DOI:10.32604/biocell.2021.014754

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.014754 |  www.techscience.com/journal/biocell |
| Review |
Lactate reloaded–reevaluation of the importance of lactate monitoring in the management of adult sepsis in the emergency department
1Cardiff University, Cardiff, CF10 3AT, UK
2Semmelweis University, Budapest, 1085, Hungary
3Dalhuis University Halifax, Halifax, NS B3H 4R2, Canada
4EpiConsult Biomedical Consulting and Medical Communications Agency, Dover, DE 19901, USA
5Pecs University, Clinical Centre, Pecs, 7626, Hungary
*Address correspondence to: Gabor Xantus, gabor.xantus@gmail.com
Received: 27 October 2020; Accepted: 21 December 2020
Abstract: For about a quarter of a century, monitoring lactate levels and/or lactate clearance has been an unquestionable cornerstone in sepsis management. The elevated lactate level appeared to be an independent predictor of mortality, and the consequent metabolic acidosis was thought to explain a number of pathophysiological changes seen in septic shock. Recent physiological and clinical findings seem to challenge the adverse role of lactic acidosis in sepsis. Evidence suggests that lactate levels are not necessarily directly proportional to either tissue or cellular hypoxia, and conversely, despite high lactate levels, increased peripheral tissue oxygen pressure can be measured in adult patients with septic shock. According to the most recent understanding of in vitro and in vivo evidence, the elevated lactate level in sepsis might be a normal reaction due to adrenergic stress with potential beneficial/protective physiological effects, as well. On the one hand, burning lactic may help fuel the body during critical illness, but on the other hand, with a slight drop in pH, the body may counteract certain deleterious changes during the dysregulated host response; reduce the chances of reperfusion myocardial injury, and improve tissue oxygenation by shifting the haemoglobin dissociation curve to the right. Understanding the pathophysiological process in sepsis resulting in elevated lactate levels may aid management in an emergency, medicine, and intensive care settings. With more in-depth physiological knowledge, physicians may inevitably surpass normalisation heuristics and deliver personalized rather than protocolised sepsis resuscitation.
Keywords: Reevaluation; Lactate; Sepsis; Resuscitation
Sepsis resuscitation (i.e., interventions aimed at restoring tissue perfusion/oxygenation) in the present emergency and intensive care practice is guided mostly on indirect parameters like absolute arterial or venous lactate levels and/or lactate clearance (temporal changes in serial lactate assessment). Over the past 25 years, several studies have demonstrated that elevated lactate levels, and consequent metabolic acidosis, in sepsis, is an independent predictor of mortality, similar to other states of shock (Szabó et al., 2017; Jenei et al., 2019; Villar et al., 2019). However, recent research seems to challenge the prognostic accuracy of lactate monitoring/clearance during the assessment of tissue perfusion (Singh et al., 2019).
Until now, sepsis-associated hyperlactataemia (SAHL) was considered to be equivalent to tissue hypoperfusion (hypoxia-induced anaerobic glycolysis) (Dellinger et al., 2013; Sterling et al., 2013). Metabolic acidosis was thought to aggravate vasoplegia (pathologic relaxation in the wall of the small blood vessels) and therefore worsen septic shock by three main mechanisms. First, a drop in pH results is not only downregulating the number of surface adrenoceptors (Levy et al., 2012; Ives et al., 2013) of the vascular smooth muscle cells (VSMC) but at the same time is decreasing the intracellular calcium levels (which opens the ATP-sensitive potassium channels, prompting smooth muscle relaxation) (Ishizaka and Kuo, 1996; Kuo et al., 2009); increasing the expression of inducible nitric oxide synthase both in the endothelium and in VSMC (Yaghi et al., 1993; Pedoto et al., 1999; Pedoto et al., 2001; Fernandes and Assreuy, 2008). Our primary goal is to review the process of lactate production in sepsis to better understand the prognostic value of it in SAHL.
There is a growing body of (overlooked) evidence challenging the unquestionable dogma of “lactic acidosis = tissue hypoxia”. Physiologically, a rise in the lactate level could be explained by adrenergic stimulation and secondary aerobic glycolysis in the muscles than by systematic tissue hypoxia driven anaerobic glycolysis. Beta2 stimulation–even in low perfusion shock–is known to increase the NaK-ATPase activity in the skeletal muscles resulting in lactate release (Xantus et al., 2020b). In light of recent findings, SAHL is most likely an integral part of the systemic stress response but could also be a marker of impaired mitochondrial respiration (Garcia-Alvarez et al., 2014).
Lactic acid production in sepsis is a very complex mechanism not completely explained by either primary or secondary glycolysis. It seems that lactate production may also depend on mitochondrial pyruvate dehydrogenase (dys)function, with a not completely understood link with increased glycolysis (Park et al., 2018). Lactate (in anaerobic states) converted to pyruvate during the Cori/Krebs cycle serves as a precursor for glucogenesis (Nuttall et al., 2008) (Fig. 1). Based on the above, it seems that lactate might play a pivotal role in maintaining the homeostasis in sepsis, as it may serve as extra fuel in the oxidation processes (Fig. 2).
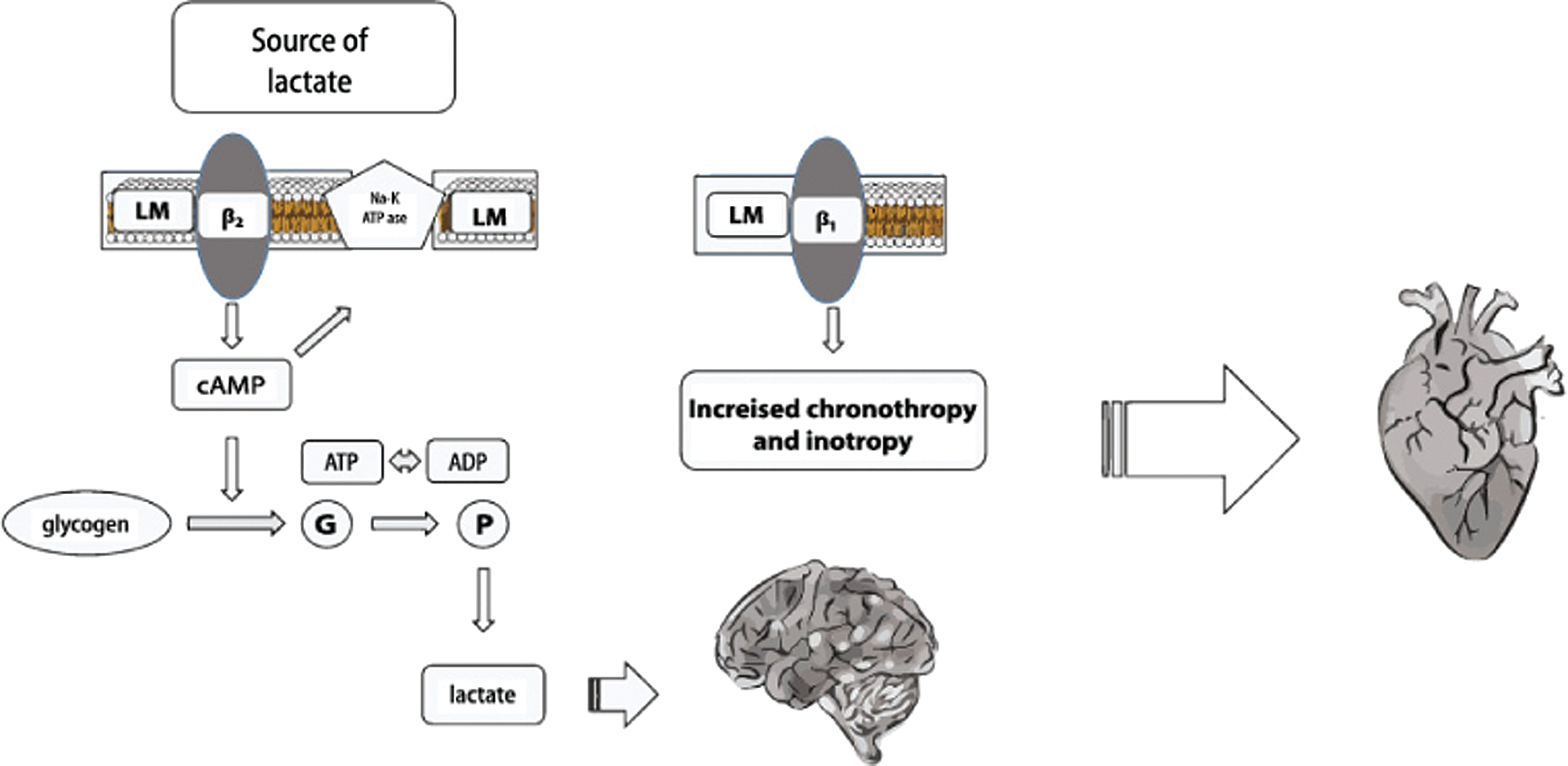
Figure 1: Lactate production under aerobic condition.
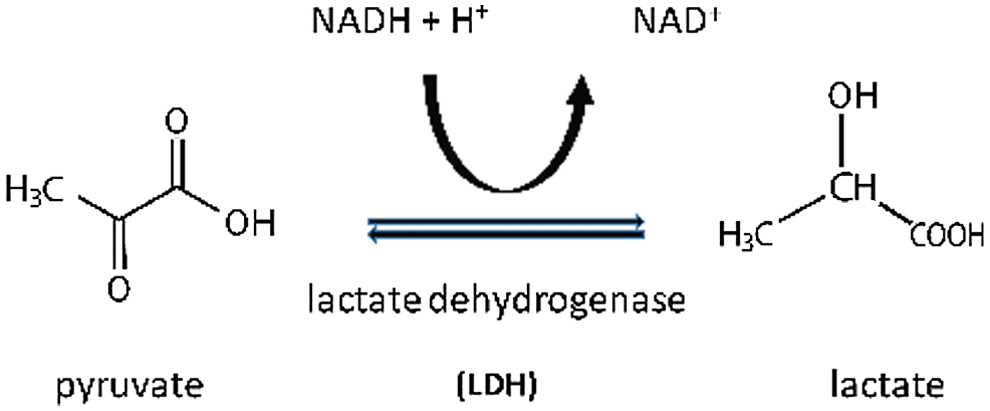
Figure 2: Lactate production under anaerobic condition.
Human clinical observations also seemingly challenge the role of lactate in predicting tissue hypoxia. Boekstegers et al. (1994) found no association between higher lactate levels nor tissue or cellular hypoxia. Partial oxygen pressure (PtO2) of the biceps muscle was measured in three groups of adult ICU patients. Their status ranged from severe sepsis to septic shock (with a third subgroup in cardiogenic shock). The results demonstrated no statistically significant correlation between serum lactate levels and brachial PtO2. The clinical significance of this observation is yet to be explored fully. However, their findings were confirmed later by Sair et al. (2001), who compared PtO2 values in both the forearm musculature and subcutaneous tissue of patients with severe sepsis vs healthy volunteers. This study showed that in septic patients, despite increased serum lactate concentration, an increase in tissue oxygenation was measured compared to those healthy volunteers. Both papers concluded that elevated lactate levels do not correlate with upper limb peripheral tissue hypoxia.
Another approach used fluoromomisonidazole isotope to detect hypoxia. This agent was originally developed for Positron Emission Tomography however, the isotope is now also widely used outside radiology both in translational and clinical diagnostics (Hotchkiss et al., 1991). Animal studies of induced sepsis found no evidence of tissue hypoxia in either skeletal or myocardial muscle, lung, or brain despite higher lactate concentrations (Hotchkiss and Karl, 1992). A recent review of in-vitro and animal evidence (Jeger et al., 2013) concluded that mitochondrial dysfunction does not necessarily result in multi-organ failure in severe sepsis and/or septic shock. Unfortunately, the inferential value of these conclusions (based on observations of mostly young and healthy animals) is unknown in the elderly septic population.
Regueira et al. (2012) further demonstrated that the expression of the hypoxia-induced factor (HIF-1) was not proportionate with the rise in lactate level during the course of sepsis, in either skeletal and cardiac muscle or in other vital organs such as the liver, pancreas, lung, kidney. It seemed that even though serum lactate level doubled up during disease progression, in the porcine model the mitochondrial respiration remained intact regardless of sepsis severity (Regueira et al., 2012). HIF-1 is a protein complex that plays a key role in the body’s response to low oxygen concentrations; numerous processes like hypoxia-related re-vascularization, systemic immune response, HIF-1 assay may not only be useful in experimental models, but it is also likely to be a feasible diagnostic method in human practice as well, this is because HIF-1 related mRNA serum concentrations are consistently and significantly higher in patients with septic shock vs healthy controls (Textoris et al., 2012). One clinical study assessed the correlation between serum lactate and HIF-1 mRNA level in septic patients and found no significant association between the two (van Hall, 2010), questioning the predictive role of lactate.
Reinterpretation of lactate in sepsis
Clinical and in vitro models seem to suggest that SAHL is not exclusively a sign of mitochondrial hypoxia, but elevated lactate production could be a part of the systemic response to sepsis. Furthermore, hyperlactataemia is not directly proportional to tissue level hypoxia, as presumed previously. Animal experiments investigating muscle metabolism in induced sepsis, by means of phosphorus 31 nuclear magnetic resonance spectroscopy, found no evidence of a drop in either the intracellular NDPH level or overall pH, even in significant hyperlactataemia (Gilles et al., 1994). However, the clinical significance of this finding is yet to be determined.
Clinical observations consistently found elevated lactate levels in asthmatics on beta-agonist treatment, which could not be explained with the previously unquestioned “hypoxia = lactate elevations” theory (Jee and Brownlow, 2007). A recently published placebo-controlled, randomized clinical trial investigated the hemodynamic effects of esmolol infusion (an ultra-short acting beta-blocker) in adult patients with sepsis. The administration of esmolol was associated with a significant decrease in serum lactate level. This finding was unexpected in view of the fact that esmolol reduces cellular oxygen demand (Bouglé and Mira, 2014). Should SAHL be caused by abnormal perfusion/oxygenation only, hyperlactatemia would have been corrected by supranormal values of systemic and/or regional oxygen delivery; however, this study proved the contrary.
A strong correlation between adrenergic stress and lactic acidosis was also confirmed on isolated human left ventricular cardiac myocytes (Schotola et al., 2012). This is in line with previous observations that proved that mild metabolic acidosis reduced inotrope provoked beta-adrenergic response (Marsh et al., 1988; Keiichi et al., 2013), even when serum lactate levels were elevated. It seems that a slight drop in pH may positively affect cardiac relaxation in diastole (Toller et al., 2005; McCaul et al., 2006). Such a response may explain the beneficial effect of beta-antagonist on mortality in septic shock.
In addition to mitigating the adverse effects of adrenergic stress, metabolic acidosis may also play a protective role in preventing reperfusion injury. Fujita et al. (2007) reported that transient episodes of metabolic acidosis may reduce early cardiac reperfusion injury (Mitchell et al., 1972). Lastly, metabolic acidosis may also be important in meeting the increased tissue O2 demand in the critically ill, as at lower pH, the haemoglobin (HbO2) dissociation curve shifts to the right, resulting in a decrease in SaO2 and consequently increase PO2. Due to the sigmoid shape of the HbO2 curve, the effect of such a shift is generally insignificant at normal PO2 levels. Such a shift may be particularly important at low PO2 levels seen in septic shock (Refsum et al., 1997; Fujita et al., 2007). In-depth physiological knowledge may inevitably surpass normalisation heuristics, correct certain parameters to physiological levels in a pathophysiological situation, and deliver personalized rather than protocolised sepsis resuscitation (Xantus et al., 2020a).
To the best of our knowledge, elevated lactate levels in septic adults with consequent mild metabolic acidosis are likely to be a consequence of a not yet completely understood systemic adrenergic response to stress as opposed to a simple marker of tissue hypoxia. Resuscitative interventions in sepsis aimed to correct lactate levels and subsequent metabolic acidosis may deprive the body of additional energy and may counteract certain potential defensive responses intended to mitigate some of the potentially detrimental effects of the dysregulated adrenergic response and secondary reperfusion injury; or conversely, even impaired tissue oxygenation by shifting the haemoglobin dissociation curve to the right. Understanding the complex physiological basis of SAHL can help emergency physicians to revisit sepsis resuscitation goals aiming at correcting serum lactate levels at all costs.
Acknowledgement: The authors are grateful to the editorial team for the invaluable help in improving the article.
Author Contribution: Gabor Xantus incepted the idea, Balint Kiss performed the necessary search and drafted the first version, Gyula Molnar reviewed the work in progress, V. Anna Gyarmathy reviewed the work in progress, Peter L Kanizsai recommended changes in the concept to shape the final draft, Candice Matheson proofread and edited the final version.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Boekstegers P, Weidenhofer S, Kapsner T, Werdan K. (1994). Skeletal muscle partial pressure of oxygen in patients with sepsis. Critical Care Medicine 22: 640–650. DOI 10.1097/00003246-199404000-00021. [Google Scholar] [CrossRef]
Bouglé A, Mira JP. (2014). Short-acting β-blocker administration in patients with septic shock--reply. JAMA 311: 736–737. DOI 10.1001/jama.2014.315. [Google Scholar] [CrossRef]
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. (2013). Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock. Intensive Care Medicine 39: 165–228. DOI 10.1007/s00134-012-2769-8. [Google Scholar] [CrossRef]
Fernandes D, Assreuy J. (2008). Nitric oxide and vascular reactivity in sepsis. Shock 30: 10–13. DOI 10.1097/SHK.0b013e3181818518.
Fujita M, Asanuma H, Hirata A, Wakeno M, Takahama H, Sasaki H, Kim J, Takashima S, Tsukamoto O, Minamino T, Shinozaki Y, Tomoike H, Hori M, Kitakaze M. (2007). Prolonged transient acidosis during early reperfusion contributes to the cardioprotective effects of postconditioning. American Journal of Physiology-Heart and Circulatory Physiology 292: H2004–H2008. DOI 10.1152/ajpheart.01051.2006. [Google Scholar]
Garcia-Alvarez M, Marik P, Bellomo R. (2014). Sepsis-associated hyperlactatemia. Critical Care 18: 503. DOI 10.1186/s13054-014-0503-3. [Google Scholar] [CrossRef]
Gilles RJ, D’Orio V, Ciancabilla F, Carlier PG. (1994). In vivo 31P nuclear magnetic resonance spectroscopy of skeletal muscle energetics in endotoxemic rats: A prospective, randomized study. Critical Care Medicine 22: 499–505. DOI 10.1097/00003246-199403000-00022.
Hotchkiss RS, Karl IE. (1992). Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis. JAMA: The Journal of the American Medical Association 267: 1503–1510. DOI 10.1001/jama.1992.03480110079038. [Google Scholar] [CrossRef]
Hotchkiss RS, Rust RS, Dence CS, Wasserman TH, Song SK, Hwang DR, Karl IE, Welch MJ. (1991). Evaluation of the role of cellular hypoxia in sepsis by the hypoxic marker [18F]fluoromisonidazole. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 261: R965–R972. DOI 10.1152/ajpregu.1991.261.4.R965. [Google Scholar]
Ishizaka H, Kuo L. (1996). Acidosis-induced coronary arteriolar dilation is mediated by ATP-sensitive potassium channels in vascular smooth muscle. Circulation Research 78: 50–57. DOI 10.1161/01.RES.78.1.50. [Google Scholar] [CrossRef]
Ives SJ, Andtbacka RH, Noyes RD, Morgan RG, Gifford JR, Park SY, Symons JD, Richardson RS. (2013). α1-Adrenergic responsiveness in human skeletal muscle feed arteries: The impact of reducing extracellular pH. Experimental Physiology 98: 256–267. DOI 10.1113/expphysiol.2012.066613.
Jee R, Brownlow H. (2007). Hyperlactaemia due to nebulised salbutamol. Anaesthesia 62: 751–752. DOI 10.1111/j.1365-2044.2007.05160.x. [Google Scholar] [CrossRef]
Jeger V, Djafarzadeh S, Jakob SM, Takala J. (2013). Mitochondrial function in sepsis. European Journal of Clinical Investigation 43: 532–542. DOI 10.1111/eci.12069. [Google Scholar] [CrossRef]
Jenei K, Szatmári I, Szabó E, Mariam A, Luczay A, Zsidegh P, Péter TH. (2019). Laktátszintváltozások diabeteses ketoacidosisban és frissen diagnosztizált 1-es típusú diabetes mellitusban. Orvosi Hetilap 160: 1784–1790. DOI 10.1556/650.2019.31533. [Google Scholar] [CrossRef]
Keiichi H, Hiroshi T, Yumi I, Shinichi I, Makoto T. (2013). Influence of acidosis on cardiotonic effects of colforsin and epinephrine: A dose-response study. Journal of Cardiothoracic and Vascular Anesthesia 27: 925–932. DOI 10.1053/j.jvca.2012.09.019. [Google Scholar] [CrossRef]
Kuo JH, Chen SJ, Shih CC, Lue WM, Wu CC. (2009). Abnormal activation of potassium channels in aortic smooth muscle of rats with peritonitis-induced septic shock. Shock 32: 74–79. DOI 10.1097/SHK.0b013e31818bc033. [Google Scholar] [CrossRef]
Levy B, Collin S, Sennoun N, Ducrocq N, Kimmoun A, Asfar P, Perez P, Meziani F. (2012). Vascular hyporesponsiveness to vasopressors in septic shock: From bench to bedside. Applied Physiology in Intensive Care Medicine. Berlin, Heidelberg: Springer, 2: 251–261. [Google Scholar] [CrossRef]
Marsh JD, Margolis TI, Kim D. (1988). Mechanism of diminished contractile response to catecholamines during acidosis. American Journal of Physiology-Heart and Circulatory Physiology 254: H20–H27. DOI 10.1152/ajpheart.1988.254.1.H20. [Google Scholar] [CrossRef]
McCaul CL, McNamara P, Engelberts D, Slorach C, Hornberger LK, Kavanagh BP. (2006). The effect of global hypoxia on myocardial function after successful cardiopulmonary resuscitation in a laboratory model. Resuscitation 68: 267–275. DOI 10.1016/j.resuscitation.2005.06.018. [Google Scholar]
Mitchell JH, Wildenthal K, Johnson RLJr. (1972). The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney International 1: 375–389. DOI 10.1038/ki.1972.48. [Google Scholar]
Nuttall FQ, Ngo A, Gannon MC. (2008). Regulation of hepatic glucose production and the role of gluconeogenesis in humans: Is the rate of gluconeogenesis constant? Diabetes/Metabolism Research and Reviews 24: 438–458. DOI 10.1002/dmrr.863. [Google Scholar] [CrossRef]
Park S, Jeon JH, Min BK, Ha CM, Thoudam T, Park BY, Lee IK. (2018). Role of the pyruvate dehydrogenase complex in metabolic remodeling: Differential pyruvate dehydrogenase complex functions in metabolism. Diabetes & Metabolism Journal 42: 270–281. DOI 10.4093/dmj.2018.0101. [Google Scholar] [CrossRef]
Pedoto A, Caruso JE, Nandi J, Oler A, Hoffmann SP, Tassiopoulos AK, McGraw DJ, Camporesi EM, Hakim TS. (1999). Acidosis stimulates nitric oxide production and lung damage in rats. American Journal of Respiratory and Critical Care Medicine 159: 397–402. DOI 10.1164/ajrccm.159.2.9802093. [Google Scholar] [CrossRef]
Pedoto A, Nandi J, Oler A, Camporesi EM, Hakim TS, Levine RA. (2001). Role of nitric oxide in acidosis-induced intestinal injury in anesthetized rats. Journal of Laboratory and Clinical Medicine 138: 270–276. DOI 10.1067/mlc.2001.118176. [Google Scholar] [CrossRef]
Refsum HE, Opdahl H, Leraand S. (1997). Effect of extreme metabolic acidosis on oxygen delivery capacity of the blood-an in vitro investigation of changes in the oxyhemoglobin dissociation curve in blood with pH values of approximately 6.30. Critical Care Medicine 25: 1497–1501. DOI 10.1097/00003246-199709000-00016. [Google Scholar] [CrossRef]
Regueira T, Djafarzadeh S, Brandt S, Gorrasi J, Borotto E, Porta F, Takala J, Bracht H, Shaw S, Lepper PM, Jakob SM. (2012). Oxygen transport and mitochondrial function in porcine septic shock, cardiogenic shock, and hypoxaemia. Acta Anaesthesiologica Scandinavica 56: 846–859. DOI 10.1111/j.1399-6576.2012.02706.x. [Google Scholar] [CrossRef]
Sair M, Etherington PJ, Winlove CP, Evans TW. (2001). Tissue oxygenation and perfusion in patients with systemic sepsis. Critical Care Medicine 29: 1343–1349. DOI 10.1097/00003246-200107000-00008. [Google Scholar] [CrossRef]
Schotola H, Toischer K, Popov AF, Renner A, Schmitto JD, Gummert J, Quintel M, Bauer M, Maier LS, Sossalla S. (2012). Mild metabolic acidosis impairs the β-adrenergic response in isolated human failing myocardium. Critical Care 16: R153. DOI 10.1186/cc11468. [Google Scholar] [CrossRef]
Singh G, Sharma H, Rachoin JS, Patel S. (2019). Common cognitive biases in nephrology critical care: A plea for metacognition. Cureus 11: e6304.
Sterling S, Puskarich M, Shapiro N, Trzeciak S, Kline J, Summers RL, Jones AE. (2013). Emergency Medicine Shock Research Network (EMSHOCKNET) Characteristics and outcomes of patients with vasoplegic versus tissue dysoxic septic shock. Shock 40: 11–14. DOI 10.1097/SHK.0b013e318298836d.
Szabó E, Balogh L, Szabó A, Szatmári I. (2017). Diagnostics of inborn errors of metabolism: Laboratory approaches. Orvosi Hetilap 158: 1903–1907. DOI 10.1556/650.2017.30899.
Textoris J, Beaufils N, Quintana G, Ben Lassoued A, Zieleskiewicz L, Wiramus S, Blasco V, Lesavre N, Martin C, Gabert J, Leone M. (2012). Hypoxia-inducible factor (HIF1α) gene expression in human shock states. Critical Care 16: R120. DOI 10.1186/cc11414.
Toller W, Wölkart G, Stranz C, Metzler H, Brunner F. (2005). Contractile action of levosimendan and epinephrine during acidosis. European Journal of Pharmacology 507: 199–209. DOI 10.1016/j.ejphar.2004.11.049.
van Hall G. (2010). Lactate kinetics in human tissues at rest and during exercise. Acta physiologica 199: 499–508. DOI 10.1111/j.1748-1716.2010.02122.x.
Villar J, Short JH, Lighthall G. (2019). Lactate predicts both short-and long-term mortality in patients with and without sepsis. Infectious Diseases: Research and Treatment 12: 1178633719862776. DOI 10.1177/1178633719862776.
Xantus G, Allen P, Norman S, Kanizsai P (2020a). Antibiotics administered within 1 hour to adult emergency department patients screened positive for sepsis: A systematic review. European Journal of Emergency Medicine 27: 260–267. DOI 10.1097/MEJ.0000000000000654.
Xantus G, Penny A, Norman SE, Kanizsai PL (2020b). A korai krisztalloidbolus előnyei felnőtt szeptikus betegek sürgősségi kezelésében. Orvosi Hetilap 161: 1668–1674. DOI 10.1556/650.2020.31864.
Yaghi A, Paterson NAM, McCormack DG. (1993). Nitric oxide does not mediate the attenuated pulmonary vascular reactivity of chronic pneumonia. American Journal of Physiology-Heart and Circulatory Physiology 265: H943–H948. DOI 10.1152/ajpheart.1993.265.3.H943.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |