DOI:10.32604/biocell.2021.012137

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.012137 |  www.techscience.com/journal/biocell |
| Article |
Study on the clinical significance of TRPV2 and MMP2 expressions in ovarian cancer
Department of Obstetrics and Gynecology, Affiliated Hospital of Hebei University, Baoding, 071000, China
*Address correspondence to: Huiling Xue, osqhhv@163.com
#These authors contributed equally to this work
Received: 26 June 2020; Accepted: 30 September 2020
Abstract: Ovarian cancer was one of the most common malignant tumors in female reproductive organs. Moreover, epithelial ovarian cancer showed the highest mortality rate in gynecological tumors, posing serious threats to women’s life and health. Transient Receptor Potential Cation Channel Subfamily V Member 2 (TRPV2) and matrix metalloproteinase-2 MMP-2 have been found to play important roles in regulating the pathogenesis of most tumors, but there were few studies exploring the relationships of TRPV2 and MMP-2 in OC. Therefore, we evaluated the expression of TRPV2 and MMP-2 proteins in cancer tissues and adjacent normal tissues of OC patients. Immunohistochemistry was used to analyze the expressions of TRPV2 and MMP-2 in cancer tissues (N = 70) and adjacent normal tissues (N = 70) of OC patients, and the correlation of TRPV2 and MMP-2 with the occurrence and development of OC was analyzed with the combination of clinicopathological parameters of OC patients. The results showed that the expressions of TRPV2 and MMP-2 in OC tissues were significantly higher than those in adjacent normal tissues, and there were significant differences in TRPV2 and MMP-2 expressions in terms of tumor stage, differentiation, and lymph node metastasis of OC. Taken together, our results showed that protein expressions of TRPV2 and MMP-2 were closely related associated with the occurrence and development of OC.
Keywords: Ovarian cancer; TRPV2; MMP-2; Immunohistochemistry
Ovarian cancer (OC) was one of the most common malignant tumors in the female reproductive system, with morbidity ranking third place in gynecologic malignancies (second only to cervical cancer and endometrial adenocarcinoma) and mortality ranking first place in gynecological tumors. Therefore, OC was considered a serious threat to women’s life and health (Webb and Jordan, 2017; Stewart et al., 2019). OC was difficult to be confirmed due to the special location (deep in the pelvic cavity) and the small size of the ovary, as well as the fact that OC is hidden in pathogenetic condition and difficult in early diagnosis. In addition, OC was suggested to be related to gynecological diseases, genetics, environment, and hormones; but the etiology of OC has been not clear yet (Roett and Evans, 2009; Kujawa and Lisowska, 2015). At present, the general principle for the treatment of OC was dominated by surgery, with the combination of chemotherapy and other means for comprehensive treatment. With the progress of science and technology, targeted therapy was believed to play a key role in the clinical treatment of OC in recent years. Generally speaking, patients diagnosed at the early stage could be completely cured by surgery combined with other treatments, but the recurrence rate was still higher. Therefore, it was of great concern to clarify the pathogenesis of OC and to explore the occurrence and development processes from the molecular level, in order to carry out the molecular-targeted therapy (Dong et al., 2014).
Organisms could sense the changes of the external environment sensitively, mainly depending on some special proteins on the epidermis, especially the transient receptor potential channel proteins (TRPs) on the cell membrane (Kojima and Nagasawa, 2014). When stimulated by physical and chemical factors, TRPs could maintain the body’s homeostasis by regulating the intracellular environment. As a member of the TRPs family, TRPV2 was a calcium channel protein. In recent years, it has been found that TRPV2 played crucial roles in neuron development, cardiac function regulation, immune response, and tumorigenesis (Iwata and Matsumura, 2019; Zhang et al., 2016). Zoppoli et al. carried out an in-depth study on the TRPV2 calcium channel gene in gastric cancer (GC) patients, differential gene expression was compared between the gastric tissue of GC patients and the normal gastric tissue, and the results showed that patients with a high expression level of TRPV2 had shorter overall survival, and the TRPV2 expression increased according to tumor stage, revealing that TRPV2 may be used as a biomarker or a potential target for further study (Zoppoli et al., 2019). Additionally, TRPV2 was also highly expressed in melanoma, esophageal cancer, prostate cancer, and breast cancer, but TRPV2 expression has not been reported in OC. Therefore, the TRPV2 expression in OC was detected in the study, and the relationship between the TRPV2 expression and OC was analyzed with the combination of the clinicopathological characteristics of OC patients (Zheng et al., 2019; Kudou et al., 2019; Oulidi et al., 2013).
Matrix metalloproteinases (MMP), a large family, have the capability of decomposing extracellular matrix and of contributing to cell migration and invasion; thus, MMP was widely concerned in the occurrence and development of tumors. At present, there were more in-depth studies on the relationships of MMP-2 and MMP-9 with tumors. Furthermore, MMP-2 has been reported to be highly expressed in bladder cancer, pancreatic cancer, lung cancer, and liver cancer, indicating that MMP-2 may be related to tumorigenesis, but with the failure of defining the relationship between MMP-2 and OC (Ghosh et al., 2017; Han et al., 2019).
From February 2019 to March 2020, 70 pairs of specimens were selected from OC patients confirmed by pathologic diagnosis admitted to Affiliated Hospital of Hebei University, including 70 cancer tissue specimens and 70 adjacent normal tissue specimens (3 cm away from the cancer tissues).
Inclusion criteria: (1) Postoperative pathological diagnosis of primary OC; (2) No treatment before the operation, including radiotherapy and chemotherapy; (3) Complete case data and follow-up data; (4) No other tumor diseases or immune system diseases; and (5) Informed consent of family members. Exclusion criteria: (1) Relevant treatments before operation; (2) History of other malignant tumors; and (3) Incomplete follow-up data.
All the 70 patients were newly diagnosed, with an average age of 48.5 years old, ranging from 24 to 72 years old. According to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system, there were 28 patients in Stage I and Stage II, and 42 patients in Stage III and Stage IV. The OC tissues and the adjacent normal tissues obtained by surgical excision were placed in 4% paraformaldehyde for immunohistochemistry (IHC).
Ultra-Thin Semiautomatic Microtome
1. DHG Series Heating and Drying Oven (Shanghai Jinghong Experimental Equipment Co., Ltd.)
2. Electro-heating standing-temperature cultivator (Tianjin Taisite Instrument Co., Ltd.)
3. Ultra-pure water system (Heal Force Co., Hong Kong)
4. Microscope Model (OLYMPUS, Japan)
5. Microscope photograph system (OLYMPUS, Japan)
6. TRPV2 antibody (Boster Biological Technology Co., Ltd.)
7. MMP-2 antibody (Wuhan Servicebio Technology Co., Ltd.)
8. HRP-labeled goat anti-rabbit IgG (Beijing Solarbio Science & Technology Co., Ltd.)
9. Absolute alcohol (Sinopharm Chemical Reagent Co., Ltd.)
10. Hematoxylin (Sinopharm Chemical Reagent Co., Ltd.)
11. Goat serum (Beijing Solarbio Science & Technology Co., Ltd.)
12. Color-developing solution (Beijing Solarbio Science & Technology Co., Ltd.)
13. Hydrogen peroxide (Beijing Solarbio Science & Technology Co., Ltd.)
The tissue samples fixed in 4% paraformaldehyde were dehydrated and soaked in wax. The tissue samples were embedded into paraffin blocks by an embedding machine; the paraffin blocks were cut into 3–4 μm slices, followed by unfolding and baking. The slices were subjected to dewaxing to replace paraffin with water and antigen retrieval and incubated with 3% hydrogen peroxide for 25 min. After serum blocking, TRPV2 and MMP-2 primary antibodies (1:1000 dilution, BA4959, Boster; and 1:500 dilution, GB11130, Servicebio) were added for incubation overnight at 4°C; and after PBS washing, secondary antibodies (1:1000 dilution; G1213, Servicebio) were added for incubation at room temperature for 50 min. DAB color-developing solution was dripped to cover the tissue samples. Hematoxylin (G1004-100, Servicebio) was used for redyeing for 2 min, followed by dehydrating and sealing. The prepared sections were observed with the use of a microscope carrier. The target protein was stained brown–yellow. Each section was photographed at three different fields of vision, and the photographs were preserved. The Image-Pro Plus software was used to analyze the optical density (OD) of each photograph, and the average OD was calculated.
SPSS 20.0 was used for data analysis, and the results were expressed as mean ± SD. Chi-square test was applied to enumeration data. A p-value less than 0.05 was considered to be statistically significant (*p < 0.05, **p < 0.01, and ***p < 0.001). GraphPad Prism 8.0 software was used for charting.
The study was approved by the Institutional Ethics Committee (No. HDFY-LL-2020-021) of Affiliated Hospital of Hebei University, and written informed consent was obtained from all participants.
Analysis of TRPV2 expression in ovarian cancer
To study the relationship between the expression of TRPV2 protein and ovarian cancer, the expression levels of TRPV2 protein in 70 pairs of OC tissues and adjacent normal tissues were analyzed, as well as the average OD. The results showed that TRPV2 protein was highly expressed in cancer tissues and was 9 times as much as that in adjacent normal tissues (Fig. 1).
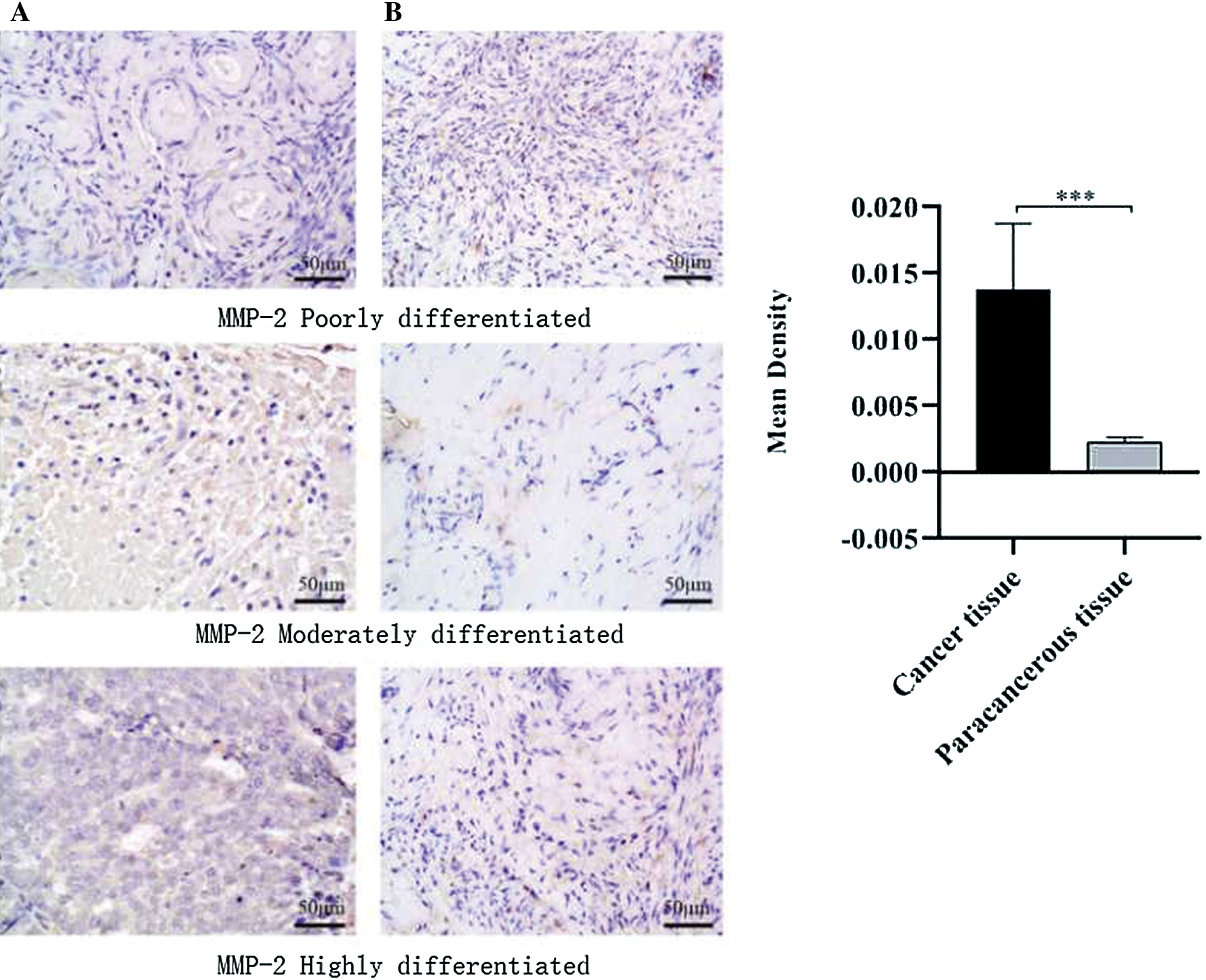
Figure 1: TRPV2 protein expressions in ovarian cancer tissues and adjacent normal tissues.
Analysis of MMP-2 expression in ovarian cancer
In order to explore whether MMP-2 is differentially expressed in ovarian cancer tissues and adjacent tissues, the MMP-2 protein expression in OC tissues and adjacent normal tissues were analyzed. The results showed that the expression of MMP-2 protein in cancer tissue was 4.7 times as much as that in adjacent normal tissues (Fig. 2).
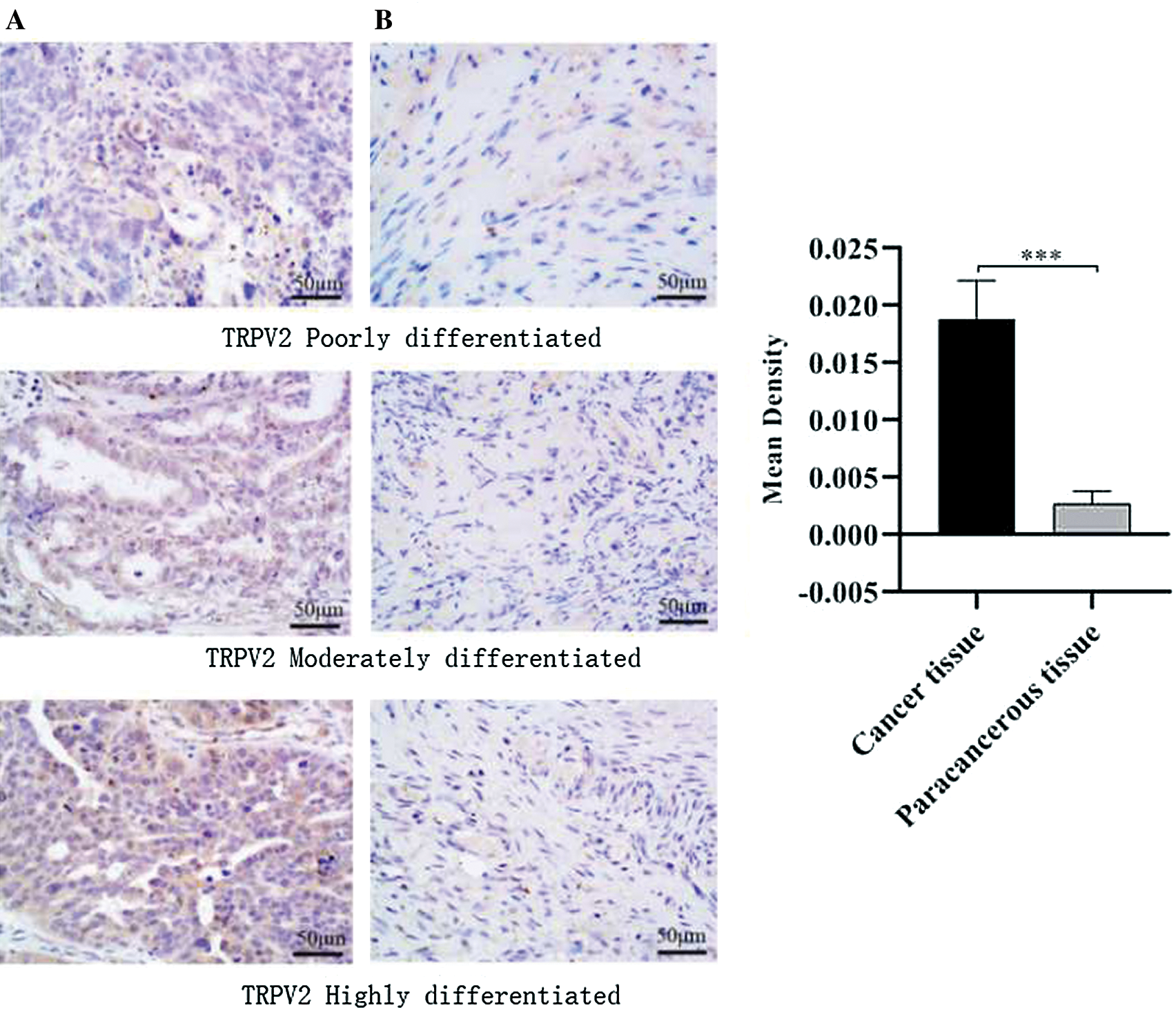
Figure 2: MMP-2 protein in ovarian cancer tissues and adjacent normal tissues.
Relationships between TRPV2 and MMP-2 expressions in ovarian cancer and the clinicopathological characteristics of ovarian cancer patients
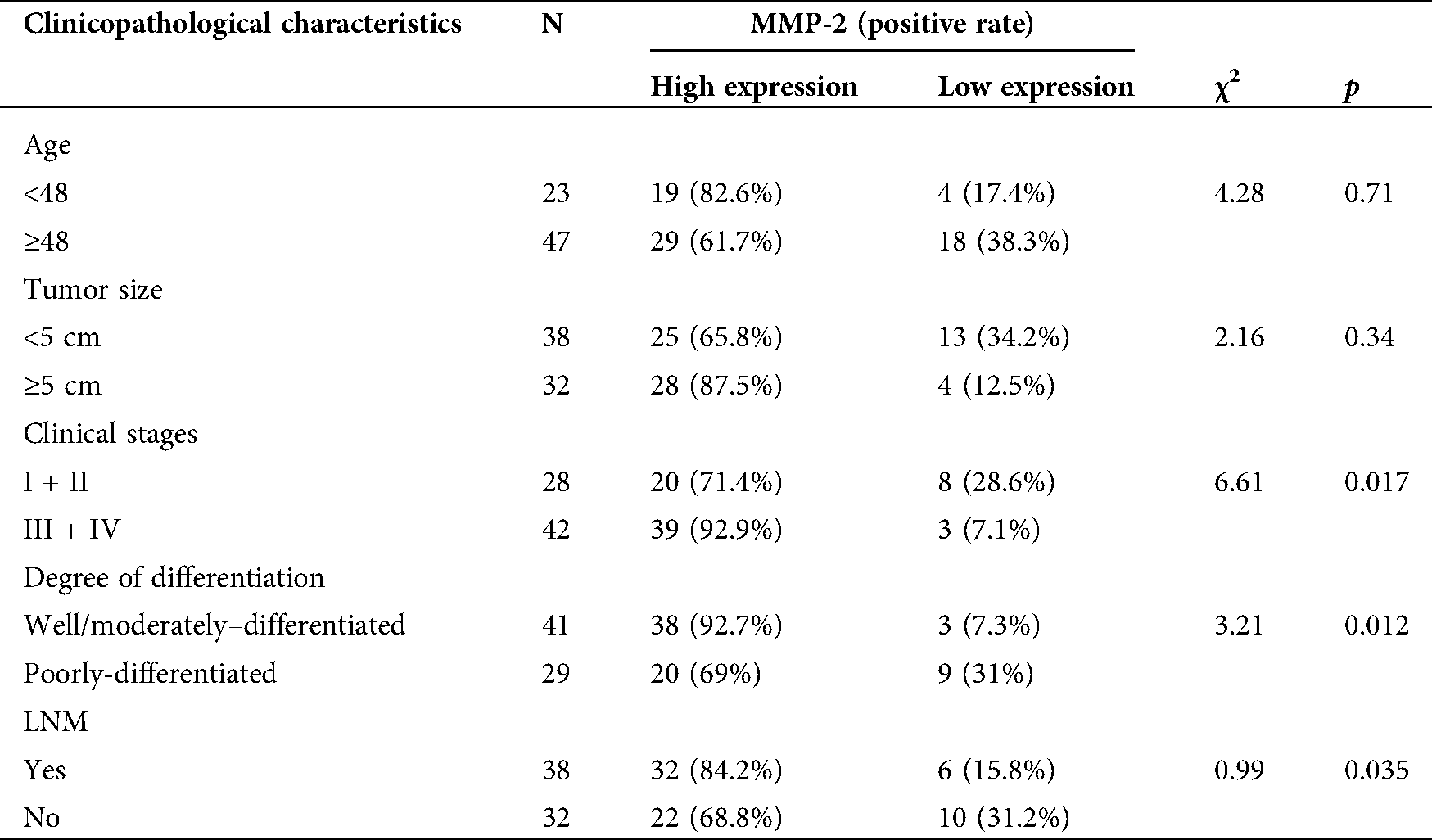
To further collect the clinicopathological information of patients and explore the relationship between TRPV2, MMP-2, and ovarian cancer. Combined with basic information of OC patients, the relationships between the expression levels of TRPV2 and MMP-2 and the clinicopathological characteristics were analyzed (Tabs. 1 and 2). The results showed that the expressions of TRPV2 and MMP-2 were significantly different in terms of tumor stage, tumor differentiation, and lymph node metastasis (LNM) of OC.
Table 1: Correlation analysis between the TRPV2 expression and clinicopathological characteristics in ovarian cancer
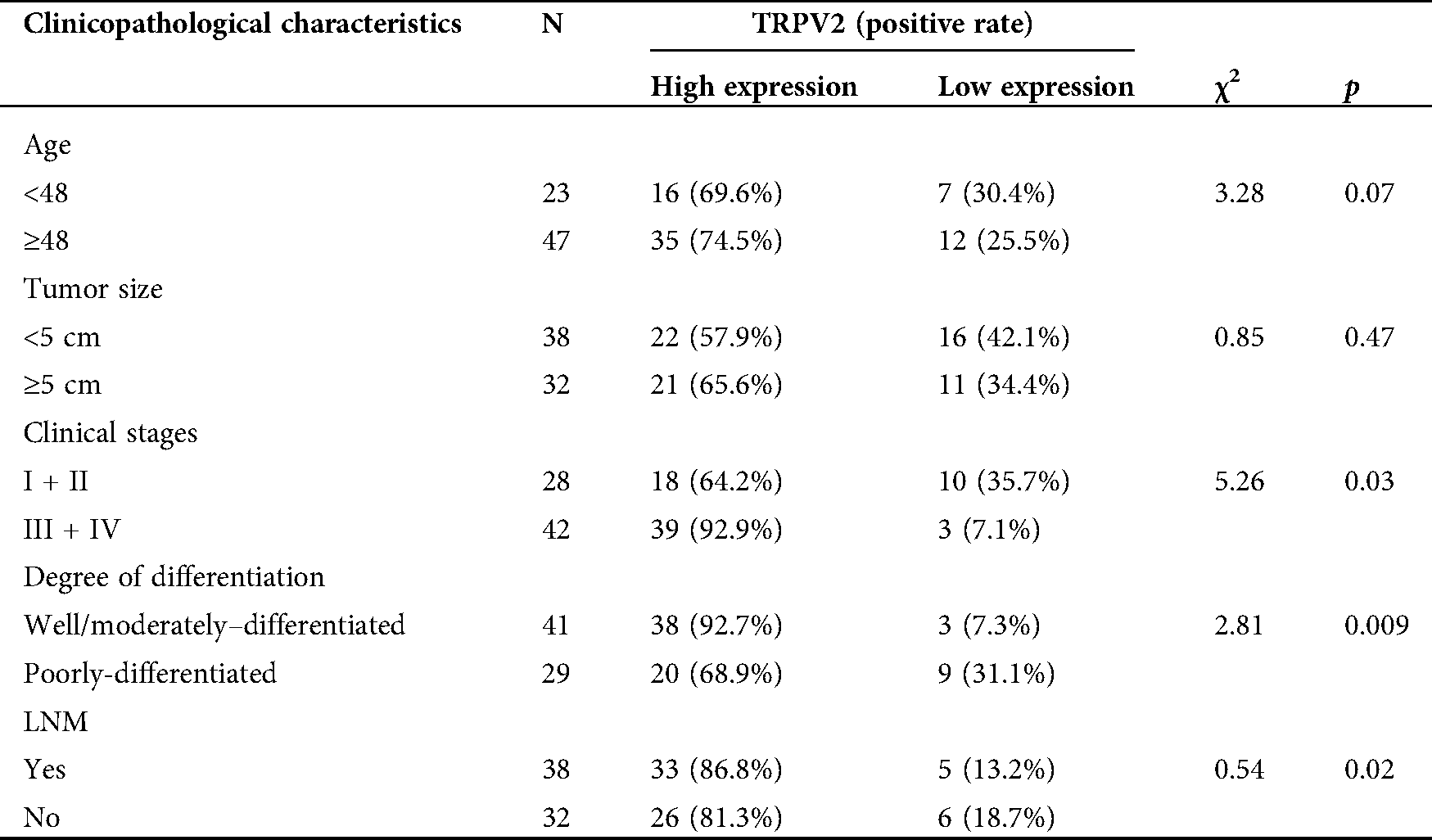
Table 2: Correlation analysis between the MMP-2 expression and clinicopathological characteristics in ovarian cancer
As a malignant tumor with high mortality in gynecological diseases, OC was advanced in most patients by the time of confirmed diagnosis due to the unclear early symptoms and the lack of effective early diagnosis methods. The 5-year survival rate of patients receiving early effective treatments was about 80%, while the 5-year survival rate of patients with advanced OC was only about 20%. Therefore, it was particularly important to carry out early diagnosis and early treatment of OC (Menon et al., 2018). In recent years, molecular targeted therapy has attracted people’s attention, and an increasing number of studies found that the targeted therapy could accurately intervene in the occurrence and development of diseases and played a good role in the prevention and treatment of diseases. In our study, proteins that may be differentially expressed and may play a regulatory role in OC were detected from the molecular level, hoping to clarify the occurrence process of OC at the molecular mechanism level and to provide theoretical basis and practical significance for the accurately targeted therapy of OC (Au et al., 2015).
The organism could sense the external stimulation mainly depending on the ion channel protein on the cell membrane. There were 7 subfamilies in the TRP channel, and the most studied one was the TRPV subfamily, among which the roles of TRPV2 have been widely reported. As found by a previous study, TRPV2 played a key role in cardiac structure and function and participated in the compensatory mechanism of the heart to pathological and exercise-induced stress. These results were helpful to lay the foundation for the subsequent studies on the TRPV2 as a cardiac drug target (Aguettaz et al., 2017). It has been confirmed that TRPV1 and TRPV2 were also involved in the pathogenesis of myocardial infarction and ensured the myocardial tolerance to ischemia-reperfusion, thus participating in the pathogenesis of cardiomyopathy (Gorbunov et al., 2019). It was found that the functional expression of TRPV2 was altered by culturing multiple myeloma cells in a high-calcium environment, and TRPV2 was revealed to be involved in osteolysis (Bai et al., 2018). In esophageal squamous cell carcinoma (ESCC), TRPV2 had the role of regulating the tumor progression by affecting WNT/β-catenin signal transduction, and over-expression of TRPV2 was found to be related to poor prognosis of ESCC patients, revealing that TRPV2 may be a new therapeutic target and biomarker for ESCC (Kudou et al., 2019). Our study also found that TRPV2 was highly expressed in OC, which was consistent with the above-mentioned study, speculating that TRPV2 may be used as a new target for the prevention and treatment of OC in the future. As a member of the MMP family, MMP-2 mainly had the roles of degrading extracellular matrix and participating in signal transduction, while the first step of tumor metastasis was ECM degradation, contributing to tumor cell migration and invasion. Therefore, in this study, the expression of MMP-2 was also detected in OC tissues and adjacent normal tissues, and we found that the expression of MMP-2 in cancer tissues was significantly higher than that in adjacent normal tissues, indicating that MMP-2 may also play crucial roles in the occurrence and development of OC. Our findings were consistent with many studies; it has been reported that abnormally high expressions of MMP-2 were found in breast cancer, liver cancer, lung cancer, pancreatic cancer, and GC (Gogebakan et al., 2014; Wu et al., 2018; Wang et al., 2018; Avădanei et al., 2013; Zhang et al., 2019). At the same time, we also found that the differential expressions of TRPV2 and MMP-2 were closely related to tumor stage, tumor differentiation, and LNM in OC patients. These results may provide a theoretical basis and practical significance for the early cure of OC and the search for targeted therapy drugs.
In addition, the relationship between TRPV2 and MMP-2 has been reported. For example, Monet et al. (2010) found that inhibition of TRPV2 expression with RNA interference could inhibit the growth and invasion of prostate cancer xenograft in nude mice and decreased the expressions of MMP-2 and MMP-9 (Monet et al., 2010). Liu and Wang (2013) demonstrated that transfection of the TRPV2 gene into bladder cancer cells could promote the migration and invasion of bladder cancer cells, and it was speculated that TRPV2 might play the role through MMP-2 mediation. In rheumatoid arthritis, it was found that TRPV2 stimulation could effectively inhibit the expressions of MMP-2 and MMP-3, which may provide a new therapy for the treatment of rheumatoid arthritis (Laragione et al., 2015). In the future, we would further explore the possible interaction and relationship between TRPV2 and MMP-2 in OC in order to provide new ideas and new methods for the early cure of OC.
Availability of Data and Materials: The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Funding Statement: This research was supported Government-sponsored clinical medicine excellent talents training and basic research projects (No. 361007).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Au KK, Josahkian JA, Francis JA, Squire JA, Koti M. (2015). Current state of biomarkers in ovarian cancer prognosis. Future Oncology 11: 3187–3195. DOI 10.2217/fon.15.251. [Google Scholar] [CrossRef]
Aguettaz E, Bois P, Cognard C, Sebille S. (2017). Stretch-activated TRPV2 channels: role in mediating cardiopathies. Progress in Biophysics and Molecular Biology 130: 273–280. DOI 10.1016/j.pbiomolbio.2017.05.007. [Google Scholar] [CrossRef]
Avădanei R, Căruntu ID, Amălinei C, Lozneanu L, Balan R, Grigoraş A, Ciobanu AD, Giuşcă SE. (2013). High variability in MMP2/TIMP2 and MMP9/TIMP1 expression in secondary liver tumors. Romanian Journal of Morphology and Embryology 54: 479–485. [Google Scholar]
Bai H, Zhu H, Yan Q, Shen X, Lu X, Wang J, Li J, Chen L. (2018). TRPV2-induced Ca2+-calcineurin-NFAT signaling regulates differentiation of osteoclast in multiple myeloma. Cell Communication and Signaling 16: 21. DOI 10.1186/s12964-018-0280-8. [Google Scholar] [CrossRef]
Dong X, Men X, Zhang W, Lei P. (2014). Advances in tumor markers of ovarian cancer for early diagnosis. Indian Journal of Cancer 51: 72. DOI 10.4103/0019-509X.154049. [Google Scholar] [CrossRef]
Ghosh A, Dasgupta D, Ghosh A, Roychoudhury S, Kumar D, Gorain M, Butti R, Datta S, Agarwal S, Gupta S, Krishna Dhali G, Chowdhury A, Schmittgen TD, Kundu GC, Banerjee S. (2017). MiRNA199a-3p suppresses tumor growth, migration, invasion and angiogenesis in hepatocellular carcinoma by targeting VEGFA, VEGFR1, VEGFR2, HGF and MMP2. Cell Death & Disease 8: e2706. DOI 10.1038/cddis.2017.123. [Google Scholar] [CrossRef]
Gorbunov AS, Maslov LN, Jaggi AS, Singh N, De Petrocellis L, Boshchenko AA, Roohbakhsh A, Bezuglov VV, Oeltgen PR. (2019). Physiological and pathological role of TRPV1, TRPV2 and TRPV4 channels in heart. Current Cardiology Reviews 15: 244–251. DOI 10.2174/1573403X15666190307112326. [Google Scholar] [CrossRef]
Gogebakan B, Bayraktar R, Suner A, Balakan O, Ulasli M, Izmirli M, Oztuzcu S, Camci C. (2014). Do Fasudil and Y-27632 affect the level of transient receptor potential (TRP) gene expressions in breast cancer cell lines? Tumor Biology 35: 8033–8041. DOI 10.1007/s13277-014-1752-0. [Google Scholar] [CrossRef]
Han Y, Ma L, Zhao L, Feng W, Zheng X. (2019). Rosmarinic inhibits cell proliferation, invasion and migration via up-regulating miR-506 and suppressing MMP2/16 expression in pancreatic cancer. Biomedicine & Pharmacotherapy 115: 108878. DOI 10.1016/j.biopha.2019.108878. [Google Scholar] [CrossRef]
Iwata Y, Matsumura T. (2019). Blockade of TRPV2 is a novel therapy for cardiomyopathy in muscular dystrophy. International Journal of Molecular Sciences 20: 3844. DOI 10.3390/ijms20163844. [Google Scholar] [CrossRef]
Kujawa KA, Lisowska KM. (2015). Rak jajnika--od biologii do kliniki [Ovarian cancer--from biology to clinic]. Postepy Higieny i Medycyny Doswiadczalnej 69: 1275–1290. DOI 10.5604/17322693.1184451. [Google Scholar] [CrossRef]
Kojima I, Nagasawa M. (2014). TRPV2. Handbook of Experimental Pharmacology 222: 247–272. [Google Scholar]
Kudou M, Shiozaki A, Yamazato Y, Katsurahara K, Kosuga T, Shoda K, Arita T, Konishi H, Komatsu S, Kubota T, Fujiwara H, Okamoto K, Kishimoto M, Konishi E, Marunaka Y, Otsuji E. (2019). The expression and role of TRPV2 in esophageal squamous cell carcinoma. Scientific Reports 9: 4964. DOI 10.1038/s41598-019-52227-0. [Google Scholar] [CrossRef]
Liu Q, Wang X. (2013). Effect of TRPV2 cation channels on the proliferation, migration and invasion of 5637 bladder cancer cells. Experimental and Therapeutic Medicine 6: 1277–1282. DOI 10.3892/etm.2013.1301. [Google Scholar] [CrossRef]
Laragione T, Cheng KF, Tanner MR, He M, Beeton C, Al-Abed Y, Gulko PS. (2015). The cation channel Trpv2 is a new suppressor of arthritis severity, joint damage, and synovial fibroblast invasion. Clinical Immunology 158: 183–192. DOI 10.1016/j.clim.2015.04.001. [Google Scholar] [CrossRef]
Menon U, Karpinskyj C, Gentry-Maharaj A. (2018). Ovarian cancer prevention and screening. Obstetrics & Gynecology 131: 909–927. DOI 10.1097/AOG.0000000000002580. [Google Scholar] [CrossRef]
Monet M, Lehen’kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, Gkika D, Pourtier A, Bidaux G, Slomianny C, Delcourt P, Rassendren F, Bergerat JP, Ceraline J, Cabon F, Humez S, Prevarskaya N. (2010). Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Research 70: 1225–1235. DOI 10.1158/0008-5472.CAN-09-2205. [Google Scholar] [CrossRef]
Oulidi A, Bokhobza A, Gkika D, Vanden Abeele F, Lehen’kyi V, Ouafik L, Mauroy B, Prevarskaya N. (2013). TRPV2 mediates adrenomedullin stimulation of prostate and urothelial cancer cell adhesion, migration and invasion. PLoS One 8: e64885. DOI 10.1371/journal.pone.0064885. [Google Scholar] [CrossRef]
Roett MA, Evans P. (2009). Ovarian cancer: an overview. American Family Physician 80: 609–616. [Google Scholar]
Stewart C, Ralyea C, Lockwood S. (2019). Ovarian cancer: an integrated review. Seminars in Oncology Nursing 35: 151–156. DOI 10.1016/j.soncn.2019.02.001. [Google Scholar] [CrossRef]
Webb PM, Jordan SJ. (2017). Epidemiology of epithelial ovarian cancer. Best Practice & Research Clinical Obstetrics & Gynaecology 41: 3–14. DOI 10.1016/j.bpobgyn.2016.08.006. [Google Scholar] [CrossRef]
Wu D-M, Deng S-H, Liu T, Han R, Zhang T, Xu Y. (2018). TGF-β-mediated exosomal lnc-MMP2-2 regulates migration and invasion of lung cancer cells to the vasculature by promoting MMP2 expression. Cancer Medicine 7: 5118–5129. DOI 10.1002/cam4.1758. [Google Scholar] [CrossRef]
Wang T, Hou J, Jian S, Luo Q, Wei J, Li Z, Wang X, Bai P, Duan B, Xing J, Cai J. (2018). miR-29b negatively regulates MMP2 to impact gastric cancer development by suppress gastric cancer cell migration and tumor growth. Journal of Cancer 9: 3776–3786. DOI 10.7150/jca.26263. [Google Scholar] [CrossRef]
Zhang H, Xiao J, Hu Z, Xie M, Wang W, He D. (2016). Blocking transient receptor potential vanilloid 2 channel in astrocytes enhances astrocyte-mediated neuroprotection after oxygen-glucose deprivation and reoxygenation. European Journal of Neuroscience 44: 2493–2503. DOI 10.1111/ejn.13352. [Google Scholar] [CrossRef]
Zoppoli P, Calice G, Laurino S, Ruggieri V, La Rocca F, La Torre G, Ciuffi M, Amendola E, De Vita F, Petrillo A, Napolitano G, Falco G, Russi S. (2019). TRPV2 calcium channel gene expression and outcomes in gastric cancer patients: a clinically relevant association. Journal of Clinical Medicine 8: 662. DOI 10.3390/jcm8050662. [Google Scholar] [CrossRef]
Zheng J, Liu F, Du S, Li M, Wu T, Tan X, Cheng W. (2019). Mechanism for regulation of melanoma cell death via activation of thermo-TRPV4 and TRPV2. Journal of Oncology 2019: 1–14. DOI 10.1155/2019/7362875. [Google Scholar] [CrossRef]
Zhang X, Shi G, Gao F, Liu P, Wang H, Tan X. (2019). TSPAN1 upregulates MMP2 to promote pancreatic cancer cell migration and invasion via PLCγ. Oncology Reports 41: 2117–2125. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |