DOI:10.32604/biocell.2021.014439

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.014439 |  www.techscience.com/journal/biocell |
| Review |
Application of photodynamic therapy in cancer: challenges and advancements
1National Center for International Research of Bio-Targeting Theranostics, Guangxi Key Laboratory of Bio-Targeting Theranostics, Collaborative Innovation Center for Targeting Tumor Diagnosis and Therapy, Guangxi Talent Highland of Bio-Targeting Theranostics, Guangxi Medical University, Nanning, 530021, China
2Scientific Research and Education Department, The First People’s Hospital of Changde City, Changde, 415000, China
3The First People’s Hospital of Changde City, Changde, 415000, China
4Guangxi University of Chinese Medicine, Nanning, 530001, China
*Address correspondence to: Zhiming Deng, cdzm_deng@yeah.net; Yong Huang, Huangyong503@126.com
#These authors contributed equally to this work
Received: 27 September 2020; Accepted: 11 November 2020
Abstract: Although great achievements have been made in the past decades in medicine, cancer remains a worldwide public health issue. Surgery is usually accompanied by shortcomings such as residual lesions and poor treatment effects, and the successive appearance of other treatment methods, such as radiotherapy and chemotherapy, has not changed the postoperative recurrence rate, toxicity, and side effects. However, the advent of photodynamic therapy has greatly improved this situation. Photodynamic therapy is an emerging tumor diagnosis and treatment technology with good application prospects, photodynamic therapy uses a specific wavelength of light to excite a photosensitizer to generate reactive oxygen species, damage tumor blood vessels and promote tumor cell apoptosis, exerting an anti-tumor effect. Photodynamic therapy has become a new clinical anti-tumor therapy due to its clear efficacy, few side effects, and easy use in combination with other therapies. In this review, we summarized the main mechanism, current challenges, and advancements of photodynamic therapy.
Keywords: Photodynamic therapy; Reactive oxygen species; Photosensitizer; Cancer; Mechanism
According to the World Health Organization (WHO), in 2018, cancer has claimed 9.6 million lives, and the global cancer burden will rise to 18.1 million new cases (Siegel et al., 2018). Over the years, researchers have studied the mechanism of cancer cell proliferation, infiltration, and migration to provide new ideas for treatment. Presently, cancer treatments mainly include surgery, radiotherapy, chemotherapy, and immunotherapy. For most patients with solid tumors, surgery is considered the most common and effective way to cure tumors. However, surgery may cause local metastases and positive margins, and radiation therapy and chemotherapy can lead to side effects such as fatigue, nausea and vomiting, hair loss, and blood cell reduction (Balcer-Kubiczek and Eley, 2018; Schirrmacher, 2019).
Photodynamic therapy (PDT) originated in 1900 by Oscar Raab (Raab, 1900), who stumbled on the finding that paramecia exposed to acridine orange and light can be quickly killed, thus, the theory of combining chemical and light energy to induce cell death was proposed. PDT uses a combination of a photosensitizer (PS), light, and oxygen molecules to selectively kill diseased tissues. When the PS absorbs sufficient energy from a specific light wavelength, they activate oxygen molecules in the surrounding environment of the tumor tissue, exerting cytotoxic effects to treat malignant lesions (Yanovsky et al., 2019). In 1975, Kelly et al. (1975) successfully used hematoporphyrin derivative (HpD) as a PS to treat bladder cancer, setting a precedent for PDT in cancer treatment. Although PDT is still a new and non-invasive method of tumor treatment, it has been approved by the U.S. Food and Drug Administration (FDA) and Europe, the Middle East, and Africa (EMEA) to treat tumors and non-malignant diseases (Baskaran et al., 2018; Gong et al., 2016; Song et al., 2018) (Fig. 1).
In recent decades, PDT has become an effective method for cancer treatments in scientific research and clinical practice due to its many advantages, including the following: (1) PDT can kill tumor cells locally by producing reactive oxygen species (ROS) in a short time, without causing tumor resistance; (2) PS produces apoptosis-inducing ROS only after light exposure, and the time and location of the illumination are controllable, minimizing, the toxicity of the PS to the body; (3) PDT can avoid minor lesions that are invisible to the eyes during surgery, improve the prognosis and prevent recurrence; (4) PDT can be used multiple times for the same tumor site and can be combined with other therapies such as chemotherapy, radiation and gene therapy (Cheong et al., 2015; Hwang et al., 2018; Ozog et al., 2016; Wachowska et al., 2015a). Therefore, PDT is a new clinical method with huge potential advantages.
This review discussed the brief history and development of the first-generation to existing third-generation photosensitizers, and the principles and mechanisms of PDT, current challenges, and future perspectives.
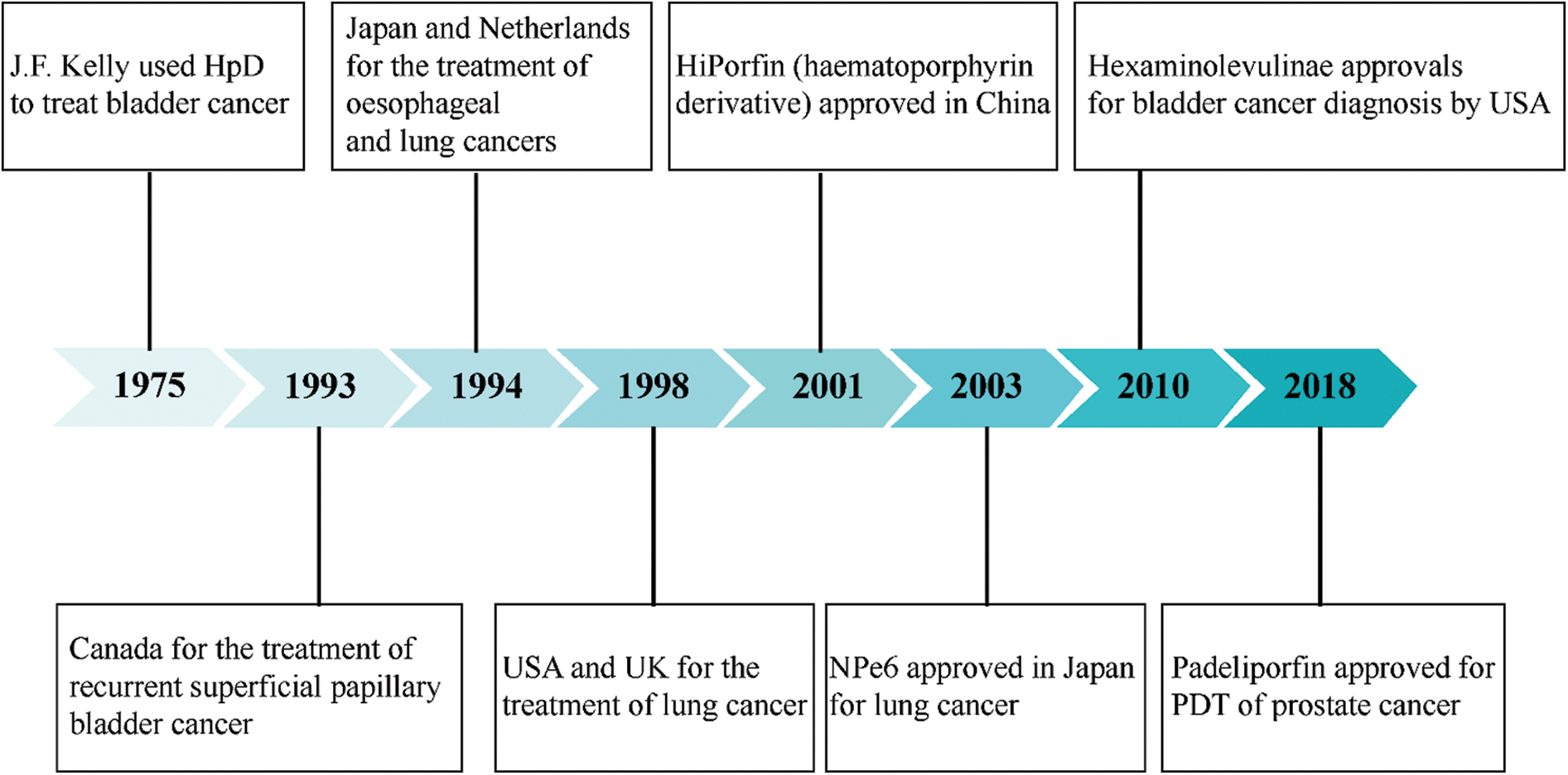
Figure 1: Timeline of select approvals of photodynamic therapy photosensitizers for cancer indications.
Principle of Photodynamic Therapy
Photodynamic therapy mainly comprises three elements: A light source that provides energy for the photodynamic response; PS that can absorb the corresponding light and perform the photodynamic response; and a large amount of ROS produced by electron or energy transfer through excited PS, particularly singlet oxygen (1O2) and superoxide (Ancona et al., 2018; Shi et al., 2019). Consequently, the role of PDT is based on the synergistic effect of excitation light of a precise wavelength, PS, and ROS (Dabrowski and Arnaut, 2015; Zhang and Li, 2018).
In the process of optical imaging in vivo, the penetration depth of photons mainly depends on the absorption and scattering of tissue. Because the wavelength of the light source of traditional is mostly in the visible light region, the tissue penetration of photon is weak and easily interfered by endogenous substances; thus, they cannot be used in the photodynamic therapy of deep tumors (Mansoori et al., 2019; Xiao et al., 2018). Correspondingly, the near-infrared (NIR) light has low absorption and strong scattering in biological tissues, indicating that it has a large optical penetration depth. In the range of 650–950 nm, the NIR radiation absorption of tissue components is significantly lower than that of visible light, which is the “first optical window” (NIR-I). The NIR-II window for bioimaging applications is typically from 1000 to 1700 nm (Cao et al., 2019). At the same time, the factors influencing clinical PDT include the intensity of the light source and light frequency. Hence, specific excitation wavelengths need to be selected according to the tumor type and PS for PDT.
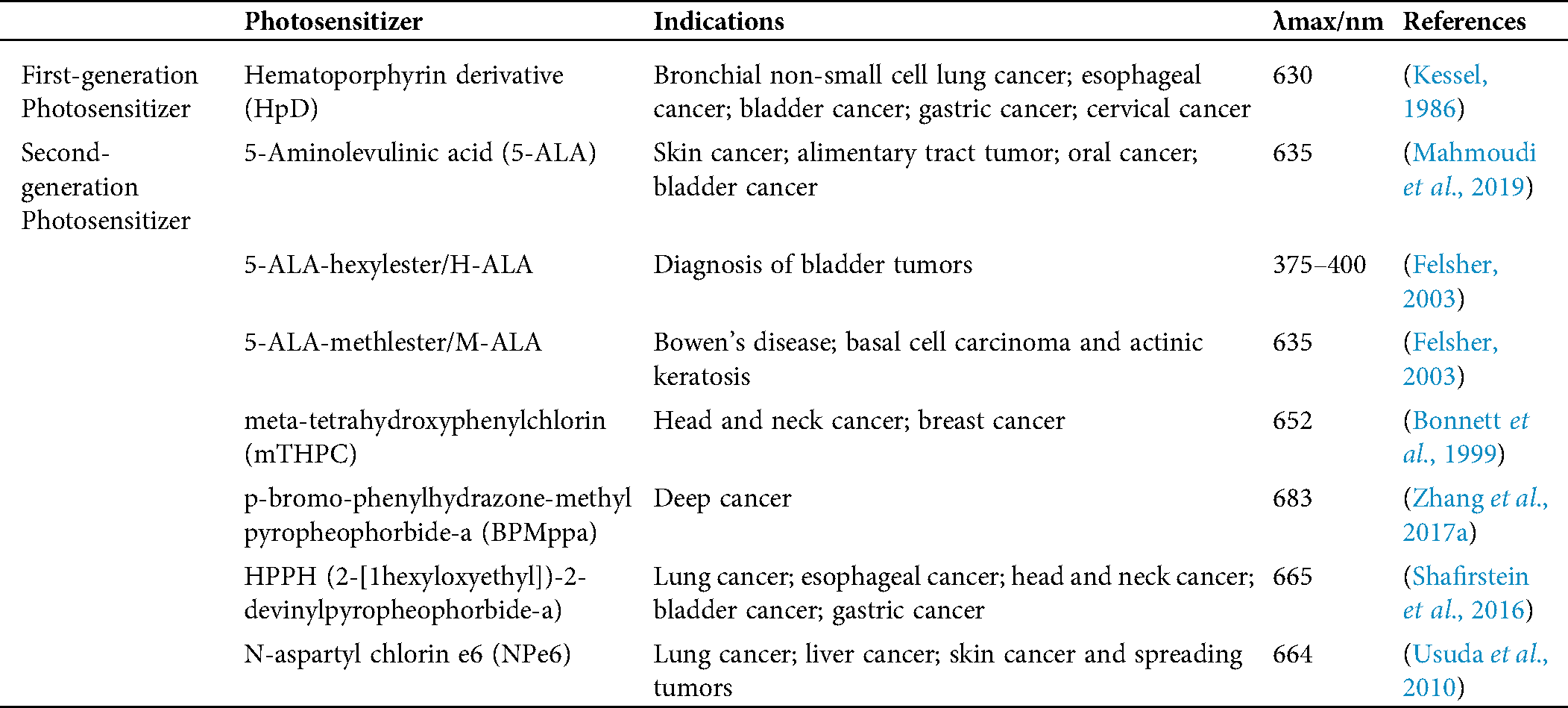
A PS is a photosensitive compound that can transit from the ground state to the excited state after being irradiated at a specific wavelength. As the core element of PDT, the characteristics of the PS determine the therapeutic effect. Currently, many PSs belonging to the first and second generations have been approved by the FDA for clinical treatment and trials (Tab. 1).
The first-generation PS, hematoporphyrin derivative (HpD), was isolated and identified in 1980 (Zhang et al., 2018a). Compared with healthy cells, tumor cells can absorb more PS; thus, HpD was considered a suitable cancer diagnosis and PDT drug for a long period (Gray et al., 1967). Peng and his group (Peng et al., 2016) combined HpD with calcitriol to provide an effective and selective way to improve the treatment of breast cancer cells by PDT. However, HpD also has disadvantages such as a long half-life, poor tissue permeability, a low chemical purity, a weak effect on deep pathological tissues, and a long blood circulation time, leading to a poor therapeutic effect and limiting its wide clinical application (Li et al., 2018b; Zhang et al., 2018a).
The second-generation PS is an optimized monomer compound developed based on the first-generation PS, and most of them are heterocyclic porphyrins (Morgan, 1989; Morgan et al., 1985), including bacteriochlorins (Ethirajan et al., 2011), bacterial chlorophyll (Zhang et al., 2017a), chlorin (Zhou et al., 2016), protoporphyrins (Singh et al., 2015), phthalocyanines (Kinsella et al., 2001), and their derivatives. Some compounds also have a non-porphyrin structure, such as 5-aminolevulinic acid, curcumin, quinone, phenothiazine, and psoralen (Harris and Pierpoint, 2012). Most of the porphyrin PS have been developed from the structure of hematoporphyrin, mainly including chlorine and chlorophyll derivatives. For PDT to act on deeper tumor tissues during treatment, modifying groups on porphyrin rings will shift the maximum absorption wavelength of PS toward the NIR region (James et al., 2013). Chlorin e6 (Ce6) is a type of natural chlorophyll extracted from algae and silkworm sand that, is obtained via a series of separation, purification, and modification steps (James et al., 2018). As an excellent new PS, Ce6 has a maximum absorption wavelength of approximately 660 nm and has fewer side effects on healthy tissues, making it a good choice for PDT. Feng et al. (2019) developed a co-delivery albumin nano-system based on bovine serum albumin, cyclopamine and diselenide-containing amphiphilic hyaluronic acid-chlorin e6 polymers. This nano-system had the advantages of targeting tumor cells and releasing Ce6 through redox reaction in the cell and proved its anti-tumor efficacy in in vitro and in vivo experiments. 5-ALA is a non-fluorescent endogenous biochemical substance in PS with a non-porphyrin structure (Harris and Pierpoint, 2012; He et al., 2017a; Mahmoudi et al., 2019), serving as a precursor to the synthesis of protoporphyrin IX (PpIX), which is photosensitive in vivo and does not accumulate in large quantities under normal conditions. However, PpIX is generated in rapidly dividing cells in vivo in the presence of a large amount of exogenous 5-ALA, and PDT could be performed after light exposure (Arnaut et al., 2014). The advantages of 5-ALA include its relatively fixed excitation wavelength, lower toxicity, higher enrichment in tumor tissues, and rapid removal from healthy tissues and plasma (Champeau et al., 2019). Presently, ALA-mediated photodynamic therapy has been widely used in neoplastic and non-tumor skin diseases, such as psoriasis (Yi et al., 2019), acne (Barbaric et al., 2018), squamous cell carcinoma (Morton and Braathen, 2018), basal cell carcinoma (Trafalski et al., 2019), and mycosis fungoides (Han et al., 2016). However, the second-generation of PS cannot be injected intravenously due to their poor water solubility (Lucky et al., 2015).
Table 1: First and second-generation photosensitizers approved for clinical use or undergoing clinical trials
Photosensitizers have many innate advantages, but their inherent disadvantages greatly limit their development space in medicine. More specifically, most PSs have a lower solubility in water and higher polymerization capacity, resulting in a lower photodynamic activity. Additionally, these PSs lack selectivity toward diseased tissue or tumor cells, causing serious side effects in humans (Li et al., 2017b; Zhang et al., 2020). However, activatable PSs can be designed to be activated only in the context of specific tumor markers or the tumor microenvironment to effectively avoid light damage to normal tissue and skin, thus improving the precision of PDT (Liu and Li, 2020). Therefore, the development of third-generation PS mostly focuses on novel derivatives of second-generation PSs that enlarges the functional group or molecule, such as linking biospecific targeted molecules (Abrahamse and Hamblin, 2016), binding to molecules that increase biocompatibility (Li et al., 2018b), introducing complexes formed from compounds with specific physiological functions (Li et al., 2017a), and loading on multifunctional carriers (Chilakamarthi and Giribabu, 2017; Shen et al., 2016). They accumulate less in healthy tissues, reducing the effective concentration, and improving safety (Kataoka et al., 2017). Ruiyun Zhang and others (Zhang et al., 2016b) treated breast cancer with nanoparticles (NPs), which are formed by the self-assembly of Ce6 and DOX. Thus, a new type of carrier-free nano-drug was manufactured that plays a synergistic role in tumor treatment. Molina et al. (2016) adopted the method of nanoprecipitation to obtain abundant human serum albumin (HSA) NPs with a diameter of about 295 ± 5 nm, loaded with Ce6, which can reduce the extracellular residence time of the NPs, and play a key role in the drug metabolism and transportation. Core-shell NPs with liposome and polymer compounds, which were loaded with PS, chemotherapeutic drug oxaliplatin, and programmed death-1 (PD-1) antibody, were synthesized by He et al. (2016); in subsequent experiments, the synergistic effect of PDT, chemotherapy, and immunotherapy on colon cancer was confirmed. A novel nanocluster was synthesized by Cai et al. (2020) that can be hydrolyzed by the tumor-specific carboxylesterase (CE) and penetrate through the deep tumor. PDT can be promoted by enhancing the singlet oxygen generation capacity. Su’s team (Su et al., 2019b) presented a strategy using an amphiphilic diblock copolymer and a stimulus-responsive dye as components to distinguish between healthy and diseased cells. Zhai’s team (Zhai et al., 2019) successfully developed a new class of activated photosensitizers to achieve the transformation of photosensitivity performance from complete inhibition to stress initiation.
A PS has two ground state electrons that rotate in opposite directions (Zhang and Feng, 2018). When the light absorbed by a PS is excited by appropriate energy, an electron will be excited into a higher energy orbit, forming an excited state (Moan et al., 1998; Ochsner, 1997). Excited PSs are extremely unstable. After reacting with surrounding oxygen molecules, they can react with non-oxygen substrates, transfer protons or electrons to generate free radicals, and further interact with ground oxygen to generate ROS (Type I reaction) (Hong et al., 2016; Hu et al., 2018). However, activated PSs can also directly react with the ground state oxygen and then return to the ground state, transfer energy to the ground state oxygen to generate ROS (Type II reaction) (Lucky et al., 2015). Regardless of the Type I reaction or Type II reaction, a large amount of ROS will be produced, leading to apoptosis and necrosis after oxidative stress (Abrahamse and Hamblin, 2016; Gdovin et al., 2017; Jiang et al., 2019; Mansoori et al., 2019; Xiao et al., 2018) (Fig. 2). In the presence of oxygen, most PSs work primarily in Type II reactions. At the same time, it is generally believed that the photodynamic destruction of 1O2 is dominant. Thus, Type II reaction is considered the main reaction in PDT (Lange and Bednarski, 2016; Xiao et al., 2018; Zou et al., 2020).
Reactive oxygen species are by-products of the body’s metabolism in an aerobic environment. During the process of oxidative phosphorylation of mitochondria to produce ATP, oxygen molecules are generated through Complex I and Complex III of the inner mitochondrial membrane (IMM) respiratory chain (Battogtokh et al., 2018; Zhang et al., 2018b). When the body is in a pathological state, electrons escape from the mitochondrial respiratory chain, and oxygen molecules are catalyzed by peroxidase to form peroxide ions or superoxide ions. After continuous transformation in the cell, oxygen radicals with active chemical reactions are formed, including superoxide radicals (·O2−), hydroxyl radicals (·OH), hydrogen peroxide (H2O2), and singlet oxygen (1O2) (Battogtokh et al., 2018; Dryden, 2018). Moreover, cytochromeP450 (CYP450), lipoxidase (LOX), cyclooxygenase (COX), and xanthine oxidase (XO) are the pathways of endogenous ROS production (Bouzid et al., 2015; Costa et al., 2016). Some external environments (such as drugs, chemical pollutants, and radiation therapy) can also induce the production of exogenous ROS.
Under normal circumstances, the oxidation and anti-oxidation systems in the cell are in equilibrium (Fig. 3). On the one hand, ROS are continuously generated during the metabolism of healthy cells and participates in many physical functions, such as defense functions and the synthesis of specific physiologically active substances. On the other hand, even if a large amount of ROS is synthesized, it will be adjusted to balance by the body’s enzyme and non-enzyme systems. ROS enzymatic scavengers mainly comprise antioxidant enzymes, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and glutathione S-transferase (GST). They will continuously clear the ROS produced in the cells (He et al., 2017b). Non-enzymatic ROS scavengers can provide an electron to oxygen free radicals to change their unpaired state to neutralize it, such as glutathione (GSH), thioredoxin (TrxA), vitamin C, and vitamin E (Pizzino et al., 2017).
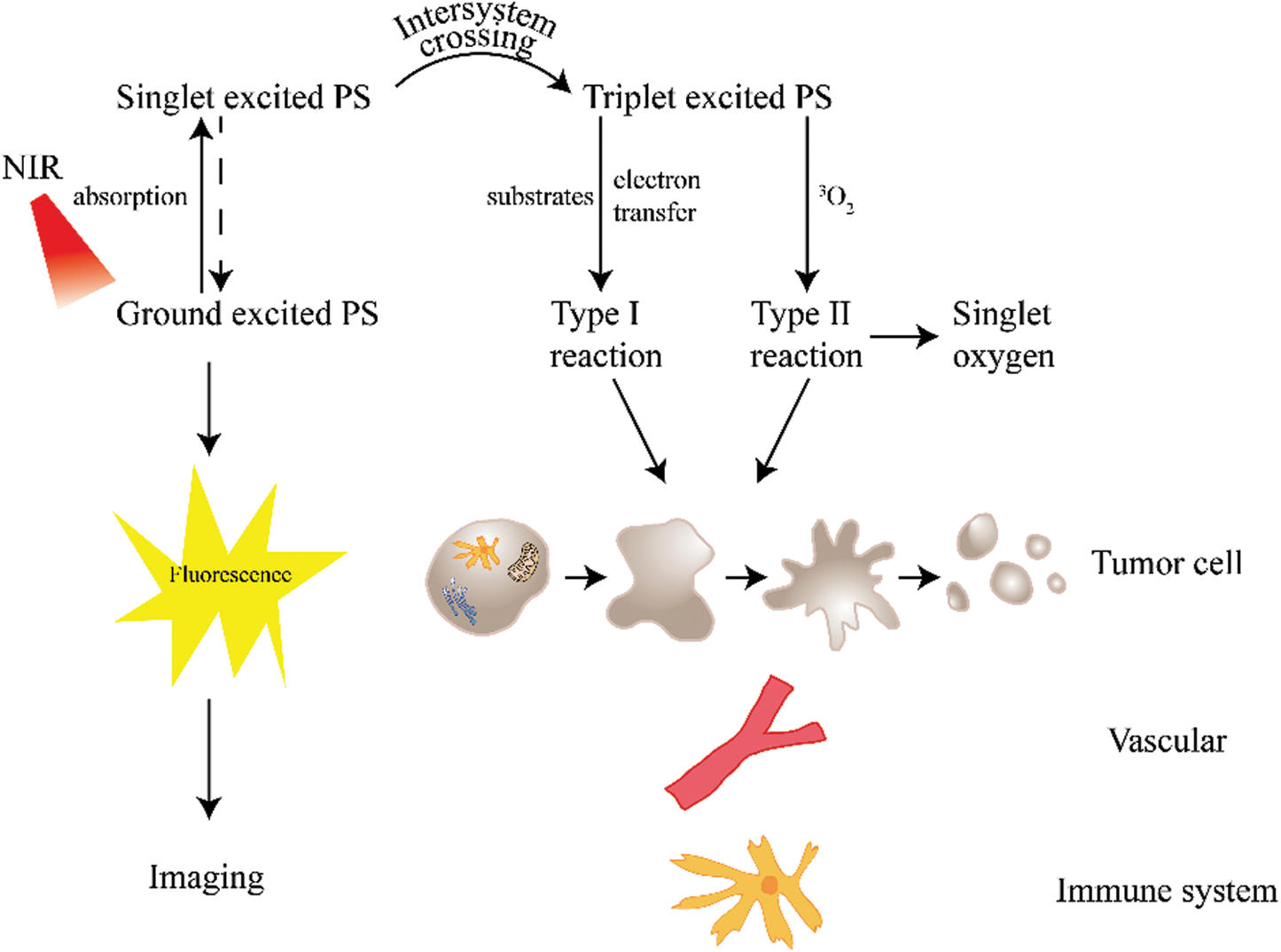
Figure 2: Mechanism of action of PDT.
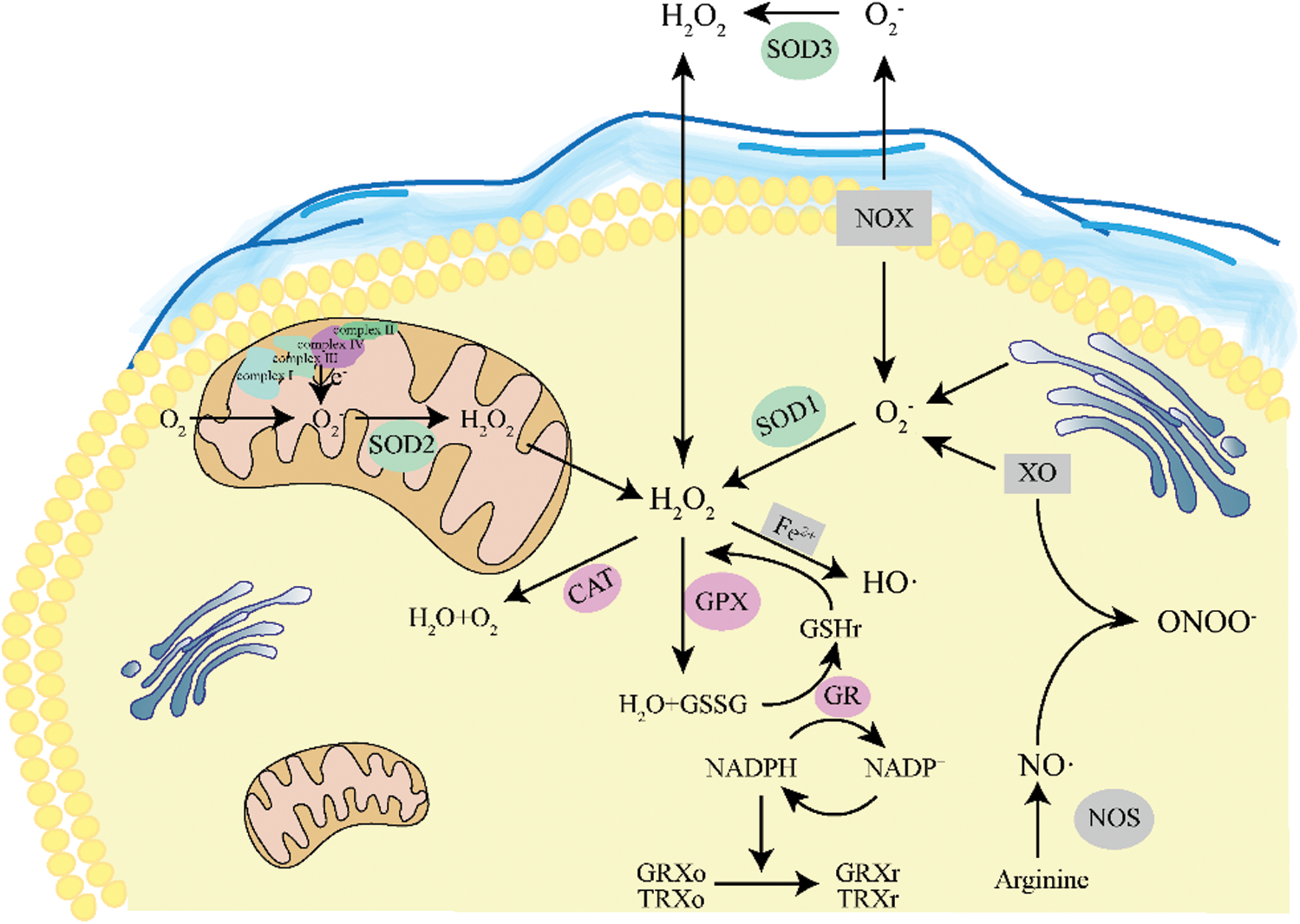
Figure 3: Schematic diagram of intracellular redox.
However, when the body is in a state of disease or stimulated by foreign substances, this dynamic balance may be affected. Excessive ROS accumulation and weakening of the antioxidant capacity will lead to mitochondria dysfunction, which will lead to oxidative stress (Diaz-Vivancos et al., 2015) and excessive oxidation of DNA (Milisav et al., 2018; Singh et al., 2019; Taverne et al., 2018), proteins (Sies, 2018) and lipids (Su et al., 2019a) in cells, inducing cancer (Klaunig, 2018; Moloney and Cotter, 2018). Kasai and Nishimura (Kasai, 1984) first reported the formation of 8-OHdG by oxygen radicals in 1984. ROS attack the 8th carbon atom of the guanine nucleobase in the DNA molecule to produce 8-hydroxy-2 deoxyguanosine (8-OHdG), leading to the translocation of G:C→T:A, which mediates the activation of proto-oncogenes or inactivation of tumor suppressor genes and is involved in tumorigenesis. Chernigina’s team (Chernigina et al., 2017) used the DNA comet assay to measure the DNA levels of leukocytes in tumor-carrying rats treated with PDT to indirectly assess and predict the rate of malignant tumor growth. ROS will change the tertiary structure of proteins, facilitate the formation of protein-protein cross-linking, and degrade proteins and inactivate enzymes. Polyunsaturated fatty acids (PUFAs) in the phospholipid of biofilm are very sensitive to ROS attack due to the multiple double bonds with active chemical properties. The oxidation and decomposition of lipids easily occur, causing damage to cells and leading to cell death and body damage (Hauck et al., 2019).
Mechanism of Action of Photodynamic Therapy
Studies have shown that the anti-tumor effect of PDT stems from three interrelated mechanisms: (1) Direct cytotoxic effect of ROS on tumor cells; (2) Targeting the tumor vasculature, leading to vascular damage; and (3) Activating the immune system, inducing and regulating immune responses (Lan et al., 2019; Van Straten et al., 2017). Cell death and apoptosis mediated by ROS are the fundamental mechanisms for the effect of PDT (Dobson et al., 2018; Olsen et al., 2017; Zou et al., 2017).
Cytotoxic effects of reactive oxygen species
Gapeyev et al. (2001) proposed that calcium channels of the plasma membrane could be chosen as the target by external periodic signals under the influence of some external factors. A high concentration of ROS can lead to the opening of the mitochondrial membrane permeability transition pore (mPTP), causing a reduced the mitochondrial membrane potential (Δψm), the release of cytochrome C, the triggering of the caspase cascade, the destruction of DNA, and accelerate of cell apoptosis. A close relationship exists between the increased ROS content and increased Ca2+ concentration in the process of apoptosis (Bertero and Maack, 2018; Zhang et al., 2016a; Zhang et al., 2016c). Cao’s study (Cao et al., 2011) showed that, in the apoptosis pathway of breast cancer cells, the Ca2+ released in the endoplasmic reticulum and mitochondria was caused by ROS generation, increased Ca2+ resulting in the opening of mPTP and decreased of Δψm. The opening of mPTP and decreased Δψm increased the content of Ca2+ in the mitochondria, further intensifying the opening of the pores in the form of positive feedback and amplifying the apoptosis signal in turn, thus forming a cycle favorable to the mitochondrial apoptosis pathway.
As a multi-potent cytokine, tumor necrosis factor-alpha (TNF-α) not only induces cell differentiation, survival, and apoptosis but also changes the intracellular redox state in the cell and increases ROS content (Blaser et al., 2016; Pinegin et al., 2018). TNF-α can change the structure of mitochondrial membrane receptors, inhibit the initial stage of the respiratory chain, and leak unpaired electrons to oxygen molecules so that the intracellular mitochondrial permeability and ROS increase, eventually leading to apoptosis (Zhang et al., 2017b). NOX is one of the most important enzymes responsible for ROS formation. It generates superoxide through the assembly of a multisubunit protein complex utilizing nicotinamide adenine dinucleotide (NADH) or nicotinamide adenine dinucleotide phosphate (NADPH) as the electron donor (Musicki et al., 2010). Studies have shown that FasL can activate Racl protein expression by regulating Ras signaling, and Racl protein can transfer electrons from NADPH to oxygen molecules to form ·O2−. The expression of inhibitor of apoptosis proteins was then down-regulated by the ROS generated through the ubiquitination pathway, causing the activation of downstream caspase-8 and cell apoptosis (Wang, 2008).
Blood vessels carry nutrients and oxygen to tumor cells, which are essential for tumors to survive and metastasize to other parts of the body. The large blood vessel wall space, poor structure, and insufficient lymphatic circulation in tumor tissue are conducive to PS accumulation (Baluk et al., 2005). However, the tumor area surrounding the normal vasculature may help remove PS. Many specific receptors have been identified in vascular endothelial cells, and the binding of PS to specific vectors such as albumin, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) will enhance the affinity of receptors and uptake of endothelial cells (Bin et al., 2006). When the light source irradiates the PS, singlet oxygen acts on the blood vessel and produces a series of physiological responses such as leukocyte adhesion, platelet aggregation, increased vascular permeability, vasoactive molecule release, and vasoconstriction to prevent tumor vascular remodeling (Dolmans et al., 2002; Hamblin and Abrahamse, 2020; Yanovsky et al., 2019). Vascular endothelial growth factor (VEGF) is a key protein that is a major signaling molecule of hypoxic cells (Chatterjee et al., 2013); PDT can down-regulate VEGF (Bucher et al., 2014; Tong et al., 2016), leading to blood stasis, vascular obstruction, and the lack of oxygen and nutrition in tumor tissue, to achieve the photodynamic treatment effect of the tumor. Aleksandra et al. (Kawczyk-Krupka et al., 2018) confirmed that, under hypoxia conditions, ALA-PDT reduces the release of VEGF in SW620 colon cancer cell line, producing an anti-angiogenesis effect.
Photodynamic therapy also triggers an immune response when it induces necrosis of the tumor and its vasculature. PDT activates the body’s innate immunity by triggering inflammation-related signaling pathways, promoting cytokine release, inducing neutrophils and macrophages to accumulate at the tumor site, and activating complement (Maeding et al., 2016; Nath et al., 2019; Showalter et al., 2017). At the same time, macrophages present tumor antigens to CD4+ T lymphocytes by phagocytosis of the killed tumor cells and then activates CD8+ T lymphocytes to induce necrosis and apoptosis of nearby tumor cells, further stimulating specific anti-tumor immunity (Dobson et al., 2018; Wachowska et al., 2015b; Yang et al., 2016). The immune response may occur not only in the PDT site but also in local and distant lymphoid tissues. Kousis’s team (Kousis et al., 2007) reported that PDT induces neutrophilic infiltration into the tumor cells and enhances the strength of the tumor-specific primary immune response and establishment of memory antitumor immune responses. Canti et al. (2010) confirmed the mechanism by which tumor-bearing mice treated with PDT induce a specific immune response against reattack by tumor cells. Therefore, compared with the immunosuppressive effects of chemotherapy, radiotherapy, and other treatment methods, PDT shows superiority to activate the immune response and control tumor recurrence.
Challenges and Future Perspectives
The anti-cancer effect of PDT has been discovered for more than a century. PDT, which is a non-invasive and harmless, involving tumor phototherapy tools, plays an increasingly important role in cancer treatment. However, due to complex biological systems and individual differences in patients, different measures should be taken in different clinical situations, such as, changing the light source, designing new photosensitizers to relieve tumor hypoxia microenvironment and combining chemotherapy, photothermal therapy (PTT), and immunotherapy (Liang et al., 2020). It will be a long time and challenge before PDT becomes a first-line treatment strategy for cancer. Researchers hope that synergistically enhanced interactions will have an effect greater than the sum of the treatments, improving the overall outcome and even achieving the optimal outcome of eradicating malignant solid tumors.
The depth of light penetration is one of the prevalent limitations of the applications PDT. Thus, in recent years, the efficacy of PDT has been improved by using electromagnetic radiation X-rays or fluorescence rays (Mallidi et al., 2016). A series of photodynamic reactions occurs after a PS absorbs light of a specific wavelength, but different irradiation schemes will also produce different results under the same light source. Photons at high fluence rates of light transmission can penetrate the skin to about 3 mm of subcutaneous tissue, but the oxygen content of tumor tissue can also be consumed too quickly. Therefore, the light dosage regimen may also affect the anti-tumor effect, and the optimal dosage regimen may depend on the situation (Keereweer et al., 2014).
Although the enhanced permeability and retention effect (EPR) can increase the concentration of PSs in tumors, the size distribution, morphology, and surface modification can affect the location. HSA consists of three homologous domains, two of which are the major drug binding sites. In clinical application, PS can be administered intravenously or locally, and different PSs will bind to HSA at different sites, so their distribution and pharmacokinetics will change accordingly. From the moment the drug is administered until the PS reaches the target location, various physical, chemical, and biological events occur that together affect the final location of PS.
The Type I reaction in the PDT process and limited range of singlet oxygen causes a hypoxia microenvironment of the tumor, which enhances the resistance of the tumor to PDT. The combination of PS and a catalyst can alleviate hypoxia. Li et al. (2018a) assembled a new type of nanostructured phthalocyanine that can efficiently promote ROS production through a Type I reaction. Luo’s team (Luo et al., 2020) designed a nano-scale metal-organic framework based on bacterial chlorine, which can perform efficient PDT through Type I and Type II reactions. Deng et al. (2020) synthesized IR780 and catalase-co-loaded liposomes and promoted tumor oxygenation by utilizing the high catalytic efficiency of CAT when it encountered H2O2.
Simple PDT treatment cannot eradicate solid tumors accurately and completely due to its limitations. Chemotherapy drugs inhibit cell division and cause cancer cell death. However, the serious side effects of the drug on the whole body limit its clinical application. Chen et al. (2017) described the covalent coupling of HSA to Ce6 after the self-assembly and encapsulation of catalase and the chemotherapy drug paclitaxel (PTX). This effect significantly regulates TME and enhances the anti-tumor effect.
Photothermal therapy is a technique that converts energy from near-infrared radiation absorbed by certain molecules into heat that destroys cancer cells. Different from PDT using external ROS and other oxygen free radicals to kill cancer cells, PTT uses the combination of light and a special light absorption system to induce local overheat, leading to cell death. Li Wei’s team (Li et al., 2019) generated a nanosystem comprising indocyanine green conjugated to hollow gold nanospheres and oxygen-transporting hemoglobin liposomes that modify targeted peptides to achieve PDT and PTT.
The immune response induced by PDT involves almost all aspects of the immune system, which may become the target of treatment. Gollnick et al. (2002) proposed that, unlike other traditional vaccines, the PDT-generated tumor cell lysates do not require a combination of adjuvants. Ishida et al. (2016) used a monoclonal antibody that binds to epidermal growth factor receptors (EGFRs) with PS to allow deeper penetration into tissue and specifically target and eliminate tumors.
The importance of this review is to briefly introduce the development process, treatment mechanism, current challenges, and prospects for the future of photodynamic therapy. PDT has made great progress with its unique advantages; however, its full potential has yet to be shown. Additionally, the photodynamic reaction is a complex process involving molecular, subcellular, and vascular changes that cause tumor necrosis or apoptosis. There are still many problems in its mechanism of action and clinical application, and clinicians still use a double-edged sword to target and treat tumors. However, as nano-medicine, biology and cutting-edge optical technology development and integration, researchers are working on mechanisms to improve the anti-tumor effect, while reducing the side effects and improving the safety of patients. The combination of knowledge from these fields will ultimately lead to the development of a powerful and immediate treatment for cancer.
We hope this work will raise awareness of the cellular biomechanical aspects of PDT in health and disease, as a direction for the future development of PDT in combination with other therapies, individualized treatment planning, and real-time monitoring, let it play an increasingly important role in the treatment of various malignant tumors and becoming one of the important means of clinical treatment.
Author Contribution: Zixuan Wang, Hongmei Peng and Shi Wei wrote the paper and prepared figures and table. Lu Gan, Linlin Xie and Pan Wu conceived the review. Liping Zhong, Jian He and Yongxiang Zhao reviewed drafts of the paper. Zhiming Deng, Hongliang Tang and Yong Huang analyzed and corrected the paper. All authors read and approved the final paper.
Ethics Approval: Not applicable.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Funding Statement: This review was supported by the National Natural Science Foundation of China (No. 82072340), the Major National Science and Technology Projects–Major New Drug Creation (2019ZX09301-132); Changjiang Scholars and Innovative Research Team in University (No. IRT_15R13); Guangxi Science and Technology Base and Talent Special Project (No. AD17129003).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Morgan AR. (1989). New sensitizers for photodynamic therapy: Controlled synthesis of purpurins and their effect on normal tissue. Journal of Medicinal Chemistry 4: 904–908. [Google Scholar]
Abrahamse H, Hamblin MR. (2016). New photosensitizers for photodynamic therapy. Biochemical Journal 473: 347–364. DOI 10.1042/BJ20150942. [Google Scholar] [CrossRef]
Ancona A, Dumontel B, Garino N, Demarco B, Chatzitheodoridou D, Fazzini W, Engelke H, Cauda V. (2018). Lipid-coated zinc oxide nanoparticles as innovative ROS-generators for photodynamic therapy in cancer cells. Nanomaterials (Basel) 8: 143. DOI 10.3390/nano8030143. [Google Scholar] [CrossRef]
Arnaut LG, Pereira MM, Dabrowski JM, Silva EF, Schaberle FA, Abreu AR, Rocha LB, Barsan MM, Urbanska K, Stochel G, Brett CM. (2014). Photodynamic therapy efficacy enhanced by dynamics: The role of charge transfer and photostability in the selection of photosensitizers. Chemistry-A European Journal 20: 5346–5357. DOI 10.1002/chem.201304202. [Google Scholar] [CrossRef]
Balcer-Kubiczek EK, Eley JG. (2018). Secondary malignancies in the era of high-precision radiation therapy. Critical Reviews in Oncogenesis 23: 93–112. DOI 10.1615/CritRevOncog.2018025830. [Google Scholar] [CrossRef]
Baluk P, Hashizume H, Mcdonald DM. (2005). Cellular abnormalities of blood vessels as targets in cancer. Current Opinion in Genetics & Development 15: 102–111. DOI 10.1016/j.gde.2004.12.005. [Google Scholar] [CrossRef]
Barbaric J, Abbott R, Posadzki P, Car M, Gunn LH, Layton AM, Majeed A, Car J. (2018). Light therapies for acne: Abridged Cochrane systematic review including GRADE assessments. British Journal of Dermatology 178: 61–75. DOI 10.1111/bjd.15495. [Google Scholar] [CrossRef]
Baskaran R, Lee J, Yang SG. (2018). Clinical development of photodynamic agents and therapeutic applications. Biomaterials Research 22: 25. DOI 10.1186/s40824-018-0140-z. [Google Scholar] [CrossRef]
Battogtokh G, Choi YS, Kang DS, Park SJ, Shim MS, Huh KM, Cho YY, Lee JY, Lee HS, Kang HC. (2018). Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: Current strategies and future perspectives. Acta Pharmaceutica Sinica B 8: 862–880. DOI 10.1016/j.apsb.2018.05.006. [Google Scholar] [CrossRef]
Bertero E, Maack C. (2018). Calcium signaling and reactive oxygen species in mitochondria. Circulation Research 122: 1460–1478. DOI 10.1161/CIRCRESAHA.118.310082. [Google Scholar] [CrossRef]
Bin C, W. PB, Jack HP, Tayyaba H. (2006). Vascular and cellular targeting for photodynamic therapy. Critical Reviews in Eukaryotic Gene Expression 16: 279–306. DOI 10.1615/CritRevEukarGeneExpr.v16.i4.10. [Google Scholar] [CrossRef]
Blaser H, Dostert C, Mak TW, Brenner D. (2016). TNF and ROS crosstalk in inflammation. Trends in Cell Biology 26: 249–261. DOI 10.1016/j.tcb.2015.12.002. [Google Scholar] [CrossRef]
Bonnett R, Charlesworth P, Djelal BD, Foley S, Mcgarvey DJ, Truscott TG. (1999). Photophysical properties of 5,10,15,20-tetrakis(m-hydroxyphenyl)porphyrin (m-THPP5,10,15,20-tetrakis(m-hydroxyphenyl)chlorin (m-THPC) and 5,10,15,20-tetrakis(m-hydroxyphenyl)bacteriochlorin (m-THPBCA comparative study. Journal of Photochemistry and Photobiology B: Biology 53: 136–143. DOI 10.1016/S1011-1344(99)00139-6. [Google Scholar] [CrossRef]
Bouzid MA, Filaire E, Mccall A, Fabre C. (2015). Radical oxygen species, exercise and aging: An update. Sports Medicine 45: 1245–1261. DOI 10.1007/s40279-015-0348-1. [Google Scholar] [CrossRef]
Bucher F, Bi Y, Gehlsen U, Hos D, Cursiefen C, Bock F. (2014). Regression of mature lymphatic vessels in the cornea by photodynamic therapy. British Journal of Ophthalmology 98: 391–395. DOI 10.1136/bjophthalmol-2013-303887. [Google Scholar] [CrossRef]
Cai Y, Ni D, Cheng W, Ji C, Wang Y, Mullen K, Su Z, Liu Y, Chen C, Yin M. (2020). Enzyme-triggered disassembly of perylene monoimide-based nanoclusters for activatable and deep photodynamic therapy. Angewandte Chemie 132: 14118–14122. DOI 10.1002/ange.202001107. [Google Scholar] [CrossRef]
Canti G, Calastretti A, Bevilacqua A, Reddi E, Palumbo G, Nicolin A. (2010). Combination of photodynamic therapy + immunotherapy + chemotherapy in murine leukiemia. Neoplasma 57: 184–188. DOI 10.4149/neo_2010_02_184. [Google Scholar] [CrossRef]
Cao J, Zhu B, Zheng K, He S, Meng L, Song J, Yang H. (2019). Recent progress in NIR-II contrast agent for biological imaging. Frontiers in Bioengineering and Biotechnology 7: 487. DOI 10.3389/fbioe.2019.00487. [Google Scholar] [CrossRef]
Cao XH, Zhao SS, Liu DY, Wang Z, Niu LL, Hou LH, Wang CL, Cao XH, Zhao SS, Liu DY, Wang Z, Niu LL, Hou LH, Wang CL. (2011). ROS-Ca2+ is associated with mitochondria permeability transition pore involved in surfactin-induced MCF-7 cells apoptosis, ROS-Ca2+ is associated with mitochondria permeability transition pore involved in surfactin-induced MCF-7 cells apoptosis. Chemico-Biological Interactions 190: 16–27. DOI 10.1016/j.cbi.2011.01.010. [Google Scholar] [CrossRef]
Lange C, Bednarski PJ. (2016). Photosensitizers for photodynamic therapy: Photochemistry in the service of oncology. Current Pharmaceutical Design 22: 6956–6974. [Google Scholar]
Costa JPD, Vitorino R, Silva GM, Vogel C, Duarte AC, Rocha-Santos T. (2016). A synopsis on aging—Theories, mechanisms and future prospects. Ageing Research Reviews 29: 90–112. DOI 10.1016/j.arr.2016.06.005. [Google Scholar] [CrossRef]
Champeau M, Vignoud S, Mortier L, Mordon S. (2019). Photodynamic therapy for skin cancer: How to enhance drug penetration? Journal of Photochemistry and Photobiology B: Biology 197: 111544. DOI 10.1016/j.jphotobiol.2019.111544. [Google Scholar] [CrossRef]
Chatterjee S, Heukamp LC, Siobal M, Schottle J, Wieczorek C, Peifer M, Frasca D, Koker M, Konig K, Meder L, Rauh D, Buettner R, Wolf J, Brekken RA, Neumaier B, Christofori G, Thomas RK, Ullrich RT. (2013). Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. Journal of Clinical Investigation 123: 1732–1740. DOI 10.1172/JCI65385. [Google Scholar] [CrossRef]
Chen Q, Chen J, Liang C, Feng L, Dong Z, Song X, Song G, Liu Z. (2017). Drug-induced co-assembly of albumin/catalase as smart nano-theranostics for deep intra-tumoral penetration, hypoxia relieve, and synergistic combination therapy. Journal of Controlled Release 263: 79–89. DOI 10.1016/j.jconrel.2016.11.006. [Google Scholar] [CrossRef]
Cheong TC, Shin EP, Kwon EK, Choi JH, Wang KK, Sharma P, Choi KH, Lim JM, Kim HG, Oh K, Jeon JH, So I, Kim IG, Choi MS, Kim YK, Seong SY, Kim YR, Cho NH. (2015). Functional manipulation of dendritic cells by photoswitchable generation of intracellular reactive oxygen species. ACS Chemical Biology 10: 757–765. DOI 10.1021/cb5009124. [Google Scholar] [CrossRef]
Chernigina IA, Plekhanova ES, Scherbatyuk TG. (2017). The DNA comet assay for evaluating damage to leukocyte DNA after photodynamic therapy. Sovremennye Tehnologii v Medicine 9: 89. DOI 10.17691/stm2017.9.4.11. [Google Scholar] [CrossRef]
Chilakamarthi U, Giribabu L. (2017). Photodynamic therapy: Past, present and future. Chemical Record 17: 775–802. DOI 10.1002/tcr.201600121. [Google Scholar] [CrossRef]
Dabrowski JM, Arnaut LG. (2015). Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photochemical & Photobiological Sciences 14: 1765–1780. DOI 10.1039/C5PP00132C. [Google Scholar] [CrossRef]
Deng L, Sheng D, Liu M, Yang L, Ran H, Li P, Cai X, Sun Y, Wang Z. (2020). A near-infrared laser and H2O2 activated bio-nanoreactor for enhanced photodynamic therapy of hypoxic tumors. Biomaterials Science 8: 858–870. DOI 10.1039/C9BM01126A. [Google Scholar] [CrossRef]
Diaz-Vivancos P, De Simone A, Kiddle G, Foyer CH. (2015). Glutathione–linking cell proliferation to oxidative stress. Free Radical Biology and Medicine 89: 1154–1164. DOI 10.1016/j.freeradbiomed.2015.09.023. [Google Scholar] [CrossRef]
Dobson J, De Queiroz GF, Golding JP. (2018). Photodynamic therapy and diagnosis: Principles and comparative aspects. Veterinary Journal 233: 8–18. DOI 10.1016/j.tvjl.2017.11.012. [Google Scholar] [CrossRef]
Dolmans DEJGJ, Kadambi A, Hill JS, Waters CA, Robinson BC, Walker JP, Fukumura D, Jain RK. (2002). Vascular accumulation of a novel photosensitizer, MV6401, causes selective thrombosis in tumor vessels after photodynamic therapy. Cancer Research 62: 2151–2156. [Google Scholar]
Dryden M. (2018). Reactive oxygen species: A novel antimicrobial. International Journal of Antimicrobial Agents 51: 299–303. DOI 10.1016/j.ijantimicag.2017.08.029. [Google Scholar] [CrossRef]
Ethirajan M, Chen Y, Joshi P, Pandey RK. (2011). The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chemical Society Reviews 40: 340–362. DOI 10.1039/B915149B. [Google Scholar] [CrossRef]
Felsher DW. (2003). Cancer revoked: Oncogenes as therapeutic targets. Nature Reviews Cancer 3: 375–380. DOI 10.1038/nrc1070. [Google Scholar] [CrossRef]
Feng C, Chen L, Lu Y, Liu J, Liang S, Lin Y, Li Y, Dong C. (2019). Programmable Ce6 delivery via cyclopamine based tumor microenvironment modulating nano-system for enhanced photodynamic therapy in breast cancer. Frontiers in Chemistry 7: 853. DOI 10.3389/fchem.2019.00853. [Google Scholar] [CrossRef]
Gapeyev AB, Sokolov PA, Chemeris NK. (2001). Response of membrane-associated calcium signaling systems of the cell to extremely low-frequency external signals with different waveform parameters. Electro- and Magnetobiology 20: 107–122. DOI 10.1081/JBC-100103163. [Google Scholar] [CrossRef]
Gdovin MJ, Kadri N, Rios L, Holliday S, Jordan Z. (2017). Focal photodynamic intracellular acidification as a cancer therapeutic. Seminars in Cancer Biology 43: 147–156. DOI 10.1016/j.semcancer.2017.02.005. [Google Scholar] [CrossRef]
Gollnick SO, Vaughan L, Henderson BW. (2002). Generation of effective antitumor vaccines using photodynamic therapy. Cancer Research 62: 1604–1608. [Google Scholar]
Gong H, Chao Y, Xiang J, Han X, Song G, Feng L, Liu J, Yang G, Chen Q, Liu Z. (2016). Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Letters 16: 2512–2521. DOI 10.1021/acs.nanolett.6b00068. [Google Scholar] [CrossRef]
Gray MJ, Lipson R, Maeck JVS, Parker L, Romeyn D. (1967). Use of hematoporphyrin derivative in detection and management of cervical cancer. American Journal of Obstetrics and Gynecology 99: 766–771. DOI 10.1016/0002-9378(67)90392-4. [Google Scholar] [CrossRef]
Hamblin M, Abrahamse H. (2020). Factors affecting photodynamic therapy and anti-tumor immune response. Anti-Cancer Agents in Medicinal Chemistry 21: 123–136. [Google Scholar]
Han D, Xue J, Wang T, Liu Y. (2016). Observation of clinical efficacy of photodynamic therapy in 3 patients with refractory plaque-stage mycosis fungoides. Photodiagnosis and Photodynamic Therapy 16: 9–11. DOI 10.1016/j.pdpdt.2016.07.011. [Google Scholar] [CrossRef]
Harris F, Pierpoint L. (2012). Photodynamic therapy based on 5-aminolevulinic acid and its use as an antimicrobial agent. Medicinal Research Reviews 32: 1292–1327. DOI 10.1002/med.20251. [Google Scholar] [CrossRef]
Hauck AK, Huang Y, Hertzel AV, Bernlohr DA. (2019). Adipose oxidative stress and protein carbonylation. Journal of Biological Chemistry 294: 1083–1088. DOI 10.1074/jbc.R118.003214. [Google Scholar] [CrossRef]
He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, Lin W. (2016). Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nature Communications 7: 12499. DOI 10.1038/ncomms12499. [Google Scholar] [CrossRef]
He J, Yang L, Yi W, Fan W, Wen Y, Miao X, Xiong L (2017a). Combination of fluorescence-guided surgery with photodynamic therapy for the treatment of cancer. Molecular Imaging 16: 153601211772291. DOI 10.1177/1536012117722911. [Google Scholar] [CrossRef]
He L, He T, Farrar S, Ji L, Liu T, Ma X (2017b). Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cellular Physiology and Biochemistry 44: 532–553. DOI 10.1159/000485089. [Google Scholar] [CrossRef]
Hong EJ, Choi DG, Shim MS. (2016). Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharmaceutica Sinica B 6: 297–307. DOI 10.1016/j.apsb.2016.01.007. [Google Scholar] [CrossRef]
Hu F, Xu S, Liu B. (2018). Photosensitizers with aggregation-induced emission: Materials and biomedical applications. Advanced Materials 30: e1801350. DOI 10.1002/adma.201801350. [Google Scholar] [CrossRef]
Hwang HS, Shin H, Han J, Na K. (2018). Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. Journal of Pharmaceutical Investigation 48: 143–151. DOI 10.1007/s40005-017-0377-x. [Google Scholar] [CrossRef]
Ishida M, Kagawa S, Shimoyama K, Takehara K, Noma K, Tanabe S, Shirakawa Y, Tazawa H, Kobayashi H, Fujiwara T. (2016). Trastuzumab-based photoimmunotherapy integrated with viral HER2 transduction inhibits peritoneally disseminated HER2-negative cancer. Molecular Cancer Therapeutics 15: 402–411. DOI 10.1158/1535-7163.MCT-15-0644. [Google Scholar] [CrossRef]
James NS, Cheruku RR, Missert JR, Sunar U, Pandey RK. (2018). Measurement of cyanine dye photobleaching in photosensitizer cyanine dye conjugates could help in optimizing light dosimetry for improved photodynamic therapy of cancer. Molecules 23: 1842. DOI 10.3390/molecules23081842. [Google Scholar] [CrossRef]
Jiang C, Yang W, Wang C, Qin W, Ming J, Zhang M, Qian H, Jiao T. (2019). Methylene blue-mediated photodynamic therapy induces macrophage apoptosis via ROS and reduces bone resorption in periodontitis. Oxidative Medicine and Cellular Longevity 2019: 1529520. DOI 10.1155/2019/1529520. [Google Scholar] [CrossRef]
Kasai H. (1984). Detection and identification of mutagens and carcinogens as their adducts with guanosine derivatives. Nucleic Acids Research 12: 2127–2136. DOI 10.1093/nar/12.4.2127. [Google Scholar] [CrossRef]
Kataoka H, Nishie H, Hayashi N, Tanaka M, Nomoto A, Yano S, Joh T. (2017). New photodynamic therapy with next-generation photosensitizers. Annals of Translational Medicine 5: 183. DOI 10.21037/atm.2017.03.59. [Google Scholar] [CrossRef]
Kawczyk-Krupka A, Kwiatek B, Czuba ZP, Mertas A, Latos W, Verwanger T, Krammer B, Sieron A. (2018). Secretion of the angiogenic factor VEGF after photodynamic therapy with ALA under hypoxia-like conditions in colon cancer cells. Photodiagnosis and Photodynamic Therapy 21: 16–18. DOI 10.1016/j.pdpdt.2017.10.020. [Google Scholar] [CrossRef]
Keereweer S, Van Driel PB, Robinson DJ, Lowik CW. (2014). Shifting focus in optical image-guided cancer therapy. Molecular Imaging and Biology 16: 1–9. DOI 10.1007/s11307-013-0688-x. [Google Scholar] [CrossRef]
Kelly JF, Snell ME, Berenbaum MC. (1975). Photodynamic destruction of human bladder carcinoma. British Journal of Cancer 31: 237–244. DOI 10.1038/bjc.1975.30. [Google Scholar] [CrossRef]
Kessel D. (1986). Photosensitization with derivatives of haematoporphyrin. International Journal of Radiation Biology and Related Studies in Physics, Chemistry and Medicine 49: 901–907. DOI 10.1080/09553008514553131. [Google Scholar] [CrossRef]
Kinsella TJ, Colussi VC, Oleinick NL, Sibata CH. (2001). Photodynamic therapy in oncology. Expert Opinion on Pharmacotherapy 2: 917–927. DOI 10.1517/14656566.2.6.917. [Google Scholar] [CrossRef]
Klaunig JE. (2018). Oxidative stress and cancer. Current Pharmaceutical Design 24: 4771–4778. DOI 10.2174/1381612825666190215121712. [Google Scholar] [CrossRef]
Kousis PC, Henderson BW, Maier PG, Gollnick SO. (2007). Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Research 67: 10501–10510. DOI 10.1158/0008-5472.CAN-07-1778. [Google Scholar] [CrossRef]
Lan M, Zhao S, Liu W, Lee CS, Zhang W, Wang P. (2019). Photosensitizers for photodynamic therapy. Advanced Healthcare Materials 8: e1900132. DOI 10.1002/adhm.201900132. [Google Scholar] [CrossRef]
Li W, Tan G, Zhang H, Wang Z, Jin Y (2017a). Folate chitosan conjugated doxorubicin and pyropheophorbide acid nanoparticles (FCDP–NPs) for enhance photodynamic therapy. RSC Advances 7: 44426–44437. DOI 10.1039/C7RA08757H. [Google Scholar] [CrossRef]
Li W, Yang J, Luo L, Jiang M, Qin B, Yin H, Zhu C, Yuan X, Zhang J, Luo Z, Du Y, Li Q, Lou Y, Qiu Y, You J. (2019). Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nature Communications 10: 3349. DOI 10.1038/s41467-019-11269-8. [Google Scholar] [CrossRef]
Li X, Kolemen S, Yoon J, Akkaya EU (2017b). Activatable photosensitizers: Agents for selective photodynamic therapy. Advanced Functional Materials 27: 1604053. DOI 10.1002/adfm.201604053. [Google Scholar] [CrossRef]
Li X, Lee D, Huang JD, Yoon J (2018a). Phthalocyanine-assembled nanodots as photosensitizers for highly efficient Type I photoreactions in photodynamic therapy. Angewandte Chemie International Edition 57: 9885–9890. DOI 10.1002/anie.201806551. [Google Scholar] [CrossRef]
Li X, Lee S, Yoon J (2018b). Supramolecular photosensitizers rejuvenate photodynamic therapy. Chemical Society Reviews 47: 1174–1188. DOI 10.1039/C7CS00594F. [Google Scholar] [CrossRef]
Liang C, Zhang X, Wang Z, Wang W, Yang M, Dong X. (2020). Organic/inorganic nanohybrids rejuvenate photodynamic cancer therapy. Journal of Materials Chemistry B 8: 4748–4763. DOI 10.1039/D0TB00098A. [Google Scholar] [CrossRef]
Liu M, Li C. (2020). Recent advances in activatable organic photosensitizers for specific photodynamic therapy. ChemPlusChem 85: 948–957. DOI 10.1002/cplu.202000203. [Google Scholar] [CrossRef]
Lucky SS, Soo KC, Zhang Y. (2015). Nanoparticles in photodynamic therapy. Chemical Reviews 115: 1990–2042. DOI 10.1021/cr5004198. [Google Scholar] [CrossRef]
Luo T, Ni K, Culbert A, Lan G, Li Z, Jiang X, Kaufmann M, Lin W. (2020). Nanoscale metal–organic frameworks stabilize bacteriochlorins for Type I and Type II photodynamic therapy. Journal of the American Chemical Society 142: 7334–7339. DOI 10.1021/jacs.0c02129. [Google Scholar] [CrossRef]
Maeding N, Verwanger T, Krammer B. (2016). Boosting tumor-specific immunity using PDT. Cancers 8: 91. DOI 10.3390/cancers8100091. [Google Scholar] [CrossRef]
Mahmoudi K, Garvey KL, Bouras A, Cramer G, Stepp H, Jesu Raj JG, Bozec D, Busch TM, Hadjipanayis CG. (2019). 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. Journal of Neuro-Oncology 141: 595–607. DOI 10.1007/s11060-019-03103-4. [Google Scholar] [CrossRef]
Mallidi S, Anbil S, Bulin AL, Obaid G, Ichikawa M, Hasan T. (2016). Beyond the barriers of light penetration: Strategies, perspectives and possibilities for photodynamic therapy. Theranostics 6: 2458–2487. DOI 10.7150/thno.16183. [Google Scholar] [CrossRef]
Mansoori B, Mohammadi A, Amin Doustvandi M, Mohammadnejad F, Kamari F, Gjerstorff MF, Baradaran B, Hamblin MR. (2019). Photodynamic therapy for cancer: Role of natural products. Photodiagnosis and Photodynamic Therapy 26: 395–404. DOI 10.1016/j.pdpdt.2019.04.033. [Google Scholar] [CrossRef]
Milisav I, Ribaric S, Poljsak B. (2018). Antioxidant vitamins and ageing. Biochemistry and Cell Biology of Ageing: Part I Biomedical Science 90: 1–23. [Google Scholar]
Moan J, Peng Q, Sorensen R, Iani V, Nesland JM. (1998). The biophysical foundations of photodynamic therapy. Endoscopy 30: 387–391. DOI 10.1055/s-2007-1001288. [Google Scholar] [CrossRef]
Molina AM, Morales-Cruz M, Benitez M, Berrios K, Figueroa CM, Griebenow K. (2016). Redox-sensitive cross-linking enhances albumin nanoparticle function as delivery system for photodynamic cancer therapy. Journal of Nanomedicine & Nanotechnology 6: 294. [Google Scholar]
Moloney JN, Cotter TG. (2018). ROS signalling in the biology of cancer. Seminars in Cell & Developmental Biology 80: 50–64. DOI 10.1016/j.semcdb.2017.05.023. [Google Scholar] [CrossRef]
Morgan AR, Pangka VS, Dolphin D. (1985). ChemInform Abstract: Ready syntheses of benzoporphyrins via Diels-Alder reactions with protoporphyrin IX. Chemischer Informationsdienst 16: 1047–1048. [Google Scholar]
Morton CA, Braathen LR. (2018). Daylight photodynamic therapy for actinic keratoses. American Journal of Clinical Dermatology 19: 647–656. DOI 10.1007/s40257-018-0360-y. [Google Scholar] [CrossRef]
Musicki B, Liu T, Lagoda GA, Strong TD, Sezen SF, Johnson JM, Burnett AL. (2010). Hypercholesterolemia-induced erectile dysfunction: Endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. Journal of Sexual Medicine 7: 3023–3032. DOI 10.1111/j.1743-6109.2010.01880.x. [Google Scholar] [CrossRef]
Nath S, Obaid G, Hasan T. (2019). The course of immune stimulation by photodynamic therapy: Bridging fundamentals of photochemically induced immunogenic cell death to the enrichment of T-cell repertoire. Photochemistry and Photobiology 95: 1288–1305. DOI 10.1111/php.13173. [Google Scholar] [CrossRef]
Ochsner M. (1997). Photophysical and photobiological processes in the photodynamic therapy of tumours. Journal of Photochemistry and Photobiology B: Biology 39: 1–18. DOI 10.1016/S1011-1344(96)07428-3. [Google Scholar] [CrossRef]
Olsen CE, Weyergang A, Edwards VT, Berg K, Brech A, Weisheit S, Hogset A, Selbo PK. (2017). Development of resistance to photodynamic therapy (PDT) in human breast cancer cells is photosensitizer-dependent: Possible mechanisms and approaches for overcoming PDT-resistance. Biochemical Pharmacology 144: 63–77. DOI 10.1016/j.bcp.2017.08.002. [Google Scholar] [CrossRef]
Ozog DM, Rkein AM, Fabi SG, Gold MH, Goldman MP, Lowe NJ, Martin GM, Munavalli GS. (2016). Photodynamic therapy. Dermatologic Surgery 42: 804–827. DOI 10.1097/DSS.0000000000000800. [Google Scholar] [CrossRef]
Peng ZZ, Liu RG, Li YB, Zhang QY, Cai XJ, Li LB. (2016). Calcitriol enhances the effect of photodynamic therapy in human breast cancer. Journal of BUON: Official Journal of the Balkan Union of Oncology 21: 1068–1075. [Google Scholar]
Pinegin B, Vorobjeva N, Pashenkov M, Chernyak B. (2018). The role of mitochondrial ROS in antibacterial immunity. Journal of Cellular Physiology 233: 3745–3754. DOI 10.1002/jcp.26117. [Google Scholar] [CrossRef]
Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. (2017). Oxidative stress: Harms and benefits for human health. Oxidative Medicine and Cellular Longevity 2017: 8416763. DOI 10.1155/2017/8416763. [Google Scholar] [CrossRef]
Raab O. (1900). Uber die Wirkung Fluoreszierender Stoffe auf Infusorien. Zeitschrift für Biologie 39: 524–546. [Google Scholar]
James NS, Chen Y, Joshi P, Ohulchanskyy TY, Ethirajan M, Henary M, Strekowsk L, Pandey RK. (2013). Evaluation of polymethine dyes as potential probes for near infrared fluorescence imaging of tumors: Part-1. Theranostics 3: 692–702. DOI 10.7150/thno.5922. [Google Scholar] [CrossRef]
Schirrmacher V. (2019). From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. International Journal of Oncology 54: 407–419. [Google Scholar]
Shafirstein G, Rigual NR, Arshad H, Cooper MT, Bellnier DA, Wilding G, Tan W, Merzianu M, Henderson BW. (2016). Photodynamic therapy with 3-(1’-hexyloxyethyl) pyropheophorbide-a for early-stage cancer of the larynx: Phase Ib study. Head & Neck 38: E377–E383. [Google Scholar]
Shen Y, Shuhendler AJ, Ye D, Xu JJ, Chen HY. (2016). Two-photon excitation nanoparticles for photodynamic therapy. Chemical Society Reviews 45: 6725–6741. DOI 10.1039/C6CS00442C. [Google Scholar] [CrossRef]
Shi X, Zhang CY, Gao J, Wang Z. (2019). Recent advances in photodynamic therapy for cancer and infectious diseases. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 11: e1560. DOI 10.1002/wnan.1560. [Google Scholar] [CrossRef]
Showalter A, Limaye A, Oyer JL, Igarashi R, Kittipatarin C, Copik AJ, Khaled AR. (2017). Cytokines in immunogenic cell death: Applications for cancer immunotherapy. Cytokine 97: 123–132. DOI 10.1016/j.cyto.2017.05.024. [Google Scholar] [CrossRef]
Siegel RL, Miller KD, Jemal A. (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 68: 7–30. DOI 10.3322/caac.21442. [Google Scholar] [CrossRef]
Sies H. (2018). On the history of oxidative stress: Concept and some aspects of current development. Current Opinion in Toxicology 7: 122–126. DOI 10.1016/j.cotox.2018.01.002. [Google Scholar] [CrossRef]
Singh A, Kukreti R, Saso L, Kukreti S. (2019). Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 24: 1583. DOI 10.3390/molecules24081583. [Google Scholar] [CrossRef]
Singh S, Aggarwal A, Bhupathiraju NV, Arianna G, Tiwari K, Drain CM. (2015). Glycosylated porphyrins, phthalocyanines, and other porphyrinoids for diagnostics and therapeutics. Chemical Reviews 115: 10261–10306. DOI 10.1021/acs.chemrev.5b00244. [Google Scholar] [CrossRef]
Song W, Kuang J, Li CX, Zhang M, Zheng D, Zeng X, Liu C, Zhang XZ. (2018). enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano 12: 1978–1989. DOI 10.1021/acsnano.7b09112. [Google Scholar] [CrossRef]
Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng ZY (2019a). Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Medicine and Cellular Longevity 2019: 1–13. [Google Scholar]
Su M, Li S, Zhang H, Zhang J, Chen H, Li C (2019b). Nano-assemblies from J-aggregated dyes: A stimuli-responsive tool applicable to living systems. Journal of the American Chemical Society 141: 402–413. DOI 10.1021/jacs.8b10396. [Google Scholar] [CrossRef]
Taverne YJ, Merkus D, Bogers AJ, Halliwell B, Duncker DJ, Lyons TW. (2018). Reactive oxygen species: Radical factors in the evolution of animal life. BioEssays 40: 1700158. DOI 10.1002/bies.201700158. [Google Scholar] [CrossRef]
Tong Y, Zhao KK, Feng D, Biswal M, Zhao PQ, Wang ZY, Zhang Y. (2016). Comparison of the efficacy of anti-VEGF monotherapy versus PDT and intravitreal anti-VEGF combination treatment in AMD: A Meta-analysis and systematic review. International Journal of Ophthalmology 9: 1028–1037. [Google Scholar]
Trafalski M, Kazubowska K, Jurczyszyn K. (2019). Treatment of the facial basal cell carcinoma with the use of photodynamic therapy: A case report. Dental and Medical Problems 56: 105–110. DOI 10.17219/dmp/100507. [Google Scholar] [CrossRef]
Usuda J, Ichinose S, Ishizumi T, Hayashi H, Ohtani K, Maehara S, Ono S, Honda H, Kajiwara N, Uchida O, Tsutsui H, Ohira T, Kato H, Ikeda N. (2010). Outcome of photodynamic therapy using NPe6 for bronchogenic carcinomas in central airways >1.0 cm in diameter. Clinical Cancer Research 16: 2198–2204. DOI 10.1158/1078-0432.CCR-09-2520. [Google Scholar] [CrossRef]
Van Straten D, Mashayekhi V, De Bruijn HS, Oliveira S, Robinson DJ. (2017). Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 9: 19. DOI 10.3390/cancers9020019. [Google Scholar] [CrossRef]
Wachowska M, Muchowicz A, Demkow U (2015a). Immunological aspects of antitumor photodynamic therapy outcome. Central European Journal of Immunology 40: 481–485. DOI 10.5114/ceji.2015.56974. [Google Scholar] [CrossRef]
Wachowska M, Muchowicz A, Golab J (2015b). Targeting epigenetic processes in photodynamic therapy-induced anticancer immunity. Frontiers in Oncology 5: 176. DOI 10.3389/fonc.2015.00176. [Google Scholar] [CrossRef]
Wang L. (2008). The fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. Journal of Immunology 180: 3072–3080. DOI 10.4049/jimmunol.180.5.3072. [Google Scholar] [CrossRef]
Xiao Q, Wu J, Pang X, Jiang Y, Wang P, Leung AW, Gao L, Jiang S, Xu C. (2018). Discovery and development of natural products and their derivatives as photosensitizers for photodynamic therapy. Current Medicinal Chemistry 25: 839–860. DOI 10.2174/0929867324666170823143137. [Google Scholar] [CrossRef]
Yang Y, Hu Y, Wang H. (2016). Targeting antitumor immune response for enhancing the efficacy of photodynamic therapy of cancer: Recent advances and future perspectives. Oxidative Medicine and Cellular Longevity 2016: 5274084. DOI 10.1155/2016/5274084. [Google Scholar] [CrossRef]
Yanovsky RL, Bartenstein DW, Rogers GS, Isakoff SJ, Chen ST. (2019). Photodynamic therapy for solid tumors: A review of the literature. Photodermatology, Photoimmunology & Photomedicine 35: 295–303. DOI 10.1111/phpp.12489. [Google Scholar] [CrossRef]
Yi F, Zheng X, Fang F, Zhang J, Zhou B, Chen X. (2019). ALA-PDT alleviates the psoriasis by inhibiting JAK signalling pathway. Experimental Dermatology 28: 1227–1236. DOI 10.1111/exd.14017. [Google Scholar] [CrossRef]
Zhai W, Zhang Y, Liu M, Zhang H, Zhang J, Li C. (2019). Universal scaffold for an activatable photosensitizer with completely inhibited photosensitivity. Angewandte Chemie International Edition 58: 16601–16609. DOI 10.1002/anie.201907510. [Google Scholar] [CrossRef]
Zhang H, Cheng J, Li W, Tan G, Wang Z, Jin Y (2017a). Facile synthesis of a highly water-soluble graphene conjugated chlorophyll-a photosensitizer composite for improved photodynamic therapy in vitro. New Journal of Chemistry 41: 10069–10082. DOI 10.1039/C7NJ01696D. [Google Scholar] [CrossRef]
Zhang J, Jiang C, Figueiro Longo JP, Azevedo RB, Zhang H, Muehlmann LA (2018a). An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharmaceutica Sinica B 8: 137–146. DOI 10.1016/j.apsb.2017.09.003. [Google Scholar] [CrossRef]
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W (2016a). ROS and ROS-mediated cellular signaling. Oxidative Medicine and Cellular Longevity 2016: 4350965. DOI 10.1155/2016/4350965. [Google Scholar] [CrossRef]
Zhang L, Qin Y, Chen M (2018b). Viral strategies for triggering and manipulating mitophagy. Autophagy 14: 1665–1673. DOI 10.1080/15548627.2018.1466014. [Google Scholar] [CrossRef]
Zhang R, Xing R, Jiao T, Ma K, Chen C, Ma G, Yan X (2016b). Carrier-free, chemophotodynamic dual nanodrugs via self-assembly for synergistic antitumor therapy. ACS Applied Materials & Interfaces 8: 13262–13269. DOI 10.1021/acsami.6b02416. [Google Scholar] [CrossRef]
Zhang SY, Li XB, Hou SG, Sun Y, Shi YR, Lin SS (2016c). Cedrol induces autophagy and apoptotic cell death in A549 non-small cell lung carcinoma cells through the P13K/Akt signaling pathway, the loss of mitochondrial transmembrane potential and the generation of ROS. International Journal of Molecular Medicine 38: 291–299. DOI 10.3892/ijmm.2016.2585. [Google Scholar] [CrossRef]
Zhang XF, Feng N. (2018). Attaching naphthalene derivatives onto BODIPY for generating excited triplet state and singlet oxygen: Tuning PET-based photosensitizer by electron donors. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 189: 13–21. DOI 10.1016/j.saa.2017.08.005. [Google Scholar] [CrossRef]
Zhang Y, Su SS, Zhao S, Yang Z, Zhong CQ, Chen X, Cai Q, Yang ZH, Huang D, Wu R, Han J (2017b). RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nature Communications 8: 14329. DOI 10.1038/ncomms14329. [Google Scholar] [CrossRef]
Zhang Y, Wang B, Zhao R, Zhang Q, Kong X. (2020). Multifunctional nanoparticles as photosensitizer delivery carriers for enhanced photodynamic cancer therapy. Materials Science and Engineering: C 115: 111099. DOI 10.1016/j.msec.2020.111099. [Google Scholar] [CrossRef]
Zhang QY, Li YB. (2018). Photodynamic combinational therapy in cancer treatment. Journal of BUON: Official Journal of the Balkan Union of Oncology 23: 561–567. [Google Scholar]
Zhou Z, Song J, Nie L, Chen X. (2016). Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chemical Society Reviews 45: 6597–6626. DOI 10.1039/C6CS00271D. [Google Scholar] [CrossRef]
Zou H, Wang F, Zhou JJ, Liu X, He Q, Wang C, Zheng YW, Wen Y, Xiong L. (2020). Application of photodynamic therapy for liver malignancies. Journal of Gastrointestinal Oncology 11: 431–442. DOI 10.21037/jgo.2020.02.10. [Google Scholar] [CrossRef]
Zou Z, Chang H, Li H, Wang S. (2017). Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 22: 1321–1335. DOI 10.1007/s10495-017-1424-9. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |