DOI:10.32604/biocell.2021.014350

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.014350 |  www.techscience.com/journal/biocell |
| Article |
Synergetic effects of shock waves with polydeoxyribonucleotides on rotator cuff tendon tear in a rabbit model
1Department of Rehabilitation Medicine, Catholic University of Daegu School of Medicine, Daegu, 42472, South Korea
2Department of Anatomy, Catholic University of Daegu School of Medicine, Daegu, 42472, South Korea
*Address correspondence to: Dong Rak Kwon, coolkwon@cu.ac.kr
Received: 19 September 2020; Accepted: 20 December 2020
Abstract: This work aimed to investigate the synergetic therapeutic effects of polydeoxyribonucleotides (PDRN) combined with extracorporeal shock waves therapy (ESWT) and the effects of the therapy according to ESWT sequences on a chronic traumatic full-thickness rotator cuff tear (RCT) in rabbit models. For this purpose, thirty-two rabbits were randomly allocated into 4 groups. An excision was made to create a 5-mm sized full-thickness RCT right proximal to the insertion site on the supraspinatus. After 6 weeks, 4 different procedures (normal saline, Group 1; PDRN injection, Group 2; PDRN injection before ESWT, Group 3; PDRN injection after ESWT, Group 4) were performed. PDRN injection and radial type ESWT were each performed 4 times per week. Gross morphology, Immunohistochemistry, and motion analysis were performed at 4 weeks after treatments. All parameters including tear size, Masson’s trichrome (MT) staining, anti-collagen 1 monoclonal antibody immunostaining (COL-1), proliferating cell nuclear antigen (PCNA), vascular endothelial growth factor (VEGF), anti-platelet endothelial cell adhesion molecule-1 polyclonal antibody (PECAM-1), and motion analysis, were significantly greater in Group 2, Group 3, and Group 4 than in Group 1. In Group 4, all parameters were significantly greater than in Group 2, and parameters including MT staining, COL-1, PCNA, PECAM-1, and fast walking time were significantly greater than Group 3. There were no significant differences between Group 2 and Group 3. This study demonstrated that PDRN injection alone and combined with ESWT were more effective than control treatment and applying ESWT before PDRN injection was also shown to yield better outcomes in parameters including angiogenesis, cell proliferation, and fast walking time in a rabbit model with chronic traumatic full-thickness RCT. Therefore, the application of ESWT before PDRN injection could be recommended for optimal outcomes of the conservative treatments when the patients with full-thickness RCT are not suitable in surgical treatment.
Keywords: Shoulder; Rotator cuff; Polydeoxyribonucleotides; Extracorporeal shock waves
A rotator cuff tear (RCT) is a pathologic condition that causes weakness, pain, and disability in shoulder joints, which may result in a considerable decrease in quality of life (Kwon et al., 2019). There are no agreements on the strategies in managing RCT to date. Conservative management, including non-steroidal anti-inflammatory drugs, various combinations of physical therapy, local anesthesia, and steroid injections, are often selected as initial treatment approaches. When these non-operative treatments fail, surgical tendon repair may be attempted (Schwitzguebel et al., 2019). However, failure rates of the surgical tendon repair are currently being reported to be 13–94% (Castricini et al., 2011), and since, more than half of the patients who underwent RCT suffer from persistent and recurrent pain (Lewis, 2009), various materials such as platelet-rich plasma and hyaluronic acid are being considered to be used in such refractory cases (Schwitzguebel et al., 2019; Osti et al., 2015).
Chronic tendon degeneration found after surgical treatment is considered a major pathologic cause of these adverse events (Baydar et al., 2009). However, unlike traumatic rotator cuff tears, the features and healing processes of chronic degenerative rotator cuff tears are not clearly understood (Killian et al., 2015). Therefore, allowing for these limitations of current treatments, more fundamental approaches to tendon regeneration are required.
Biological adjuvants, which contribute to the microenvironment of regeneration, have great potential to improve healing rates and the function of injured rotator cuff tendons (Murray et al., 2014). Due to its anti-inflammatory properties with relatively fewer side effects, polydeoxyribonucleotides (PDRN), which has advantages in terms of commercial mass production, has recently been suggested as one of the viable alternative treatment options to corticosteroid (Huh et al., 2019). PDRN, which is extracted from salmon sperm and purified in a high percentage of DNA, is a mixture of deoxyribonucleic acid polymers with chain lengths of 50 to 2000 bp. PDRN may serve as a source of purines and pyrimidines, stimulating nucleic acid synthesis by a salvage pathway (Galeano et al., 2008). In a previous study, Kim and Chung (2015) reported that PDRN is shown to induce angiogenesis and collagen synthesis, as well as anti-inflammatory action. Several studies on the efficacy of PDRN injection have recently been published. Yoon et al. (2017) has reported on the therapeutic use of PDRN in patients with chronic supraspinatus tendinopathy, while Huh et al. (2019) reported on PDRN injection in patients with carpal tunnel syndrome.
Extracorporeal shock wave therapy (ESWT) is a non-invasive therapeutic method in which a specific target area is treated with a series of single sound wave pulses generated by a proper generator (Guo et al., 2017). ESWT has been therapeutically used in the clinic for numerous musculoskeletal disorders, including plantar fasciitis, tennis elbow, and calcific tendinitis of the shoulder (Pan et al., 2003; Zhong et al., 2019). Even a single application of ESWT may induce increased blood flow, which can be prolonged by repeated application of ESWT on muscle and tendon tissues (Kisch et al., 2016). It is also suggested that ESWT may increase collagen synthesis of tenocytes, while it may decrease the expression of inflammatory cytokines that are associated with tendinopathy in tendon tissues (van der Worp et al., 2013). Several physical methods to permeabilize mammalian cells have been developed as an alternative to viral and chemical techniques. One of the most appealing physical methods to deliver chemical materials such as genes into cells is shock wave-induced poration (López-Marín et al., 2018). It would be ideal if a combination of ESWT and PDRN could yield synergetic regenerative effects on injured rotator cuff tendons. To the best of our knowledge, there are no current studies investigating the effects of ESWT sequences combined with PDRN injection on a chronic traumatic full-thickness rotator cuff tear (RCT). In addition, ESWT and PDRN are clinically more easily accessible than surgery and can be used as one of the conservative treatment modalities considering their action mechanism when the patients are not suitable in surgical treatment. Therefore, this study aims to investigate the synergetic therapeutic effects of PDRN combined with ESWT and the effects of the therapy on a chronic traumatic full-thickness RCT according to ESWT sequences in a rabbit model.
A total of 32 skeletally mature male New Zealand white rabbits (twelve-week-old) were used. The rabbits were separately housed in metal cages with a temperature of 24 ± 2°C, and a humidity of 45 ± 10%. The rabbits were fed with a commercial rabbit diet and provided with free access to tap water. All rabbits were allowed to do daily activities in a cage (65 cm × 45 cm × 30 cm). None of these animals were put on additional exercise. Experiments that include animals were conducted according to internationally accredited guidelines. This study is approved by the Institutional Animal Care and Use Committee (IACUC) of the Catholic University of Daegu School of Medicine. Isoflurane (Forane; JW Pharmaceutical) vaporized in oxygen was used to induce general anesthesia, and the rabbits were delivered using a large animal cycling system. With punch biopsy (SFM, Wächtersbach, Germany), 5 mm × 5 mm sized full-thickness RCT was created proximal to the insertion site of the right supraspinatus tendon under general anesthesia. In order to induce chronic full-thickness RCT, the excision wound was immediately closed with a non-absorbable round-shaped silicone Penrose drainage tube (Sewoon Medical Co., Ltd., Cheonan-si, Korea) (Coleman et al., 2003). Nylon monofilament (AILEE Co., Ltd., Busan, Korea) was used for suturing the subcutaneous and skin incision.
At 6 weeks after the excision procedure, inserted silicone tubes were removed to induce chronic full-thickness supraspinatus tendon tear. After all full-thickness supraspinatus tendon tear was confirmed, the incision site was sutured with nylon monofilament. A total of 32 rabbits were randomly allocated to four treatment groups (n = 8 per group). Group 1 (G1-SAL) was injected with 0.2 mL of normal saline into full-thickness RCT, while group 2 (G2-PDRN) with commercially obtained 0.2 mL of PDRN (Placentex Inj.; Mastelli SRL, San Remo, Italy; Fig. 1a). Group 3 (G3-PDRN+ESWT) and group 4 (ESWT+PDRN) was applied with radial type ESWT (BTL-5000; BTL, Columbia, SC, USA) to the full-thickness RCT after and before the injection, respectively. ESWT was applied with a transmission gel used as a contact medium according to the following parameters: Impulse count 2000 shocks per intervention, 2.5 bar at 3 Hz. After 7 days had passed from the initial intervention, these treatment procedures were repeated four times. All rabbits were euthanized at 4 weeks after each treatment (Fig. 2). All injections were performed by an expert physiatrist under ultrasound (US) guide with a 5–13-MHz multi-frequency linear transducer (Antares; Siemens Healthcare, Erlangen, Germany; Figs. 1b–1d). All rabbits were immobilized in equinus position using an elastic bandage for two days after treatment, and no additional medications were administered.
Rabbits were euthanized under general anesthesia. The tear area of the supraspinatus tendon was segmented and then, fixed with neutral-buffered formalin solution for 24 h. The specimens were embedded in paraffin (Paraplast, Oxford, St. Louis, Missouri, USA) and cut sagittally into 5 μm-thick serial sections. These specimens were stained with Masson’s trichrome (MT) and examined by light microscopy.
Immunohistochemical staining of serial tendon sections was performed to observe collagen fibers using mouse anti-collagen 1 monoclonal antibody (COL-1; Abcam, Cambridge, UK). The tendon sections were stained on proliferating cells using mouse anti-proliferating cell nuclear antigen monoclonal antibody (PCNA, PC10; Santa Cruz Biotechnology, CA, USA), with angiogenetic markers of anti-vascular endothelial growth factor polyclonal antibody (VEGF, A-20; Santa Cruz Biotechnology) and anti-platelet endothelial cell adhesion molecule-1 polyclonal antibody (PECAM-1, M-20; Santa Cruz Biotechnology). Then the paraffin-embedded sections were cleared, dehydrated, and washed using phosphate-buffered saline (PBS). Antigen was retrieved with citrate buffer (pH 6.0) at 95°C for 30 min and then was followed by cooling. Pre-incubation for preventing endogenous peroxidases was conducted with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min. These sections were blocked for non-specific protein binding for 30 min using PBS with 10% normal horse serum or normal goat serum or normal rabbit serum (Vector Laboratories), incubated for 2 h with primary antibodies (1:100–1:200) at room temperature, and washed three times with PBS. The sections were incubated at room temperature for one hour with secondary antibody (1:100), biotinylated anti-mouse IgG, anti-rabbit IgG or anti-goat IgG (Vector Laboratories). Washed with PBS three times, the sections were put on exposure to avidin-biotin-peroxidase complex (ABC, Vector Laboratories) for 1 h and washed three times with PBS. 0.05 M Tris-HCl (pH 7.6) containing 0.01% H2O2 and 0.05% 3,3’-diaminobenzidine (DAB; Sigma-Aldrich) was used to induce peroxidase reaction on the sections, which were then counterstained with hematoxylin and mounted. An Axiophot Photomicroscope (Carl Zeiss, Germany) equipped with an AxioCam MRc5 (Carl Zeiss, Germany) was used for examining the slides, which were respectively evaluated according to its intensity of positive immunostaining.
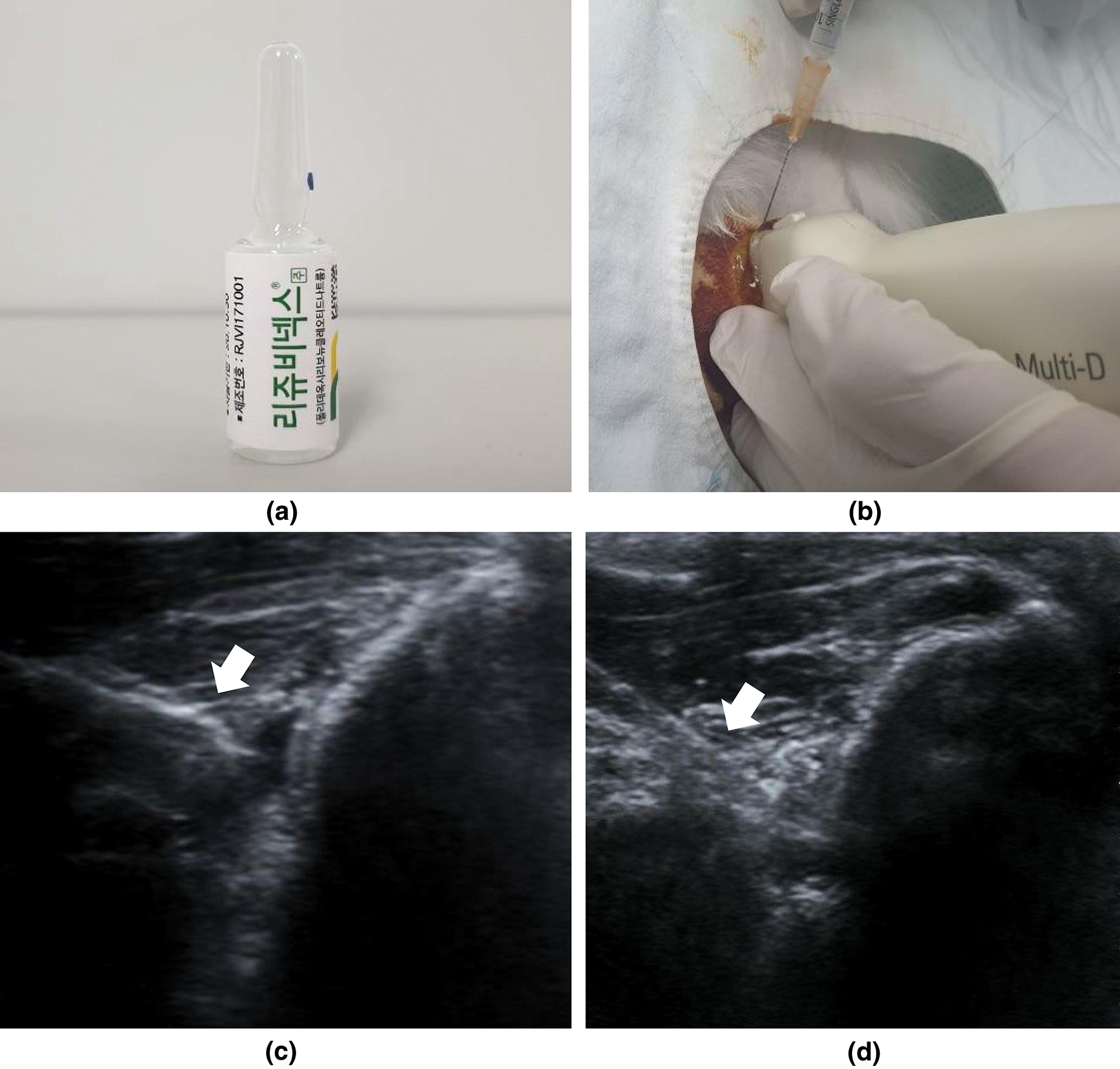
Figure 1: PDRN, injection under ultrasound guidance, and ultrasound images.
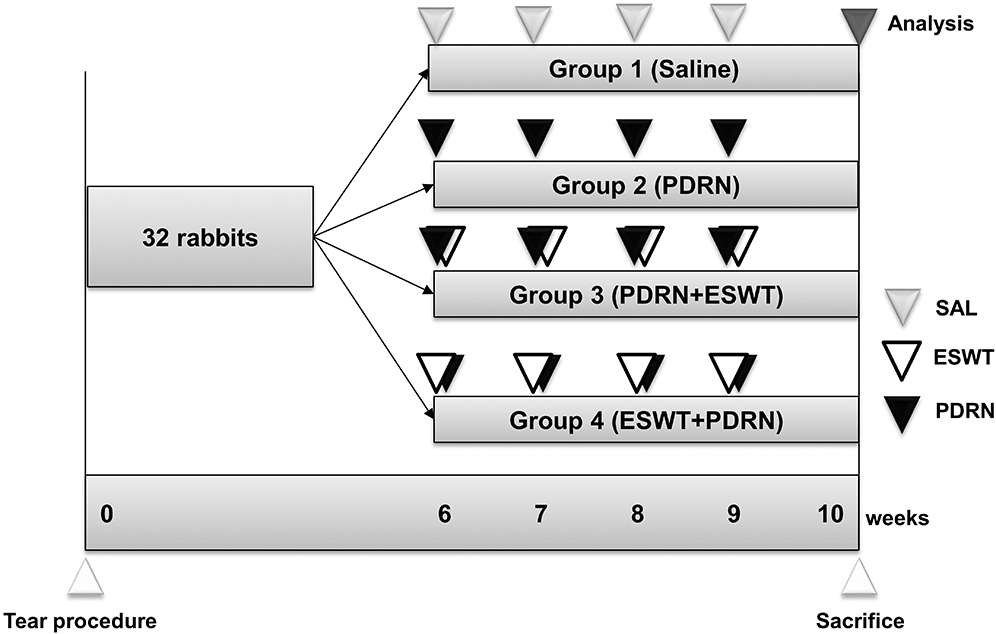
Figure 2: Timeline of experiments.
Gross morphologic examinations were performed after euthanizing each rabbit. Each supraspinatus tendon tear was classified as completely healed, partial-thickness, and full-thickness. Gross morphologic supraspinatus tendon tears were photographed with a solid ruler near the center of the tear site. The size of tendon tears was calculated by tracing the outlined tear edge at pre-treatment and 4 weeks after the treatment using Image J macro software (National Institutes of Health, Bethesda, MD).
Evaluation of immunohistochemical staining
AxioCam MRc5 interfaced with Axiophot Photomicroscope (Carl Zeiss, Germany) was used to photograph thirty fields randomly selected in each group. AxioVision SE64 (Carl Zeiss, Germany) program was used for immunohistochemical analysis. A semi-quantitative scoring system was used for cytoplasmic or nuclear markers PCNA, VEGF, and PECAM-1, with consideration of the staining extent and intensity of the area, which is a previously accepted method used in recent studies (Han et al., 2009; Henriksen et al., 2007). The proportion of positive-stained cells was classified as score 0 to 4, each indicating no cells stained positive, 1–10% positive-stained cells, 11–33%, positive-stained cells, 34–66% positive-stained cells, and 67–100% positive-stained cells. The intensity of MT staining or COL-1 immunostaining was scored as 0 to 3, each indicating negative staining, slight positive staining, moderately positive staining, and strongly positive staining.
Motion analysis of the rabbits was performed at pre-treatment and 4 weeks after the treatment. Before the analysis, the rabbits were habituated to the open field for more than 30 min. They were placed on a 3 × 3 m-sized flat-file and allowed to freely move around for 5 min. Movements of each animal were assessed with a video-tracking system equipped with a camera (SMART 3.0; Panlab, Barcelona, Spain) recording two-dimensional activities of the animal. Three parameters, fast walking time, five-minute walking distance, and mean walking speed, were measured. This assessment had been used in previous studies to evaluate functional outcomes after treatment of RCT (Murray et al., 2016; Kwon et al., 2018a; Kwon et al., 2018b).
Statistical analysis was performed using SPSS for Windows program, version 25.0 (SPSS Inc., Chicago, IL, USA). As well as standard descriptive statistical measures (means and standard deviation), analysis of variance (ANOVA) were used to determine statistical differences among inter- and intra-groups. When ANOVA has shown significant differences between the groups, Tukey’s post-hoc test and Mann–Whitney’s U-test were also performed. 95% confidence intervals were applied for mean values. All data were expressed as mean ± standard deviation. Statistical significance was predetermined to be p < 0.05.
In gross morphologic evaluation, full-thickness tear was observed in eight (100%) rabbits among G1-SAL. In G2-PDRN, full thickness tear was found in five (62.5%) rabbits, while partial-thickness tear in three (37.5%) at 4 weeks after treatment. In G3- PDRN+ ESWT, full thickness tear was found in four (50.0%) rabbits, while partial-thickness tear in four (50.0%). In G4-ESWT+PDRN, full thickness tear was found in three (37.5%) rabbits, while partial-thickness tear in five (62.5%). Significant differences in gross morphologic changes after the treatment were found in all groups except G1-SAL when compared with baseline evaluation (Fig. 4a). In gross morphologic evaluation, mean supraspinatus tendon tear size at 4 weeks after treatment was 13.79 mm2 in G1-SAL, 12.42 mm2 in G2-PDRN, 11.71 mm2 in G3-PDRN+ESWT, and 10.60 mm2 in G4-ESWT+PDRN. There were significant differences in tendon tear size between G1-SAL and the other three groups and between G2-PDRN and G4-ESWT+PDRN (Tab. 1; Figs. 3a and 4b).
Histology and immunohistochemistry
In MT staining assessed with light microscopy, no signs of inflammation were found in all groups. Newly regenerated collagen fibers were observed in MT staining, and these regenerated tendon fibers were stained with COL-1 in G2-PDRN, G3-PDRN+ESWT, and G4-ESWT+PDRN (Fig. 3b, A to H). With the intensity of MT staining, numerous MT-stained cells were revealed, and it is shown that COL-1 positive-cell densities were significantly greater in G2-PDRN, G3-PDRN+ESWT, and G4-ESWT+PDRN than in G1-SAL. MT and COL-1 staining were also found to be significantly greater in values in G4-ESWT+PDRN than in G2-PDRN and G3-PDRN+ESWT (Tab. 1, Fig. 4c). Extensive PCNA staining was observed in regenerated collagen fibers in G2-PDRN, G3-PDRN+ESWT, and G4-ESWT+PDRN (Fig. 3b, I to L). Significant differences in PCNA staining intensities were also found between G2-PDRN and G4-ESWT+PDRN, and between G3-PDRN+ESWT and G4-ESWT+PDRN. Immunohistochemistry staining revealed numerous VEGF-positive cells in G2-PDRN, G3-PDRN+ESWT, and G4-ESWT+PDRN (Fig. 3b, M to P). There were also significant differences in VEGF staining intensities between G1-SAL and the other groups (Tab. 1, Fig. 4c). However, there were no significant differences in VEGF staining intensities between G2-PDRN and G3-PDRN+ESWT, and between G3-PDRN+ESWT and G4-ESWT+PDRN (Tab. 1, Fig. 4c). PECAM-1 positive microvascular densities were shown to be significantly higher in G2-MSC, G3-3D, and G4-3D+MSC than in G1-SAL (Fig. 3b, Q to T). In PECAM-1 staining, significant differences in PECAM-1 positive microvascular densities were also found between G2-PDRN and G4-ESWT+PDRN and between G3-PDRN+ESWT and G4-ESWT+PDRN groups (Tab. 1, Fig. 4c).
Table 1: Tear size, semiquantitative score of histological findings, immunoreactivity of staining, and motion analysis according to treatment groups at 4 weeks after treatments
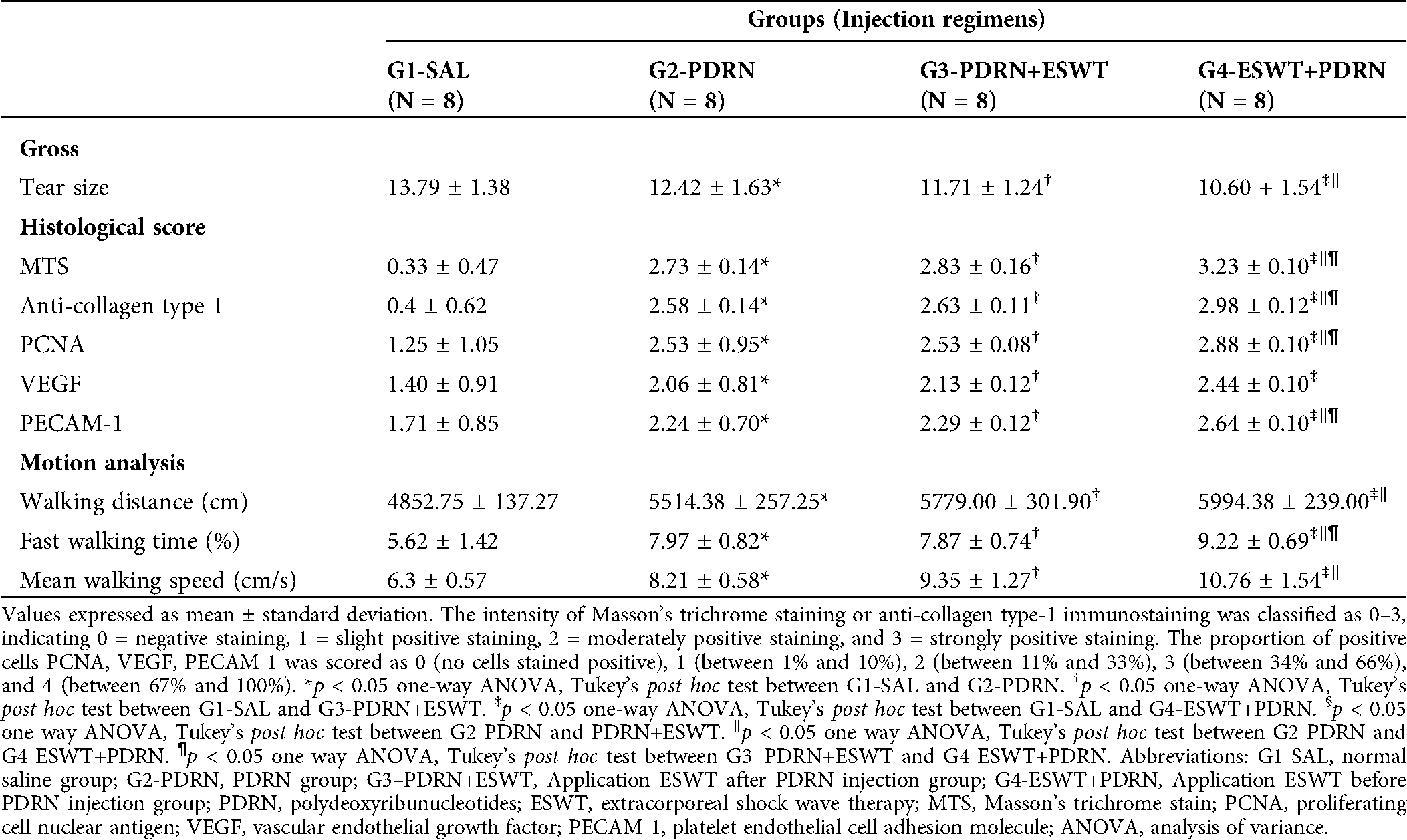
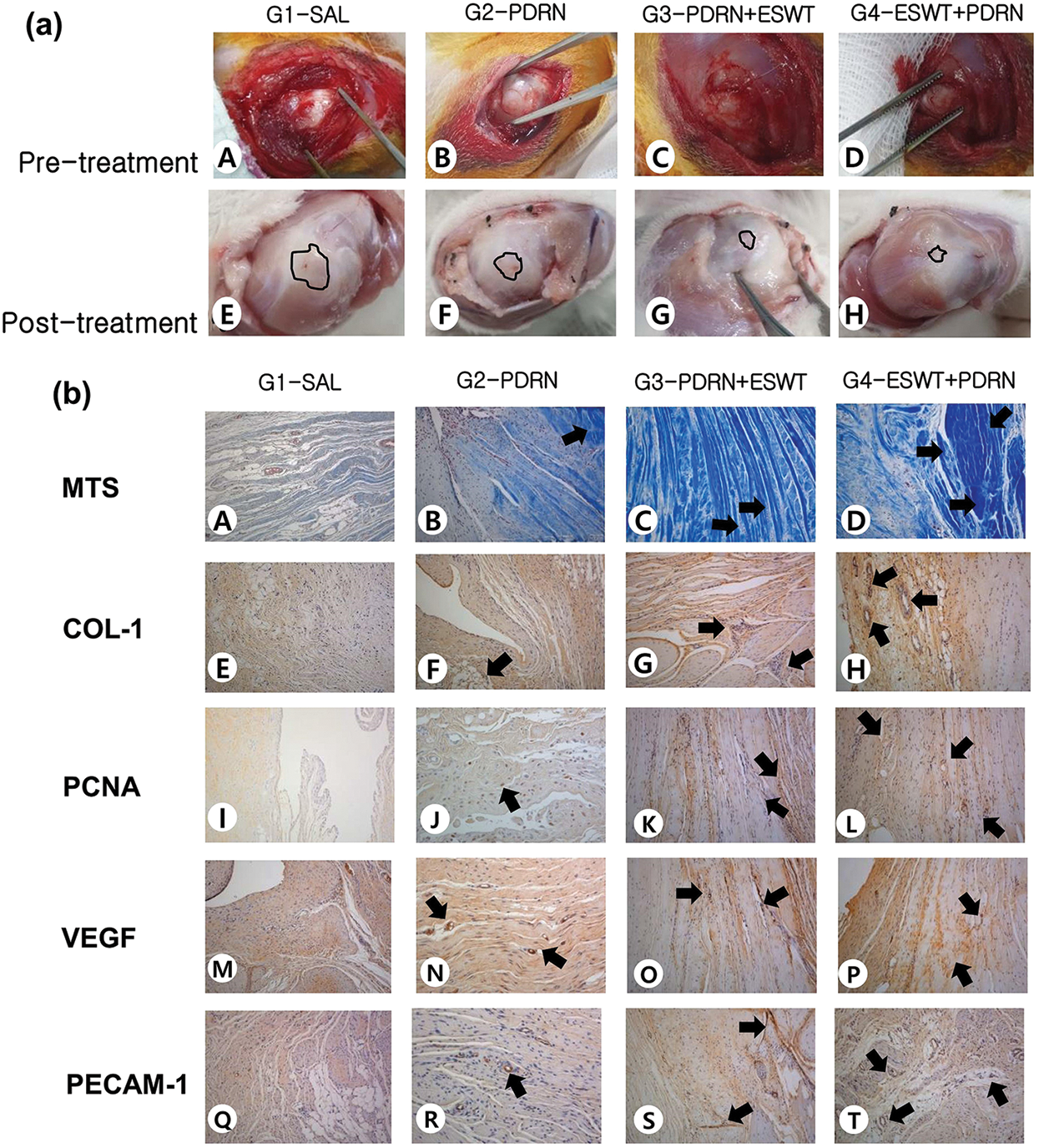
Figure 3: (a) Gross morphological findings of the supraspinatus in G1-SAL, G2-PDRN, G3-PDRN+ESWT, and G4-ESWT+PDRN. Pre-treatment images. FTT (circle) is observed in all four groups (A to D). Post-treatment images. FTT is shown. No gross morphologic changes are found between before and after the treatment (at four weeks post-treatment) in (E) G1-SAL. Significant differences in gross morphologic changes are found between pre-treatment and four weeks after post-treatment in G2-PDRN, G3-PDRN+ESWT, and G4-ESWT+PDRN. (b) Gross morphology and histologic findings of the supraspinatus tendon in Groups 1, 2, 3, and 4. Gross morphological (A–T) findings of the supraspinatus tendons in G1-SAL, G2-PDRN, G3-PDRN+ESWT, and G4-ESWT+PDRN. Newly regenerated tendon fibers were seen in blue (Masson’s trichrome stain; x 100) in Groups 2, 3, and 4. Few regenerated collagen fibers were shown in Group 1 (A to D). Regenerated tendon fibers (x 100) stained with anti-type 1 collagen antibody were shown in Groups 2, 3, and 4. Few regenerated tendon fibers were also observed in group 1 (E to H). Numerous cell proliferating PCNA stained cells (black arrow, x 100) were observed in regenerated tendon fibers in G4-ESWT+PDRN. Lesser PCNA stained cells were observed in G2-PDRN, G3-PDRN+ESWT and few PCNA stained cells were observed in G1-SAL (I to L). VEGF-positive cells and PECAM-1 positive microvascular angiogenesis densities (black arrows, x 100) were numerously observed in G4-ESWT+PDRN. Lesser VEGF-positive cells and PECAM-1 positive microvascular angiogenesis densities were observed in G2-PDRN, and G3-PDRN+ESWT, few in G1-SAL (M to T). In G4-ESWT+PDRN, PCNA, and PECAM-1 positive densities are significantly greater than those of G3-PDRN+ESWT. Abbreviations: G1-SAL, normal saline group; G2-PDRN, PDRN group; G3-PDRN+ESWT, ESWT after PDRN injection group; G4-ESWT+PDRN, ESWT before PDRN injection group; SAL, normal saline; PDRN, polydeoxyribonucleotides; ESWT, Extracorporeal shock wave therapy; FTT, full-thickness tendon tear; MTS, Masson’s trichrome stain; COL-1; collagen type 1; PCNA, proliferating cell nuclear antigen; VEGF, vascular endothelial growth factor; PECAM-1, anti-platelet endothelial cell adhesion molecule-1 polyclonal antibody.
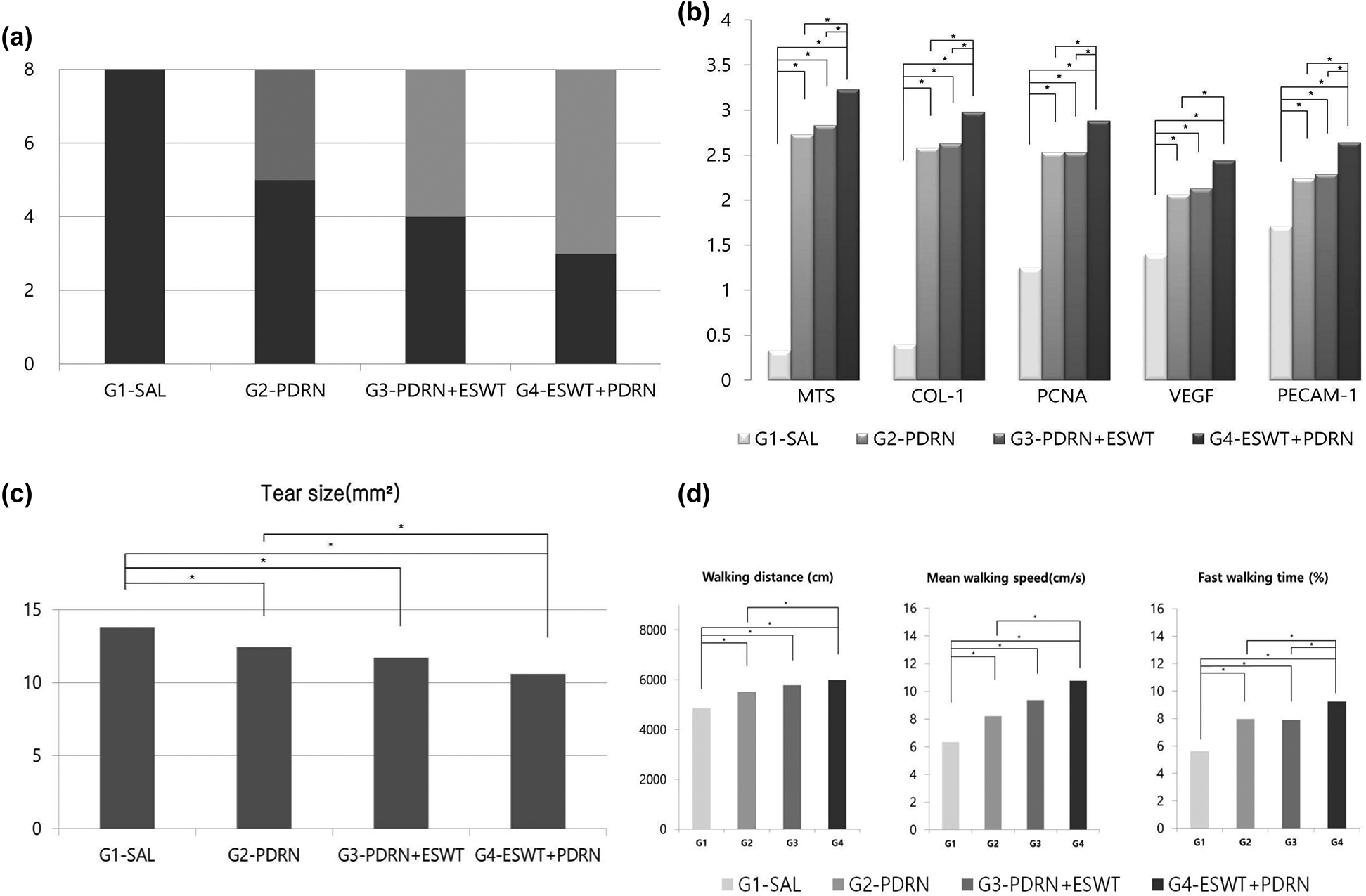
Figure 4: (a) Gross morphology of tear site 4 weeks after treatments. In G1-SAL, FTT was observed in every rabbit. In G2-PDRN, a PTT was found in three rabbits, while FTT in five rabbits. In G3-PDRN+ESWT, Each PTT and FTT was observed in four rabbits. In G4-ESWT+PDRN, a PTT was found in five rabbits, while FTT in three rabbits. (b) Semiquantitative score of histological findings, immunoreactivity of stain. The intensity of MT staining and the proportion of COL-1, PCNA-, VEGF-, and PECAM-1-positive cells were scored as detailed above in methods. (c) Supraspinatus tendon tear size 4 weeks after treatment. No gross morphologic changes between pre-treatment and at 4 weeks after treatment G1-SAL. There are significant differences in gross morphologic changes between pre-treatment and four weeks after treatment in G2-PDRN, G3-PDRN+ESWT, and G4-ESWT+PDRN. (d) Motion analysis of the rabbits at 4 weeks after treatments. In G2-PDRN, G3-PDRN+ESWT, and G4-ESWT+PDRN, walking distance, fast walking time, and mean walking speed are shown to be greater than those in G1-SAL on motion analysis. In addition, G4-ESWT+PDRN showed a significant difference in fast walking time than those in G2-PDRN, G3-PDRN+ESWT. There were no significant differences in walking distance or fast walking time between G2-PDRN, and G3-PDRN+ESWT. *p < 0.05 by one-way ANOVA, Tukey’s post hoc test. Abbreviations: G1-SAL, normal saline group; G2-PDRN, PDRN group; G3-PDRN+ESWT, ESWT after PDRN injection group; G4-ESWT+PDRN, ESWT before PDRN injection group; SAL, normal saline; PDRN, polydeoxyribonucleotides; ESWT, Extracorporeal shockwave therapy; CH, complete healing; PTT, partial-thickness tendon tear; FTT, full-thickness tendon tear; MTS, Masson’s trichrome stain; COL-1, collagen type 1; PCNA, proliferating cell nuclear antigen; VEGF, vascular endothelial growth factor; PECAM-1, anti-platelet endothelial cell adhesion molecule-1 polyclonal antibody; ANOVA, analysis of variance.
In motion analysis, three parameters, walking distance, fast walking time, and mean walking speed were found to be significantly greater in G2-PDRN, G3-PDRN+ESWT, and G4-ESWT+PDRN than in G1-SAL. There were no significant differences in walking distance, fast walking time, and mean walking speed between G2-PDRN and G3-PDRN+ESWT. In motion analysis, walking distance, fast walking time, and mean walking speed were found to be greater in G4-ESWT+PDRN than in the G2-PDRN and G3-PDRN+ESWT (Tab. 1, Fig. 4d).
In this study, local injection of PDRN alone or combined with ESWT application was found to be more effective than normal saline administration in the regeneration of rotator cuff tendon in the rabbit model. In terms of ESWT sequences, it is also shown that the ESWT application may yield better outcomes in several parameters when performed before PDRN injection than later. Therefore, it is suggested that local injection of PDRN alone or combined with ESWT application could improve the microenvironment for regenerative processes of full-thickness RCT without any surgical repair.
PDRN, a mixture of deoxyribonucleic acid polymers, may activate wound healing by stimulating adenosine A2 receptor and thereby enhancing the production of VEGF and angiogenesis (Galeano et al., 2008). Adenosine 2A receptor has specifically been shown to inhibit the inflammatory cytokine, tumor necrosis factor-α (TNF-α) production in human peripheral blood mononuclear cells (Altavilla et al., 2009). Moreover, in another animal study, it has been demonstrated that PDRN may lower the circulating levels and expression of the pro-inflammatory cytokines interleukin 6 (IL-6) and TNF-a in a mouse model of rheumatoid arthritis (Bitto et al., 2011). Animal studies have shown that PDRN is not fatal and is non-toxic to the organs such as the brain, liver, lungs, skeletal muscle, and heart (Polito et al., 2012). The safety of PDRN could be attributed to its non-antigenic properties due to its composition with deoxyribonucleotide linear polymers (Bitto et al., 2008). These biochemical properties of PDRN may have helped the tendon healing process in degenerative tissue.
In this study, 0.2 mL of PDRN was injected into the right full-thickness RCT under US guidance in a rabbit model. There was no established optimal dose of PDRN required for rotator cuff tendon regeneration. In previous clinical studies, 1.5 mL of PDRN was injected three times per week in the patients with plantar fasciitis, while 3 mL of PDRN in the patient with rotator cuff tendinopathy (Kim and Chung, 2015; Yoon et al., 2017). For animal studies, Kwon et al. have reported that successful rotator cuff tendon regeneration was observed with a dose of 0.2 mL PDRN injection in a rabbit model with chronic traumatic full-thickness RCT (Kwon et al., 2018a; Kwon et al., 2018b). Based on these previous findings, we determined to administer 0.2 mL of PDRN per week for four weeks.
We used radial type ESWT to evaluate the synergetic regenerative effects of PDRN. Compared to the commonly used focused type of ESWT, radial type ESWT could be characterized with larger therapeutic areas and simplified applications with the reflection of pathologic zones rather than a point because full-thickness RCT is superficial and larger areas than focal tear (Gollwitzer et al., 2013). Since the radial type ESWT is advantageous in that it does not require accurate targeting with larger treatment area and lower cost (Chang et al., 2012). A previous study showed that Radial shock waves do not appear to structurally damage articular cartilage but do impact chondrocyte viability and membrane permeability in equine cartilage explants (Byron et al., 2005).
In our study, we decided to set the energy level of radial type ESWT to 2.5 bars and 2000 pulses, which is, according to a previous study, equivalent to 0.12 mJ/mm2, when converted into Joules (Kwon, 2016). Unfortunately, no optimal application parameters have been determined that would ensure ESWT effectiveness in this condition. The treatment with low-energy shock wave is recommended for patients with tendinopathies (Rompe et al., 1998). 1000 to 2000 impulses of an energy flux density from 0.01 up to 0.28 mJ/mm2 are usually recommended (Ko et al., 2001; Rompe et al., 1996). Previous trials with high methodological quality ratings (2000 pulses, 2.5–3 bars) in patients with Achilles tendinopathy and (Rompe et al., 2009; Rompe et al., 2007). Moreover, Rompe et al. (1998) reported that the degree of ESWT strength can be classified based on the energy-flux density, as low, medium, and high, indicating <0.08 mJ/mm2, <0.28 mJ/mm2, and <0.60 mJ/mm2, respectively. Several adverse events including tendon edema, fibrillar degeneration, peritendinous fibrosis, and inflammatory cell infiltration may occur in animal experiments with energy flux density over 0.28 mJ/mm2 (Rompe et al., 1998). Antalgic, anti-inflammatory, and angiogenic effects with low and medium energy flux density could be greatly beneficial in clinical treatment (Notarnicola and Moretti, 2012).
With ESWT being applied therapeutically for the musculoskeletal system, successful therapeutic effects have been reported in various disorders such as lateral epicondylitis, plantar fasciitis, patellar tendinitis, and calcific tendinitis of the shoulder (Kisch et al., 2016). However, only a few studies investigating the effects of ESWT on tendon healing were found, and the mechanism of ESWT acting on the tendon is not clearly verified yet. ESWT had previously been reported to enhance the level of nitric oxide, which is related to vasodilation, and VEGF, related to angiogenesis, the collateral vessel formation (Feichtinger et al., 2019). It may also seem to increase cell growth and collagen synthesis, as well as to decrease the expression of inflammatory cytokines, including interleukins and matrix metalloproteases (van der Worp et al., 2013). The recent literature also reported a central mechanism of ESWT related to biomolecular and cellular processes, in which purinergic signaling through the extracellular signal-regulated kinases 1 and 2 pathway and its various growth factors could play an important role (Feichtinger et al., 2019). In this study, the immunohistochemistry has demonstrated that the effects of ESWT mentioned above have affected the tendon healing process.
The exact mechanism of the combined effects of ESWT and PDRN still remains unclear. The combination of ESWT and PDRN could be possibly advantageous in that the ESWT hits the tissue and forms cavitation with conflicting pressures (Notarnicola and Moretti, 2012). The highly localized property of cavitation may exert suppressed stress on endothelial cell membranes. This so-called “shearing stress” increases cell membrane permeability and leads to gene expression by activating growth factors (Apfel, 1982). A previous study (Gambihler and Delius, 1992) showed that no enhanced effect of the combined treatment could be demonstrated when the cells were exposed to cisplatin before or after shock wave treatment. The authors suggested that the result was due to a short-lived ESWT effect about 15-second period from the last shock wave, the cell membranes were permeable (López-Marín et al., 2017) On the contrary, another study indicated that ESWT accelerated the anesthetic effects of the EMLA cream on the rat caudal nerve since shock wave-mediated transdermal drug delivery is possible during the ESWT period (Luh et al., 2018). The results of this study are consistent with our results, the synergetic regenerative effect. Our study demonstrates superior effects when the ESWT is applied before PDRN injection. However, the EMLA with concurrent ESWT group had more anesthetic effect than the EMLA application after ESWT group. In view of sequential exposure to ESWT, there is a difference from our results, which is assumed to be due to the delivery method. Our research injected PDRN directly into full-thickness RCT immediately after ESWT, and in previous study EMLA application after ESWT is transmitted through the skin, it takes a lot of time; therefore, it is presumed that the result difference has occurred.
There are several limitations in this study. First, we created full-thickness RCT just proximal to the insertion site on the supraspinatus tendon, but enthesis healing was not evaluated. Second, the follow-up duration after the treatment was limited to only 4 weeks. Tendon regeneration was identified, but complete healing was not observed. If the observation period were longer than four weeks, more tendon healing or decrement of tear size could have been found. Therefore, further studies on long-term treatment effects are required. Third, motion analysis was used to evaluate rotator cuff function after treatments instead of biomechanical testing. Although motion analysis has not been proven to be better in evaluation than biomechanical testing, it may provide important information distinct from those of histologic examination regarding the therapeutic effect on RCT in both human and animal studies (Kwon et al., 2018a; Kwon et al., 2018b; Keener et al., 2014; Park et al., 2015). Finally, we did not contemplate the influence of PDRN injection dosage that was determined based on the previous studies, and further studies for the optimal dose of those treatment methods should be done.
In conclusion, PDRN injection alone and application of ESWT combined with PDRN injection were found to be effective in the treatment of rabbit model with chronic traumatic full-thickness RCT. Moreover, applying ESWT before PDRN injection has shown significant improvements in parameters indicating angiogenesis, cell proliferation, and fast walking time when compared with ESWT application after PDRN injection. Therefore, the application of ESWT before PDRN injection could be recommended for optimal outcomes of the conservative treatments when the patients with full-thickness RCT are not suitable in surgical treatment.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: This study was approved by the Animal Care Committee (IACUC) of the Animal Hospital of Daegu Catholic University School of Medicine on 22 November, 2018 in the name of DCIAFCR-181108-26.
Author Contribution: The authors confirm contribution to the paper as follows: Study conception and design: Dong Rak Kwon; data collection: Dong Han Kim, Dong Rak Kwon; analysis and iterpretation of results: Dong Han Kim, Dong Rak Kwon, Yong Suk Moon; draft manuscript preparation: Dong Han Kim, Dong Rak Kwon, Gi-Young Park. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B01014260).
Conflicts of Interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Altavilla D, Bitto A, Polito F, Marini H, Minutoli L, Di Stefano V, Irrera N, Cattarini G, Squadrito F. (2009). Polydeoxyribonucleotide (PDRNA safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovascular & Hematological Agents in Medicinal Chemistry 7: 313–321. DOI 10.2174/187152509789541909. [Google Scholar] [CrossRef]
Apfel RE. (1982). Acoustic cavitation: A possible consequence of biomedical uses of ultrasound. The British Journal of Cancer Supplement 5: 140–146. [Google Scholar]
Baydar M, Akalin E, El O, Gulbahar S, Bircan C, Akgul O, Manisali M, Torun Orhan B, Kizil R. (2009). The efficacy of conservative treatment in patients with full-thickness rotator cuff tears. Rheumatology International 29: 623–628. DOI 10.1007/s00296-008-0733-2. [Google Scholar] [CrossRef]
Bitto A, Polito F, Altavilla D, Minutoli L, Migliorato A, Squadrito F. (2008). Polydeoxyribonucleotide (PDRN) restores blood flow in an experimental model of peripheral artery occlusive disease. Journal of Vascular Surgery 48: 1292–1300. DOI 10.1016/j.jvs.2008.06.041. [Google Scholar] [CrossRef]
Bitto A, Polito F, Irrera N, D’Ascola A, Avenoso A, Nastasi G, Campo GM, Micali A, Bagnato G, Minutoli L, Marini H, Rinaldi M, Squadrito F, Altavilla D. (2011). Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A2A receptor. Arthritis & Rheumatism 63: 3364–3371. DOI 10.1002/art.30538. [Google Scholar] [CrossRef]
Byron CR, Benson BM, Stewart AA, Stewart MC. (2005). Effects of radial shock waves on membrane permeability and viability of chondrocytes and structure of articular cartilage in equine cartilage explants. American Journal of Veterinary Research 66: 1757–1763. DOI 10.2460/ajvr.2005.66.1757. [Google Scholar] [CrossRef]
Castricini R, Longo UG, De Benedetto M, Panfoli N, Pirani P, Zini R, Maffulli N, Denaro V. (2011). Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: A randomized controlled trial. American Journal of Sports Medicine 39: 258–265. DOI 10.1177/0363546510390780. [Google Scholar] [CrossRef]
Chang KV, Chen SY, Chen WS, Tu YK, Chien KL. (2012). Comparative effectiveness of focused shock wave therapy of different intensity levels and radial shock wave therapy for treating plantar fasciitis: A systematic review and network meta-analysis. Archives of Physical Medicine and Rehabilitation 93: 1259–1268. DOI 10.1016/j.apmr.2012.02.023. [Google Scholar] [CrossRef]
Coleman SH, Fealy S, Ehteshami JR, MacGillivray JD, Altchek DW, Warren RF, Turner AS. (2003). Chronic rotator cuff injury and repair model in sheep. Journal of Bone and Joint Surgery. American Volume 85: 2391–2402. DOI 10.2106/00004623-200312000-00018. [Google Scholar] [CrossRef]
Feichtinger X, Monforte X, Keibl C, Hercher D, Schanda J, Teuschl AH, Muschitz C, Redl H, Fialka C, Mittermayr R. (2019). Substantial biomechanical improvement by extracorporeal shockwave therapy after surgical repair of rodent chronic rotator cuff tears. American Journal of Sports Medicine 47: 2158–2166. DOI 10.1177/0363546519854760. [Google Scholar] [CrossRef]
Galeano M, Bitto A, Altavilla D, Minutoli L, Polito F, Calò M, Lo Cascio P, Stagno d’Alcontres F, Squadrito F. (2008). Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair and Regeneration 16: 208–217. DOI 10.1111/j.1524-475X.2008.00361.x. [Google Scholar] [CrossRef]
Gambihler S, Delius M. (1992). In vitro interaction of lithotripter shock waves and cytotoxic drugs. British Journal of Cancer 66: 69–73. DOI 10.1038/bjc.1992.218. [Google Scholar] [CrossRef]
Gollwitzer H, Gloeck T, Roessner M, Langer R, Horn C, Gerdesmeyer L, Diehl P. (2013). Radial extracorporeal shock wave therapy (rESWT) induces new bone formation in vivo: Results of an animal study in rabbits. Ultrasound in Medicine & Biology 39: 126–133. DOI 10.1016/j.ultrasmedbio.2012.08.026. [Google Scholar] [CrossRef]
Guo P, Gao F, Zhao T, Sun W, Wang B, Li Z. (2017). Positive effects of extracorporeal shock wave therapy on spasticity in poststroke patients: A meta-analysis. Journal of Stroke and Cerebrovascular Diseases 26: 2470–2476. DOI 10.1016/j.jstrokecerebrovasdis.2017.08.019. [Google Scholar] [CrossRef]
Han CP, Kok LF, Wang PH, Wu TS, Tyan YS, Cheng YW, Lee MY, Yang SF. (2009). Scoring of p16(INK4a) immunohistochemistry based on independent nuclear staining alone can sufficiently distinguish between endocervical and endometrial adenocarcinomas in a tissue microarray study. Modern Pathology 22: 797–806. DOI 10.1038/modpathol.2009.31. [Google Scholar] [CrossRef]
Henriksen KL, Rasmussen BB, Lykkesfeldt AE, Moller S, Ejlertsen B, Mouridsen HT. (2006). Semi-quantitative scoring of potentially predictive markers for endocrine treatment of breast cancer: A comparison between whole sections and tissue microarrays. Journal of Clinical Pathology 60: 397–404. DOI 10.1136/jcp.2005.034447. [Google Scholar] [CrossRef]
Huh J, Shim KS, Cho HJ, Lee BJ, Park D. (2019). Polydeoxyribonucleotide injection in the treatment of patients with carpal tunnel syndrome: Retrospective preliminary study. Medicine 98: e17522. DOI 10.1097/MD.0000000000017522. [Google Scholar] [CrossRef]
Keener JD, Galatz LM, Stobbs-Cucchi G, Patton R, Yamaguchi K. (2014). Rehabilitation following arthroscopic rotator cuff repair: A prospective randomized trial of immobilization compared with early motion. Journal of Bone and Joint Surgery 96: 11–19. DOI 10.2106/JBJS.M.00034. [Google Scholar] [CrossRef]
Killian ML, Cavinatto LM, Ward SR, Havlioglu N, Thomopoulos S, Galatz LM. (2015). Chronic degeneration leads to poor healing of repaired massive rotator cuff tears in rats. The American Journal of Sports Medicine 43: 2401–2410. DOI 10.1177/0363546515596408. [Google Scholar] [CrossRef]
Kim JK, Chung JY. (2015). Effectiveness of polydeoxyribonucleotide injection versus normal saline injection for treatment of chronic plantar fasciitis: A prospective randomised clinical trial. International Orthopaedics 39: 1329–1334. DOI 10.1007/s00264-015-2772-0. [Google Scholar] [CrossRef]
Kisch T, Wuerfel W, Forstmeier V, Liodaki E, Stang FH, Knobloch K, Mailaender P, Kraemer R. (2016). Repetitive shock wave therapy improves muscular microcirculation. Journal of Surgical Research 201: 440–445. DOI 10.1016/j.jss.2015.11.049. [Google Scholar] [CrossRef]
Kwon DR. (2016). Regenerative medicine in the treatment of sports injuries: Prolotherapy and extracorporeal shock wave therapy. Korean Journal of Sports Medicine 34: 1–9. DOI 10.5763/kjsm.2016.34.1.1. [Google Scholar] [CrossRef]
Kwon DR, Park GY, Lee SC (2018a). Treatment of full-thickness rotator cuff tendon tear using umbilical cord blood-derived mesenchymal stem cells and polydeoxyribonucleotides in a rabbit model. Stem Cells International 2018: 1–11. DOI 10.1155/2018/7146384. [Google Scholar] [CrossRef]
Kwon DR, Park GY, Moon YS, Lee SC (2018b). Therapeutic effects of umbilical cord blood-derived mesenchymal stem cells combined with polydeoxyribonucleotides on full-thickness rotator cuff tendon tear in a rabbit model. Cell Transplantation 27: 1613–1622. DOI 10.1177/0963689718799040. [Google Scholar] [CrossRef]
Kwon J, Kim SH, Lee YH, Kim TI, Oh JH. (2019). The rotator cuff healing index: A new scoring system to predict rotator cuff healing after surgical repair. American Journal of Sports Medicine 47: 173–180. DOI 10.1177/0363546518810763. [Google Scholar] [CrossRef]
Ko JY, Chen HS, Chen LM. (2001). Treatment of lateral epicondylitis of the elbow with shock waves. Clinical Orthopaedics and Related Research 387: 60–67. DOI 10.1097/00003086-200106000-00008. [Google Scholar] [CrossRef]
Lewis JS. (2009). Rotator cuff tendinopathy/subacromial impingement syndrome: Is it time for a new method of assessment? British Journal of Sports Medicine 43: 259–264. DOI 10.1136/bjsm.2008.052183. [Google Scholar] [CrossRef]
López-Marín LM, Millán-Chiu BE, Castaño-González K, Aceves C, Fernández F, Varela-Echavarría A, Loske AM. (2017). Shock wave-induced damage and poration in eukaryotic cell membranes. Journal of Membrane Biology 250: 41–52. DOI 10.1007/s00232-016-9921-2. [Google Scholar] [CrossRef]
López-Marín LM, Rivera AL, Fernández F, Loske AM. (2018). Shock wave-induced permeabilization of mammalian cells. Physics of Life Reviews 26: 1–38. [Google Scholar]
Luh JJ, Huang WT, Lin KH, Huang YY, Kuo PL, Chen WS. (2018). Effects of extracorporeal shock wave-mediated transdermal local anesthetic drug delivery on rat caudal nerves. Ultrasound in Medicine & Biology 44: 214–222. DOI 10.1016/j.ultrasmedbio.2017.09.010. [Google Scholar] [CrossRef]
Murray IR, LaPrade RF, Musahl V, Geeslin AG, Zlotnicki JP, Mann BJ, Petrigliano FA. (2016). Biologic treatments for sports injuries II think tank-current concepts, future research, and barriers to advancement, part 2: Rotator cuff. Orthopaedic Journal of Sports Medicine 4: 2325967116636586. [Google Scholar]
Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C, Peault B. (2014). Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cellular and Molecular Life Sciences 71: 1353–1374. DOI 10.1007/s00018-013-1462-6. [Google Scholar] [CrossRef]
Notarnicola A, Moretti B. (2012). The biological effects of extracorporeal shock wave therapy (eswt) on tendon tissue. Muscles, Ligaments and Tendons Journal 2: 33–37. [Google Scholar]
Osti L, Buda M, Buono AD, Osti R, Massari L. (2019). Clinical evidence in the treatment of rotator cuff tears with hyaluronic acid. Muscles Ligaments and Tendons Journal 5: 270–275. DOI 10.32098/mltj.04.2015.03. [Google Scholar] [CrossRef]
Pan PJ, Chou CL, Chiou HJ, Ma HL, Lee HC, Chan RC. (2003). Extracorporeal shock wave therapy for chronic calcific tendinitis of the shoulders: A functional and sonographic study. Archives of Physical Medicine and Rehabilitation 84: 988–993. DOI 10.1016/S0003-9993(03)00010-8. [Google Scholar] [CrossRef]
Park GY, Kwon DR, Lee SC. (2015). Regeneration of full-thickness rotator cuff tendon tear after ultrasound-guided injection with umbilical cord blood-derived mesenchymal stem cells in a rabbit model. Stem Cells Translational Medicine 4: 1344–1351. DOI 10.5966/sctm.2015-0040. [Google Scholar] [CrossRef]
Polito F, Bitto A, Galeano M, Irrera N, Marini H, Calo M, Squadrito F, Altavilla D. (2012). Polydeoxyribonucleotide restores blood flow in an experimental model of ischemic skin flaps. Journal of Vascular Surgery 55: 479–488. DOI 10.1016/j.jvs.2011.07.083. [Google Scholar] [CrossRef]
Rompe JD, Furia J, Maffulli N. (2017). Eccentric loading versus eccentric loading plus shock-wave treatment for midportion Achilles tendinopathy: A randomized controlled trial. American Journal of Sports Medicine 37: 463–470. DOI 10.1177/0363546508326983. [Google Scholar] [CrossRef]
Rompe JD, Hopf C, Küllmer K, Heine J, Bürger R, Nafe B. (1996). Low-energy extracorporal shock wave therapy for persistent tennis elbow. International Orthopaedics 20: 23–27. DOI 10.1007/s002640050021. [Google Scholar] [CrossRef]
Rompe JD, Kirkpatrick CJ, Kullmer K, Schwitalle M, Krischek O. (1998). Dose-related effects of shock waves on rabbit tendo Achillis. A sonographic and histological study. Journal of Bone and Joint Surgery. British Volume 80: 546–552. DOI 10.1302/0301-620X.80B3.0800546. [Google Scholar] [CrossRef]
Rompe JD, Nafe B, Furia JP, Maffulli N. (2017). Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: A randomized controlled trial. American Journal of Sports Medicine 35: 374–383. DOI 10.1177/0363546506295940. [Google Scholar] [CrossRef]
Schwitzguebel AJ, Kolo FC, Tirefort J, Kourhani A, Nowak A, Gremeaux V, Saffarini M, Ladermann A. (2019). Efficacy of platelet-rich plasma for the treatment of interstitial supraspinatus tears: A double-blinded, randomized controlled trial. American Journal of Sports Medicine 47: 1885–1892. DOI 10.1177/0363546519851097. [Google Scholar] [CrossRef]
van der Worp H, van den Akker-Scheek I, van Schie H, Zwerver J. (2013). ESWT for tendinopathy: Technology and clinical implications. Knee Surgery, Sports Traumatology, Arthroscopy 21: 1451–1458. DOI 10.1007/s00167-012-2009-3. [Google Scholar] [CrossRef]
Yoon YC, Lee DH, Lee MY, Yoon SH. (2017). Polydeoxyribonucleotide injection in the treatment of chronic supraspinatus tendinopathy: A case-controlled, retrospective, comparative study with 6-month follow-up. Archives of Physical Medicine and Rehabilitation 98: 874–880. DOI 10.1016/j.apmr.2016.10.020. [Google Scholar] [CrossRef]
Zhong Z, Liu B, Liu G, Chen J, Li Y, Chen J, Liu X, Hu Y. (2019). A randomized controlled trial on the effects of low-dose extracorporeal shockwave therapy in patients with knee osteoarthritis. Archives of Physical Medicine and Rehabilitation 100: 1695–1702. DOI 10.1016/j.apmr.2019.04.020. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |