DOI:10.32604/biocell.2021.014569

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.014569 |  www.techscience.com/journal/biocell |
| Article |
Human adipose, placenta, and umbilical cord-derived mesenchymal stem cells ameliorate imiquimod-induced psoriatic mice via reducing T cells infiltration
1Cellular Biomedicine Group, Shanghai, 200233, China
2Translational Medicine Center, Shanghai General Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, 200025, China
3Shanghai Institute of Immunology, Shanghai Jiaotong University School of Medicine, Shanghai, 200025, China
*Address correspondence to: Chengxiang Dai, morgile@126.com
#These authors contributed equally to this work
Received: 09 October 2020; Accepted: 21 December 2020
Abstract: Psoriasis is an autoimmune-related chronic inflammatory disease with an approximate prevalence of 2–3% around the world, involving increased keratinocyte proliferation. Indeed, Th17 cells and IL-17 play critical roles in the pathogenesis of psoriasis. The monoclonal antibodies against cytokines have been shown to have effectively immunosuppressive effects on human psoriasis. However, there are still some patients that have no response to these treatments. Some patients have even serious side-effects which may affect their life. Mesenchymal stem cells have the ability of immunosuppressive and anti-inflammatory effects, which may be an alternative therapy with more safety and efficacy for human psoriasis. Moreover, the underlying mechanisms by which the MSCs prevent or ameliorate psoriasis are still poorly understood. Here, we first isolated and characterized human adipose, placenta, and umbilical cord-derived mesenchymal stem cells (haMSCs, hpMSCs, and huMSCs). After that, the animal model of imiquimod (IMQ)-induced psoriasis in C57BL/6 mice was confirmed. We investigated the impact of haMSCs, hpMSCs, and huMSCs on this model by H&E staining, immunohistochemistry staining, and quantitative real-time PCR. Data analysis showed that mice subcutaneously injected with these MSCs had a significantly decreased epidermal thickness, which was caused by obviously reduced hyper-proliferation of keratinocytes. Furthermore, our findings revealed that the infiltration of T cells to psoriatic lesions in IMQ-induced psoriasis mice was markedly downregulated by intradermal administration of haMSCs, hpMSCs, and huMSCs, respectively. Consequently, the production of IL-17 from Th17 cells was reduced, which inhibits the proliferation of keratinocytes in lesioned skin of IMQ-induced psoriasis mice. These data suggest that haMSCs, hpMSCs, and huMSCs can inhibit the effects of proinflammatory Th17 cells on the development of psoriasis, which may be potential therapeutic candidates for skin inflammatory disease or other autoimmune diseases.
Keywords: Mesenchymal stem cells; Psoriasis; T cells; Skin inflammation; Cell therapy
Psoriasis is an autoimmune-related chronic inflammatory disease, which can represent various phenotypes on the skin, and has a complex genetic architecture (Lebwohl, 2003). Psoriatic patients are widely distributed around the world, with an approximate prevalence of 2–3% (Greb et al., 2016). Generally, psoriasis manifest as various phenotypes that include vulgaris psoriasis, guttate psoriasis, inverse psoriasis, pustular psoriasis, palmoplantar psoriasis, and erythrodermic psoriasis, in which vulgaris psoriasis is the main manifestation (Greb et al., 2016). The pathological characteristics of psoriatic skin mainly manifest as epidermal hyperplasia, increased angiogenesis, dilated capillary vessels, increased immune cell infiltration, and related cytokines and chemokines (Lowes et al., 2007; Griffiths and Barker, 2007). Psoriasis is strongly associated with proinflammatory T cells, in which T-helper 1 (Th1) and T-helper 17 (Th17) cells play an important role in the pathogenesis of psoriasis (Lowes et al., 2008). However, Th17 cells are proposed to be a major player in that IL-17 secreted from Th17 cells is a crucial proinflammatory cytokine in psoriasis (Di Cesare et al., 2009). The skin inflammation in psoriasis is caused by T cells and neutrophils infiltration into the dermis and epidermis, which reduces the antioxidant capacity of the skin (Sah et al., 2016).
Imiquimod (IMQ) is a ligand for toll-like receptor 7 and 8 (TLR7/8) and has been reported to induce psoriasis-like inflammatory skin disease via activating the IL23/IL17 axis, which partially dependent on the presence of T cells (van der Fits et al., 2009). Moreover, the topical application of IMQ increases the level of relative chemokines and pro-inflammatory cytokines in the derma. These features are in accordance with that of psoriasis in patients. Therefore, IMQ-induced psoriasis in mice can closely resemble human psoriasis (van der Fits et al., 2009), and this mouse model is generally used to evaluate the therapeutic effects of some drugs on psoriasis (Li et al., 2020). Additionally, there are also many psoriatic mouse models, which included xenograft models, spontaneous models, transgenic models, and IL-23-induced models (Bocheńska et al., 2017).
Currently, therapeutic approaches of biologics and cell transplantation are being developed and explored. In previous reports, the drugs of monoclonal antibodies against cytokines have been shown to have effectively immunosuppressive effects on human psoriasis (Di Cesare et al., 2009). However, some patients have no response to the treatment of monoclonal antibody drugs, and their therapeutic effect is low or ineffective (Gedebjerg et al., 2013). Moreover, during these antibody drugs treatment, some patients have common side-effects, even serious side-effects which may affect their life, including hypertension, heart failure, and liver dysfunction (Jeon et al., 2017a). Thus, it is urgent to develop an alternative therapy with more safety and efficacy for human psoriasis.
Mesenchymal stem cells (MSCs) are multipotent progenitor cells, which have strong immunosuppressive and anti-inflammatory effects through the interaction with immune cells or soluble factors secreted from MSCs (Su et al., 2011). Various studies have indicated that the MSCs derived from bone marrow, human umbilical cord blood, human palatine tonsil, and human embryonic stem cell could be used for prevention or treatment of autoimmune diseases in mice, such as encephalomyelitis (Zappia et al., 2005), arthritis (Augello et al., 2007), atopic dermatitis (Na et al., 2014), psoriasis (Sah et al., 2016; Lee et al., 2017; Cho et al., 2017; Kim et al., 2019). However, above these MSCs are not easily available, which does not benefit for the large-scale production and MSCs-based therapeutics for clinical use. In addition, the underlying mechanisms by which the MSCs prevent or ameliorate psoriasis are still poorly understood. In this study, we aimed to explore the effects of human adipose, placenta, and umbilical cord-derived mesenchymal stem cells (haMSCs, hpMSCs, and huMSCs) on IMQ-induced psoriasis in mice and their possible mechanisms.
Isolation and culture of haMSCs, hpMSCs, and huMSCs
The haMSCs were isolated from lipoaspirates and cultured as the previous description (Wang et al., 2015). The human placenta and umbilical cord were obtained with informed consent from healthy mothers undergoing cesarean section. The procedure was approved by the Institutional Patients and Ethics Committee of Shanghai Baijia Maternity Hospital (Shanghai, China). Human placenta and umbilical cord-derived mesenchymal stem cells (hpMSCs and huMSCs) were isolated according to the modified method (Ikhsan et al., 2020; Putra et al., 2020). In brief, the tissue of the human placenta and umbilical cord were rinsed to remove the blood cells with phosphate-buffered saline (PBS) containing 1% penicillin/streptomycin solution and then cut into ~25 mm2 pieces with scissors. Subsequently, these pieces were resuspended in 20 mL PBS containing 0.2% Type II collagenase (Gibco, #17101-015, USA) digestion and incubated on a shaker at 37°C for 2 h. The supernatant was filtered through a 100 μm cell sieve (BD Biosciences, San Jose, CA, USA). The filtrate was centrifuged at 300 × g for 10 min. Finally, the cell pellets were resuspended and cultured in a T75 flask with α-MEM medium (Gibco, C12571500BT, USA) supplemented with 5% EliteGro (EliteCell, EPAGMP-500, USA) in a humidified incubator at 37°C with 5% CO2. After culturing for 24 h, the unattached cells were removed. The fresh medium was added to the remaining cells, which were defined as passage 0 (P0). The medium was changed every 2 days. When they were cultured to the confluence of 80–90%, the cells were trypsinized and sub-cultured. The hpMSCs and huMSCs at P3-P4 were harvested for animal study.
The MSCs of 1 × 106 were resuspended in 100 μL PBS and incubated with 1 μg phycoerythrin (PE)-conjugated, allophycocyanin (APC)-conjugated, fluorescein isothiocyanate (FITC)-conjugated, or peridinin-chlorophyll-protein (PerCP)-Cy5.5- conjugated anti-human monoclonal antibodies in the dark for 30 min at 4°C. These antibodies included the following: CD34 (Biolegend, #343503, USA), CD45 (Biolegend, #304008, USA), CD73 (Biolegend, #344016, USA), CD90 (Biolegend, #328118, USA), CD105 (Biolegend, #800508, USA), and HLA-DR (Biolegend, #307603, USA). Subsequently, These MSCs were washed with cold PBS and then analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA).
Trilineage differentiation of haMSCs, hpMSCs, and huMSCs in vitro
Adipogenesis, osteogenesis, and chondrogenesis of haMSCs, hpMSCs, and huMSCs were respectively determined as the previous description (Ma et al., 2019). Briefly, the MSCs at passage 4 were seeded onto 6-well plates at 1 × 104 cells per cm2 for adipogenesis and osteogenesis and maintained with the culture medium in a humidified incubator at 37°C with 5% CO2. When the MSCs had a 90% confluency, the culture medium was replaced by adipogenic or osteogenic differentiation medium (Gibco, USA) and kept for 3 weeks. For chondrogenic differentiation, 1 × 106 cells resuspended in 1 mL chondrogenic differentiation medium were centrifuged at 300×g for 5 min and kept in conical tubes for 4 weeks. Samples were fixed in 4% paraformaldehyde solution for 30 min. Positive induction of adipogenesis and osteogenesis was respectively confirmed by Oil Red O staining (Sciencell, SC-0843, USA) and Alizarin Red staining (Sciencell, SC-0223, USA). Cryosection of a chondrogenic pellet was carried out, and the section was stained with Safranin O staining (Sciencell, SC-8348, USA).
C57BL/6 wild-type male mice were purchased from Shanghai SLAC laboratory animal center (Shanghai, China). All mice used for the study at 8 weeks of age were provided with food and water under specific pathogen-free conditions on a 12-hours light/dark cycle. All experiments were performed in accordance with the animal ethics committee of the Scientific Investigation Board of Shanghai Jiaotong University School of Medicine, Shanghai, China (IACUC approval No. 2019-A021-01). Age- and sex-matched mice were randomly divided into five groups of six mice each. Mice were received a daily topical dose of 62.5 mg of commercially available IMQ cream (5% the active compound) (MedShine, #120503, China) on shaved back skin for 7 consecutive days (van der Fits et al., 2009), excluded the blank control mice. The 100 μL of 2 × 106 MSCs suspended in the saline (Lee et al., 2017) or saline was subcutaneously injected into the back of IMQ-treated mice using a 26-gauge needle on day −1, 3, and 5, respectively. Meanwhile, negative control mice received a subcutaneous injection of an equal volume of saline. Finally, after mice were sacrificed on day 7, the samples of dorsal skin were collected for analysis.
Histological analysis and immunohistochemistry
Skin samples were fixed in 4% paraformaldehyde for 24 h. After washed with distilled water, the samples were dehydrated in a graded ethanol series and then embedded in paraffin (Jeon et al., 2017b). 5 μm-thick sections on slides were used for hematoxylin and eosin (H&E) and immunohistochemistry staining. Epidermal hyperplasia (acanthosis) was assessed using the average area of measurements of five random fields per section. Briefly, the epidermal area was outlined and measured by the lasso tool in Adobe Photoshop CS4 (Yan et al., 2015). And the epidermal thickness was measured as the average of 20 random measures of five random fields per section between stratum basale and stratum granulosum, excluding the stratum corneum and hair follicles.
For immunohistochemistry staining, sections were incubated with primary rabbit monoclonal anti-mouse CD3 (1:150, Abcam, ab16669, USA) or rabbit polyclonal anti-mouse Ki67 (1:200, Abcam, ab15580, USA) antibody at 4°C overnight, and then with diluted biotin-conjugated anti-rabbit IgG at room temperature following the manufacturer’s instructions. The twenty-four images of stained sections of each sample were randomly captured by light microscopy (Axioscope A1, Zeiss). The number of dermal infiltrating cells, CD3+ cells, and epidermal Ki67+ cells per field was calculated using Adobe Photoshop CS4.
Total RNA of skin samples was extracted using the RNAiso Plus (TaKaRa, #9108/9109, Japan) and quantified by NanoDrop spectrophotometer (ND-1000). And then, 1 μg of total RNA was reverse transcribed into complementary DNA (cDNA) using HiScript® III RT SuperMix (Vazyme, R323-01, China). 50 ng of cDNA per well was distributed on plates and run in triplicate. Real-time PCR was carried out with TaqMan® Gene Expression Master Mix (ThermoFisher, #4369016, USA) and TaqMan probes (ThermoFisher, USA) of IL-17 (Mm00439618_m1) and GAPDH (Mm99999915_g1) in a QuantStudioTM DX real-time PCR instrument (Applied Biosystems). The PCR program consisted of 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The relative expression of target genes was calculated and normalized to GAPDH expression by the 2−ΔΔCT method (Yan et al., 2015).
The data were analyzed with GraphPad Prism 5.0 (GraphPad Software Inc.) and expressed as the mean ± SEM. The unpaired two-tailed Student’s t-test was used for statistical comparisons between two groups. One-way analysis of variance (ANOVA) followed by post hoc Tukey test was used for multiple groups. p values of <0.05 were considered statistically significant.
Isolation and characterization of human adipose, placenta, and umbilical cord-derived mesenchymal stem cells
To investigate the effects of haMSCs, huMSCs, and hpMSCs on IMQ-induced psoriasis in mice, we first isolated and characterized these mesenchymal stem cells. Flow cytometry analysis showed that the population of haMSCs, huMSCs, and hpMSCs positively expressed (≥95%) CD73, CD90, and CD105, and negatively expressed (≤2%) CD34, CD45, and HLA-DR (Fig. 1A). Furthermore, the results of trilineage differentiation of haMSCs, huMSCs, and hpMSCs displayed that they could be differentiated toward adipocytes, osteocytes, and chondrocytes in specified differentiation media, respectively (Fig. 1B). Therefore, these findings demonstrate that the isolated cells were confirmed to the characterization of haMSCs, huMSCs, and hpMSCs, respectively.
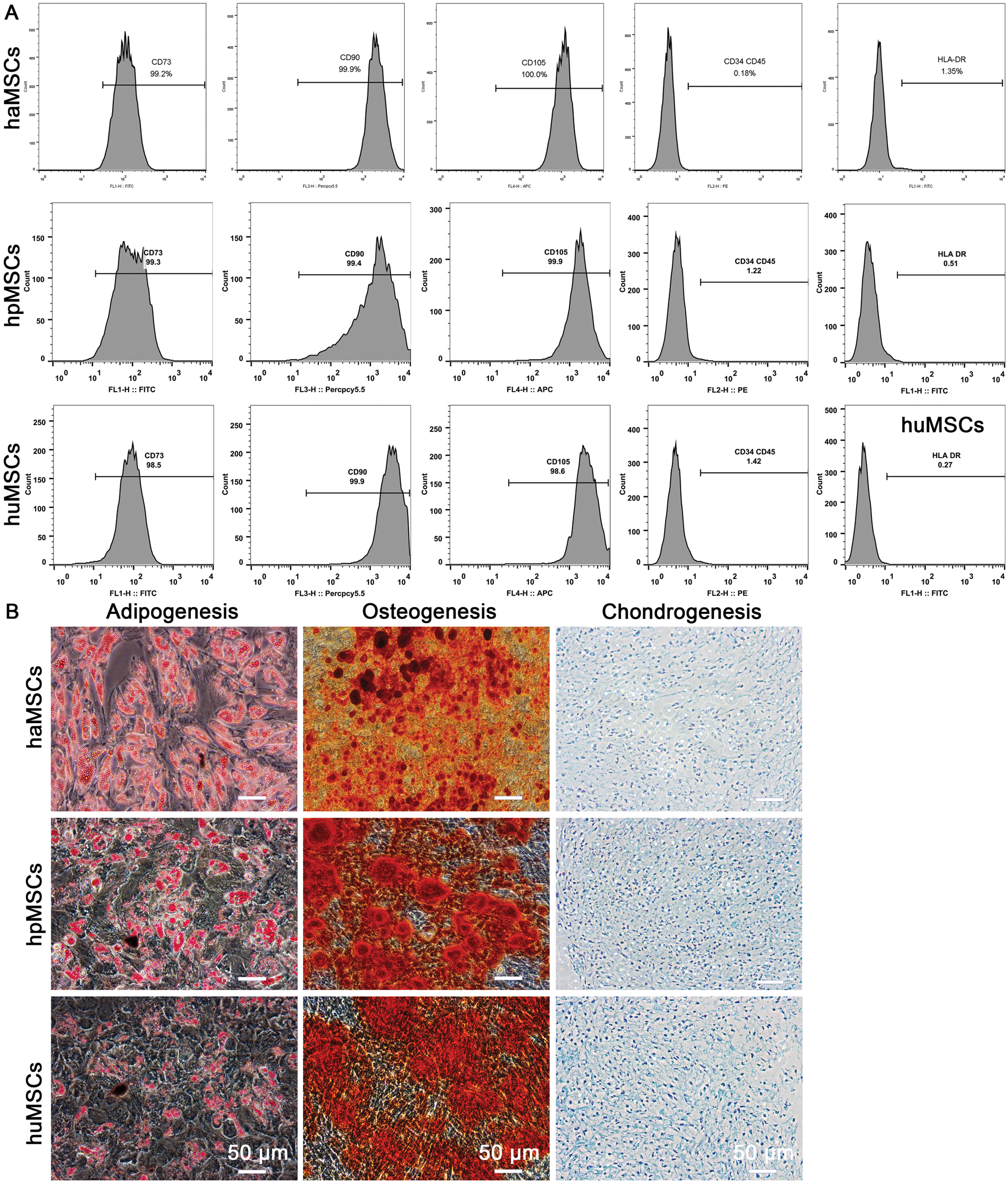
Figure 1: Isolation and characterization of human adipose, placenta, and umbilical cord-derived mesenchymal stem cells (haMSCs, hpMSCs, and huMSCs).
Establishment of IMQ-induced psoriasis in mice
To determine whether haMSCs, huMSCs, and hpMSCs can prevent psoriasis-like skin inflammation, we first confirmed the animal model of IMQ-induced psoriasis in C57BL/6 mice. (Fig. 2A). Our results showed the back skin became scaly and thickened in IMQ-induced psoriasis mice (Fig. 2B). Quantitative analysis of H&E-stained sections showed that the epidermal thickness was significantly increased in IMQ-induced psoriasis mice compared with control mice (p < 0.001, Figs. 2C and 2D). Moreover, in contrast with control mice, the number of dermal infiltrating cells was also markedly enhanced in IMQ-induced psoriasis mice (p < 0.001, Fig. 2E). Thus, these results indicate that the animal model of IMQ-induced psoriasis in C57BL/6 mice was successfully confirmed.
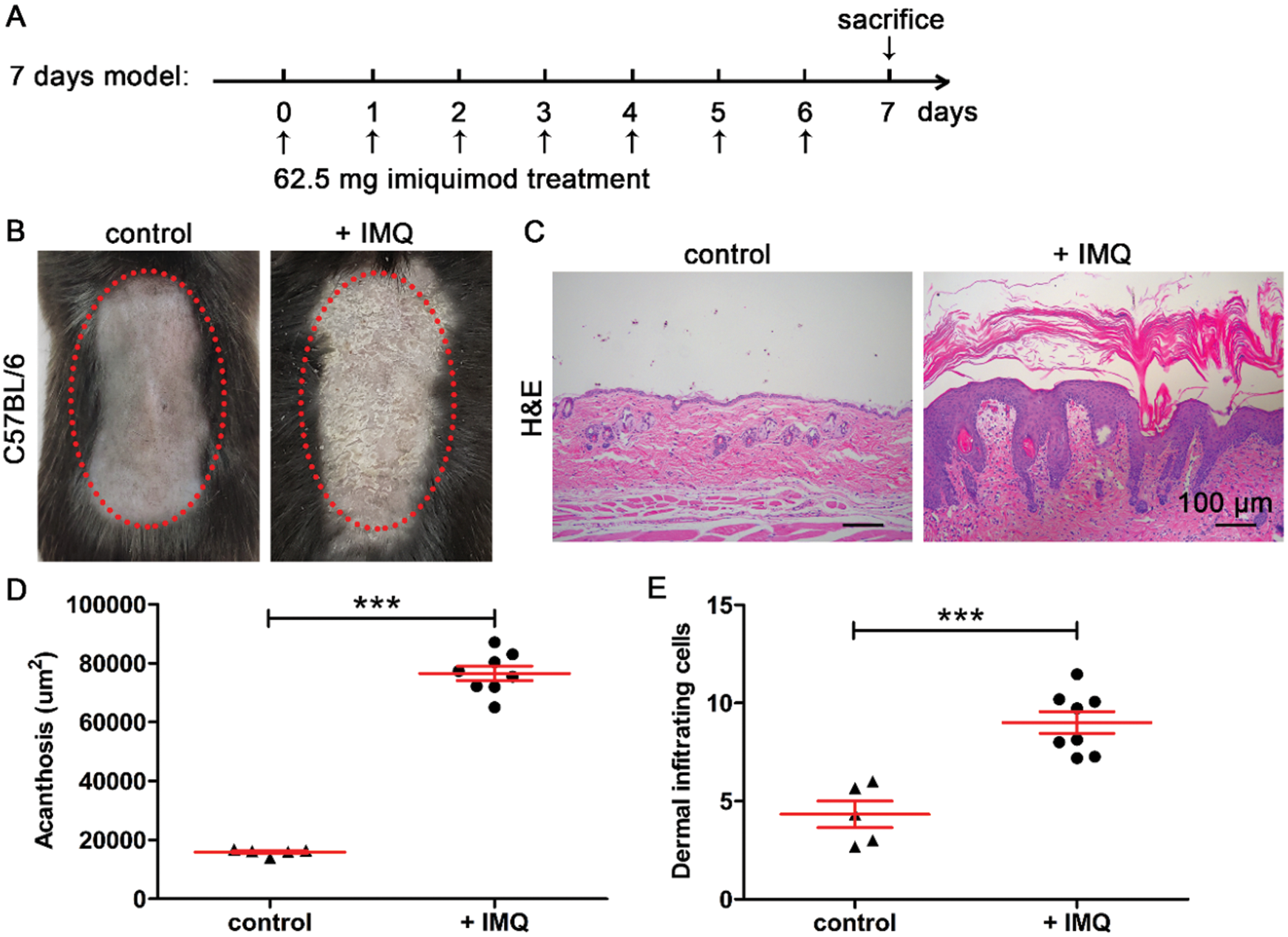
Figure 2: Establishment of IMQ-induced psoriasis model in mice.
Human adipose, placenta, and umbilical cord-derived mesenchymal stem cells inhibit epidermal hyperplasia in the skin of IMQ-induced psoriasis mice
In order to examine the inhibitory effects of haMSCs, huMSCs, and hpMSCs on psoriasis development, these MSCs were subcutaneously injected on days −1, 3, and 5, respectively, and psoriasis was induced by imiquimod for 7 consecutive days (Fig. 3A). The observation of the back skin in mice showed that these MSCs may improve the appearance of psoriasis to a certain extent (Fig. 3B). Subsequently, histopathological analysis of dorsal skin samples was performed by H&E staining and immunohistochemistry staining. Compared with IMQ and saline-treated mice, a reduced epidermal thickness of IMQ-induced mice subcutaneously injected with haMSCs, huMSCs or hpMSCs was observed (Fig. 3C). Moreover, quantitative analysis of epidermal thickness showed that mice treated with haMSCs (p < 0.05), huMSCs (p < 0.01) or hpMSCs (p < 0.01) had a significantly decreased epidermal thickness, compared with saline-treated mice (Fig. 3D). Interestingly, ameliorative effects of huMSCs and hpMSCs on IMQ-induced epidermal thickness seemed to be more effective than that of haMSCs-treated mice, but there were no significant differences among them (p > 0.05, Fig. 3D).
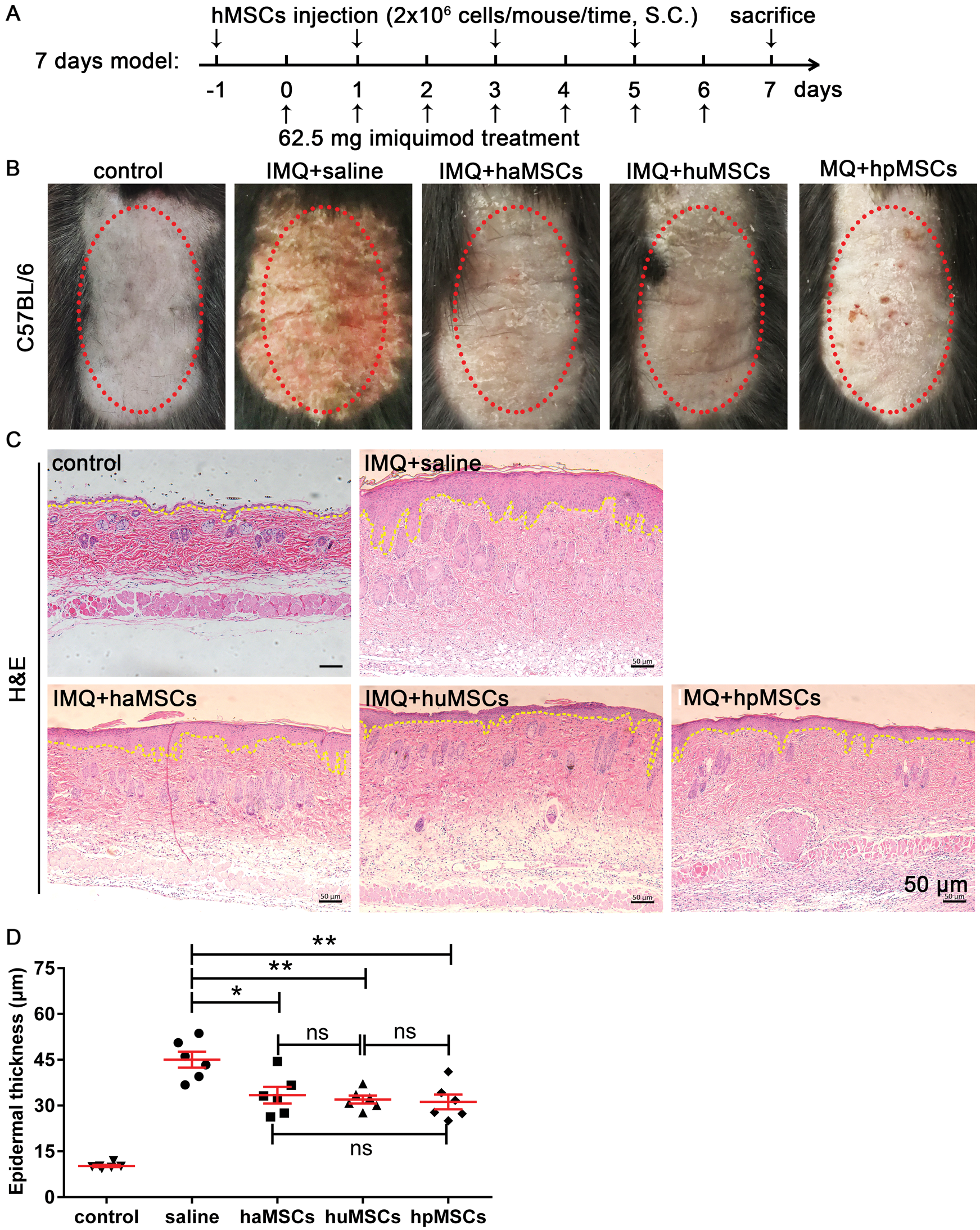
Figure 3: Human adipose, placenta, and umbilical cord-derived mesenchymal stem cells reduce epidermal thickness in lesional skin of IMQ-induced psoriasis mice.
Human adipose, placenta, and umbilical cord-derived mesenchymal stem cells inhibit keratinocyte proliferation in the skin of IMQ-induced psoriasis mice
Psoriasis is characterized by keratinocyte proliferation (Perera et al., 2012). Thus, we explored the level of keratinocyte proliferation in the epidermis of IMQ-induced psoriasis mice by immunohistochemistry staining of Ki67. The data revealed that mice subcutaneously injected with haMSCs, huMSCs or hpMSCs displayed a reduced hyper-proliferation of keratinocytes in the basal layer of the epidermis, compared with saline-treated mice (Fig. 4A). And quantitative analysis of the number of Ki67+ cells per field in the epidermis of haMSCs, huMSCs or hpMSCs-treated mice also revealed hyper-proliferation of keratinocytes was obviously reduced, compared with saline-treated mice (p < 0.01, Fig. 4B). These results are consistent with that of H&E staining analysis of epidermal hyperplasia. Consequently, these findings indicate that the administration of haMSCs, huMSCs or hpMSCs could effectively inhibit the epidermal hyperplasia of IMQ-induced psoriasis mice.
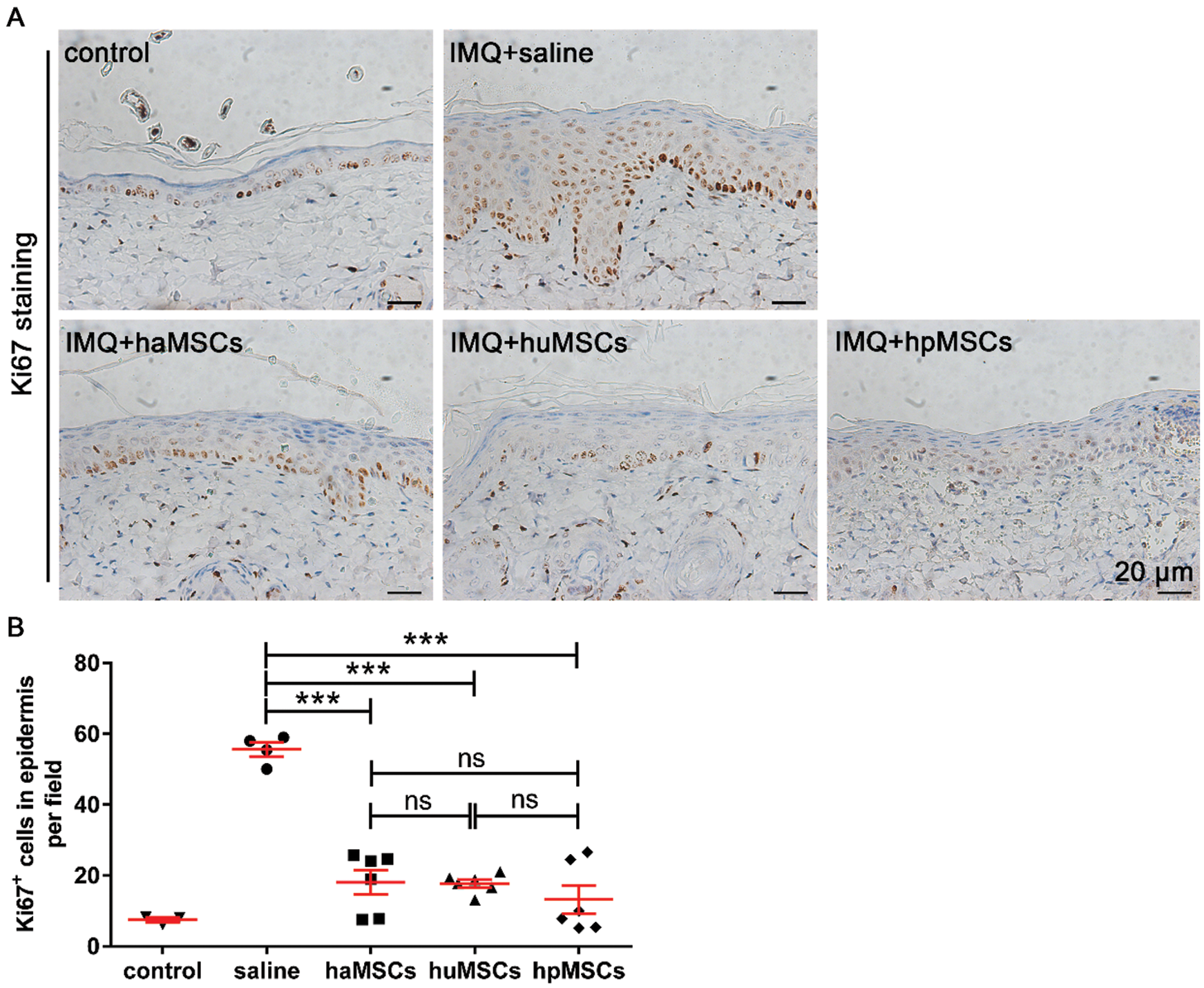
Figure 4: Human adipose, placenta, and umbilical cord-derived mesenchymal stem cells decrease proliferation of keratinocytes in the epidermis of IMQ-induced psoriasis mice.
Human adipose, placenta, and umbilical cord-derived mesenchymal stem cells reduce inflammatory infiltration of T lymphocyte in the derma of IMQ-induced psoriasis mice
To further confirm the ameliorative effects of human adipose, placenta, and umbilical cord-derived mesenchymal stem cells on IMQ-induced psoriasis mice, histopathological analysis of CD3+ T cells infiltration in the derma of dorsal skin samples was conducted by immunohistochemistry staining. We found that mice treated with haMSCs, huMSCs or hpMSCs showed a much lower number of CD3+ cells infiltration in the derma than that of saline-treated mice (Fig. 5A). Furthermore, quantitative analysis of the number of CD3+ T cells infiltration per field in the derma of haMSCs (p < 0.01), huMSCs (p < 0.001) or hpMSCs (p < 0.001)-injected mice also indicated CD3+ cells infiltration was markedly reduced, compared with saline-treated mice (Fig. 5B). Of note, we observed that the inhibited effects of huMSCs and hpMSCs on CD3+ T cells infiltration in the derma of IMQ-induced mice tended to be more obvious in contrast to haMSCs-treated mice (Fig. 5B). However, the statistical differences among them were no significant (p > 0.05, Fig. 5B).
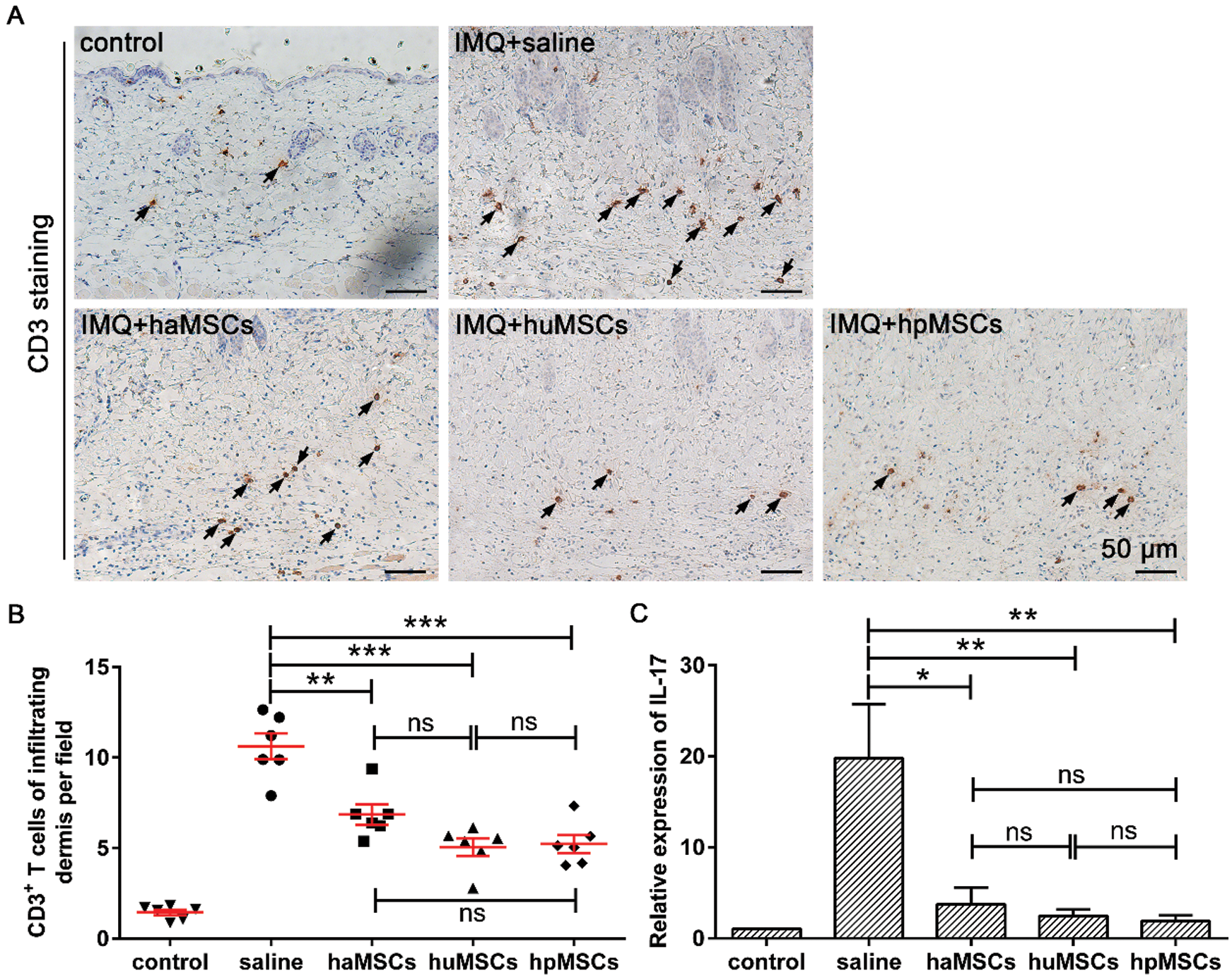
Figure 5: Human adipose, placenta, and umbilical cord-derived mesenchymal stem cells lower inflammatory infiltration of T lymphocyte in the derma of IMQ-induced psoriasis mice.
Human adipose, placenta, and umbilical cord-derived mesenchymal stem cells reduce IL-17 expression of T lymphocyte in the derma of IMQ-induced psoriasis mice
To examine the levels of IL-17 expression in lesional skin of IMQ-induced psoriasis mice, we carried out a quantitative real-time PCR assay. The data showed that the levels of IL-17 expression were markedly decreased in lesional skin of IMQ-induced psoriasis mice, which were treated with haMSCs (p < 0.05), huMSCs (p < 0.01) or hpMSCs (p < 0.01), compared with saline-treated psoriatic mice (Fig. 5C). Similarly, we also found that the reduced levels of IL-17 expression tended to be more marked in lesioned skin of IMQ-induced psoriasis mice with huMSCs or hpMSCs treatment, compared to haMSCs-treated mice, but the statistical differences among them were no significant (p > 0.05, Fig. 5C).
Psoriasis is an autoimmune-mediated chronic inflammatory disease. Its important feature is the epidermal hyperplasia resulted from keratinocyte excessive proliferation, which is mainly caused by the cytokines released from activated T cells infiltrated into the dermis and epidermis (Lebwohl, 2003). T cells have a critical role in the pathogenesis of psoriasis (Gudjonsson et al., 2004). Psoriasis is strongly associated with the balance of the Th1 and Th17 cells, which play an important role in the pathogenesis of psoriasis (Lowes et al., 2008). However, Th17 cells are proposed to play a pivotal role in psoriatic inflammation in that IL-17 secreted from Th17 cells is a crucial proinflammatory cytokine in psoriasis (Di Cesare et al., 2009).
A series of studies have revealed that the MSCs derived from human umbilical cord blood (Sah et al., 2016; Lee et al., 2017), palatine tonsil (Cho et al., 2017), and embryonic stem cell (Kim et al., 2019) be applicated to prevent or treat psoriasis in mice by means of their immunosuppressive and anti-inflammatory effects. Additionally, the MSCs isolated from the peripheral blood of sheep also exhibit anti-inflammatory activity in the skin wound healing process in a sheep model (Martinello et al., 2018). However, the above tissue source of human MSCs limits their applications for human psoriasis. Human mesenchymal stem cells (MSCs) derived from adipose, placenta, and umbilical cord are easily available and may be extracted in large volume. These characteristics make them ideally suited for a wide spectrum of clinical applications. Thus, we comparatively assessed the effects of haMSCs, hpMSCs, and huMSCs on IMQ-induced psoriasis in mice. The preliminary investigation of our study revealed that the therapeutical effects of these MSCs on the IMQ-induced psoriatic mice model were significant. Our results indicate there is a tendency that the treatment of hpMSCs and huMSCs may be better than that of the haMSCs. However, the statistical differences were not significant among them. As is known to all, haMSCs derived from adipose tissue belong to adult cells, and both hpMSCs and huMSCs, respectively isolated from the placenta and umbilical cord belong to perinatal stem cells. The differentiation potential of the haMSCs may be weak compared with that of the hpMSCs and huMSCs. Therefore, we suggest that it is more beneficial for the treatment of psoriasis using perinatal mesenchymal stem cells.
The previous study has reported that the MSCs could alleviate psoriasis via reducing type I interferon (IFN-I) secreted by plasmacytoid dendritic cells (Chen et al., 2019). In our study, we found that hyper-proliferation of keratinocytes in the skin of IMQ-induced psoriasis mice could be effectively inhibited by the haMSCs, huMSCs, and hpMSCs, respectively. Thus, the epidermal hyperplasia of IMQ-induced psoriasis mice could be impaired. Our results revealed that it was also impaired that inflammatory infiltration of T lymphocytes to the derma of IMQ-induced psoriasis mice which were individually administrated of haMSCs, huMSCs, and hpMSCs. Subsequently, quantitative real-time PCR analysis showed that the levels of IL-17 expression were markedly suppressed in the lesioned skin of these mice. The previous study has shown that MSCs could decrease the levels of interleukin 6 (IL-6) and transforming growth factor (TGF-β) in vitro assay (Darlan et al., 2020). It is inferred that the level of IL-17 produced from Th17 cells could also be reduced by above MSCs treatment in vivo. Hence, we conclude that these human MSCs could inhibit the activity of Th17 cells, reducing the level of IL-17 production, which inhibits the proliferation of keratinocytes in lesioned skin of IMQ-induced psoriasis mice. Taken together, IMQ-induced psoriasis mice could be significantly ameliorated by the treatment of haMSCs, huMSCs or hpMSCs through reducing T cell infiltration.
A previous report has shown that the benefits of MSCs therapy could be due to their secretome, which is defined as a series of soluble factors and exosomes secreted to the extracellular space (Vizoso et al., 2017). Exosomes may exhibit various advantages over mesenchymal stem cells, including manufacturing, storage, product shelf life, and their potential as a ready-to-go biologic product (Vizoso et al., 2017). Moreover, the application of exosomes could avoid several risks such as tumorigenicity, emboli formation, immune compatibility, and infection transmission (Vizoso et al., 2017). These strengths will be more conducive to the development of cell-free therapy products. Heat shock protein 70 (HSP70) can prevent IMQ-induced psoriasis by regulating T cell activation in mice (Seifarth et al., 2018). Proteomics analysis has revealed that exosomes derived from human MSCs contain various proteins, which include HSP70 in the previous work (Lai et al., 2012). In addition, numerous miRNAs involving the immunomodulatory efficacy were examined by RNA sequencing in exosomal cargo, which includes miR-146 (Song et al., 2017), miR-223 (He et al., 2019), and miR-106b-5p (Shi et al., 2020). Thus, we speculate that human MSCs-derived exosomes maybe contribute to the treatment of psoriasis. However, further studies are needed to clarify whether IMQ-induced psoriasis could be ameliorated by the exosomes derived from the haMSCs, huMSCs, and hpMSCs.
In summary, these data suggest that the treatments of haMSCs, huMSCs or hpMSCs could effectively suppress T lymphocytes recruitment to lesioned skin and reduce the production of IL-17 from Th17 cells in lesioned skin of IMQ-induced psoriasis mice. The results demonstrated that these human MSCs could be applied to the prevention or treatment of IMQ-induced psoriasis in mice. The findings also imply that these human MSCs may be potential therapeutic candidates for skin inflammatory disease or other autoimmune diseases.
Acknowledgement: We are grateful to Mr. Bizuo Liu for supporting this project and Dr. Honglin Wang (Shanghai Institute of Immunology, Shanghai Jiaotong University School of Medicine, China) for technical assistance and scientific advice.
Availability of Data and Materials: All the data supporting these findings is contained within this manuscript.
Author Contributions: Chengxiang Dai, Suke Li, Meng Li, and Jigang Lei designed the research. Jigang Lei, Zhenyao Xu, and Zhikai Wang performed the most experiments. Ping Li, Jing Wang, and Yinglu Chen helped with cell isolation and flow cytometry. Xiaole Song, Chengjie Ren, and Meiping Shen performed cell culture and the trilineage differentiation of MSCs. Chengxiang Dai and Jigang Lei analyzed the data. Jigang Lei wrote the manuscript. Chengxiang Dai, Jigang Lei, and Zhikai Wang revised the manuscript. All authors have read and approved the final manuscript.
Ethics Approval: All experiments were performed in accordance with the animal ethics committee of the Scientific Investigation Board of Shanghai Jiaotong University School of Medicine, Shanghai, China (IACUC Approval No. 2019-A021-01).
Funding Statement: The research funding is provided by Cellular Biomedicine Group, Ltd., and this work is also supported by grants from the National Natural Science Foundation of China (No. 81703118).
Conflicts of Interest: Jigang Lei, Suke Li, Meng Li, Ping Li, Jing Wang, Yinglu Chen, Xiaole Song, Chengjie Ren, Meiping Shen, and Chengxiang Dai are current employees of Cellular Biomedicine Group, Ltd. All authors have no conflict of interest.
Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. (2007). Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis & Rheumatism 56: 1175–1186. DOI 10.1002/art.22511. [Google Scholar] [CrossRef]
Bocheńska K, Smolińska E, Moskot M, Jakóbkiewicz-Banecka J, Gabig-Cimińska M. (2017). Models in the research process of psoriasis. International Journal of Molecular Sciences 18: 2514. DOI 10.3390/ijms18122514. [Google Scholar] [CrossRef]
Cho KA, Park M, Kim YH, Ryu KH, Woo SY. (2017). Mesenchymal stem cells inhibit RANK-RANKL interactions between osteoclasts and Th17 cells via osteoprotegerin activity. Oncotarget 8: 83419–83431. DOI 10.18632/oncotarget.21379. [Google Scholar] [CrossRef]
Chen M, Peng J, Xie Q, Xiao N, Su X, Mei H, Lu Y, Zhou J, Dai Y, Wang S, Li C, Lin G, Cheng L. (2019). Mesenchymal stem cells alleviate moderate-to-severe psoriasis by reducing the production of type I interferon (IFN-I) by plasmacytoid dendritic cells (pDCs). Stem Cells International 2019: 6961052. DOI 10.1155/2019/6961052. [Google Scholar] [CrossRef]
Di Cesare A, Di Meglio P, Nestle FO. (2009). The IL-23/Th17 axis in the immunopathogenesis of psoriasis. Journal of Investigative Dermatology 129: 1339–1350. DOI 10.1038/jid.2009.59. [Google Scholar] [CrossRef]
Darlan DM, Munir D, Jusuf NK, Putra A, Ikhsan R, Alif I. (2020). In vitro regulation of IL-6 and TGF-β by mesenchymal stem cells in systemic lupus erythematosus patients. Medicinski Glasnik (Zenica) 17: 408–413. DOI 10.17392/1186-20. [Google Scholar] [CrossRef]
Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, Mehta NN, Finlay AY, Gottlieb AB. (2016). Psoriasis. Nature Reviews Disease Primers 2: 16082. DOI 10.1038/nrdp.2016.82. [Google Scholar] [CrossRef]
Griffiths CE, Barker JN. (2007). Pathogenesis and clinical features of psoriasis. Lancet 370: 263–271. DOI 10.1016/S0140-6736(07)61128-3. [Google Scholar] [CrossRef]
Gedebjerg A, Johansen C, Kragballe K, Iversen L. (2013). IL-20, IL-21 and p40: Potential biomarkers of treatment response for ustekinumab. Acta Dermato Venereologica 93: 150–155. DOI 10.2340/00015555-1440. [Google Scholar] [CrossRef]
Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. (2004). Immunopathogenic mechanisms in psoriasis. Clinical and Experimental Immunology 135: 1–8. DOI 10.1111/j.1365-2249.2004.02310.x. [Google Scholar] [CrossRef]
He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, Jin Y, Yuan L, Li B. (2019). MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells International 2019: 7132708. DOI 10.1155/2019/7132708. [Google Scholar] [CrossRef]
Ikhsan R, Putra A, Munir D, Darlan DM, Suntoko B, Retno A. (2020). Mesenchymal stem cells induce regulatory T-cell population in human SLE. Bangladesh Journal of Medical Science 19: 743–748. DOI 10.3329/bjms.v19i4.46635. [Google Scholar] [CrossRef]
Jeon C, Sekhon S, Yan D, Afifi L, Nakamura M, Bhutani T (2017a). Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis. Human Vaccines & Immunotherapeutics 13: 2247–2259. DOI 10.1080/21645515.2017.1356498. [Google Scholar] [CrossRef]
Jeon YJ, Sah SK, Yang HS, Lee JH, Shin J, Kim TY (2017b). Rhododendrin inhibits toll-like receptor-7-mediated psoriasis-like skin inflammation in mice. Experimental & Molecular Medicine 49: e394. DOI 10.1038/emm.2017.173. [Google Scholar] [CrossRef]
Kim CH, Lim CY, Lee JH, Kim KC, Ahn JY, Lee EJ. (2019). Human embryonic stem cells-derived mesenchymal stem cells reduce the symptom of psoriasis in imiquimod-induced skin model. Tissue Engineering and Regenerative Medicine 16: 93–102. DOI 10.1007/s13770-018-0165-3. [Google Scholar] [CrossRef]
Lebwohl M. (2003). Psoriasis. Lancet 361: 1197–1204. DOI 10.1016/S0140-6736(03)12954-6. [Google Scholar] [CrossRef]
Lowes MA, Bowcock AM, Krueger JG. (2007). Pathogenesis and therapy of psoriasis. Nature 445: 866–873. DOI 10.1038/nature05663. [Google Scholar] [CrossRef]
Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueqer JG. (2008). Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. Journal of Investigative Dermatology 128: 207–1211. DOI 10.1038/sj.jid.5701213. [Google Scholar] [CrossRef]
Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK. (2012). Proteolytic potential of themsc exosome proteome: Implications for an exosome-mediated delivery of therapeutic proteasome. International Journal of Proteomics 2012: 971907. DOI 10.1155/2012/971907. [Google Scholar] [CrossRef]
Lee YS, Sah SK, Lee JH, Seo KW, Kang KS, Kim TY. (2017). Human umbilical cord blood-derived mesenchymal stem cells ameliorate psoriasis-like skin inflammation in mice. Biochemistry and Biophysics Reports 9: 281–288. DOI 10.1016/j.bbrep.2016.10.002. [Google Scholar] [CrossRef]
Li L, Zhang HY, Zhong XQ, Lu Y, Wei J, Li L, Chen H, Lu C, Han L. (2020). PSORI-CM02 formula alleviates imiquimod-induced psoriasis via affecting macrophage infiltration and polarization. Life Sciences 243: 117231. DOI 10.1016/j.lfs.2019.117231. [Google Scholar] [CrossRef]
Martinello T, Gomiero C, Perazzi A, Iacopetti I, Gemignani F, DeBenedictis GM, Ferro S, Zuin M, Martines E, Brun P, Maccatrozzo L, Chiers K, Spaas JH, Patruno M. (2018). Allogeneic mesenchymal stem cells improve the wound healing process of sheep skin. BMC Veterinary Research 14: 202. DOI 10.1186/s12917-018-1527-8. [Google Scholar] [CrossRef]
Ma J, Wu J, Han L, Jiang X, Yan L, Hao J, Wang H. (2019). Comparative analysis of mesenchymal stem cells derived from amniotic membrane, umbilical cord, and chorionic plate under serum-free condition. Stem Cell Research & Therapy 10: 19. DOI 10.1186/s13287-018-1104-x. [Google Scholar] [CrossRef]
Na K, Yoo HS, Zhang YX, Choi MS, Lee K, Yi TG, Song SU, Jeon MS. (2014). Bone marrow-derived clonal mesenchymal stem cells inhibit ovalbumin-induced atopic dermatitis. Cell Death & Disease 5: e1345. DOI 10.1038/cddis.2014.299. [Google Scholar] [CrossRef]
Perera GK, Meglio PD, Nestle FO. (2012). Psoriasis. Annual Review of Pathology: Mechanisms of Disease 7: 385–422. DOI 10.1146/annurev-pathol-011811-132448. [Google Scholar] [CrossRef]
Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD. (2011). Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells 29: 1849–1860. DOI 10.1002/stem.738. [Google Scholar] [CrossRef]
Putra A, Rosdiana I, Darlan DM, Alif I, Hayuningtyas F, Wijaya I, Aryanti R, Makarim FR, Antari AD. (2020). Intravenous administration is the best route of mesenchymal stem cells migration in improving liver function enzyme of acute liver failure. Folia Medica 62: 52–58. DOI 10.3897/folmed.62.e47712. [Google Scholar] [CrossRef]
Sah SK, Park KH, Yun CO, Kang KS, Kim TY. (2016). Effects of human mesenchymal stem cells transduced with superoxide dismutase on imiquimod-induced psoriasis-like skin inflammation in mice. Antioxidants & Redox Signaling 24: 233–248. DOI 10.1089/ars.2015.6368. [Google Scholar] [CrossRef]
Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, Ji J, Liu F, Ding L, Ni Y, Hou Y. (2017). Exosomal miR-146a contributes to the enhanced therapeutic efficacy of Interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cell 35: 1208–1221. DOI 10.1002/stem.2564. [Google Scholar] [CrossRef]
Seifarth FG, Lax JM, Harvey J, DiCorleto PE, Husni ME, Chandrasekharan UM, Tytell M. (2018). Topical heat shock protein 70 prevents imiquimod-induced psoriasis-like inflammation in mice. Cell Stress and Chaperones 23: 1129–1135. DOI 10.1007/s12192-018-0895-0. [Google Scholar] [CrossRef]
Shi Y, Zhang B, Zhu J, Huang W, Han B, Wang Q, Qi C, Wang M, Liu F. (2020). miR-106b-5p inhibits IRF1/IFN-β signaling to promote M2 macrophage polarization of glioblastoma. OncoTargets and Therapy 13: 7479–7492. DOI 10.2147/OTT.S238975. [Google Scholar] [CrossRef]
Van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. (2009). Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. Journal of Immunology 182: 5836–5845. DOI 10.4049/jimmunol.0802999. [Google Scholar] [CrossRef]
Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. (2017). Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. International Journal of Molecular Sciences 18: 1852. DOI 10.3390/ijms18091852. [Google Scholar] [CrossRef]
Wang W, He N, Feng C, Liu V, Zhang L, Wang F, He J, Zhu T, Wang S, Qiao W, Li S, Zhou G, Zhang L, Dai C, Cao W. (2015). Human adipose-derived mesenchymal progenitor cells engraft into rabbit articular cartilage. International Journal of Molecular Sciences 16: 12076–12091. DOI 10.3390/ijms160612076. [Google Scholar] [CrossRef]
Yan S, Xu Z, Lou F, Zhang L, Ke F, Bai J, Liu Z, Liu J, Wang H, Zhu H, Sun Y, Cai W, Gao Y, Su B, Li Q, Yang X, Yu J, Lai Y, Yu X, Zheng Y, Shen N, Eugene Chin Y, Wang H. (2015). NF-κB-induced microRNA-31 promotes epidermal hyperplasia by repressing protein phosphatase 6 in psoriasis. Nature Communications 6: 7652. DOI 10.1038/ncomms8652. [Google Scholar] [CrossRef]
Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravole A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. (2005). Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T cell anergy. Blood 106: 1755–1761. DOI 10.1182/blood-2005-04-1496. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |