DOI:10.32604/biocell.2021.012851

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.012851 |  www.techscience.com/journal/biocell |
| Article |
Upregulation of microRNA-451a improves endothelial cell function in atherosclerosis by direct targeting of macrophage migration inhibitory factor
Department of Laboratory Medicine, Henan Provincial Hospital, Zhengzhou, 450000, China
*Address correspondence to: Hongxia Hu, hongxiahu@126.com
Received: 15 July 2020; Accepted: 21 December 2020
Abstract: This study aims to detect the expression of selected circulating microRNAs (miRNA), including miRNA-451a, miRNA-486-5p and miR-10b-5p, and their prospective roles as biomarkers in patients with atherosclerosis. For this purpose, levels of miRNAs were detected by real-time quantitative polymerase chain reaction (RT-qPCR) in case (N = 30) and healthy control (N = 30) groups. Receiver operating characteristic (ROC) curve analysis was carried out to evaluate the diagnostic ability of miRNAs. The correlations of miR-451a with lipid parameters were evaluated using Pearson’s correlation coefficients. HUVEC tested by ox-LDL was used as a cellular model of atherosclerosis. Cell viability and apoptosis were detected by methyl thiazolyl tetrazolium (MTT) and flow cytometry (FC) assays. Luciferase assay was used to determine the miRNA binding site on target genes. The results showed that miRNA-451a expression level was significantly lower in patients than controls. ROC curve analysis showed that the areas under the curve (AUC) of plasma miRNA-451a was 0.90. The miRNA-451a expression level exhibited significant negative correlations with cholesterol (TC), triglyceride (TG), and low-density lipoprotein (LDL). Besides, miRNA-451a specifically binds to the 3’UTR of macrophage migration inhibitory factor (MIF) and mediated the cell proliferation and apoptosis of HUVECs exposed to ox-LDL. Furthermore, overexpression of miRNA-451a promoted the proliferation and alleviated apoptosis of HUVECs exposed to ox-LDL, while this result was attenuated by macrophage migration inhibitory factor (MIF) overexpression. Therefore, miRNA-451a could be considered as a potential biomarker for atherosclerosis and miRNA-451a might contribute to regulating atherosclerosis through targeting MIF.
Keywords: miRNA-451a; Biomarker; Atherosclerosis; MIF; ox-LDL
Atherosclerosis is the major cause of coronary artery disease (CAD), cerebral infarction, and peripheral vascular disease (Gupta et al., 2020). In the pathogenesis of atherosclerosis, dysfunction of endothelial cells has been linked as a very early action adhered to by the intrusion of proinflammatory cells, proliferation, and the migration of smooth muscle mass cells (Li et al., 2018). Nowadays, heart disease makes up roughly 30% of all health-related deaths worldwide, making atherosclerotic lesions a common cause of death (Poznyak et al., 2020). Early medical diagnosis and therapy can slow down or halt the worsening of atherosclerosis. It is hence essential to create effective techniques for the detection of atherosclerosis at an early stage.
Endothelial injury plays an important role in the initiation and also the development of coronary atherosclerosis.
It is normally believed that the state of the endothelial cell is influenced by several cardio risk variables and various stimulation (Dong et al., 2017; Huang et al., 2019). A low-density lipoprotein (LDL) is one of the most important risk factors of atherosclerosis. After oxidation, LDL obtains changed right into oxidized LDL (ox-LDL) and participates in atherosclerosis progression (Miller et al., 2010).
The microRNAs (miRNAs) are a class of small, non-coding, single-stranded RNAs identified as important post-transcriptional regulators (Solly et al., 2019). Most studies have confirmed that miRNAs are key regulators of the cardiovascular system. They are involved in regulating almost all cell types related to the cardiovascular system, such as endothelial cells (Widlansky et al., 2018), cardiomyocytes (Liu et al., 2019), smooth muscle cells (Karvande et al., 2018), fibroblasts (Krakowsky et al., 2018), etc., so they are associated with the occurrence and development of various cardiovascular diseases and their molecular mechanisms (van Rooij, 2012; Solly et al., 2019; Peters et al., 2020). One recent study has described miRNAs differentially expressed between coronary atherosclerotic plaques (CAP) and healthy arteries. miRNA microarray chip analysis showed that miRNA-451a, miRNA-486-5p, and miR-10b-5p were downregulated in CAP (Berkan et al., 2019). However, little data were found on the association of atherosclerosis with ox-LDL, as well as with these miRNAs. And the mechanisms underlying ox-LDL and 451a for atherosclerosis remained less well studied. Therefore, the present study aimed to use RT-qPCR to identify the relative expression levels of these miRNAs in patients with atherosclerosis and healthy people. We also evaluated the effects of these miRNAs on cell proliferation and apoptosis in ox-LDL induced HUVECs.
A total of 60 patients diagnosed with atherosclerosis and 30 healthy control subjects were enrolled from the Henan provincial hospital for this study. Atherosclerosis group defined as having CAD were documented by angiography. Angiographic Inclusion criteria were: ≥50% stenosis of at least one major coronary vessel because of atherosclerosis. Tab. 1 shows the detailed characteristic of the patients. All requirements were met according to the Declaration of Helsinki. Patients were fully informed of the study procedures before they provided their consent. Experiments were conducted according to the Declaration of Helsinki. This study was supported and approved by the Ethics Committee of Henan provincial hospital (2019-11).
Human Umbilical Vein Endothelial Cells (HUVECs) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma, Shanghai, China) supplemented with 10% fetal bovine serum (FBS) under humidified incubator containing 5% CO2 at 37°C. The cell transfection was performed using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer’s instructions. The miRNA-451a mimic/inhibitor, negative control (NC), and pcDNA3.1/pcDNA3.1-MIF were obtained from RiboBio (Guangzhou, China).
Cell viability and cytotoxicity assay were measured with CCK8 Kit (Solarbio) according to the instructions of the manufacturers. HUVECs cells were seeded in 96-well plates at a density of 5.0 × 103 cells/well and treated with different concentrations of ox-LDL for 24/48/72 h. At the end of the experiments, CCK-8 solution was added to each culture well and incubated for 30 min at 37°C. It was then measured using a microplate reader (Bio-Rad, USA) at 490 nm.
3’-UTR luciferase reporter assays
HUVECs cells were seeded in 6-well plates at a density of 1 × 105 cells/well and co-transfected with 20 ng luciferase reporter plasmid and the indicated miRNAs (final concentration, 25 nmol/L) using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. Luciferase assays were performed using the Dual-Luciferase Assay System (Promega). Transfection efficiency was normalized by calculating the Luciferase/Renilla activity ratio. All experiments were done in triplicate, and three independent experiments were performed.
Detection of apoptosis by flow cytometry
The cell apoptosis was detected by flow cytometry using an Annexin V-FITC Apoptosis Detection kit (Beyotime, China) following the manufacturer’s instructions. Briefly, after treatment, cells were trypsinized at 37°C for 5 min and incubated with PI and Annexin V-FITC for 15 min at 37°C in the dark. Then, the rates of apoptosis were analyzed in an Accuri C6 flow cytometer (Becton Dickinson) and determined using FlowJo software.
Detection and quantification of miRNAs by RT-qPCR
The expression level of miRNAs (miRNA-451a, miRNA-486-5p, and miR-10b-5p) was detected by qRT-PCR analysis using an Applied Biosystems (ABI) 7300 system. Briefly, total RNA was extracted from 60 samples using a miRcute miRNA isolation kit (Tiangen, China), according to the manufacturer’s instructions. Reverse transcription was performed using miRNA RT Reaction Buffer and miRNA RT Enzyme Mix (Tiangen, China). The sequences of primer pairs are listed in Tab. 1. mRNA and miRNA expression levels were quantified using the 2−ΔΔCq method and normalized to the internal reference genes GAPDH and U6, respectively.
Table 1: Primer sequences for quantitative real-time PCR

Protein was extracted from HUVECs in RIPA lysis buffer (Beyotime, Shanghai, China) and separated on 8–10% SDS-PAGE to quantify the level of MIF, Bax, Bcl-2, Caspase 3. Then the proteins were transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, USA). After blocking with 5% milk powder diluted in TBS containing 0.05% Tween 20 (TBST), membranes were with being probed with MIF, Bax, Bcl-2, Caspase 3 (Proteintech), GAPDH (Abcam) for 24 h at 4°C. Afterwards, the proteins were incubated with appropriate secondary antibodyies for 2 h at about 25°C. All Western blots were repeated at least three times. The density of the immunoreactive bands was quantified using ImageJ software. Protein levels were normalized to endogenous control of GAPDH protein.
All quantitative data were reported at least three separate experiments performed in triplicate. Statistical significance was conducted by GraphPad Prism 5, and One-way ANOVA followed by Tukey’s post hoc test, and Student’s t-test was used for comparisons between groups, and p < 0.05 was considered statistically significant. Multivariate regression analysis and the rank-sum test were also used to analyze and process-related data. The area under the ROC curve (AUC) is the average sensitivity of the biomarker over the range of specificities. It is often used as a summary statistic representing the overall performance of the biomarker. GraphPad Prism 8.0 software was used for ROC curve analyses to evaluate the AUC values. The ROC analysis was performed to evaluate the diagnostic performance of parameters showing significant differences between low-grade and high-grade rectal cancer groups together with their combinations by comparing the AUCs. Pearson correlations were performed to characterize the bivariate relationship between the dependent and independent variables.
Clinical characteristics of the study subjects
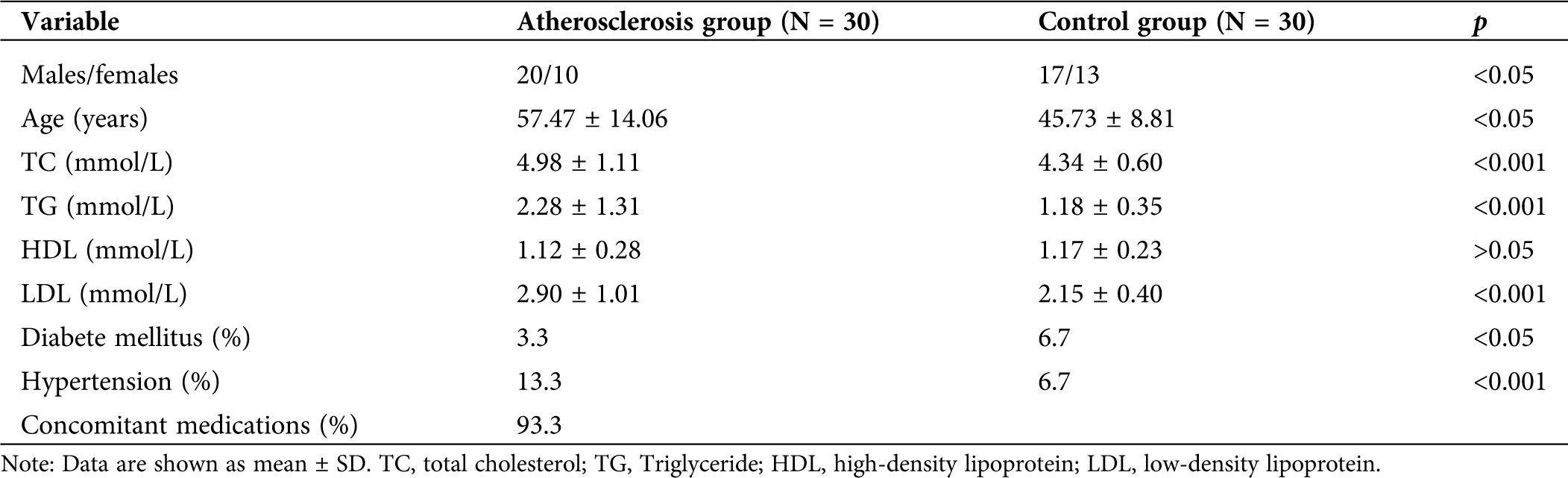
The patient’s basic information was recorded before the patients were admitted into the hospital. The clinical characteristics of the patients were summarized in Tab. 2. Among them, several variables showed significant differences, including age, TC, TG, LDL, and diabetes and high blood pressure between the two groups.
Differentially expression of miRNAs in patients with atherosclerosis
Table 2: The clinical characteristics of the study subjects of both the atherosclerosis patients and the control group
To explore the expression of miRNAs in the patients with atherosclerosis, the blood of atherosclerosis and control group was selected, and the expression of miRNA-451a, miRNA-486-5p, and miRNA-10b-5p were detected by RT-qPCR. The results showed that circulating miRNA-451a was significantly lower expressed in atherosclerosis than controls (Fig. 1A), while miRNA-486-5p and miRNA-10b-5p levels did not significantly differ between the two groups. To further explore the diagnostic potential of circulating miRNAs, we determined the discriminatory power between the groups by ROC analysis, using the RT-PCR results from the validation study. As shown in Fig. 1B, the ROC curve analysis of miRNA-451a exhibited strong differentiation power between patients with atherosclerosis and healthy people, evidenced by an AUC of 0.900 (95%CI, 0.827–0.973; p < 0.001). The AUC of miRNA-486-5p or miRNA-10b-5p in atherosclerosis patients was 0.636 (95%CI, 0.494–0.778; p = 0.071), 0.616 (95%CI, 0.470–0.761; p = 0.124). Our results indicate that miRNA-451a could be eligible candidate biomarkers for the diagnosis of atherosclerosis. Besides, correlations of miR-145a with the lipid parameters were determined using Pearson’s correlation analyses (Tab. 3). The results demonstrated that the miR-145a expression level exhibited negative correlations with TC.
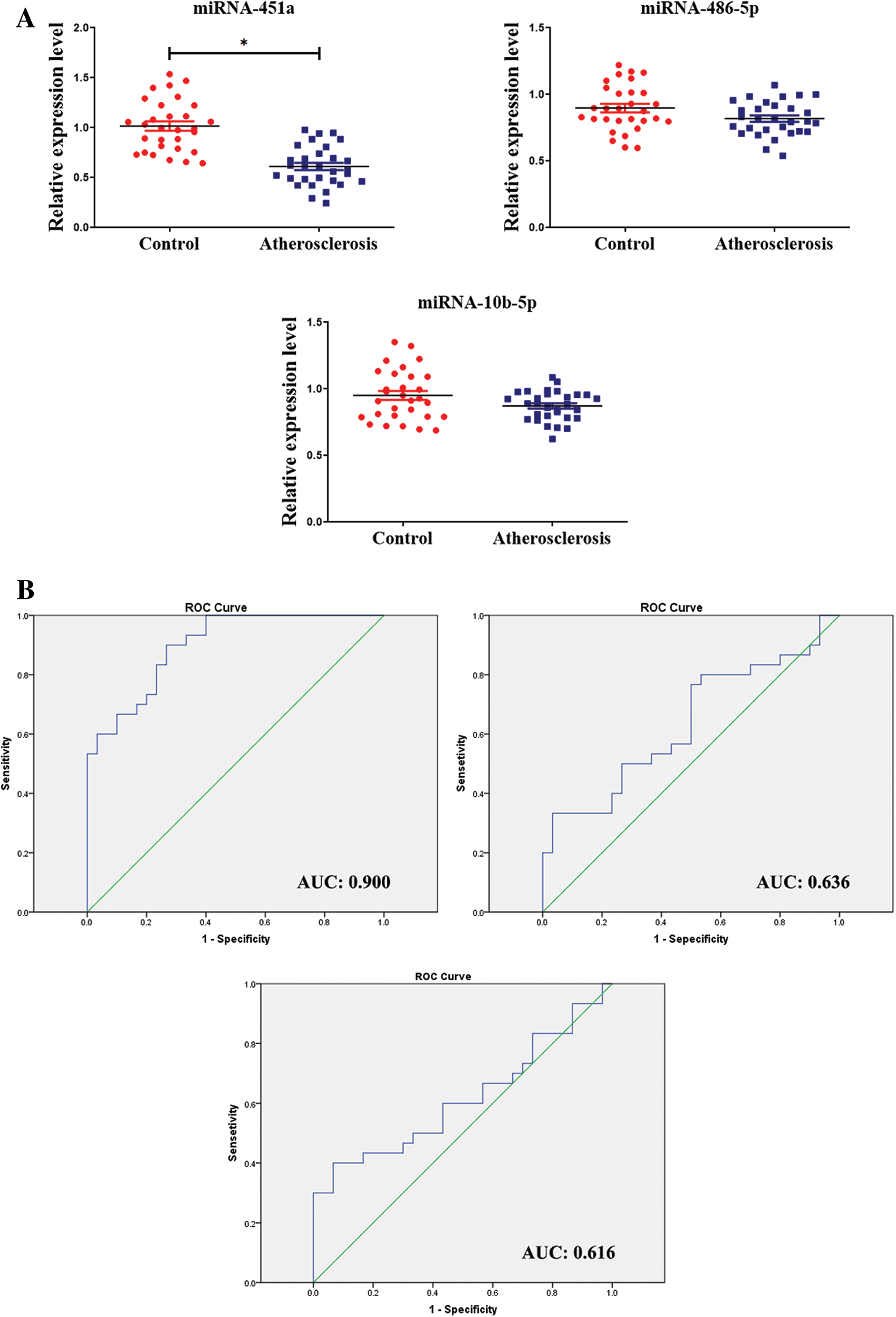
Figure 1: The expression level of circulating miRNAs in patients with atherosclerosis.
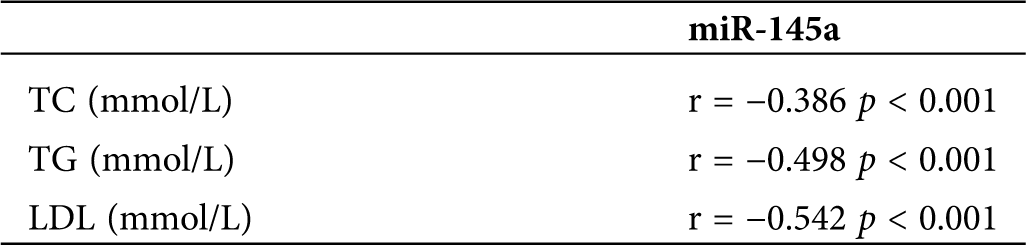
Table 3: Pearson correlation analysis of the association between miR-145a and lipid parameters
Identification of MIF as a target of miRNA-451a in HUVECs
It has been well known that miRNA exerts its It has been well known that miRNA exerts its function via binding to the 3’-UTR of target genes through partial sequence homology. We sought to identify the direct target of miRNA-451a involved in atherosclerosis. We used the most commonly used bioinformatics prediction tools (TargetScan, starBase, and miRanda) to identify the target. Among the top list, one of the potential targets was MIF, an inflammatory cytokine that also exerts chemokine-like function in cell proliferation and organ development (Bernhagen et al., 2007). To verify this possibility, we employed a luciferase gene report system fused downstream to a segment of the MIF 3’-UTR containing either the WT putative miRNA-451a-binding sequence (MIF-3’-UTR-WT) or a point MUT putative miRNA-451a-binding sequence (MIF-3;-UTR-MUT) (Fig. 2A). 48 h after co-transfection, luciferase reporter activity was significantly reduced by miRNA-451a mimic, indicating that MIF is a downstream target gene of miRNA-451a (Fig. 2B). Furthermore, transfection of miRNA-451a mimics into HUVEC cells downregulated both MIF mRNA and protein levels, as shown by real-time PCR and Western blot analysis (Figs. 2C and 2D). Meanwhile, the transfection of miRNA-451a inhibitor resulted in the upregulation of mRNA and protein levels of MIF (Figs. 2C and 2D). Taken together, these results confirmed that MIF is a direct downstream target of miRNA-451a.
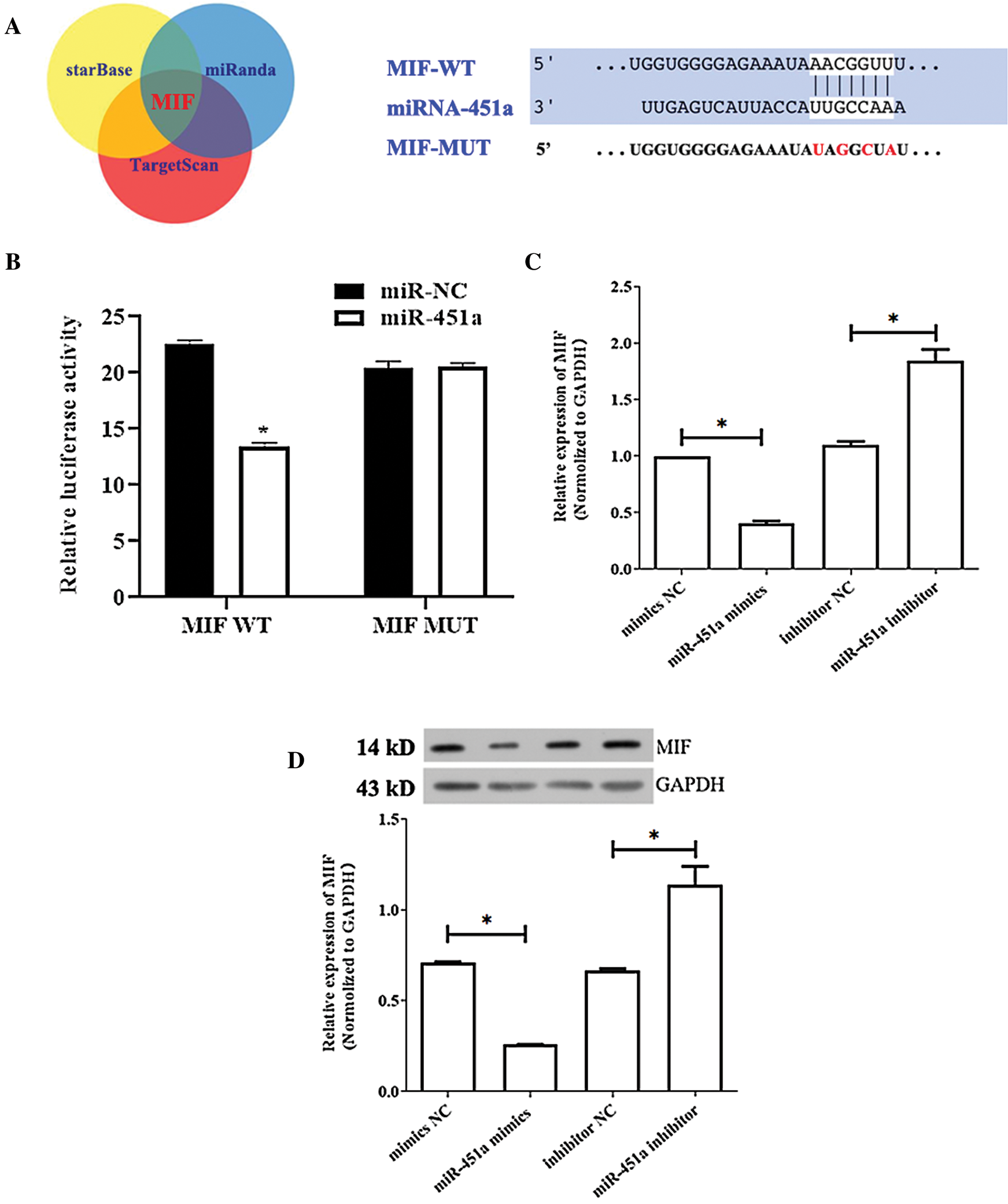
Figure 2: miRNA-451a directly targets MIF in HUVECs.
MiRNA-451a regulates proliferation and apoptosis through targeting on MIF treated with ox-LDL in HUVECs
We consequently conducted further analysis to explore whether miR-451a regulated MIF expression in ox-LDL-treated HUVECs. HUVEC cell survival rates were examined by the CCK8 assay after treatment with increasing concentrations of ox-LDL (0, 25, 50, and 100 μg/mL) at different time periods (12, 24, or 48 h). Ox-LDL caused HUVECs death as compared with the control group in a dose and time-dependent manner. Cell viability decreased to 54.91 ± 2.23% after treated with 100 μg/mL ox-LDL (Fig. 3A). Therefore, the ox-LDL concentration of 100 μg/mL was used for further experiments. Results showed that miRNA-451a expression was decreased in HUVEC after stimulation of ox-LDL in a concentration-dependent manner (Fig. 3B). Simultaneously, the mRNA level of MIF increased after ox-LDL treatment (Fig. 3C). In the pathogenesis of atherosclerosis, ECs dysfunction has been implicated as an early step, and the following HUVECs proliferation and apoptosis are particularly important associated with atherosclerotic progression. We observed that the overexpression of miRNA-451a significantly promoted cell proliferation in ox-LDL-treated cells compared with the ox-LDL+mimics NC group, whereas the effect was reversed by co-transfection with miRNA-451a mimics and pcDNA3.1-MIF (Fig. 4A).
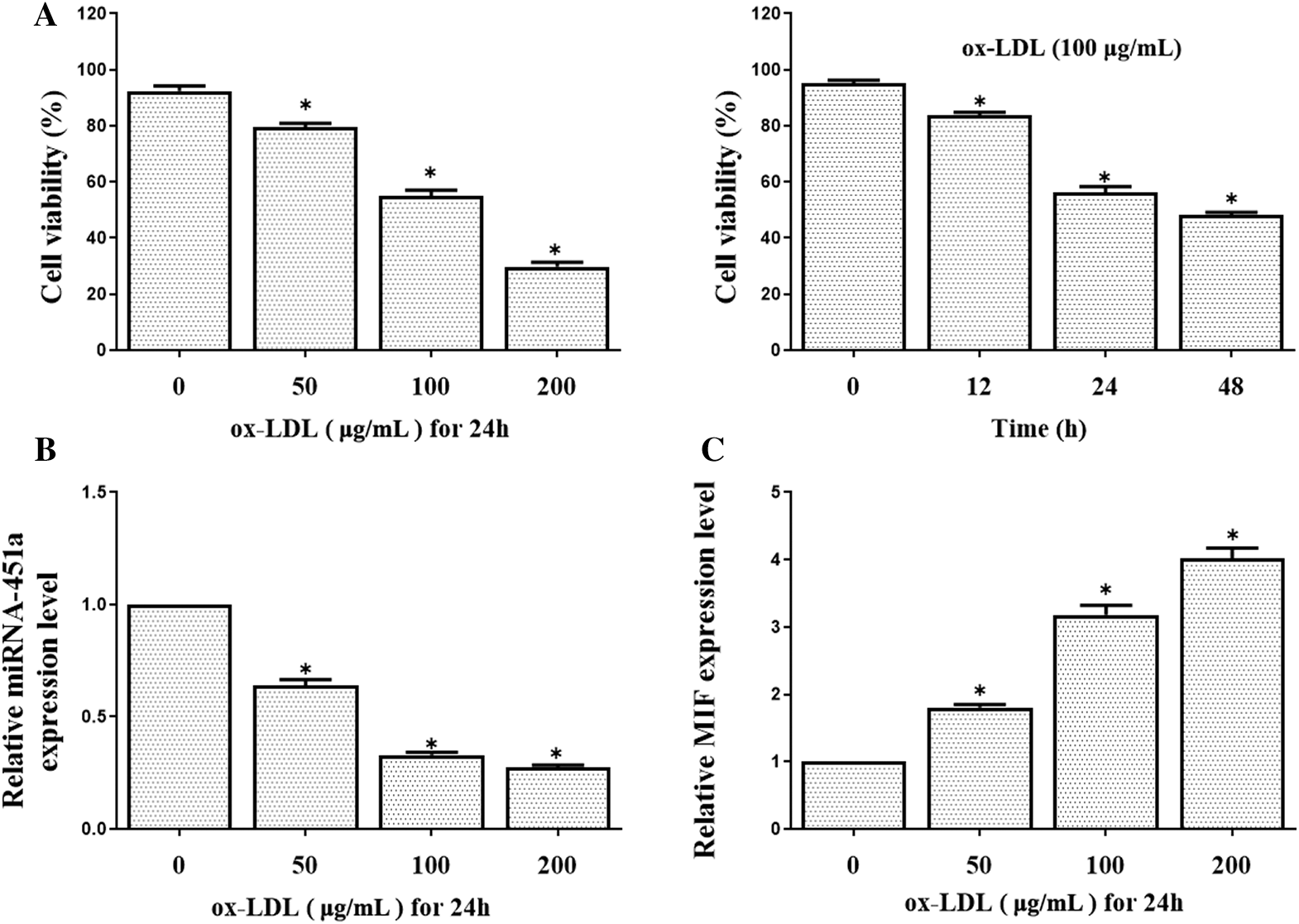
Figure 3: (A) HUVECs were exposed to various concentrations of ox-LDL (0, 50, 100, 200 μg/mL) for different periods (12, 24, 48 h).
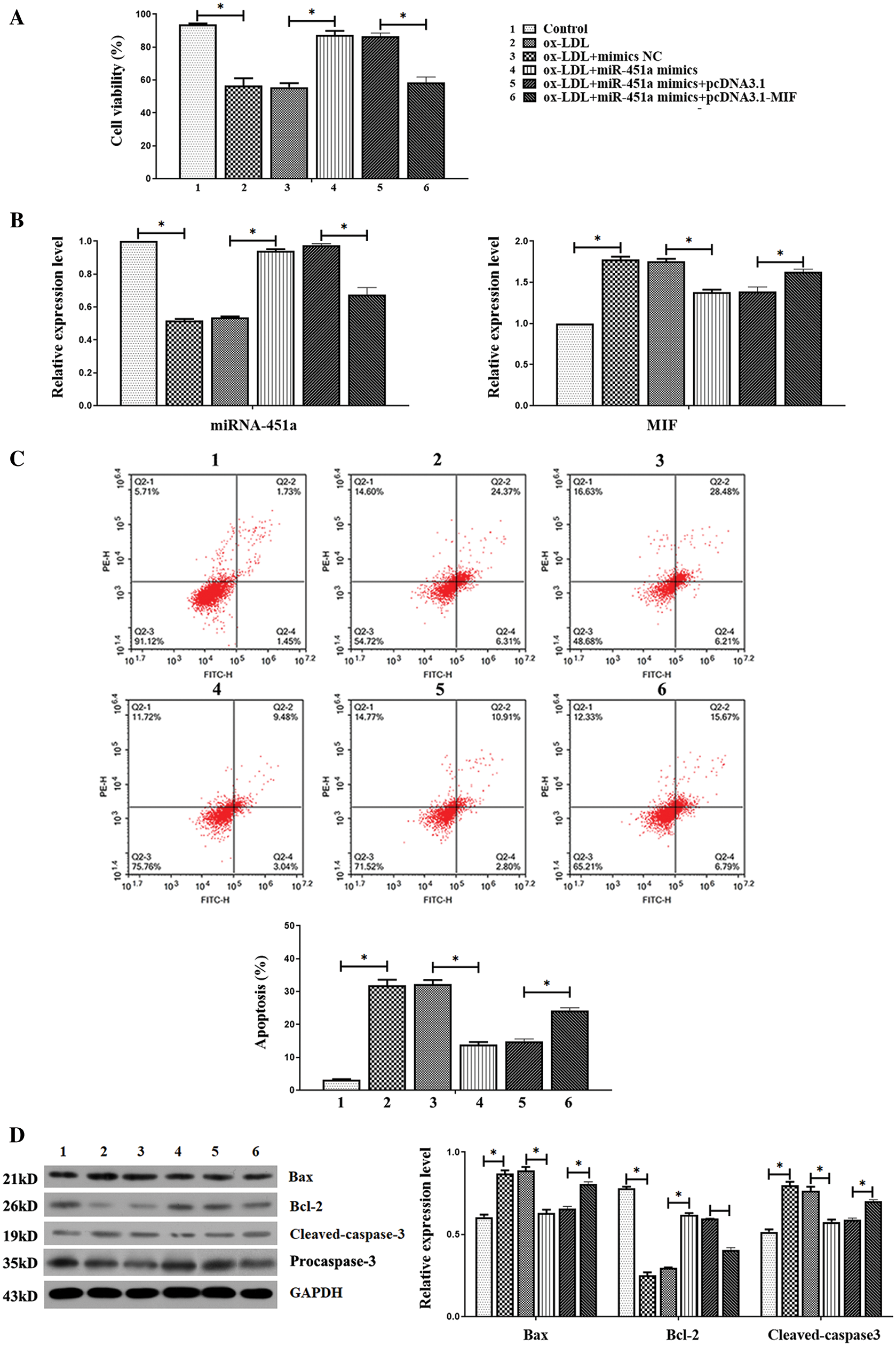
Figure 4: The up-regulation of MIF reverses the effect of miRNA-451a overexpression on proliferation and apoptosis in HUVEC stimulated by ox-LDL.
Moreover, qRT-PCR results indicated that MIF mRNA levels in HUVEC under ox-LDL treatment could be significantly reduced by miRNA-451a overexpressing, which was largely restored by MIF overexpressing plasmid transfection (Fig. 4B). Also, in ox-LDL-treated HUVECs, the increased apoptotic rate, upregulated Bax, and cleaved-caspase-3 protein expressions, as well as decreased protein Bcl-2 protein, were ameliorated by miRNA-451a mimics, but these changes were eliminated with co-transfection with miRNA-451a mimics and pcDNA3.1-MIF in ox-LDL-treated HUVEC cells (Figs. 4C and 4D). Thus, miRNA-451a upregulation could stimulate HUVECs proliferation and apoptosis by directly targeting MIF.
Appropriate statistical treatment of the data is essential. Heart disease is a consequence of epigenetic and genetic interactions. In the last few years, emerging evidence has indicated that miRNAs participated in the progression of various diseases, and studies had proved that some miRNAs are involved in different biological functions of atherosclerosis (Hartmann et al., 2016; Lu et al., 2018). Serum miRNAs have been considered a promising novel biomarker for CAD (Du et al., 2020a; Gupta et al., 2020). The results of this study uphold the basic hypothesis that miRNAs are present in serum and represent useful clinical biomarkers. Our study tested whether serum levels of three miRNAs (miRNA-451a, miRNA-486-5p, and miR-10b-5p) were different among subjects of different severities of cerebrovascular disease. We found that atherosclerosis subjects had significantly lower miRNA-451a levels than healthy controls, while the miRNA-486-5p and miR-10b-5p levels did not show a significant difference.
miRNA-451a is located in chromosome 17q11.2 and is widely dysregulated in numerous human malignancies (Bus et al., 2016; Sun and Jiang, 2018). Currently, the function of miRNA-451a in cardiovascular diseases has not been well identified. Most studies provided evidence of the effects of miRNA-451a, showing its capability to inhibit cell proliferation and induce apoptosis in numerous cancer cell lines. Previous studies show that miRNA-451a aggravates lipotoxicity and cardiac hypertrophy in high fat diet-induced diabetic cardiomyopathy (Yamada et al., 2013). miR-451 was very closely related to nonalcoholic fatty liver illness (NAFLD), which led to the rising danger of heart diseases, metabolic diseases, and type 2 diabetes (Zeng et al., 2018). Besides, epidemiological studies had also shown that the expression level of miR-451 was positively correlated with coronary heart disease (CHD) (Ren et al., 2013). We have demonstrated that the serum levels of miRNA-451a can serve as risk or diagnostic markers for AS. Given that circulating miRNAs are a potential key feature of atherosclerosis-associated dysfunction of gene regulatory networks (Zernecke et al., 2009; Zhang et al., 2017), our study focused on the potential cellular properties regulated by miRNA-451a. The MIF-encoded mRNA contains a 3’-UTR element that is partially complementary to miRNA-451a, indicating that the miRNAs might directly target the related sites.
The ox-LDL plays a more important role in the genesis and progression of atherosclerosis. Some studies have shown that the adhesion molecules of endothelial cells increased under the stimulation of ox LDL, which promoted the adhesion between leukocytes and vascular endothelium and induced atherosclerosis (Trpkovic et al., 2015). It has been confirmed that ox-LDL causes the activation of the endothelial disorder (Parthasarathy et al., 2010). Our studies used ox-LDL-induced HUVEC cells to establish an in vitro model of atherosclerosis and investigated the role of miRNA-451a.
Macrophage migration inhibitory factor (MIF), an inflammatory cytokine, is widely expressed in various cell types, including endothelial cells (Zhang et al., 2019). Previous studies show that MIF is a key modulator that modulates cardiovascular disease, such as acute coronary syndrome (Du et al., 2020b), ST-elevation myocardial infarction (Zhao et al., 2019), ischemic heart disease (Xiao et al., 2019), and atherosclerosis (Uchida et al., 2019). Recent studies have demonstrated that atherosclerosis development requires a dialogue between vascular smooth muscle cells (VSMCs), endothelial cells, and immune cells (Ramel et al., 2019). In the current study, MIF expression was found to be significantly higher in ox-LDL caused HUVECs. MIF overexpression also reversed the effects of miRNA-451a overexpressing on proliferation and apoptosis of HUVEC under ox-LDL treatment. Thus, miR-451a/MIF may play potential roles in the regulation of immune cells and VSMCs in relation to atherosclerosis. These findings support that miR-451 can target MIF and highlight its importance in the pathogenesis of atherosclerosis. A limitation of this study is the lack of an independent validation cohort to confirm the association between miR-451a and atherosclerosis. Moreover, we did not detect the miRNA expression level in peripheral blood cells as serum miRNAs are not necessarily reflecting the intracellular ones.
Our study suggested that miRNA-451a, which is down-regulated in atherosclerosis, might be a novel diagnostic marker for atherosclerosis patients. Furthermore, we discovered that miRNA-451a upregulation could stimulate HUVECs proliferation and apoptosis by directly targeting MIF.
Availability of Data and Materials: The data is available upon the request from the corresponding author
Author Contribution: Study conception and design: Hongxia Hu, Ping Guo, Qian Zhao; data collection: Hongxia Hu, Ping Guo, Qian Zhao, Haoran Li, Hualei Liu, Caihong Ma; analysis and interpretation of results: Hongxia Hu1, Ping Guo, Qian Zhao, Haoran Li, Hualei Liu, Caihong Ma; draft manuscript preparation: Hongxia Hu. All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: This study was supported and approved by the Ethics Committee of Henan provincial hospital (2019-11) at 9 November 2020.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Berkan O, Arslan S, Lalem T, Zhang L, Şahin NO, Aydemir EI, Korkmaz O, Eğilmez HR, Çekin N, Devaux Y (2019). Regulation of microRNAs in coronary atherosclerotic plaque. Epigenomics 11: 1387–1397. DOI 10.2217/epi-2019-0036. [Google Scholar] [CrossRef]
Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C (2007). MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nature Medicine 13: 587–596. DOI 10.1038/nm1567. [Google Scholar] [CrossRef]
Bus P, Kestens C, Ten Kate F, Peters W, Drenth J, Roodhart J, Siersema P, Baal JV (2016). Profiling of circulating microRNAs in patients with Barrett’s esophagus and esophageal adenocarcinoma. Journal of Gastroenterology 51: 560–570. DOI 10.1007/s00535-015-1133-5. [Google Scholar] [CrossRef]
Dong Y, Fernandes C, Liu Y, Wu Y, Wu H, Brophy ML, Deng L, Song K, Wen A, Wong S, Yan D, Towner R, Hong C (2017). Role of endoplasmic reticulum stress signalling in diabetic endothelial dysfunction and atherosclerosis. Diabetes and Vascular Disease Research 14: 14–23. DOI 10.1177/1479164116666762. [Google Scholar] [CrossRef]
Du L, Xu Z, Wang X, Liu F (2020a). Integrated bioinformatics analysis identifies microRNA-376a-3p as a new microRNA biomarker in patient with coronary artery disease. American Journal of Translational Research 12: 633–648. [Google Scholar]
Du GL, Luo JY, Wang D, Li YH, Fang BB, Li XM, Gao XM, Yang YN (2020b). MIF gene rs755622 polymorphism positively associated with acute coronary syndrome in Chinese Han population: Case–control study. Scientific Reports 10: 140. DOI 10.1038/s41598-019-56949-z. [Google Scholar] [CrossRef]
Gupta M, Blumenthal C, Chatterjee S, Bandyopadhyay D, Jain V, Lavie CJ, Virani SS, Ray KK, Aronow WS, Ghosh RK (2020). Novel emerging therapies in atherosclerosis targeting lipid metabolism. Expert Opinion on Investigational Drugs 29: 611–622. DOI 10.1080/13543784.2020.1764937. [Google Scholar] [CrossRef]
Hartmann P, Zhou Z, Natarelli L, Wei Y, Schober A (2016). Corrigendum: Endothelial Dicer promotes atherosclerosis and vascular inflammation by miRNA-103-mediated suppression of KLF4. Nature Communications 7: 11907. DOI 10.1038/ncomms11907. [Google Scholar] [CrossRef]
Huang R, Hu Z, Cao Y, Li H, Zhang H (2019). MiR-652-3p inhibition enhances endothelial repair and reduces atherosclerosis by promoting Cyclin D2 expression. EBioMedicine 40: 685–694. DOI 10.1016/j.ebiom.2019.01.032. [Google Scholar] [CrossRef]
Karvande A, Kushwaha P, Ahmad N, Adhikary S, Kothari P, Tripathi AK, Khedgikar V, Trivedi R (2018). Glucose dependent miR-451a expression contributes to parathyroid hormone mediated osteoblast differentiation. Bone 117: 98–115. DOI 10.1016/j.bone.2018.09.007. [Google Scholar] [CrossRef]
Krakowsky RHE, Wurm AA, Gerloff D, Katzerke C, Brauer-Hartmann D, Hartmann J, Wilke F, Thiede C, Müller-Tidow C, Niederwieser D, Behre G (2018). miR-451a abrogates treatment resistance in FLT3-ITD-positive acute myeloid leukemia. Blood Cancer Journal 8: 36. DOI 10.1038/s41408-018-0070-y. [Google Scholar] [CrossRef]
Li W, Huang H, Li L, Wang L, Li Y, Wang Y, Guo S, Li L, Wang D, He Y, Chen L (2018). The pathogenesis of atherosclerosis based on human signaling networks and stem cell expression data. International Journal of Biological Sciences 14: 1678–1685. DOI 10.7150/ijbs.27896. [Google Scholar] [CrossRef]
Liu L, Zhang H, Mao H, Li X, Hu Y (2019). Exosomal miR-320d derived from adipose tissue-derived MSCs inhibits apoptosis in cardiomyocytes with atrial fibrillation (AF). Artificial Cells, Nanomedicine, and Biotechnology 47: 3976–3984. DOI 10.1080/21691401.2019.1671432. [Google Scholar] [CrossRef]
Lu Y, Thavarajah T, Gu W, Cai J, Xu Q (2018). Impact of miRNA in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 38: e159–e170. [Google Scholar]
Miller YI, Choi SH, Fang L, Tsimikas S (2010). Lipoprotein modification and macrophage uptake: Role of pathologic cholesterol transport in atherogenesis. Subcellular Biochemistry 51: 229–251. [Google Scholar]
Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N (2010). Oxidized low-density lipoprotein. Methods in Molecular Biology 610: 403–417. [Google Scholar]
Peters LJF, Biessen EAL, Hohl M, Weber C, van der Vorst EPC, Santovito D (2020). Small things matter: Relevance of microRNAs in cardiovascular disease. Frontiers in Physiology 11: 793. DOI 10.3389/fphys.2020.00793. [Google Scholar] [CrossRef]
Poznyak AV, Grechko AV, Orekhova VA, Chegodaev YS, Wu WK, Orekhov AN (2020). Oxidative stress and antioxidants in atherosclerosis development and treatment. Biology 9: 60. DOI 10.3390/biology9030060. [Google Scholar] [CrossRef]
Ramel D, Gayral S, Sarthou MK, Augé N, Nègre-Salvayre A, Laffargue M (2019). Immune and smooth muscle cells interactions in atherosclerosis: How to target a breaking bad dialogue? Frontiers in Pharmacology 10: 1276. DOI 10.3389/fphar.2019.01276. [Google Scholar] [CrossRef]
Ren J, Jing Z, Ning X, Han G, Qiang G, Song J, Li S, Zhao J, Chen H (2013). Signature of circulating microRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS One 8: e80738. DOI 10.1371/journal.pone.0080738. [Google Scholar] [CrossRef]
Solly E, Dimasi C, Bursill C, Psaltis P, Tan J (2019). MicroRNAs as therapeutic targets and clinical biomarkers in atherosclerosis. Journal of Clinical Medicine 8: 2199. DOI 10.3390/jcm8122199. [Google Scholar] [CrossRef]
Sun H, Jiang P (2018). MicroRNA-451a acts as tumor suppressor in cutaneous basal cell carcinoma. Molecular Genetics & Genomic Medicine 6: 1001–1009. DOI 10.1002/mgg3.473. [Google Scholar] [CrossRef]
Trpkovic A, Resanovic I, Stanimirovic J, Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D, Isenovic ER (2015). Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Critical Reviews in Clinical Laboratory Sciences 52: 70–85. DOI 10.3109/10408363.2014.992063. [Google Scholar] [CrossRef]
Uchida A, Seki N, Mizuno K, Yamada Y, Misono S, Sanada H, Kikkawa N, Kumamoto T, Suetsugu T, Inoue H (2019). Regulation of KIF2A by antitumor miR-451a inhibits cancer cell aggressiveness features in lung squamous cell carcinoma. Cancers 11: 258. DOI 10.3390/cancers11020258. [Google Scholar] [CrossRef]
van Rooij E (2012). Introduction to the series on microRNAs in the cardiovascular system. Circulation Research 110: 481–482. DOI 10.1161/CIRCRESAHA.111.257311. [Google Scholar] [CrossRef]
Widlansky ME, Jensen DM, Wang J, Liu Y, Geurts AM, Kriegel AJ, Liu P, Ying R, Zhang G, Casati M, Chu C, Malik M, Branum A, Tanner MJ, Tyagi S, Usa K, Liang M (2018). contributes to normal endothelial function and can restore it in cardiometabolic disorders. EMBO Molecular Medicine 10: e8046. DOI 10.15252/emmm.201708046. [Google Scholar] [CrossRef]
Xiao XB, Gu Y, Sun DL, Ding LY, Yuan XG, Jiang HW, Wu ZX (2019). Effect of rituximab combined with chemotherapy on the expression of serum exosome miR-451a in patients with diffuse large b-cell lymphoma. European Review for Medical and Pharmacological Sciences 23: 1620–1625. [Google Scholar]
Yamada H, Suzuki K, Ichino N, Ando Y, Sawada A, Osakabe K, Sugimoto K, Ohashi K, Teradaira R, Inoue T, Hamajima N, Hashimoto S (2013). Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clinica Chimica Acta 424: 99–103. DOI 10.1016/j.cca.2013.05.021. [Google Scholar] [CrossRef]
Zeng N, Huang R, Li N, Jiang H, Li R, Wang F, Chen W, Xia M, Wang Q (2018). MiR-451a attenuates free fatty acids–mediated hepatocyte steatosis by targeting the thyroid hormone responsive spot 14 gene. Molecular and Cellular Endocrinology 474: 260–271. DOI 10.1016/j.mce.2018.03.016. [Google Scholar] [CrossRef]
Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C (2009). Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Science Signaling 2: ra81. DOI 10.1126/scisignal.2000610. [Google Scholar] [CrossRef]
Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D (2017). Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548: 52–57. DOI 10.1038/nature23282. [Google Scholar] [CrossRef]
Zhang Y, Zhu W, He H, Fan B, Deng R, Hong Y, Liang X, Zhao H, Li X, Zhang F (2019). Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging 11: 12641–12660. [Google Scholar]
Zhao Q, Men L, Li X, Liu F, Shan C, Zhou X, Song N, Zhu J, Gao X, Ma Y, Du X, Gao X, Yang Y (2019). Circulating MIF levels predict clinical outcomes in patients with ST-elevation myocardial infarction after percutaneous coronary intervention. Canadian Journal of Cardiology 35: 1366–1376. DOI 10.1016/j.cjca.2019.04.028. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |