DOI:10.32604/biocell.2021.012504

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.012504 |  www.techscience.com/journal/biocell |
| Article |
Thymidylate synthase confers pemetrexed resistance of non-small cell lung cancer cells by EGFR/PI3K/AKT pathway
1Department of Oncology, Center People’s Hospital of Zhanjiang, Zhanjiang, 524000, China
2Department of Respiration, Center People’s Hospital of Zhanjiang, Zhanjiang, 524000, China
3Department of Oncology, Yijishan Hospital Affiliated to Wannan Medical College, Wuhu, 241001, China
4Department of Oncology, The Affiliated Hospital of Guangdong Medical University, Zhanjiang, 524001, China
5Department of Thoracic Surgery, Center People’s Hospital of Zhanjiang, Zhanjiang, 524000, China
6Cardiovascular Medicine Center, Affiliated Hospital of Guangdong Medical University, Zhanjiang, 524001, China
*Address correspondence to: Zhihui Zhang, chestmirror@126.com; Wei Lei, leiwei2006@126.com
Received: 02 July 2020; Accepted: 28 September 2020
Abstract: Chemotherapy drug resistance is the main cause leading to the relapse and metastasis of non-small cell lung cancer (NSCLC) patients. Our study aimed to investigate the mechanism of pemetrexed resistance in NSCLC. Firstly, the pemetrexed (PEM)-resistant PC-9 and A549 lung adenocarcinoma cell lines (PC-9/PEM and A549/PEM) were established. The expression of thymidylate synthase (TS) in PC-9/PEM, A549/PEM, A549, and PC-9 cells were analyzed by qRT-PCR and western blot. Then, cell viability, colony formation, migration, and invasion were performed on PEM-resistant cells transfected with TS siRNA. The role of EGFR in PEM resistance of PEM-resistant cells was investigated using EGFR siRNA. The effects of gefitinib and EGFR siRNA on EGFR/PI3K/AKT pathway and downstream signaling Cyclin D1 and E2F1 in PEM-resistant cells were analyzed. Results showed that the protein level of TS was significantly increased in A549/PEM and PC-9/PEM. TS knockdown inhibited the potency of proliferation, colony-forming potential, migration, and invasion in PEM-resistant cells. EGFR knockdown abrogated the resistance to PEM of PEM-resistant cells and suppressed the migration and invasion of PEM-resistant cells. Gefitinib treatment and EGFR knockdown respectively inhibited the EGFR/PI3K/AKT pathway and downregulated Cyclin D1 and E2F1 in PEM-resistant cells. Thus, TS might be a predictive marker for PEM resistance in NSCLC. Inhibition of the EGFR pathway abrogated the resistance to PEM and inhibited the EGFR/PI3K/AKT and downstream signaling of PEM-resistant NSCLC cell lines.
Keywords: NSCLC cell lines; Pemetrexed; EGFR; PI3K; AKT; Thymidylate synthase
As one of the most common cancer types among women and men, non-small cell lung cancer (NSCLC) is one of the main causes of morbidity and mortality in the entire world (Ren et al., 2015; Sandler et al., 2019). Although early diagnosis and therapeutic approaches for NSCLC have considerably progressed, the 5-year survival rate of NSCLC patients is still less than 15% (Chang, 2011; Zhang, 2015; Siegel et al., 2018). Importantly, the resistance of chemotherapy drugs limits the effectiveness of therapies for NSCLC, which leads to the relapse and metastasis of NSCLC (Chang, 2011; Dong et al., 2017). Although progression-free survival and response rate were better than chemotherapy in targeted therapy in first-line therapy, the overall survival did not improve. The second-line targeted therapy still has a higher response rate and survival benefit after the failure of first-line chemotherapy. Only a 15% response rate of the second-line chemotherapy was achieved after the failure of first-line targeted therapy (Mistry et al., 2019; Yamaguchi et al., 2018). A previous study showed that first-line targeted therapy would reduce the sensitivity of second-line chemotherapy drugs (Zeng et al., 2014). The development of novel strategies for NSCLC is still critical.
Pemetrexed (PEM) is an inhibitor of thymidylate synthase (TS), which is used worldwide for NSCLC excluding squamous cell lung cancer by decreasing the de novo biosynthesis of the essential thymidine (Calvert and Bunn, 2002; Schultz et al., 1999; Hanna et al., 2004). TS regulates the de novo biosynthesis of thymidine and purine nucleotides by catalyzing the methylation of fluoro dUMP into dTMP (Wu et al., 2010). TS expression is a significant association with outcomes of pemetrexed for NSCLC, which is a promising predictor for the efficacy of pemetrexed-based chemotherapy for NSCLC (Liu et al., 2013).
The pemetrexed resistance in NSCLC is a challenge for NSCLC therapy. It is clinically important for NSCLC patients to overcome the resistance of pemetrexed in NSCLC cells. Nevertheless, the mechanisms underlying the resistance of pemetrexed in NSCLC need to be elucidated. Currently, the increased expression of TS in pemetrexed resistant cells and the acquisition of pemetrexed resistance were found in colon and breast cancer (Chiu et al., 2017; Takezawa et al., 2011). In NSCLC, a meta-analysis showed that the increased TS expression might be an independent risk factor of pemetrexed resistance (Liu et al., 2013). The epidermal growth factor receptor/phosphoinositide 3-kinase/protein kinase B (PKB), also known as Akt (EGFR/PI3K/Akt) pathway, is associated with gefitinib resistance in NSCLC cells (Liu et al., 2020). However, it is still not clear about the role and molecular mechanisms of TS in the PEM-resistance of NSCLC.
Here, we found the enhanced level of TS in pemetrexed-resistant NSCLC cell lines. TS knockdown inhibited the potency of proliferation, colony-forming potential, migration, and invasion of pemetrexed-resistant NSCLC cell lines. Inhibition of the EGFR pathway abrogated the resistance to pemetrexed and inhibited the EGFR/PI3K/AKT and downstream signaling of pemetrexed-resistant NSCLC cell lines. We have revealed that EGFR signaling pathways regulated the PEM-induced resistance in NSCLC cell lines. Our study provided the potential treatment strategies to combine gefitinib and PEM for overcoming the pemetrexed resistance of NSCLC.
Cell culture and treatment agents
Human NSCLC cell lines (PC-9 and A549) were purchased from American Type Culture Collection (ATCC, USA) and cultured in Roswell Park Memorial Institute 1640 medium (Procell Life Science & Technology, China) supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA). The PEM-resistant NSCLC cells (i.e., PC-9/PEM and A549/PEM) were generated by continuous exposing cells with a stepwise increase in the concentration of pemetrexed for over six months. Whenever 70–80% confluency was reached, cells were passaged. The gefitinib (Selleckchem, TX, USA) and PEM (LC Laboratories, MA, USA) reagents were used in the study.
Three thousand cells each well were seeded and exposed to the indicated treatment. 90 μL fresh media was used to replace the media, and 10 µL of CCK-8 solution (CCK-8, Dojin, Japan) was added into each well; 2.5 h later, absorbance at 450 nm was measured by the Infinite M200 FA plate reader (TECAN, Männedorf, Switzerland) (Deng et al., 2020).
1.5 × 103 PC-9/PEM or A549/PEM lung adenocarcinoma cell lines per well were cultured in complete medium for 12 to 16 days. Afterward, the cells were stained with 0.2% crystal violet after fixed by 4% paraformaldehyde, and then the cells were washed with PBS, and the representative images were photographed (McQuillan et al., 2020).
The apoptosis of PC-9/PEM or A549/PEM cells was analyzed by Annexin V-FITC/PI detection kit (Sigma-Aldrich). Briefly, the cells in the logarithmic growth stage were seeded in a 6-well plate with a density of 2 × 105/well and cultured for 48 h. Washed with pre-cooled PBS twice, the supernatant was discarded after centrifugation for 3 min, and 400 μL Annexin V-FITC staining solution was added and incubated on ice under dark for 15 min. Then 10 μL propidium iodide (PI) dye solution was added and mixed and incubated under dark for 5 min. The apoptosis was detected by flow cytometry by BD FACS Calibur Aria (BD, USA). The experiment was repeated three times, and the average value of the data from three experiments was taken out (Majtnerová and Roušar, 2018).
Quantitative real-time PCR (qRT-PCR)
The cells were homogenized in 1 mL of TRIzol (Invitrogen, CA) to extract the total RNA and then reversed-transcribed by the QuantiTect Reverse Transcription Kit (#205313) (Qiagen, Shanghai, China). SYBR® Green (BioRad Laboratories Inc., USA) was used for qRT–PCR analysis of TS and GAPDH. The relative expressions were normalized to GAPDH using the comparative cycle threshold method and fold change. The primer used as followed. TS forward primer, 5’-AAGCCTGTCTGGAAGTTACTTGT-3’; TS reverse primer, 5’-GCAGGGTATCCTTGTGCCA-3’; GAPDH forward primer, 5’-GGAGCGAGATCCCTCCAAAAT-3’; GAPDH reverse primer, 5’-GGCTGTTGTCATACTTCTC ATGG-3’ (Hawkins and Guest, 2017).
Cells were homogenized in ice-cold lysis buffer and then were centrifuged for 5 min at 10000 × g. Then, equivalent amounts of proteins (20 µg) were subjected to 12% SDS-PAGE and transferred onto a nitrocellulose membrane. Membranes were blocked for 60 min by phosphate-buffered saline (PBS). Then, the membranes were incubated with respective primary antibodies: Anti- TS (Santa Cruz, 1:1000), EGFR (Santa Cruz, 1:1000), p-EGFR (CST, 1:1000), p-PI3K (Abcam, 1:1000), p-AKT (Abcam, 1:1000), Cyclin D1 (Sigma, 1:1000), E2F1 (Santa Cruz, 1:1000) and anti-rabbit IgG-HRP (Santa Cruz, 1:1000). Bands were visualized with the enhanced chemiluminescent system (Thermo Fisher Scientific, Inc. USA) (Taylor and Posch, 2014).
The small interfering RNAs (siRNAs) and scrambled negative controls (NCs) for TS and EGFR were designed and purchased from Genepharma (Co., Ltd., China). Lipofectamine™ 2000 Transfection Reagent (Invitrogen, Shanghai, China) was used for transfection of siRNA in PC-9/PEM and A549/PEM (Longo et al., 2013).
Transwell migration and invasion assay
For transwell migration assay, 2 × 105 cells/well were seeded onto the upper well of a millipore transwell chambers (8 μm) in serum-free medium, and then 650 μL media containing 10% FBS were added in the lower chamber. 24 h later, the non-penetrated cells in the upper surface of the membrane were removed. The cells adhering to the lower chamber were fixed with formaldehyde, stained with 0.1% Crystal Violet, and photographed in different filed by inverted microscope (CX41, Olympus) and counted (Zeng et al., 2019). For transwell invasion assay, all the steps were carried out similarly to those in the migration assay except for the Matrigel coating: Serum-free DMEM/RPMI-1640 (1:1) medium containing 0.2 mg/mL type I-collagen and 2.5 mg/mL matrigel matrix (refer to the below recipe) was laid onto the upper chamber of transwells (150 μL/ well), and solidify in a CO2 incubator at 37°C for 30 min (Justus et al., 2013).
All experiments were performed at least three times. p < 0.05 was considered statistically significant. Unpaired Student’s t-tests were used by Prism statistical software package (Version 8.0, Graphpad Software Inc.).
Enhanced expression of TS in PC9/PEM and A549/PEM
Firstly, we developed the pemetrexed-resistant NSCLC cells PC-9/PEM and A549/PEM cells. To analyze the relative pemetrexed resistance of PC-9/PEM cells and A549/PEM cells compared to the corresponding parental cell line, the cells were exposed to various concentrations of pemetrexed, and the viability profile was analyzed. As shown in Fig. 1A, the PC-9/PEM cells showed higher resistance to pemetrexed than the corresponding parental cell line. A similar result was found in A549/PEM cells compared with A549 cells (Fig. 1B). Then, we assessed the TS mRNA level in PC-9/PEM, A549/PEM, PC-9, and A549 cells. The mRNA levels of TS in PC-9/PEM and A549/PEM were significantly increased compared with PC-9 and A549 cells, respectively (Fig. 1C). The protein level of TS was also markedly increased inA549/PEM cells and PC-9/PEM (Fig. 1D).
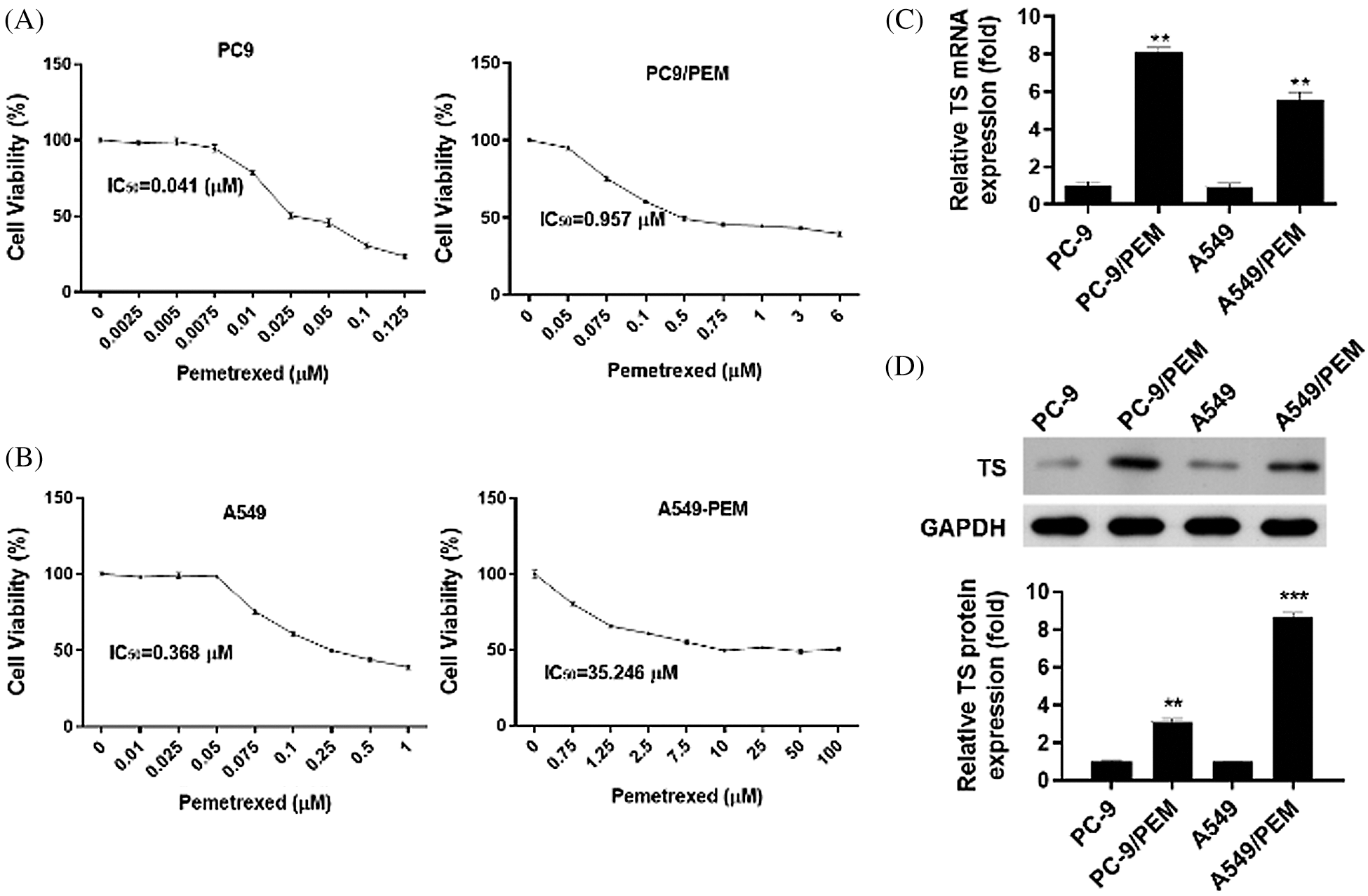
Figure 1: Characteristics of pemetrexed-resistant lung cancer cell lines such as TS expression. (A and B) Pemetrexed (PEM)-resistant NSCLC cell lines. PC-9/PEM and A549/PEM cells developed and the sensitivity to pemetrexed of PC-9/PEM, A549/PEM, PC-9, and A549 cells was determined by using CCK-8 assays. (C) q-PCR analyses of TS mRNA levels (change in folds) of in PC-9/PEM, A549/PEM, PC-9, and A549 cells. (D) The protein levels of thymidylate synthase in PC-9/PEM, A549/PEM, PC-9, and A549 cells were analyzed by Western Blot. All experiments were repeated three times. Data represent mean ± SEM. **p < 0.01. ***p < 0.001.
TS knockdown inhibited the potency of proliferation, colony forming potential, migration, and invasion in pemetrexed-resistant NSCLC cell lines
To further explore the role of TS in pemetrexed induced resistance, we knocked down the TS by siRNA transfection. The interference efficiency of siRNA-TS was shown (Figs. 2A and 2B). The knockdown of TS significantly inhibited the cell viability of PC-9/PEM and A549/PEM cells (Figs. 3A and 3B). The cell colony-forming potential of PC-9/PEM and A549/PEM cells was abrogated by TS knockdown (Fig. 3C). In addition, TS knockdown significantly induced apoptosis in both PC-9/PEM and A549/PEM cells (Fig. 3D). The migration and invasion potency of PC-9/PEM and A549/PEM cells were also assessed. Compared with the negative control group, siRNA-TS significantly decreased the migration of PC-9/PEM and A549/PEM cells (Fig. 3E). The invasion potency of PC-9/PEM and A549/PEM cells was markedly inhibited by TS knockdown (Fig. 3F). The results showed that TS knockdown inhibited the potency of proliferation, colony-forming potential, migration, and invasion in pemetrexed-resistant NSCLC cell lines.
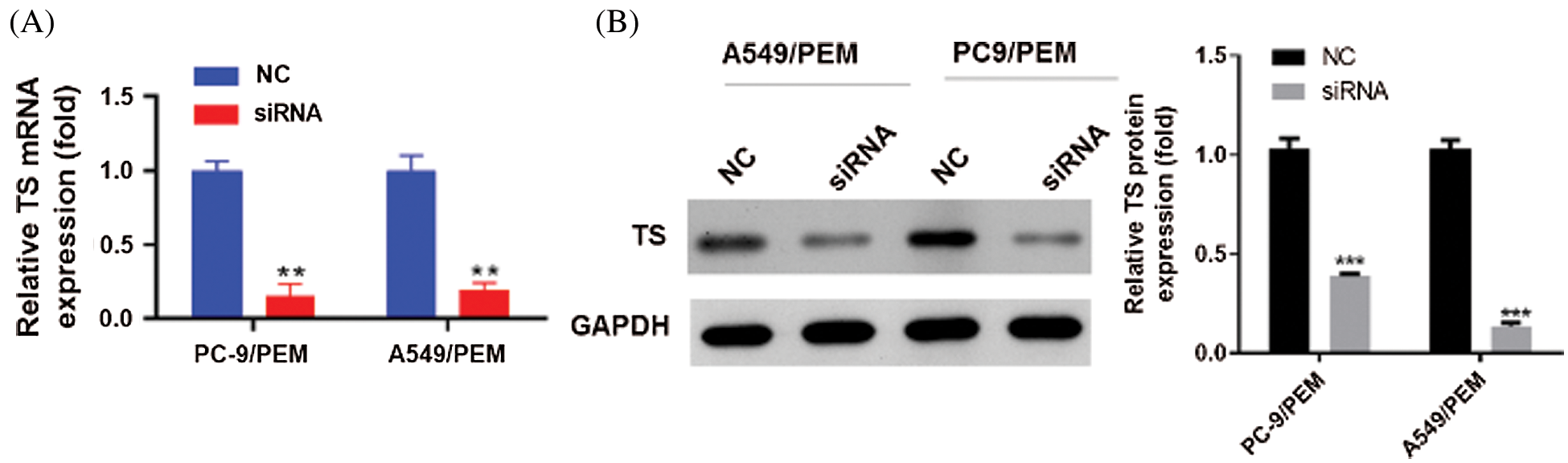
Figure 2: Thymidylate synthase knockdown in pemetrexed-resistant NSCLC cell lines. The PC-9/PEM and A549/PEM cells were transfected by siRNA targeting TS Iα or siRNA negative control. (A) q-PCR analyses of TS mRNA levels in PC-9/PEM and A549/PEM cells after transfection. (B) The protein levels of TS in PC-9/PEM and A549/PEM cells after transfection were analyzed by Western Blot. Data represent mean ± SEM. **p < 0.01. ***p < 0.001.
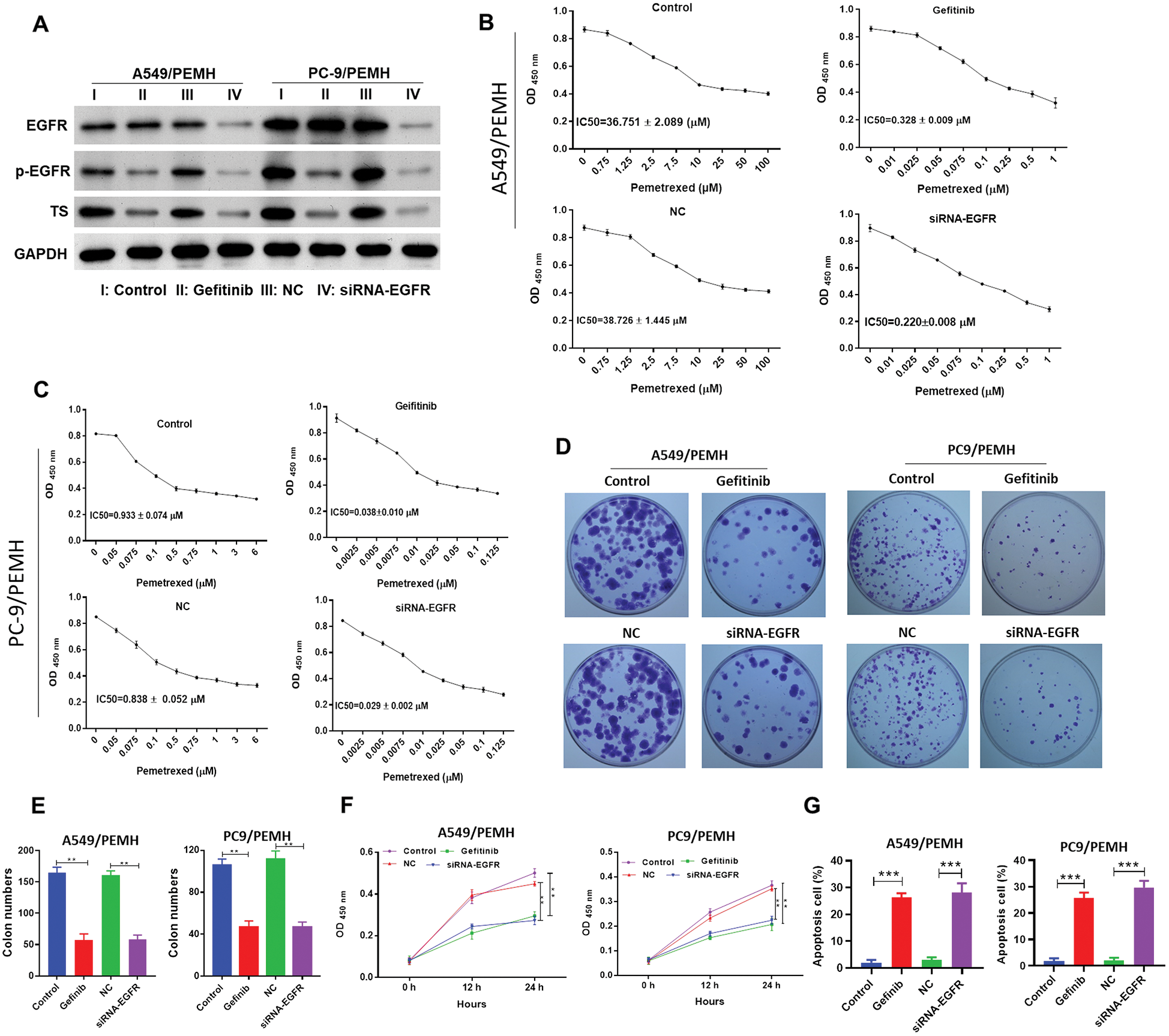
Figure 3: Thymidylate synthase knockdown inhibited the potency of proliferation, colony-forming potential, migration, and invasion in pemetrexed-resistant NSCLC cell lines. The PC-9/PEM and A549/PEM cells were transfected by siRNA targeting TS Iα or siRNA negative control (NC). (A–B) The cell viability of PC-9/PEM (A) and A549/PEM cells (B) after transfection was analyzed by CCK8. (C) The potential of PC-9/PEM and A549/PEM cells to form colonies after transfection was analyzed by colony-forming assay, scale bar: 1 cm. (D) Flow cytometry analysis of cell apoptosis of PC-9/PEM and A549/PEM cells after transfection. (E) Migration assay was performed to assess the migration potency of PC-9/PEM and A549/PEM cells after transfection. Scale bar: 100 μm. (F) Cell invasion assay was performed to assess the invasion potency of PC-9/PEM and A549/PEM cells after transfection. Scale bar: 100 μm. All experiments were repeated three times. Data represent mean ± SEM. **p < 0.01. ***p < 0.001.
Inhibition of EGFR pathway abrogated the resistance to pemetrexed of pemetrexed-resistant NSCLC cell lines
Previous studies reported that EGFR-TKI can down-regulate TS expression and activity in breast cancer cells (Budman et al., 2006; Tung et al., 2017). Next, we wondered whether the expression of TS and the role of TS in PC9/PEM and A549/PEM was regulated by the EGFR pathway. As the orally active inhibitor of EGFR tyrosine kinase with activity in non-small-cell NSCLC, gefitinib was used to block the EGFR pathway in our study. The expression of EGFR in PC-9/PEM and A549/PEM cells was knocked down by siRNA targeting EGFR (Fig. 4A). Western blots showed that both the level of phosphorylated-EGFR in PC-9/PEM and A549/PEM cells was significantly decreased by gefitinib treatment or EGFR knockdown (Fig. 4A). Gefitinib treatment had no effect on the EGFR protein level and significantly decreased the expression of TS in PC-9/PEM and A549/PEM cells. EGFR knockdown also decreased the expression of TS in PC-9/PEM and A549/PEM cells (Fig. 4A). The pemetrexed IC50 values for A549/PEM cells under gefitinib treatment were reduced from 36.751 μM to 0.328 μM. The pemetrexed IC50 values for A549/PEM cells under EGFR knockdown were reduced from 38.728 μM to 0.220 μM (Fig. 4B). Similar results were found in PC-9/PEM cells about pemetrexed IC50 values with inhibition of the EGFR pathway (Fig. 3C). The cell colony-forming potential of PC-9/PEM and A549/PEM cells was abrogated by gefitinib treatment or EGFR knockdown (Figs. 4D and 4E). In the PC-9/PEM and A549/PEM cells, both the gefitinib treatment and EGFR knockdown significantly inhibited the cell proliferation (Fig. 4F). The cell apoptosis of PC-9/PEM and A549/PEM cells induced by pemetrexed was also significantly promoted by gefitinib treatment or EGFR knockdown (Fig. 4G).
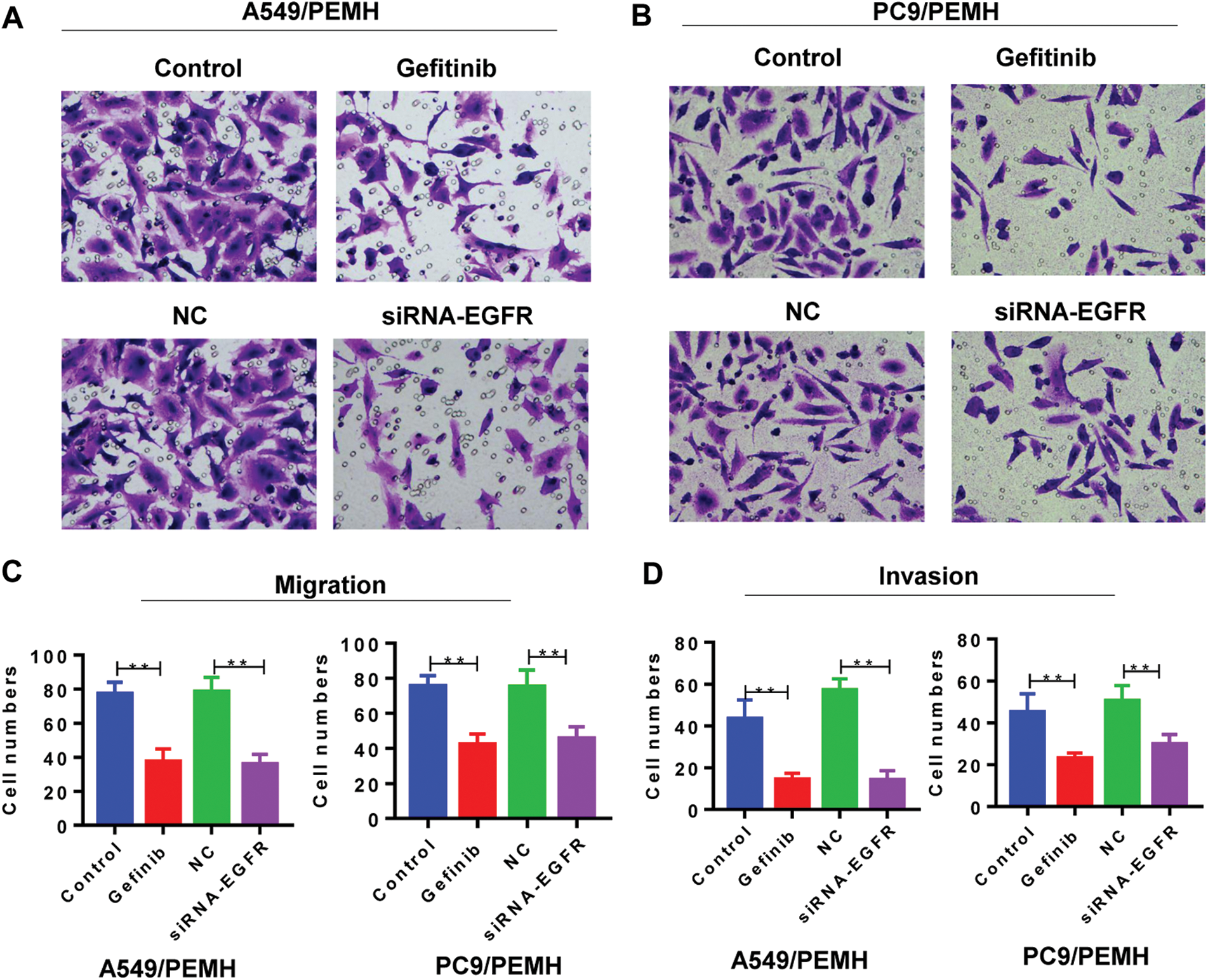
Figure 4: Inhibition of the EGFR pathway abrogated the resistance to pemetrexed in PEM-resistant NSCLC cell lines. The PC-9/PEM and A549/PEM cells were transfected by siRNA targeting EGFR Iα or siRNA negative control (NC). The PC-9/PEM (0.953 μM) and A549/PEM cells (35.640 μM) were treated with gefitinib. (A) Western blot analyzed the protein levels of EGFR, p-EGFR, and TS in PC-9/PEM and A549/PEM cells after gefitinib treatment or EGFR knockdown. (B) The pemetrexed IC50 values for A549/PEM cells under gefitinib treatment or EGFR knockdown. (C) The pemetrexed IC50 values for PC-9/PEM cells under gefitinib treatment or EGFR knockdown. (D–E) The EGFR pathway in PC-9/PEM and A549/PEM cells were inhibited by gefitinib treatment or EGFR knockdown. The cell colony-forming potential was analyzed by colony-forming assay. Scale bar: 1 cm. (F) EGFR pathway inhibition decreased the PC-9/PEM and A549/PEM cell potency of proliferation. The EGFR pathway in PC-9/PEM and A549/PEM cells were inhibited by gefitinib treatment or EGFR knockdown. Then, the cells were treated with pemetrexed and cell viability was analyzed by CCK8. (G) EGFR pathway inhibition increased the PC-9/PEM and A549/PEM cells apoptosis. The EGFR pathway in PC-9/PEM and A549/PEM cells was inhibited by gefitinib treatment or EGFR knockdown. Then, the cells were treated with pemetrexed, and cell apoptosis was analyzed by Flow cytometry. All experiments were repeated three times. Data represent Mean ± SEM. **p < 0.01. ***p < 0.001.
Inhibition of EGFR pathway suppressed the migration and invasion of pemetrexed-resistant NSCLC cell lines
We also assessed the migration and invasion of PC-9/PEM and A549/PEM cells. Compared with the negative control group, siRNA-EGFR significantly inhibited the migration of A549/PEM cells (Figs. 5A and 5C). Gefitinib also inhibited the migration of A549/PEM cells (Figs. 5A and 5C). Similar results were found in PC-9/PEM cells under EGFR knockdown or gefitinib treatment (Fig. 5B). In addition, we performed the invasion assay of PC-9/PEM and A549/PEM cells and found that the invasion potency of PC-9/PEM and A549/PEM cells was also suppressed by inhibition of the EGFR pathway (Fig. 5D).
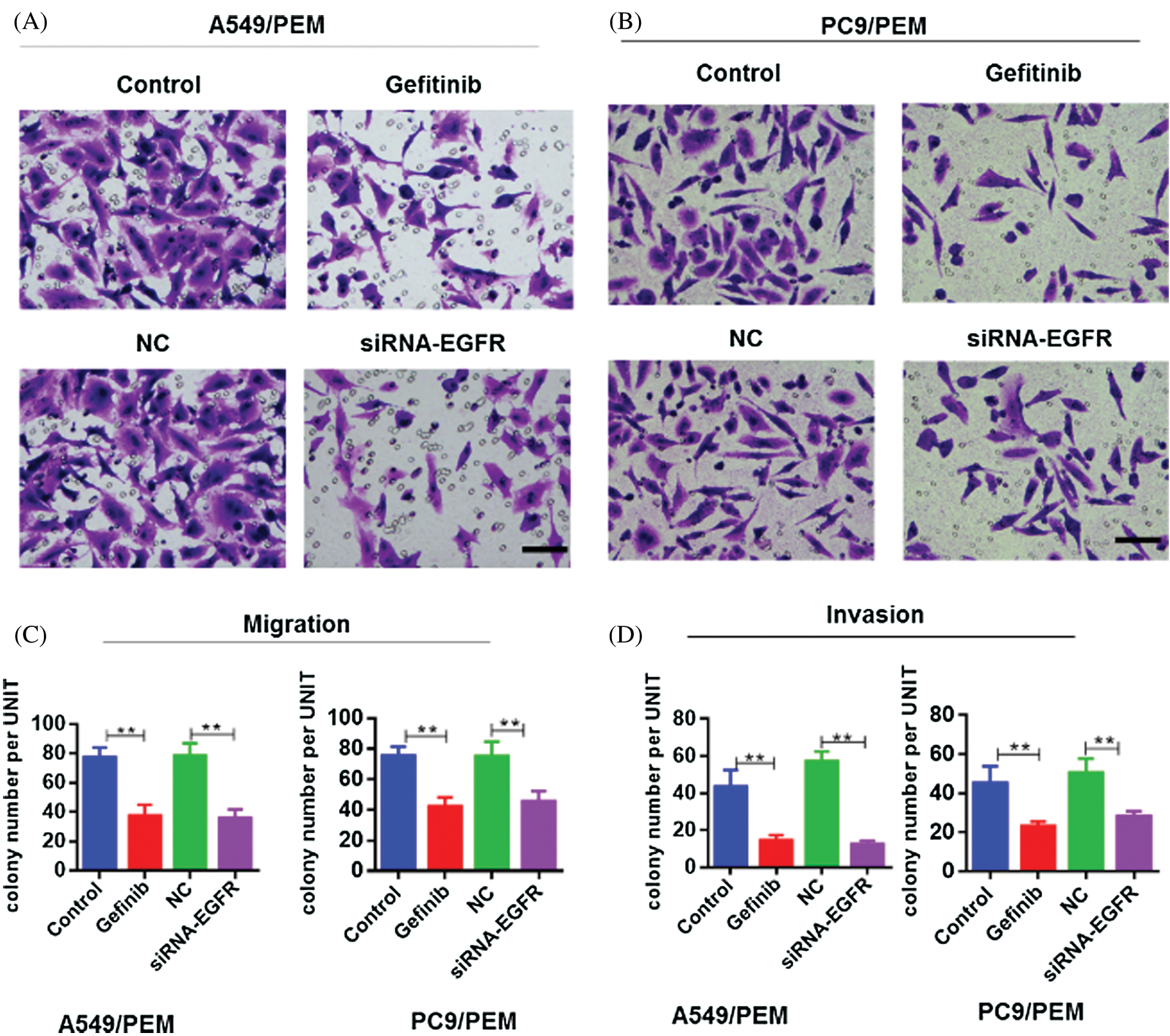
Figure 5: Inhibition of the EGFR pathway suppressed the migration and invasion of pemetrexed-resistant NSCLC cell lines. The PC-9/PEM and A549/PEM cells were transfected by siRNA targeting EGFR Iα or siRNA negative control. The PC-9/PEM and A549/PEM cells were treated with gefitinib. (A) Migration assay was performed to assess the migration potency of A549/PEM cells after indicated treatment. Representative images of migration assay are shown. Scale bar: 100 μm. (B) Migration assay was performed to assess the migration potency of PC-9/PEM cells after indicated treatment. Representative images of migration assay are shown. Scale bar: 100 μm. (C) Quantitative assay of migrated A549/PEM and PC-9/PEM cells in the migration assay was performed. (D) Invasion assay was performed to assess the invasion potency of A549/PEM cells and PC-9/PEM after indicated treatment. Quantitative assay of invaded A549/PEM and PC-9/PEM cells in the invasion assay was performed. All experiments were repeated three times. Data represent mean ± SEM. *p < 0.05, **p < 0.01. ***p < 0.001.
The EGFR/PI3K/AKT and downstream signaling was inhibited by gefitinib or EGFR knockdown in PC-9/PEM and A549/PEM cells
As a key factor that activates the PI3K signaling pathway, EGFR with continuous activation promotes the cell proliferation and progression of cancers (Sun et al., 2018; Xia et al., 2018). We also assessed the EGFR/PI3K/AKT signaling in PC-9/PEM and A549/PEM cells after gefitinib treatment and EGFR knockdown. As shown in Fig. 6, the levels of phosphorylated AKT and phosphorylated PI3K in PC-9/PEM cells were significantly decreased by gefitinib treatment or EGFR knockdown. A similar result was also found in A549/PEM cells (Fig. 6). As Cyclin D1and E2F1 were the downstream signal molecule, the protein levels of Cyclin D1 and E2F1 in PC-9/PEM and A549/PEM cells after gefitinib treatment and EGFR knockdown were also analyzed. As expected, the protein levels of Cyclin D1 and E2F1 in both PC-9/PEM and A549/PEM cells were decreased by EGFR inhibition (Fig. 6).
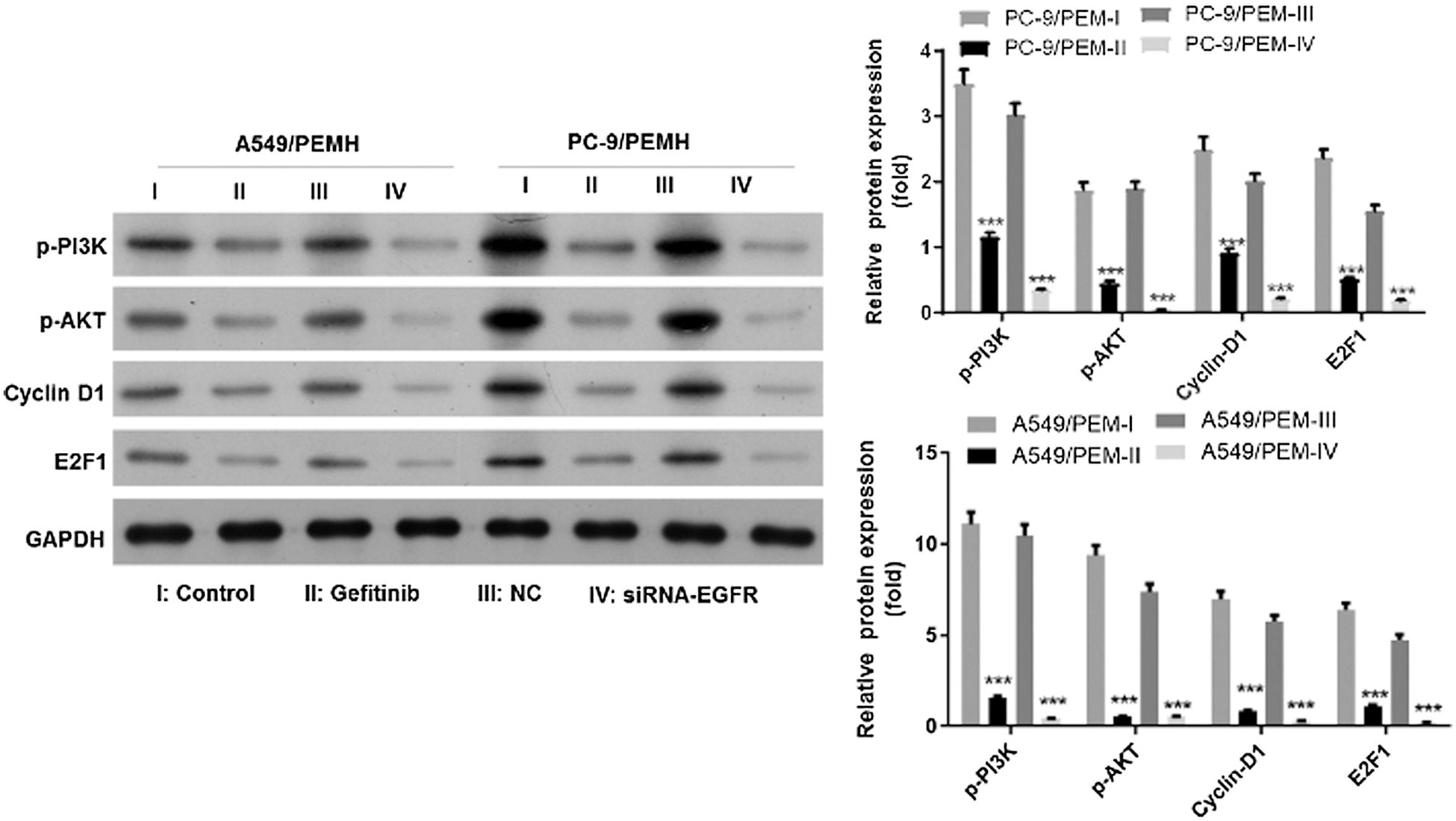
Figure 6: The EGFR/PI3K/AKT and downstream signaling was inhibited by gefitinib treatment or EGFR knockdown in PC-9/PEM and A549/PEM cells. Cells (i.e., A549/PEM cells PC-9/PEM) were transfected by siRNA targeting EGFR Iα or siRNA negative control or treated with gefitinib. I: Control, II: Gefitinib, III: Negative control (NC), and IV: siRNA-EGFR. Western blot analyzed the protein levels of p-AKT, p-EGFR Cyclin D1, and E2F1 in PC-9/PEM cells after gefitinib treatment or EGFR knockdown. All experiments were repeated three times. ***p < 0.001.
Pemetrexed is a multi-target antifolic acid agent that is essential for the first- and second-line as well as for maintenance’s treatment of non-squamous histological non-small cell lung cancer. A comprehensive assessment of drug toxicity and resistance to pemetrexed is still required when combined therapy is used in patients. To understand the complex mechanisms of non-small cell lung cancer patients and to individualize the use of pemetrexed in clinical applications, we explored the role of TS involved in pemetrexed resistance. TS is the main biological target of pemetrexed.
Ozasa et al. (2010) found that the expression of TS was significantly increased in PEM-resistant PC-9 cells. This was consistent with our results that the expression of TS in PC-9/PEM and A549/PEM were significantly increased compared with PC-9 and A549 cells, respectively. A meta-analysis demonstrated that the enhanced expression of TS might be the risk factor of pemetrexed resistance, and NSCLC patients with lower TS expression had the better outcomes of PEM-based chemotherapy (Liu et al., 2013). A shorter median progression-free survival was found in TS highly expressed NSCLC patients compared with NSCLC patients, which had low TS expression (Chen et al., 2011). We further proved the enhanced expression of TS in PEM -resistant A549 cells. TS knockdown inhibited the potency of proliferation and increased apoptosis.
A reduction in cell viability and an increase in apoptosis of TS knockdown without PEM indicate that TS itself is particularly important for cell viability, and its knockdown results in an excellent increase in apoptotic cells. In addition, TS knockdown inhibited the potency of proliferation. Thus, inhibition of the EGFR pathway inhibited the potency of proliferation, colony-forming potential, migration, and invasion in PEM-resistant NSCLC cell lines. Because A549 cells are grown faster than that of PC9 cells, A549 cells are overcrowding when PC9 cells can see obvious clones.
For PEM treatment of adenocarcinomas ATP-binding cassette-transporter 11 was reported as a biomarker (Uemura et al., 2010). In our study, we found the inhibition of EGFR/PI3K/AKT and downstream signaling abrogated the resistance of PEM-resistant NSCLC cell lines. Migration and invasion of PEM-resistant NSCLC cell lines changes with the resistance to PEM. The TS knockdown inhibited the potency of migration and invasion in PC-9/PEM and A549/PEM cells. Inhibition of the EGFR pathway also inhibited the potency of migration and invasion in PEM-resistant NSCLC cell lines. Many studies have reported that the epithelial–mesenchymal transition (EMT) played an important role in the chemoresistance of cancer cells (Zeng et al., 2016), which promoted potency of migration and invasion with the remodeling of the cytoskeleton of cancer cells (Bure et al., 2019; Lu and Kang, 2019). In the PEM resistance lung cancer cells, Chiu et al. found that the EMT was happened with the enhanced expression of ZEB1 and fibronectin, and decreased expression of E-cadherin (Chiu et al., 2017). EMT might play an important role in the acquisition of PEM resistance. We will further explore the association of PEM resistance with EMT alteration in PC9/PEM and A549/PEM.
Our observations indicate that the expression of TS in PEM-resistant NSCLC cell lines was increased compared with the corresponding parental cells. Thus, TS might be a predictive marker for PEM resistance in NSCLC. Inhibition of the EGFR pathway abrogated the resistance to PEM and inhibited the EGFR/PI3K/AKT and downstream signaling of PEM-resistant NSCLC cell lines. We showed the potential of combining gefitinib and PEM in preventing the acquisition of resistance to PEM in NSCLC. Future studies will be required to determine the exact molecular mechanism in the EGFR signaling pathways and TS activity in preventing the acquisition of resistance to PEM in NSCLC.
Acknowledgement: This work was supported by Center People’s Hospital of Zhanjiang.
Availability of Data and Material: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions: DZ, ZZ and WL conceived and designed the experiments. DZ, HL, ZY, YL, YC and WS performed the experiments. DZ, HL and WL analyzed the data. DZ, HL, ZZ and WL wrote the paper.
Funding Statement: The authors received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Budman DR, Soong R, Calabro A, Tai J, Diasio R. (2006). Identification of potentially useful combinations of epidermal growth factor receptor tyrosine kinase antagonists with conventional cytotoxic agents using median effect analysis. Anti-Cancer Drugs 17: 921–928. DOI 10.1097/01.cad.0000224457.36522.60. [Google Scholar] [CrossRef]
Bure IV, Nemtsova MV, Zaletaev DV. (2019). Roles of E-cadherin and noncoding RNAs in the epithelial-mesenchymal transition and progression in gastric cancer. International Journal of Molecular Science 20: 2870. DOI 10.3390/ijms20122870. [Google Scholar] [CrossRef]
Calvert H, Bunn PA. (2002). Future directions in the development of pemetrexed. Seminars in Oncology 29: 54–61. DOI 10.1053/sonc.2002.30761. [Google Scholar] [CrossRef]
Chang A. (2011). Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer 71: 3–10. DOI 10.1016/j.lungcan.2010.08.022. [Google Scholar] [CrossRef]
Chen CY, Chang YL, Shih JY, Lin JW, Chen KY, Yang CH, Yu CJ, Yang PC. (2011). Thymidylate synthase and dihydrofolate reductase expression in non-small cell lung carcinoma: the association with treatment efficacy of pemetrexed. Lung Cancer 74: 132–138. DOI 10.1016/j.lungcan.2011.01.024. [Google Scholar] [CrossRef]
Chiu LY, Hsin IL, Yang TY, Sung WW, Chi JY, Chang JT, Ko JL, Sheu GT. (2017). The ERK–ZEB1 pathway mediates epithelial-mesenchymal transition in pemetrexed resistant lung cancer cells with suppression by vinca alkaloids. Oncogene 36: 242–253. DOI 10.1038/onc.2016.195. [Google Scholar] [CrossRef]
Deng J, Zhang N, Chen F. (2020). Irisin ameliorates high glucose-induced cardiomyocytes injury via AMPK/mTOR signal pathway. Cell Biology International 44: 2315–2325. DOI 10.1002/cbin.11441. [Google Scholar] [CrossRef]
Dong L, Lei D, Zhang H. (2017). Clinical strategies for acquired epidermal growth factor receptor tyrosine kinase inhibitor resistance in non-small-cell lung cancer patients. Oncotarget 8: 64600–64606. DOI 10.18632/oncotarget.19925. [Google Scholar] [CrossRef]
Hawkins SF, Guest PC. (2017). Multiplex analyses using real-time quantitative PCR. Methods Mol Biol 1546: 125–133. [Google Scholar]
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TCY, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar, Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PAJr. (2004). Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. Journal of Clinical Oncology 22: 1589–1597. DOI 10.1200/JCO.2004.08.163. [Google Scholar] [CrossRef]
Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. (2013). In vitro cell migration and invasion assays. JoVE (Journal of Visualized Experiments) 88: e51046. DOI 10.3791/51046. [Google Scholar] [CrossRef]
Liu X, Jiang T, Li X, Zhao C, Li J, Zhou F, Zhang L, Zhao S, Jia Y, Shi J, Gao G, Li W, Zhao J, Chen X, Su C, Ren S, Zhou C. (2019). Exosomes transmit T790M mutation‐induced resistance in EGFR‐mutant NSCLC by activating PI3K/AKT signalling pathway. Journal of Cellular and Molecular Medicine 24: 1529–1540. DOI 10.1111/jcmm.14838. [Google Scholar] [CrossRef]
Liu Y, Yin TJ, Zhou R, Zhou S, Fan L, Zhang RG. (2013). Expression of thymidylate synthase predicts clinical outcomes of pemetrexed-containing chemotherapy for non-small-cell lung cancer: a systemic review and meta-analysis. Cancer Chemotherapy and Pharmacology 72: 1125–1132. DOI 10.1007/s00280-013-2299-2. [Google Scholar] [CrossRef]
Lu W, Kang Y. (2019). Epithelial-mesenchymal plasticity in cancer progression and metastasis. Developmental Cell 49: 361–374. DOI 10.1016/j.devcel.2019.04.010. [Google Scholar] [CrossRef]
Longo PA, Kavran JM, Kim MS, Leahy DJ. (2013). Transient mammalian cell transfection with polyethylenimine (PEI). Methods in Enzymology 529: 227–240. [Google Scholar]
Mistry HB, Helmlinger G, Al-Huniti N, Vishwanathan K, Yates J. (2019). Resistance models to EGFR inhibition and chemotherapy in non-small cell lung cancer via analysis of tumour size dynamics. Cancer Chemotherapy and Pharmacology 84: 51–60. DOI 10.1007/s00280-019-03840-3. [Google Scholar] [CrossRef]
McQuillan K, Gargoum F, Murphy MP, McElvaney OJ, McElvaney NG. (2020). Targeting IgG autoantibodies for improved cytotoxicity of bactericidal permeability increasing protein in cystic fibrosis. Frontiers in Pharmacology 11: 832. DOI 10.3389/fphar.2020.01098. [Google Scholar] [CrossRef]
Majtnerová P, Roušar T. (2018). An overview of apoptosis assays detecting DNA fragmentation. Molecular Biology Reports 45: 1469–1478. DOI 10.1007/s11033-018-4258-9. [Google Scholar] [CrossRef]
Ozasa H, Oguri T, Uemura T, Miyazaki M, Maeno K, Sato S, Ueda R. (2010). Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Science 101: 161–166. DOI 10.1111/j.1349-7006.2009.01358.x. [Google Scholar] [CrossRef]
Ren J, Nie Y, Lv M, Shen S, Tang R, Xu Y, Hou Y, Zhao S, Wang T. (2015). Estrogen upregulates MICA/B expression in human non-small cell lung cancer through the regulation of ADAM17. Cellular & Molecular Immunology 12: 768–776. DOI 10.1038/cmi.2014.101. [Google Scholar] [CrossRef]
Sandler JE, D’Aiello A, Halmos B. (2019). Changes in store for early-stage non-small cell lung cancer. Journal of Thoracic Disease 11: 2117–2125. DOI 10.21037/jtd.2019.05.34. [Google Scholar] [CrossRef]
Schultz RM, Patel VF, Worzalla JF, Shih C. (1999). Role of thymidylate synthase in the antitumor activity of the multitargeted antifolate, LY231514. Anticancer Research 19: 437–443. [Google Scholar]
Siegel RL, Miller KD, Jemal A. (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 68: 7–30. DOI 10.3322/caac.21442. [Google Scholar] [CrossRef]
Sun S, Wu Y, Guo W, Yu F, Kong L, Ren Y, Wang Y, Yao X, Jing C, Zhang C, Liu M, Zhang Y, Zhao M, Li Z, Wu C, Qiao Y, Yang J, Wang X, Zhang L, Li M, Zhou X. (2018). STAT3/HOTAIR signaling axis regulates HNSCC growth in an EZH2-dependent manner. Clinical Cancer Research 24: 2665–2677. DOI 10.1158/1078-0432.CCR-16-2248. [Google Scholar] [CrossRef]
Taylor SC, Posch A. (2014). The design of a quantitative western blot experiment. BioMed Research International 2014: 1–8. DOI 10.1155/2014/361590. [Google Scholar] [CrossRef]
Takezawa K, Okamoto I, Okamoto W, Takeda M, Sakai K, Tsukioka S, Kuwata K, Yamaguchi H, Nishio K, Nakagawa K. (2011). Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. British Journal of Cancer 104: 1594–1601. DOI 10.1038/bjc.2011.129. [Google Scholar] [CrossRef]
Tung CL, Chen JC, Wu CH, Peng YS, Chen WC, Zheng HY, Jian YJ, Wei CL, Cheng YT, Lin YW. (2017). Salinomycin acts through reducing AKT-dependent thymidylate synthase expression to enhance erlotinib-induced cytotoxicity in human lung cancer cells. Experimental Cell Research 357: 59–66. DOI 10.1016/j.yexcr.2017.04.026. [Google Scholar] [CrossRef]
Uemura T, Oguri T, Ozasa H, Takakuwa O, Miyazaki M, Maeno K, Sato S, Ueda R. (2010). ABCC11/MRP8 confers pemetrexed resistance in lung cancer. Cancer Science 101: 2404–2410. DOI 10.1111/j.1349-7006.2010.01690.x. [Google Scholar] [CrossRef]
Wu MF, Hsiao YM, Huang CF, Huang YH, Yang WJ, Chan HW, Chang JT, Ko JL. (2010). Genetic determinants of pemetrexed responsiveness and nonresponsiveness in non-small cell lung cancer cells. Journal of Thoracic Oncology 5: 1143–1151. DOI 10.1097/JTO.0b013e3181e0b954. [Google Scholar] [CrossRef]
Xia H, Dai X, Yu H, Zhou S, Fan Z, Wei G, Tang Q, Gong Q, Bi F. (2018). EGFR-PI3K-PDK1 pathway regulates YAP signaling in hepatocellular carcinoma: the mechanism and its implications in targeted therapy. Cell Death & Disease 9: 421. DOI 10.1038/s41419-018-0302-x. [Google Scholar] [CrossRef]
Yamaguchi K, Masuda T, Fujitaka K, Miwata K, Sakamoto S, Horimasu Y, Hamai K, Miyamoto S, Nakashima T, Okamoto Y, Iwamoto H, Ishikawa N, Miyata Y, Okada M, Hamada H, Hattori N. (2018). Bevacizumab with single-agent chemotherapy in previously treated non-squamous non-small-cell lung cancer: phase II study. In Vivo 32: 1155–1160. [Google Scholar]
Zeng Y, Yao X, Chen L, Yan Z, Liu J, Zhang Y, Feng T, Wu J, Liu X. (2016). Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/syndecan-1/TGF-03B2; autocrine loop. Oncotarget 7: 63324–63337. DOI 10.18632/oncotarget.11450. [Google Scholar] [CrossRef]
Zeng Y, Yao X, Liu X, He X, Li L, Liu X, Yan Z, Wu J, Fu B. (2019). Anti-angiogenesis triggers exosomes release from endothelial cells to promote tumor vasculogenesis. Journal of Extracellular Vesicles 8: 1629865. DOI 10.1080/20013078.2019.1629865. [Google Scholar] [CrossRef]
Zeng Z, Yan HH, Zhang XC, Zhong WZ, He YY, Guan JL, Niu FY, Xie Z, Huang YS, Xu CR, Dong S, Wu YL. (2014). Reduced chemotherapy sensitivity in EGFR-mutant lung cancer patient with frontline EGFR tyrosine kinase inhibitor. Lung Cancer 86: 219–224. DOI 10.1016/j.lungcan.2014.09.008. [Google Scholar] [CrossRef]
Zhang H. (2015). Apatinib for molecular targeted therapy in tumor. Drug Design, Development and Therapy 9: 6075–6081. DOI 10.2147/DDDT.S97235. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |