DOI:10.32604/biocell.2021.014233

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.014233 |  www.techscience.com/journal/biocell |
| Article |
Overloading of differentiated Caco-2 cells during lipid transcytosis induces glycosylation mistakes in the Golgi complex
1Department of Anatomy, Saint Petersburg State Paediatric Medical University, Petersburg, 194100, Russia
2Department of Biochemistry, Saint Petersburg State Paediatric Medical University, Petersburg, 194100, Russia
3Department of Heart and Vessel Surgery, Saint Petersburg State Paediatric Medical University, Petersburg, 194100, Russia
4The FIRC Institute of Molecular Oncology, Milan, 20139, Italy
*Address correspondence to: Alexander A. Mironov, alexandre.mironov@ifom.eu
Received: 12 September 2020; Accepted: 27 December 2020
Abstract: Overloading the intestine enterocytes with lipids induced alteration of the Golgi complex (GC; Sesorova et al., 2020) and could cause glycosylation errors. Here, using differentiated Caco-2 cells with the established 0[I] blood group phenotype (no expression of the blood antigens A and B [AgA, AgB] under normal conditions) as a model of human enterocytes we examined whether the overloading of these cells with lipids could cause errors in the Golgi-dependent glycosylation. We demonstrated that under these conditions, there were alterations of the GC and the appearance of lipid droplets in the cytoplasm. Rare cells produced AgA and AgB. This suggested that after overloading of enterocytes with lipids, AgA were mistakenly synthesized in individual enterocytes by the Golgi glycosyltransferases. These mistakes could explain why in the absence of AgA and AgB antibodies against them exist in the blood.
Keywords: Golgi; Blood group antigens; Glycosylation errors; Enterocyte; Lipid transcytosis; Caco-2 cells
The mechanism responsible for the formation of the antibodies against the human blood group polysaccharide (PS) antigens is unknown. The blood group is determined by the presence of antigens A and/or B (AgA and AgB) on the surface of erythrocytes and on the basolateral plasma membrane of other cells (Ravn and Dabelsteen, 2000; de Mattos, 2016). People with group 0(I) do not synthesize AgA and AgB but have antibodies against AgA (AbA) and AgB (AbB). Persons with the blood group A produce AgA and AbB. Individuals with the blood group B synthesize AgB and AbA. Finally, people with the blood group AB synthesize AgA and AgB but have no AbA and AbB. AgA and AgB are the final parts of PS attached to proteins or lipids (Hakomori, 1999; de Mattos, 2016). AgA and AgB are synthesized by glycosyltransferases A and B (GTA, GTB) (Ravn and Dabelsteen, 2000). GTA and GTB are transmembrane proteins of Type II containing C-terminal catalytic domains in the lumen of the medial Golgi cisternae Antibodies against AgA and AgB belong mostly to the IgM class. They are generated against antigens, which do not exist in the organism (White et al., 1990; Sheffield et al., 2005; Milland and Sandrin, 2006; Branch, 2015).
GTA catalyses the transfer of a monosaccharide residue from UDP-GalNAc (UDP-N-acetylgalactosamine) to Fuc alpha1-2Gal beta-R (H)-terminating acceptors, whereas GTB catalyses the transfer of a monosaccharide residue from UDP-Gal (UDP-galactose) to Fuc alpha1-2Gal beta-R (H)-terminating acceptors (Yamamoto, 2004; Milland and Sandrin, 2006). AgA and AgB are localized at the end of the long oligosaccharide chains, which are attached to the heavily glycosylated proteins such as Band-3 or sphingolipids (glycolipids are formed) (Hakomori, 1999; de Mattos, 2016). It seems that other Golgi glycosylation enzymes could synthesize antigens AgA and AgB although with significant difficulties (Varki, 1998; Varki et al., 1999). In newborn children, their titre of AbA and AbB is very low (Wuttke et al., 1997), it increases rapidly in 3 months after the birth and becomes maximal at 18–20 years of age, and then, their titre is reduced.
Several hypotheses were proposed in order to explain this discrepancy. The first hypothesis suggests that the appearance of AgA and AgB in the blood and subsequent generation of antibody poses that epitopes similar to AgA and AgB are delivered to the blood from the intestine after the digestion of food. For instance, many bacteria express similar polysaccharide chains on their cell surfaces. However, AgA and AgB per se are polysaccharides, whereas, in adults, human enterocytes cannot transcytose the polysaccharide molecules (Sesorova et al., 2020). Humans lost the ability to absorb PS from the lumen of the intestine soon after their birth. Only immediately after birth and only a small amount of proteins and PS could be transported. Most of these proteins represent antibodies of the IgG class due to the presence of anti-Fc receptors (He et al., 2008).
Currently, there are two models for the formation of antibodies against non-existing antigens A and B. (1) These antibodies could originate from antibodies produced against Gram-negative bacteria, such as Escherichia coli, cross-reacting with the α-D-galactose on the B glycoprotein (van Oss, 2004). (2) These antibodies could be caused by viral infection because most viruses contain membranes filled with proteins passing through the host GC (Glingston et al., 2019) where enzymes producing AgA or AgB could be. Appearance of the membranes composed of proteins and lipids containing PS with AgA or AgB could induce an immune reaction.
Here, we tested these hypotheses and also our one, which is based on the assumption that Golgi glycosylation could make mistake especially when the intra-Golgi transport is overloaded. Indeed, after overloading the Golgi with cargo, the synthesis of sugar chains occurs with low preciseness inducing a change in glycosylation (Marra et al., 2007). Caco-2 cells express H Type 1 blood group antigen on the basolateral plasma membrane and chylomicrons (ChMs; Amano and Oshima, 1999). We loaded the differentiated Caco-2 cells with a high concentration of lipids. This led to the alterations of the GC and the appearance of lipid droplets suggesting that enterocytes were overloaded with lipids (Sesorova et al., 2020). Under these conditions, some of these cells produced AgA suggesting that non-existing polysaccharide antigens could be synthesized under some conditions by the human own Golgi glycosidases.
It is impossible to perform these experiments on laboratory animals because they do not have the human blood group antigens. One possibility is to use biopsies of the human small intestine, but it is technically and ethically very difficult to do. Finally, we could use Caco-2 cells able to become enterocytes. On the other hand, Caco-2 cells were from a person with the blood group 0(I) and they express H Type 1 blood group antigen on the basolateral PM and on ChMs (Amano and Oshima, 1999) and due to the absence of both GTA and GTB these cells do not synthesise AgA and AgB. Caco-2 cells derive from human colorectal adenocarcinomas and can spontaneously polarize in vitro when cultured in a tight monolayer for 3 weeks. They form a mature brush border, express small intestine-specific enzymes, and use trafficking routes specific for polarized cells, including direct biosynthetic trafficking and transcytosis (Fleet et al., 2003; Schneeberger et al., 2018). Caco-2 cells synthesize both ApoB-100 and ApoB-48 and are able to take up lipids. The ApoB-48/ApoB-100 ratio is maximal in proliferating Caco-2 cells (Amano and Oshima, 1999; Santos et al., 2016; Schneeberger et al., 2018). In non-differentiated Caco-2 cells, apical endocytosis exists. After differentiation, these cells behave as human enterocytes and transcytose lipids (Santos et al., 2016). Several leukocyte markers, namely, CD10, CD13, CD14, CD18, CD21, CD25, CD26, CD28, CD31, CD35, CD47, CD59, CD61, and CD63 are present on both human enterocytes and Caco-2 cells. In contrast to enterocytes in Caco-2 cells, HLA-class 11 molecules are not found, synthesis of fat via the mono-acyl-glycerol pathway is much lower, the mono-acyl-glycerol pathway is inactive, and despite abundant production of ApoB, the secretion of newly synthesized triglyceride-rich lipoproteins is restricted (Trotter and Storch, 1993; Levy et al., 1995). Caco-2 cells express lower levels of intestinal fatty acid-binding protein than enterocytes and cannot induce proliferation of allogeneic lymphocytes (Darimont et al., 1998; Rodriguez-Juan et al., 2001; Hiebl et al., 2020). However, since our primary interest is to utilize the ability of Caco-2 cells to assemble and secrete chylomicrons from a source of fatty acids, it is a useful model cell line for this study. In other mammals, the monosaccharide’s very short branches (forks) similar to human AgA or AgB are not found. Therefore, here we used Caco-2 cells.
Unless otherwise stated, all chemicals and reagents were obtained from the previously indicated sources (Beznoussenko et al., 2014, Beznoussenko et al., 2016; Sesorova et al., 2020). Cholic (bile) acid (Catalogue number: №C1129) and cholesterol (Catalogue number: №C8667) were from Sigma-Aldrich (Milan, Italy). Protein kinase inhibitor H-89 was from MedChemExpress (Catalogue number: №HY-15979). PKI-1422 (Subramanian et al., 2019) was from EMD Millipore/Calbiochem (Catalogue number: №476485100). Blood Group A Antigen Monoclonal Antibody (HE-193. Invitrogen. Catalogue number: №MA1-19693) and Blood Group B Antigen Monoclonal Antibody (HEB-29, Catalogue № MA1-19691) was from ThermoFisher Scientific. The blood-group antibodies were used at a dilution of 1:100 for 1 h at room temperature, as was described by Gehrie et al. (2014). Treatment of cells with NEM was performed exactly as described by Kweon et al. (2004). Brefeldin A used at concentration 1 µm/mL (Mironov et al., 2004).
Caco-2 cells were cultivated as it was described (Townley et al., 2012) with the addition of recommendations by Wu et al. (2013). Briefly: Cells were seeded on MatTek Petri dishes at a density of 2 × 105 cells per insert and cultured for 3 weeks with media changes every other day. The cell viability was always higher than 95%, evaluated with Trypan Blue solution. We used 12% BSA or adult serum as the replacements for embryonic serum.
In order to test whether the transcytosis through the Caco2 differentiated cells occurred, we used the mixture of fatty acids proposed by Townley et al. (2012) and Santos et al. (2016) with the addition of 1% of bile acid and 0.5% of cholesterol. The mixture was heated and intermixed. Then it was diluted 5-fold and used in the dilution condition and without dilution. In one MatTek, we added 150 µL of this pseudochyme over the central cavity of the MatTek Petri dish where Caco-2 cells were grown, and cells were incubated for 20 min.
The applied concentration of H89 was equal to 50 µM, whereas the concentration of PKI1422 was equal to 150 µM (Subramanian et al., 2018). In the experiments with COPII, BeFx complexes were prepared according to Antonny et al. (2001); namely, BeFx solution contained 10 mM KF, and 250 mM BeCl2 was used for the preparation of the pseudo-chyme. 1 µM NEM was added to the pseudo-chyme in 5 min and 20 min after the beginning of the incubation according to the protocol described by Kweon et al. (2004).
We took isolated washed erythrocytes obtained from the person with the blood group AB (department of blood transfusion of S. Petersburg). These cells were fixed with 0.05% glutaraldehyde in PBS (pH 7.4) or 10 min and then and these cells were incubated with endoglycosidase H for 4 h. Erythrocytes were supplemented with 1 U/μL endoglycosidase H (NEB) and were incubated for 2 h at 37°C. Then samples were centrifuged and heated at 90°C for 5 min to induce denaturation of endo H. Next this supernatant was used for the dilution of the monoclonal anti-AgA and anti-AgB antibodies. We found that heating for 5 min at 85°C was sufficient for the denaturation of endoglycosidase-H. In control experiments, where the epitope was already glued to the most-sticky part of the antibody, there was no labelling in the Golgi area and in post-Golgi carriers operating between the Golgi apparatus and basolateral plasma membrane.
Preparation of reagents, immune fluorescent microscopy, conventional electron microscopy (EM), correlative video-light EM, nano-gold immune EM labelling, ultra-thin cryo-sectioning, counting of labelling density, and analyses by electron microscopic tomography were all carried out as previously described (Beznoussenko et al., 2015). Sections were examined using electron microscopes Tecnai-12 and 20 (ThermoFisher, Eindhoven, The Netherlands) as described previously (Beznoussenko et al., 2016).
After 21 days of cultivation, Caco-2 formed a brush border (Figs. 1A–1D) and interdigitating contacts (Fig. 1E). The most complete differentiation of Caco-2 cells into enterocytes was found when the pH of the medium was equal to 7.5: The percentage of cells with microvilli reached 91 ± 7% (Fig. 1D). We also found the cisternae of the SER attached to the basolateral PM. This feature was similar to that observed in enterocytes in the intestine (Sesorova et al., 2020). The Golgi complex (GC) was situated above the nucleus. On optical sections passed perpendicularly to the long axis of a Caco-2 cell GC appeared as dashed rings (Fig. 1F).
When the diluted artificial chyme (pseudo-chyme) was added, these Caco-2 cells exhibited similar phenotypes to those described earlier (Sesorova et al., 2020). After the addition of the diluted pseudo-chyme, we observed the appearance of ChMs in the distensions of Golgi cisternae (Fig. 2A). Lipid droplets were not formed. The IF analysis did not reveal a significant number of spots positive for AgA and AgB (this parameter was at the level of background (Figs. 1G: left, 1I: left).
In order to overload Caco2 cells with lipids, we added the non-diluted pseudo–chyme. The overloading phenotype appeared (Sesorova et al., 2020). The main evidence that we reached the overloading of the Caco2 cells with lipids was the appearance of lipid droplets in the cytosol and accumulation of many ChMs at the trans-side of the GC (Figs. 2B–2D).
Several cells acquired spots positive for anti-AgA and Anti-AgB with the Golgi area (Figs. 1G–1J, Figs. 2E–2G, 2I and 2J). In order to control the specificity of antibodies, we used AgA and AgB obtained from isolated erythrocytes from the AB blood group. The addition of AgA and AgB blocked staining with antibodies (Figs. 1H and 1J). Thus, overloading of enterocytes with lipids could induce rare errors of the protein glycosylation.
Role of SNAREs, COPII and COPI
We tried to identify at what level there could be glycosylation errors. To this end, we tested the role of the most important molecular machines operating at the ER-GC interface COPII, COPI, and SNARE. To inhibit COPI we used BFA, the inhibitor of the COPI assembly (Klausner et al., 1992). In order to inhibit COPII BeFx, H89 and PKI (permeable protein kinase A inhibitor) 1422, inhibitors of COPII assembly (Aridor and Balch, 2000; Omari et al., 2018; Subramanian et al., 2019) were applied. Finally, we treated cells with NEM for 5 min on ice immediately after the moment when ChMs appeared in the smooth ER (5 min and after their appearance in the GC (20 min). Then cells were washed and observed for 5 additional min.
Our analysis revealed that BeFx, 50 µM H89, and 100 µM PKI1422 did not interfere with the ER-Golgi transport of pre-ChMs (Figs. 3H–3J). In all these cases, ChMs were visible inside the GC. In 20 min after the addition of the pseudo-chyme. In contrast, 1 mg/mL BFA blocked the delivery of pre-ChMs from the ER at the Golgi and formed giant ChMs and the tubulated GC (Figs. 3K and 3L). Thus, the errors in the synthesis of AgA were not dependent on COPII but could be dependent on COPI.
In order to evaluate the role of membrane fusion for different steps of intracellular transcytosis, we added the undiluted pseudo-chyme to differentiated Caco-2 cells and, in 5 min, when ChMs were already visible within the SER, we treated cells with 1 mM NEM on ice for 15 min and, after its washout, we incubated cells for additional 5 min (the time when the effect of NEM on mitochondria is minimal, see Kweon et al., 2004) and examined whether ChMs are detectable within the GC. Although in control experiments we found ChMs within the GC, after such treatment with NEM the GC almost did not contain ChMs (Fig. 3M). Also, we treated cells with NEM in 15 min after the pseudo-chyme addition and then incubated additionally for 5 min. In control cells, ChMs were already visible within the space between the basolateral PM (BLPM) of interdigitating contacts (IDCs), whereas after treatment of cells with NEM CHMs were observed less frequently there (Fig. 3N). However, the size of ChMs was not changed (our unpublished observations). Thus, our experiments with NEM indicated that the ER-Golgi and the post-Golgi transport routes were SNARE dependent. Altogether our analysis suggests that the incorrect glycosylation was caused by alterations of the GC.
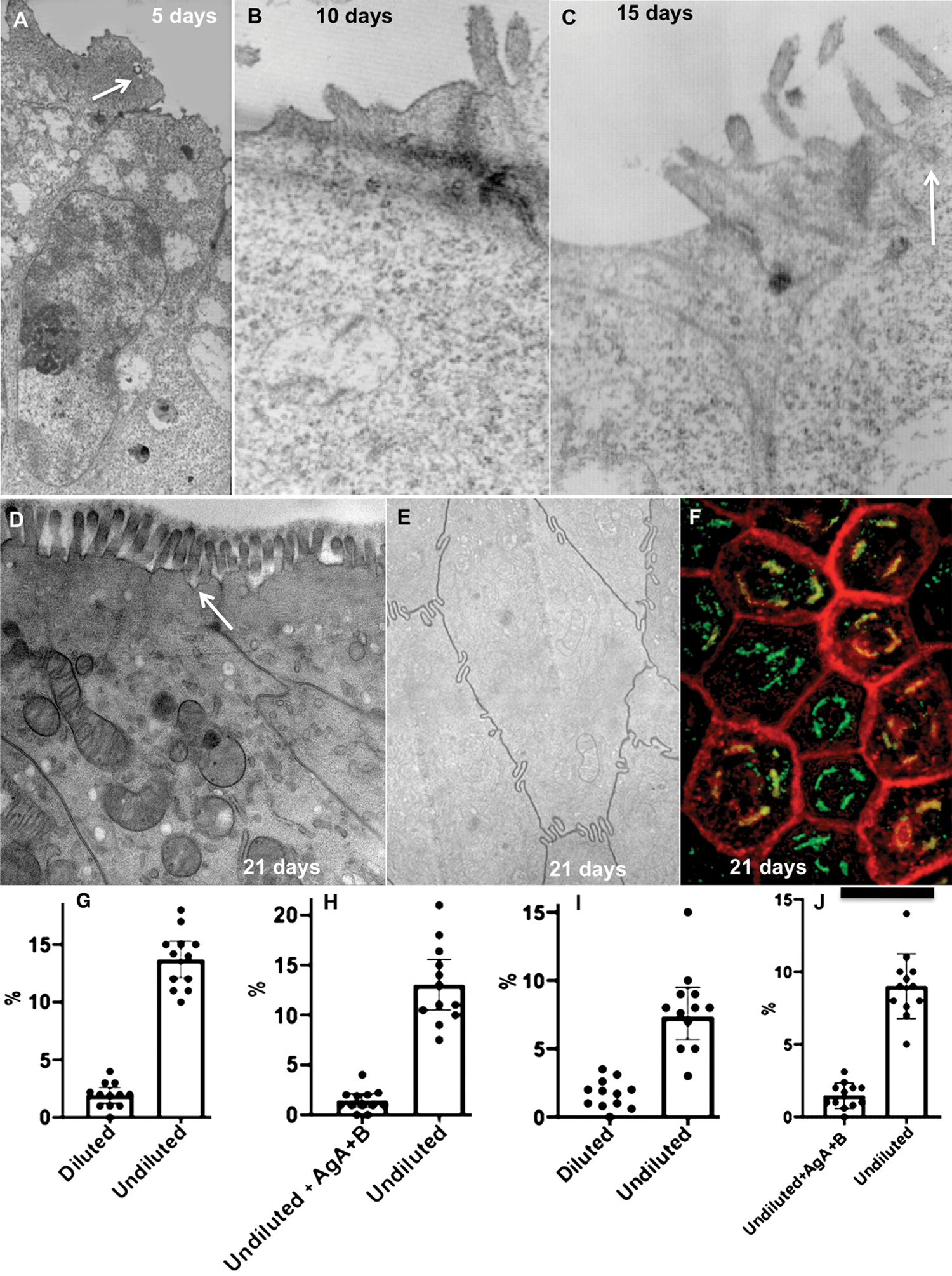
Figure 1: Dynamics of the Caco-2 cell differentiation.
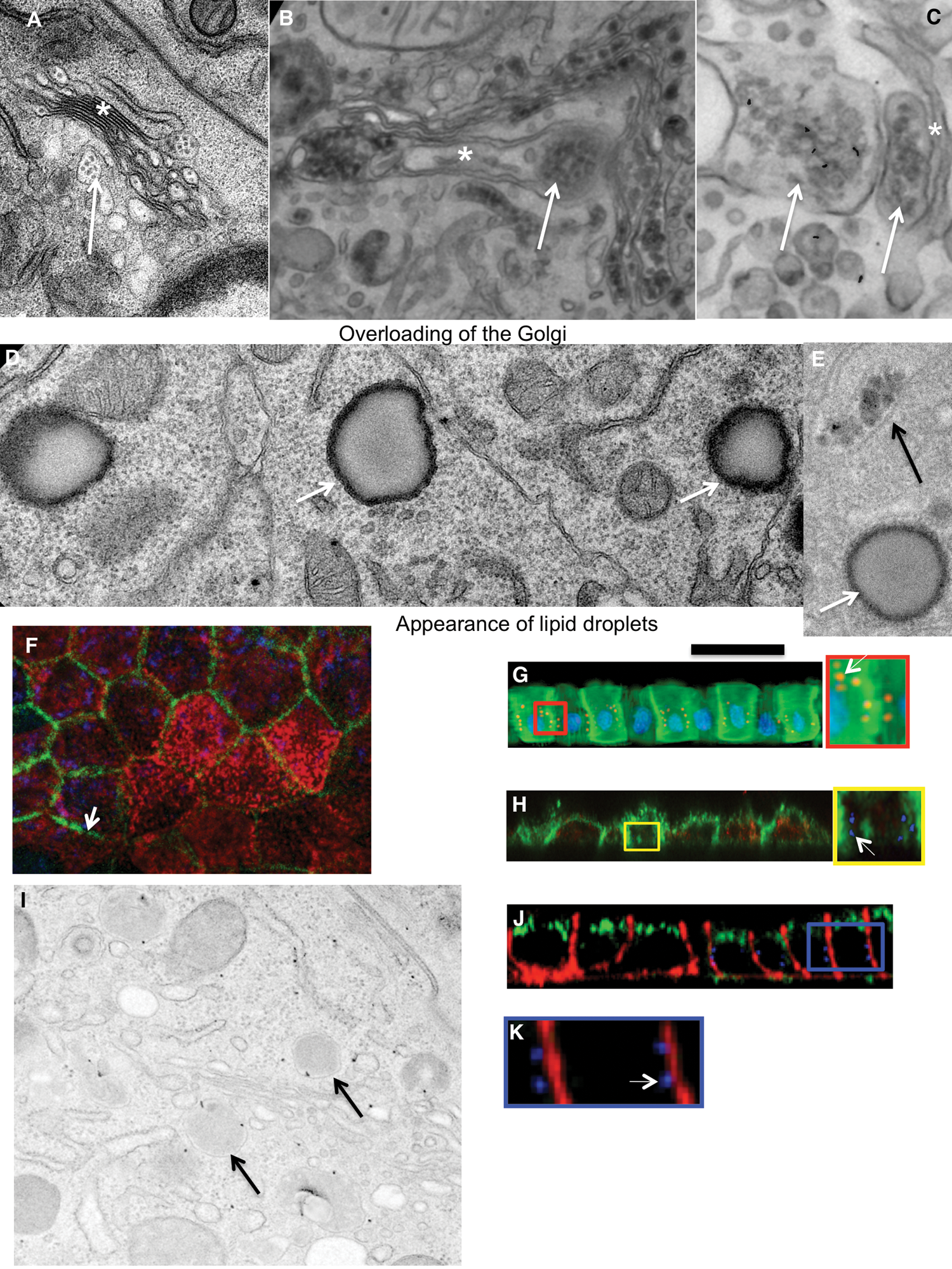
Figure 2: Loading of Caco-2 cells with lipid.
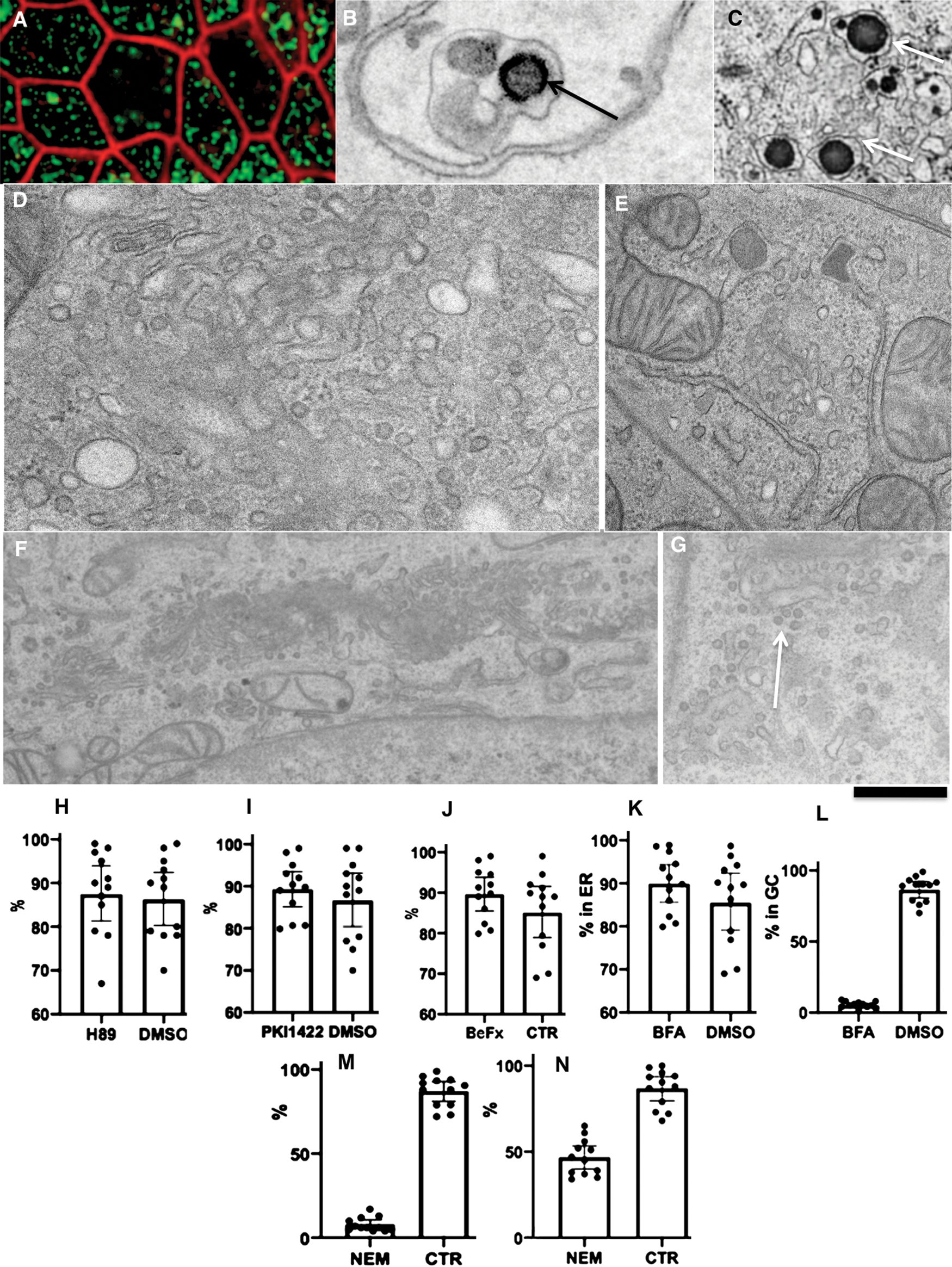
Figure 3: Effect of the treatment of Caco-2 cells with different drugs affecting COPII, COPI and SNAREs.
Although morphology and functional characteristics of Cavo-2 cells are rather similar to enterocytes, Caco-2 cells slightly differ from standard enterocytes in the organism. For instance, even after differentiation, these cells express lower levels of intestinal fatty acid-binding protein (I-FABP) than enterocytes suggesting that I-FABP is not necessary for Caco-2 cells to absorb and metabolize long-chain FFAs (Darimont et al., 1998). After 7 days of cultivation of filter, the relative apical and basolateral membrane surface areas of the Caco-2 cells was found to be only 1:3 (Trotter and Storch, 1993). Application of an adult serum and BSA accelerated differentiation of Caco-2 cells. Of interest, in undifferentiated Caco-2 cells, the apical endocytosis is visible after differentiation it became less evident as in enterocytes from adult animals (our unpublished observations; see Sesorova et al., 2020).
However, iCaco-2 cells represent a useful model for this study because our primary interest was to utilize the ability of Caco-2 cells to assemble and secrete ChMs (Townley et al., 2012; Santos et al., 2016). Not only enterocytes but also other cells are very sensitive to lipid overloading. Indeed, monocytes from mice incubated with LDL taken from normal animals did not form foam cells. However, when these monocytes were incubated with LDL taken from humans, they are transformed into foam cells (Rekhter et al., 1993).
The appearance of lipid droplets and alter and overloading of the GC suggests that the addition of a lot of pseudo-chyme could induce over-loading of Caco-2 cells with ChMs. Earlier, we demonstrated that when enterocytes were overloaded with lipids containing bile acids and fatty acids, lipid droplets appeared in the cytoplasm, the structure of the GC changes and the transcytosis of ChMs was altered (Sesorova et al., 2020). Experiments with overloading of the differentiated Caco-2 cells with the pseudo-chyme allowed us to find the errors in glycosylation of proteins that are present inside ChMs. This was similar to the situation that occurs during the transport of massive amounts of membrane proteins through the GC and leads to a change in standard glycosylation processes (Marra et al., 2007). Our observation is not unique. The altered terminal glycosylation is a common feature of cancer cells conferring new phenotypic properties to the cells (Groux-Degroote et al., 2018).
Glycosyltransferases acting on the histo-blood group carbohydrate biosynthesis have redundancy and degeneration. Redundancy is observed when two separate enzymes synthesize the same antigen (Groux-Degroote et al., 2018). Degeneration occurs when the same enzyme synthesizes different carbohydrate structures. For instance, FUT3 gene-defined fucosyltransferase is capable of synthesizing at least four different blood groups of carbohydrates (de Mattos, 2016). Also after Gal-knockout (KO), pigs were produced in several institutes by knocking out the α(1,3)galactosyl transferase (GGTA1), another transferase, and the GGTA2 remains in pigs (Keusch et al., 2000). Several researchers reported this possibility in Gal (GGTA1)-KO pigs (Taylor et al., 2003; Milland et al., 2006; Sandrin, 2007; Kiernan et al., 2008). ß-D-mannoside, ß 1,4-N-acetylglucosaminyltransferase III (GnT-III) catalyses the branching of N-linked oligosaccharides, producing a bisecting N-acetylglucosamine (GlcNAc) residue. Once a bisecting GlcNAc residue is added to the core mannose by GnT-III, the action of other competitive enzymes such as GnT-IV and GnT-V is prevented from introducing any additional structures into the Golgi stack. As a result, it is likely that all levels of N-linked sugar, including Gal and non-Gal antigens, are decreased. As a strong point of this strategy, overexpression of GnT-III clearly works on non-Gal antigens relate to N-linked sugar (Miyagawa et al., 2001, 2012).
We think that other Golgi enzymes are involved in the synthesis of AgB because GTA and GTB do not exist in Caco-2 cells. Indeed, there is a significant overlapping in functional characteristics among different Golgi enzymes (Diswall et al., 2007, 2010, 2011). Importantly, the altered terminal glycosylation is a common feature of cancer cells conferring new phenotypic properties to the cells (Groux-Degroote et al., 2018). In the same individual various tissues express AgB and AgA in a different way (Oriol et al., 1992). Sialylation increased and fucosylation decreased with age (Lityńska and Przybyło, 1998). This explains why with age the titre of AbA and AbB decreased. In any case, this question deserves additional analysis.
On the other hand, in differentiated Caco-2 cells, we did not find ER exit sites, COPII-coated buds and mega-buds, and apical clathrin-coated buds or ChM-containing vesicles near the APM before their loading and overloading with lipids. Also, in rat adult enterocytes, we did not find COPII-coated buds and mega-buds. Also, no mega-vesicles ferrying ChMs were observed (Sesorova et al., 2020).
Therefore in order to check whether COPII is necessary for the ER-Golgi transport of ChMs, we treated cells with BeFx, H89 or PKI-1422. These substances impaired the function of COPII. However, this did not block ER-Golgi transport of ChMs, whereas brefeldin A inhibited this delivery leading to augmentation of ChM size and Golgi tubulation. Nevertheless, several authors claim that COPII-coated vesicles exist. However, careful analysis of these papers revealed that after initiation of synchronized transcytosis through enterocytes generated from Caco-2 cells labelling for TANGO1 were never surrounded ApoB-containing BODIPI-positive ChM (Fig. 6C in Santos et al., 2016).
After treatment of the differentiated Caco-2 cells with 1 µm/mL brefeldin A, we found partial fragmentation of the GC at the level of light microscopy but did not observe ChM immune EM labelling for AgA and AgB within the GC. Similarly, Santos et al. (2016) demonstrated that loading of differentiated Caco-2 cells with lipids in the presence of brefeldin A, induced the formation of giant ChMs with their diameter of up to 2.3 µm. Thus, in Caco-2 cells, the ER-GC transport of ChMs is dependent on COPI but independent on COPII.
The first hypothesis of the AbA and AbB formation poses that epitopes on gut flora or plant materials similar to AgA and AgB are delivered to the blood from food (van Oss, 2004). Chickens kept in a germ-free environment would produce anti-B but not anti-A when fed bacteria expressing high levels of a B-like antigen and lower levels of an A-like antigen (Springer et al., 1959, 1961; Springer GF).
However, there are several problems with this hypothesis. Indeed, bacterial lipo-PS (LPS) are rather toxic, being able to alter the jejunal absorption of amino acids (Abad et al., 2001) and ascorbic acids (Subramanian et al., 2018), and serotonin (Mendoza et al., 2009). After prolonged exposure of the intestine to LPS, there is a loss of epithelial integrity and could induce detachment of epithelial cells (Wells et al., 1993).
Antibodies against AgA and AgB could be found even in neonates where consumption of bacterial PS is not possible. These antibodies are not of maternal origin (De Biasi, 1923; Thomaidis et al., 1967; Chattoraj et al., 1968; Godzisz, 1979; Mencarini et al., 1982; Wuttke et al., 1997; Merbl et al., 2007). Only in newborn mammals, enterocytes can transcytose macromolecules and polysaccharides (PS) from the intestine lumen (Gossrau, 1975a, 1975b). Moreover, only a small amount of proteins are transcytosed, and most of these proteins represent antibodies of the IgG class due to the presence of anti-Fc receptors (He et al., 2008). Moreover, soon after birth, this capacity disappears and enterocyte form a strong tissue-blood barrier, which does not allow proteins and oligosaccharides to be transcytosed through enterocytes. For instance, in rats, mice and pigs already in 7 days after the birth, such transcytosis was not found (Ekström et al., 1988, Ekström and Weström, 1992). In humans, the level of the maternally transferred IgG1-4 declined over the first week of life (Bennike et al., 2020). In adults, PS is hydrolysed by pancreatic α-amylase (Asanuma-Date et al., 2012).
Large PS molecules could be absorbed by enterocytes from the gut only through apical endocytosis because PS cannot jump through the lipid bilayer and tight junctions between enterocytes are impermeable for PS. In the intestine, carbohydrates undergo digestion and newly formed monosaccharides are absorbed through the APM (Bu et al., 2010; Asanuma-Date et al., 2012; Goto et al., 2012; Howe et al., 2014). However, we did not find clathrin-coated buds on the APM of the rat adult enterocytes (Sesorova et al., 2020). In spite of this inhibition of endocytosis of PS (which occurs very soon after birth) concentration of anti-A and anti-B antibodies increases until 18 years (Springer et al., 1959, 1961; Springer and Horton, 1969). Finally, linear PS’s have low antigenic properties. Specific extremely short double-edged fork (branches where each branch from two is composed of only one monosaccharide) at the tips of the bacterial PS and LPS are not found.
The cross-reaction hypothesis poses that these antibodies originate from the immune response towards the influenza virus, whose epitopes are similar enough to the α-D-N-galactosamine on the A glycoprotein could elicit a cross-reaction (Hakomori, 1999; Christen et al., 2010; Arend, 2013; Mujahid and Dickert, 2015). Finally, viruses could transfer AgA and AgB formed within their membrane deliver antigens A and B carry from one person to another. Viruses form the external membrane of their viral envelope from the portions of the plasma membrane. After infection, the virus delivers the antigen into the PM of human cells. Therefore, a person begins to synthesize antibodies against AgA introduced by the virus. However, at the beginning of life, a child often has a very small number of contacts with other people. Also,a the amount of antigen delivered by a virus is too small to stimulate the relevant lymphocyte clones. Moreover, antigen A and B are synthesized on the basolateral plasma membrane and not on the apical plasma membrane. Therefore, viruses bearing these antibodies have no direct access to the external environment, and their infection occurs through the blood. Constant synthesis of AgA or AgB in person with Groups B and A correspondingly could induce immune conflict. Of interest, the altered terminal glycosylation is a common feature of cancer cells conferring new phenotypic properties to the cells (Groux-Degroote et al., 2018). Similarly, alterations of glycosylation of chylomicrons could be one of the main atherogenesis mechanisms (Mironov et al., 2019).
Thus, overloading of enterocytes with lipids induces rare glycosylation errors in the structure of PS and the formation of PS antigens within the sugar chain. This could explain the mechanism of the formation of blood group antibodies. Our hypothesis could be tested using the predictions, which it generates. For instance, it predicts that (1) The titre of AgA and AgB would be higher in those who eat a lot of fatty foods. A similar study could be performed using biopsies from the intestine of humans. Biopsies of jejunum could be obtained during operation for cancer. These samples could be taken from the tumour containing part of the intestine just near the border. Then, the samples could be placed into a culture medium and delivered at 37°C to the laboratory. Next, the samples could be placed epithelial back on the large droplet of chyme from rats and incubated for 1 h. Further, samples could be washed and immune labelled with an antibody against AgA. In turn, the knowledge about mechanisms of formation of AgA and AgB in an organism where these antigens should not be important for organ transplantation especially of kidneys (Irving et al., 2012) because waitlist mortality continues to be a limiting factor for all solid-organ transplant programs (Grasemann et al., 2012). This observation could be useful for the explanation of atherogenesis. The role of glycosylation mistakes in the development of atherosclerosis is explained in our review (Mironov et al., 2020). For instance, alteration of glycosylation, especially sialylation, could induce aggregation of ChMs, ILDLs and LDLs and lead to the formation of antibodies against these apo-lipoproteins.
Acknowledgement: We thank all of scientists who sent us their reagents and Dr. A. Ivanova for the help in EM preparations.
Availability of Data and Materials: This study did not produce new reagents. We are ready to provide all new materials to any scientist.
Ethics Approval: We did not perform experiments on animals and human.
Author Contributions: The authors confirm contribution to the paper as follows: Study conception and design: GND, IDD. AVZ; data collection: GND, NRK, IDD, AAM; Y. Author; analysis and interpretation of results: LJA. AVZ. IDD; draft manuscript preparation: AAM. All authors reviewed the results and approved the final version of the manuscript. The author confirms sole responsibility for the following: Study conception and design, data collection, analysis and interpretation of results, and manuscript preparation.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Abad B, Mesonero JE, Salvador MT, Garcia-Herrera J, Rodriguez-Yoldi MJ. (2001). Effect of lipopolysaccharide on small intestinal L-leucine transport in rabbit. Digestive Diseases and Sciences 46: 1113–1119. DOI 10.1023/A:1010782600380. [Google Scholar] [CrossRef]
Amano J, Oshima M. (1999). Expression of the H Type 1 blood group antigen during enterocytic differentiation of Caco-2 cells. Journal of Biological Chemistry 274: 21209–21216. DOI 10.1074/jbc.274.30.21209. [Google Scholar] [CrossRef]
Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. (2001). Dynamics of the COPII coat with GTP and stable analogues. Nature Cell Biology 3: 531–537. DOI 10.1038/35078500. [Google Scholar] [CrossRef]
Arend P. (2013). Ancestral gene and “complementary” antibody dominate early ontogeny. Immunobiology 218: 755–761. DOI 10.1016/j.imbio.2012.08.277. [Google Scholar] [CrossRef]
Aridor M, Balch WE. (2000). Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. Journal of Biological Chemistry 275: 35673–35676. DOI 10.1074/jbc.C000449200. [Google Scholar] [CrossRef]
Asanuma-Date K, Hirano Y, Le N, Sano K, Kawasaki N, Hashii N, Hiruta Y, Nakayama K, Umemura M, Ishikawa K, Sakagami H, Ogawa H. (2012). Functional regulation of sugar assimilation by N-glycan-specific interaction of pancreatic α-amylase with glycoproteins of duodenal brush border membrane. Journal of Biological Chemistry 287: 23104–23118. DOI 10.1074/jbc.M111.314658. [Google Scholar] [CrossRef]
Bennike TB, Fatou B, Angelidou A, Diray-Arce J, Falsafi R, Ford R, Gill EE, van Haren SD, Idoko OT, Lee AH, Ben-Othman R, Pomat WS, Shannon CP, Smolen KK, Tebbutt SJ, Ozonoff A, Richmond PC, AHJvd Biggelaar, Hancock REW, Kampmann B, Kollmann TR, Levy O, Steen H. (2020). Preparing for life: Plasma proteome changes and immune system development during the first week of human life. Frontiers in Immunology 11: 578505. DOI 10.3389/fimmu.2020.578505. [Google Scholar] [CrossRef]
Beznoussenko GV, Parashuraman S, Rizzo R, Polishchuk R, Martella O, Di Giandomenico D, Fusella A, Spaar A, Sallese M, Capestrano MG, Pavelka M, Vos MR, Rikers YG, Helms V, Mironov AA, Luini A. (2014). Transport of soluble proteins through the Golgi occurs by diffusion via continuities across cisternae. eLife 3, 10.7554/eLife.02009. [Google Scholar] [CrossRef]
Beznoussenko GV, Pilyugin SS, Geerts WJ, Kozlov MM, Burger KN, Luini A, Derganc J, Mironov AA. (2015). Trans-membrane area asymmetry controls the shape of cellular organelles. International Journal of Molecular Sciences 16: 5299–5333. DOI 10.3390/ijms16035299. [Google Scholar] [CrossRef]
Beznoussenko GV, Ragnini-Wilson A, Wilson C, Mironov AA. (2016). Three-dimensional and immune electron microscopic analysis of the secretory pathway in Saccharomyces cerevisiae. Histochemistry Cell Biology 146: 515–527. DOI 10.1007/s00418-016-1483-y. [Google Scholar] [CrossRef]
Branch DR. (2015). Anti-A and anti-B: What are they and where do they come from? Transfusion 55: S74–S79. DOI 10.1111/trf.13087. [Google Scholar] [CrossRef]
Bu HF, Wang X, Tang Y, Koti V, Tan XD. (2010). Toll-like receptor 2-mediated peptidoglycan uptake by immature intestinal epithelial cells from apical side and exosome-associated transcellular transcytosis. Journal of Cell Physiology 222: 658–668. [Google Scholar]
Chattoraj A, Gilbert R, Josephson AM. (1968). Serological demonstration of fetal production of blood group isoantibodies. Vox Sanguinis 14: 289–291. DOI 10.1159/000464703. [Google Scholar] [CrossRef]
Christen U, Hintermann E, Holdener M, von Herrath MG. (2010). Viral triggers for autoimmunity: Is the ‘glass of molecular mimicry’ half full or half empty? Journal of Autoimmunity 34: 38–44. DOI 10.1016/j.jaut.2009.08.001. [Google Scholar] [CrossRef]
Darimont C, Gradoux N, Cumin F, Baum HP, De Pover A. (1998). Differential regulation of intestinal and liver fatty acid-binding proteins in human intestinal cell line (Caco-2Role of collagen. Experimental Cell Research 244: 441–447. DOI 10.1006/excr.1998.4186. [Google Scholar] [CrossRef]
De Biasi B. (1923). Studies on iso-agglutinins in the blood of the new-born. JAMA 81: 1776–1778. DOI 10.1001/jama.1923.02650210042011. [Google Scholar] [CrossRef]
de Mattos LC. (2016). Structural diversity and biological importance of ABO, H, Lewis and secretor histo-blood group carbohydrates. The Revista Brasileira de Hematologia e Hemoterapia 38: 331–340. DOI 10.1016/j.bjhh.2016.07.005. [Google Scholar] [CrossRef]
Diswall M, Angström J, Schuurman HJ, Dor FJ, Rydberg L, Breimer ME. (2007). Studies on glycolipid antigens in small intestine and pancreas from alpha1,3-galactosyltransferase knockout miniature swine. Transplantation 84: 1348–1356. DOI 10.1097/01.tp.0000287599.46165.15. [Google Scholar] [CrossRef]
Diswall M, Angström J, Karlsson H, Phelps CJ, Ayares D, Teneberg S, Breimer ME. (2010). Structural characterization of alpha1,3-galactosyltransferase knockout pig heart and kidney glycolipids and their reactivity with human and baboon antibodies. Xenotransplantation 17: 48–60. DOI 10.1111/j.1399-3089.2009.00564.x. [Google Scholar] [CrossRef]
Diswall M, Gustafsson A, Holgersson J, Sandrin MS, Breimer ME. (2011). Antigen-binding specificity of anti-αGal reagents determined by solid-phase glycolipid-binding assays. A complete lack of αGal glycolipid reactivity in α1,3GalT-KO pig small intestine. Xenotransplantation 18: 28–39. DOI 10.1111/j.1399-3089.2011.00623.x. [Google Scholar] [CrossRef]
Ekström GM, Weström BR, Telemo E, Karlsson BW. (1988). The uptake of fluorescein-conjugated dextran 70,000 by the small intestinal epithelium of the young rat and pig in relation to macromolecular transmission into the blood. Journal of Developmental Physiology 10: 227–233. [Google Scholar]
Ekström GM, Weström BR. (1992). Intestinal uptake and transmission of macromolecules into the blood in the young guinea pig. Journal of Pediatric Gastroenterology and Nutrition 14: 71–78. DOI 10.1097/00005176-199201000-00013. [Google Scholar] [CrossRef]
Fleet JC, Wang L, Vitek O, Craig BA, Edenberg HJ. (2003). Gene expression profiling of Caco-2 BBe cells suggests a role for specific signaling pathways during intestinal differentiation. Physiological Genomics 13: 57–68. DOI 10.1152/physiolgenomics.00152.2002. [Google Scholar] [CrossRef]
Gehrie E, Muranyi A, Walk E, Young PP. (2014). Commercially available blood-group antibodies may be used to visualize blood group A antigen in paraffin-embedded tissue sections. Archives of Pathology & Laboratory Medicine 138: 155. DOI 10.5858/arpa.2013-0302-LE. [Google Scholar] [CrossRef]
Glingston RS, Deb R, Kumar S, Nagotu S. (2019). Organelle dynamics and viral infections: At cross roads. Microbes and Infection 21: 20–32. DOI 10.1016/j.micinf.2018.06.002. [Google Scholar] [CrossRef]
Godzisz J. (1979). Synthesis of natural allohemagglutinins of the ABO system in healthy children aged 3 months to 3 years. Revue Francaise de Transfusion et Immuno-hematologie 22: 399–412. DOI 10.1016/S0338-4535(79)80034-3. [Google Scholar] [CrossRef]
Gossrau R (1975a). Moulting of enterocytes during intestinal development. Verhandlungen der Anatomischen Gesellsschaft 69: 209–213. [Google Scholar]
Gossrau R (1975b). Lysosomes of the intestinal epithelium. An embryological investigation. Advances in Anatomy, Embryology, and Cell Biology 51: 1–95. [Google Scholar]
Goto T, Horita M, Nagai H, Nagatomo A, Nishida N, Matsuura Y, Nagaoka S. (2012). Tiliroside, a glycosidic flavonoid, inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract. Molecular Nutrition & Food Research 56: 435–445. DOI 10.1002/mnfr.201100458. [Google Scholar] [CrossRef]
Grasemann H, de Perrot M, Bendiak GN, Cox P, van Arsdell GS, Keshavjee S, Solomon M. (2012). ABO-incompatible lung transplantation in an infant. American Journal of Transplantology 12: 779–781. DOI 10.1111/j.1600-6143.2011.03861.x. [Google Scholar] [CrossRef]
Groux-Degroote S, Schulz C, Cogez V, Noël M, Portier L, Vicogne D, Solorzano C, Dall’Olio F, Steenackers A, Mortuaire M, Gonzalez-Pisfil M, Henry M, Foulquier F, Héliot L, Harduin-Lepers A. (2018). The extended cytoplasmic tail of the human B4GALNT2 is critical for its Golgi targeting and post-Golgi sorting. FEBS Journal 285: 3442–3463. DOI 10.1111/febs.14621. [Google Scholar] [CrossRef]
Hakomori S. (1999). Antigen structure and genetic basis of histo-blood groups A, B and O: Their changes associated with human cancer. Biochimica et Biophysica Acta 1473: 247–266. DOI 10.1016/S0304-4165(99)00183-X. [Google Scholar] [CrossRef]
He W, Ladinsky MS, Huey-Tubman KE, Jensen GJ, McIntosh JR, Björkman PJ. (2008). FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature 455: 542–546. DOI 10.1038/nature07255. [Google Scholar] [CrossRef]
Hiebl V, Schachner D, Ladurner A, Heiss EH, Stangl H, Dirsch VM. (2020). Caco-2 cells for measuring intestinal cholesterol transport-possibilities and limitations. Biological Procedures Online 11: 27. [Google Scholar]
Howe SE, Lickteig DJ, Plunkett KN, Ryerse JS, Konjufca V. (2014). The uptake of soluble and particulate antigens by epithelial cells in the mouse small intestine. PLoS One 9: e86656. DOI 10.1371/journal.pone.0086656. [Google Scholar] [CrossRef]
Irving C, Gennery A, Kirk R. (2012). Pushing the boundaries: The current status of ABO-incompatible cardiac transplantation. Journal of Heart and Lung Transplantation 31: 791–796. DOI 10.1016/j.healun.2012.03.007. [Google Scholar] [CrossRef]
Keusch JJ, Manzella SM, Nyame KA, Cummings RD, Baenziger JU. (2000). Expression cloning of a new member of the ABO blood group glycosyltransferases, iGb3 synthase, that directs the synthesis of isoglobo-glycosphingolipids. Journal of Biological Chemistry 275: 25308–25314. [Google Scholar]
Kiernan K, Harnden I, Gunthart M, Gregory C, Meisner J, Kearns-Jonker M. (2008). The anti-nonGal xenoantibody response to xenoantigens on gal knockout pig cells is encoded by a restricted number of germline progenitors. American Journal of Transplantation 8: 1829–1839. DOI 10.1111/j.1600-6143.2008.02337.x. [Google Scholar] [CrossRef]
Klausner RD, Donaldson JG, Lippincott-Schwartz J. (1992). Brefeldin A: Insights into the control of membrane traffic and organelle structure. Journal of Cell Biology 116: 1071–1080. DOI 10.1083/jcb.116.5.1071. [Google Scholar] [CrossRef]
Kweon HS, Beznoussenko GV, Micaroni M, Polishchuk RS, Trucco A, Martella O, Di Giandomenico D, Marra P, Fusella A, Di Pentima A, Berger EG, Geerts WJ, Koster AJ, Burger KN, Luini A, Mironov AA (2004). Golgi enzymes are enriched in perforated zones of golgi cisternae but are depleted in COPI vesicles. Molecular Biology of the Cell 15: 4710–4724. [Google Scholar]
Levy E, Mehran M, Seidman E. (1995). Caco-2 cells as a model for intestinal lipoprotein synthesis and secretion. FASEB Journal 9: 626–635. DOI 10.1096/fasebj.9.8.7768354. [Google Scholar] [CrossRef]
Lityńska A, Przybyło M. (1998). Age-related profile of beta-N-acetylhexosaminidase glycosylation in rat liver. Acta Biochimica Polonica 45: 791–797. DOI 10.18388/abp.1998_4273. [Google Scholar] [CrossRef]
Marra P, Salvatore L, Mironov AJr, Di Campli A, Di Tullio G, Trucco A, Beznoussenko G, Mironov A, De Matteis MA. (2007). The biogenesis of the Golgi ribbon: The roles of membrane input from the ER and of GM130. Molecular Biology of the Cell 18: 1595–1608. DOI 10.1091/mbc.e06-10-0886. [Google Scholar] [CrossRef]
Mencarini L, Tozzi C, Arachi S, Tonelli C. (1982). Autochthonous anti-A/B allohemaggluntinins in umbilical cord blood. Minerva Pediatrica 34: 83–85. [Google Scholar]
Mendoza C, Matheus N, Iceta R, Mesonero JE, Alcalde AI. (2009). Lipopolysaccharide induces alteration of serotonin transporter in human intestinal epithelial cells. Innate Immunity 15: 243–250. DOI 10.1177/1753425909104781. [Google Scholar] [CrossRef]
Merbl Y, Zucker-Toledano M, Quintana FJ, Cohen IR. (2007). Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. Journal of Clinical Investestigation 117: 712–718. DOI 10.1172/JCI29943. [Google Scholar] [CrossRef]
Milland J, Sandrin MS. (2006). ABO blood group and related antigens, natural antibodies and transplantation. Tissue Antigens 68: 459–466. DOI 10.1111/j.1399-0039.2006.00721.x. [Google Scholar] [CrossRef]
Milland J, Christiansen D, Lazarus BD, Taylor SG, Xing PX, Sandrin MS. (2006). The molecular basis for gal alpha (1,3) gal expression in animals with a deletion of the alpha 1,3 galactosyltransferase gene. Journal of Immunology 176: 2448–2454. DOI 10.4049/jimmunol.176.4.2448. [Google Scholar] [CrossRef]
Mironov AA, Colanzi A, Polishchuk RS, Beznoussenko GV, Mironov AAJr, Fusella A, Di Tullio G, Silletta MG, Corda D, De Matteis MA, Luini A. (2004). Dicumarol, an inhibitor of ADP-ribosylation of CtBP3/BARS, fragments Golgi non-compact tubular zones and inhibits intra-Golgi transport. European Journal of Cell Biology 83: 263–279. DOI 10.1078/0171-9335-00377. [Google Scholar] [CrossRef]
Mironov AA, Dimov ID, Beznoussenko GV (2019). Role of intracellular transport in the centriole-dependent formation of golgi ribbon. Results Probl Cell Differ 67: 49–79. DOI 10.1007/978-3-030-23173-6_4. [Google Scholar] [CrossRef]
Mironov AA, Sesorova IS, Dimov ID, Karelina NR, Beznoussenko GV. (2020). Intracellular transports and atherogenesis. Frontiers in Bioscience 25: 1230–1258. DOI 10.2741/4854. [Google Scholar] [CrossRef]
Miyagawa S, Murakami H, Takahagi Y, Nakai R, Yamada M, Murase A, Koyota S, Koma M, Matsunami K, Fukuta D, Fujimura T, Shigehisa T, Okabe M, Nagashima H, Shirakura R, Taniguchi N. (2001). Remodeling of the major pig xenoantigen by N-acetylglucosaminyltransferase III in transgenic pig. Journal of Biological Chemistry 276: 39310–39319. DOI 10.1074/jbc.M104359200. [Google Scholar] [CrossRef]
Miyagawa S, Ueno T, Nagashima H, Takama Y, Fukuzawa M. (2012). Carbohydrate antigens. Current Opinion in Organ Transplantation 17: 174–179. DOI 10.1097/MOT.0b013e3283508189. [Google Scholar] [CrossRef]
Mujahid A, Dickert FL. (2015). Blood group typing: From classical strategies to the application of synthetic antibodies generated by molecular imprinting. Sensors 16: E51. DOI 10.3390/s16010051. [Google Scholar] [CrossRef]
Omari S, Makareeva E, Roberts-Pilgrim A, Mirigian L, Jarnik M, Ott C, Lippincott-Schwartz J, Leikin S. (2018). Noncanonical autophagy at ER exit sites regulates procollagen turnover. Proceedings of the National Academy of Sciences of the United States of America 115: E10099–E10108. DOI 10.1073/pnas.1814552115. [Google Scholar] [CrossRef]
Oriol R, Mollicone R, Coullin P, Dalix AM, Candelier JJ. (1992). Genetic regulation of the expression of ABH and Lewis antigens in tissues. APMIS 27: 28–38. [Google Scholar]
Ravn V, Dabelsteen E. (2000). Tissue distribution of histo-blood group antigens. APMIS 108: 1–28. DOI 10.1034/j.1600-0463.2000.d01-1.x. [Google Scholar] [CrossRef]
Rekhter MD, Tertov VV, Andreeva ER, Kolpakov VA, Mironov AA, Orekhov AN. (1993). Lipid accumulation in the subendothelial cells of human aortic intima impairs cell-to-cell contacts. A comparative study in situ and in vitro. Cardiovascular Pathology 2: 53–62. DOI 10.1016/1054-8807(93)90013-R. [Google Scholar] [CrossRef]
Rodriguez-Juan C, Pérez-Blas M, Valeri AP, Aguilera N, Arnaiz-Villena A, Pacheco-Castro A, Martin-Villa JM. (2001). Cell surface phenotype and cytokine secretion in Caco-2 cell cultures: Increased RANTES production and IL-2 transcription upon stimulation with IL-1beta. Tissue and Cell 33: 570–579. DOI 10.1054/tice.2001.0212. [Google Scholar] [CrossRef]
Sandrin MS. (2007). Gal knockout pigs: Any more carbohydrates? Transplantation 84: 8–9. DOI 10.1097/01.tp.0000269728.11879.f6. [Google Scholar] [CrossRef]
Santos AJ, Nogueira C, Ortega-Bellido M, Malhotra V. (2016). TANGO1 and Mia2/cTAGE5(TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum. Journal of Cell Biology 213: 343–354. DOI 10.1083/jcb.201603072. [Google Scholar] [CrossRef]
Schneeberger K, Roth S, Nieuwenhuis EES, Middendorp S. (2018). Intestinal epithelial cell polarity defects in disease: Lessons from microvillus inclusion disease. Disease Models & Mechanisms 11: dmm031088. DOI 10.1242/dmm.031088. [Google Scholar] [CrossRef]
Sesorova IS, Karelina NR, Kazakova TE, Parashuraman S, Zdorikova MA, Dimov ID, Seliverstova EV, Beznoussenko GV, Mironov AA. (2020). Structure of the enterocyte transcytosis compartments during lipid absorption. Histochemistry and Cell Biology 153: 413–429. DOI 10.1007/s00418-020-01851-3. [Google Scholar] [CrossRef]
Sheffield WP, Tinmouth A, Branch DR. (2005). Blood group biochemistry: A canadian blood services research and development symposium. Transfusion Medicine Reviews 19: 295–307. DOI 10.1016/j.tmrv.2005.04.005. [Google Scholar] [CrossRef]
Springer GF, Horton RE, Forbes M. (1959). Origin of anti-human blood group B agglutinins in white Leghorn chicks. Journal of Experimental Medicine 110: 221–244. DOI 10.1084/jem.110.2.221. [Google Scholar] [CrossRef]
Springer GF, Williamson P, Brandes WC. (1961). Blood group activity of gram-negative bacteria. Journal of Experimental Medicine 113: 1077–1093. DOI 10.1084/jem.113.6.1077. [Google Scholar] [CrossRef]
Springer GF, Horton RE. (1969). Blood group isoantibody stimulation in man by feeding blood group-active bacteria. Journal of Clinical Investigation 48: 1280–1291. DOI 10.1172/JCI106094. [Google Scholar] [CrossRef]
Subramanian VS, Sabui S, Moradi H, Marchant JS, Said HM. (2018). Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochimica et Biophysica Acta-Biomembranes 1860: 556–565. DOI 10.1016/j.bbamem.2017.10.010. [Google Scholar] [CrossRef]
Subramanian A, Capalbo A, Iyengar NR, Rizzo R, di Campli A, Di Martino R, Lo Monte M, Beccari AR, Yerudkar A, Del Vecchio C, Glielmo L, Turacchio G, Pirozzi M, Kim SG, Henklein P, Cancino J, Parashuraman S, Diviani D, Fanelli F, Sallese M, Luini A. (2019). Auto-regulation of secretory flux by sensing and responding to the folded cargo protein load in the endoplasmic reticulum. Cell 176: 1461–1476. DOI 10.1016/j.cell.2019.01.035. [Google Scholar] [CrossRef]
Taylor SG, McKenzie IF, Sandrin IF. (2003). Characterization of the rat alpha(1,3)-galactosyltransferase: Evidence for two independent genes encoding glycosyltransferases that synthesize Gal alpha(1,3)Gal by two separate glycosylation pathways. Glycobiology 13: 327–337. DOI 10.1093/glycob/cwg030. [Google Scholar] [CrossRef]
Thomaidis T, Fouskaris G, Matsaniotis N. (1967). Isohemagglutinin activity in the first day of life. American Journal of Diseases of Children 113: 654–657. [Google Scholar]
Townley AK, Schmidt K, Hodgson L, Stephens DJ. (2012). Epithelial organization and cyst lumen expansion require efficient Sec13-Sec31-driven secretion. Journal of Cell Science 125: 673–684. DOI 10.1242/jcs.091355. [Google Scholar] [CrossRef]
Trotter PJ, Storch J. (1993). Fatty acid esterification during differentiation of the human intestinal cell line Caco-2. Journal of Biological Chemistry 268: 10017–10023. DOI 10.1016/S0021-9258(18)82166-4. [Google Scholar] [CrossRef]
van Oss CJ. (2004). “Natural” versus regular antibodies. Protein Journal 23: 357–360. DOI 10.1023/B:JOPC.0000039625.56296.6e. [Google Scholar] [CrossRef]
Varki A. (1998). Factors controlling the glycosylation potential of the Golgi apparatus. Trends in Cell Biology 8: 34–40. DOI 10.1016/S0962-8924(97)01198-7. [Google Scholar] [CrossRef]
Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth J. (1999). Essentials of glycobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press. [Google Scholar]
Wells CL, Jechorek RP, Olmsted SB, Erlandsen SL. (1993). Effect of LPS on epithelial integrity and bacterial uptake in the polarized human enterocyte-like cell line Caco-2. Circulatory Shock 40: 276–288. [Google Scholar]
White T, Mandel U, Orntoft TF, Dabelsteen E, Karkov J, Kubeja M, Hakomori S, Clausen H. (1990). Murine monoclonal antibodies directed to the human histo-blood group A transferase (UDP-GalNAc: Fuc alpha 1-2Gal alpha 1-3-N-acetylgalactosaminyltransferase) and the presence therein of N-linked histo-blood group A determinant. Biochemistry 29: 2740–2747. DOI 10.1021/bi00463a017. [Google Scholar] [CrossRef]
Wu XW, Wang RF, Yuan M, Xu W, Yang XW. (2013). Dulbecco’s modified eagle’s medium and minimum essential medium—Which one is more preferred for establishment of Caco-2 cell monolayer model used in evaluation of drug absorption? Pharmazie 68: 805–810. [Google Scholar]
Wuttke NJ, Macardle PJ, Zola H. (1997). Blood group antibodies are made by CD5+ and by CD5- B cells. Immunology and Cell Biology 75: 478–483. DOI 10.1038/icb.1997.74. [Google Scholar] [CrossRef]
Yamamoto F. (2004). Review: ABO blood group system—ABH oligosaccharide antigens, anti-A and anti-B, A and B glycosyltransferases, and ABO genes. Immunohematology 20: 3–22. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |