DOI:10.32604/biocell.2021.014694

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.014694 |  www.techscience.com/journal/biocell |
| Article |
Addition of peroxiredoxin 6 (PRDX6) to IVF fertilization medium maintains motility and longevity of human spermatozoa
1Reproductive Medical Center, Department of Obstetrics and Gynecology, Peking University International Hospital, Beijing, 102206, China
2Reproductive Medicine Centre, Peking University Second Affiliated Hospital, Beijing, 100044, China
3Center of Reproductive Medicine and Genetics, Seventh Medical Center of PLA General Hospital, Beijing, 100027, China
*Address correspondence to: Xi Chen, chenxi@pkuph.edu.cn; Li Tian, tianli@pkuih.edu.cn
#These authors contributed equally
Received: 20 October 2020; Accepted: 23 December 2020
Abstract: This study aims to investigate the protective effects of peroxiredoxin 6 on the total motility and progressive motility of human spermatozoa. Semen samples with normal parameters were collected from 23 males and supplemented with different concentrations of peroxiredoxin 6. All the semen samples were measured according to the WHO 5th manual, and the motile spermatozoa were extracted using IVF fertilization medium supplemented with different peroxiredoxin 6 concentrations. Total motility and progressive motility were observed at different time-points of culture at room temperature. After peroxiredoxin 6 supplementation, all groups had a significant increase in total motility and progressive motility compared to the control group. The difference in total motility and progressive motility between the 0 and 10−7 mM groups was observed at 24 and 48 h of culture at room temperature. At 24 h, the total motility increased by 30% in the control group (16.03 ± 11.91 vs. 11.51 ± 7.84), and progressive motility increased by 21% (10.53 ± 9.4 vs. 8.31 ± 6.04). A similar trend was observed in the 48 h group. In addition, we also found that peroxiredoxin 6 had a well protective effect on sperm kinetic parameters at 10−7 mM. The findings of this study suggest that peroxiredoxin 6 can enhance sperm total motility and progressive motility in IVF fertilization medium. Peroxiredoxin 6 may have potential benefits for sperm preparation in assisted reproductive technology.
Keywords: Peroxiredoxin 6; Sperm motility; Progressive motility; Sperm kinetic parameters
Sperm parameters, including semen concentration, general volume, color, consistency, motility, vitality, and morphology, are used to determine the quality of an ejaculate and for in vitro fertilization (IVF) and intracytoplasmic sperm injection (Alessandro et al., 2018; Shu et al., 2013; Ohlweiler et al., 2019). Human sperm motility is of utmost importance for improving the efficacy of IVF and intracytoplasmic sperm injection (Stanic et al., 2002; Sun et al., 2018). In particular, maximizing the clinical pregnancy rate following artificial insemination necessitates the development of strategies to manage sperm metabolism so that human sperm cells retain full structural and functional characteristics during their collection, in vitro culture, and subsequent insemination (Lee et al., 2018). Therefore, hyperactivity is one of the necessary processes for IVF efficiency and increasing embryonic development in assisted reproductive technologies (Stauss et al., 1995; Suarez and Ho, 2010).
PRDX6 (one of six members of the peroxiredoxins (PRDXs) family) plays an important role in protecting spermatozoa against oxidative stress (Gong et al., 2012; Liu and O’Flaherty, 2017). O’Flaherty and his colleagues observed that PRDX6 could affect the viability of spermatozoa and promote oxidative stress, thus increasing the levels of lipid peroxidation, and hence increasing sperm motility in vivo (O’Flaherty and Souza, 2011). Studies have also found that PRDX6 has peroxidase and calcium-independent phospholipase A2 activities and is a major factor in the protection of sperm motility, fertilization, and blastocyst development (Fisher, 2017; Moawad et al., 2017).
Studies have demonstrated that the peroxidase and phospholipase A2 activities of PRDX6 are important for sperm quality in vivo (Moawad et al., 2017; Ozkosem et al., 2016; O’Flaherty, 2018). In our previous study, we found that PRDX6 promoted total and progressive motility of human spermatozoa after cryopreservation (Sun et al., 2020). However, its effect on sperm motility in vitro is not known. Therefore, in the present investigation, we examined the hypothesis that PRDX6 can exert beneficial effects on total sperm motility and progressive motility under in vitro conditions.
Twenty-three semen samples were collected from 23 healthy donors at the clinical laboratory of the infertility center between December 2019 and January 2020. The mean age of healthy donors was 32.88 ± 4.64 years old. All men were asked to maintain abstinence for 2–7 days before sample collection and to release semen into sterile containers by masturbation. The study was approved by the Institutional Ethical Committee of Peking University International Hospital.
The semen characteristics were assessed according to World Health Organization (WHO) criteria (volume ≥1.5 mL, total motility ≥40%, sperm concentration ≥15 × 106 sperm/mL, and ≥4% normal). If all semen parameters meet the WHO criteria, the untreated semen was washed twice using IVF fertilization medium (G-IVF™ PLUS, Vitrolife, Sweden). Then, the semen samples were mixed with 2 mL of IVF fertilization medium and divided into five groups.
PRDX6 (Sigma-Aldrich, Saint Louis, MO, USA) at the concentration of control, 10−3, 10−5, 10−7, and 10−9 mM was added into the IVF fertilization medium of the five groups of semen samples, respectively. The influence of PRDX6 supplementation on motility was assessed at 1 h, 12 h, 24 h and 48 h at room temperature. At least 200 spermatozoa were scored for motility evaluation under 200× magnification using a computer-assisted sperm analysis program (CASA, WeiLi, Beijing, China) and graded as rapid progressive (PR), non-progressive (NP), and immotility (IM) spermatozoa. All kinetic parameters including straight-line velocity (VSL) (μm/s), curvilinear velocity (VCL) (μm/s), average path velocity (VAP) (μm/s), linearity (LIN) (%), straightness (STR) (%) and wobble (WOB) (%) were recorded.
Data were analyzed by two-way repeated-measures ANOVA using SPSS 3.0 software (USA). The Student’s paired t-test was performed to compare data between groups. Multiple comparisons were made using the Bonferroni procedure. P < 0.05 was considered statistically significant.
Initial seminal analysis after ejaculation
All semen characteristics (volume, concentration, progressive rate, non-progressive rate, immotility, and morphology) were found to be normal, according to the WHO 5th edition (Tab. 1). The mean sperm concentration was 120.03 ± 19.07 × 106/mL; volume was 3.1 ± 0.56 (mL); total motility was 75.66 ± 8.96%; progressive motility was 60.91 ± 7.95%; non-progressive motility was 14.75 ± 3.01%; and the percentage of spermatozoa with normal morphology was 5.13 ± 1.13%.
Effects of different concentrations of PRDX6 on sperm total and progressive motility
The classic negative effects of long-term in vitro culture on sperm motility were clearly observed (Tab. 2). In particular, we observed a significant decrease in total motility and progressive motility in all sperm samples (Fig. 1). In vitro culture of spermatozoa resulted in approximately 25%–75% reduction in total and progressive motility compared to the 12 h group after the incubation. The difference in total and progressive motility was also observed at 24 h and 48 h after the incubation. At 1 h, the total motility (PR+NP) was the highest, but with the prolongation of culture time, sperm motility decreased significantly (Fig. 1A). A similar trend was observed in progressive (PR) motility (Fig. 1B). As we observed, it seems that the decline speed of total motility is more obvious than that of progressive (PR) motility. At 12 h, the total motility decreased by 60% in the 1 h group (20.69 ± 1.96 vs. 56.99 ± 2.3); in the 24 and 48 h groups, total motility decreased by 75% (13.78 ± 2.03 vs. 56.99 ± 2.3) and 90% (5.56 ± 1.45 vs. 56.99 ± 2.3), respectively (P < 0.001 compared to the 1 h).
Optimum concentration of PRDX6 on total and progressive motility
The total and progressive motility in all samples decreased gradually with culture time (Fig. 2). Although there was a significant decrease in sperm motility with the prolongation of culture time, we found that regardless of total motility or progressive motility, 10−7 mM and 10−9 mM PRDX6 were the best concentrations to protect sperm motility at 24 h and 48 h. Even though the other PRDX6 groups had a marginally higher percentage of total or progressive motility, statistically, it was not found to be significant compared to the 10−7 mM group at any time point.
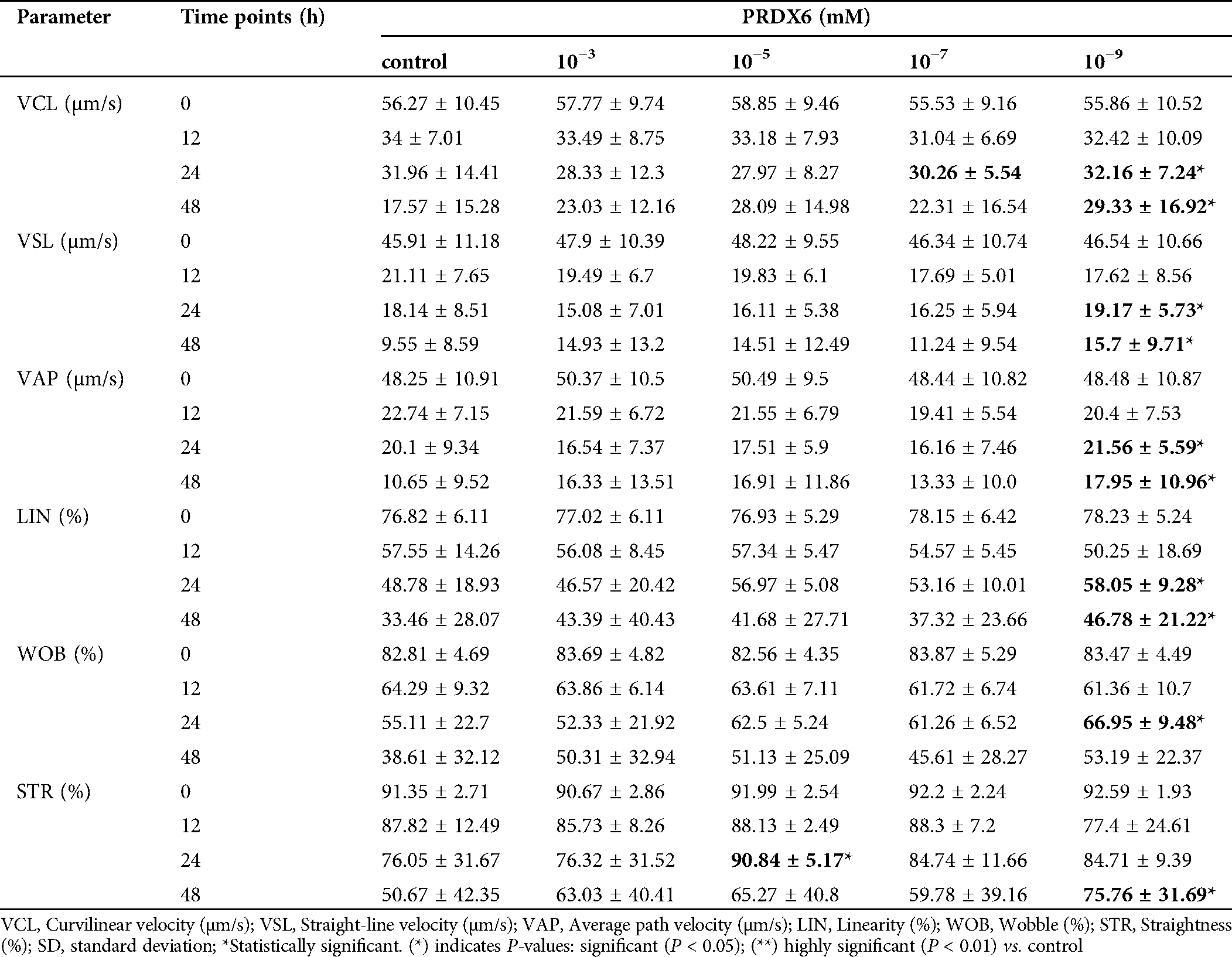
PRDX6 on sperm kinetic parameters
With the powerful analysis capability of CASA, we recorded almost all dynamic parameters, including VCL (μm/s), VSL (μm/s), VAP (μm /s), LIN (%), STR (%) and WOB (%). In our study, most individual sperm velocity parameters (VSL, VCL, VAP, LIN, WOB and STR) were significantly decreased along with the extension of culture time (Tab. 3). Surprisingly, our results showed that the sperm showed a high VCL and VSL at concentrations of 10−7 mM and 10−9 mM (Tab. 3). In addition, we also found that sperm velocity parameters of STR and LIN also had the same trend at concentrations of 10−7 mM and 10−9 mM (Tab. 3). The above results suggest that PRDX6 has a better protective effect on sperm kinetic parameters at 10−7 mM and 10−9 mM concentrations than at other concentrations.
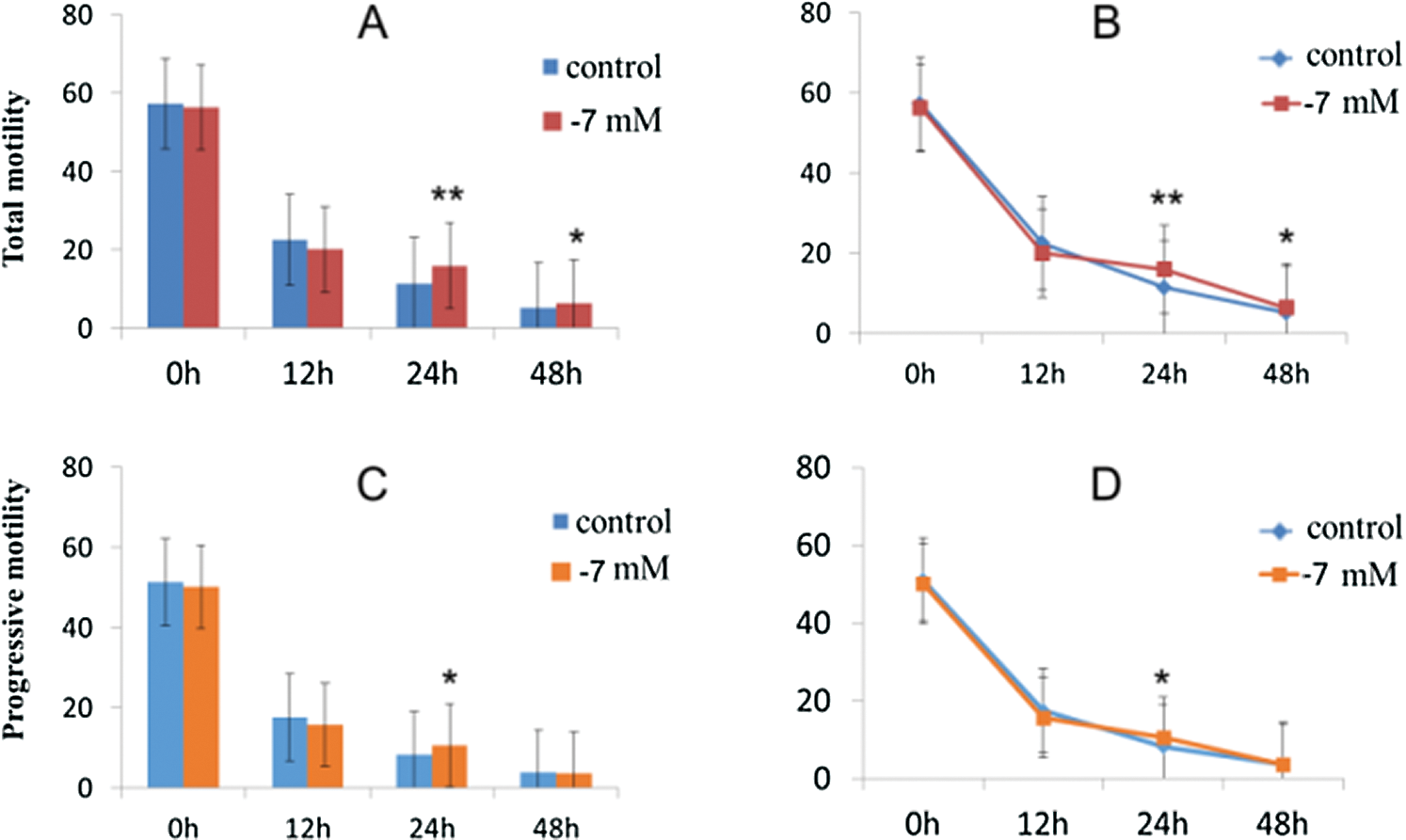
Figure 1: Effects of PRDX6 on total and progressive motility at different hours after culture in vitro.
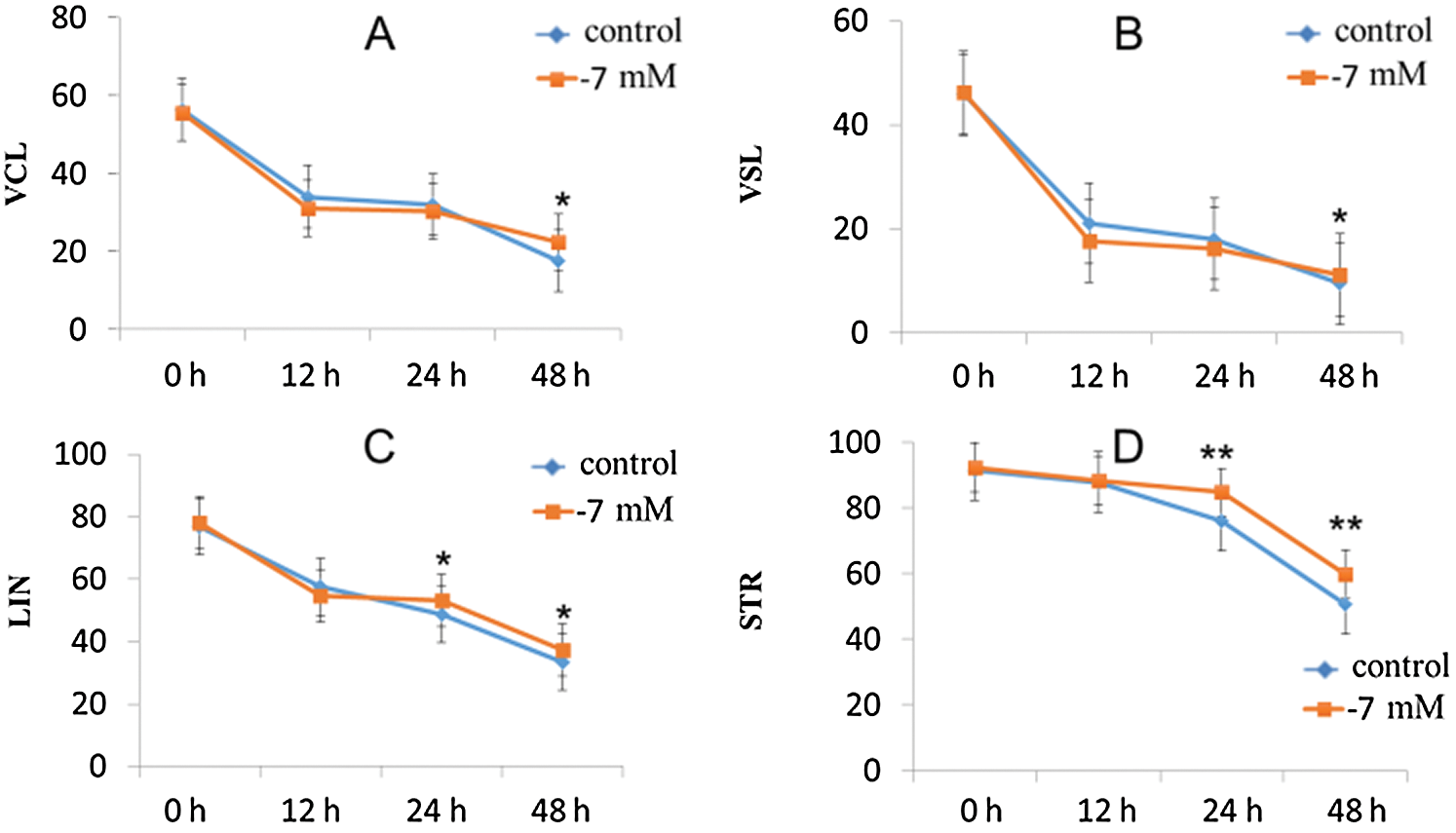
Figure 2: Effects of PRDX6 on sperm kinetic parameters (VCL, VSL, LIN and STR).
Table 1: The characteristics of the patients who underwent semen analyses (N = 23)
Table 2: Effects of different concentrations of PRDX6 supplemented to IVF fertilization medium on total and progressive motility
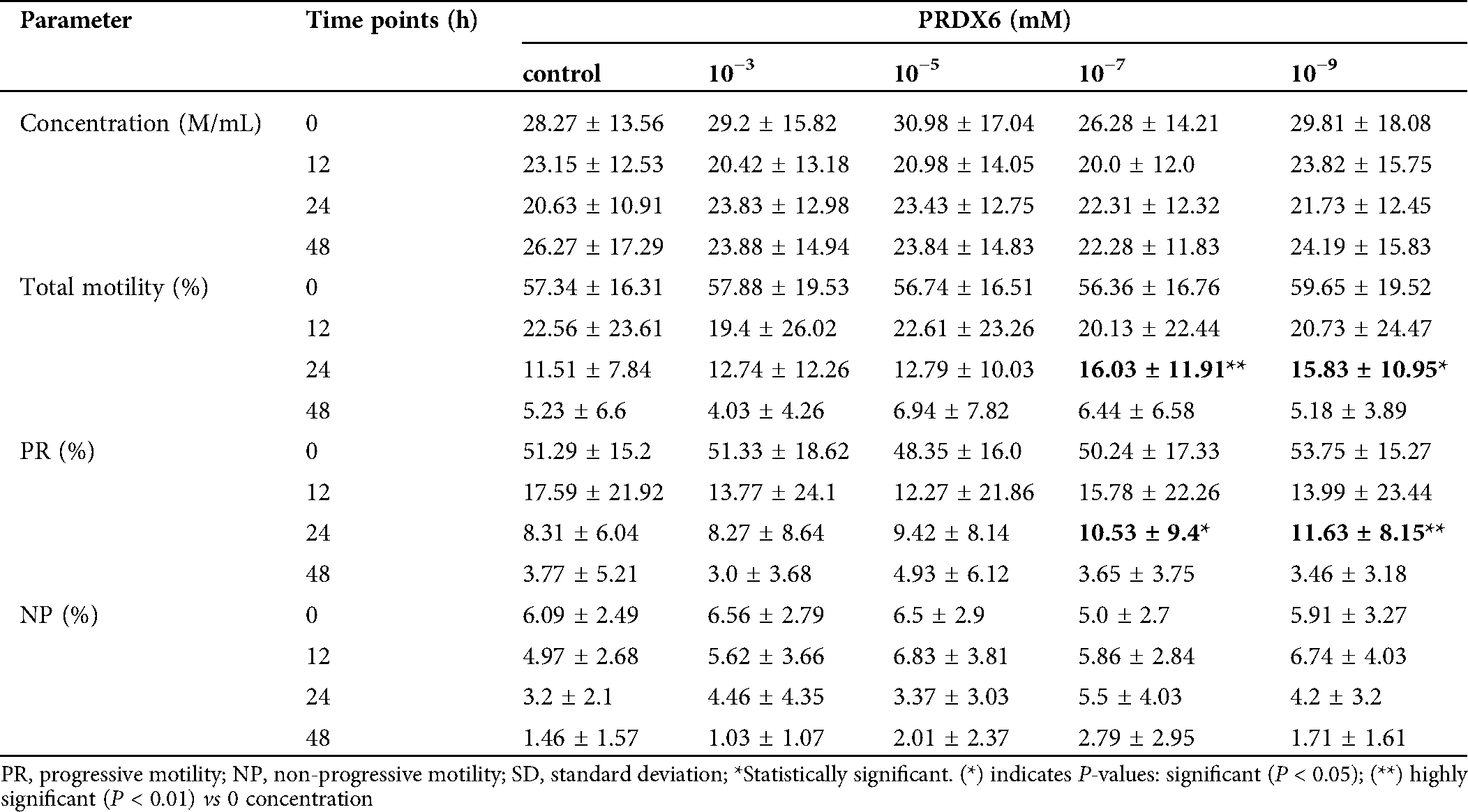
Table 3: Effects of PRDX6 on sperm kinetic parameters (mean ± SD)
Our study was carried out to investigate the effects of different concentrations of PRDX6 on human sperm motility using a CASA system. The results showed that PRDX6 increased human sperm total and progressive motility. The positive effects of PRDX6 on sperm motility have also been demonstrated previously (Shi et al., 2018). The beneficial effect of PRDX6 was observed at 10−3, 10−5, 10−7, and 10−9 mM, respectively.
It has been reported that PRDX6 can promote sperm movement, improve sperm fertilization rate, and increase the penetration rate of boar spermatozoa and zona pellucida (Fisher, 2017; Moawad et al., 2017). The total and progressive motility of human sperms were increased by using PRDX6 at 10−7 mM and 10−9 mM at different time-points (1 h, 12 h, 24 h, and 48 h) after culture at room temperature, as revealed in our study. However, other concentrations of PRDX6 did not dramatically affect sperm movement. Therefore, the effect of PRDX6 on sperm characteristics may be specific; sperm motility may be adversely affected by other concentrations of PRDX6.
The optimum value of sperm motility is a key factor for successful fertilization. According to the WHO 5th manual, less than 40% of the proportion of motile spermatozoa is an essential prognostic fertility factor (Shu et al., 2013). Sperm motility parameters, including ALH, VCL, VAP, and LIN, have been shown to be important markers of sperm motility and fertilizing ability (Lee et al., 2018; Shih et al., 2016; Rondanino et al., 2015). To our knowledge, no study has found a significant improvement in motility after PRDX6 treatment in vitro. Some have observed either no improvement in sperm total motility or VCL and VSL. Our results for the first time indicate that PRDX6 can not only improve the total sperm motility but also improve progressive motility.
Oxidative stress has a negative effect on sperm motility through reactive oxygen species (ROS) and ROS-dependent proteins. Before assisted reproductive technology, human spermatozoa were processed through in vitro preparation and cultured for a long time, which could induce certain levels of ROS (Shih et al., 2016). PRDXs have been confirmed to have antioxidant enzyme activities to control the levels of ROS production and avoid oxidative damage in the spermatozoa (O’Flaherty, 2018). Previous studies have shown that PRDX6 is the primary antioxidant defense in human spermatozoa and maintains viability by regulating the phosphoinositide 3-kinase/AKT pathway (Fernandez and O’Flaherty, 2018; Fernandez et al., 2019). Our results showed that PRDX6 significantly enhanced the sperm total motility of sperm under oxidative stress. As for the protective effect of PRDX6 on sperm motility, our results were consistent with those reported by O’Flaherty C et al. (Moawad et al., 2017). The enhanced total and progressive motility induced by PRDX6 is due to its inhibitory effect on ROS. It is difficult to elucidate the exact mechanism of action of PRDX6 from this preliminary data. However, through indirect methods, we attempted to know whether this effect is mediated by the inhibition of ROS and ROS-related pathways. Surprisingly, we observed that the addition of different concentrations of PRDX6 to the IVF fertilization medium not only significantly improved sperm motility but also maintained 48 h of motility. Moreover, the 10−7 mM or 10−9 mM PRDX6 supplemented group maintained a significantly higher percentage of total and progressive motility than the other groups at all time- points. Although our research shows that PRDX6 may have potential application value as human sperm motility “vigor” in vitro, further research is needed to clarify the exact molecular mechanism of PRDX6-induced sperm motility enhancement.
Collectively, our novel finding has demonstrated that supplementing PRDX6 to IVF fertilization medium can increase sperm total and progressive motility in vitro. Additionally, lower concentrations of PRDX6 have better protective effects than higher concentrations. Therefore, we recommend the addition of PRDX6 at 10−7 mM concentration to the IVF fertilization medium.
Acknowledgement: We thank Beijing Chunfenglv Bio-medical Technologies, Inc., Beijing, China for editing this manuscript. We apologize to some authors for not citing their interesting work. Our choice was not intended to be exclusive. The authors also thank Dr. Yao Gu for her continuous support.
Availability of Data and Materials: The data used to support the findings of this study are available from the corresponding author upon request.
Author Contribution: The authors confirm contribution to the paper as follows: Study conception and design: (X. Chen and L. Tian); data collection: (Xin Ping Sun, Hong Yu and Ling Li Song); analysis and interpretation of results: (Tie Cheng Sun, Yan Dong Zhang and Jian Hua Li); draft manuscript preparation: (Tie Cheng Sun, Yan Dong Zhang and Jian Hua Li). All authors reviewed the results and approved the final version of the manuscript.
Ethics Approval: The study was approved by the Institutional Ethical Committee of Peking University International Hospital, ethical approval code: S2020-04.
Funding Statement: This work was supported by the Peking Post-doctoral Research Fund (EE2019-50) and Peking University International Hospital Research Funds (No. YN2019QN13).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Alessandro B, Luca P, Sofia M, Andrea S Enrico P et al. (2018). Abnormal sperm concentration and motility as well as advanced paternal age compromise early embryonic development but not pregnancy outcomes: A retrospective study of 1266 ICSI cycles. Journal of Assisted Reproduction and Genetics 35: 1897–1903. DOI 10.1007/s10815-018-1256-8. [Google Scholar] [CrossRef]
Fernandez MC, O’Flaherty C. (2018). Peroxiredoxin 6 is the primary antioxidant enzyme for the maintenance of viability and DNA integrity in human spermatozoa. Human Reproduction 33: 1394–1407. DOI 10.1093/humrep/dey221. [Google Scholar] [CrossRef]
Fernandez MC, Yu A, Moawad AR, O’Flaherty C. (2019). Peroxiredoxin 6 regulates the phosphoinositide 3-kinase/AKT pathway to maintain human sperm viability. Molecular Human Reproduction 25: 787–796. [Google Scholar]
Fisher BA (2017). Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Archives of Biochemistry & Biophysics 617: 68–83. [Google Scholar]
Gong S, San Gabriel MC, Zini A, Chan P, O’Flaherty C. (2012). Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. Journal of Andrology 33: 1342–1351. DOI 10.2164/jandrol.111.016162. [Google Scholar] [CrossRef]
Lee J, Hwang S, Lee J, Yoo J, Jang D et al. (2018). Effect of insemination timing on pregnancy outcome in association with female age, sperm motility, sperm morphology and sperm concentration in intrauterine insemination. Journal of Obstetrics and Gynaecology Research 44: 1100–1106. DOI 10.1111/jog.13625. [Google Scholar] [CrossRef]
Liu Y, O’Flaherty C. (2017). In vivo oxidative stress alters thiol redox status of peroxiredoxin 1 and 6 and impairs rat sperm quality. Asian Journal of Andrology 19: 73–79. [Google Scholar]
Moawad AR, Fernandez MC, Scarlata E, Dodia C, Feinstein SI et al. (2017). Deficiency of peroxiredoxin 6 or inhibition of its phospholipase A2 activity impair the in vitro sperm fertilizing competence in mice. Scientific Reports 7: 1506. DOI 10.1038/s41598-017-13411-2. [Google Scholar] [CrossRef]
O’Flaherty C. (2018). Peroxiredoxin 6: The protector of male fertility. Antioxidants 7: 173. DOI 10.3390/antiox7120173. [Google Scholar] [CrossRef]
O’Flaherty C, Souza ARD. (2011). Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biology of Reproduction 84: 238–247. DOI 10.1095/biolreprod.110.085712. [Google Scholar] [CrossRef]
Ohlweiler LU, Mezzalira JC, Mezzalira A. (2019). Porcine IVF embryo development and estrogen receptors are influenced by the concentration of percoll gradients during sperm selection. Molecular Reproduction and Development 87: 135–141. DOI 10.1002/mrd.23290. [Google Scholar] [CrossRef]
Ozkosem B, Feinstein SI, Fisher AB, O’Flaherty C. (2016). Absence of peroxiredoxin 6 amplifies the effect of oxidant stress on mobility and SCSA/CMA3 defined chromatin quality and impairs fertilizing ability of mouse spermatozoa. Biology of Reproduction 94: 325. DOI 10.1095/biolreprod.115.137646. [Google Scholar] [CrossRef]
Rondanino C, Duchesne V, Escalier D, Jumeau F, Verhaeghe F et al. (2015). Evaluation of sperm nuclear integrity in patients with different percentages of decapitated sperm in ejaculates. Reproductive BioMedicine Online 31: 89–99. DOI 10.1016/j.rbmo.2015.04.002. [Google Scholar] [CrossRef]
Shi H, Liu J, Zhu P, Wang H, Zhao Z et al. (2018). Expression of peroxiredoxins in the human testis, epididymis and spermatozoa and their role in preventing H2O2-induced damage to spermatozoa. Folia Histochemica et Cytobiologica 56: 141–150. DOI 10.5603/FHC.a2018.0019. [Google Scholar] [CrossRef]
Shih YF, Tzeng SLI, Chen WJ, Huang CC, Chen HH, Lee TH, Lee MS. (2016). Effects of synthetic serum supplementation in sperm preparation media on sperm capacitation and function test results. Oxidative Medicine and Cellular Longevity, 1027158. [Google Scholar]
Shu JH, Feng GX, Jin LI, Li JX, Gan XY et al. (2013). Predictive value of sperm morphology according to WHO Laboratory Manual for the Examination and Processing of Human Semen (5th Ed.) on the outcomes of IVF-ET. National Journal of Andrology 19: 414. [Google Scholar]
Stanic P, Sonicki Z, Suchanek E. (2002). Effect of pentoxifylline on motility and membrane integrity of cryopreserved human spermatozoa. International Journal of Andrology 25: 186–190. DOI 10.1046/j.1365-2605.2002.00348.x. [Google Scholar] [CrossRef]
Stauss CR, Votta TJ, Suarez SS. (1995). Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biology of Reproduction 53: 1280–1285. DOI 10.1095/biolreprod53.6.1280. [Google Scholar] [CrossRef]
Suarez SS, Ho HC. (2003). Hyperactivated motility in sperm. Reproduction in Domestic Animals 38: 119–124. DOI 10.1046/j.1439-0531.2003.00397.x. [Google Scholar] [CrossRef]
Sun T, Cheng L, Ma J, Zhou S, Zhang Y et al. (2020). Effect of peroxiredoxin 6 on total and progressive motility of human spermatozoa after cryopreservation. BIOCELL 44: 323–327. DOI 10.32604/biocell.2020.011890. [Google Scholar] [CrossRef]
Sun TC, Zhang Y, Li HT, Liu XM, Yi DX et al. (2018). Sperm DNA fragmentation index, as measured by sperm chromatin dispersion, might not predict assisted reproductive outcome. Taiwanese Journal of Obstetrics and Gynecology 57: 493–498. DOI 10.1016/j.tjog.2018.06.003. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |