DOI:10.32604/biocell.2021.014280

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.014280 |  www.techscience.com/journal/biocell |
| Article |
SENEX gene promotes cell proliferation by activating RB/E2F pathway in diffuse large B-cell lymphoma cells
Department of Hematology, The Second Hospital of Anhui Medical University, Hefei, 230601, China
*Address correspondence to: Zhimin Zhai, zzzm889@163.com
Received: 15 September 2020; Accepted: 18 January 2021
#These two authors contributed equally to this study
Abstract: The present study aimed to clarify the role of SENEX in malignant cell proliferation in diffuse large B-cell lymphoma (DLBCL). 22 DLBCL patients (6 newly diagnosed cases, 7 cases at complete remission, and 9 relapsed cases) were included in the study. Our results indicated that both SENEX gene and protein were significantly increased in peripheral blood mononuclear cells (PBMCs) and tumor cells of relapsed DLBCL patients, accompanied by overexpression of p21, p16, and phosphorylated retinoblastoma (Rb). Silencing the SENEX gene in a DLBCL cell line caused a significant decrease in cell proliferation and inhibited cell cycle progression in the G1 phase. Phosphorylated Rb and E2F1 were also decreased, and activation of the Rb/E2F1 pathway was obviously suppressed. To conclude, the SENEX gene promotes proliferation in PBMC and tumor cells of DLBCL patients by activating the Rb/E2F1 pathway, in a manner suggesting that increased SENEX expression affects the relapse of DLBCL and may serve as an important target for DLBCL therapy.
Keywords: SENEX; DLBCL; Cell proliferation; Rb; E2F
As the most frequent subtype of non-Hodgkin’s lymphoma (NHL), diffuse large B-cell lymphoma (DLBCL) is often aggressive and fatal (Miao et al., 2019). Although some clinical advances, including monoclonal antibodies targeting CD20 and hematopoietic stem cell transplantation (HSCT), have been applied over the past years, the malignancy outcome remains a significant challenge, as ∼30% of patients are not cured and succumb to their disease (Miao et al., 2019; Quan et al., 2018; Rovira et al., 2015). Clearly, therapeutic intervention for these patients needs further improvement. Cell immunotherapy, especially Chimeric Antigen Receptor T (CAR-T) therapy, has recently advanced rapidly for clinical treatment; however, the efficacy of CAR-T therapy on DLBCL is not as good as in acute lymphoblastic leukemia due to the tumor immune micro-environment barrier (Batlevi et al., 2016). Further understanding the pathogenesis of DLBCL and how malignant lymphoma cells escape the immune surveillance might provide a basis for future rational combinatorial treatment approaches.
SENEX, based on the Latin for “old man”, is a new gene associated with cellular senescence that was identified and cloned successfully in 2004. It is located at 4q31.23, with a total length of 2901 bp, and encodes a protein (Named ARHGAP18 in the RefSeq system) of approximately 75 kD. ARHGAP18 (encoded by SENEX gene) has RhoGAP determinants and belongs to the GTPase activating protein (GAP) family (Calvisi et al., 2011; Coleman et al., 2010; Dransart et al., 2005; Katoh and Katoh, 2004; Sahai and Marshall, 2002). Analysis of Panel expression profile shows that SENEX gene expresses in different kinds of tissues such as kidney, spleen, brain, and even in peripheral blood leukocytes (Sahai and Marshall, 2002). The gene functions as an important regulator of cell shape, spreading, migration and angiogenesis, and has been demonstrated to be involved in cell senescence, cell cycle, and even in cancer (Humphries et al., 2017; Li et al., 2018; Sahai and Marshall, 2002). A previous study has shown the expression level of the SENEX gene in gastric tumor tissue was lower than that of adjacent normal tissue, and SENEX acted as a tumor suppressor by restraining the over-activation of MAPK signaling pathways in gastric cancer (Li et al., 2018). While in breast cancer and mammary tumors, the SENEX gene was over-expressed (Kim et al., 2011). In hematological malignancies, research on SENEX were very few. Our previous study found ARHGAP18 was significantly increased in hydrogen peroxide-induced senescent DLBCL cells, and the p16 pathway was activated in senescent DLBCL cells (Wang et al., 2019a). However, SENEX gene expression, role, and related molecular mechanism in DLBCL tumorigenesis and development are unknown.
Retinoblastoma (Rb) is the first identified tumor suppressor. The loss of it generally promotes tumor development and progression (Hutcheson et al., 2015). It is a regulator of cellular proliferation, and the G1-to-S cell cycle progression is regulated by the Rb/E2F pathway. Phosphorylation (inactivation) of the Rb protein results in the release of the transcription factor E2F1 and will activate S-phase entry (Yaswen et al., 2015). Decreased expression of Rb has been found in DLBCL (Monti et al., 2012), and SENEX gene can activate the p16/pRb pathway in endothelial cells (Coleman et al., 2010). However, the role of SENEX in DLBCL and the related molecular pathway are unclear and need to be elucidated. In this study, clinical specimens and DLBCL cell line were used to detect SENEX expression and the possible molecular pathways.
22 patients diagnosed with DLBCL and 7 healthy volunteers from April 2017 to April 2019 at the Second Hospital of Anhui Medical University, Anhui, China, were enrolled in this study. The detailed criteria for exclusion of patients are listed as follows: (1) Less than 18 years old; (2) Pregnant or lactating women; (3) Congenital/acquired immunodeficiency; (4) Human immunodeficiency virus (HIV) or syphilis; (5) Other hematological or non-hematological tumors; (6) Autoimmune diseases such as systemic lupus erythematosus. According to Chinese guidelines for diagnosis and treatment of DLBCL (2013) (Chinese Society of Hematology CMA and Chinese Society of Lymphoma Ca-CA, 2013), patients were diagnosed and divided into the newly diagnosed group, complete remission group, and relapsed group. The detailed clinical data of the patients were shown in Tab. 1. This study was approved by the Institutional Review Board (IRB) Institutional of the Second Hospital of Anhui Medical University. All patients and healthy volunteers enrolled in the study have signed informed consent.
Table 1: Characteristics of healthy control and DLBCL patients
The Human DLBCL cell line, OCI-LY8 (abbreviation in this article: LY8), was cultured in RPMI-1640 supplemented with 10% FBS. Cell cultures were maintained and incubated at 37°C in humidified air with 5% CO2.
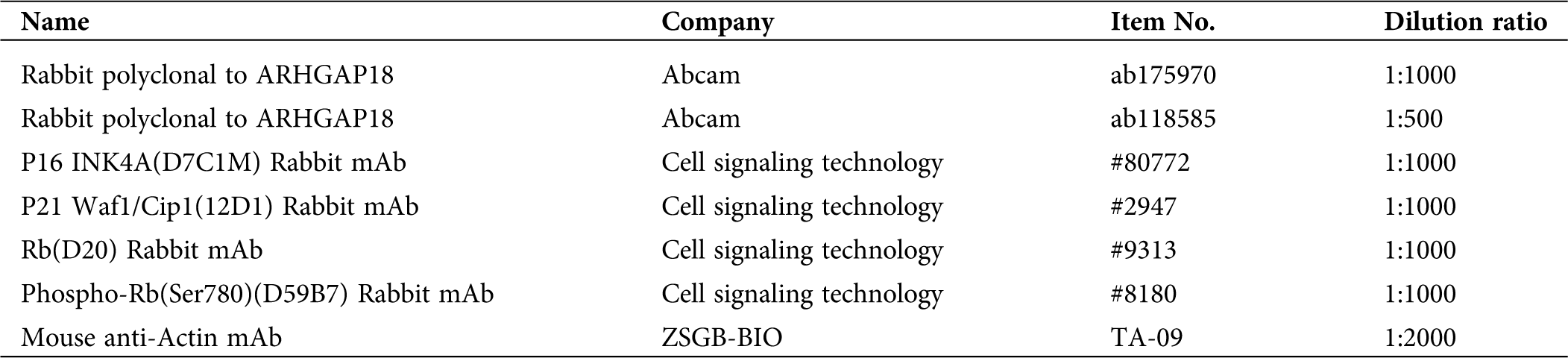
6 newly diagnosed and 3 relapsed DLBCL patients were collected and embedded in paraffin for HE-staining and Immunohistochemistry (IHC) analysis. 4 m thick paraffin sections and monoclonal antibodies against human ARHGAP18 (1:100 dilution) (Tab. 2) were used in the study. The sample sections were deparaffinized in xylene and rehydrated in a series of grades of alcohol. Then pre-treat them in citric acid antigen retrieval solution (pH 6.0). After inhibiting the internal peroxidase activity with 3% hydrogen peroxide, the sections were incubated with an anti-ARHGAP18 antibody at 4°C overnight. Then the slides were incubated with goat anti-rabbit IgG secondary antibody at 37°C for 10 min. Finally, the sections were visualized with DAB solution (DAKO, Carpinteria, USA) and counterstained with hematoxylin (DAKO). The yellow-brown positive staining was mainly located in the nucleus, cytoplasm, and cell membrane. Semi-quantitative methods were used for IHC positive staining scoring: (1) Cell staining intensity: 0 for negative, 1 score for light brown stained cells, 2 scores for brown, 3 scores for dark brown; (2) Selected 10 high-power fields, 400×, and counted the percentage of positive-stained lymphoma cells to the total number of tumor cells: 0 ≤ 5%, 1 score for 5–33%, 2 scores for 34–67%, 3 scores for 68–100%; (3) Assessment: ≤1 was Negative, >1 was Positive. 2–3 scores as Low expression; 4–6 as high expression.
Table 2: Primary antibodies used for Western-blot and Immunohistochemistry
SiRNA synthesis and transfection
The scramble negative control siRNA (NC) and individual small interfering RNA target SENEX gene (SENEX-siRNA) were synthesized by Sangon (Sangon, Shanghai, China). The final siRNA concentration was 33 nM (Chen et al., 2014). Ly8 cells (4 × 105/well) were transfected with SENEX-SiRNA or NC for 12 and 48 h by using Lipofectamine 2000 (Invitrogen, CA, USA). Sequences used for SiRNA transfection have been previously published (Wang et al., 2019a).
Enzyme linked immunosorbent assay (ELISA)
Ly8 cells were transfected with SENEX-SiRNA or NC for 24 h and then removed precipitation by centrifugation (1500 rpm for 5 min) and store samples at −20°C. Avoid repeated freeze-thaw cycles. The concentration of Lecithin-Cholesterol Acyltransferase (E2F1) was measured using Human E2F Transcription Factor 1 (E2F1) ELISA Kit (OmnimAbs, USA). Use the stock solution (8000 pg/mL) to produce a 2-fold dilution series (including 4000 pg/mL, 2000 pg/mL, 1000 pg/mL, 500 pg/mL and 250 pg/mL). All Standards and Samples were added in duplicate to the microtiter plate according to standard procedures. And take blank well as zero, measured the optical density (OD) at 450 nm after Adding Stop Solution and within 15 min.
RNA extraction and quantitative real time polymerase chain reaction (qRT-PCR) analysis
Gene expression levels were detected by qRT-PCR. Peripheral blood was collected from DLBCL patients for evaluation of SENEX gene levels via qRT-PCR. Total RNA in peripheral blood was extracted by TRIzol (Invitrogen, Carlsbad, CA, USA). RNA was reverse-transcribed using a Transcript RT Kit (Sangon, Shanghai, China) according to the manufacturer’s protocol. Real-time PCR was performed on the ABI 7500 Real-Time PCR System (Life Technologies, Grand Island, NY, USA) by SYBR Green PCR Master Mix (TaKaRa, Dalian, China). All primers were synthesized by Sangon (Shanghai, China). The relative SENEX expression level was calculated using the 2−△△Ct method. Sequences used for qRT-PCR primers were listed in a previous study (Wang et al., 2019a).
Protein expression levels were detected by Western blot. Total proteins from Peripheral blood mononuclear cells (PBMCs) and LY8 cells were extracted by Western blot with IP cell lysis liquid (Beyotime, Shanghai, China) in accordance with standard procedures. Primary antibodies used for Western blot are shown in Tab. 2. Proteins were detected with the SuperSignal West Femto Trial Kit (Thermo Fisher Scientific, Shanghai, China) as previously described (Wang et al., 2017).
To further investigate the effect of the SENEX gene on DLBCL cell proliferation, we performed proliferation analysis. LY8 cells transfected with SENEX-SiRNA or NC were plated at a density of 5000 cells/well.
At 12 or 48 h after transfection, cell proliferation was measured with the CCK-8 Kit (BestBio, Shanghai, China). Each assay was performed with 5 replicates in 3 independent experiments.
The cell cycle distribution was detected at 48 h after transfection with COULTER DNA PREP Reagents kit (Beckman). Cells were washed with PBS twice, stained with DNA Pre Stain and DNA Prep LPR according to standard procedures. And the cell cycle was detected by flow cytometer FC-500 (Beckman Coulter, Miami, FL).
The Student’s t-test was employed for analysis of two-sample and two-tailed comparisons by SPSS 16.0 (SPSS Inc., Chicago, IL). Pearson correlation was used to measure the degree of dependence between variables by SPSS 16.0. p-values were calculated, and p < 0.05 was considered statistically significant.
SENEX expression is significantly increased in relapsed DLBCL patients
To investigate the expression level of SENEX in DLBCL patients, we extracted peripheral blood mononuclear cells (PBMCs) from 7 healthy volunteers, 6 newly diagnosed, 7 in complete remission, and 9 relapsed DLBCL patients. As tumor cells were not available in healthy volunteers and complete response patients, we chose PBMCs for analysis. In addition, the lymph node tissues of 6 newly diagnosed and 3 relapsed DLBCL patients were collected and embedded in paraffin for HE-staining and immunohistochemical analysis. The detailed clinical data of all enrolled patients are shown in Tab. 1.
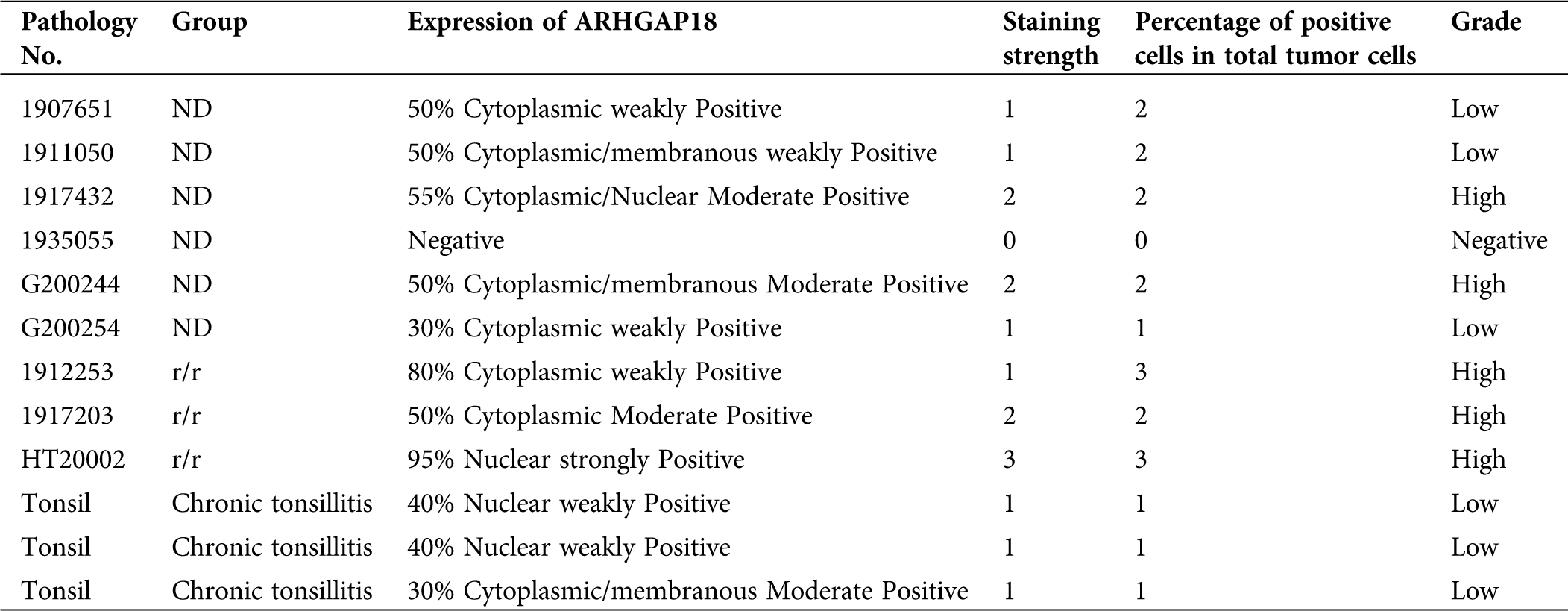
Total RNA and protein extracted from PBMCs were detected. As shown in Fig. 1A, SENEX mRNA expression level was significantly increased in the PBMCs of relapsed patients with lymphoma cell infiltration in peripheral blood compared to healthy control, newly diagnosed, and complete response patients. Consistent with this, ARHGAP18 expression in the PBMCs of relapsed patients also increased significantly (Fig. 1B). Then, we detected the expression of ARHGAP18 in the lymph node tissues of newly diagnosed and relapsed DLBCL patients. ARHGAP18 expression was significantly increased in the lymphoma cells of relapsed DLBCL patients compared with newly diagnosed patients (Figs. 1C and 1D and Tab. 3).

Figure 1: SENEX expression is significantly increased in relapsed DLBCL patients.
Table 3: Immunohistochemical score of 9 DLBCL patients
Furthermore, we found that expressions of p21 and p16 were also significantly increased in PBMCs of relapsed DLBCL patients compared to healthy control, newly diagnosed, and complete response patients (Fig. 1E). On the other hand, the expression level of phosphorylated-RB had an increasing trend, but the expression of RB was significantly decreased in relapsed DLBCL patients compared to healthy control, newly diagnosed, and complete response patients (Fig. 1E). These results indicate that SENEX expression is significantly increased in relapsed DLBCL patients, and p21, p16, and Rb, were also activated. The overexpressed SENEX gene is closely related to the relapse of DLBCL.
In this study, LY8 cells were transfected with the individual small interfering RNA (SENEX-siRNA, SS-RNA) to silence SENEX gene, and the scramble negative siRNA (NC) group was set as control. Next, the efficiency of transfection both in genetic and protein levels was verified. Expression of SENEX mRNA was significantly decreased in the SENEX-siRNA group compared with the control group (LY8 cells without any treatment) and the NC group (Fig. 2A). And ARHGAP18 expression level was also obviously reduced in the SENEX-siRNA group compared to the control and NC groups (Fig. 2B). These results suggested that transfection with SENEX-siRNA can inhibit the expression SENEX gene in DLBCL cells. On this basis, we investigated the effect of intervening SENEX gene on proliferation and related pathways in DLBCL cells.

Figure 2: Transfection with SENEX-SiRNA significantly suppresses the expression of SENEX in LY8 cells.
SENEX promotes the proliferation of DLBCL cells in vitro
To further investigate the function of SENEX gene in DLBCL, we transfected the LY8 cell line with SENEX-SiRNA or NC (as a control), and then analyzed the cell morphology and proliferation. Compared with the control and NC groups, the number of LY8 cells after transfection was reduced (Figs. 3A–3C).

Figure 3: Transfection with SENEX-SiRNA significantly decreases cell proliferation rates in LY8 cells at 12 and 48 h.
Cell proliferation was detected at 12 and 48 h after transfection. Consistent with the above result, cell proliferation was decreased in the SENEX-SiRNA group when compared with the unprocessed group and the NC group (Fig. 3D). In addition, cell proliferation in the SENEX-SiRNA group was more markedly decreased than in the unprocessed group and the NC group at 48 h after transfection (Fig. 3E). Thus, these results suggest that SENEX promotes the proliferation of DLBCL cells in vitro.
As compared with the control and NC group cells, cells transfected with SENEX-SiRNA were arrested at the G1 phase, and the percentage of cells in the G2 phase was decreased in the SS group (C: 28.32%, NC: 28.17%, SS: 21.28%) (p < 0.01) (Fig. 4). The percentage of cells in the G2/M phase was also significantly decreased in the SS group compared to the control and NC groups (p < 0.01) (Fig. 4). These results indicated that inhibiting the expression of SENEX arrested the G1 phase, resulting in inhibition of cell growth.
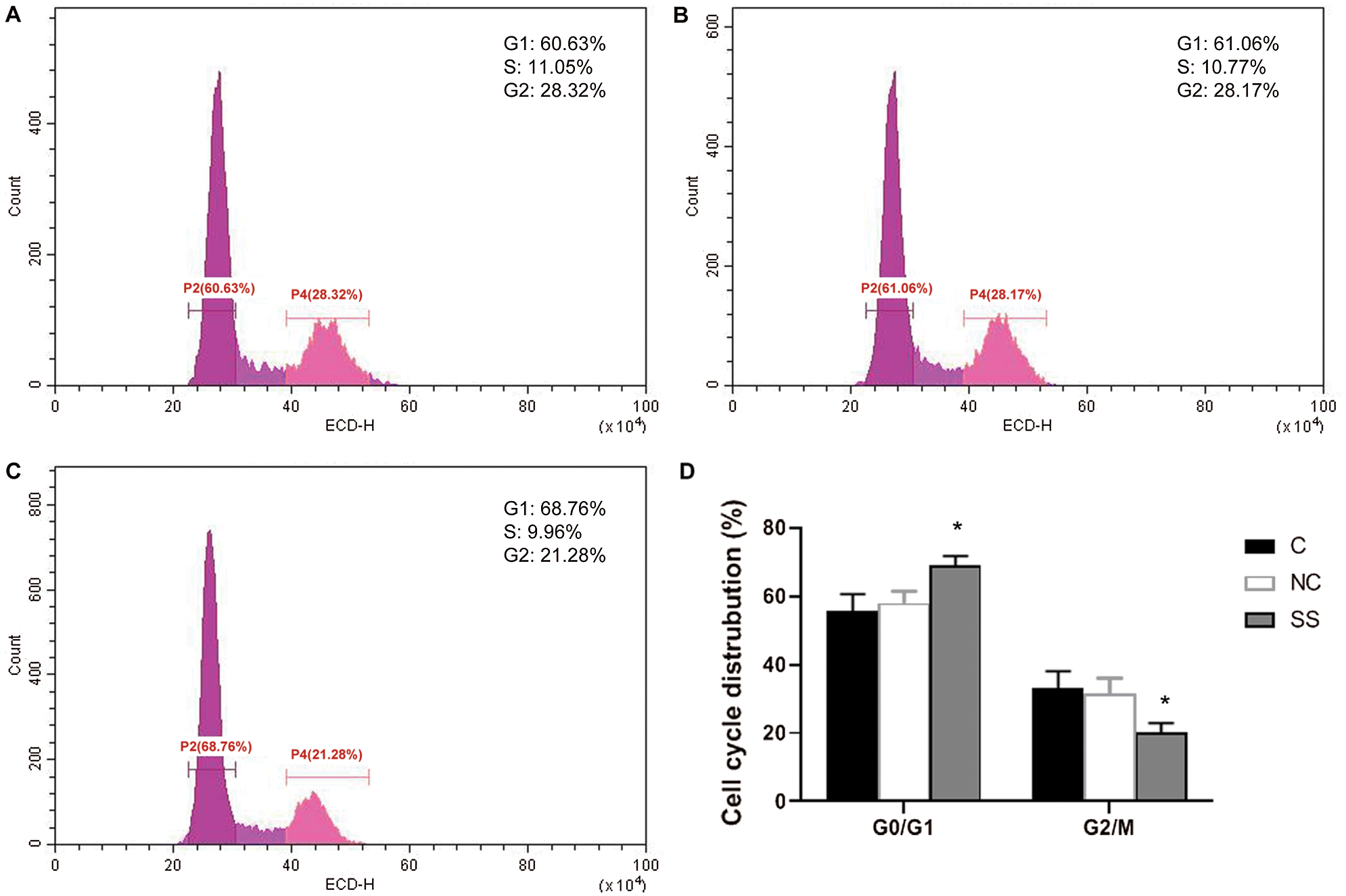
Figure 4: Effect of SENEX-SiRNA on the cell cycle distribution of LY8 cells.
SENEX promotes RB/E2F1 activation in DLBCL cells
The E2F transcription factor is thought to regulate the transition of the cell cycle from G to S by interacting with various cell cycle-dependent proteins and kinases such as RB/pRB (van den Heuvel and Dyson, 2008). RB/pRB protein is one of the major constituent proteins of human E2F regulatory protein, and E2F regulatory protein can regulate the activity of the E2F transcription factor (van den Heuvel and Dyson, 2008). To further explore the effect of SENEX in DLBCL cell proliferation, RB/E2F1 pathway was analyzed. The concentration of E2F1 was significantly decreased in the SENEX-SiRNA group as compared to the NC group (Fig. 5A). Expressions of p21, p16, and phosphorylated-RB were significantly reduced in the SENEX-SiRNA group compared to the control and NC groups (Fig. 5B). These results suggest that SENEX promotes RB/E2F1 activation in DLBCL cells.
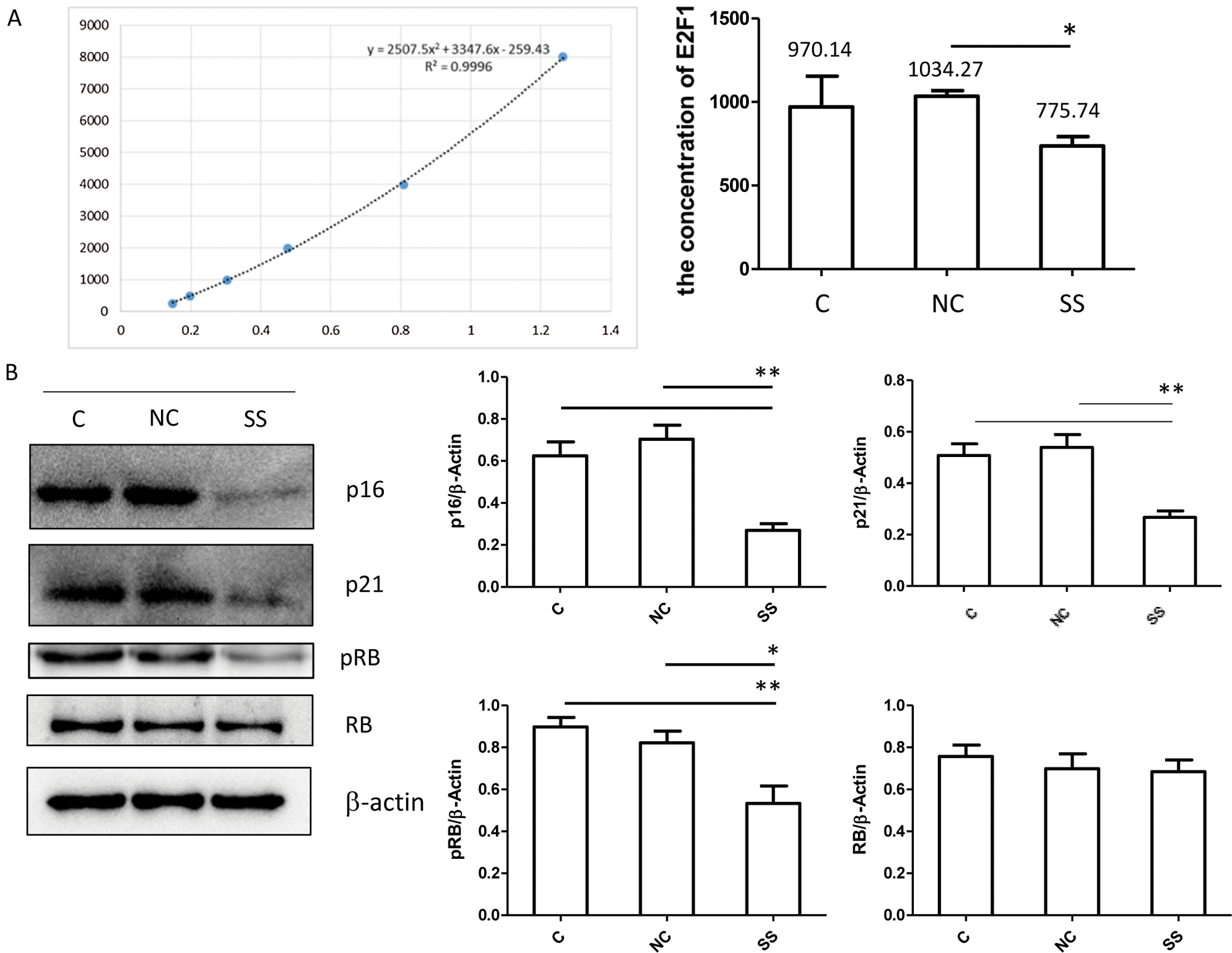
Figure 5: Transfection with SENEX-siRNA significantly inhibits RB/E2F1 activation in LY8 cells. LY8 cells were transfected with SENEX-SiRNA or NC.
Tumor immune escape is a key factor in tumorigenesis and progression of malignant tumors (Pan et al., 2014; Tao et al., 2015; Wang et al., 2019b). DLBCL is a genetically and clinically heterogeneous disorder, and the efficacy of CAR-T immunotherapy in DLBCL is not as powerful as in acute leukemia, suggesting that DLBCL has a more rigorous and complex ability to evade immune destruction (Quan et al., 2018). DLBCL lymphoma cells escaping the immune system is a pivotal problem and needs to be clarified and solved urgently (Li et al., 2014). Multiple genes and molecular pathways may involve in this complicated progress. SENEX, a new gene associated with cellular senescence, was found to regulate cell spreading, migration in the life cycle of a cell and may be involved in the growth and metastasis of tumors (Humphries et al., 2017; Li et al., 2018). Our previous study indicated that increased SENEX expression promoted tumorigenesis and metastasis of urinary bladder cancer (Chen et al., 2014). Moreover, we found that SENEX activates the p16 pathway in hydrogen peroxide-induced senescent DLBCL cells (Wang et al., 2019a). However, the expression, role, and related molecular mechanisms of SENEX in DLBCL tumorigenesis and development have not been precisely described to date.
In the present study, we found that the expression level of SENEX gene and protein in the PBMC of newly diagnostic DLBCL patients was higher than that of normal controls, it decreased when the patients reached complete remission. However, while the tumor relapsed, SENEX expression increased significantly. Besides, ARHGAP18 expression in lymphoma cells in lymph nodes of relapsed DLBCL patients was increased than that of newly diagnosed patients, indicating that SENEX might play a role during this process, especially at relapse of DLBCL. Therefore, the function of SENEX needed to be further explored in DLBCL at the cellular level. OCI-LY8 cells, a human diffuse large B-cell lymphoma cell line was used for further studies. Following transfection of OCI-LY8 cells with SENEX-SiRNA, RT-PCR, and Western blot detections found that SENEX expression was highly decreased than that of the control and NC groups. Moreover, cellular proliferative activity and the percentage of cells in the G2/M phase were apparently decreased in the SENEX-siRNA group compared with another two groups at different times after transfection, indicating SENEX can also promote the proliferation of DLBCL at the cellular level. The related role and possible molecular pathway in this process at this point need to be further investigated.
Studies (Hutcheson et al., 2015; Shats et al., 2017) have shown that Rb plays an important role in controlling cell cycle progression as well as cell fate decisions. It was the first identified tumor suppressor in view of germline predisposition to the pediatric eye tumor (Hutcheson et al., 2015). Although Rb can act as a transcriptional activator or repressor, the function of Rb is usually attributed to its ability to inhibit transcription or regulate cell cycle progression. In this regard, the interaction between Rb and the E2F transcription factor family is a typical example of Rb function. Generally, in the process of normal cell differentiation and proliferation, cyclin Dl and CDK4/6 form a complex, which makes Rb protein phosphorylation, and then relieves the effect of Rb protein. Phosphorylated Rb can dissociate the crucial transcription factor E2F1, then activate important cyclin E and cyclin A, and last initiate DNA replication and proliferation. However, dephosphorylated Rb can directly mask the transcriptional activation domain of E2F by interacting with these proteins and indirectly by recruiting additional co-repressors and lead to cell growth arrest. Dysfunction of the Rb/E2F network is a common event in many human malignancies (Hutcheson et al., 2015; Shats et al., 2017). In our study, we found the expression level of pRb was significantly increased in relapsed DLBCL patients as compared with healthy volunteers, newly diagnosed, and complete response DLBCL patients. However, Rb expression showed opposite trends. Next, we investigated the effect of SENEX on pRb/Rb and E2F1 expressions in DLBCL cells. After transfection with SENEX-SiRNA in LY8 cells, there is a significantly reduced trend of pRb level in the SS group than in the control. The concentration of free E2F1 was significantly decreased in the SENEX-SiRNA group compared to controls. These results indicate that SENEX-induced Rb/E2F1 activation may play a vital role in the proliferation and relapse of DLBCL. Besides, the role of CDK in cell proliferation is regulated by CDK inhibitors (CKIs), while p16 and p21 are two of the CKIs. In endothelial cells, overexpression of SENEX resulted in an increase of p16, but no significant change of p21 (Coleman et al., 2010). In this study, we also detected p16 and p21 expression levels, and we found p16 and p21 were also activated in relapsed DLBCL patients.
There are still some limitations of this study. First, in in vitro assays, we used the LY8 cell, and this type of lymphoma cell is derived from germinal cancer and may not represent the nongerminal center source lymphoma. Next, the sample size of patients and lymph nodes in this study was limited. Last, how SENEX regulates Rb/E2F1 pathway in DLBCL was not included in this study. A previous study has shown that SENEX can induce stress-induced senescence and specific senescence-associated secretory phenotype (SASP), making endothelial cells escape from immune attack and obtain the ability to resist apoptosis (Coleman et al., 2010). Whether this mechanism also exists in DLBCL is unknown. In our future research, we will also try to clarify the mechanism, and we will also expand the sample size and use more DLBCL cell lines to validate our findings in this study.
In conclusion, our study provides the first evidence that SENEX is essential for the proliferation and relapse of DLBCL. Clinically, overexpression of SENEX is associated with the relapse of DLBCL. At the cellular level, our study demonstrates that the SENEX gene promotes cell proliferation in DLBCL cells via activating the Rb/E2F1 pathway. Increased SENEX expression level affects the relapse of DLBCL and may serve as an important target for DLBCL therapy.
Ethics Statement: This studies was conducted ethically inaccordance with the World Medical Association Declarationof Helsinki, and approved by the Ethics Committee of the Second Hospital of Anhui Medical University. All patients enrolled in the study have signed informed consent.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author’s Contributions: Z Zhai designed this research; J Wang, Z Wanyan, and Y Wan performed experiments; J Wang and Y Pan contributed to the writing of the manuscript; Y Wan analyzed all the immunohistochemistry results. Z Wang and Q Tao collected clinical specimens; J Wang analyzed the data.
Ethics Approval: This study was approved by the Institutional Review Board (IRB) Institutional of the Second Hospital of Anhui Medical University (LLSC20160082). All patients enrolled in the study have signed informed consent.
Funding Statement: This work was supported by the National Natural Science Foundation of China (Grant No. 81670179), the Research Fund Project of Anhui Medical University (No. 2018xkj026) and the National Natural Science Foundation Incubation Project of the Second Hospital of Anhui Medical University (No. 2019GQFY11).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Batlevi CL, Matsuki E, Brentjens RJ, Younes A (2016). Novel immunotherapies in lymphoid malignancies. Nature Reviews Clinical Oncology 13: 25–40. DOI 10.1038/nrclinonc.2015.187. [Google Scholar] [CrossRef]
Calvisi DF, Ladu S, Conner EA, Seo D, Hsieh JT, Factor VM, Thorgeirsson SS (2011). Inactivation of Ras GTPase-activating proteins promotes unrestrained activity of wild-type Ras in human liver cancer. Journal of Hepatology 54: 311–319. DOI 10.1016/j.jhep.2010.06.036. [Google Scholar] [CrossRef]
Coleman PR, Hahn CN, Grimshaw M, Lu Y, Li X, Brautigan PJ, Beck K, Stocker R, Vadas MA, Gamble JR (2010). Stress-induced premature senescence mediated by a novel gene, SENEX, results in an anti-inflammatory phenotype in endothelial cells. Blood 116: 4016–4024. DOI 10.1182/blood-2009-11-252700. [Google Scholar] [CrossRef]
Chen T, Wang H, Zhang Z, Li Q, Yan K, Tao Q, Ye Q, Xiong S, Wang Y, Zhai Z, Rameshwar P (2014). A novel cellular senescence gene, SENEX, is involved in peripheral regulatory T cells accumulation in aged urinary bladder cancer. PLoS One 9: e87774. DOI 10.1371/journal.pone.0087774. [Google Scholar] [CrossRef]
Chinese Society of Hematology CMA, Chinese Society of Lymphoma Ca-CA (2013). Chinese guidelines for diagnosis and treatment of diffuse large B cell lymphoma. Zhonghua Xue Ye Xue Za Zhi 34: 816–819. [Google Scholar]
Dransart E, Olofsson B, Cherfils J (2005). RhoGDIs revisited: Novel roles in Rho regulation. Traffic 6: 957–966. DOI 10.1111/j.1600-0854.2005.00335.x. [Google Scholar] [CrossRef]
Humphries B, Wang Z, Li Y, Jhan JR, Jiang Y, Yang C (2017). ARHGAP18 downregulation by miR-200b suppresses metastasis of triple-negative breast cancer by enhancing activation of RhoA. Cancer Research 77: 4051–4064. DOI 10.1158/0008-5472.CAN-16-3141. [Google Scholar] [CrossRef]
Hutcheson J, Witkiewicz AK, Knudsen ES (2015). The RB tumor suppressor at the intersection of proliferation and immunity: Relevance to disease immune evasion and immunotherapy. Cell Cycle 14: 3812–3819. DOI 10.1080/15384101.2015.1010922. [Google Scholar] [CrossRef]
Katoh M, Katoh M (2004). Characterization of human ARHGAP10 gene in silico. International Journal of Oncology 25: 1201–1206. [Google Scholar]
Kim HH, van den Heuvel AP, Schmidt JW, Ross SR (2011). Novel common integration sites targeted by mouse mammary tumor virus insertion in mammary tumors have oncogenic activity. PLoS One 6: e27425. DOI 10.1371/journal.pone.0027425. [Google Scholar] [CrossRef]
Li Y, Ji S, Fu L, Jiang T, Wu D, Meng F (2018). Over-expression of ARHGAP18 suppressed cell proliferation, migration, invasion, and tumor growth in gastric cancer by restraining over-activation of MAPK signaling pathways. OncoTargets and Therapy 11: 279–290. [Google Scholar]
Li YL, Gu KS, Pan YY, Jiao Y, Zhai ZM (2014). Peripheral blood lymphocyte/monocyte ratio at the time of first relapse predicts outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. BMC Cancer 14: 341. DOI 10.1186/1471-2407-14-341. [Google Scholar] [CrossRef]
Miao Y, Medeiros LJ, Xu-Monette ZY, Li J, Young KH (2019). Dysregulation of cell survival in diffuse large B cell lymphoma: Mechanisms and therapeutic targets. Frontiers in Oncology 9: 107. DOI 10.3389/fonc.2019.00107. [Google Scholar] [CrossRef]
Monti S, Chapuy B, Takeyama K, Rodig SJ, Hao Y, Yeda KT, Inguilizian H, Mermel C, Currie T, Dogan A, Kutok JL, Beroukhim R, Neuberg D, Habermann TM, Getz G, Kung AL, Golub TR, Shipp MA (2012). Integrative analysis reveals an outcome-associated and targetable pattern of p53 and cell cycle deregulation in diffuse large B cell lymphoma. Cancer Cell 22: 359–372. DOI 10.1016/j.ccr.2012.07.014. [Google Scholar] [CrossRef]
Pan Y, Tao Q, Wang H, Xiong S, Zhang R, Chen T, Tao L, Zhai Z, Zhang SG (2014). Dendritic cells decreased the concomitant expanded Tregs and Tregs related IL-35 in cytokine-induced killer cells and increased their cytotoxicity against leukemia cells. PLoS One 9: e93591. DOI 10.1371/journal.pone.0093591. [Google Scholar] [CrossRef]
Quan L, Lan X, Meng Y, Guo X, Guo Y, Zhao L, Chen X, Liu A (2018). BTLA marks a less cytotoxic T-cell subset in diffuse large B-cell lymphoma with high expression of checkpoints. Experimental Hematology 60: 47–56.e1. DOI 10.1016/j.exphem.2018.01.003. [Google Scholar] [CrossRef]
Rovira J, Valera A, Colomo L, Setoain X, Rodríguez S, Martínez-Trillos A, Giné E, Dlouhy I, Magnano L, Gaya A, Martínez D, Martínez A, Campo ED, López-Guillermo A (2015). Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Annals of Hematology 94: 803–812. DOI 10.1007/s00277-014-2271-1. [Google Scholar] [CrossRef]
Sahai E, Marshall CJ (2002). RHO–GTPases and cancer. Nature Reviews Cancer 2: 133–142. DOI 10.1038/nrc725. [Google Scholar] [CrossRef]
Shats I, Deng M, Davidovich A, Zhang C, Kwon JS, Manandhar D, Gordân R, Yao G, You L (2017). Expression level is a key determinant of E2F1-mediated cell fate. Cell Death & Differentiation 24: 626–637. DOI 10.1038/cdd.2017.12. [Google Scholar] [CrossRef]
Tao Q, Pan Y, Wang Y, Wang H, Xiong S, Li Q, Wang J, Tao L, Wang Z, Wu F, Zhang R, Zhai Z (2015). Regulatory T cells-derived IL-35 promotes the growth of adult acute myeloid leukemia blasts. International Journal of Cancer 137: 2384–2393. DOI 10.1002/ijc.29563. [Google Scholar] [CrossRef]
Van Den Heuvel S, Dyson NJ (2008). Conserved functions of the pRB and E2F families. Nature Reviews Molecular Cell Biology 9: 713–724. DOI 10.1038/nrm2469. [Google Scholar] [CrossRef]
Wang J, Wang Z, Wang H, Wanyan Z, Pan Y, Zhu FF, Tao Q, Zhai Z (2019a). Stress-induced premature senescence promotes proliferation by activating the SENEX and p16(INK4a)/Retinoblastoma (Rb) pathway in diffuse large B-cell lymphoma. Turkish Journal of Haematology 36: 247–254. [Google Scholar]
Wang JY, Fang M, Boye A, Wu C, Wu JJ, Ma Y, Hou S, Kan Y, Yang Y (2017). Interaction of microRNA-21/145 and Smad3 domain-specific phosphorylation in hepatocellular carcinoma. Oncotarget 8: 84958–84973. DOI 10.18632/oncotarget.17709. [Google Scholar] [CrossRef]
Wang Z, Zhu F, Wang J, Tao Q, Xu X, Wang H, Xiong S, Wang Y, Zhai Z (2019b). Increased CD14+HLA-DR-/low myeloid-derived suppressor cells correlate with disease severity in systemic lupus erythematosus patients in an iNOS-dependent manner. Frontiers in Immunology 10: 1202. DOI 10.3389/fimmu.2019.01202. [Google Scholar] [CrossRef]
Yaswen P, MacKenzie KL, Keith WN, Hentosh P, Rodier F, Zhu J, Firestone GL, Matheu A, Carnero A, Bilsland A, Sundin T, Honoki K, Fujii H, Georgakilas AG, Amedei A, Amin A, Helferich B, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Bhakta D, Halicka D, Niccolai E, Aquilano K, Ashraf SS, Nowsheen S, Yang X (2015). Therapeutic targeting of replicative immortality. Seminars in Cancer Biology 35: S104–S128. DOI 10.1016/j.semcancer.2015.03.007. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |