AI (1991). A spectrophotometric modification of a sensitive densitometric Safranin O assay for glycosaminoglycans. Biochemistry International 24: 485–495. [Google Scholar]
Cheng J, Gao J, Tao K (2016). Prognostic role of Gli1 expression in solid malignancies: A meta-analysis. Scientific Reports 6: 22184. [Google Scholar]
Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH (2007). A single type of progenitor cell maintains normal epidermis. Nature 446: 185–189. [Google Scholar]
Colmont CS, Harding KG, Piguet V, Patel GK (2012). Human skin cancer stem cells: A tale of mice and men. Experimental Dermatology 21: 576–580. [Google Scholar]
Ding DC, Liu HW, Chu TY (2016). Interleukin-6 from ovarian mesenchymal stem cells promotes proliferation, sphere and colony formation and tumorigenesis of an ovarian cancer cell line SKOV3. Journal of Cancer 7: 1815–1823. [Google Scholar]
Engle KM, Mei TS, Wasa M, Yu JQ (2012). Weak coordination as a powerful means for developing broadly useful C-H functionalization reactions. Accounts of Chemical Research 45: 788–802. [Google Scholar]
Faustino RS, Arrell DK, Folmes CD, Terzic A, Perez-Terzic C (2013). Stem cell systems informatics for advanced clinical biodiagnostics: Tracing molecular signatures from bench to bedside. Croatian Medical Journal 54: 319–329. [Google Scholar]
Gailani M, Bale A (1997). Developmental genes and cancer: Role of patched in basal cell carcinoma of the skin. Journal of the National Cancer Institute 89: 1103–1109. [Google Scholar]
Grando S, Schofield O, Skubitz AP, Kist DA, Zelickson BD, Zachary CB (1996). Nodular basal cell carcinoma in vivo vs. in vitro. Establishment of pure cell cultures, cytomorphologic characteristics, ultrastructure, immunophenotype, biosynthetic activities, and generation of antisera. Archives of Dermatology 132: 1185–1193. [Google Scholar]
Hausman GJ (1981). Techniques for studying adipocytes. Stain Technology 56: 149. [Google Scholar]
Kyrgidis A, Vahtsevanos K, Tzellos TG, Xirou P, Kitikidou K et al. (2010). Clinical, histological and demographic predictors for recurrence and second primary tumours of head and neck basal cell carcinoma. A 1062 patient-cohort study from a tertiary cancer referral hospital. European Journal of Dermatology 20: 276–282. [Google Scholar]
Lin DL, Tarnowski CP, Zhang J, Dai J, Rohn E et al. (2001). Bone metastatic LNCaP-derivative C4-2B prostate cancer cell line mineralizes in vitro. Prostate 47: 212–221. [Google Scholar]
Meng M, Zhao XH, Ning Q, Hou L, Xin GH, Liu LF (2012). Tumor stem cells: A new approach for tumor therapy (Review). Oncology Letters 4: 187–193. [Google Scholar]
Milosevic M, Lazarevic M, Toljic B, Simonovic J, Trisic D et al. (2018). Characterization of stem-like cancer cells in basal cell carcinoma and its surgical margins. Experimental Dermatology 27: 1160–1165. [Google Scholar]
Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A et al. (2013). Antidiabetic drug metformin prevents progression of pancreatic cancer by targeting in part cancer stem cells and mTOR signaling. Translational Oncology 6: 649–659. [Google Scholar]
O’Brien CA, Pollett A, Gallinger S, Dick JE (2007). A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445: 106–110. [Google Scholar]
Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA et al. (2015). Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 16: 400–412. [Google Scholar]
Raasch BA, Buettner PG, Garbe C (2006). Basal cell carcinoma: Histological classification and body site distribution. British Journal of Dermatology 155: 401–407. [Google Scholar]
Reya T, Morrison SJ, Clarke MF, Weissman IL (2001). Stem cells, cancer, and cancer stem cells. Nature 414: 105–111. [Google Scholar]
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M et al. (2007). Identification and expansion of human colon-cancer-initiating cells. Nature 445: 111–115. [Google Scholar]
Saeed MA, El-Rahman MA, Helal ME, Zaher AR, Grawish ME (2017). Efficacy of human platelet rich fibrin exudate vs. fetal bovine serum on proliferation and differentiation of dental pulp stem cells. International Journal of Stem Cells 10: 38–47. [Google Scholar]
Sancho E, Batlle E, Clevers H (2004). Signaling pathways in intestinal development and cancer. Annual Review of Cell and Developmental Biology 20: 695–723. [Google Scholar]
Seigel GM (2011). Differentiation potential of human retinoblastoma cells. Current Pharmaceutical Biotechnology 12: 213–216. [Google Scholar]
Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG et al. (2002). Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 20: 530–541. [Google Scholar]
Sell S (2006). Cancer stem cells and differentiation therapy. Tumour Biology 27: 59–70. [Google Scholar]
Silapunt S, Chen L, Migden MR (2016). Hedgehog pathway inhibition in advanced basal cell carcinoma: latest evidence and clinical usefulness. Therapeutic Advances in Medical Oncology 8: 375–382. [Google Scholar]
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J et al. (2004). Identification of human brain tumour initiating cells. Nature 432: 396–401. [Google Scholar]
Song JH, Kim SH, Cho KM, Hwang SY, Kim HJ, Kim TS (2014). Analysis of gene profiles involved in the enhancement of all-trans retinoic acid-induced HL-60 cell differentiation by sesquiterpene lactones identifies asparagine synthetase as a novel target for differentiation-inducing therapy. International Journal of Oncology 44: 970–976. [Google Scholar]
Spychala J (2000). Tumor-promoting functions of adenosine. Pharmacology & Therapeutics 87: 161–173. [Google Scholar]
Thapa R, Wilson G (2016). The importance of CD44 as a stem cell biomarker and therapeutic target in cancer. Stem Cells International 2016: 2087204. [Google Scholar]
Yunze X, Bin J, Sayan D, Mengmeng L, Zaixian S et al. (2020). Enhanced osteogenic differentiation of human periodontal ligamentstem cells by suberoylanilide hydroxamic acid. Biocell 44: 389–400. [Google Scholar]
Zakaria N, Yusoff NM, Zakaria Z, Lim MN, Baharuddin PJ et al. (2015). Human non-small cell lung cancer expresses putative cancer stem cell markers and exhibits the transcriptomic profile of multipotent cells. BioMed Central Cancer 15: 84. [Google Scholar]
Zhang Z, Zhou Y, Qian H, Shao G, Lu X et al. (2013). Stemness and inducing differentiation of small cell lung cancer NCI-H446 cells. Cell Death & Disease 4: 633. [Google Scholar]
Zhau HE, He H, Wang CY, Zayzafoon M, Morrissey C et al. (2011). Human prostate cancer harbors the stem cell properties of bone marrow mesenchymal stem cells. Clinical Cancer Research 17: 2159–2169. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |