DOI:10.32604/biocell.2021.013913

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.013913 |  www.techscience.com/journal/biocell |
| Article |
Ultrastructural analysis shows persistence of adhesion and tight junction proteins in mature human hair
1Comparative Histolab Padova and Department of Biology, University of Bologna, Bologna, 40126, Italy
2KAO Europe Research Laboratories, Kao Germany GmbH, Darmstadt, 64297, Germany
*Address correspondence to: Lorenzo Alibardi, lorenzo.alibardi@unibo.it
Received: 26 August 2020; Accepted: 16 November 2020
Abstract: The differentiation of cells composing mature human hairs produces layers with different corneous characteristics that would tend to flake away one from another, as in the corneous layer of the epidermis, without anchoring junctions. It is likely that cell junctions established in the forming cells of the hair bulb are not completely degraded like in the corneous layer of the epidermis but instead remain in the hair shaft to bind mature cuticle, cortex, and medulla cells into a compact hair shaft. During cell differentiation in hairs, cell junctions seem to disappear, and little is known about the fate of junctional proteins present in the mature human hair shaft. The present ultrastructural immunogold study has detected some marker proteins of adhesion junction (cadherin and beta-catenin) and tight junctions (occludin and cingulin) that are still present in cornified hairs where numerous isopeptide bonds are detected, especially in the medulla. This qualitative ultrastructural study indicates that aside from the cell membrane complex, a long corneo-desmosome bonding cortex and cuticle cells, also sparse adherens and tight junction remnants are present. It is suggested that the cornification of these junctions with the incorporation of their proteins within the mature corneous material of the hair shaft likely contributes to maintaining the integrity of the mature hair. This information will also allow us to evaluate the effects of different chemical components present in hair formulations and stains on these junctional proteins and the consequent integrity of the hair shaft.
Keywords: Human hair; Cornification; Adhesion junctions; Tight junctions; Immunogold; Ultrastructure
Hairs derive from a unique process of cornification since cells originated in the hair matrix gives rise to different layers forming an onion-like organization inside the follicle (Rogers, 2004; Morioka, 2005; Langbein and Schweizer, 2005). Hair keratinocytes originate in the bulb, the bottom region of the follicle that is deeply inserted in the dermis and gives rise to the hair shafts composed of differently cornified layers, the cuticle and cortex, and in some cases also to a central medulla. During their maturation, cells of the hair increase their content in keratins and associated proteins (keratogenous zone, above the bulb), and their cell junctions are affected by this process. After full cornification in the consolidation zone, located above the keratogenous zone, most of the cortex cells’ boundaries are no longer distinguished (Orwin et al., 1973a; Orwin et al., 1973b; Orwin et al., 1973c; Orwin, 1979). Extraction of mature hairs with sodium dodecyl sulfate alone or in conjunction with reducing agents such as dithiothreithol at high temperature (80–100°C) determines a marked hair swelling and the extraction of most macro-fibrillar material from cortical cells with some preservation of the hard material present along the plasma membrane (Rice et al., 1994). It is likely that most of the resistance to extractive solvents is derived from the presence of numerous iso-peptide bonds while disulfide bonds from keratins and Keratin Associated Proteins (KAPs) are cleaved (Lee et al., 2006; Barthelemy et al., 2012). Cuticle cells appear a little altered after this treatment, but also medullary cells are more resistant than cortical cells, and a much thicker layer of corneous material is deposited along the cell periphery, apparently the more resistant structure of hairs.
Desmosomes, adherens and tight junctions are established from the different lineages of hair keratinocytes originated in the matrix region of the follicle (Orwin et al., 1973a; Orwin et al., 1973b; Orwin, 1979; Kurzen et al., 1998; Muller-Rover et al., 1999; Brandner et al., 2003; Tinkle et al., 2003; Young et al., 2003; Zhorn-Kruppa et al., 2018). In human and porcine hairs, tight junctions are formed above the bulb and between the keratinizing Henle and Huxley layer of the Inner Root Sheath (IRS) and between the Henle layer and companion layer of the Outer Root Sheath (ORS) (Zhorn-Kruppa et al., 2018). In pre-keratinized cortical cells of the wool follicle, gap junctions occupy 4–5% of the entire surface of cortical cells, desmosomes about 5%, while tight junctions are lower in percentage (Orwin et al., 1973a; Orwin et al., 1973b; Orwin, 1979). A remarkable change in cell junction architecture during cuticle, cortex, and IRS maturation is the formation of a penta-laminar structure, the “cell membrane complex,” formed by two dense thickenings in the plasma membrane of apposed cells and by two pale spaces, the beta-layers, separated by a central darker band, the delta-layer (Rogers, 1959, 2004; Bryson et al., 1992; Robbins, 2009; Jones et al., 2010). The cell membrane complex has a uniform thickness in cuticle cells, but the delta-layer becomes more irregular in cortical cells and contains desmosomal proteins such as plakophilins 1 and 3, and desmoglein 4 (Alibardi and Noecker, 2013; Alibardi et al., 2013). Some desmosomal proteins remain incorporated in the cornified cell membrane complex that appears as the longest junction formed in hairs for maintaining the cohesiveness in mature cuticle and cortical cells.
In addition to desmosomes, also adhering and tight junctions further strengthen cohesiveness among hair keratinocytes for maintaining hair integrity. Adherens junctions have been implicated in processes of cell-cell recognition and later of selective adhesion to form epithelial layers (Niessen, 2007), and possibly also during hair morphogenesis and cycling (Hardy and Vielkind, 1996; Kurzen et al., 1998; Muller-Rover et al., 1999; Young et al., 2003). Immunolabeling studies showed that also tight junctions tend to disappear in the hair shaft as cortical and cuticle cells mature (Langbein et al., 2002), but the fine details at the ultrastructural level for the human hair are not known.
The present TEM immunogold investigation aims to detect marker proteins for adherens and tight junctions in the keratinizing and consolidation regions of the human hair when hair keratinocytes are in an advanced stage of differentiation and their cytoplasm is mainly occupied by keratins and other proteins that accumulate and give rise to the mature corneous material (corneous proteins, Rogers, 2004; Langbein and Schweizer, 2005). Cadherins are detected using Pan-cadherin and beta-catenin antibodies as general markers, while tight junctional proteins are identified using anti-occludin and anti-cingulin antibodies. The qualitative analysis also includes the detection of isopeptide bond in the keratogenous and consolidating zone of the hair follicle, a chemical bond derived from the action of transglutaminases on some protein substrates (Kalinin et al., 2002; Rogers, 2004; Thibaut et al., 2005; Yamane et al., 2010). As a practical fall-out, the present study may produce useful information about the damage to the hair compactness in natural conditions during aging or after perms and heat treatments to hairs, treatments that might negatively affect these junctional proteins and the compactness of hairs.
Hairs from the scalp or the beard were collected from four informed healthy individuals of mid-age (46–53) by direct pulling the hairs from the follicles using tweezers. These people gave their permit for sampling and the use of hairs for the present and previous studies on similar samples (Alibardi and Noecker, 2013). Only the hairs that contained a bulb at their base were utilized for the study. In total, we analyzed the ultrastructure of the bulb in eleven medullated hairs (8 beard and 3 scalps hairs) and six non-medullated hairs (scalp hairs).
The hairs were cut in 1–2 mm pieces from the bulb and immediately fixed in cold (0–4°C) fixative freshly made (4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4) for 6–8 h, then rinsed in the buffer for about 30 min, and dehydrated in ethanol (70, 90, and 100%, changes of 30 min each) at 0–4°C. The hairs were then infiltrated in ethanol-Lowicryl Resin K4M (1:1) for about 1 h, then in pure Lowicryl Resin (Polyscience, USA) for 6–8 h, and later exposed to UV-light at 0–4°C for three days. The use of this hydrophilic resin for electron microscopy is suited for maintaining the antigenicity of hair proteins for the following immunocytochemical procedures (Alibardi and Noecker, 2013; see below). The samples were sectioned in transverse or longitudinal section using an LKB-Novae ultramicrotome (LKB, Bromma, Sweden), and the sections were attached to glass slides for light microscopy or copper or nickel grids for electron microscopy.
Histology and ultrastructural immunocytochemistry
Semithin sections were utilized for light microscopy in order to localize areas of interest where to collect thin sections (60–90 nm thick) for the immunocytochemical analysis under the electron microscope. The thin sections to be studied under TEM were collected on nickel grids, and some sections were stained with uranyl acetate (10 min) and lead citrate (6 min) for the morphological study according to standard methods. Other thin sections were instead utilized for immunogold labeling after they were pre-incubated for 10 min in 0.1 M phosphate buffer at pH 7.6, containing 2% cold water fish gelatin and 1% Triton-X to block non-specific antigenic sites.
Some grids were incubated with a rabbit Pan-cadherin antibody (C3678, Sigma, USA) for the general labeling of adherens junctions using a 1:50–1:100 dilution in the above buffer. A rabbit antibody against beta-catenin (C2206, Sigma-Aldrich) was also utilized with a 1:50–1:100 dilution in the buffer. The cingulin rabbit antibody, C-532, was a kind gift from Dr. S. Citi, University of Geneva (see Citi et al., 1988), and it was also diluted 1:50–1:100 in the buffer. A mouse antibody against occludin (8N-19, sc-8145, Santa Cruz, USA) was diluted 1:50–1:100 in buffer. Finally, a mouse antiserum against iso-peptide bond (ab422, Abcam, Cambridge, USA) was employed at a dilution of 1:50–1:100 in buffer. The sections were incubated overnight with the primary antibodies in the phosphate buffer indicated above at 4°C. Some sections were instead utilized as control sections, and they were incubated overnight as above, omitting the specific primary antibodies in the incubation medium. After incubation, the grids were rinsed in the buffer for three times within 10 min and incubated for 1 h at room temperature with the secondary antibody (Sigma, USA) diluted 1:100 in the buffer, anti-rabbit (for P-cadherin, Cingulin, Beta-catenin) or anti-mouse (for iso-peptide and occludin) IgG conjugated to 10 nm gold particles.
After washing in the buffer, grids were rinsed in distilled water (filtered with a 0.2 μm sterile filter) and dried at room temperature. These grids were then stained in 2% aqueous uranyl acetate for 4 min, rinsed in distilled water, dried, and observed under a Philips CM100 or Zeiss 10C/CR transmission electron microscopes. Pictures were collected with films or by a digital camera, and plates were made using the Adobe Photoshop program 8.0.
Histology and Immunofluorescence for isopeptide bonds
The available stages of hairs here examined are illustrated in Figs. 1A-1D, and they were medullated or non-medullated hairs. The sample included stages varying from follicles at the level of the keratogenous zone, showing vacuolating medullary cells (when present), and cortical and cuticle cells still accumulating keratin bundles (Figs. 1A, 1B), and hairs containing also the consolidation zone, where most cuticle and cortical cells were merged into a compact corneous mass and only some nuclei were still seen (Figs. 1C, 1D).
The immunostaining of hairs using the isopeptide antibody (ε-bond) indicated that the proteins that are cross-linked with this type of chemical bond were more numerous in the medulla of hairs (Figs. 1E, 1F). In the cortex, a diffuse, sometimes finely punctiform immunolabeling was seen, but it appeared, in general, low to absent. Although the cuticle appeared also fluorescent, the controls often showed that the cuticle was auto-fluorescent, indicating that the cuticle labeling was, at least in part, non-specific (Fig. 1G). The IRS was immunopositive in the sections still containing this tissue, but the IRS was often absent in our samples (data not shown, see Alibardi and Noecker, 2013).
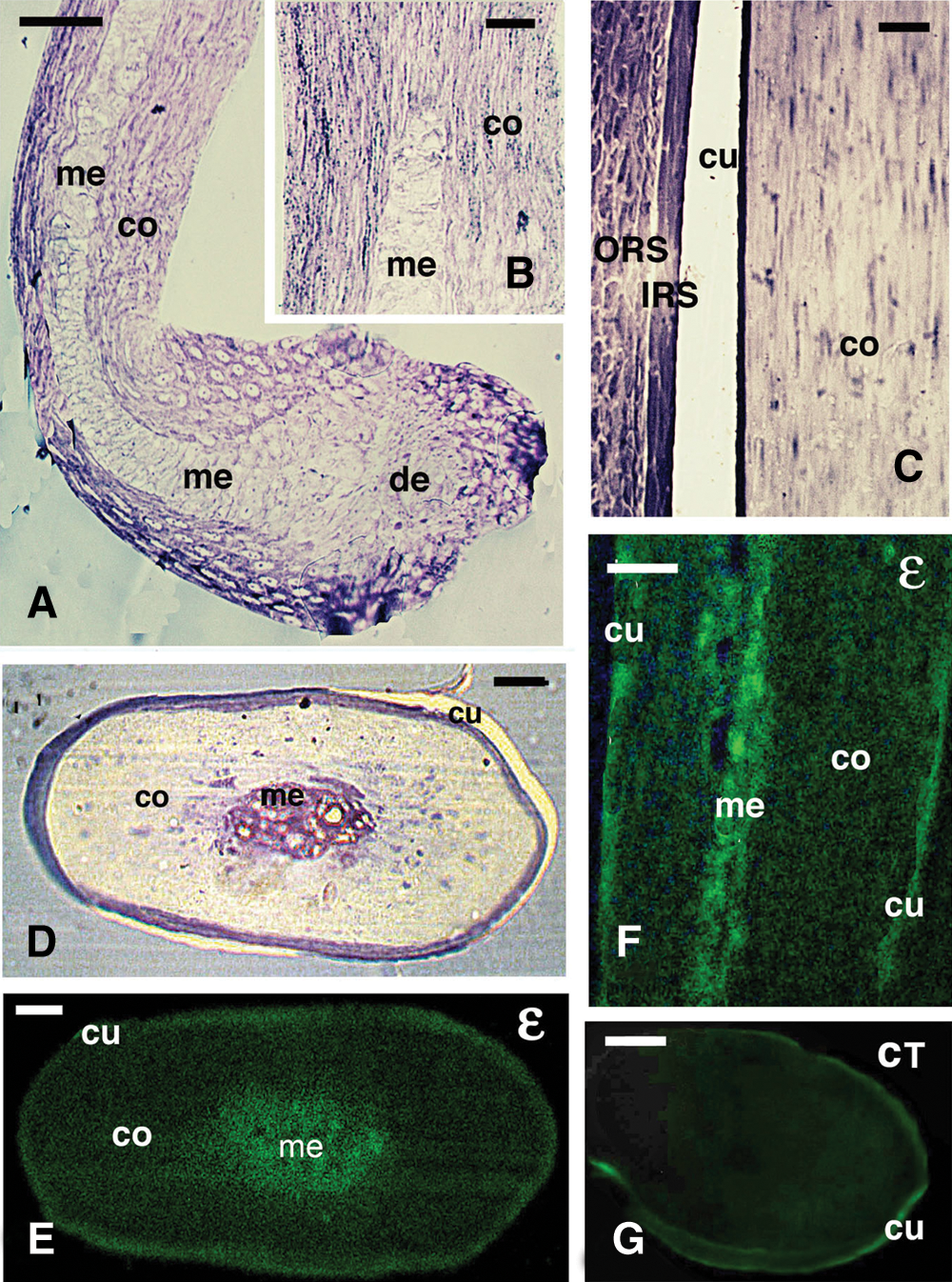
Figure 1: Histology of keratinizing (A, B) and cornified (C, D) hair, and immunofluorescence for isopeptide-bond (ε) detection (F–G). A, hair bulb (beard) with forming medulla and cortex in the keratogenous zone. Toluidine blue stain. Bar, 20 μm. B, detail on pale medulla cells and differentiating fusiform cortical cells in the keratogenous zone. Toluidine blue. Bar, 10 μm. C, detail of largely cornified, upper hair shaft region (consolidation zone) showing the denser cuticle, IRS and ORS cells. Bar, 10 μm. D, cross-section of a cornified hair shaft in the consolidation zone with a central medulla. Bar, 20 μm. E, parallel cross-section of the previous figure (consolidation zone), showing a diffuse immunofluorescence in the cortex and cuticle, but more intense in the medulla. Bar, 20 μm. F, longitudinal section of cornified hair shaft (consolidating zone), showing most of the immunolabeling located in the medulla. Bar, 10 μm. G, cross-sectioned control section of hair in the consolidating zone, showing a weak (auto-)fluorescence in the cuticle but no labeling in the shaft. Bar, 20 μm. Legends: de, dermal papilla; co, cortex; cu, cuticle; IRS, inner root sheath; me, medulla; ORS, outer root sheath.
Immunogold labeling for isopeptide-bond
The present analysis mainly considered the areas of the cuticle, cortex, and medulla (when present, Fig. 2), observed after immunogold labeling (Figs. 3–7) during cornification. In normally stained sections with uranyl acetate and lead citrate, derived from Lowicryl embedded hairs, cortical cells accumulated medium to low electron-dense keratin bundles (macrofibrils) in the keratogenous zone (Figs. 2A–2C), that were eventually packed into a compact and electron-pale mass in the consolidation zone shown in Figs. 2A, 2B. Medulla cells became electron-pale before degenerating into vacuolated cells and were intensely labeled for isopeptide bond (Fig. 2C). The mature cuticle cells accumulated a mass of corneous material subdivided into a denser region (endocuticle) and an electron-paler region (exocuticle) that were surrounded by a thickened plasma membrane (Fig. 2D). Both nuclei and cell membranes largely disappeared in fully cornified cortical cells in the consolidation zone, and only a few regions with fusions in the plasma membrane remained (Figs. 2D–2E). Nucleo-cytoplasmic remnants appeared as small denser regions located among the electron-pale corneous material (macrofibrils).
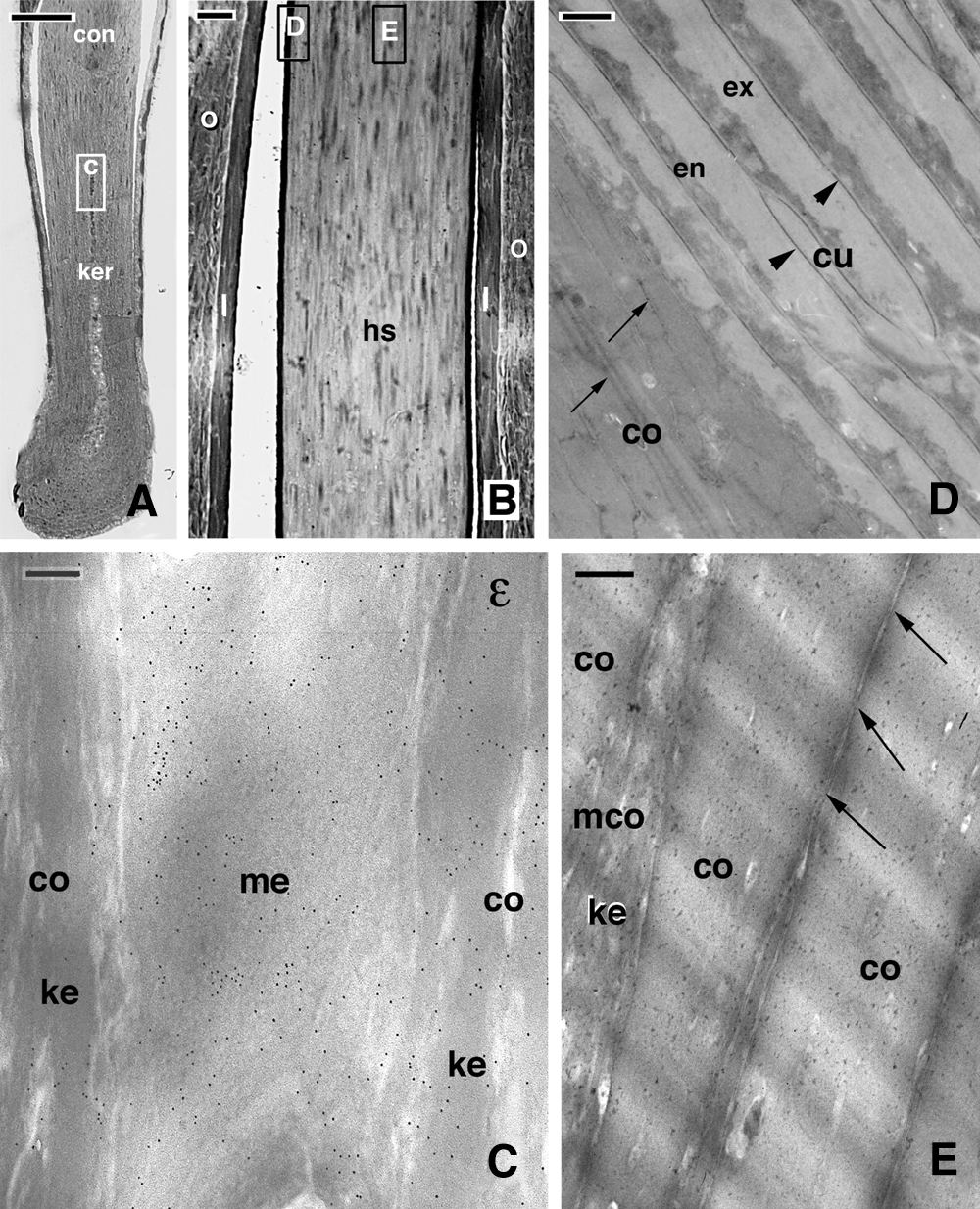
Figure 2: Light (A, B) and electron microscopy (C–E) images of the general morphology in representative hair sections showing the regions of the hair bulb that have been mainly analyzed in the present study by immunogold labeling. A, hair bulb and supra-bulb region including the keratogenous zone and the consolidation zone at the top. The square C indicates a corresponding area of the medulla as presented in figure C. Bar, 50 μm. B, close-up to the consolidation zone where the squares D and E are indicating corresponding regions shown in figures D and E. Bar, 10 μm. C, detail on a medullary cell intensely labeled for isopeptide-bond (ε). Bar, 200 nm. D, detail of mature cuticle (arrowheads indicate the thickened plasma membrane) and cortical cells (arrows indicate some boundaries/membranes still present among the merged and electron-pale macrofibrils). Bar, 200 nm. E, detail of cortical cells in the consolidating zone (arrows indicate fusion spots). A maturing cortical cell with still visible keratin bundles is also present. Bar, 500 nm. Legends: co, cortical cell; con, consolidation zone; cu, cuticle cell; en, endocuticle; ex, exocuticle; hs, hair shaft; I, Inner Root Sheath; ke, keratin bundles; ker, keratogenous zone; mco, maturing cortical cells with not completely packed keratin bundles; me, medulla; ORS, Outer Root Sheath.
Immunogold-labeling for isopeptide-bonds indicated a variable but even distribution of gold particles over the cuticle and cortex in the keratogenous and consolidation zones (Fig. 3A). The cuticle showed a thin electron-pale layer (A-layer), a pale exocuticle, and a dense endocuticle (Figs. 3A, 3B). In the cuticle, the labeling was low to absent over the A-layer and the membrane complex joining the strata of cuticle cells (Fig. 3B). The exo-cuticle and the endo-cuticle showed a diffuse but even labeling.
Also, the cortex was diffusely but evenly labeled, and gold particles were present over the keratin bundles (macrofibrils), as observed in both cross- and longitudinal-sections (Figs. 3C, 3D). Occasional labeling was also seen in the delta-layer of the irregular cell membrane complex joining these cells, the denser line present among cortical cells (Figs. 3C, 3D). A higher immune-labeling for the iso-peptide bond was seen in the corneous material of the medulla regions confining with the cortex (Figs. 2C and 3E), but the highest labeling was noted in more internal trabeculae of medullary cells, located among the corneous cytoplasm still present between the vacuoles of mature medullary cells (Fig. 3F). This corneous material of inner medullary cells appeared made of a meshwork of fine filaments (8–10 nm thick), and the labeling was uniformly intense. In controls sections, few gold particles were seen in the cuticle, cortex, and medulla (Figs. 3G–3H).
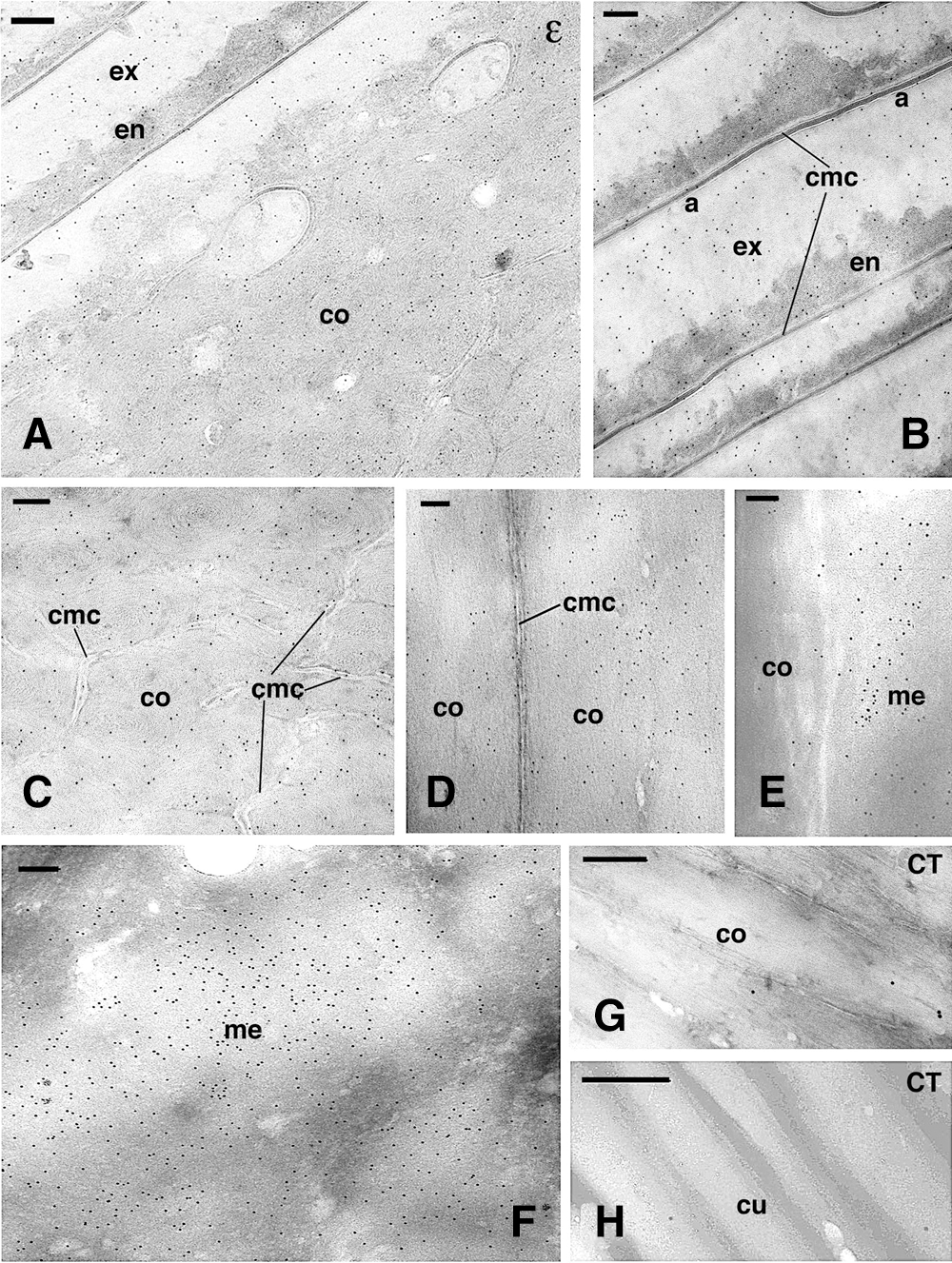
Figure 3: Immunogold labeling for isopeptide-bond (ε) in cornified cuticle-cortex (A–D), medulla (E–F) and control sections (G–H) in the consolidating zone. A, diffuse labeling in the cuticle and cortex observed in cross-section. Bar, 200 nm. B, high magnification showing the low labeling present in cuticle cells and in the membrane complex. Bar, 150 nm. C, detail on cross-sectioned cortex with extended membrane complex and diffuse labeling. Bar, 200 nm. D, detail on a longitudinal section of two adjacent cortical cells showing the unlabeled membrane complex. Bar, 200 nm. E, detail on immunolabeled external part of the medulla that contacts a low labeled cortical cell (position similar to that in Fig. 2C). Bar, 100 nm. F, central region of medullary cell with intensely immunolabeled fibrillar network. Bar, 200 nm. G, immunonegative control (CT) of cortical cells observed in longitudinal section. Bar, 150 nm. H, immunonegative control (CT) of cuticle cells seen in cross section. Bar, 200 nm. Legends: a, A-layer of the exocuticle; co, cortex; cmc, cell membrane complex; cu, cuticle cell; en, endocuticle; ex, exocuticle; me, medulla.
Immunogold-labeling for adhesion junction proteins
The immunolabeling for P-cadherin appeared in the form of small clusters of gold particles sparsely detected in all compartments of the hair cuticle, cortex, and medulla. The accumulated keratin bundles and the corneous material of keratinizing and mature cells were unlabeled, especially in the consolidation zone, and no labeling of the cell membrane complex of cortical and cuticle cells was observed. In cornified cortical cells, gold particles were seen along the membrane of some cells, but most cell membranes were immuno-negative (Fig. 4A). The labeling appeared in the form of clusters or linear aggregates of 4–10 gold particles located near or along the cell membrane when the membrane was still visible (Figs. 4B–4C). No cell membrane specializations such as dense thickenings, cell membrane complex, or intercellular material in cortical cells were seen associated with gold particles. Clusters of gold particles were detected in spots that represented tangentially sectioned cell membranes where filaments of 10–12 nm in diameter, likely made of keratin, were present. Some labeling was detected in the corneous material bordering the cell membrane complex in internal cortical cells in contact with medullary cells (Fig. 4D).
Cuticle cells in the keratogenous and consolidation zone appeared largely unlabeled for P- cadherin, aside from rare clusters of gold particles present near the cell membrane (Figs. 4E–4F). In medullary cells, sparse clusters of gold particles were seen near the cell periphery and desmosomal remnants. Controls were immunonegative or showed few random-scattered gold particles but no clusters. Immunolabeling distribution in differentiating cortical cells localized in the lower, keratinizing zone showed cadherin labeling external or along the plasma membrane but also in denser regions of cortical cells, likely representing tangentially sections of the plasma membrane (Figs. 5A, 5B). Control sections did not show any clustering of gold particles but only occasionally sparse gold particles (Fig. 5C).
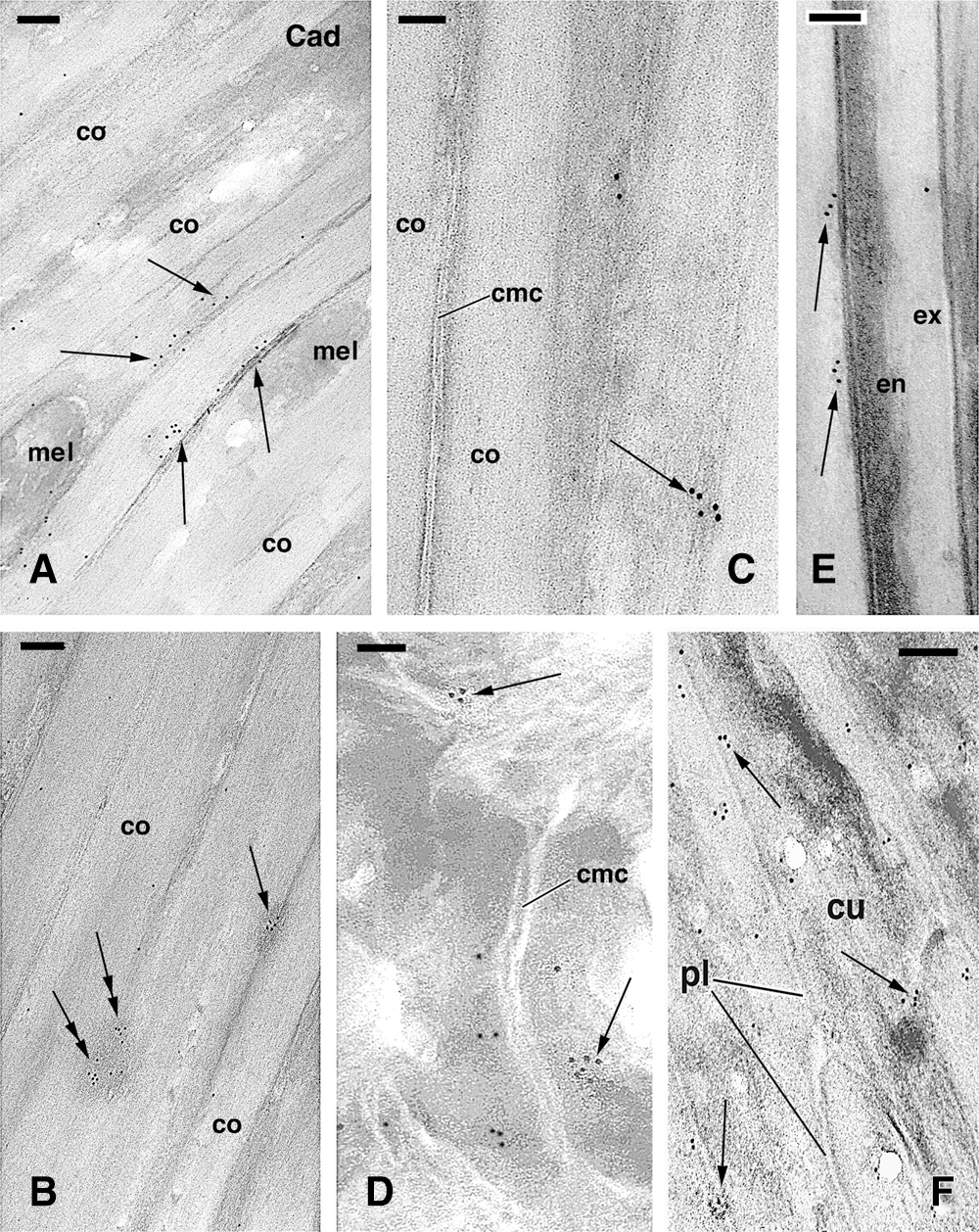
Figure 4: TEM immunolabeling for P-chaderin in mature cortical (A–C) and cuticle (E–F) cells in consolidating zone of the hair. A, cornified cortical cells cut in the in longitudinal plane, showing clustered labeling near and along the plasma membrane (arrows). Bar, 150 nm. B, other region of the cortex in longitudinal section showing clusters of gold particles along visible plasma membranes (arrow) or in areas that represent tangentially-cut membranes (double arrows). Bar, 200 nm. C, high magnification detail on a cluster of gold particles (arrow) on an area likely representing a tangentially cut membrane remnant of a cortical cell. No labeling is seen in the cell corneous membrane. Bar, 100 nm. D, detail on clusters of gold particles (arrows) detected near the membrane complex of inner cortical cells. Bar, 150 nm. E, detail on clusters of gold particles (arrows) localized near the cell membrane complex of mature cuticle cells. Bar, 100 nm. F, high magnification detail of the labeling (arrows) present along the plasma membrane of differentiating cuticle cells. Bar, 200 nm. Legends: co, cortex; cmc, cell membrane complex; cr, cell remnants; cu, cuticle cell; en, endocuticle; ex, exocuticle; pl, plasma membrane.
The immunolabeling for beta-catenin in the elongated cortical cells of the keratogenous zone was distributed within the cytoplasm among keratin bundles (Fig. 5D) and in the nucleus of these differentiating cells (data not shown). However, in upper levels of the follicle, in the consolidation zone, the beta-catenin labeling in cortical cells tended to concentrate near their plasma membranes or their likely remnants (Fig. 5E) or was detected close to the cell membrane complex of mature cortical cells but not in the membrane complex itself (Fig. 5F). A low and sparse beta-catenin immunolabeling was seen in cuticle cells while some nuclei appeared intensely labeled, especially in the euchromatin (data not shown).
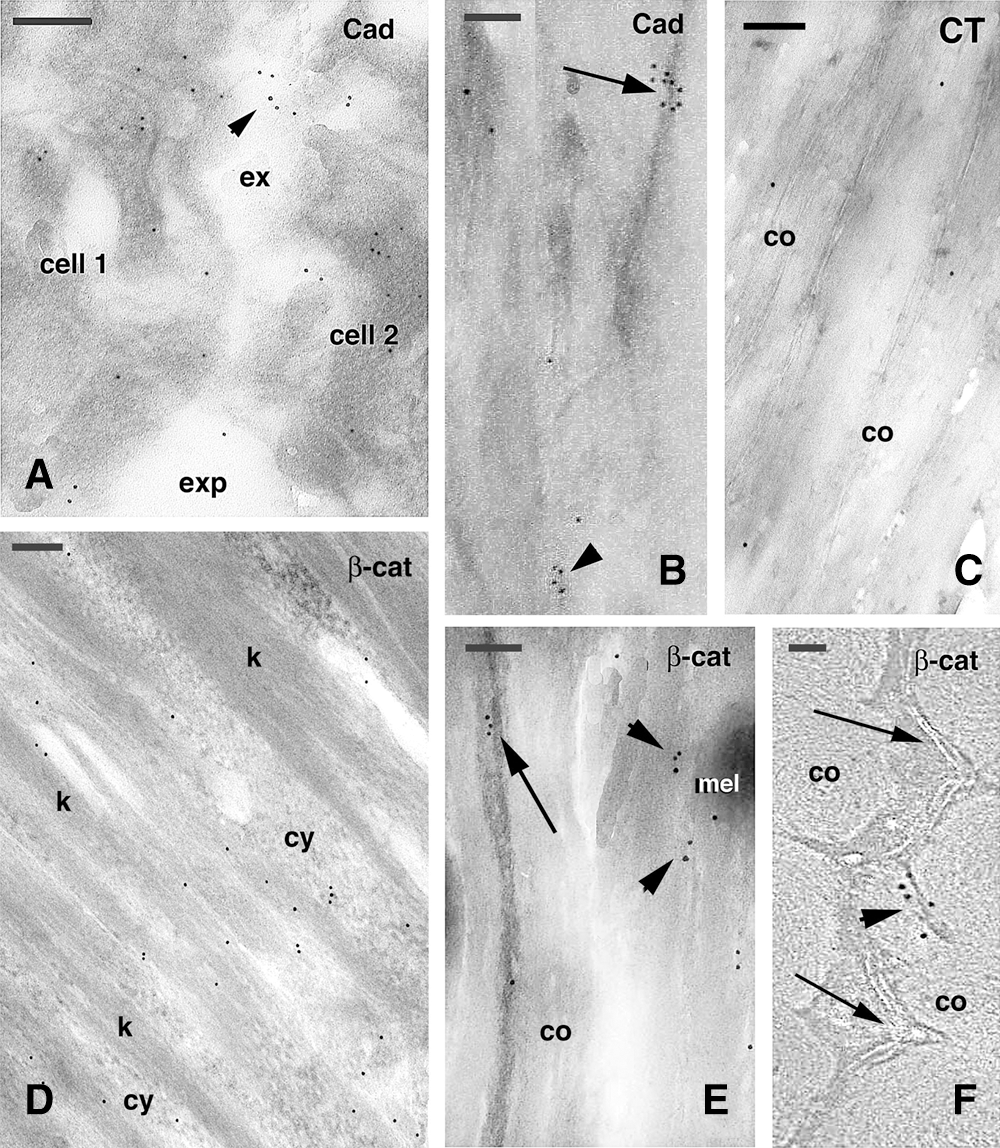
Figure 5: TEM immunogold labeling for P-cadherin (A–C, Cad) and beta-catenin (D–F, β-cat) in cortical cells. A, two cells of the keratinizing region (position like that indicated by ker in Fig. 2A) showing some labeling (arrowhead) on electron-lucent areas on their surface. Bar, 200 nm. B, detail on cornified cortical cell where no cell membranes are visible. Two clusters of gold particles are seen, one associated to a denser material (arrow) and the other to paler corneous material (arrowhead). Bar, 100 nm. C, Control section (CT) of a boundary region localized between cortex and cuticle cells and showing no labeling. Bar, 100 nm. D, beta-catenin immunolabeling present in differentiating cells of the keratogenous zone (position like that indicated by ker in Fig. 2A), which cytoplasm contains mainly sparse gold particles. Bar, 200 nm. E, cluster of gold particles close to likely remnants of plasma membranes (arrow) between two cortical cells cut in longitudinal section. Other small clusters of gold particles are seen on paler corneous material (arrowheads). Bar, 200 nm. F, cross-sectioned cornified cells showing a cluster of gold particles (arrowhead) localized outside the irregular cell membrane complex (arrows). Bar, 50 nm. Legends: co, cortex (macrofibril); cy, cytoplasm; exp, extracellular space; k, keratin bundles; mel, melanosome.
Immunogold-labeling for tight junction proteins
The labeling for occludin in cortical cells appeared as sparse rows of gold particles (Figs. 6A, 6B) or, more frequently, clusters made by 5–30 gold particles (Figs. 6C–6D). These large and roundish-shaped clusters were not seen in negative controls and were also absent in previous observations on the immunodetection for other proteins (Alibardi, 2017; Alibardi and Noecker, 2013; Alibardi et al., 2013), indicating a different labeling pattern. The clusters were closely associated with the periphery of sparse cortical and cuticle cells and their cell membrane when visible. The labeling, however, was uneven along the entire surface of the cornified cortical and cuticle cells. In cortical cells, groups of gold particles were present over scattered regions of the plasma membrane but not in desmosomal remnants or in the cell membrane complex (Figs. 6A, 6B). In some regions of the perimeter of cortical cells, gold particles appear associated with the merging membranes of contiguous cortical cells (Fig. 6B). The larger clusters of gold particles were, however, seen in longitudinal sections of cortical cells in the keratogenous and consolidation zones of hairs, likely representing tangential sections of the membrane remnants where more epitopes for occludin were available for binding (Figs. 6C, 6D).
In mature cuticle cell of the consolidation zone, few and sparse clusters of gold particles were seen, and they were localized especially in the exocuticle close to the cell membrane complex (Fig. 6E) or in darker areas within cuticle cells that likely represented tangentially sectioned membrane of adjacent cuticle cells (Fig. 6F). No clusters but instead a diffuse labeling of single gold particles or complete absence of labeling was seen in controls (Fig. 6G).
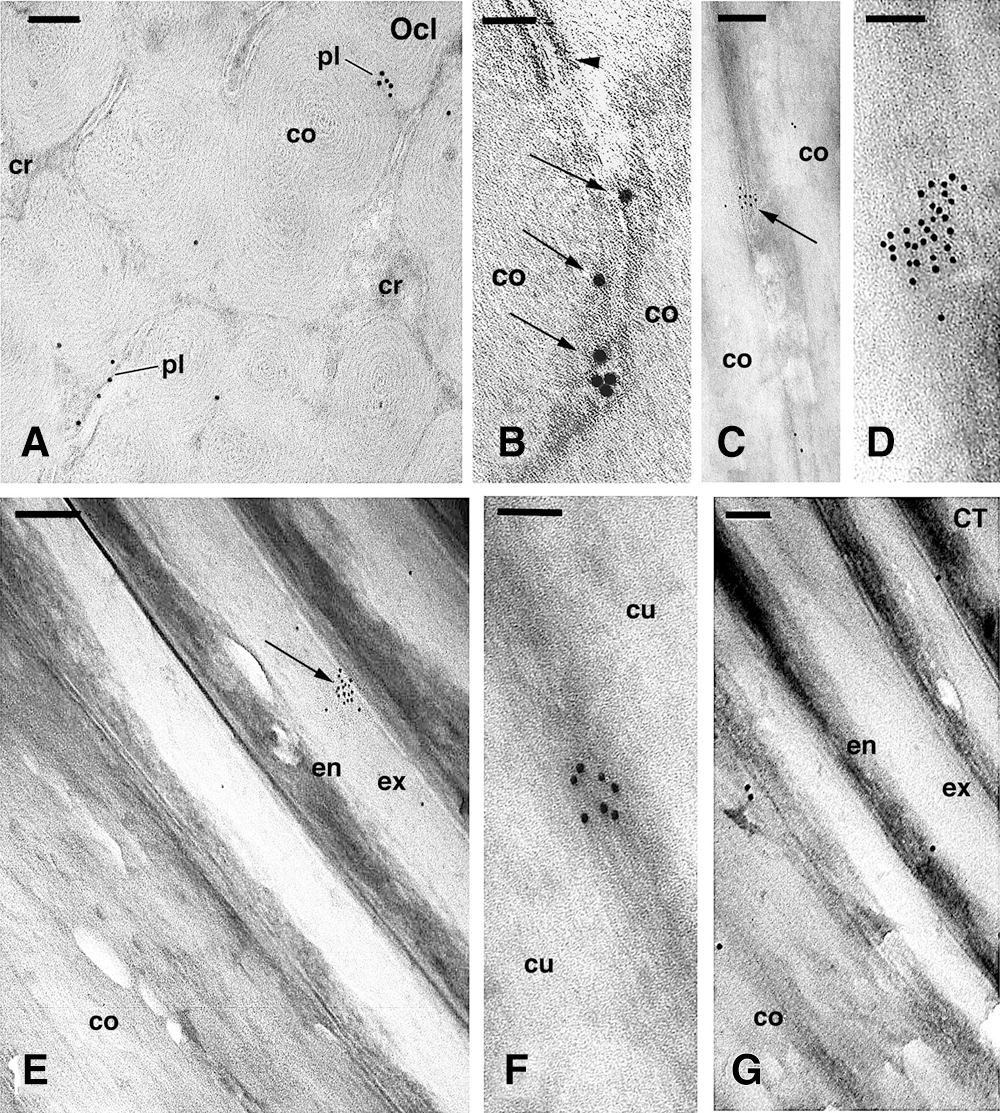
Figure 6: TEM immunogold labeling for occludin (Ocl) in cornified cortical (A–D) and cuticle (E–G) cells. A, cross-section of cortical cells showing the punctate distribution of gold particles located along or near remnants of the plasma membrane. Bar, 150 nm. B, detail of gold particles (arrows) located along a cornified tight junction along the plasma membrane of two cortical cells. A desmosmal remnant is seen (arrowhead). Bar, 50 nm. C, cluster of gold particles (arrow) localized in a darker cytoplasmic region present between pale corneous material of cortical cells. Bar, 200 nm. D, high magnification detail of a large cluster of gold particles localized in a darker region of a cortical cell, likely representing a tangentially sectioned cell membrane remnant. Bar, 100 nm. E, cross section of cuticle cells in continuation with the cortex, showing a cluster of gold particles (arrow) near the cell membrane. Bar, 200 nm. F, detail on a cluster of gold particles detected over a dense area found within cuticle cells. Bar, 100 nm. G, control section (CT) of cuticle and cortex showing few scattered gold particles. Bar, 100 nm. Legends: co, cortex; cr, cytoplasmic remnants; cu, cuticle cell; en, endocuticle; ex, exocuticle; pl, plasma membrane.
Using the cingulin antibody, very sparse labeling was observed in localized small areas of cortical and cuticle cells in the keratogenous zone. Occasional small clusters of gold particles were detected along the plasma membranes of cortical cells (Fig. 7A). No gold particles were localized over the intracellular or extracellular components of the cell membrane complex of both cortical and cuticle cells. Sparse clusters of gold particles were observed near membrane remnants or the merged membrane regions interposed between the cell membrane complex of cortical cells in the consolidation (fully cornified) zone. No ultrastructural characteristics for tight junctions were detected in these regions. Very sparse clusters made of few gold particles were seen in the membrane remnants detected between the irregular cell membrane complex of cornified cortical cells (Figs. 7B, 7C). In some areas of the mature cortex, some gold particles were seen close to electron-dense material localized among the electron-lucent macrofibrils, representing the few areas where nucleus-cytoplasm remnants of cortical cells are present (Fig. 7C). No or occasional clusters of gold particles were observed in mature cuticle cells of the consolidation zone, and only in the endocuticle or along the border of the endocuticle with the cell membrane complex (Fig. 7D). Finally, no clusters of gold particles were seen in control sections of cortical and cuticle cells (Fig. 7E).
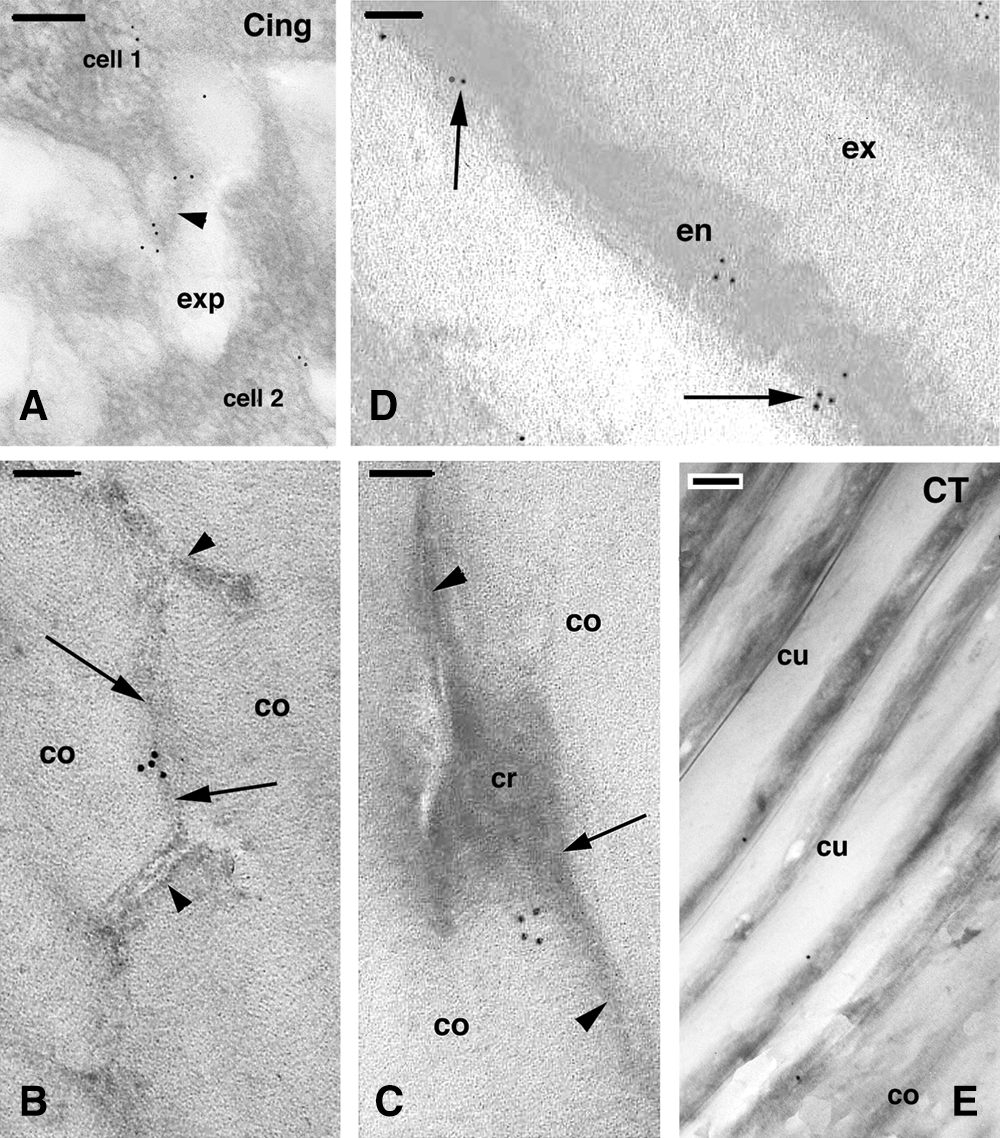
Figure 7: Immunolabeling for cingulin in the keratogenous zone (A) and consolidation zone (B–E) of the hair. A, immunolabeling present on the surface (arrowhead) of two cortical cells (area like that indicated by ker in Fig. 2A). Bar, 200 nm. B, detail on a cross-sectioned area of a cornified cell of the cortex. No labeling is seen in the membrane complex (arrowheads) while sparse gold particles are present over a denser line (arrows) that likely represents a junction remnant. Bar, 200 nm. C, detail showing a cluster of gold particles associated to an electron-dense area (arrow) representing cytoplasm remnant associated to a residual membrane (arrowheads) located between two cortical cells. Bar, 100 nm. D, gold particles found in the endocuticle or at the boundary with the exocuticle (arrows) in mature cuticle cells. Bar, 100 nm. E, immunonegative control section (CT) of cuticle and cortex in cross section showing few scattered gold particles. Bar, 200 nm. Legends: co, cortex; cr, cell remnants; cu, cuticle cell; en, endocuticle; ex, exocuticle; exp, extracellular space.
Junctional proteins contribute to hair stability
In comparison to hair fixed and stained with osmium, uranyl acetate, and lead citrate that produce a better contrast but block most antigenic sites of proteins, hair sections in Lowicryl resin appear electron-lucent and poorly contrasted while cell membranes are poorly detectable. Despite this shortage, some antigens have been sufficiently preserved to be detected by the employed methods. Another limitation to protein identification derives from their likely natural alteration and coating with other proteins during the process of cornification. In addition, the much lower content in junctional proteins detected in hairs (Barthelemy et al., 2012) made their immunodetection quite difficult. During cornification epitope masking or degradation occurs and no procedures to retrieve the antigens or etching the surface of the sections were attempted, as previously done with human epidermis (Ishida-Yamamoto et al., 1999). In fact, these treatments could have further deteriorated the ultrastructural details. Despite the above shortages and the lower resolution of ultrastructural details using Lowicryl, all cell compartments of hairs were clearly seen: medulla, cortex, and cuticle. The formation of clusters of immunogold particles near or along cell membranes or areas of the membranes cut in tangential section is not seen in control sections or using immunogold labeling for other non-junctional proteins (Alibardi, 2017; Alibardi and Noecker, 2013; Alibardi et al., 2013). This unique pattern of labeling, therefore, supports the identification of areas of corneous material where junctional proteins are still present in mature cortical and cuticle cells. The present study indicates that in addition to desmosomal proteins, adhesion and tight junction proteins remain in few and sparse regions likely located near degenerated cell membranes of mature cortical and cuticle cells (Fig. 8). These proteins undergo a re-distribution from their initial organization in well-differentiated cell junctions but appear not to participate in the formation of the cell membrane complex that instead is mostly derived from desmosome remnants (Alibardi and Noecker, 2013). In the cuticle, sparse areas of the endocuticle or localized near the exocuticle appear to be the site of accumulation of remnants of tight and adhesion proteins.
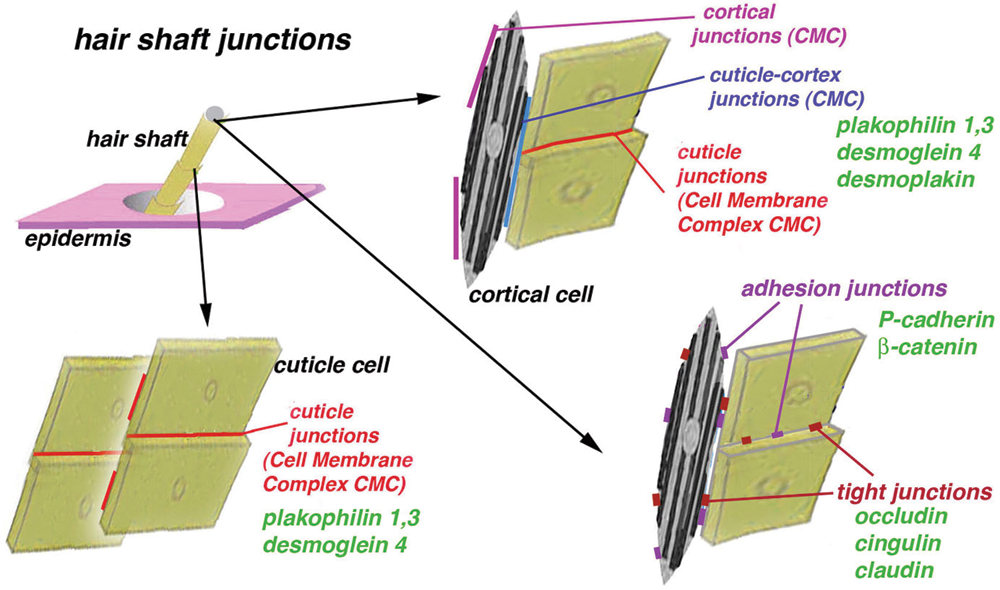
Figure 8: Summarizing schematic drawing to evidence the residual junctions and the few proteins so far detected in the mature shaft of the human hair. The three-dimensional distribution of these junctions is only indicated but it remains to be fully determined in detail, together the presence of other junctional proteins.
According to the scanty data available, desmosomes represent the prevalent type of cell junction present in hair keratinocytes (Orwin et al., 1973a; Franke and Heid, 1988; Langbein et al., 2002), and during hair cornification, they give rise to residual proteins incorporated in the cell membrane complex that likely sustains most of the cohesion of cortex and cuticle cells (Alibardi and Noecker, 2013; Fig. 8). Among others, desmosomal proteins such as desmoglein 4 and plakophilins 1 and 3 remain in the cell membrane complex of mature cortex and cuticle cells, a structure that represents a long and stable corneodesmosome (Fig. 8). The present qualitative study suggests that also residual adhesion and tight junction proteins still present in mature hairs may contribute to maintaining united cortical and cuticle cells. Lack of immunolabeling of the intercellular components or the delta-layer of the cell membrane complex indicates that adhesion and tight junctional proteins do not participate in the composition of these long corneodesmosomes.
The labeling for iso-peptide bond inside hair keratinocytes and occasionally in intercellular remnants of cortical and cuticle cells suggests the absence of transglutaminase substrates in the delta layer of the cell membrane complex. Also, the diffuse labeling for isopeptide bonds in the cortex and cuticle indicates that these cells contain a relatively low amount of this covalent bond while most of their corneous material contains disulfide bonds generated by sulfhydryl oxidase on keratins and KAPs (Rogers, 2004; Langbein and Schweizer, 2005; Alibardi, 2017). Although diffuse, the presence of isopeptide bonds in the cortex and cuticle, in addition to the heavier labeling of the IRS and medulla, confirms that TGase is involved in part of the consolidation of the corneous material, as it was previously indicated by the biochemical and immunohistochemical detection at the light microscope level (Yamane et al., 2010). Although previous studies indicate that the cuticle contains isopeptide-bonds (Rogers, 2004; Lee et al., 2006), the present study suggests a limited contribution of proteins containing lysyl-and glutamyl-residues as substrates for transglutaminase catalysis (Kalinin et al., 2002; Rogers, 2004; Thibaut et al., 2005), in particular, due to TGase 3 (Yamane et al., 2010). As opposed, medulla and IRS contain high levels of proteins capable to form isopeptide bonds, such as trichohyalin and involucrin (de Viragh et al., 1994; Thibaut et al., 2005; Alibardi, 2012). In these two compartments, medulla and IRS, a higher localization of TGase 1 has been reported to be the most active enzyme forming isopeptide bonds (Yamane et al., 2010). Therefore, previous and the present study explain the high reactivity for iso-peptide bonds detected in the fibrous network forming the trabeculae of vacuolated medullary cells in association with specific medullary keratins and associated proteins (Langbein and Schweizer, 2005). During the keratinization of medullary cells, dense and amorphous fibrils are deposited along the plasma membrane (Orwin, 1979; Rogers, 2004; Langbein and Schweizer, 2005; Morioka, 2005). This material largely derives from the coalescence of the medullary granules along the plasma membrane during the late stages of medullary differentiation.
Previous light microscopy immunohistochemistry indicated that E- and P-cadherins disappear in hardening cells of both developing and cycling hairs and IRS (Hardy and Vielkind, 1996; Kurzen et al., 1998; Muller-Rover et al., 1999; Young et al., 2003). The present study indicates the presence of residual immunoreactivity for P-cadherin and beta-catenin during keratin accumulation in cortical cells, and less frequently also in cuticle cells. The immunolabeling was observed despite no specific morphological differentiation for adherens junctions was observed, probably due to the junctional alteration in the areas under cornification and for the relatively poor preservation of fine ultrastructural details needed to maintain antigens for immunogold analysis. The sparse immuno-gold labeling for cadherins indicates that in mature cortical and cuticle cells, only a few epitopes remain available for reacting with the employed antibodies, suggesting that small quantities of adherens proteins are incorporated, degraded, or masked in the membrane of these mature cells.
E-cadherins are present in most cells of the epidermis, while P-cadherins are expressed in epithelial cells destined to give rise to the hair peg and the matrix of the bulb, suggesting that they determine a selective cell adhesion responsible for hair morphogenesis (Hardy and Vielkind, 1996) or cycling (Muller-Rover et al., 1999). Cadherins have been detected during the initial stages of cell-cell interaction in the matrix and in the differentiation zone in forming hairs (Hardy and Vielkind, 1996; Muller-Rover et al., 1999; Young et al., 2003). E-cadherins are present in the outer root sheath, while P-cadherins remain in the matrix of epithelial cells and in the central cells forming the medullary and cortical cells precursors. In cadherin-deficient mice, the IRS is most affected by the absence of E- and P-cadherins and its cells become loose, the companion layer detaches from the Henle layer leading to the distortion of the follicle and eventually to hair loss (Tinkle et al., 2003; Young et al., 2003). Cadherins apparently disappear in keratinizing cells of the medulla, cortex, cuticle, and IRS, a process that takes place in the keratogenous zone of the hair follicle. The present study suggests that adherens junctional proteins become restricted to few small regions in cortical and cuticle cells during cornification (Fig. 8), and likely are less important than desmosomal proteins for the integrity of the hair shaft.
The present TEM results confirm previous LM studies on the expression of tight junctional proteins in hairs, which become scarce or completely absent in the keratogenous zone (Brandner et al., 2003). Tight junctions were seen in the ORS and IRS, but they were less abundant in cells of the medulla and cortex and became sparse or absent in mature cells forming the hair shaft. The corneous mass connected through cornified junctions likely ensures cohesion among cortical and in few punctiform regions located among cuticle cells after complete maturation of the hair.
The weak but localized immunolabeling for occludin, still observed in cortical and cuticle cells in the keratogenous zone, largely disappears in the consolidation zone. The present study has shown poor reactivity in the residual junctions present between keratin-filled cells and in cell membrane complexes. The presence of tight junctions in differentiating cells suggests that their proteins are later incorporated into the keratin mass of cortical cells (Orwin et al., 1973a, 1973b, 1973c; Fig. 8). The observations suggest that occludin is embedded in the corneous material formed in the keratogenous zone, a process that determines the loss of reactive epitopes that become unavailable for antibody recognition after being coated-bonded with keratins and KAPs.
Tight junctional proteins such as ZO-1, occluding, and claudin were mainly localized in the ORS of the hair follicle (Tebbe et al., 2002; Zhorn-Kruppa et al., 2018). These junctions were rare in differentiating cortical and cuticle cells (Brandner et al., 2003). Occludin was especially found in the membranes of cells of the Henle layer in the IRS, where numerous tight junctions were detected (Orwin et al., 1973b; Langbein et al., 2002). Immunoreactivity for tight junctional proteins almost disappeared in keratinizing cortical and cuticle cells. The higher intensity of labeling and the more numerous tight junctions recorded in the ORS and IRS over those present in the hair shaft (Orwin et al., 1973b) suggests a function of sealing the upper epithelium of the follicle from the penetration of external molecules and microbes into the hair follicle (Brandner et al., 2003; Zhorn-Kruppa et al., 2018). The formation of tight junctions between Huxley-Henle and companion layers is also important for the cohesion of these layers that form the slippage plane during the growth (anagen) of the hair shaft (Alibardi and Noecker, 2013).
The ultrastructural immunolocalization for cingulin (Citi et al., 1988; Niessen, 2007) further confirms the persistence of few tight junction proteins in the modified junctional remnants of mature cortical and cuticle cells, except in the cell membrane complex (Langbein et al., 2002, Fig. 8). Different proteins of cell junctions might be degraded and removed once cells become keratinized or fully cornified in the upper regions of the hair follicle, and also cingulin may become masked by other proteins produced during the intense process of cornification, in particular from cross-linked keratins and KAPs. The permanence of few detectable tight junctional proteins and their incorporation into the corneous material likely contributes to maintaining the cohesion of the hair shaft, although tight junctional proteins appear less important than the more abundant desmosomal proteins for hair integrity (Lee et al., 2006; Barthelemy et al., 2012; Alibardi and Noecker, 2013; Alibardi et al., 2013). Both biochemical and immunohistochemical data support the presence of various junctional proteins in the corneous shaft of mature hairs that are important to maintain hair cohesiveness.
The identification of cell junctional proteins is of practical interest to determine whether, after treatment with hair formulations for cosmetic purposes, these proteins undergo extractions and/or chemical modification that can lead to hair damage (Dawber, 1996; Cruz et al., 2016). For instance, hair treatment with “thioglycolic acid cold permanent lotion” determines the swelling of cuticle cells, alteration of the exo/endo-cuticle organization, and the reduction of overlapping layers of cuticle cells. The A-layer of the exo-cuticle becomes electron-denser, indicating the penetration and chemical alteration that negatively influence the integrity of cuticle cells. The delta-layer of the cuticle membrane complex becomes more irregular after some cosmetic treatments, and the external macrofibrils of cortical cells appear swollen and less packed than in untreated hairs. Knowledge of the localization of other junctional proteins that compose mature hairs (Lee et al., 2006; Barthelemy et al., 2012) would, therefore, be important also for cosmetic treatments.
In conclusion, the present ultrastructural study indicates that despite cornification some reactive epitopes still remain localized in the junctional remnants indicating that tight junctional and adherens proteins, in addition to those of desmosomes previously studied, are not degraded but remain incorporated within the corneous material of hairs and contribute to its stability. This is a different process from the junctional degradation that occurs during corneous desquamation at the surface of the epidermis.
Acknowledgement: The present ultrastructural study was conducted in the Comparative Histolab in Padova, Italy. KAO Corporation supported part of the initial studies of which this is a continuation.
Availability of Data and Material: All data generated or analyzed during this study are included in this published article.
Author Contribution: Study conception and design: Lorenzo Alibardi, Noecker Bernd; Data collection: Lorenzo Alibardi; Analysis and interpretation of results: Lorenzo Alibardi; Draft manuscript preparation: Lorenzo Alibardi; All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: The present biological material belongs to the first Author and family members.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: There are no conflicts of interest in the present manuscript.
Alibardi L (2012). Ultrastructural immunolocalization of involucrin in the medulla and inner root sheath of the human hair. Annals of Anatomy—Anatomischer Anzeiger 194: 345–350. DOI 10.1016/j.aanat.2011.10.012. [Google Scholar] [CrossRef]
Alibardi L (2017). Ultrastructural localization of hair keratins, high sulfur keratin-associated proteins and sulfhydryl oxidase in the human hair. Anatomical Science International 92: 248–261. DOI 10.1007/s12565-016-0330-5. [Google Scholar] [CrossRef]
Alibardi L, Noecker B (2013). Immunolocalization of junctional proteins in human hairs indicates that the membrane complex stabilizes the inner root sheath while desmosomes contact the companion layer through specific keratins. Acta Histochemica 115: 519–526. DOI 10.1016/j.acthis.2012.11.011. [Google Scholar] [CrossRef]
Alibardi L, Tsuchya M, Watanabe S, Noecker B (2013). Ultrastructural localization of desmoglein and plakophilin in the human hair suggests that the cell membrane complex is a long desmosomal remnant. Acta Histochemica 115: 879–886. DOI 10.1016/j.acthis.2013.04.008. [Google Scholar] [CrossRef]
Barthelemy NR, Bednarczyk A, Schaffer-Reiss C, Jullien D, van Dorsselaer A, Cavusoglu N (2012). Proteomic tools for the investigation of human hair structural proteins and evidence of weakness sites on hair keratin coil segments. Analytical Biochemistry 421: 43–55. DOI 10.1016/j.ab.2011.10.011. [Google Scholar] [CrossRef]
Brandner JM, McIntyre M, Kief S, Wladykowski E, Moll I (2003). Expression and localization of tight junction-associated proteins in human hair follicles. Archives of Dermatological Research 295: 211–221. DOI 10.1007/s00403-003-0418-3. [Google Scholar] [CrossRef]
Bryson WG, McNeil SJ, McKinnon AJ, Rankin DA (1992). The cell membrane complex of wool. Wool Research Organization of New Zealand Communications C123: 1–43. [Google Scholar]
Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J (1988). Cingulin, a new peripheral component of tight junctions. Nature 333: 272–276. DOI 10.1038/333272a0. [Google Scholar] [CrossRef]
Cruz C, Costa C, Gomes A, Matamá T, Cavaco-Paulo A (2016). Human hair and impact of cosmetic procedures: A review of cleansing and shape-modulating cosmetics. Cosmetics 3: 26. DOI 10.3390/cosmetics3030026. [Google Scholar] [CrossRef]
Dawber R (1996). Hair: Its structure and response to cosmetical preparations. Clinics in Dermatology 14: 105–112. DOI 10.1016/0738-081X(95)00117-X. [Google Scholar] [CrossRef]
de Viragh PA, Huber M, Hohl D (1994). Involucrin mRNA is more abundant in human hair follicles than in normal epiudermis. Journal of Investigative Dermatology 103: 815–819. DOI 10.1111/1523-1747.ep12413482. [Google Scholar] [CrossRef]
Franke WW, Heid H 1988. Desmosomal proteins and cytokeratins in the hair follicle. In: Rogers G, (eds.) The Biology of Wool and Hair. pp. 403–416. London-New York: Chapman & Hall. [Google Scholar]
Hardy MH, Vielkind U (1996). Changing patterns of cell adhesion molecules during mouse pelage hair follicle development. Cells Tissues Organs 157: 169–182. DOI 10.1159/000147879. [Google Scholar] [CrossRef]
Kalinin AE, Kajava AV, Steinert PM (2002). Epithelial barrier function: Assembly and structural features of the cornified cell envelope. BioEssays 24: 789–800. DOI 10.1002/bies.10144. [Google Scholar] [CrossRef]
Kurzen H, Moll I, Moll R, Schafer S, Simics E, Amagai M, Wheelock MJ, Franke WW (1998). Compositionally different desmosomes in the various compartments of the human hair follicle. Differentiation 63: 295–304. DOI 10.1046/j.1432-0436.1998.6350295.x. [Google Scholar] [CrossRef]
Ishida-Yamamoto A, Tanaka H, Nakatane H, Takahashi H, Iizuka H (1999). Antigen retrieval of loricrin epitopes at desmosomal areas of cornified cell envelopes: An immunoelectron microscopic analysis. Experimental Dermatology 8: 402–406. DOI 10.1111/j.1600-0625.1999.tb00389.x. [Google Scholar] [CrossRef]
Jones LN, Rogers GE, Rufaut N, Sinclair RD (2010). Location of keratin-associated proteins in developing fiber cuticle cells using immunoelectron microscopy. International Journal of Trichology 2: 89–95. DOI 10.4103/0974-7753.77512. [Google Scholar] [CrossRef]
Langbein L, Schweizer J (2005). The Keratins of the human hair follicle. International Review of Cytology 243: 1–78. [Google Scholar]
Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, Moll I, Franke WW (2002). Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. European Journal of Cell Biology 81: 419–435. DOI 10.1078/0171-9335-00270. [Google Scholar] [CrossRef]
Lee YJ, Rice RH, Lee YM (2006). Proteome analysis of human hair shaft. Molecular Cell Proteomics 5: 789–800. DOI 10.1074/mcp.M500278-MCP200. [Google Scholar] [CrossRef]
Morioka K (2005). Hair Follicle. Differentiation Under the Electron Microscope. An Atlas. Tokyo: Springer. [Google Scholar]
Muller-Rover S, Tokura Y, Welker P, Furukawa F, Wakita H, Takigawa M, Paus R (1999). E- and P-cadherin expression during murine hair follicle morphogenesis and cycling. Experimental Dermatology 8: 237–246. DOI 10.1111/j.1600-0625.1999.tb00377.x. [Google Scholar] [CrossRef]
Niessen CM (2007). Tight junctions/Adherens junctions: Basic structure and function. Journal of Investigative Dermatology 127: 2525–2532. DOI 10.1038/sj.jid.5700865. [Google Scholar] [CrossRef]
Orwin DFG (1979). The cytology and cytochemistry of the wool follicle. International Review of Cytology 60: 331–374. [Google Scholar]
Orwin DFG, Thomson RW, Flower NF (1973a). Plasma membrane differentiation of keratinizing cells into the wool follicle: II. Desmosomes. Journal of Ultrastructural Research 45: 15–29. DOI 10.1016/S0022-5320(73)90029-4. [Google Scholar] [CrossRef]
Orwin DFG, Thomson RW, Flower NF (1973b). Plasma membrane differentiation of keratinizing cells into the wool follicle: III. Tight junctions. Journal of Ultrastructural Research 45: 30–40. DOI 10.1016/S0022-5320(73)90030-0. [Google Scholar] [CrossRef]
Orwin DFG, Thomson RW, Flower NF (1973c). Plasma membrane differentiation of keratinizing cells into the wool follicle: IV. Further membrane differentiation. Journal of Ultrastructural Research 45: 41–49. DOI 10.1016/S0022-5320(73)90031-2. [Google Scholar] [CrossRef]
Rice RH, Wong VJ, Pinkerton KE (1994). Ultrastructural visualization of cross-linked protein features in epidermal appendages. Journal of Cell Science 107: 1985–1992. [Google Scholar]
Robbins C (2009). The cell membrane complex: Three related but different cellular cohesion components of mammalian hair fibers. Journal of Cosmetic Sciences 60: 437–465. [Google Scholar]
Rogers GE (1959). Electron microscopy of wool. Journal of Ultrastructure Research 2: 309–330. DOI 10.1016/S0022-5320(59)80004-6. [Google Scholar] [CrossRef]
Rogers GE (2004). Hair follicle differentiation and regulation. International Journal of Developmental Biology 48: 163–170. DOI 10.1387/ijdb.15272381. [Google Scholar] [CrossRef]
Tebbe B, Mankertz J, Schwarz C, Amasheh S, Fromm M, Assaf C, Schultz-Ehrenburg U, Sanchez Ruderisch H, Schulzke JD, Orfanos CE (2002). Tight junction proteins: A novel class of integral membrane proteins. Archives of Dermatological Research 294: 14–18. DOI 10.1007/s00403-001-0290-y. [Google Scholar] [CrossRef]
Thibaut S, Candi E, Pietroni V, Melino G, Schmidt R, Bernard BA (2005). Transglutaminase 5 expression in human hair follicle. Journal of Investigative Dermatology 125: 581–585. DOI 10.1111/j.0022-202X.2005.23868.x. [Google Scholar] [CrossRef]
Tinkle CL, Lechler T, Pasolli HA, Fuchs E (2004). Conditional targeting of E-cadherin in skin: Insights into hyperproliferative and degenerative responses. Proceedings of the National Academy of Sciences of the United States of America 101: 552–557. DOI 10.1073/pnas.0307437100. [Google Scholar] [CrossRef]
Yamane A, Fukui M, Sugimura Y, Itoh M, Alea MP, Thomas V, El Alaoui S, Akiyama M, Hitomi K (2010). Identification of a preferred substrate peptide for transglutaminase 3 and detection of in situ activity in skin and hair follicles. FEBS Journal 277: 3564–3574. DOI 10.1111/j.1742-4658.2010.07765.x. [Google Scholar] [CrossRef]
Young P, Boussadia O, Halfter H, Grose R, Berger P, Leone DP, Robenek H, Charnay P, Kemler R, Suter U (2003). E-cadherin controls adherens junctions in the epidermis and renewal of hair follicles. EMBO Journal 22: 5723–5733. DOI 10.1093/emboj/cdg560. [Google Scholar] [CrossRef]
Zhorn-Kruppa M, Vidal-Y-Sy S, Houdek P, Wladykowski E, Grzybowski S, Gruber R, Gorzelanny C, Hardcup J, Schneider SW, Majumbdar A, Brandner JM (2018). Tight junction barriers in human hair follicles. Role of claudin-1. Scientific Reports 8: 89. DOI 10.1038/s41598-018-30341-9. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |