DOI:10.32604/biocell.2021.015752

| BIOCELL DOI:10.32604/biocell.2021.015752 |  |
| Article |
TaVNS reduces inflammatory responses in a L-NAME-induced rat model of pre-eclampsia
1Department of Obstetrics, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, 570100, China
2Department of General Surgery, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, 570100, China
3Department of ICU, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, 570100, China
*Address correspondence to: Lei Shi, 13198907396@163.com
Received: 28 March 2021; Accepted: 08 May 2021
Abstract: Pre-eclampsia is characterized by an excessive maternal inflammatory response. The cholinergic anti-inflammatory pathway (CAP) has been shown as the efferent arm of a vagal reflex with the potential to limit inflammatory responses. Therefore, in this study, the CAP regulation through the nervous vagal stimulation (VNS) reduced the severity of NG-nitro-L-arginine methyl ester (L-NAME)-induced pre-eclampsia was determined in a rat model. Rats were given 125 mg/kg/day of L-NAME via subcutaneous injection on gestational day (GD) 10–16. In addition, the rats were treated by active or sham electrical stimulation once a day during GD 13–19. Systolic blood pressure (SBP), urinary albumin, and pregnancy outcomes were documented for each rat. The average fetal weights and crown-rump length (CRL) as well as the placental weights of rats in both control and experimental groups were recorded onthe 13th day, 16th day and 20th day of gestation. Subsequently, placentas were collected from the rats on GD20 to measure the level of cytokines. In addition, qRT-PCR and Western blot analysis were used to detect the mRNA and protein expression of α7 nicotinic acetylcholine receptor (α7nAChR) and nuclear factor-κB (NF-κB), respectively. Immunohistochemistry assays were also carried out to determine the location and level of α7nAChR and NF-κB in placentas. CAP regulation through the transcutaneous auricular nerve stimulation alleviated the clinical symptoms in the rats after L-NAME induction, including hypertension, proteinuria, fetal growth retardation and fetal death. In addition, TaVNS also increased α7nAChR expression, reduced NF-κB p65 expression, and reversed L-NAME-induced proinflammatory cytokines in the placenta tissues, including tumor necrosis factor-alpha (TNF-α), high mobility group box 1 (HMGB-1) and interleukin-6 (IL-6). The findings of this study showed that TaVNS might be used as a promising tool to attenuate pre-eclampsia-like symptoms. In addition, the protective effect of TaVNS was associated with the improvement of α7nAChR expression and the inhibition of inflammatory reactions at the maternal-fetal interface through activating cholinergic anti-inflammation pathway.
Keywords: Pre-eclampsia; Cholinergic anti-inflammatory pathway; TaVNS; Inflammation
Pre-eclampsia is a disease during pregnancy and is featured by hypertension, proteinuria, maternal endothelial dysfunctions and chronic immune activation. As a type of pregnancy-related disorders, pre-eclampsia is the leading cause of neonatal and maternal morbidity and mortality worldwide, and can affect up to 5–7% of pregnancies (Henderson et al., 2017; Hutcheon et al., 2011; Say et al., 2014). Currently, there are no medical therapies available to halt the progression of this disease after its onset. Therefore, delivery remains the main treatment for pre-eclampsia, thus leading to an increased rate of iatrogenic preterm birth.
Pre-eclampsia can be diagnosed after 20 weeks of gestation characterized by systemic inflammation, endothelial dysfunction, and oxidative stress (Chaiworapongsa et al., 2014; Roberts et al., 2013). Although the definite pathophysiology of pre-eclampsia is still unknown, existing evidence suggests that the endothelial dysfunction may act as a leading cause of pre-eclampsia (Ahmed, 2011). Both, observational and experimental studies have revealed a correlation between inflammation and endothelial dysfunction (Mantovani and Dejana, 1989; Zimmerman et al., 1992). More evidence of excessive inflammation responses in pre-eclampsia has also been demonstrated by the uncontrolled elevation in the activation of the complement system during pre-eclampsia (Lynch et al., 2010).
The vagus nerve is a key component of the autonomic nervous system and plays a pivotal role in the modulation of inflammatory responses (Bonaz et al., 2016; Koopman et al., 2016). Tracey (2002) have demonstrated that vagus nerve stimulation (VNS) could inhibit immune activation and could successfully reduce the production of pro-inflammatory cytokines in a murine model of septic shock. In fact, through the activation of α7nAChR and the cholinergic anti-inflammatory pathway (CAP), the VNS inflammatory reflex is considered as a main neural control mechanism to prevent the production of proinflammatory mediators from innate immune cells (Borovikova et al., 2000). In a previous study, we reported that treatment with VNS significantly improved adverse pregnancy outcomes through anti-inflammatory pathway in L-NAME -induced PE model rats (Zheng et al., 2021). However, the clinical application of VNS is limited by its invasiveness. Recently, devices have been developed to noninvasively stimulate the vagus nerve. For example, functional magnetic resonance imaging of brain has demonstrated that the transcutaneous auricular VNS (TaVNS) could activate the same areas in the brain as those activated by surgically implanted VNS (Frangos et al., 2015).
It was demonstrated that TaVNS could evoke parasympathetic excitation and potentially reduce vagus-mediated symptoms, thus establishing a safer and more affordable treatment modality (Hsu et al., 2006; Wu et al., 2009). Zhao et al. (2012) have exhibited that Ta-VNS could be utilized to suppress inflammatory responses via α7nAChR-mediated CAP in an experimental model of sepsis. Aberrant α7nAChR and NF-κB protein expression in placenta and pre-eclampsia has been reported previously (Zheng et al., 2018). In the present study, it has been hypothesized that TaVNS might play a role in activating the vagus nerve-based CAP. In particular, the effect of TaVNS on the levels of NF-κB p65 and pro-inflammatory cytokines was explored in this study to clarify the role of TaVNS in the regulation of inflammatory diseases.
Female Sprague-Dawley rats at 8 weeks (220–240 g in body weight) were obtained from the SPF Animal Laboratory of Hainan, China. Rats were housed in a temperature-controlled room (23 ± 1°C) and kept under a 12:12-h light/dark cycle with food and water ad libitium. Female rats were mated with fertile male rats overnight. Subsequently, the female rats with timed pregnancy (the first day of positive vaginal smear results was considered as gestational day 0 (G0)) were used for the following experiments.
We randomly divide the pregnant rats into control (VC group, n = 21) and L-NAME groups (n = 63). The preeclamptic model was established as previously described (Tian et al., 2016; Zhu et al., 2017). In brief, 125 mg/kg/day of L-NAME(Sigma, St Louis, MO, USA) was used to cause pre-eclampsia-like symptoms in the pregnant rats by injecting subcutaneously from day 10 to day 16 of gestation. The rats were provided an equivalent volume of saline solution as in the control group. The L-NAME groups contained three sub-groups (n = 21 per group): (1) L-NAME group (LN), receiving no stimulation. (2) L-NAME+sham group (LN+sham), treated with sham stimulation from GD 13 to 19. (3) L-NAME+TaVNS group (LN+TaVNS), treated with TaVNS from GD 13 to 19.
After a low level of anesthesia achieved by inhalation of 3% isoflurane, electrodes were placed on the auricular conchae bilaterally. A daily transcutaneous electrical stimulation was applied via a stimulator (SEN-7203, Nihon Kohden) for 7 days at 8:00–10:00 h with the frequencies of 2/15 Hz switched every second and an intensity of 2 mA (Wang et al., 2015). In the sham group, similar procedures were adopted except that the power of the stimulator was turned off, thus no electrical stimulation was generated (Fig. 1).
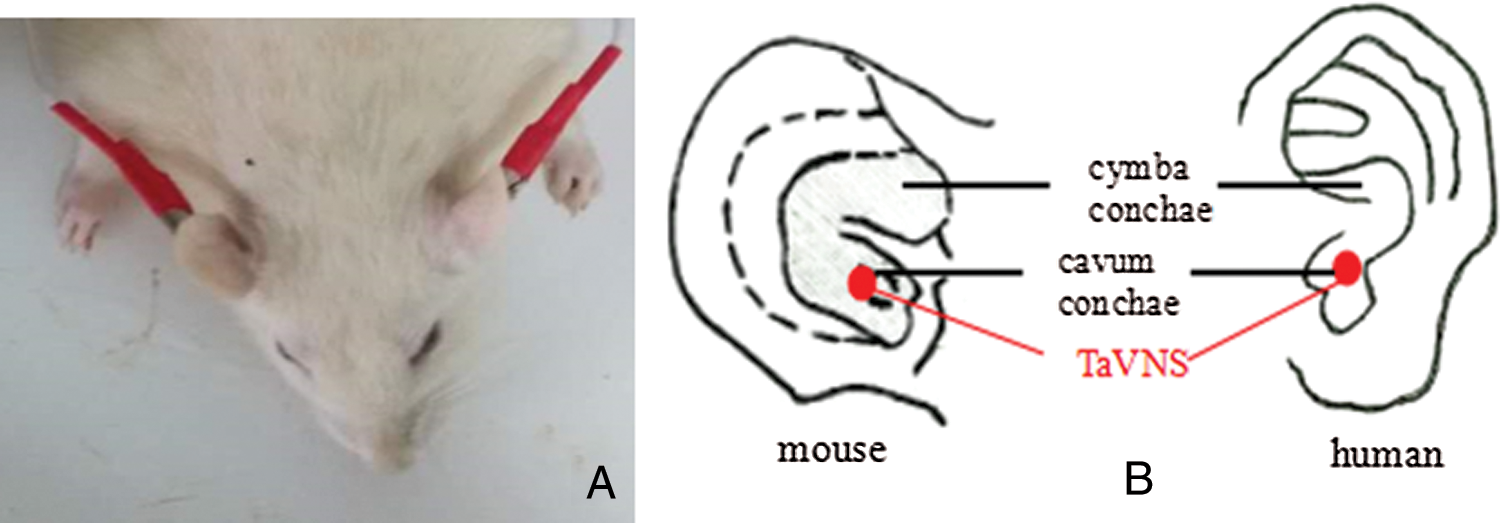
Figure 1: Transcutaneous auricular vagus nerve stimulation posture (A) and position (B) of the procedure.
Measurement of systolic blood pressure (SBP) and 24h-urinary protein
The SBP of the rats was measured using a noninvasive automatic blood pressure analyzer (Zhenghua Biological Instrument Inc., China) on the 10th, 13th, 16th, 18th and 20th day of GD as described previously (Agarwal et al., 2009). The measurement was repeated at least three times to record the mean value. As a key criterion in evaluating the successful establishment of the pre-eclampsia model, the SBP should be increased by at least 20 mmHg after L-NAME treatment.
The rats were placed in separate metabolic cages for 24 h to obtain their urine samples on the 10th, 13th, 16th, 18th, and 20th day of GD. Urinary levels of albumin were measured by an automatic analyzer (HIACHI 7600-020, Japan).
Measurement of biochemical parameters
The experimental animals were observed daily for mortality, morbidity, general appearance, and behavior. On the 13th, 16th, and 20th day of GD, 7 rats were collected from each of the four groups respectively and euthanized by cardiac puncture after being anesthetized by 3% isoflurane inhalation. All fetuses were assessed for appearance, body weight and size (crown-rump length). In addition, placenta weights were recorded in each group. On the 20th day of GD, the number of alive, dead, and resorbed fetuses was recorded. Some of placenta samples were fixed in 4% paraformaldehyde and embedded in paraffin for immunohistochemistry. The remaining placenta samples were stored in a −80°C freezer for subsequent real time PCR and Western blot analysis.
In brief, protein extracts of the samples were prepared using a RIPA lysis buffer (Beyotime, Shanghai, China), resolved on 10% SDS-PAGE, electro-transferred onto polyvinylidene fluoride (PVDF) membranes, and blocked with PBST containing 5% nonfat-dried milk for 2 h. The membranes were then incubated with primary antibodies against α7nAChR (Cat. No. ab10096; Abcam, MA, USA) and NF-κB p65 (Cat. No. ab207297; Abcam, MA, USA) overnight at 4°C, followed by the incubation with HRP-labeledsecondary antibodies (Cell Signaling Technology). Finally, the protein bands on the membranes were detected by an enhanced chemiluminescence (ECL) kit (Sevenseapharmtech Co., Ltd., Shanghai, China). β-actin served as endogenous control for the Western blot analysis.
RNA extraction and real-time qPCR
According to the manufacturer’s instructions, TRIzol® reagent (Invitrogen, CA, USA) was used to extract total RNA from placenta. Following the manufacturer’s protocols, reverse transcription was performed using 5 µg of total RNA in conjunction with a reverse transcription primer and RevertAidTM M-MuLV Reverse Transcriptase (Thermo, USA). Subsequently, qPCR was performed using a 20 μL system containing 10 μL of TaqMan® Universal PCR Master Mix (TliRNaseH Plus; Takara Bio, Shiga, Japan), 1 μL each of forward and reverse primers, 2 μL of cDNA template, and 7 μL of ultrapure water. The reaction conditions are as follows: 50°C for 2 min and 94°C for 10 min, followed by PCR which consisted of 40 cycles of 95°C for 15 s, and 60°C for 1 min. Primer sequences designed by Shanghai Biotechnology Co., Ltd., Shanghai, China. The sequences of the primers for α7nAChR, NF-κB p65 and β-Actin were as follows (forward and reverse, respectively): α7nAChR (ACCTCGTGTGATCCAAAGCC and GGTTTCCTCTTGCTCAGGGT); NF-κB p65 (CGACGTATTGCTGTGCCTTC and TGAGATCTGCCCAGGTGGTAA) and β-Actin (CCTCTATGCCAACACAGTGC and GTACTCCTGCTTGCTGATCC). β-Actin was used as internal control. Relative mRNA expression was adjusted by using 2−ΔΔCt.
Placental tissues were paraffin-embedded and subsequently cut into 3–4 μm-thick serial sections. The sections were pretreated with 0.3% hydrogen peroxide, followed by incubation in a humidified chamber with a purified rabbit polyclonal antibody against α7nAChR and NF-κB p65 (Zhongshan Jinqiao Co., Ltd., Beijing, China) at 4°C overnight. After being washing with PBS, the slides were incubated with a solution containing a suitable secondary antibody (Biogottechnology Co., Ltd., Nanjing, China). Finally, the sections were incubated in streptavidin-peroxidase, stained with diaminobenzidine, washed with hematoxylin, dehydrated, and determined under an optical microscope (OLYMPUS, BX60).
According to the manufacturer’s instructions, the expression of TNF-α, HGMB-1 and IL-6 in placenta was tested utilizing an ELISA kit (R&D Systems, Minneapolis, USA).
The data were analyzed by version 13.0 (SPSS Inc, Chicago, IL) using two-tailed Student’s t-test (for comparison between two groups), one-way ANOVA (for comparison among three or more groups), or two-way ANOVA (when two-variables were involved). All data were expressed as mean ± SEM. P < 0.05 indicated significant difference.
TaVNS ameliorated L-NAME-induced pre-eclampsia-like symptoms in rats
As expected, L-NAME-induced pre-eclampsia-like symptoms in rats. In addition, the rats in LN and LN+sham groups showed a continuously increased level of SBP from GD14 to delivery. In contrast, the TaVNS treatment significantly reduced the magnitude of SBP elevation in pre-eclamsia rats. After the TaVNS treatment (Fig. 2A), the SBP in pre-eclamsia rats on GD 16 was significantly lower compared to the SBP in non-treated pre-eclampsia rats, although the SBP in TaVNS treated rats was still higher than that in the normal control group. However, the SBP in TaVNS treated rats was similar to that in normal rats on GD18 and GD20. Interestingly, the SBP between sham-treated and non-treated L-NAME groups showed no significant difference from GD10 to GD 20.
Proteinuria is closely related with pre-eclampsia and is used as a marker of renal malfunction. In this study, the level of proteinuria was also evaluated in the rat model. Although the levels of urine protein were similar in all groups before pregnancy and on GD 10, the L-NAME group showed a significantly increased level of proteinuria in pre-eclamptic rats (P < 0.01) (Fig. 2B), indicating the presence of renal dysfunction in this group. The treatment with sham stimulation failed to alleviate this condition of renal dysfunction. However, after the TaVNS treatment, the level of urinary protein significantly decreased by 11% on GD 18 and 20 (P < 0.01), suggesting that the TaVNS treatment partly reduced kidney injuries.
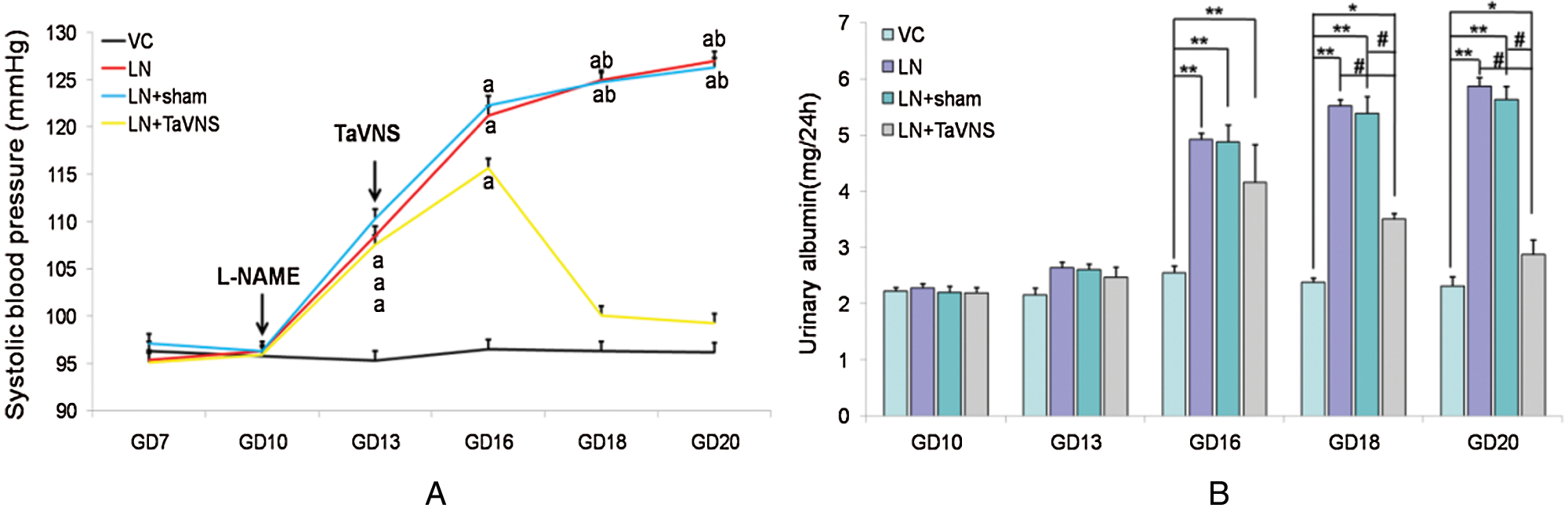
Figure 2: (A) The SBP was measured on gestational days (GDs) 7, 10, 13, 16, 18, and 20 in pregnant groups.
TaVNS attenuated L-NAME-induced restriction on fetal growth
The effects of L-NAME on fetal growth in the utero are shown in Fig. 3. Daily L-NAME administration on GD 10–16 significantly decreased fetal weight and placental weight. However, after the TaVNS treatment starting from GD 13, both fetal weight and placental weight were significantly increased (P < 0.01) compared to those in the LN and LN+sham groups (P < 0.05) on GD 20. However, there was no significant difference in terms of CRL among these groups.
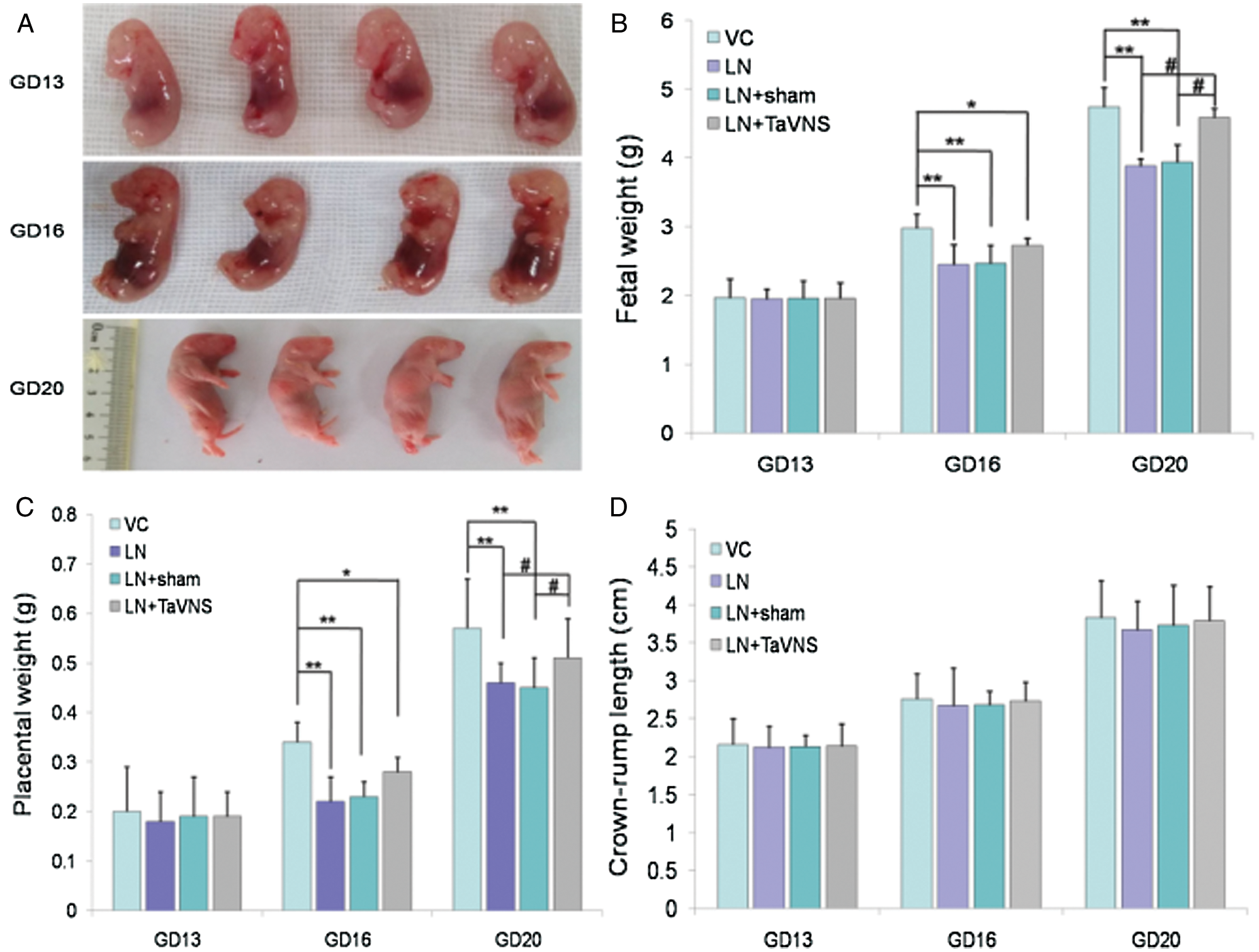
Figure 3: Fetal weights, placental weights and CRL (crown-rump length) are shown on GDs 13, 16 and 20.
TaVNS improved pregnancy outcomes in L-NAME-treated rats
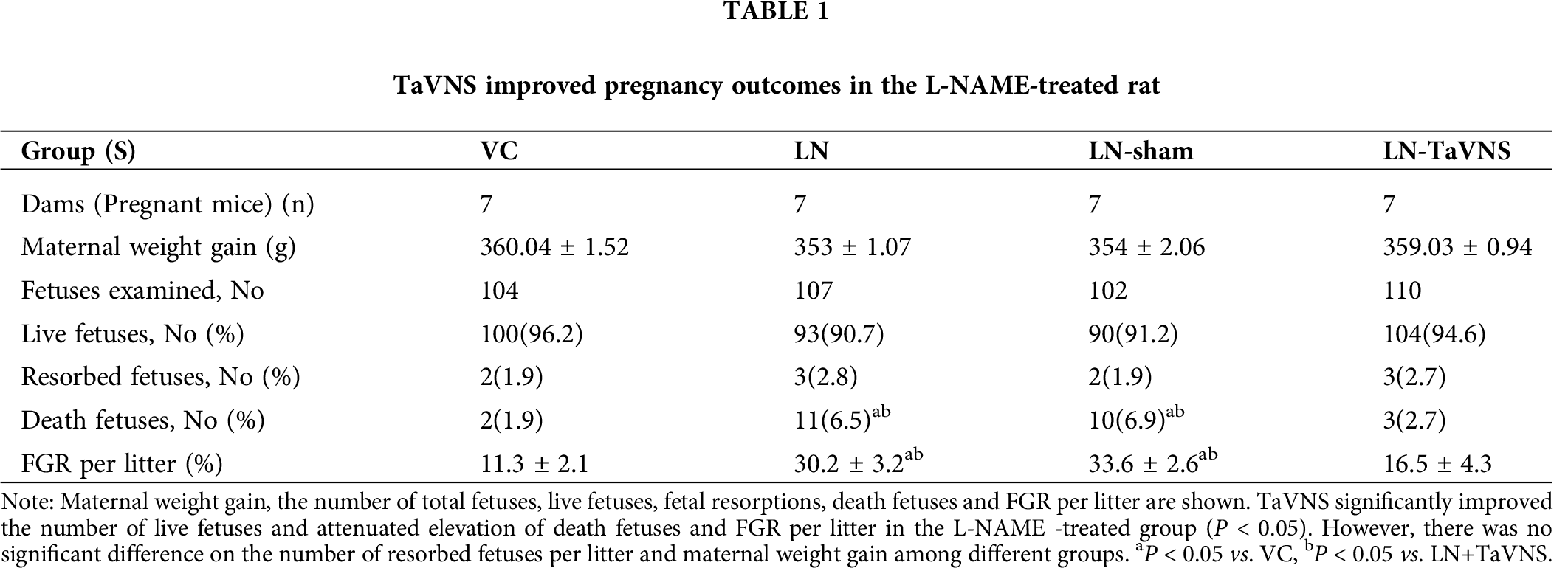
No pregnant rats were dead, and no preterm delivery were observed among pregnant rats intraperitoneally injected with L-NAME. As shown in Tab. 1, there was no significant difference in weight gain of pregnant rats among the groups. The injection of L-NAME resulted in an 4.6% increase in dead fetuses and led to a 5.5% decrease in live fetuses (P < 0.05), further analysis showed that TaVNS treatment significantly blocked the effect of L-NAME. Fetal growth restriction (FGR) per litter was approximately 20% increased significantly in the LN+sham and LN groups compared to the VC group (P < 0.05). While the administration of TaVNS reduced the rate of FGR per litter. In addition, there was no significant difference among different groups in resorbed fetuses.
TaVNS reduced the expression of inflammatory factors in the placenta of L-NAME-treated rats
To explore the mechanism for the protective action of TaVNS on pre-eclampsia rats, we measured IL-6, TNF-α and HMGB1 levels on GD20 by ELISA. As shown in Fig. 4, we found that control rats expressed low levels of IL-6 (6.18 ± 0.37 pg/mg), TNF-α (3.25 ± 0.1 pg/mg) and HMGB1 (13.2 ± 0.49 pg/mg). Levels of IL-6 (10.11 ± 0.14 pg/mg), TNF-α (6.9 ± 0.09 pg/mg) and HMGB1 (24.23 ± 0.61 pg/mg) were enhanced markedly after L-NAME compared with the VC group (P < 0.05). Treatment with TaVNS prevented the increases in IL-6 (7.51 ± 0.12 pg/mg), TNF-α (5.56 ± 0.33 pg/mg) and HMGB1 (17.5 ± 0.75 pg/mg) in the placenta following L-NAME administration (P < 0.05).
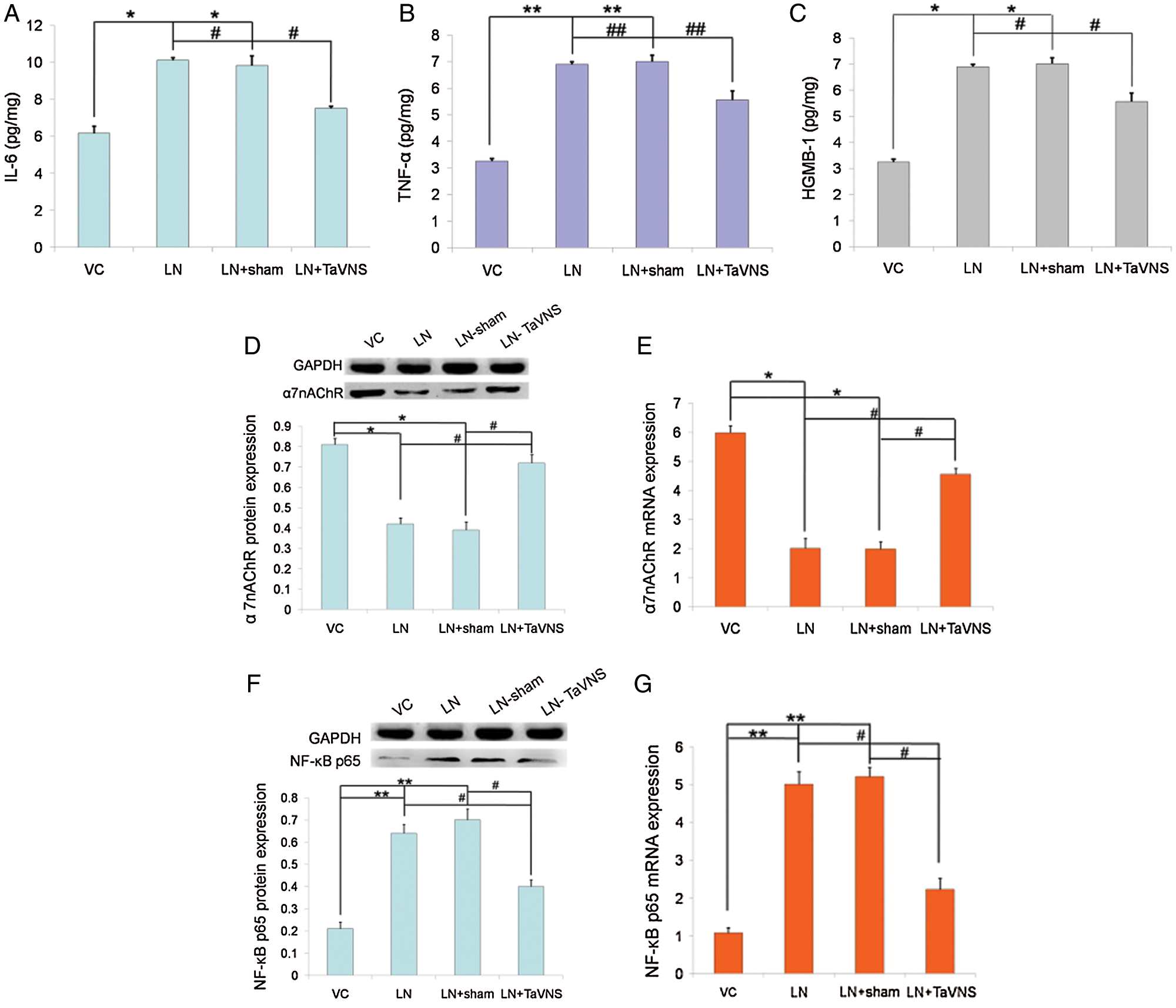
Figure 4: Graphical representations of (A) IL-6, (B) TNF-α, and (C) HGMB-1 expression in the placentae of pregnant dams on GD20.
TaVNS up-regulated α7nAChR expression in the placenta of L-NAME-treated rats
As shown in Fig. 4, the expression of α7nAChR in experimental groups was detected by qRT-PCR and Western blot. Analysis showed that the protein level of α7nAChR decreased in L-NAME-treated rats and increased significantly after TaVNS treatment (Fig. 4D). To investigate the cause, we conducted an mRNA-level verification and found that the mRNA level of α7nAChR was significantly increased (Fig. 4E). No significant difference was found between the VC group and LN+TaVNS group in the protein and mRNA levels (both P > 0.05).
TaVNS down-regulated NF-κB p65 expression in the placenta of L-NAME-treated rats
The effects of TaVNS on the protein and mRNA expression of NF-κB p65 in the placenta are shown in Figs. 4G and 4F. The data showed that the protein expression of NF-κB p65 was significantly lower in the LN+TaVNS group (0.4 ± 0.03) than that in the LN (0.64 ± 0.04) and LN+sham (0.7 ± 0.05) groups (P < 0.05). In agreement with the results from Western blotting, NF-κB p65 mRNA expression were significantly increased in the LN (5.01 ± 0.33) and LN+sham (5.21 ± 0.25) groups (P < 0.05), while TaVNS significantly decreased the elevated levels (2.23 ± 0.3) (P < 0.05). There was no statistical significance between the VC group (0.21 ± 0.03; 1.08 ± 0.14) and LN+TaVNS group in the protein and mRNA levels (both P > 0.05).
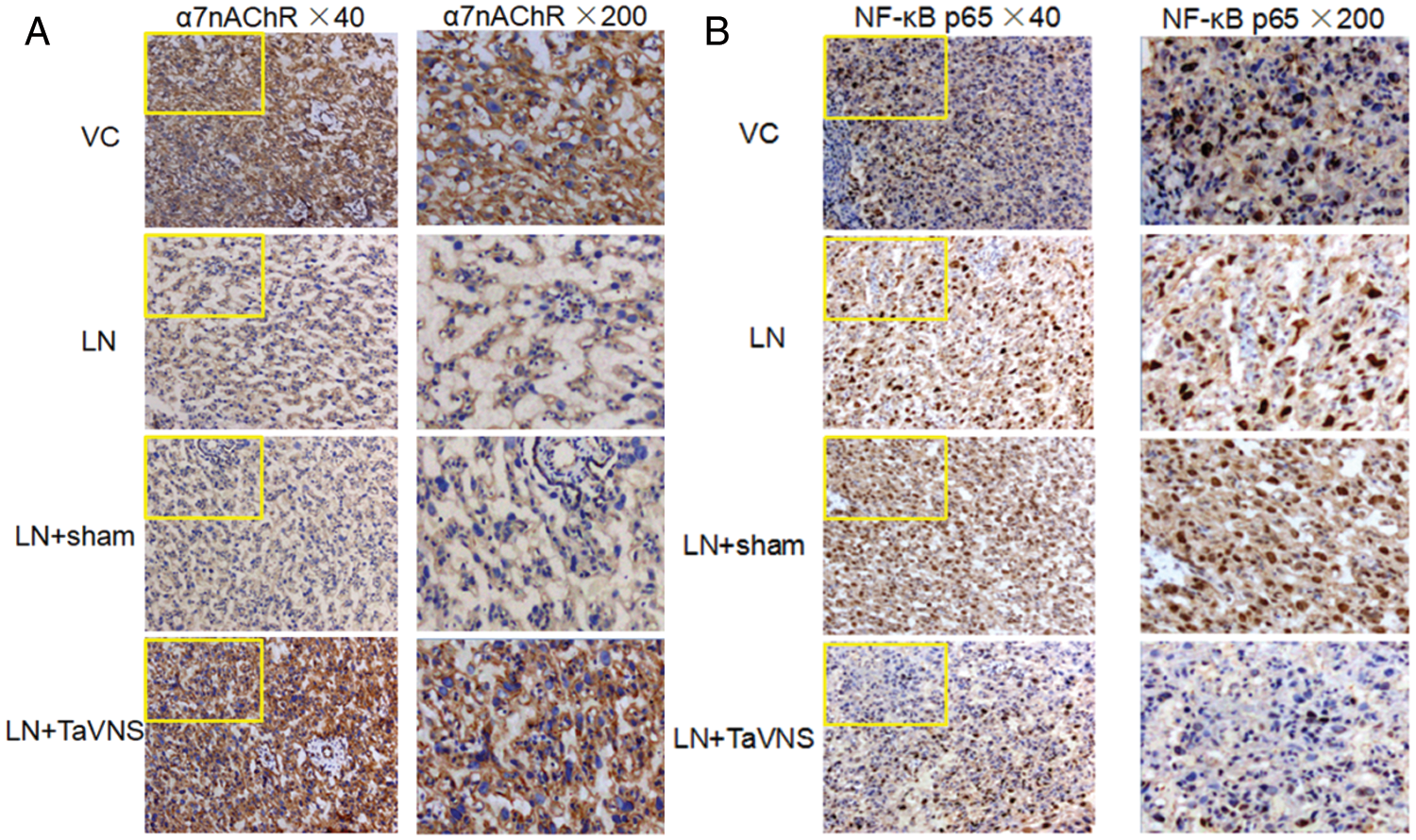
Figure 5: In the placental tissue, the expression of NF-κB p65 and α7nAChR was significantly up- and down-regulated in the LN and LN+sham group, respectively (P < 0.01), as compared with the VC control.
Effect of TaVNS on placental levels of α7nAChR and NF-κB p65 by immunohistochemistry
Expression of α7nAChR and NF-κB p65 in the placenta was also assessed by immunohistochemistry (Fig. 5). The locations of the expression of the α7nAChR in experiment groups were mainly in the cell membrane, and partly in the cytoplasm. Low but detectable levels of α7nAChR were observed in the LN and LN+sham groups. Intense α7nAChR staining was seen after TaVNS treatment on GD20 (Fig. 5A). NF-κB p65 immunohistochemical staining was predominantly observed in the nuclei. The level of NF-kB p65 was significantly elevated in the LN and LN+sham groups, whereas TaVNS treatment significantly lowered the number of positive immunostaining cells (Fig. 5B).
This study demonstrates, for the first time, that TaVNS treatment alleviated some of the pre-eclampsia-like symptoms, such as adverse hypertension, proteinuria, and fetal growth restriction in a pre-eclampsia rat model. Furthermore, this study showed that the effect of TaVNS treatment was exerted by promoting α7nAChR expression via activating the CAP activity and by inhibiting the activity of NF-κB p65 and other pro-inflammatory cytokines in the placenta. Chronic activation of the immune system and persistently high levels of pro-inflammatory cytokines are some of know pathological pathways of pre-eclampsia. The abnormal changes in cytokines can result in chronic systemic inflammation as well as local inflammation in placenta, thus contributing to the symptoms of pre-eclampsia (Baumann et al., 1990; Gadonski et al., 2006). Previous reports have revealed that the maternal exposure to L-NAME, an inhibitor of NO synthesis, could stimulate the production of pro-inflammatory cytokines, which in turn triggered severe systemic inflammatory responses during pregnancy (Soobryan et al., 2017; Yallampalli and Garfield, 1993). Therefore, a rat model of L-NAME-induced pre-eclampsia was used in this study to observe the effects of TaVNS on placental inflammation.
In this study, a rat model of pre-eclampsia was successfully established by the infusion of L-NAME into pregnant rats. Subsequently, maternal hypertension and proteinuria were observed. Results from this study were consistent with those in previous reports demonstrating that NOS inhibition could result in a pre-eclampsia-like syndrome (Tian et al., 2016; Zhu et al., 2017; Zou et al., 2014). The present study showed no effect of L-NAME on litter size in the rat model. In contrast, a few previous studies have shown reduced the size of litter in L-NAME-induced rats (Celadilla et al., 2005; Isler et al., 2003; Mayr et al., 2005). Furthermore, in the present study, the administration of L-NAME showed an approximately 20% decrease in pup weight, suggesting that pre-eclampsia might cause intrauterine growth retardation. These findings were in line with the results of other studies reporting lower fetal weights in L-NAME treated rats (Kiliç et al., 2003; Tsukimori et al., 2008). In contrast, some studies reported higher fetal weights (Tanir et al., 2005) or no changes in fetal weights (Yang et al., 2011) among L-NAME treated rats. In addition, although no significant difference was observed in terms of fetal crown-rump length between the TaVNS treated L-NAME group and the VC group in this study, previous studies have shown that human fetuses suffered from asymmetrical growth restriction under chronic hypoxia conditions (Chauhan et al., 2006).Moreover, this study also demonstrated that the placental weight in L-NAME-treated rats was approximately 20% lower than that in the VC group. This result was consistent with the studies reported by Ramesar et al. (2010) and Ma et al. (2010) who also found a dramaticreduction in the placental weight in the Sprague Dawley rats treated with L-NAME (Ma et al., 2010; Ramesar et al., 2010). In addition, a higher percentage of fetal resorption was observed in pre-eclampsia rats both in this study and other studies (Xue et al., 2015).
Recently, most studies have focused on the inhibition of inflammatory responses in pre-eclampsia (Amaral et al., 2015; Lin et al., 2012). Low carbon monoxide (CO) exposure could suppress the production of pro-inflammatory cytokines and attenuate the severity of pre-eclampsia in a rat model (Venditti et al., 2014). In addition, the anti-inflammatory cytokine IL-10 secreted during gestation could ameliorate the symptoms of pre-eclampsia and decrease the IFN-γ level in pregnant DOCA/saline treated (PDS) rats (Tinsley et al., 2010). Furthermore, LXA4 weakened inflammation and alleviated the clinical symptoms in endotoxin administrated pregnant rats (Lin et al., 2012).
As a mixed nerve, the vagus nerve (VN) plays a key role in the neuroendocrine–immune axis and provides an important first-line innate defense against inflammation (Johnston and Webster, 2009). The VN is sensitive to pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6 and TNF-α, which are released by macrophages and other immune cells (Hosoi et al., 2005; Werner et al., 2003). Vagus nerve stimulation (VNS) achieved via a surgically implanted device has been approved as an adjunctive epilepsy and depression therapy for more than 20 years (Fan et al., 2019; Flesler et al., 2017), and its anti-inflammatory properties is currently investigated in a potential therapy for a range of inflammatory disease, such as inflammatory bowel disease (IBD) and rheumatoid arthritis (RA) (Bonaz et al., 2017; Koopman et al., 2016).VNS could reduce the serum levels of IL-6 and increase the serum levels of IL-10 (Aalbers et al., 2012; Majoie et al., 2011). In addition, VNS could reduce the production of TNF, IL-1B, IL-6 and IL-8 in isolated peripheral blood mononuclear cells (De Herdt et al., 2009; Koopman et al., 2016). Yang et al. (2000) found vagus nerve dysfunction in pre-eclampsia women. In all previous cases, the safety of VNS was confirmed through its use during pregnancy, labor, and delivery, and no morphological abnormalities of the fetus were reported (Salerno et al., 2016). However, surgical operation risks, adverse effects and expensive treatment have limited the application of VNS (Fitzgerald, 2013; Ventureyra, 2000).
To deal with obstacles limiting the application of invasive VNS (iVNS) or non-invasive transcutaneous VNS (tVNS) has been started to appear in clinic studies. According to the report in a neural anatomy study, the auricular branch of the VN is mainly distributed on the meatus acusticus externus (Peuker and Filler, 2002). Therefore, TaVNS may increase the discharge of Nucleus TractusSolitarii (NTS) (Frangos et al., 2015). In addition, the stimulation on the auricular branch of the VN may produce a modulation effect similar to that of iVNS (Carreno and Frazer, 2016; Hein et al., 2013). TaVNS was used to treat many disorders, such as epilepsy (Rong et al., 2014), chronic tinnitus (Shim et al., 2015) and diabetic neuropathy development (Li et al., 2018), which also boosts associative memory (Jacobs et al., 2015). In experiments conducted on non-pregnant rats, Zhao et al. (2012) found that TaVNS inhibited LPS-induced inflammation through CAP mediated by α7nAChR. The present study illustrated that L-NAME administration significantly elevated the placenta levels of several pro-inflammatory cytokines, such as TNF-α, HMGB-1 and IL-6, whereas the TaVNS treatment during pregnancy significantly inhibited the expression of above pro-inflammatory cytokines. It is important to note that no changes in the fetal number and no evidence of malformation were observed in all groups. It was also found that the maternal behavior and mental status of rats were not significantly affected by TaVNS, indicating that the applied parameters of TaVNS in this study could be considered as in the safe range.
The CAP is a vagal neuro-immune circuit (termed the ‘inflammatory reflex’) and may lead to inflammatory reactions. This reflex is mediated through the link of Acetylcholine (ACh), the principal vagal neuro transmitter with α7nAChR in macrophages could inhibit the synthesis and release of pro-inflammatory cytokines (Borovikova et al., 2000). Many therapies are based on the cholinergic stimulation, which induces the efferent vagal nerve signaling to reduce the activation of immune cells and to suppress the overproduction of pro-inflammatory cytokines. Pre-treatment with nicotine, a selective agonist of α7nAChR, protects rats against LPS-induced inflammatory responses via the CAP activation (Liu et al., 2017). The administration of choline also ameliorated pre-eclampsia-like symptoms and protected against inflammatory symptoms through the functions of α7nAChR and CAP (Zhang et al., 2018). In models of sepsis, α7nAChR has been repeatedly shown to mediate the beneficial effect of VNS (Rana et al., 2018; Zhao et al., 2013).
In the present study, it was found that TaVNS improved pregnancy outcomes in pre-eclampsia rats, presumably by significantly inhibiting the release of pro-inflammatory cytokines in the placenta. Moreover, the mRNA and protein levels of α7nAChR in the placenta of pre-eclampsia rats were elevated by the TaVNS treatment. In summary, these data indicated that TaVNS might exert its anti-inflammatory role in pre-eclampsia placentas by activating the CAP and by regulating the level of α7nAChR.
To address the mechanisms by which TaVNS exerts its anti-inflammatory effects in pre-eclampsia rats, the placental NF-κB activation in pre-eclampsia rats was examined. It was found that TaVNS treatment led to significant inhibition of NF-κB activity. Previous studies have shown that NF-κB plays critical roles in the transcriptional regulation of pro-inflammatory genes. A study by Zhao et al. (2013) demonstrated that VNS suppressed cytokine release through α7nAChR and inhibited NF-κB signaling in a rat model of myocardial ischemia/reperfusion. It was also found in this study that TaVNS improved the placental expression of α7nAChR in pre-eclampsia rats and significantly inhibited the activity of NF-κB p65 in the placenta. The stimulation of auricular branch of the vagus nerve (ABVN) ascends to the NTS, where they cause the activation of efferent vagus nerve fibers to inhibit cytokine release through the activation of α7nAChR (Zhao et al., 2012).
In conclusion, our data show that TaVNS reversed the impairments resulting from L-NAME induced pre-eclampsia. The underlying mechanisms of TaVNS treatment may include the activation of α7nAChR, the inhibition of NF-κB activities, and the downregulation in the expression of pro-inflammatory cytokines. This study provided new insights for TaVNS in the treatment of inflammatory responses during pre-eclampsia without inducing adverse effects. Nevertheless, further studies are needed to optimize the potential use of TaVNS in clinics.
Acknowledgement: The authors thank Zhicheng Lu and Fujin Liu for their help with the animal experiments.
Availability of Data and Materials: The datasets used and/or analysed during the current study are available upon reasonable request from the corresponding author.
Author Contribution: The study was conceived by Lei Shi, Linmei Zheng and Rong Tang carried out the experiment and analysed the data. Linmei Zheng wrote the first version of the paper. All authors contributed to revisions of the paper and approved of the final manuscript.
Ethics Approval: All animal procedures were approved by the Animal Ethics Committee of Hainan Medical University. (Ratification No. 2020-185; Approval Date Jun. 04, 2020.)
Funding Statement: Funding was provided by Provincial Natural Science Foundation of China Hainan (819MS119).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Aalbers MW, Klinkenberg S, Rijkers K, Verschuure P, Kessels A et al. (2012). The effects of vagus nerve stimulation on pro-and anti-inflammatory cytokines in children with refractory epilepsy: An exploratory study. Neuroimmunomodulation 19: 352–358. [Google Scholar]
Agarwal D, Haque M, Sriramula S, Mariappan N, Pariaut R, Francis J (2009). Role of proinflammatory cytokines and redox homeostasis in exercise-induced delayed progression of hypertension in spontaneously hypertensive rats. Hypertension 54: 1393–1400. [Google Scholar]
Ahmed A (2011). New insights into the etiology of preeclampsia: Identification of key elusive factors for the vascular complications. Thrombosis Research 127: S72–S75. [Google Scholar]
Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin Jr, JN, Lamarca B (2015). 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension 65: 225–231. [Google Scholar]
Baumann M, Becker S, Krüger HJ, Vogler H, Maurer T, Beck-Bornholdt HP (1990). Flow cytometric determination of the time of metastasis during fractionated radiation therapy of the rat rhabdomyosarcoma R1H. International Journal of Radiation Biology 58: 361–369. [Google Scholar]
Bonaz B, Sinniger V, Pellissier S (2016). Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. Journal of Physiology 594: 5781–5790. [Google Scholar]
Bonaz B, Sinniger V, Pellissier S (2017). Vagus nerve stimulation: A new promising therapeutic tool in inflammatory bowel disease. Journal of Internal Medicine 282: 46–63. [Google Scholar]
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI et al. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462. [Google Scholar]
Carreno FR, Frazer A (2016). The allure of transcutaneous vagus nerve stimulation as a novel therapeutic modality. Biological Psychiatry 79: 260–261. [Google Scholar]
Celadilla LF, Rueda MC, Rodríguez MM (2005). Prolonged inhibition of nitric oxide synthesis in pregnant rats: Effects on blood pressure, fetal growth and litter size. Archives of Gynecology and Obstetrics 271: 243–248. [Google Scholar]
Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R (2014). Pre-eclampsia part 1: Current understanding of its pathophysiology. Nature Reviews Nephrology 10: 466–480. [Google Scholar]
Chauhan SP, Cole J, Sanderson M, Magann EF, Scardo JA (2006). Suspicion of intrauterine growth restriction: use of abdominal circumference alone or estimated fetal weight below 10%. Journal of Maternal-Fetal & Neonatal Medicine 19: 557–562. [Google Scholar]
De Herdt V, Bogaert S, Bracke KR, Raedt R, De Vos M et al. (2009). Effects of vagus nerve stimulation on pro-and anti-inflammatory cytokine induction in patients with refractory epilepsy. Journal of Neuroimmunology 214: 104–108. [Google Scholar]
Fan JJ, Shan W, Wu JP, Wang Q (2019). Research progress of vagus nerve stimulation in the treatment of epilepsy. CNS Neuroscience & Therapeutics 25: 1222–1228. [Google Scholar]
Fitzgerald PB (2013). Non-pharmacological biological treatment approaches to difficult-to-treat depression. Medical Journal of Australia 199: S48–S51. [Google Scholar]
Flesler S, Reyes G, Fortini S, Ramos B, Cersosimo R et al. (2017). Vagus nerve stimulation: Treatment of 158 pediatric patients with a long-term follow-up. Revista de Neurologia 64: 496–501. [Google Scholar]
Frangos E, Ellrich J, Komisaruk BR (2015). Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimulation 8: 624–636. [Google Scholar]
Gadonski G, Lamarca BBD, Sullivan E, Bennett W, Chandler D, Granger JP (2006). Hypertension produced by reductions in uterine perfusion in the pregnant rat: Role of interleukin 6. Hypertension 48: 711–716. [Google Scholar]
Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K et al. (2013). Auricular transcutaneous electrical nerve stimulation in depressed patients: A randomized controlled pilot study. Journal of Neural Transmission 120: 821–827. [Google Scholar]
Henderson JT, Thompson JH, Burda BU, Cantor A (2017). Preeclampsia screening: Evidence report and systematic review for the US Preventive Services Task Force. JAMA 317: 1668–1683. [Google Scholar]
Hosoi T, Okuma Y, Matsuda T, Nomura Y (2005). Novel pathway for LPS-induced afferent vagus nerve activation: Possible role of nodose ganglion. Autonomic Neuroscience 120: 104–107. [Google Scholar]
Hsu CC, Weng CS, Liu TS, Tsai YS, Chang YH (2006). Effects of electrical acupuncture on acupoint BL15 evaluated in terms of heart rate variability, pulse rate variability and skin conductance response. American Journal of Chinese medicine 34: 23–36. [Google Scholar]
Hutcheon JA, Lisonkova S, Joseph K (2011). Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Practice & Research Clinical Obstetrics & Gynaecology 25: 391–403. [Google Scholar]
Isler CM, Bennett WA, Rinewalt AN, Cockrell KL, Martin Jr, JN et al. (2003). Evaluation of a rat model of preeclampsia for HELLP syndrome characteristics. Journal of the Society for Gynecologic Investigation 10: 151–153. [Google Scholar]
Jacobs HI, Riphagen JM, Razat CM, Wiese S, Sack AT (2015). Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiology of Aging 36: 1860–1867. [Google Scholar]
Johnston G, Webster N (2009). Cytokines and the immunomodulatory function of the vagus nerve. British Journal of Anaesthesia 102: 453–462. [Google Scholar]
Kiliç İ., Güven C, Kilinç K (2003). Effect of maternal NG-nitro-l-arginine administration on fetal growth and hypoxia-induced changes in newborn rats. Pediatrics International 45: 375–378. [Google Scholar]
Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S et al. (2016). Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America 113: 8284–8289. [Google Scholar]
Li S, Sun C, Rong P, Zhai X, Zhang J et al. (2018). Auricular vagus nerve stimulation enhances central serotonergic function and inhibits diabetic neuropathy development in Zucker fatty rats. Molecular Pain 14: 1744806918787368. [Google Scholar]
Lin F, Zeng P, Xu Z, Ye D, Yu X et al. (2012). Treatment of Lipoxin A4 and its analogue on low-dose endotoxin induced preeclampsia in rat and possible mechanisms. Reproductive Toxicology 34: 677–685. [Google Scholar]
Liu Y, Yang J, Bao J, Li X, Ye A et al. (2017). Activation of the cholinergic anti-inflammatory pathway by nicotine ameliorates lipopolysaccharide-induced preeclampsia-like symptoms in pregnant rats. Placenta 49: 23–32. [Google Scholar]
Lynch A, Murphy J, Gibbs R, Levine R, Giclas P et al. (2010). The interrelationship of complement-activation fragments and angiogenesis-related factors in early pregnancy and their association with pre-eclampsia. BJOG: An International Journal of Obstetrics & Gynaecology 117: 456–462. [Google Scholar]
Ma RQ, Sun MN, Zi Y (2010). Effects of preeclampsia-like symptoms at early gestational stage on feto-placental outcomes in a mouse model. Chinese Medical Journal 123: 707–712. [Google Scholar]
Majoie H, Rijkers K, Berfelo M, Hulsman J, Myint A et al. (2011). Vagus nerve stimulation in refractory epilepsy: Effects on pro-and anti-inflammatory cytokines in peripheral blood. Neuroimmunomodulation 18: 52–56. [Google Scholar]
Mantovani A, Dejana E (1989). Cytokines as communication signals between leukocytes and endothelial cells. Immunology Today 10: 370–375. [Google Scholar]
Mayr AJ, Lederer W, Wolf HJ, Dünser M, Pfaller K, Mörtl MG (2005). Morphologic changes of the uteroplacental unit in preeclampsia-like syndrome in rats. Hypertension in Pregnancy 24: 29–37. [Google Scholar]
Peuker ET, Filler TJ (2002). The nerve supply of the human auricle. Clinical Anatomy 15: 35–37. [Google Scholar]
Ramesar S, Mackraj I, Gathiram P, Moodley J (2010). Sildenafil citrate improves fetal outcomes in pregnant, l-NAME treated, Sprague-Dawley rats. European Journal of Obstetrics & Gynecology and Reproductive Biology 149: 22–26. [Google Scholar]
Rana M, Fei-Bloom Y, Son M, La Bella A, Ochani M et al. (2018). Constitutive vagus nerve activation modulates immune suppression in sepsis survivors. Frontiers in Immunology 9: 2032. [Google Scholar]
Roberts JM, August PA, Bakris G, Barton JR, Bernstein IM et al. (2013). Hypertension in pregnancy: Executive summary. Obstetrics and Gynecology 122: 1122–1131. [Google Scholar]
Rong P, Liu A, Zhang J, Wang Y, Yang A et al. (2014). An alternative therapy for drug-resistant epilepsy: Transcutaneous auricular vagus nerve stimulation. Chinese Medical Journal 127: 300–303. [Google Scholar]
Salerno G, Passamonti C, Cecchi A, Zamponi N (2016). Vagus nerve stimulation during pregnancy: An instructive case. Child’s Nervous System 32: 209–211. [Google Scholar]
Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB et al. (2014). Global causes of maternal death: A WHO systematic analysis. Lancet Global Health 2: e323–e333. [Google Scholar]
Shim HJ, Kwak MY, An YH, Kim DH, Kim YJ, Kim HJ (2015). Feasibility and safety of transcutaneous vagus nerve stimulation paired with notched music therapy for the treatment of chronic tinnitus. Journal of Audiology & Otology 19: 159. [Google Scholar]
Soobryan N, Murugesan S, Phoswa W, Gathiram P, Moodley J, Mackraj I (2017). The effects of sildenafil citrate on uterine angiogenic status and serum inflammatory markers in an L-NAME rat model of pre-eclampsia. European Journal of Pharmacology 795: 101–107. [Google Scholar]
Tanir HM, Sener T, Inal M, Akyuz F, Uzuner K, Sivri E (2005). Effect of quercetine and glutathione on the level of superoxide dismutase, catalase, malonyldialdehyde, blood pressure and neonatal outcome in a rat model of pre-eclampsia induced by NG-nitro-L-arginine-methyl ester. European Journal of Obstetrics & Gynecology and Reproductive Biology 118: 190–195. [Google Scholar]
Tian M, Zhang Y, Liu Z, Sun G, Mor G, Liao A (2016). The PD-1/PD-L1 inhibitory pathway is altered in pre-eclampsia and regulates T cell responses in pre-eclamptic rats. Scientific Reports 6: 27683. [Google Scholar]
Tinsley JH, South S, Chiasson VL, Mitchell BM (2010). Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 298: R713–R719. [Google Scholar]
Tracey KJ (2002). The inflammatory reflex. Nature 420: 853–859. [Google Scholar]
Tsukimori K, Komatsu H, Fukushima K, Kaku T, Nakano H, Wake N (2008). Inhibition of nitric oxide synthetase at mid-gestation in rats is associated with increases in arterial pressure, serum tumor necrosis factor-α, and placental apoptosis. American Journal of Hypertension 21: 477–481. [Google Scholar]
Venditti CC, Casselman R, Young I, Karumanchi SA, Smith GN (2014). Carbon monoxide prevents hypertension and proteinuria in an adenovirus sFlt-1 preeclampsia-like mouse model. PLoS One 9: e106502. [Google Scholar]
Ventureyra EC (2000). Transcutaneous vagus nerve stimulation for partial onset seizure therapy. Child’s Nervous System 16: 101–102. [Google Scholar]
Wang S, Zhai X, Li S, Mccabe MF, Wang X, Rong P (2015). Transcutaneous vagus nerve stimulation induces tidal melatonin secretion and has an antidiabetic effect in Zucker fatty rats. PLoS One 10: e0124195. [Google Scholar]
Werner M, Fraga D, Melo M, Souza G, Zampronio A (2003). Importance of the vagus nerve for fever and neutrophil migration induced by intraperitoneal LPS injection. Inflammation Research 52: 291–296. [Google Scholar]
Wu JH, Chen HY, Chang YJ, Wu HC, Chang WD et al. (2009). Study of autonomic nervous activity of night shift workers treated with laser acupuncture. Photomedicine and Laser Surgery 27: 273–279. [Google Scholar]
Xue P, Zheng M, Gong P, Lin C, Zhou J et al. (2015). Single administration of ultra-low-dose lipopolysaccharide in rat early pregnancy induces TLR4 activation in the placenta contributing to preeclampsia. PLoS One 10: e0124001. [Google Scholar]
Yallampalli C, Garfield RE (1993). Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. American Journal of Obstetrics and Gynecology 169: 1316–1320. [Google Scholar]
Yang CC, Chao TC, Kuo TB, Yin CS, Chen HI (2000). Preeclamptic pregnancy is associated with increased sympathetic and decreased parasympathetic control of HR. American Journal of Physiology-Heart and Circulatory Physiology 278: H1269–H1273. [Google Scholar]
Yang X, Guo L, Sun X, Chen X, Tong X (2011). Protective effects of hydrogen-rich saline in preeclampsia rat model. Placenta 32: 681–686. [Google Scholar]
Zhang M, Han X, Bao J, Yang J, Shi SQ et al. (2018). Choline supplementation during pregnancy protects against gestational lipopolysaccharide-induced inflammatory responses. Reproductive Sciences 25: 74–85. [Google Scholar]
Zhao M, He X, Bi XY, Yu XJ, Wier WG, Zang WJ (2013). Vagal stimulation triggers peripheral vascular protection through the cholinergic anti-inflammatory pathway in a rat model of myocardial ischemia/reperfusion. Basic Research in Cardiology 108: 345. [Google Scholar]
Zhao YX, He W, Jing XH, Liu JL, Rong PJ et al. (2012). Transcutaneous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysaccharide-induced inflammation. Evidence-Based Complementary and Alternative Medicine 2012: 627023. [Google Scholar]
Zheng L, Shi L, Zhou Z, Chen X, Wang L et al. (2018). Placental expression of AChE, α7nAChR and NF-κB in patients with preeclampsia. Ginekologia Polska 89: 249–255. [Google Scholar]
Zheng L, Tang R, Shi L, Zhong M, Zhou Z (2021). Vagus nerve stimulation ameliorates L-NAME-induced preeclampsia-like symptoms in rats through inhibition of the inflammatory response. BMC Pregnancy and Childbirth 21: 177. [Google Scholar]
Zhu H, Zhu W, Hu R, Wang H, Ma D, Li X (2017). The effect of pre-eclampsia-like syndrome induced by L-NAME on learning and memory and hippocampal glucocorticoid receptor expression: A rat model. Hypertension in Pregnancy 36: 36–43. [Google Scholar]
Zimmerman GA, Prescott SM, Mcintyre TM (1992). Endothelial cell interactions with granulocytes: Tethering and signaling molecules. Immunology Today 13: 93–100. [Google Scholar]
Zou Y, Zuo Q, Huang S, Yu X, Jiang Z et al. (2014). Resveratrol inhibits trophoblast apoptosis through oxidative stress in preeclampsia-model rats. Molecules 19: 20570–20579. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |