DOI:10.32604/biocell.2021.013864

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.013864 |  www.techscience.com/journal/biocell |
| Review |
Cell cycle regulation through primary cilium: A long-forgotten story
Institute of Cell and Developmental Biology, College of Life Sciences, Zhejiang University, Hangzhou, 310058, China
*Address correspondence to: Xiao Huang, xh220@zju.edu.cn
Received: 25 August 2020; Accepted: 21 December 2020
Abstract: Protruded from cytomembrane, primary cilium is a widespread cell organelle that can be found in almost all cell types in Mammalia. Because of its comprehensive requirement in various cellular activities and various functions in different organs, primary cilium has been a valuable research area of human pathology research since the turn of the millennium. And the potential application of the interaction between primary cilia and cell cycle regulation may be the most promising direction as many primary cilium-caused diseases are found to be caused by cell cycle dysregulation resulted from primary cilia defects. Therefore, a deep understanding of the interaction between primary cilia and the cell cycle is in great need. Hence in this review, we mainly described how the interaction between primary cilia and cell cycle proceeds and demonstrated three hypotheses raised from much different research. These hypotheses include (1) Primary cilium as a cellular signaling hub to regulate the cell cycle, (2) Primary cilium as a reservoir of cell cycle regulation-related factors, and (3) Primary cilium as a cell cycle checkpoint or a brake. Nonetheless, we also call for more attention on research of interaction between cell cycle and primary cilia and tried to point out some possible research directions for those who are interested.
Keywords: Signaling pathway; Cell cycle checkpoint; Ciliopathy; Cancer
Abbreviation
| DNA: | DeoxyriboNucleic Acid |
| PDGF: | Platelet Derived Growth Factor |
| TGF-β: | Transforming Growth Factor-β |
| Mtor: | Mammalian Target of Rapamycin |
| GPCRs: | G Protein-Coupled Receptors |
| PTCH1: | Patched 1 |
| PDGFR: | Platelet-Derived Growth Factor Receptors |
| 5-HTr6: | 5-Hydroxytryptamine (serotonin) Receptor 6 |
| SSTR3: | Somatostatin Receptor 3 |
| MCHR1: | Melanin-Concentrating Hormone Receptor 1 |
| MSC: | Mesenchymal Stem Cell |
| PKD: | Polycystic Kidney Disease |
| cAMP: | Cyclic Adenosine Monophosphate |
| STAT: | Signal Transducer and Activator of Transcription |
| RTK: | Receptor Protein Tyrosine Kinase |
| SHP2: | Src Homology Phosphotyrosyl Phosphatase 2 |
| PI3K: | Phosphatidyl Inositol 3-OH kinase |
| Mek1/2: | MAPK Kinase ½ |
| MAPK: | Mitogen-Activated Protein Kinase |
| Erk1/2: | Extracellular Signal-Regulated Kinase ½ |
| CDK1: | Cyclin-Dependent Kinases 1 |
| CDK2: | Cyclin-Dependent Kinases 2 |
| HEF1: | Human Enhancer of Filamentation 1 |
| HDAC6: | Histone Deacetylase 6 |
| SuFu: | Suppressor of Fused |
| E2F1: | E2 Promoter Binding Factor 1 |
| IFT: | Intraflagellar Transport |
| BBS: | Bardet-Biedl Syndrome |
| ALMS: | Alstrom Syndrome |
| WNT: | Wingless/Integrated |
| NLS: | Nuclear Localization Sequence |
| Jbn: | Jouberin |
| NDE1: | Nuclear distribution protein nudE homolog 1 |
| Nek2: | NIMA Related Kinase 2 |
| NIMA: | Never in Mitosis Gene A |
| DYNLT1: | Dynein Light Chain Tctex-Type 1 |
| IGF: | Insulin-Like Growth Factors |
| LC8: | Light Chain 8 |
| ESCs: | Embryo Stem Cells |
| CPAP: | Centrosomal-P4.1-Associated Protein |
| NPCs: | Non-Parenchymal Cells |
| MB: | Medulloblastoma |
Cilium is a widespread organelle that is mainly consisted of microtubules and locating on the cytomembrane. Its discovery can be traced back to almost 350 years ago by Anthony van Leeuwenhoek (May-Simera et al., 2017). The related research on cilia has already started since the nineteenth century, but there is no significant breakthrough because of the limitations on experimental technologies. Not until the invention and wide application of the electron microscope did scientists begin to figure out the structure and function of the cilium.
Cilia can be divided into two major categories: motile-cilia (or so-called secondary cilia or flagella) and primary-cilia. Motile-cilia, as the name suggests, are capable of wobbling and thus can be found in many bio-tracts with transportation functions, such as the digestive and reproductive tracts. While primary cilia are unable to spontaneously move and thus have been considered as a vestigial version of motile-cilia in higher animal cells. Most of the research has been only focusing on motile cilia for a long period of time. However, since a bunch of unexpected functions of primary cilia was uncovered in the 1990s (Wheatley, 1995), more and more focus has begun to shift to primary cilia. And this passion reached a new level after the turn of the millennium when researchers noticed the correlation between primary cilia and a wide range of human diseases.
Primary cilia were firstly named by Sorokin (1968). And it widely exists in almost all vertebrate cells (Bangs et al., 2015). Primary cilia now have been discovered to have multiple biological functions such as biosensor, cellular signaling transducing hub, differentiation regulator and cell cycle controller et al. Take one for instance, as the bio-sensor, primary cilia have an essential role in olfaction (Tadenev et al., 2011) and optesthesia (Ramamurthy and Cayouette, 2009). Their defects usually lead to a wide range of diseases which are defined as “Ciliopathy” (Reiter and Leroux, 2017).
The structure of primary cilia has been very clear. They have the classical 9+0 structure, which contains nine doublet microtubules but without the central pair of singlet microtubules that often appear inside motile cilia. Primary cilia are unable to move by themselves since molecular motors, and axonemal dyneins are missing (Satir and Christensen, 2007). In general, the structure of primary cilia can be divided into four parts: Basal body, transition zone, axoneme, and the ciliary membrane (Elliott and Brugmann, 2019). However, it is worth noting that there is a special type of primary cilia, nodel cilia, which are capable of oscillating and playing an important role in the determination of the left and right body axes during embryonic development.
Cell cycle regulation is the most important factor influencing cell proliferation, and different cell cycle checkpoints strictly regulate the cell cycle process to ensure normal cell proliferation. In addition to the replication of genetic material during the cell cycle, cell proliferation also involves the replication of centrioles to form a spindle, which is responsible for evenly distributing the replicated genetic material to the daughter cells. As centrioles are also indispensable components for ciliation, it is supposed to have a dynamic and reciprocal interaction between primary cilia and cell cycle as shown in Fig. 1. Tucker and colleagues firstly found the mutually exclusive relation between ciliation and cell proliferation (Tucker et al., 1979). And further research unveiled more this kind of mutually exclusive connections (Breslin et al., 2014). The cell cycle is artificially divided into four phases: G1 (G0), S, G2 and M for research purposes. In most cells, primary cilia start to form during the G1 phase or the early stage of the G0 phase (Nigg and Stearns, 2011). At this point, mitosis just right finishes, and the mother centriole (the older centriole in two centrioles in the daughter cell) is released from the centrosome, an essential component in the spindle apparatus. The released centriole next relocates to the cytoplasmic membrane. Then it recruits appendages to form a basal body, which is the base of the primary cilium (Kobayashi and Dynlacht, 2011). Throughout the whole G0 phase, the primary cilia play important roles to regulate cellular activities. However, this quiescent state begins to transform when the cell prepares to re-enter into a new cell cycle at G0/G1 phase. Firstly, primary cilia initiate their disassembly during G1/S transition under the manipulation of specific proteins (like Aurora-A). Then the mother centriole in the basal body is released. Just like the reverse version of ciliogenesis, the released centriole next relocates to the nucleus and forms the centrosome at the G2/M phase to facilitate the even distribution of genetic material into two daughter cells during mitosis (M phase) (Liang et al., 2016) (Plotnikova et al., 2009). And after mitosis, the cell enters the G1 phase again and waits for the next cell cycle.
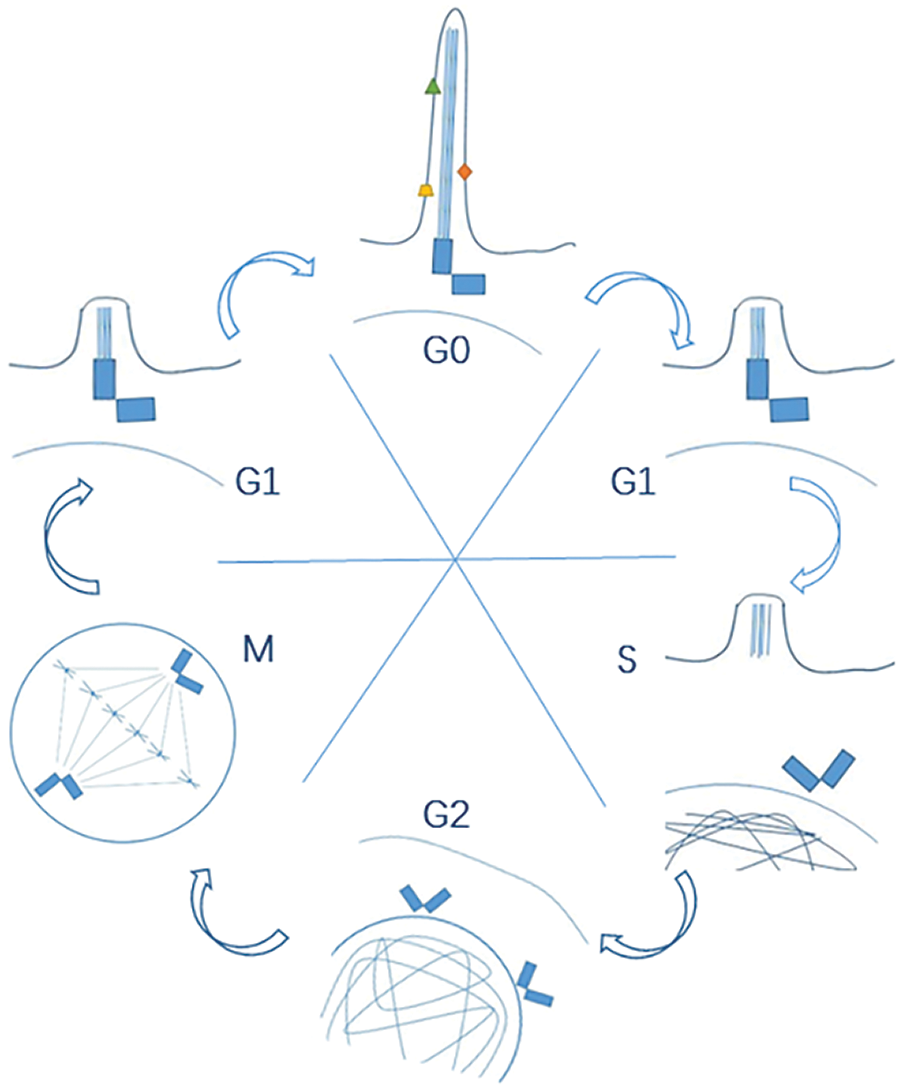
Figure 1: A dynamic and reciprocal interaction between primary cilia and cell cycle.
Thus, it is almost an instinct to propose an intimate relationship between the cell cycle and primary cilia as they share a common component. Therefore, what kind of pivotal connection between the cell cycle and cilia becomes an issue worth pondering. Up to now, no reciprocal relation between motile-cilia and cell cycle regulation has ever been reported because motile cilia usually emerge in limited highly specialized cell types, which exit from the cell cycle and undergo no proliferation, to perform specific functions. So, in this review, we only focus on the interaction between primary cilia and cell cycle regulation based on the universal presence of primary cilia.
In addition to these obviously mutually exclusive functions of centriole in both cell statuses, many other studies have also shown more aspects of the connection between cilia and cell-cycle at the beginning of this century (Quarmby and Parker, 2005) and unraveled more intricate interactions between cilia and cell cycle. For example, research also revealed a suppressing effect of primary cilia on cell proliferation in zebrafish embryos; longer primary cilia suppress cell proliferation (Kim et al., 2011). And proliferating tumor cells are considered to be cilium-free. However, a recent study has found a great proportion of tumor cells, such as HeLa and MG63, having primary cilia (Kowal and Falk, 2015). This discovery makes the possible role that primary cilia may play during tumorigenesis or tumor cell maintenance even more obscure but also more intriguing. It should point out that there could be a more sophisticated interaction between cilia and the cell cycle than we usually thought as only the mutually exclusive relation, which also leads more scientists to focus on a deep story about their interaction.
However, the exact mechanism of this interaction is still obscure after decades of research. So, in this review, we summarize the latest literature about the relationship between primary cilia and cell cycle control and put forward three major hypotheses of how the interaction between cilia and cell cycle proceeds. In the end, we also raise up possible problems and research directions to share with research communities and trigger new promotion on this long-ignored area.
Primary cilium as cellular signal hub to regulate cell cycle
Primary cilia were first discovered as an important bridge of signal transduction between the extracellular environment and intracellular activities in 2003 (Huangfu et al., 2003). After that, more signaling pathways have been found to have primary cilia as their signaling hubs or transfer stations (Pala et al., 2017). Downstream cellular activities based on primary cilia-related signaling networks include cell differentiation, proliferation, survival, metabolism, migration, and cell cycle regulation. Thus, impairment of primary cilia can cause defects in signal transduction between the extracellular environment and intercellular signals. And that defects usually result in physiological imperfections (Pruski et al., 2019). It is worth mentioning that many physiological imperfections were caused by cell cycle regulation defects resulted from cilia-related signaling pathway impairments. Under normal physiological conditions, cellular proliferation is under strict regulation through cilia-involved signaling pathways. Therefore the imperfection of cilia-involved signaling pathways caused by defects of cilia might lead to cell cycle dysregulation and then lead to ciliopathies and cancers (Nishimura et al., 2019). While a lot of signaling pathways have been found to be primary cilia-related, many of these signaling pathways lack sufficient research. Therefore, in order to make a general impression about the hypothesis on cilia and cell cycle-related signaling pathways only three relatively in-depth researched primary cilia receptors are demonstrated below. The basic information of other cilia-related signaling pathways is shown in Tab. 1.
Table 1: Multiple cilia-related signaling pathways and their basic information
The general content of three typical cilia-related signaling pathways is shown in the figure above. (1) Polycystin1/2 regulate gene expression by increasing the intracellular Ca2+ concentration to promote the interaction between Ga2+ and Calmodulins. (2) Hedgehog signaling (PTCH1) has Gli2 as a transcription factor to do this job. (3) PDGFR initiates cell cycle-related gene expression by recruiting SH2/PTB domain-containing adaptors.
In the table above, it is clearly demonstrated that most of the cilia-related signaling pathways are involved in cell proliferation. And any defects of these signaling pathways or cilia are capable of triggering diseases caused by dysregulation of cell cycle regulation, such as tumors, PKD, or cysts (Pala et al., 2017; Wheway et al., 2018; Labour et al., 2016; Tian et al., 2019; Siebel and Lendahl, 2017; Ibraghimov-Beskrovnaya and Natoli, 2011; Liu et al., 2019; Aspera-Werz et al., 2019).
Polycystin1/2 receptor complex mediated Ca2+ pathway
PKD is a common kidney disease characterized mainly by the growth of large and fluid-filled cysts (Bergmann, 2019). It is already known that PKD is caused at least in part by the dysregulated renal cell proliferation. Polycystin1/2 encoded by PKD1 and PKD2 (Cornec-Le Gall et al., 2019) respectively is the essential mediator of this process. These two receptors are locating in primary cilia in a form of complex to act as a type of mechanical sensor and ion channel, which in response to extracellular stimuli pump extracellular Ca2+ into the cytoplasm. The elevated intracellular Ca2+ lets the Ca2+ reservoir release more Ca2+ (Nauli et al., 2003). For example, in mouse embryonic renal cells, the fluid flow or so-called mechanical stimuli directly act on polycystin-1, and which then triggers polycystin-2 to pump extracellular Ca2+ into the primary cilia of the renal cells. Elevated intracellular Ca2+ level further stimulates the endoplasmic reticulum membrane to release more Ca2+. Combined with the released Ca2+, the downstream Calmodulins are then activated and keep the renal cell proliferate in a low and normal mitotic index. High-level Ca2+ promotes cyclic nucleotide catabolism and makes less cAMP, which suppresses cell proliferation as feedback (Nauli et al., 2003) (illustrated in Fig. 2). If polycystin-1 or polycystin-2 lose their functions resulting from ciliary defects and lead to no extracellular stimuli detection, it will cause low Ca2+ concentration and decrease of cyclic nucleotide catabolism inside renal cells. And those two alterations together cause activation of Ca2+ inhibitable adenyl cyclase 5/6 and accumulation of cAMP levels, which might promote cell cycle progress pathways (Delling et al., 2013). As a result, renal cells proliferate in a higher mitotic index and finally lead to cystic tissue (Lee et al., 2011). Certainly, another Ca2+-independent gene expression regulation mechanism is also possible, by which polycystin-1 undergoes proteolytic cleavage and its cytoplasmic tail translocate to the nucleus and interact with P100 or STAT to affect downstream gene expression (Low et al., 2006).
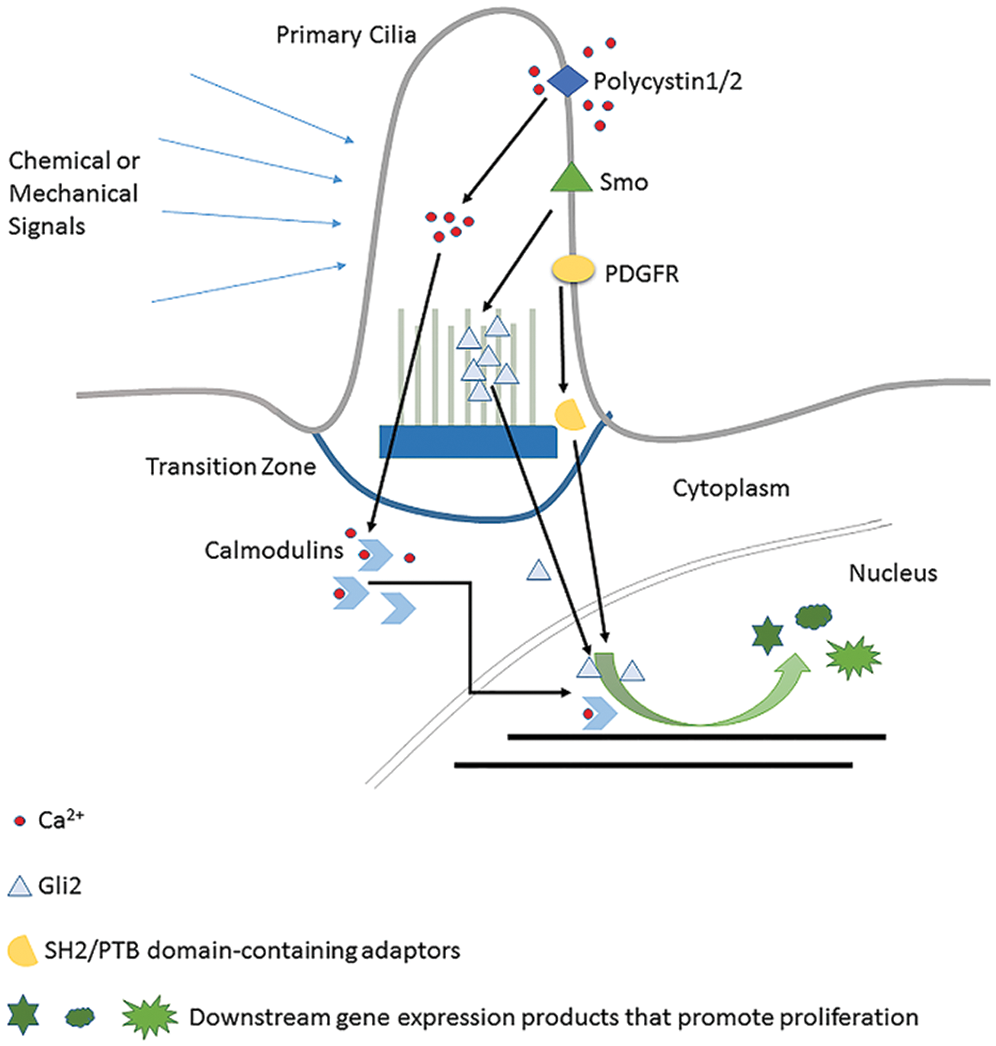
Figure 2: Details of three typical cilia-related signaling pathways.
PDGF/PDGFR related RTK pathway
PDGFR (Platelet-Derived Growth Factor Receptor) is a widely expressed RTK family receptor locating on primary cilia and reported to transmit extracellular information into the nucleus and regulate the cell proliferation in fibroblast cells (Christensen et al., 2008; Schneider et al., 2005). Autocrine activation of PDGF signaling is involved in certain tumors like gliomas, sarcomas, and leukemia (Andrae et al., 2008). The ligands firstly bind to PDGFR on cilia which let the intracellular tyrosine residues be autophosphorylated. Conformation change allows the recruitment of SH2/PTB domain-containing adaptors, such as SHP2 (Src Homology Phosphotyrosyl Phosphatase 2) and PI3K (Phosphatidyl Inositol 3-OH kinase) to bind. Subsequently, through the Ras-Mek1/2-Erk1/2 pathway, CDK1 and CDK2 are activated to promote cell cycle re-entry (see Fig. 2). This pathway will only be activated when the cell is in the ciliated quiescent status (Schneider et al., 2005). So, it is clear that primary cilia play its essential role in the PDGF signaling pathway. It is noteworthy that this pathway has crosstalk with the HEF1/Aurora A/HDAC6 pathway (Nielsen et al., 2015), which is a very important cilia status regulator. In summary, as an essential cell cycle control signaling pathway, the fulfilling of functions of the PDGF signaling pathway heavily relies on normal primary cilia.
Hedgehog signaling is an essential developmental pathway. It is also a crucial regulator of cell cycle regulation (Agathocleous et al., 2007; Lupu et al., 2018) and is involved in several diseases caused by abnormal cell cycle regulation. Thus Hedgehog has become a hot spot of tumorigenesis and cancer therapy research (Skoda et al., 2018). The primary cilium is the major site where Hedgehog signaling transduction happens.
Mutual repulsive phenomena between Ptc1 and Smo on the cilium defines different activity states of Hedgehog signaling. In the absence of ligands, the Ptc1 receptor is located on the primary cilia and repress Smo to transport onto cilium. Gli transcription factors, the downstream executant, are thus trapped at the tip of the primary cilium and suppressed by Suppressor of Fused (SuFu). When ligands bind on, Ptc is transferred to the cytomembrane, which lets Smo move onto the ciliary membrane to repress SuFu, removing repression on Gli. Gli activation finally initiates transcription of proliferation-related genes such as Cyclin D, Cyclin E, and E2F1 (Kasper et al., 2006).
It is worth emphasizing that the role of primary cilia in the Hedgehog pathway is far beyond the fact that primary cilia are the location of the receptors. The exact influence of primary cilia on Hedgehog signaling is complicated. Whether these act positively or negatively is context-dependent and also determined by appropriate coordination of ciliary proteins (Wong and Reiter, 2008; Dhekne et al., 2018; Kilander et al., 2018), especially the Intraflagellar Transport (IFT) family. Cilium enclosed space is relatively independent of the cytoplasm. The material and information exchange between them is carried out by the IFT System (Lechtreck, 2015). For the Hedgehog signaling pathway, the Gli2 move from the tip of cilia and then is released to the cytoplasm to help relocate β-catenin (Liem et al., 2012) is also IFT dependent (see Fig. 2). Thus, IFT defects can interfere with Hedgehog signaling function in cell cycle regulation (Wu et al., 2017). And this is a different situation compared with the PDGFR pathway and PKD-related pathway, whose signaling transductions are not IFT-dependent. So, from this point of perspective, Hedgehog might be the most “ciliary pathway” among all the other signaling pathways related to primary cilia.
In summary, primary cilia are associated with numerous pathways that are well known to form complex signaling networks (Christensen et al., 2017). And any defects of these complex signaling networks are about to cause a wide arrange of human diseases, such as cancers, ciliopathies, and obesity et al. Among them, the relationship between cancer and primary cilia is the most noticeable. Unregulated cell proliferation is one of the major characters of cancer cells. And as discussed above, primary cilium is a signal hub and many primary cilia mediated signaling pathways are cell cycle regulation-related. Except for the Hedgehog pathway, PDGF pathway, Ga2+ pathway mentioned above, more pathways are listed in Tab. 1. Therefore, any defects of primary cilia can give rise to cell cycle regulation defects then lead to cancer. It has been observed that many cancer cells are either non-ciliated (Cao and Zhong, 2016) or those ciliated cancer cells have abnormal primary cilia (Yasar et al., 2017). This phenomenon suggests that the ciliary loss or abnormality may cause cancers. Thus, in Tab. 1, it is intuitive to see that the diseases resulted from primary cilia mediated signal defects are mostly cancers or tumors. Furthermore, some primary cilia-mediated signaling pathway defects only induce mild cell cycle dysregulation as the mentioned PKD and many other ciliopathies with cyst (Tsang et al., 2018). In addition, defects of primary cilia mediated pathways also cause obesity. Obesity is a common character of Bardet-Biedl Syndrome (BBS) and Alstrom Syndrome (ALMS), which are both ciliopathies (Vaisse et al., 2017). This phenomenon indicates a potential intimacy between obesity (energy homeostasis of cells) and primary cilia. However, the molecular mechanism about how exactly defects in primary cilia interact with the energy homeostasis of cells is still unknown. Based on the recent literature, the interaction can be divided into two categories as far: Leptin-dependent and leptin-independent (Vaisse et al., 2017; Volta and Gerdes, 2017). These two pathways are different in molecule details from each other, but both of them are depending on the role of primary cilia as the signaling hubs in cells as many receptors of cellular energy homeostasis related signaling pathways are located in primary cilia, such as lepRb, Sstr3, 5HT6 and Npy2r et al. Therefore, any defects happening to primary cilia can damage the energy homeostasis and cause obesity in individual level.
Generally speaking, the multiplicity of the ciliary receptors system opens up a whole realm of possibilities for the primary cilium to coordinate the cross-talking between different signaling pathways, which in a concerted action, balances the biological output. And therefore, comprehensively regulates the physiological activities of cells, more than just cell cycle re-entry. Recent research shows that in keratinocytes, corneal epithelia and neuroepithelia, primary cilia are able to regulate cell proliferation and differentiation by regulating the Notch signaling pathway (Wheway et al., 2018). Although the mechanism is still intriguing, the implication of coordination from different pathways illustrates again the complicacy.
Primary cilium as a reservoir of cell cycle regulation factors
Primary cilia is a cell cycle regulation related factor reservoir structurally and compositionally separated from cytomembrane and cytoplasm (Satir and Christensen, 2007). When cilia experience physiological resorption or pathological structure damage, transcription factors or signaling pathway members originally stored inside primary cilia might be released to the cytoplasm and even end up being inside the nucleus. Gene expression profile regulating the cell cycle control may be changed. This mechanism is usually described as the “Reservoir Hypothesis” (Kim and Tsiokas, 2011) (see Fig. 3). Jouberin (Jbn) (Lancaster et al., 2011) and Gli2 (Han et al., 2009) are two typical cases of this hypothesis.
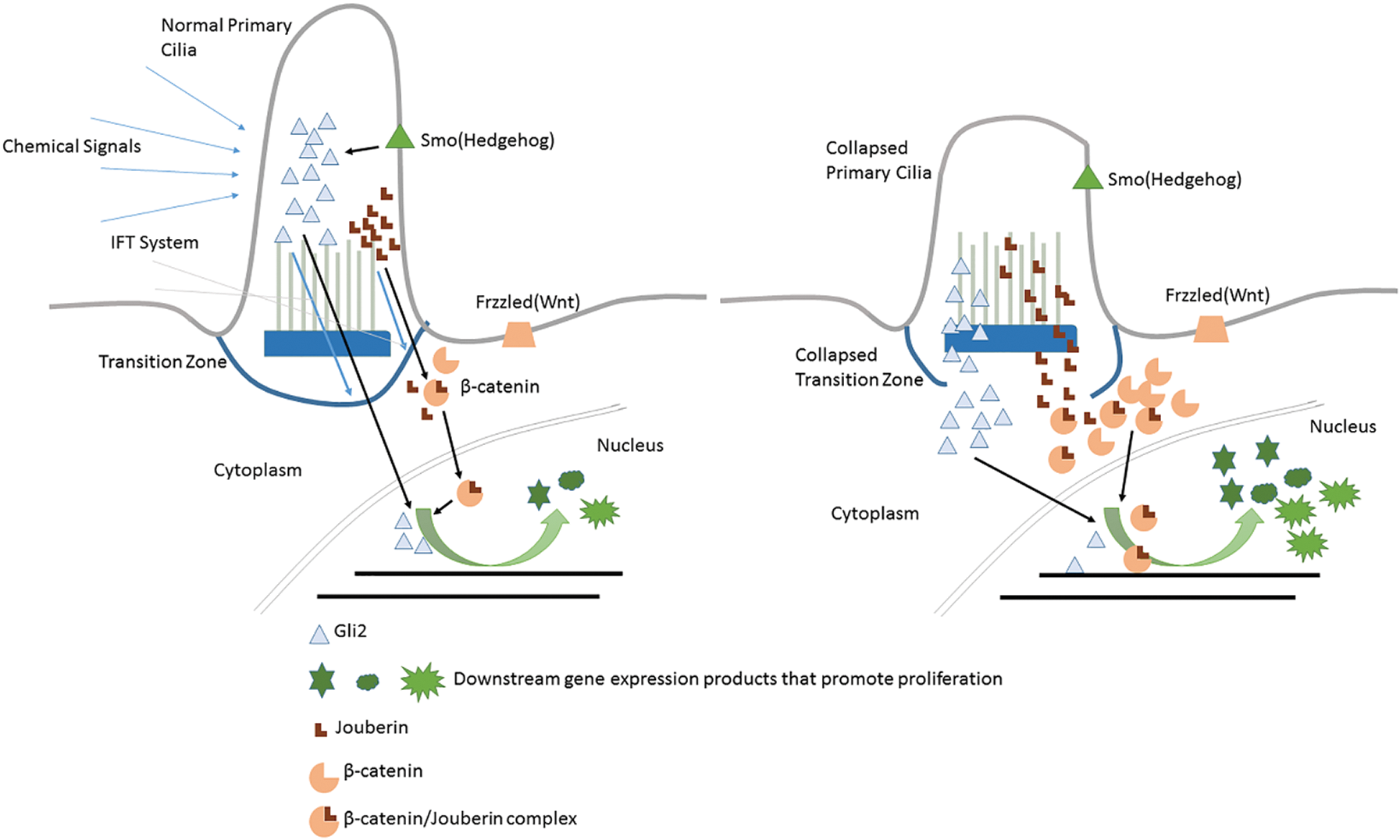
Figure 3: Primary cilium as a reservoir of cell cycle regulatory factors.
Jbn is encoded by AHI1, mutations of which mainly contribute to the Joubert Syndrome (Dixon-Salazar et al., 2004). The β-catenin is the core component of the canonical WNT pathway. Extracellular signals received by Wnt receptors (Frizzled, Lrp) are transmitted into the nucleus to regulate the downstream cellular activities including cell cycle re-entry, during which the nuclear localization of β-catenin is the most critical step. However, β-catenin itself has no nuclear localization sequence (NLS) responsible for this trans-localization. Jouberin (Jbn) is one kind of protein facilitating β-catenin’s re-localization and accumulation inside the nucleus (Lancaster et al., 2009). Jbn usually locates inside of primary cilia (Lancaster et al., 2011). However, this isolation status can be broken up under some conditions and the molecules are transferred out by the ciliary IFT system (Lancaster et al., 2009; Lancaster et al., 2011). So when cells are in quiescent status, primary cilia act as a negative regulator of Wnt signal to keep Jbn inside and away from β-catenin (Lancaster et al., 2011). While when the primary cilia experience resorption, Jbn may be released to the cytoplasm but in an IFT-independent way and promote Wnt-signaling by transferring more β-catenin into the nucleus. Then β-catenin makes proliferation-related genes express and finally leads to cell cycle re-entry (shown in Fig. 3).
As mentioned above, activation of Hedgehog signaling leads to the release of Gli factors to the cytoplasm, which are originally restricted inside primary cilium by SuFu in quiescent cells. Thus, those sequestered Gli factors (such as Gli2) use primary cilium as the reservoir. Under normal conditions, Gli2 could be released to the cytoplasm by Hedgehog ligands binding on Ptc1 to relieve Smo’s repression on SuFu. However, in some cases when primary cilia experience resorption (Han et al., 2009; Wong et al., 2009; Zhao et al., 2017), Gli2 could also be released to the cytoplasm, which makes the higher concentration of cytoplasmic Gli2. Excess Gli2 will be transferred into the nucleus and activate cell cycle promotion genes, leading to cell cycle dysregulation, which causes ciliopathies or tumors.
In conclusion, Jbn and Gli2 are originally sequestered inside primary cilia. When primary cilia are experiencing abnormal resorption or collapse, Jbn and Gli2 would be released into the cytoplasm and over-activate cell cycle-related signaling pathways. This hypothesis is different from the one that primary cilia are the cellular signal hub. Because there are no extracellular stimuli nor complete signal transduction involved. And this field calls for more attention.
Primary cilium as a cell cycle checkpoint or brake
Cell cycle checkpoints determine whether and when do cells enter the cell cycle. Many cellular events have been considered as checkpoints during the cell cycle. In fact, many characters of the interaction between primary cilia and cell cycle meet the features of being considered as a cell cycle checkpoint. First, when proteins promoting ciliogenesis such as NDE1 (Kim et al., 2011) and Tctex-1 (Li et al., 2011) are overexpressed, cells will experience cell cycle arrest. Second, when proteins suppressing the ciliogenesis such as Aurora-A and trichoplein are inhibited, the cell will experience cell cycle arrest as well (Zhu et al., 2017). And third, manipulating IFT proteins to make cell compulsorily experience ciliogenesis or ciliary resorption can reverse the effects by all the molecules. These three facts imply a similarity of primary cilia with the pattern of general checkpoint. Therefore, primary cilia might work as a physical checkpoint before cell cycle re-entry (Goto et al., 2017). This is the checkpoint hypothesis. In other words, by manipulating the length of primary cilia or the timing of ciliogenesis, researchers are able to delay the cell cycle re-entry or initiate it in advance (Hsiao et al., 2018). Many proteins functioning as ciliary regulators have been involved in this hypothesis. Such as Nde1, Tctex-1, Aurora-A-HEF1, Kif24 (Kim et al., 2015), Pifo (Kinzel et al., 2010), and IFT88 and IFT27 (Qin et al., 2007; Robert et al., 2007).
1. Aurora-A
Aurora/Ipl1-related kinases are evolutionally conserved serine/threonine kinases (Marumoto et al., 2005). And it is a key regulator during oncogenesis and a potential cancer therapeutic molecule (Katayama et al., 2003; Yan et al., 2016). Aurora-A influences the cell cycle by making the balance between ciliary resorption and ciliary disassembly towards disassembly (Izawa et al., 2015; Kasahara et al., 2018). After ciliary disassembly caused by Aurora-A, the cell would initiate its cell cycle re-entry process. Furthermore, Aurora-A is also capable of suppressing ciliogenesis during cell mitosis (Goto et al., 2017; Inaba et al., 2016). This demonstrates the dual function of Aurora-A in regulating mitosis. And it is a clear truth that being ciliated or not is a key feature before the cell cycle re-entry.
2. Nek2
Nek2 is an S/G phase kinase, which consists of two subtypes, Nek2A and Nek2b (Pfleger and Kirschner, 2000; Hames et al., 2001), and it has been found to function as an oncogene in many cancers (Cappello et al., 2014; Zhou et al., 2013; Hayward et al., 2004). There are researches demonstrating Nek2 as a suppressing factor of ciliogenesis (Spalluto et al., 2012; Kim et al., 2015), in which Nek2 and Kif24 work together to suppress ciliogenesis and promote ciliary disassembly and, next, cells are allowed to initiate cell cycle re-entry (Kobayashi et al., 2011). This phenomenon is consistent with the one described Aurora-A that when cilia are removed, cells would initiate the cell cycle re-entry.
3. Tctex-1
Tctex-1, also known as DYNLT1, is a dynein light chain protein. It is mostly discovered in neural precursor cells and plays an important role in neuron differentiation. When precursor cells lose its ability to proliferate, it begins to differentiate (Gauthier-Fisher et al., 2009). This fact suggests a possible interaction between Tctex-1 and cell cycle re-entry. And later research found that only in ciliated cells does Tctex-1 have an impact on G1/S transition (Li et al., 2011), demonstrating an essential role of cilia in the cell cycle. A further mechanism was uncovered that Thr96 Phosphorylated Tctex-1 can cause cilia resorption by IGF-1 and promote cell cycle re-entry (Yeh et al., 2013). However, only in ciliated cells can Tctex-1 be phosphorylated in Thr94 (Li et al., 2011). So phosphorylated Tctex-1 promotes ciliary resorption in a cilia-dependent way and leads to cell cycle re-entry. In summary, primary cilium is a cell cycle checkpoint and functions through Tctex-1.
4. Nde1
Nde1, interacting with dynein light chain LC8, can negatively regulate the ciliary length. It expresses at a high level during the M phase while at a low level during the G0 phase. And depletion of Nde1 leads to longer primary cilia delay in G0/G1 transition (Kim et al., 2011). Those two facts together suggest a connection between Nde1 and cell cycle regulation through cilia length. By knockdown Nde1 in zebrafish embryos, cells are found with longer primary cilia but with a lower mitosis index (Kim et al., 2011). So, Nde1 regulates cell cycle re-entry timing by controlling the length of primary cilia.
What is worth emphasizing here is that primary cilia play an essential role as the so-called checkpoint of mitosis not because of the dual functions of those proteins in both ciliogenesis/resorption and cell cycle re-entry and also not simply because both mitosis and ciliogenesis require the involvement of centriole. The quintessence of this hypothesis here is that cilia are considered as a checkpoint before and during cell cycle re-entry, just like other checkpoint parameters: Cell size, environmental nutrition, and DNA integrity. This feature is well demonstrated in Tctex-1 and Nde1: The presence or the length of cilia both influence the timing of cell cycle re-entry, just like the biological effects of other cellular checkpoint factors. Take DAN reparation, for instance, damaged DNA material without appropriate reparation leads to proliferation arrest (Karimian et al., 2016). And initiating ciliogenesis during mitosis also leads to proliferation arrest. Moreover, almost all the mentioned molecules (Aurora A, Nek1, Tctex-1, and Nde1) are oncogenesis-related. This supports the “checkpoint hypothesis” in an indirect way. Because many other cell cycle checkpoints related factors are also oncogenesis-related. It is easy to understand. These checkpoints molecules are ciliogenesis or ciliary resorption related. If they lost their functions, cells might be able to re-enter the cell cycle without appreciating regulation. Thus, all these proteins naturally can be considered as proto-oncogenes and tumor suppressor genes and should be brought to the light and considered as potentially promising cancer treatment targets.
Primary cilia and embryonic stem cell maintaining and differentiation
Regulating differentiation can be considered as a special form of cell cycle regulation. Because when a cell specializes, it loses the ability to divide while the cell non-specialized keeps its ability to divide. Primary cilia can also be discovered in embryonic stem cells (ESCs). Combining the following facts: most of the differentiated cells lost their ability to divide and primary cilia regulate the cell cycle, it is logical to assume that primary cilia may regulate cell differentiation as well. Literally, there is increasing evidence indicating that primary cilia may play an important role in ESCs maintaining and differentiation or the so-called asymmetric division (asymmetric division decide the fate of the daughter cells). Take Tctex-1, for instance, as mentioned above, it is an important neuron differentiation factor. And the possible mechanism of Tctex-1 promoting differentiation is suppressing Tctex-1 to keep cells ciliated. And these ciliated cells would lose their ability to divide and begin to differentiate. CPAP works the same way. In in vitro experiments, when CPAP is mutated, it fails to negatively regulate cilia length. This causes long cilia, retarded ciliary disassembly, and delayed cell cycle re-entry, and further leads to premature differentiation of NPCs (Gabriel et al., 2016). This is the first way of cilia regulating cell fate. Second, many embryonic stem cell makers are discovered on primary cilia in ESCs, including Oct4, Sox2, and Nanog. These factors are essential for stem cell maintenance. So this fact indicates a possibly critical role that primary cilia may play in ESCs maintaining (Vestergaard et al., 2014), but the mechanism is not known yet. Third, as the signaling hubs in cells, many signaling pathways are primary cilia-dependent. And these primary cilia-dependent signaling pathways are playing a significant role in not only cell cycle re-entry regulation but also ESCs maintaining or differentiation; these signaling pathways include PDGFR (Pébay et al., 2005), Hedgehog (Hunkapiller et al., 2011), and Wnt/β-catenin (Sato et al., 2003), and so on. Thus, by regulating the signal transduction, primary cilia become a key part of the cell differentiation manipulation center. In summary, Primary cilia regulate ESCs, both stem maintaining and differentiation, in three different ways.
In this review, three hypotheses were demonstrated in detail: (1) Primary cilium as a cellular signaling hub to regulate the expression of cell cycle control-related genes, (2) Primary cilium as a reservoir of cell cycle regulation-related transcription factors, (3) Primary cilium as a cell cycle brake or checking-point. However, this forgotten field has not been given enough attention. In terms of the first hypothesis, no further interaction has been uncovered so far. It is not clear whether primary cilia can regulate signaling pathways in a way more than just the scaffold of signaling, whether there is molecular cross-talking among different signaling pathways through or coordinated by primary cilia, and whether primary cilia can regulate signal strength and select downstream targets so to have different impacts on cell cycle. All these problems are attractive and need more attention. In the second hypothesis, the research of the molecular mechanism of Medulloblastoma genesis (MB) is the major resource on the information of Gli2 as a cilia-sequestered factor inside primary cilia so far. But there still is no direct evidence to prove that Gli2 or Jbn literally function in a way that perfectly matches the pattern of the hypothesis. And Medulloblastoma is still a promising information resource for future research so far. In the third hypothesis, it is clear to see the potential of primary cilia as a brake of cell cycle control. And these molecules mentioned above are all possible counterparts of other mature proliferation brake components such as p53 and p21. All those proteins (Aurora-A, Nek2, Tctex1, and Nde1) are promising targets for tumor treatment and require deeper research (Chen et al., 2020). And making cilia-free tumor cells ciliated may be a good way to suppress tumor spread and finally leads to complete cure. And as almost all human cells are ciliated, we would not see any potential of negative side effects. In the end, we briefly described the relation between primary cilia and stem cell maintaining/differentiation, which is similar to the pattern of interaction between the cell cycle and primary cilia. Therefore, there may be a more sophisticated interaction among these three cellular activities (ciliation, proliferation, and stem cell maintaining/differentiation).
From something being thought of as a vestigial structure in cells to a cellular organelle being considered as one of the most valuable research fields, the research on primary cilia has encountered its highlight moment in the 21st century, but the light is not bright enough yet. Many promising fields are quite away from the research spotlight, such as the interaction among primary cilia, cell cycle regulation, and tumorigenesis. Therefore, we sincerely hope that all this obscure can be clarified in future researches. And all potentials can become realities.
Author Contribution: The authors confirm contribution to the paper as follows: Study concept and design: Lin Liu, draft manuscript preparation: Lin Liu, Zhouwen Xu, Yuyan Jiang, Md. Rezaul Karim. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This study was supported by Zhejiang Provincial Natural Science Foundation of China No. LY20C120003.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Agathocleous M, Locker M, Harris WA, Perron M (2007). A general role of hedgehog in the regulation of proliferation. Cell Cycle 6: 156–159. DOI 10.4161/cc.6.2.3745. [Google Scholar] [CrossRef]
Andrae J, Gallini R, Betsholtz C (2008). Role of platelet-derived growth factors in physiology and medicine. Genes & Development 22: 1276–1312. DOI 10.1101/gad.1653708. [Google Scholar] [CrossRef]
Aspera-Werz RH, Chen T, Ehnert S, Zhu S, Frohlich T, Nussler AK (2019). Cigarette smoke induces the risk of metabolic bone diseases: Transforming growth factor beta signaling impairment via dysfunctional primary cilia affects migration, proliferation, and differentiation of human mesenchymal stem cells. International Journal of Molecular Sciences 20: 2915. DOI 10.3390/ijms20122915. [Google Scholar] [CrossRef]
Bangs FK, Schrode N, Hadjantonakis AK, Anderson KV (2015). Lineage specificity of primary cilia in the mouse embryo. Nature Cell Biology 17: 113–122. DOI 10.1038/ncb3091. [Google Scholar] [CrossRef]
Bergmann C (2019). Early and severe polycystic kidney disease and related ciliopathies: An emerging field of interest. Nephron 141: 50–60. DOI 10.1159/000493532. [Google Scholar] [CrossRef]
Breslin L, Prosser SL, Cuffe S, Morrison CG (2014). Ciliary abnormalities in senescent human fibroblasts impair proliferative capacity. Cell Cycle 13: 2773–2779. DOI 10.4161/15384101.2015.945868. [Google Scholar] [CrossRef]
Cao M, Zhong Q (2016). Cilia in autophagy and cancer. Cilia 5: 4. DOI 10.1186/s13630-016-0027-3. [Google Scholar] [CrossRef]
Cappello P, Blaser H, Gorrini C, Lin DCC, Elia AJ, Wakeham A, Haider S, Boutros PC, Mason JM, Miller NA, Youngson B, Done SJ, Mak TW (2014). Role of Nek2 on centrosome duplication and aneuploidy in breast cancer cells. Oncogene 33: 2375–2384. DOI 10.1038/onc.2013.183. [Google Scholar] [CrossRef]
Cornec-Le Gall E, Alam A, Perrone RD (2019). Autosomal dominant polycystic kidney disease. Lancet 393: 919–935. DOI 10.1016/S0140-6736(18)32782-X. [Google Scholar] [CrossRef]
Chen Q, Li J, Yang X, Ma J, Gong F, Liu Y (2020). Prdx1 promotes the loss of primary cilia in esophageal squamous cell carcinoma. BMC Cancer 20: 372. DOI 10.1186/s12885-020-06898-y. [Google Scholar] [CrossRef]
Christensen ST, Morthorst SK, Mogensen JB, Pedersen LB (2017). Primary cilia and coordination of Receptor Tyrosine Kinase (RTK) and Transforming Growth Factor beta (TGF-beta) signaling. Cold Spring Harbor Perspectives in Biology 9: a028167. DOI 10.1101/cshperspect.a028167. [Google Scholar] [CrossRef]
Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L (2008). Chapter 10 The Primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Current Topics in Developmental Biology 85: 261–301. [Google Scholar]
Delling M, Decaen PG, Doerner JF, Febvay S, Clapham DE (2013). Primary cilia are specialized calcium signalling organelles. Nature 504: 311–314. DOI 10.1038/nature12833. [Google Scholar] [CrossRef]
Dhekne HS, Yanatori I, Gomez RC, Tonelli F, Diez F, Schüle B, Steger M, Alessi DR, Pfeffer SR (2018). A pathway for Parkinson’s Disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. eLife 7: e40202. DOI 10.7554/eLife.40202. [Google Scholar] [CrossRef]
Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, Al-Gazali L, Al-Tawari AA, Kayserili H, Sztriha L, Gleeson JG (2004). Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. American Journal of Human Genetics 75: 979–987. DOI 10.1086/425985. [Google Scholar] [CrossRef]
Elliott KH, Brugmann SA (2019). Sending mixed signals: Cilia-dependent signaling during development and disease. Developmental Biology 447: 28–41. DOI 10.1016/j.ydbio.2018.03.007. [Google Scholar] [CrossRef]
Gabriel E, Wason A, Ramani A, Gooi LM, Keller P, Pozniakovsky A, Poser I, Noack F, Telugu NS, Calegari F, Šarić T, Hescheler J, Hyman AA, Gottardo M, Callaini G, Alkuraya FS, Gopalakrishnan J (2016). CPAP promotes timely cilium disassembly to maintain neural progenitor pool. EMBO Journal 35: 803–819. DOI 10.15252/embj.201593679. [Google Scholar] [CrossRef]
Gauthier-Fisher A, Lin DC, Greeve M, Kaplan DR, Rottapel R, Miller FD (2009). Lfc and Tctex-1 regulate the genesis of neurons from cortical precursor cells. Nature Neuroscience 12: 735–744. DOI 10.1038/nn.2339. [Google Scholar] [CrossRef]
Goto H, Inaba H, Inagaki M (2017). Mechanisms of ciliogenesis suppression in dividing cells. Cellular and Molecular Life Sciences 74: 881–890. DOI 10.1007/s00018-016-2369-9. [Google Scholar] [CrossRef]
Hames RS, Wattam SL, Yamano H, Bacchieri R, Fry AM (2001). APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO Journal 20: 7117–7127. DOI 10.1093/emboj/20.24.7117. [Google Scholar] [CrossRef]
Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A (2009). Dual and opposing roles of primary cilia in medulloblastoma development. Nature Medicine 15: 1062–1065. DOI 10.1038/nm.2020. [Google Scholar] [CrossRef]
Hayward DG, Clarke RB, Faragher AJ, Pillai MR, Hagan IM, Fry AM (2004). The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Research 64: 7370–7376. DOI 10.1158/0008-5472.CAN-04-0960. [Google Scholar] [CrossRef]
Hsiao CJ, Chang CH, Ibrahim RB, Lin IH, Wang CH, Wang WJ, Tsai JW (2018). Gli2 modulates cell cycle re-entry through autophagy-mediated regulation of the length of primary cilia. Journal of Cell Science 131: jcs221218. DOI 10.1242/jcs.221218. [Google Scholar] [CrossRef]
Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV (2003). Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426: 83–87. DOI 10.1038/nature02061. [Google Scholar] [CrossRef]
Hunkapiller J, Singla V, Seol A, Reiter JF (2011). The ciliogenic protein oral-facial-digital 1 regulates the neuronal differentiation of embryonic stem cells. Stem Cells and Development 20: 831–841. DOI 10.1089/scd.2010.0362. [Google Scholar] [CrossRef]
Ibraghimov-Beskrovnaya O, Natoli TA (2011). mTOR signaling in polycystic kidney disease. Trends in Molecular Medicine 17: 625–633. DOI 10.1016/j.molmed.2011.06.003. [Google Scholar] [CrossRef]
Inaba H, Goto H, Kasahara K, Kumamoto K, Yonemura S, Inoko A, Yamano S, Wanibuchi H, He D, Goshima N, Kiyono T, Hirotsune S, Inagaki M (2016). Ndel1 suppresses ciliogenesis in proliferating cells by regulating the trichoplein–Aurora A pathway. Journal of Cell Biology 212: 409–423. DOI 10.1083/jcb.201507046. [Google Scholar] [CrossRef]
Izawa I, Goto H, Kasahara K, Inagaki M (2015). Current topics of functional links between primary cilia and cell cycle. Cilia 4: 12. DOI 10.1186/s13630-015-0021-1. [Google Scholar] [CrossRef]
Karimian A, Ahmadi Y, Yousefi B (2016). Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 42: 63–71. DOI 10.1016/j.dnarep.2016.04.008. [Google Scholar] [CrossRef]
Kasahara K, Aoki H, Kiyono T, Wang S, Kagiwada H, Yuge M, Tanaka T, Nishimura Y, Mizoguchi A, Goshima N, Inagaki M (2018). EGF receptor kinase suppresses ciliogenesis through activation of USP8 deubiquitinase. Nature Communications 9: 758. DOI 10.1038/s41467-018-03117-y. [Google Scholar] [CrossRef]
Kasper M, Regl G, Frischauf AM, Aberger F (2006). GLI transcription factors: Mediators of oncogenic Hedgehog signalling. European Journal of Cancer 42: 437–445. DOI 10.1016/j.ejca.2005.08.039. [Google Scholar] [CrossRef]
Katayama H, Brinkley WR, Sen S (2003). The Aurora kinases: Role in cell transformation and tumorigenesis. Cancer and Metastasis Reviews 22: 451–464. DOI 10.1023/A:1023789416385. [Google Scholar] [CrossRef]
Kilander MBC, Wang CH, Chang CH, Nestor JE, Herold K, Tsai JW, Nestor MW, Lin YC (2018). A rare human CEP290 variant disrupts the molecular integrity of the primary cilium and impairs Sonic Hedgehog machinery. Scientific Reports 8: 17335. DOI 10.1038/s41598-018-35614-x. [Google Scholar] [CrossRef]
Kim S, Lee K, Choi JH, Ringstad N, Dynlacht BD (2015). Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nature Communications 6: 8087. DOI 10.1038/ncomms9087. [Google Scholar] [CrossRef]
Kim S, Tsiokas L (2011). Cilia and cell cycle re-entry: More than a coincidence. Cell Cycle 10: 2683–2690. DOI 10.4161/cc.10.16.17009. [Google Scholar] [CrossRef]
Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, Obara T, Tsiokas L (2011). Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nature Cell Biology 13: 351–360. DOI 10.1038/ncb2183. [Google Scholar] [CrossRef]
Kinzel D, Boldt K, Davis EE, Burtscher I, Trümbach D, Diplas B, Attié-Bitach T, Wurst W, Katsanis N, Ueffing M, Lickert H (2010). Pitchfork regulates primary cilia disassembly and left-right asymmetry. Developmental Cell 19: 66–77. DOI 10.1016/j.devcel.2010.06.005. [Google Scholar] [CrossRef]
Kobayashi T, Dynlacht BD (2011). Regulating the transition from centriole to basal body. Journal of Cell Biology 193: 435–444. DOI 10.1083/jcb.201101005. [Google Scholar] [CrossRef]
Kobayashi T, Tsang WY, Li J, Lane W, Dynlacht BD (2011). Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell 145: 914–925. DOI 10.1016/j.cell.2011.04.028. [Google Scholar] [CrossRef]
Kowal TJ, Falk MM (2015). Primary cilia found on HeLa and other cancer cells. Cell Biology International 39: 1341–1347. DOI 10.1002/cbin.10500. [Google Scholar] [CrossRef]
Labour MN, Riffault M, Christensen ST, Hoey DA (2016). TGFβ1-induced recruitment of human bone mesenchymal stem cells is mediated by the primary cilium in a SMAD3-dependent manner. Scientific Reports 6: 35542. DOI 10.1038/srep35542. [Google Scholar] [CrossRef]
Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, Willert K, Gleeson JG (2009). Impaired Wnt–β-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nature Medicine 15: 1046–1054. DOI 10.1038/nm.2010. [Google Scholar] [CrossRef]
Lancaster MA, Schroth J, Gleeson JG (2011). Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nature Cell Biology 13: 700–707. DOI 10.1038/ncb2259. [Google Scholar] [CrossRef]
Lechtreck KF (2015). IFT–cargo interactions and protein transport in cilia. Trends in Biochemical Sciences 40: 765–778. DOI 10.1016/j.tibs.2015.09.003. [Google Scholar] [CrossRef]
Lee K, Battini L, Gusella GL (2011). Cilium, centrosome and cell cycle regulation in polycystic kidney disease. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1812: 1263–1271. DOI 10.1016/j.bbadis.2011.02.008. [Google Scholar] [CrossRef]
Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, Tomizawa K, Kaitsuka T, Sung CH (2011). Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nature Cell Biology 13: 402–411. DOI 10.1038/ncb2218. [Google Scholar] [CrossRef]
Liang Y, Meng D, Zhu B, Pan J (2016). Mechanism of ciliary disassembly. Cellular and Molecular Life Sciences 73: 1787–1802. DOI 10.1007/s00018-016-2148-7. [Google Scholar] [CrossRef]
Liem KFJr., Ashe A, He M, Satir P, Moran J, Beier D, Wicking C, Anderson KV (2012). The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. Journal of Cell Biology 197: 789–800. DOI 10.1083/jcb.201110049. [Google Scholar] [CrossRef]
Liu Z, Tu H, Kang Y, Xue Y, Ma D, Zhao C, Li H, Wang L, Liu F (2019). Primary cilia regulate hematopoietic stem and progenitor cell specification through Notch signaling in zebrafish. Nature Communications 10: 1839. DOI 10.1038/s41467-019-09403-7. [Google Scholar] [CrossRef]
Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T (2006). Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Developmental Cell 10: 57–69. DOI 10.1016/j.devcel.2005.12.005. [Google Scholar] [CrossRef]
Lupu FI, Burnett JB, Eggenschwiler JT (2018). Cell cycle-related kinase regulates mammalian eye development through positive and negative regulation of the Hedgehog pathway. Developmental Biology 434: 24–35. DOI 10.1016/j.ydbio.2017.10.022. [Google Scholar] [CrossRef]
Marumoto T, Zhang D, Saya H (2005). Aurora-A—A guardian of poles. Nature Reviews Cancer 5: 42–50. DOI 10.1038/nrc1526. [Google Scholar] [CrossRef]
May-Simera H, Nagel-Wolfrum K, Wolfrum U (2017). Cilia-The sensory antennae in the eye. Progress in Retinal and Eye Research 60: 144–180. DOI 10.1016/j.preteyeres.2017.05.001. [Google Scholar] [CrossRef]
Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J (2003). Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genetics 33: 129–137. DOI 10.1038/ng1076. [Google Scholar] [CrossRef]
Nielsen BS, Malinda RR, Schmid FM, Pedersen SF, Christensen ST, Pedersen LB (2015). PDGFRbeta and oncogenic mutant PDGFRalpha D842V promote disassembly of primary cilia through a PLCgamma- and AURKA-dependent mechanism. Journal of Cell Science 128: 3543–3549. DOI 10.1242/jcs.173559. [Google Scholar] [CrossRef]
Nigg EA, Stearns T (2011). The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nature Cell Biology 13: 1154–1160. DOI 10.1038/ncb2345. [Google Scholar] [CrossRef]
Nishimura Y, Kasahara K, Shiromizu T, Watanabe M, Inagaki M (2019). Primary cilia as signaling hubs in health and disease. Advanced Science 6: 1801138. DOI 10.1002/advs.201801138. [Google Scholar] [CrossRef]
Pala R, Alomari N, Nauli SM (2017). Primary cilium-dependent signaling mechanisms. International Journal of Molecular Sciences 18: 2272. DOI 10.3390/ijms18112272. [Google Scholar] [CrossRef]
Pébay A, Wong RCB, Pitson SM, Wolvetang EJ, Peh GSL, Filipczyk A, Koh KLL, Tellis I, Nguyen LTV, Pera MF (2005). Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells 23: 1541–1548. DOI 10.1634/stemcells.2004-0338. [Google Scholar] [CrossRef]
Pfleger CM, Kirschner MW (2000). The KEN box: An APC recognition signal distinct from the D box targeted by Cdh1. Genes & Development 14: 655–665. [Google Scholar]
Plotnikova OV, Pugacheva EN, Golemis EA (2009). Primary cilia and the cell cycle. Methods in Cell Biology 94: 137–160. [Google Scholar]
Pruski M, Hu L, Yang C, Wang Y, Zhang JB, Zhang L, Huang Y, Rajnicek AM, St Clair D, McCaig CD, Lang B, Ding YQ (2019). Roles for IFT172 and primary cilia in cell migration, cell division, and neocortex development. Frontiers in Cell and Developmental Biology 7: 287. DOI 10.3389/fcell.2019.00287. [Google Scholar] [CrossRef]
Qin H, Wang Z, Diener D, Rosenbaum J (2007). Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Current Biology 17: 193–202. DOI 10.1016/j.cub.2006.12.040. [Google Scholar] [CrossRef]
Quarmby LM, Parker JD (2005). Cilia and the cell cycle? Journal of Cell Biology 169: 707–710. DOI 10.1083/jcb.200503053. [Google Scholar] [CrossRef]
Ramamurthy V, Cayouette M (2009). Development and disease of the photoreceptor cilium. Clinical Genetics 76: 137–145. DOI 10.1111/j.1399-0004.2009.01240.x. [Google Scholar] [CrossRef]
Reiter JF, Leroux MR (2017). Genes and molecular pathways underpinning ciliopathies. Nature Reviews Molecular Cell Biology 18: 533–547. DOI 10.1038/nrm.2017.60. [Google Scholar] [CrossRef]
Robert A, Margall-Ducos G, Guidotti JE, Bregerie O, Celati C, Brechot C, Desdouets C (2007). The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. Journal of Cell Science 120: 628–637. DOI 10.1242/jcs.03366. [Google Scholar] [CrossRef]
Satir P, Christensen ST (2007). Overview of structure and function of mammalian cilia. Annual Review of Physiology 69: 377–400. DOI 10.1146/annurev.physiol.69.040705.141236. [Google Scholar] [CrossRef]
Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH (2003). Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Medicine 10: 55–63. DOI 10.1038/nm979. [Google Scholar] [CrossRef]
Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen Søren T (2005). PDGFRαα signaling is regulated through the primary cilium in fibroblasts. Current Biology 15: 1861–1866. DOI 10.1016/j.cub.2005.09.012. [Google Scholar] [CrossRef]
Siebel C, Lendahl U (2017). Notch signaling in development, tissue homeostasis, and disease. Physiological Reviews 97: 1235–1294. DOI 10.1152/physrev.00005.2017. [Google Scholar] [CrossRef]
Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L (2018). The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosnian Journal of Basic Medical Sciences. Udruzenje Basicnih Mediciniskih Znanosti 18: 8–20. DOI 10.17305/bjbms.2018.2756. [Google Scholar] [CrossRef]
Sorokin SP (1968). Reconstructions of centriole formation and ciliogenesis in mammalian lungs. Journal of Cell Science 3: 207–230. [Google Scholar]
Spalluto C, Wilson DI, Hearn T (2012). Nek2 localises to the distal portion of the mother centriole/basal body and is required for timely cilium disassembly at the G2/M transition. European Journal of Cell Biology 91: 675–686. DOI 10.1016/j.ejcb.2012.03.009. [Google Scholar] [CrossRef]
Tadenev AL, Kulaga HM, May-Simera HL, Kelley MW, Katsanis N, Reed RR (2011). Loss of Bardet-Biedl syndrome protein-8 (BBS8) perturbs olfactory function, protein localization, and axon targeting. Proceedings of the National Academy of Sciences of the United States of America 108: 10320–10325. DOI 10.1073/pnas.1016531108. [Google Scholar] [CrossRef]
Tian T, Li X, Zhang J (2019). mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. International Journal of Molecular Sciences 20: 755. DOI 10.3390/ijms20030755. [Google Scholar] [CrossRef]
Tsang SH, Aycinena ARP, Sharma T (2018). Ciliopathy: Bardet-Biedl syndrome. Advances in Experimental Medicine and Biology 1085: 171–174. [Google Scholar]
Tucker RW, Pardee AB, Fujiwara K (1979). Centriole ciliation is related to quiescence and DNA-synthesis in 3t3-cells. Cell 17: 527–535. DOI 10.1016/0092-8674(79)90261-7. [Google Scholar] [CrossRef]
Vaisse C, Reiter JF, Berbari NF (2017). Cilia and obesity. Cold Spring Harbor Perspectives in Biology 9: a028217. DOI 10.1101/cshperspect.a028217. [Google Scholar] [CrossRef]
Vestergaard ML, Awan A, Warzecha CB, Christensen ST, Andersen CY (2014). Immunofluorescence microscopy and mRNA analysis of human Embryonic Stem Cells (hESCs) including primary cilia associated signaling pathways. In: Turksen K (ed.Human Embryonic Stem Cell Protocols, pp. 123–140. New York, NY: Humana Press. [Google Scholar]
Volta F, Gerdes JM (2017). The role of primary cilia in obesity and diabetes. Annals of the New York Academy of Sciences 1391: 71–84. DOI 10.1111/nyas.13216. [Google Scholar] [CrossRef]
Wheatley DN (1995). Primary cilia in normal and pathological tissues. Pathobiology 63: 222–238. DOI 10.1159/000163955. [Google Scholar] [CrossRef]
Wheway G, Nazlamova L, Hancock JT (2018). Signaling through the Primary Cilium. Frontiers in Cell and Developmental Biology 6: 8. DOI 10.3389/fcell.2018.00008. [Google Scholar] [CrossRef]
Wong SY, Reiter JF (2008). Chapter 9 The primary cilium. Current Topics in Developmental Biology 85: 225–260. [Google Scholar]
Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EHJr, Dlugosz AA, Reiter JF (2009). Primary cilia can both mediate and suppress Hedgehog pathway–dependent tumorigenesis. Nature Medicine 15: 1055–1061. DOI 10.1038/nm.2011. [Google Scholar] [CrossRef]
Wu C, Li J, Peterson A, Tao K, Wang B (2017). Loss of dynein-2 intermediate chain Wdr34 results in defects in retrograde ciliary protein trafficking and Hedgehog signaling in the mouse. Human Molecular Genetics 26: 2386–2397. DOI 10.1093/hmg/ddx127. [Google Scholar] [CrossRef]
Yan M, Wang C, He B, Yang M, Tong M, Long Z, Liu B, Peng F, Xu L, Zhang Y, Liang D, Lei H, Subrata S, Kelley KW, Lam EWF, Jin B, Liu Q (2016). Aurora-A kinase: A potent oncogene and target for cancer therapy. Medicinal Research Reviews 36: 1036–1079. DOI 10.1002/med.21399. [Google Scholar] [CrossRef]
Yasar B, Linton K, Slater C, Byers R (2017). Primary cilia are increased in number and demonstrate structural abnormalities in human cancer. Journal of Clinical Pathology 70: 571–574. DOI 10.1136/jclinpath-2016-204103. [Google Scholar] [CrossRef]
Yeh C, Li A, Chuang JZ, Saito M, Caceres A, Sung CH (2013). IGF-1 activates a cilium-localized noncanonical Gβγ signaling pathway that regulates cell-cycle progression. Developmental Cell 26: 358–368. DOI 10.1016/j.devcel.2013.07.014. [Google Scholar] [CrossRef]
Zhao X, Pak E, Ornell KJ, Pazyra-Murphy MF, Mackenzie EL, Chadwick EJ, Ponomaryov T, Kelleher JF, Segal RA (2017). A Transposon screen identifies loss of primary cilia as a mechanism of resistance to SMO inhibitors. Cancer Discovery 7: 1436–1449. DOI 10.1158/2159-8290.CD-17-0281. [Google Scholar] [CrossRef]
Zhou W, Yang Y, Xia J, Wang H, Salama ME, Xiong W, Xu H, Shetty S, Chen T, Zeng Z, Shi L, Zangari M, Miles R, Bearss D, Tricot G, Zhan F (2013). NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Cancer Cell 23: 48–62. DOI 10.1016/j.ccr.2012.12.001. [Google Scholar] [CrossRef]
Zhu Q, Yu X, Zhou ZW, Zhou C, Chen XW, Zhou SF (2017). Inhibition of Aurora A kinase by alisertib induces autophagy and cell cycle arrest and increases chemosensitivity in human hepatocellular carcinoma HepG2 cells. Current Cancer Drug Targets 17: 386–401. DOI 10.2174/1568009616666160630182344. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |