DOI:10.32604/biocell.2021.015923

| BIOCELL DOI:10.32604/biocell.2021.015923 |  |
| Article |
Invasive stratified mucin-producing carcinoma (ISMC) of the uterine cervix: An analysis of 6 cases with distinctive clinicopathological features
Department of Pathology, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, Cancer Hospital affiliate to School of Medicine, UESTC, Chengdu, 610000, China
*Address correspondence to: Yang Liu, liuyanglyon@cmu.edu.cn
Received: 24 January 2021; Accepted: 08 March 2021
Abstract: Invasive stratified mucin-producing carcinoma (ISMC) is a recently described histologic variant of high-risk human papillomavirus (HPV)-associated endocervical adenocarcinoma, as the putative invasive counterpart of the stratified mucin-producing intraepithelial lesion (SMILE). ISMC can display variable architectural patterns and usually coexists with other more conventional types of HPV-associated carcinomas, which makes diagnosis and differential diagnosis of ISMC is difficult for pathologists. Moreover, the prognosis of ISMC is still controversial. We analyzed 6 ISMCs with detailed pathological and clinical information. Intraepithelial lesion, including 1 high-grade squamous intraepithelial lesion and 1 SMILE, was found. Various architectures were observed (including nest, glandular, solid, trabecular, and single cell). Nuclear peripheral palisading, apoptotic bodies and mitoses, and variable cytoplasmic mucin vacuoles were seen in all of our cases. The predominance of neutrophils infiltration was seen in only 1 tumor. All the tumors infiltrated the cervical stroma in Silva pattern C manner. p63 and/or p40 was characteristically expressed in the peripheral cells in only 2 cases. High-risk HPV infection was observed in 3/3 detected cases. All the patients were alive during the follow-up time. Recognition of this infrequent tumor may help pathologists and oncologists for an accurate diagnosis and a better understanding of the clinicopathological behavior.
Keywords: Invasive stratified mucin-producing carcinoma; ISMC; Stratified mucin-producing intraepithelial lesion; SMILE; Cervical carcinoma; Human papillomavirus
Stratified mucin-producing intraepithelial lesion (SMILE) is a rare preinvasive neoplasm of the uterine cervix that initially described by Park et al. (2000) in 2000 and was included in the 2014 World Health Organization (WHO) Classification of Tumors of Female Reproductive Organs as a variant pattern of intracervical adenocarcinoma in situ (AIS) (Wilbur et al., 2014). It is thought to arise from the HPV-infected reserve cells of the transformation zone (Park et al., 2000; Boyle and McCluggage, 2015). Morphologically, SMILE is characterized by stratified columnar epithelial cells, consists of various quantities of intracytoplasmic mucin throughout the entire epithelial thickness (Wilbur et al., 2014). Invasive stratified mucin-producing carcinoma (ISMC), the putative invasive counterpart of SMILE, is firstly reported as a distinct morphologic variant of endocervical adenocarcinoma (ECA) by Lastra et al. (2016). This entity has been included in the International Endocervical Adenocarcinoma Criteria and Classification (IECC) and 2020 World Health Organization (WHO) Classification of Tumours of Female Genital Tumours (Parra et al., 2020) system (Stolnicu et al., 2018). ISMC displaying a wide morphologic spectrum and always coexists with other invasive and intraepithelial tumor types, which makes it difficult to distinguish from other malignant tumors of the cervix.
Some studies suggested ISMC was a poor prognostic factor compared to usual-type endocervical adenocarcinoma (UEA) (Horn et al., 2019). However, ISMC accounts for only about 2.4% of all cervical adenocarcinoma (Stolnicu et al., 2018), and the cohort sizes were relatively limited. So far, the prognosis of ISMC is still controversial and has not been clearly elucidated (Lastra et al., 2016; Onishi et al., 2016; Horn et al., 2019; Stolnicu et al., 2019).
Insufficient clinical information and histological complexity of ISMC make it difficult to be diagnosed and not fully recognized by clinicians. Hence, in this study, we additionally describe a case series of 6 cases of ISMC to provide more clinicopathological, immunohistochemical, and follow-up information of this extremely rare entity in order to help pathologists and oncologists to have a better understanding of this infrequent disease.
A retrospective search of the hospital surgical pathology files using keywords “stratified mucin-producing carcinoma” or “ISMC” involving the cervix from December 2018 to September 2020 was performed. Hematoxylin and eosin (H&E) and immunohistochemistry sections of all the cases were retrieved, and all the tumors were reviewed by two pathologists with female reproductive system tumor pathology expertise (YL and SQ) and two general surgical pathologists (TL and XG), and a consensus diagnosis was reached in all cases. During this period, we identified 336 endocervical adenocarcinomas, including 6 ISMCs, representing a frequency of 1.8%.
The cases were classified according to the IECC system (Stolnicu et al., 2018), and ISMC was defined as the carcinoma consists of stratified, immature epithelial cells displaying various quantities of intracytoplasmic mucin throughout the tumor nest with stromal invasive, which was morphologically similar to SMILE. All the tumors were divided into two types: pure ISMC and mix tumor (ISMCmixed). Tumors were classified as ISMCmixed if the ISMC portion constituted ≥10% but <90% of the entire tumor and mixed with other invasive carcinoma components (if existed), especially human papillomavirus (HPV)-associated tumors, like usual-type endocervical adenocarcinoma (UEA), villoglandular adenocarcinoma, mucinous adenocarcinoma (not otherwise specified, intestinal-type or signet ring cell type), adenosquamous carcinoma (ASC), squamous cell carcinoma and neuroendocrine carcinoma (NEC) (Stolnicu et al., 2018), and other non-HPV-associated carcinomas. Intraepithelial neoplastic components were also recorded, such as the high-grade squamous intraepithelial lesion (HSIL), adenocarcinoma in situ (AIS), and SMILE. The pattern of tumor invasion was subsequently categorized as Silva pattern A, B, or C, (Diaz de Vivar et al., 2013). The morphologic features, lymph node metastasis, margin status, tumor size, nerve and vessel invasion, and International Federation of Gynecology and Obstetrics (FIGO) stage were evaluated.
Immunohistochemistry and histochemical staining
Immunohistochemical staining was carried out on formalin-fixed paraffin-embedded (FFPE) tissue, using the EnVision Plus detection system (DAKO, Carpinteria, CA) in all cases. Standard immunohistochemical studies were performed using the antibodies of p16, p63, CK7, and Ki-67 (MIB-1) (ZSGB-Bio, China), and p40 (MXB-Biotechnologies, China), with negative and positive control. p16 was considered positive only when it showed strong, diffuse, or “blocky” expression; whereas, focal, patchy, or weak staining were considered negative. CK7 was interpreted as positive if tumor cell nuclei and cytoplasm were stained. p63 and p40 were considered positive if any nuclear staining was noted in tumor cells. Histochemical staining of periodic acid-Schiff (PAS) and Alcian blue (AB) was performed according to standard protocols.
The patients aged from 40 to 56 years (mean, 46.8; median, 45.5), and the tumor size ranged from 2.2 to 5.4 cm (median, 3 cm; mean, 3.3 cm). Three patients presented with bleeding after sexual intercourse, one with vaginal bleeding, one with vaginal bleeding accompany with atypical Pap smear, and the remaining one with vaginal discharge. The information on HPV infection was obtained from the medical record. Three out of six patients represented a co-infection of HPV-type 18 and 45, HPV-type 18 and HPV-type 16, respectively.
Four patients were 2018 FIGO stage IB2, one was stage IB3, and one was Stage IIB (case No. 6, assessed by magnetic resonance imaging). Four out of 6 patients received radical hysterectomy and adjuvant chemotherapy and/or combined radiation, 1 underwent trachelectomy after subtotal hysterectomy 12 years ago because of myoma of the uterus. Whereas the remaining one (case No. 6) refused the operation and had only received radiotherapy and chemotherapy after needle biopsy. In the surgically resected specimens, a mean number of 29 pelvic lymph nodes (range 22–36) were removed, no lymph node metastasis was found, and all specimen margins were negative. All the patients were successfully followed up; the patient who refused operation was alive with the disease in 17 months, the others were alive with no evidence of disease recurrence during the follow-up time (4–24 months; mean, 12.0 months). Clinical findings were summarized in Tab. 1.
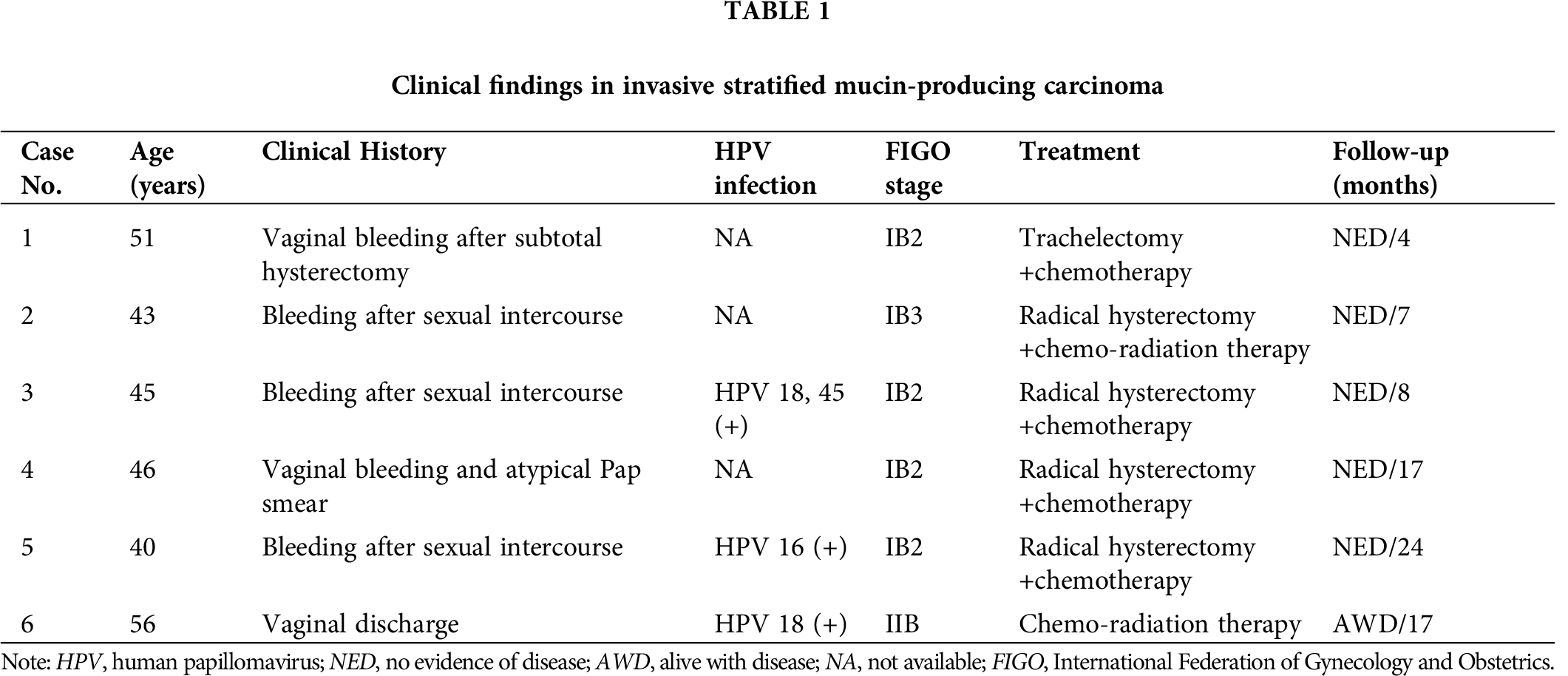
The pathological findings were summarized in Tab. 2. Four out of the five resected tumors were pure ISMC. Within these pure cases, one coexisted with HSIL (case No. 5). Only the ISMC component was observed in the needle biopsy specimen. The remaining case was an ISMCmixed (case No. 2); this case consisted mainly of UEAs, approximately 15% of ISMC, and a small focus of SMILE component.
Histologically, the tumors consisted of nests of stratified columnar cells that with bland, round to ovoid nuclei. Prominent nucleoli were absent in most tumors. Nuclear peripheral palisading was found in all cases (Fig. 1), even it only appears in parts of the tumor. The amount of the cytoplasmic mucin vacuoles varied from case to case and area to area, from abundant (“mucin-rich”) (Figs. 1A–1C) to scattered (“mucin-poor”) (Fig. 1D). Apoptotic bodies were seen in all cases; mitotic figures were ranged from 5/10HPF to 160/10HPF. Mixed inflammatory cell infiltration was seen in all tumors, peritumoral and intratumoral infiltrated with a predominance of lymphocytes and plasma cells, whereas diffuse neutrophil infiltration was seen in only one tumor.
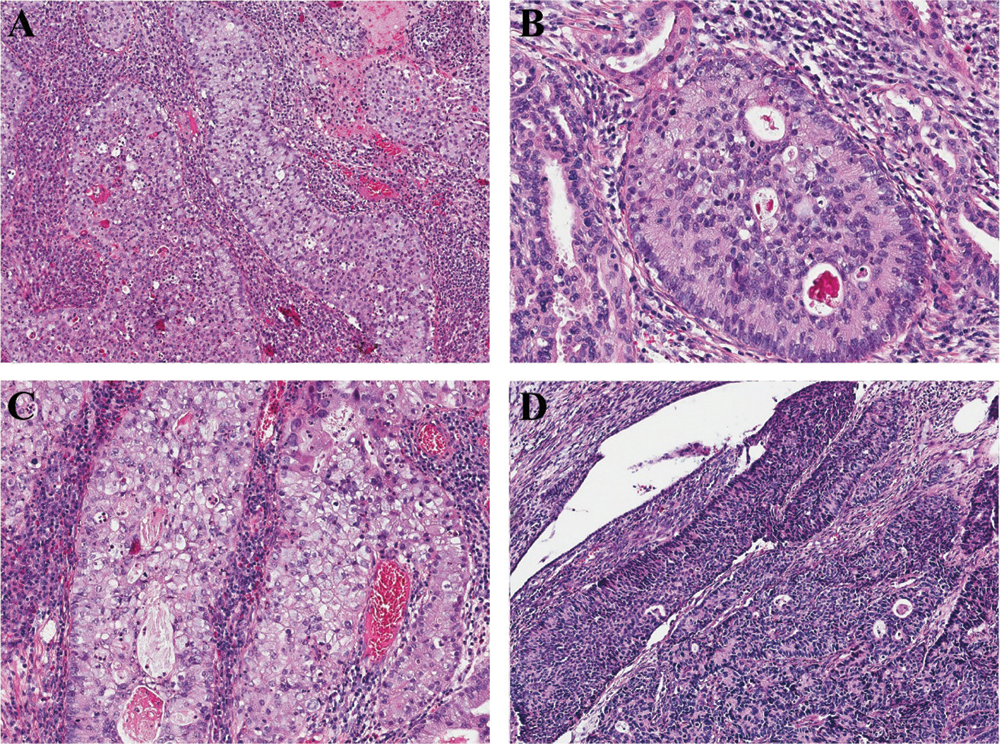
Figure 1: ISMCs consist of stratified columnar cell nests with nuclear peripheral palisading (A–D). The amount of the cytoplasmic mucin vacuoles varies from abundant (“mucin-rich”) (A–C) to scattered (“mucin-poor”) (D).
We also found some unusual architectural patterns, including glandular in 5 cases (Fig. 2A) (5/6, 83.3%; case 1–5) and solid in 2 cases (Fig. 2B) (2/6, 33.3%; cases 2 and 5). Various architectural patterns were found in a single tumor (case No. 1), including glandular, solid, trabecular (Fig. 2C), and single cells (Fig. 2D), as well as stroma mucoid degeneration was found focally in this case. In the ISMCmixed tumor, SMILE fused with ISMC component (Fig. 2E), whereas the UEA and ISMC areas were distinct and separate (Fig. 2F). All tumors exhibit diffusely destructive stromal invasion (Silva pattern C). Depth of invasion in the 5 resected specimens was from 1.0 cm to 2.1 cm. Nerve and vessel infiltration was observed in 4 and 2 tumors, respectively.

Figure 2: ISMCs display variable unusual architectural patterns: glandular (A), solid (B), trabecular (C), and single cells (D); SMILE fuses with ISMC component (E), UEA and ISMC areas are distinct and separate (F).
Immunohistochemistry and histochemical staining
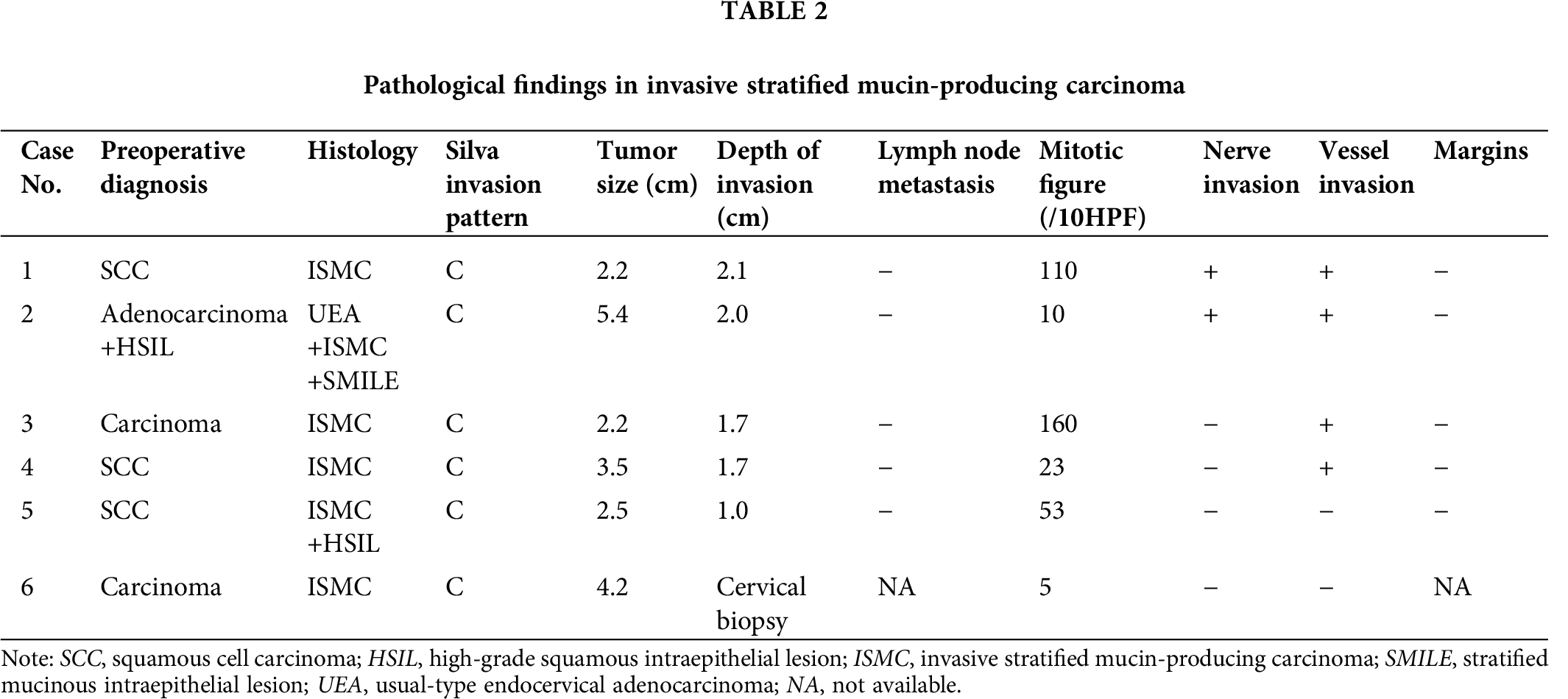
In ISMCs, p63 expressed in the peripheral cells (Fig. 3A) of the tumor cell nests in 2/6 cases, and p40 (Fig. 3B) expressed by the same pattern in 1/6 case. All tumors showed strong and diffuse immunoreactivity of p16 (Fig. 3C) and CK7 (Fig. 3D). The Ki-67 proliferation index was 15–90%.
In the mixed tumor, the expression pattern of the antibodies (p63, p40, p16, and CK7) in SMILE counterpart was the same as it is in ISMCs. The UEA component showed diffuse and strong positive reactivity for CK7 and p16, whereas p63 and p40 were entirely negative. p16, p63, and p40 were diffuse and strongly positive in HSIL component, and CK7 was negatively stained. All the cases showed intracytoplasmic positive staining for AB (Fig. 3E) and PAS (Fig. 3F) in the ISMC and UEA components, revealing various quantities of intracytoplasmic mucus (from 5% to 95%) in ISMC areas.

Figure 3: p63 (A) and p40 (B) expressed in the peripheral cells of the tumor cell nests; Strong and diffuse p16 (C) and CK7 (D) expression; AB (E) and PAS (F) show various quantities of intracytoplasmic mucus.
ISMC is a recently described endocervical adenocarcinoma of the uterine cervix (Lastra et al., 2016; Stolnicu et al., 2020). It is typically composed of stratified tumor cells with rich mitochondria, rough endoplasmic reticulum, and microvilli on the cell surface in ultrastructure, which are characteristics of glandular cells (Onishi et al., 2016). Therefore, ISMC is classified as a glandular epithelial lesion in the IECC system (Stolnicu et al., 2018). ISMC is likely arising from high-risk HPV-infected cervical reserve cells, which have the potential to differentiate into a variety of architectural and cytological patterns (Stolnicu et al., 2020). Limited report and morphologic variation of ISMC make pathologists may confuse this newly recognized subtype with other histological types. Hence it is necessary to study histological characters of ISMC for a further accurate diagnosis. Moreover, as far as is documented, the bona fide prognosis and malignance of ISMC are still uncertain.
We reviewed 6 cases (including 5 surgically resected and 1 biopsy specimens). The most common clinical presenting symptoms were bleeding after sexual intercourse, vaginal bleeding, or vaginal discharge, with or without atypical Pap smear. The median age was 45.5 years (from 40 to 56 years), which was similar to invasive squamous cell carcinoma and other histologic subtypes of HPV-associated adenocarcinoma of the uterine cervix (median, 42 years), and younger than the non-HPV-associated adenocarcinomas (median, 55 years) (Stolnicu et al., 2018; Horn et al., 2019). Three out of 6 cases accepted HPV detection, and high-risk HPV infection was detected in all the 3 cases, mainly with HPV type 16, 18, or mixed with other types, which is similar to the previous studies that found mainly with HPV type 18 (Lastra et al., 2016; Onishi et al., 2016; Horn et al., 2019; Lei, 2019; Xu et al., 2020). The result supporting the hypothesis that high-risk HPV is associated with tumorigenesis of ISMCs.
ISMCs are classically infiltrating the cervical stroma with nests of stratified mucinous cells. A variable amount of mucin vacuoles (“mucin-poor” to “mucin-rich”), mitotic adjacent to the luminal surface, and apoptotic bodies located in the basal region of the glandular, characteristic of HPV-associated adenocarcinoma, were seen in each of our cases. However, peritumoral and intratumoral neutrophilic-dominant infiltration (Lastra et al., 2016; Lei, 2019; Stolnicu et al., 2020) that reported characteristically identified in ISMC by several previous studies was not so frequently observed in our series and other reports (Onishi et al., 2016; Horn et al., 2019; Xu et al., 2020). ISMCs occur in pure form or may be accompanied by other histological tumor types, like UEA, ASC, mucinous-type adenocarcinoma, or neuroendocrine carcinoma, etc. (Stolnicu et al., 2020), which represents phenotypic instability speculated by some investigators (Park and Soslow, 2009; Boyle and McCluggage, 2015; Backhouse et al., 2016; Onishi et al., 2016; Schwock et al., 2016). The portion of pure ISMC rages from 56% to 86% in different studies. In our research, 4 out of the 5 surgically resected cases (80.0%) were pure tumors, and only ISMC component was found in the biopsy specimen. UEA is the most common component that coexisted with ISMC (Lastra et al., 2016; Stolnicu et al., 2020). The mixed invasive component was UEA in the only ISMCmixed tumor in our research. The most common intraepithelial tumor components accompanied by ISMC are AIS, HSIL, and SMILE (Stolnicu et al., 2020). In our series, no conventional AIS was observed, HSIL was seen in a pure ISMC. Prior studies reported that up to 75% of ISMCs coexisted with SMILE (Horn et al., 2019), which reflects a stepwise progression from the intraepithelial precursor to the invasive counterpart. SMILE was observed in only 1 tumor in our cases, overgrown by the invasive component that may be responsible for it. All the intraepithelial and invasive components mixed with ISMCs are HPV-associated in our series and previous studies; this phenomenon highlights that ISMC shares the same pathogenesis with other high-risk HPV-related carcinomas.
AB and PAS staining identify various acidic and neutral intracytoplasmic mucin throughout the tumor nest of ISMCs. Immunohistochemically, CK7 and p16 are strong and diffused positive in ISMCs as in other high-risk HPV-associated adenocarcinomas, which is in accordance with the prior literature (Park et al., 2000; Onishi et al., 2016). In most cases, p63 and p40 were negative or only focally and weakly positive, and they often expressed in the peripheral cells of the tumor nests in positive tumors. This special expression pattern was significantly different from other HPV-associated cervical adenocarcinomas (negative) and squamous cell carcinoma (diffuse and strongly positive).
Researchers found that ISMCs can differentiate into architectural patterns, including nest, insular, glandular, solid, papillary, trabecular, micropapillary, and single cell with variable amounts of stroma. Tumor cells usually show varying intracytoplasmic mucin, whereas delicate eosinophilic cytoplasm, cytoplasmic clearing, histiocytoid, glassy cell-like, and signet ring-like features also can be observed in some cases (Stolnicu et al., 2020). Most architectural patterns like nest, glandular, solid, trabecular, and single cell, were seen in our cases, existed alone or mixed with each other. We speculate that, whether the patterns of trabecular and single cell in our case represented poorer differentiation? More cases are needed for further study.
ISMC needs to be distinguished from other invasive carcinomas of the cervix. Among the 6 ISMCs in our research, 3 were originally diagnosed as squamous cell carcinoma, 2 were diagnosed as invasive carcinoma without clear classification, and the remaining one was diagnosed as adenocarcinoma coexisted with HSIL on preoperative biopsy specimens. The prior studies also found that ISMC was often misdiagnosed as adenosquamous carcinoma, squamous cell carcinoma, or other histological subtypes of cervical adenocarcinoma. Especially when the adenocarcinomas were poorly differentiated, it was not always possible to make a clear diagnose. Stolnicu and colleagues (Stolnicu et al., 2019) identified that 33.3% (19/57) originally diagnosed adenosquamous carcinomas were pure ISMC or ISMC coexisted with other components. The ISMCs with scarce intracytoplasmic mucin, mimicking the appearance of immature squamous metaplasia (Onishi et al., 2016) are easily diagnosed as adenosquamous carcinomas. Glassy cell carcinoma is a rare poorly differentiated variant of adenosquamous carcinoma, the tumor cells showing sharp cytoplasmic margins, “ground glass” eosinophilic cytoplasm, and large round or ovoid nuclei with prominent nucleoli. The tumor cells of ISMC show glassy cell-like cytoplasm in a few cases, which may make them difficult to be distinguished from the glassy cell carcinomas (Stolnicu et al., 2020). However, adenosquamous carcinoma is a mixed tumor with unequivocal invasive malignant glandular and squamous differentiation, and each component accounts for at least 10% of the tumor (Stolnicu et al., 2019). Whereas, ISMC lacks unequivocal squamous cell carcinoma component and peripheral nuclear palisade can be seen in most cases, even in parts of the tumor. p40 and p63 can express in the peripheral cells of the tumor cell nests in parts of the cases (regardless of the presence of palisading). The characteristic features of ISMC above are not seen in the other tumor types.
SCC is the most common malignant tumor of the cervix, it always grows in a nest pattern, and the cytoplasm can be clear due to intracytoplasmic glycogen production, which makes this tumor similar to ISMC. The presence of intercellular bridges, keratinization, intracellular tonofilaments peculiar in ultrastructure, and diffuse expression of p63 and p40 support a diagnosis of SCC.
Mucoepidermoid carcinoma is an extremely rare tumor in the cervix, with the same histological morphology as in the salivary gland. It is composed of epidermoid cells, mucin-producing cells, and intermediate cells in different proportions. Characteristic t(11;19) due to the gene fusion of CRTC1 and MAML2 was observed in this tumor type in the salivary gland. Detection of MAML2 gene rearrangement by fluorescence in situ hybridization might be useful in the differential diagnosis (Coca-Pelaz et al., 2015).
Clinically, small limited series revealed that ISMCs were potentially more aggressive and have a poorer prognosis than UEAs (Lastra et al., 2016; Hodgson et al., 2019; Horn et al., 2019). Horn et al. (2019) recognized that ISMCs had a high risk of distant metastasis, especially to the lungs. They speculated that the poorer prognosis of ISMCs might be related to more likely to exhibit diffuse destructive stromal invasion (Silva Pattern C) and a higher FIGO stage when diagnose comparing to UEAs (Takeda et al., 2002; Singh and Arif, 2004; Lastra et al., 2016; Hodgson et al., 2019; Horn et al., 2019). Diaz de Vivar et al. (2013) recognized that Silva pattern C was frequently associated with disease recurrence (22%) and death from disease (8%). All tumors infiltrated in Silva pattern C in our study; it was similar to the prior research (91.7–92.3%) (Stolnicu et al., 2020; Xu et al., 2020). The depth of invasion was higher than 10 mm in all of our cases; nerve and/or vessel invasion was observed in 4 out of 6 tumors. These aspects indicate ISMC might be a more aggressive entity.
The percentage of FIGO stage II or higher ISMC was about 30% in previous studies (Horn et al., 2019; Stolnicu et al., 2020). Whereas the percentage was 16.7% (1/6) in our research and 15.4% (2/13) in another Asian series (Xu et al., 2020), this was similar to UEAs (14% of tumors were stage II or higher in a prior study) (Stolnicu et al., 2019). Prior researchers found a clear difference in survival of cervical cancer depending on FIGO stage with near 99% 5-year survival for stage IA1, 65% for IIB, and 43% for IIIB (Rock et al., 2008). Depth of invasion, size of the tumor, lymphovascular invasion, the presence of pelvic/para-aortal lymph node metastases, and parametrial invasion were well-established prognostic factors of cervical adenocarcinomas regardless of histologic subtype (Baalbergen et al., 2004). All the tumors in our research were larger than 2 cm with a mean tumor size of 3.3 cm, which was smaller than Horn’s (Horn et al., 2019). The majority of cases in Horn et al.’s (Horn et al., 2019) series were associated with pelvic lymph node involvement at the time of diagnosis. However, no lymph node metastasis and distant metastasis were observed when diagnosing in our cases.
During the follow-up in our cases, the patients who underwent resection were alive without tumor recurrence, even the patient who only received combined chemo-radiation therapy was alive with disease. Therefore, we speculate that the lower FIGO stage, smaller tumor size, and no lymph node metastasis when diagnosing might contributes to a better prognosis in our research.
Interestingly, unlike Horn et al.’s (Horn et al., 2019) result, Hodgson et al. (2019) found that there was no significant difference of disease-free and disease-specific survival between ISMCs and UEAs on multivariate analysis based on small retrospective studies. Stolnicu and colleagues (Stolnicu et al., 2019) also identified that there was no difference in overall survival and disease-free survival between ISMC (pure and with components) and HPV-associated mucinous carcinomas exclusive of ISMC. So far, during a not long follow-up time, we did not find ISMCs presented a poorer prognosis in our series compared to UEAs previously documented (Baalbergen et al., 2004). More cases and a longer follow-up period were required to fully understand the clinical behavior of this entity. To date, the number of studies evaluating the prognosis of ISMC is limited. Moreover, results on the prognosis of this disease are inconsistent in different studies, so further research is needed to determine whether the outcome was worse in ISMC.
In conclusion, ISMC is a newly described cervical adenocarcinoma with distinct morphology and has a different immunophenotype from other more conventional types of HPV-associated carcinomas. The prognosis of ISMC is controversial. We provide additional distinct histological and clinical information by analyzing our cases. Further studies are needed to understand this kind of tumor better.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Author Contribution: The authors confirm contribution to the paper as follows: study conception and design: YL, TL; data collection: TL; analysis and interpretation of results: TL, YL, SQ, XG, JY, PZ; draft manuscript preparation: TL. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: This study was approved by the Ethics Committee of Cancer Hospital affiliate to School of Medicine, University of Electronic Science and Technology of China (Approval No. SCCHEC-03-2018-005, Approval Date: February 08, 2018).
Funding Statement: This study is financially supported by the Scientific Research Funds Project of the Science and Technology Department of Sichuan Province (Grant No. 21YYJC1616 to Liu Y).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Baalbergen A, Ewing-Graham PC, Hop WC, Struijk P, Helmerhorst TJ (2004). Prognostic factors in adenocarcinoma of the uterine cervix. Gynecologic Oncology 92: 262–267. DOI 10.1016/j.ygyno.2003.09.001. [Google Scholar] [CrossRef]
Backhouse A, Stewart CJ, Koay MH, Hunter A, Tran H, Farrell L, Ruba S (2016). Cytologic findings in stratified mucin-producing intraepithelial lesion of the cervix: A report of 34 cases. Diagnostic Cytopathology 44: 20–25. DOI 10.1002/dc.23381. [Google Scholar] [CrossRef]
Boyle DP, McCluggage WG (2015). Stratified mucin-producing intraepithelial lesion (SMILEReport of a case series with associated pathological findings. Histopathology 66: 658–663. DOI 10.1111/his.12498. [Google Scholar] [CrossRef]
Coca-Pelaz A, Rodrigo JP, Triantafyllou A, Hunt JL, Rinaldo A, Strojan P, Haigentz M,Jr, Mendenhall WM, Takes RP, Vander Poorten V, Ferlito A (2015). Salivary mucoepidermoid carcinoma revisited. European Archives of Oto-rhino-laryngology 272: 799–819. DOI 10.1007/s00405-014-3053-z. [Google Scholar] [CrossRef]
Diaz De Vivar A, Roma AA, Park KJ, Alvarado-Cabrero I, Rasty G, Chanona-Vilchis JG, Mikami Y, Hong SR, Arville B, Teramoto N, Ali-Fehmi R, Rutgers JK, Tabassum F, Barbuto D, Aguilera-Barrantes I, Shaye-Brown A, Daya D, Silva EG (2013). Invasive endocervical adenocarcinoma: proposal for a new pattern-based classification system with significant clinical implications: A multi-institutional study. International Journal of Gynecological Pathology 32: 592–601. DOI 10.1097/PGP.0b013e31829952c6. [Google Scholar] [CrossRef]
Hodgson A, Olkhov-Mitsel E, Howitt BE, Nucci MR, Parra-Herran C (2019). International Endocervical Adenocarcinoma Criteria and Classification (IECCcorrelation with adverse clinicopathological features and patient outcome. Journal of Clinical Pathology 72: 347–353. DOI 10.1136/jclinpath-2018-205632. [Google Scholar] [CrossRef]
Horn LC, Handzel R, Borte G, Siebolts U, Haak A, Brambs CE (2019). Invasive stratified mucin-producing carcinoma (i-SMILE) of the uterine cervix: report of a case series and review of the literature indicating poor prognostic subtype of cervical adenocarcinoma. Journal of Cancer Research and Clinical Oncology 145: 2573–2582. DOI 10.1007/s00432-019-02991-3. [Google Scholar] [CrossRef]
Lastra RR, Park KJ, Schoolmeester JK (2016). Invasive Stratified Mucin-producing Carcinoma and Stratified Mucin-producing Intraepithelial Lesion (SMILE15 cases presenting a spectrum of cervical neoplasia with description of a distinctive variant of invasive adenocarcinoma. American Journal of Surgical Pathology 40: 262–269. DOI 10.1097/PAS.0000000000000543. [Google Scholar] [CrossRef]
Lei R (2019). Invasive stratified mucin-producing carcinoma: A clinicopathological analysis of three cases. Cancer Biology & Therapy 20: 1403–1407. DOI 10.1080/15384047.2019.1647054. [Google Scholar] [CrossRef]
Onishi J, Sato Y, Sawaguchi A, Yamashita A, Maekawa K, Sameshima H, Asada Y (2016). Stratified mucin-producing intraepithelial lesion with invasive carcinoma: 12 cases with immunohistochemical and ultrastructural findings. Human Pathology 55: 174–181. DOI 10.1016/j.humpath.2016.05.007. [Google Scholar] [CrossRef]
Parra HC, Alvarado CI, Hoang LN (2020). Tumours of the uterine cervix. In: WHO Classification of Tumours Editorial Board (ed.WHO Classification of Tumours of Female Genital Tumours. 5th ed. Lyon, France: IARC Press. [Google Scholar]
Park JJ, Sun D, Quade BJ, Flynn C, Sheets EE, Yang A, McKeon F, Crum CP (2000). Stratified mucin-producing intraepithelial lesions of the cervix: Adenosquamous or columnar cell neoplasia? American Journal of Surgical Pathology 24: 1414–1419. DOI 10.1097/00000478-200010000-00012. [Google Scholar] [CrossRef]
Park KJ, Soslow RA (2009). Current concepts in cervical pathology. Archives of Pathology & Laboratory Medicine 133: 729–738. DOI 10.5858/133.5.729. [Google Scholar] [CrossRef]
Rock JA, Jones HW, Te Linde RW (2008). Te Linde’s operative gynecology. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
Schwock J, Ko HM, Dubé V, Rouzbahman M, Cesari M, Ghorab Z, Geddie WR (2016). Stratified mucin-producing intraepithelial lesion of the cervix: Subtle features not to be missed. Acta Cytologica 60: 225–231. DOI 10.1159/000447940. [Google Scholar] [CrossRef]
Singh N, Arif S (2004). Histopathologic parameters of prognosis in cervical cancer--A review. International Journal of Gynecological Pathology 14: 741–750. [Google Scholar]
Stolnicu S, Barsan I, Hoang L, Patel P, Terinte C, Pesci A, Aviel-Ronen S, Kiyokawa T, Alvarado-Cabrero I, Pike MC, Oliva E, Park KJ, Soslow RA (2018). International Endocervical Adenocarcinoma Criteria and Classification (IECCA new pathogenetic classification for invasive adenocarcinomas of the endocervix. American Journal of Surgical Pathology 42: 214–226. DOI 10.1097/PAS.0000000000000986. [Google Scholar] [CrossRef]
Stolnicu S, Hoang L, Chiu D, Hanko-Bauer O, Terinte C, Pesci A, Aviel-Ronen S, Kiyokawa T, Alvarado-Cabrero I, Oliva E, Park KJ, Abu-Rustum NR, Soslow RA (2019a). Clinical outcomes of HPV-associated and unassociated endocervical adenocarcinomas categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC). American Journal of Surgical Pathology 43: 466–474. DOI 10.1097/PAS.0000000000001224. [Google Scholar] [CrossRef]
Stolnicu S, Hoang L, Hanko-Bauer O, Barsan I, Terinte C, Pesci A, Aviel-Ronen S, Kiyokawa T, Alvarado-Cabrero I, Oliva E, Park KJ, Soslow RA (2019b). Cervical adenosquamous carcinoma: Detailed analysis of morphology, immunohistochemical profile, and clinical outcomes in 59 cases. Modern Pathology 32: 269–279. DOI 10.1038/s41379-018-0123-6. [Google Scholar] [CrossRef]
Stolnicu S, Segura S, Parra-Herran C, Horn LC, Hoang L, Terinte C, Pesci A, Aviel-Ronen S, Kyokawa T, Alvarado-Cabrero I, Oliva E, Soslow RA, Park KJ (2020). Invasive Stratified Mucin-producing Carcinoma (ISMC) of the cervix: A study on morphologic diversity. American Journal of Surgical Pathology 44: 873–880. DOI 10.1097/PAS.0000000000001480. [Google Scholar] [CrossRef]
Takeda N, Sakuragi N, Takeda M, Okamoto K, Kuwabara M, Negishi H, Oikawa M, Yamamoto R, Yamada H, Fujimoto S (2002). Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstetricia et Gynecologica Scandinavica 81: 1144–1151. DOI 10.1034/j.1600-0412.2002.811208.x. [Google Scholar] [CrossRef]
Wilbur DC, Colgan TJ, Ferenczy AS (2014). Tumours of the uterine cervix. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH (eds.WHO Classification of Tumours of Female Reproductive Organs. 4th ed. Lyon, France: IARC Press. [Google Scholar]
Xu H, Wang YM, Zhang J (2020). Clinicopathological features of stratified mucin-producing neoplastic lesions of the cervix. Zhonghua Bing Li Xue Za Zhi 49: 28–33. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |