DOI:10.32604/biocell.2021.013409

www.techscience.com/journal/biocell
| Biocell DOI:10.32604/biocell.2021.013409 |  www.techscience.com/journal/biocell |
| Article |
Carbon nanotubes: A review on risks assessment, mechanism of toxicity and future directives to prevent health implication
1College of Agriculture, Rani Laxmi Bai Central Agriculture University, Jhansi, 284003, India
2Department of Biological Sciences, Indian Institute of Science Education and Research Bhopal (IISERB), Bhopal, 462066, India
3Department of Biotechnology, Himachal Pradesh University, Shimla, 171005, India
*Address correspondence to: Piyoosh Kumar Babele,piyoosh.bhu06@gmail.com
Received: 12 August 2020; Accepted: 11 November 2020
#These authors contributed equally to this work
Abstract: Carbon nanotubes (CNTs) have tremendous applications in almost every walk of life; however, their harmful impacts on humans and the environment are not well addressed. CNTs have been used in various applications ranging from medical science to different engineering branches, to ease human life. Generally, the toxicological profile of CNTs under laboratory conditions cannot be assessed primarily in medical science due to the inconsistent availability of cytotoxic study data. CNT toxicity has been affected by many physicochemical properties (e.g., size, type of functionalization), concentration, the extent of exposure, mode of exposure, and even the solvents/medium used to dissolve/disperse CNTs for their application. These inconsistencies arise due to the variation in synthesis methods as well as the mode of their human exposure. Besides their unlimited use in various fields, most of CNT toxicity aspects and mechanisms remain uncertain. Additionally, in-depth knowledge of CNTs toxicity is scarce, and the available literature shows dissimilarities in experimental data and exposure studies. To understand the toxicological issues, it is the need of the hour to provide insight into the published data, post-exposure studies, and various factors that may damage the cells due to CNTs toxicity. This review article analyses the hazardous potential through toxicological implications and summarizes the detailed mechanism(s) of CNTs studied on the different model organisms, including human cell lines. In this review article, we hypothesized that thorough knowledge of various aspects, as mentioned above, helps us design and develop possible strategies to reduce the toxicity of nanomaterial to make them safer and secure for humanity’s betterment.
Keywords: Nanotechnology; CNTs; Nanotoxicity; Toxicity mechanism; Drug delivery
Science and technology to create purposeful systems and devices at a molecular scale, which is about from 1 to 100 nm, are called nanotechnology (Labib et al., 2015). Conversion of materials into nanoscale levels significantly changes their physicochemical properties and thus functions. The integration of scientific disciplines (e.g., physics, chemistry, biology, and engineering, etc.) diversifies nanoscale material applications in broad areas. Rising nanoscale materials applications are leading to their multiplication in manufacturing materials (Costa and Fadeel, 2016). Nanotechnology is likely to substantially impact our economy and society within the next 5 to 10 years. Among the wide range of nanomaterials, carbon nanotubes (CNTs) gained enormous nanotechnology popularity for their unique physiochemical properties (Turabekova et al., 2014). They have applications in various fields, including electronics (transistor and integrated circuits), energy (catalyst in a fuel cell), environment (air and water purifier, bioremediation), healthcare (sensors, drug delivery), and used in various consumer products (Li et al., 2014). CNTs have promising applications in the fields of nanotheranostics and personalized medicine (the field of delivering a suitable drug to the right individual in a specific organ with precision and accuracy) and can be proved a game-changer for cancer therapy, which is one of the promising areas in nano-theranostics (Yaari et al., 2016). The drug delivery across the blood-brain barrier (BBB) remains a major challenge; studies have shown that CNTs have potential in crossing the blood-brain barrier and the possibility to cure the ailments related to neurological disorders such as cerebral ischemia, Parkinson, Alzheimer, multiple sclerosis, etc. (Fadeel and Orrenius, 2005). The design and synthesis of CNTs, including both single-walled carbon nanotubes (SWCNTs) and multiwalled carbon nanotubes (MWCNTs), require a set of experimental procedures. Often the surface area is considered a deciding factor in the synthesis of CNTs that allow the extended application of nanodesigns in various fields (Aschberger et al., (2010) and Allegri et al. (2016)). However, opting for a synthesis method at the same remains link with toxicity as well. It has been reported that improved surface area of CNTs provides an increased rate and extent of absorption. During the design and synthesis of CNTs, several parameters that could introduce toxicity are functional group, chelating agents, coating material, contaminants, and agglomeration/sedimentation (Aschberger et al., (2010) and Allegri et al. (2016)). These parameters can be optimized to reduce the toxicity of CNTs in the purification process. However, CNTs, as a result of chemical synthesis, often possess a higher tendency of toxicity over CNTs derived from green synthesis. A summary of toxicity sources, subcellular events associated with CNTs toxicity, and the opportunity to optimize their synthesis have shown in Fig. 1.
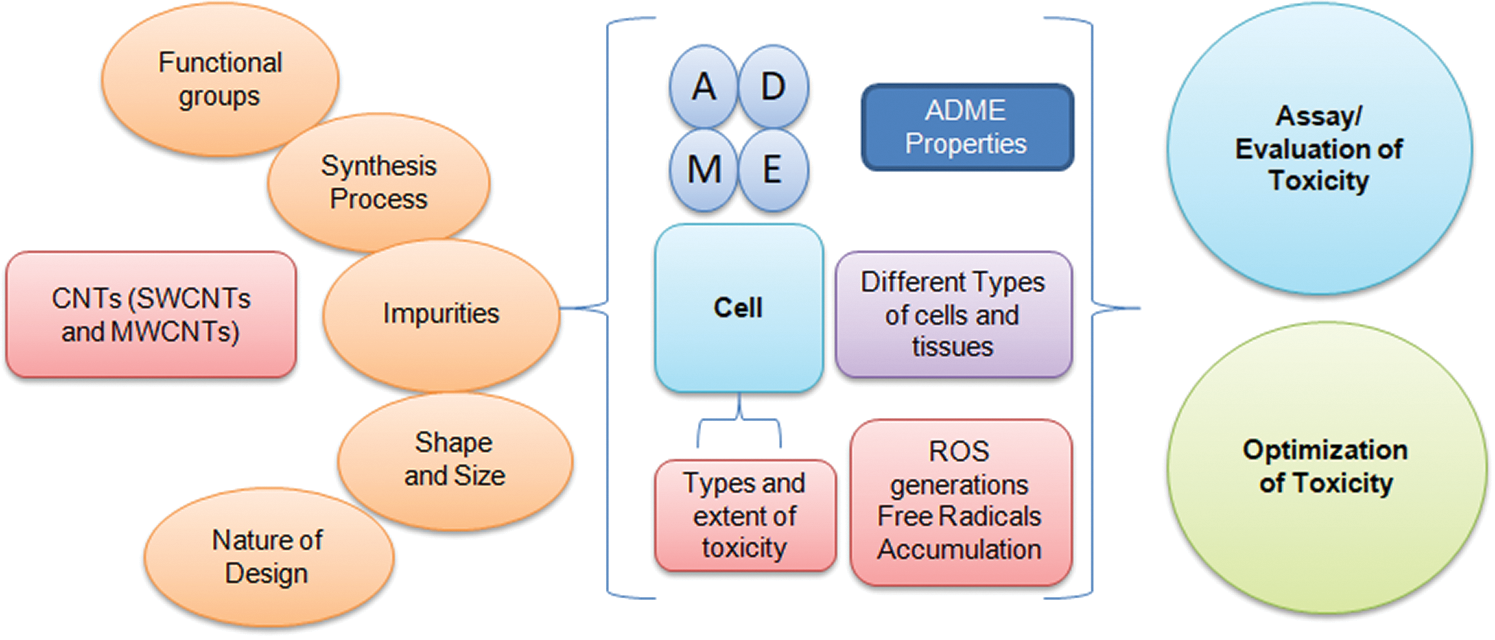
Figure 1: Sources of CNTs toxicity, subcellular events associated with toxicity, and the opportunity to optimize their synthesis using precise toxicity evaluation
Further, a higher probability of contaminants and ions as part of CNTs synthesis brings toxicity level. The level of toxicity among SWCNTs and MWCNTs differs and depends on several factors, including length, diameter, purity, and impurities associated. Comparing SWCNTs with MWCNTs, characteristics such as fictionalization, twisting capacity, ease in synthesis, use of catalyst, the arraignment of layers, and extent of accumulation provide a comprehensive overview of the nature and extent of toxicity (Kavosi et al., 2018). In general, it is challenging to predict toxicity as level and or extent among SWCNTs and MWCNTs by considering using a standard parameter. However, it has been reported SWCTNs are often more toxic than MWCNTs (Deng et al., 2007).
Although CNTs have shown promising applications in nanotherapy, their extensive and unrestricted manufacturing and disposal into the environment (soil, water, and air) may impose human concerns leading to severe health issues (Babele et al., 2018; Singh et al., 2014). There are accumulating research evidence on the toxic effects of CNTs describing the mechanism(s) of CNTs toxicity at the cellular, subcellular and molecular levels depending on the doses and mode of internalization using various model organisms, including animals but the finding of these studies are often contradictory (Gedda et al., 2019). In vivo studies proved that the toxic effects resulted from CNTs not only vary from cell to cell but also from organism to organism. Despite various drawbacks of in vitro studies, a detailed CNTs toxicity analysis is fundamental to provide new insights in this emerging area. It’s the time to explore the potential hazards and unknown issues of the CNTs toxicity on human health and the environment (Kota et al., 2017). To achieve this, a detailed understanding of CNTs toxicity is significant for better utilization of these nanomaterials in the near future. This review provides an up to date analysis of routes of exposures of CNTs and their connection to organ and tissue toxicity. We summarized different in vitro and in vivo studies and describe various toxicity mechanisms(s) at a molecular level conducted on numerous microorganisms, human cell lines, lower and higher animals. The knowledge summarized will enhance our understanding of CNT toxicity and help us find new strategies to synthesize nontoxic CNTs.
Synthesis and Functionalization of CNTs
A variety of methods have been developed to synthesize CNTs; among those, three main techniques, such as chemical vapor deposition (CVD) (require low temperature), laser ablation, and carbon arc-discharge (require high temperature), have gained significant interest. These methods can easily control various CNT properties such as length, diameter, alignment, purity, density, orientation, etc. (Singh et al., 2014; Siddiqi et al., 2018; Sasrimuang et al., 2020). CVD, which is further modified using catalysts, plasma, oxygen, water, microwave plasma, radiofrequency, hot-filament, etc., is one of the typical CNT processing methods. Catalytic chemical vapor deposition (CCVD) is currently the most standard method of carbon nanotubes synthesis (Shah and Tali, 2016). CNTs are further functionalized to improve their interfacial interaction between CNTs and polymer matrix. This can be done either by covalent bonding of functional groups onto the surface of CNTs or by non-covalent functionalization (Zhou et al., 2019). Covalent bond functionalization can be done by reaction with molecules of high chemical reactivity (Zhou et al., 2019). Initially, strong acids (HNO3, H2SO4, etc.), strong oxidants (KMnO4, ozone, etc.), or their mixtures has become popular for the covalent functionalization, but these strong agents resulted in intrinsic defects due to oxidative damage to the CNTs framework leaving behind detrimental structures, hence hampering their potential for practical applications and can also compromise the mechanical properties (Shah and Tali, 2016). Therefore non-covalent functionalization of CNTs with various other agents like zinc porphyrin derivative and some biological molecules onto CNTs surface with a high degree of control and specificity tried by multiple researchers for better functionalization between CNTs and the foreign matrix, without any structural damage to the CNTs, for various applications (Zhou et al., 2019).
Nano-Toxicity of Carbon Nanotubes
Routes of exposure, clearance and associated implications
CNTs can be administered into the body either purposely (as a part of therapy, drug delivery, and diagnostic) or unintentionally due to environmental pollution or accidental release. In intentional procedures, usually intravenous, intradermal, intramuscular, and peritoneal injections are utilized. CNTs unintentionally may enter to lungs, skin, or organs as airway exposure is one of the common routes for CNTs in the working environment. Therefore inhalation and intratracheal instillation are mainly used in animal models to imitate human exposure to CNTs. Instillation methods are well adopted than inhalation to study the CNTs toxicity as CNTs were found to accumulate in the lungs at a high-level (Morimoto et al., 2011; Ryman-Rasmussen et al., 2009). CNTs injected intravenously in animal models get concentrated near the entry site and attain diverse locations through blood circulation or biological barriers, resulting in varying accumulation levels in different organs such as the liver and spleen (Qu et al., 2009). CNTs can reach deeper organs by traversing through the standard physiological barriers; blood-air barrier, blood-testis barrier, blood-brain barrier, and blood-placental barrier (Simkó and Mattsson, 2010). The lungs are a potential route for the entrance of CNTs into the human body through the airway.
The inhaled CNTs exert their potential toxicity by damaging the pulmonary surfactant’s ultrastructure and biophysical properties, which serve as the first line of host defense (Valle et al., 2015). The agglomerated or dispersed CNTs are engulfed by alveolar macrophages and deposited on the inner alveolar surface within the alveoli (Wiemann et al., 2016). It has been found that CNTs rarely pass through the blood-brain barrier and hardly cause neurotoxicity, but Blood-brain barrier research had made substantial progress in this area to study the effect of CNTs toxicity and pathology (Jain, 2012; Kafa et al., 2015). Large-sized and oxidized MWCNTs can move across the blood-placenta barrier (BPB), enter the fetus body, and restrict the development of fetuses, and induced brain deformity (Huang et al., 2014; Qi et al., 2014). These four barriers were the most frequently mentioned barriers in the literature.
Moreover, the detailed molecular mechanism by which CNTs pass through these barriers is not well known, and more organized investigations are immediately required. The inhaled CNTs are deposited and accumulated in the respiratory tract and quickly undergo a series of site-specific clearance mechanisms. The clearance and excretion of CNTs vary in different organs. Clearance from the lungs includes fast bronchial clearance via mucociliary escalator, slow bronchial clearance by airway macrophages, transcytosis, and alveolar clearance using alveolar macrophages and endocytosis by alveolar epithelium (Sturm, 2014). Another study describes that lung macrophages digest CNTs and, therefore, get cleared from the lungs by a superoxide/NO* peroxynitrite-driven oxidative pathway (Kagan et al., 2014). Recent research describes the role of matrix metalloproteinases (MMP), a class of extracellular endopeptidases that control various processes related to tissue repair and inflammation. The role of stromelysin-2 (MMP-10), a modulator of macrophage activation and function, was assessed on MMP-10 null (Mmp10−/−) mouse model in responses to inhaled MWCNTs. It was reported that MMP-10 facilitates the clearance of MWCNTs and moderates the pro-inflammatory response of exposed alveolar and infiltrated macrophages (Vandivort et al., 2017). After administration, CNTs were removed from the lungs, but these nanoparticles can move to distant organs. Intratracheally instilled CNTs can reach the liver, spleen, and bone marrow by passing through the air-blood barrier (Czarny et al., 2014; Deng et al., 2007). Hepatic accumulation and subsequent hepatobiliary removal of functionalized CNTs have been well reported in various animal models.
Hepatic and renal clearance of covalently functionalized CNTs includes receptor-mediated endocytosis, cellular trafficking, and biliary elimination. The water-soluble functionalized CNTs prefer fenestrated sinusoidal endothelium localization instead of hepatocytes or resident macrophages of the liver. It has been found nanoparticles/nanotubes could be cleared by stabilin receptors mediating endocytosis (Alidori et al., 2016). The excretion paths of CNTs have not been well explained, but renal and fecal routes emerge to be the main removal routes for CNTs. Numerous researchers have been reported nano-toxicological excretion and spreading of CNTs in vivo models, but a systematic toxicokinetics evaluation of the CNTs is still needed. In most recent studies of CNTs toxicity, it focused only on short-term toxicological assessments, whereas assessment of long term toxicity studies is still unexplored. Therefore, to ensure the biosafety of CNTs, their long-term assessment deposition and excretion must be studied using various cell lines and animal models before their final use in medical and diagnostics (Gedda et al., 2019).
Biocompatibility and the toxicity of CNTs have been analyzed and observed by theoretical and experimental studies. CNTs can cause chronic injuries and acute inflammatory responses by interfering with essential organs’ normal physiological functions. After inhalation, CNTs translocated to lung lymph nodes and resulted in lung injury and inflammation (Aschberger et al., 2010). CNTs have primarily been used in neurosurgery to deliver drugs or genes for brain tumor treatment, biocompatible spinal devices, bio-sensing, and bio-imaging techniques (Visalli et al., 2017). Studies of graphene on the chicken embryo model showed that grapheme flakes halt the transcription and translation processes of RNA and DNA, leading to brain tissue damage (Gholamine et al., 2016). Most of the recent research of CNTs mainly focuses on synthesis and applications rather than their toxicological studies. The data of the toxic study on CNTs is underway. CNTs also impose reproductive and developmental toxicity (Gedda et al., 2019). Inhaled MWCNTs translocated to extrapulmonary organs, and their hydrophobicity allows them to cross the blood-brain barrier (BBB). Neurotoxicity and neuroinflammation of pristine functionalized MWCNTs toxicity are time- and dose-dependent and results in reactive oxygen species (ROS) overproduction, mitochondrial impairment, DNA damage, and decreased viability. It also increases the transcription levels of TNFα, IL-1β, and IL-6 that is confirmed by an ELISA test (Visalli et al., 2017; Facciola et al., 2019).
Possible toxicity mechanisms of CNTs
Although many researchers studied the toxicity of CNTs using diverse model systems such as chicken, mouse brain, lungs, embryonic and pulmonary systems, the exact mechanism(s) underlying the toxicity of CNTs remains vague. A schematic of the possible primary mechanism(s) of CNTs cytotoxicity is demonstrated in Fig. 2. Based on previous reports, the toxicity mechanism involves different targets. Here, we described various mechanisms in brief.
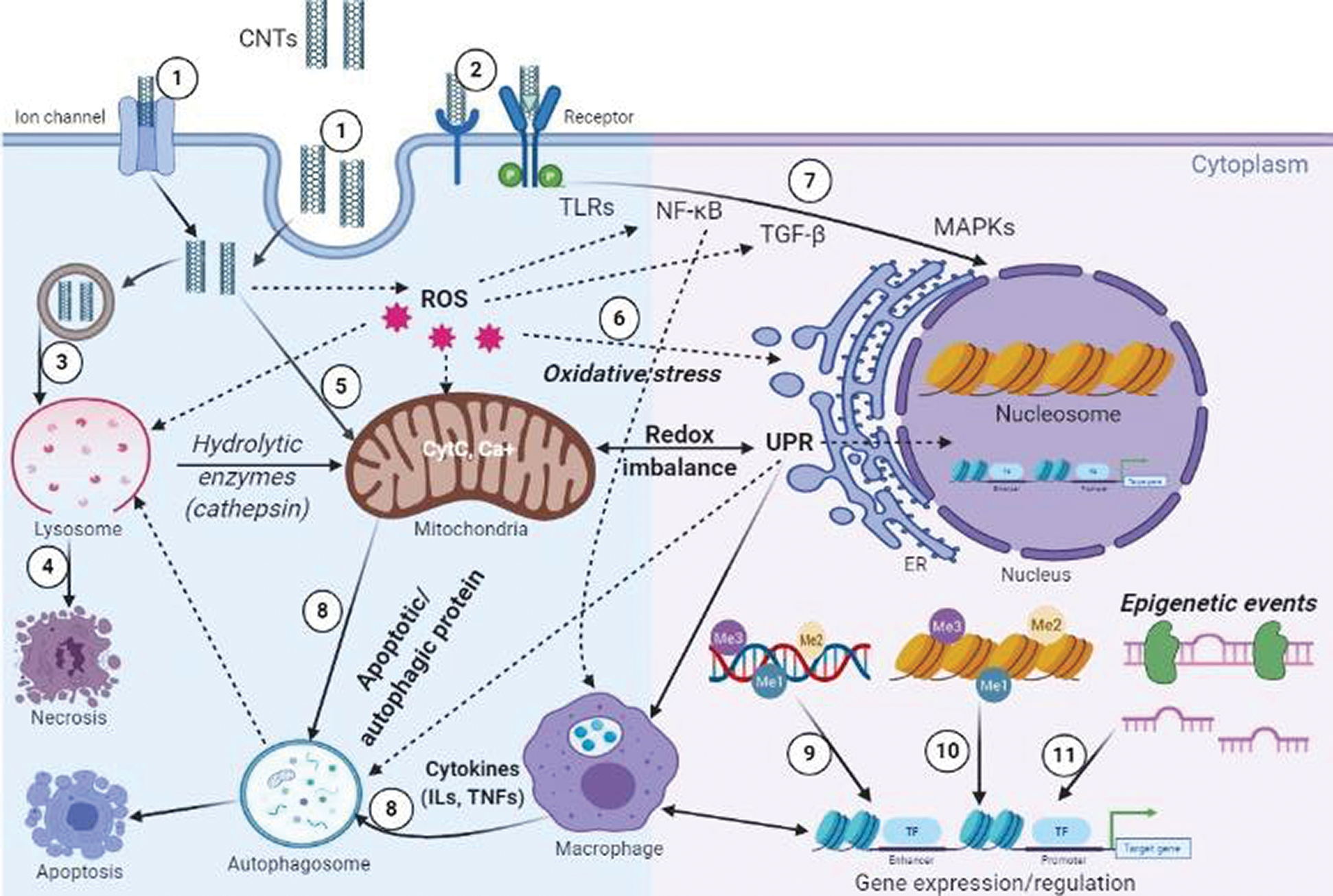
Figure 2: Schematic of cellular cross-talks and cytotoxicity mechanisms: (1) CNTs get internalized by various pathways, through ion channels and different endocytic pathways. (2, 7) CNTs can trigger several signaling cascades (NF-κB, NLRP3 inflammasome, p53, TGF-β1, and MAPK pathways), dysregulation of these signaling pathways leads to abnormal gene expression and protein functions. (3) CNTs induce ROS accumulation and lysosomal membrane permeabilization (LMP), resulting in the translocation of hydrolytic enzymes (e.g., cathepsins) to the cytoplasm. (4) ROS and LMP could potentially cause necrosis and autophagy/apoptosis dysfunction characterized by cleavage of caspase1 and downstream IL-1β release. (5, 6) Conditions resulting from effector-mediated loss of homeostasis such as oxidative stress, inflammation, mitochondrial perturbation (release of CytC, disrupted mitophagy), ER stress (unfolded protein response-UPR, accumulation of ubiquitinated protein aggregates). (8) Loss of homeostasis can result in the cell death pathways; necrosis and apoptotic and autophagic cell death. Finally, CNT may result in oxidative stress or inflammatory responses that in turn have the potential to damage DNA and alter transcriptional patterns by de-regulating various epigenetic events, (9) DNA methylation, (10) histone modification and (11) miRNA expression
Physical Destruction, Membrane Damage, and Cellular Uptake
It was postulated that nanomaterials induced physical injuries to cells, and its subcellular components are one of the major causes of nano-toxicity. The impairment of nanomaterials’ cellular structures is a determining factor of nano-toxicity (Wu et al., 2012). The physical contact of CNTs with cell walls/membranes is one of the leading causes of CNTs cytotoxicity (Liu et al., 2012; Shimizu et al., 2013). The strong interactions of CNTs with the cell membrane lead to morphological changes. CNTs, when came in direct contact with the cell membrane, lead to increase cell permeability, ultimately cause cell death as proved by various studies conducted on bacterial cells (Arias and Yang, 2009; Kang et al., 2007; Nepal et al., 2008; Rajavel et al., 2014). The effects of MWCNTs in macrophages (RAW264) cells were examined. It has been found that MWCNTs are mainly located on the surface of the plasma membrane and cause direct physical perturbation of bio-membranes by transmembrane current fluxes (Shimizu et al., 2013). It has been reported that MWCNTs traverse through the lipid bilayer membrane and form CNTs channels that allow the transport of ions from the cell membrane and ultimately destroy the cells. (Corredor et al., 2013).
The degree of dispersion, the formation of supra-molecular complexes, and the nanotube length are crucial factors that determine their cellular uptake (ADME) and toxicity (Raffa et al., 2010). A study conducted on human lung epithelial (A549) and primary macrophages suggested that the MWNT-NH3+ got internalized in these cells via membrane wrapping, translocation, etc. (Al-Jamal et al., 2011). Clathrin-mediated, caveolae-mediated, and macropinocytosis are the most common endocytic pathways associated with MWCNTs internalization into the human bronchial epithelial cells and human mesothelial cells (Maruyama et al., 2015). In human hepatocellular carcinoma cells, depending on the size of the SWCNTs, clathrin-coated pits, caseload-mediated endocytosis, and direct uptake through an energy-independent pathway involving their insertion and diffusion across the cell membrane are involved in the internalization (Kang et al., 2010). In an integrated systems toxicology study using Caenorhabditis elegans, the toxicity of pristine and hydroxylated (OH–) multi-wall CNTs (MWCNTs) was investigated. The result of pathway analyses proposed that endocytosis, phagocytosis are the potential mechanisms of MWCNTs uptake. The endocytosis- and phagocytosis- related genes (ced-10 and rab-7) were significantly up-regulated by CNTs exposure (Eom et al., 2015).
ROS Generation and Oxidative Stress-Induced Endoplasmic Reticulum (ER) and Mitochondrial Damage
CNTs induced ROS generation leading to oxidative stress, described as one of the most acceptable toxicity mechanisms (Shvedova et al., 2012). Oxidative stress occurs when increasing ROS levels surmount the activity of cellular antioxidative defense systems. ROS can cause direct damage to cellular biomolecules. Since ROS acts as second messengers, they can induce various intracellular signaling cascades leading to macromolecular damage (Genestra, 2007; Son et al., 2011). ROS break lipid membrane, cause DNA fragmentation, protein degradation, and dysfunction of mitochondria and ER that ultimately disturb the various signaling pathways (Galluzzi et al., 2012; Nel et al., 2006; Pulskamp et al., 2007). In the first step of the cytotoxicity mechanism, CNTs interact with cellular entities that lead to excessive oxidative stress and ROS generation cause mitochondrial damage to cultured human lung cells (Jacobsen et al., 2008; Pacurari et al., 2008; He et al., 2011). Studies have also shown that SWCNT induces oxidative stress and causes a significant decrease in superoxide dismutase (SOD-1 and SOD-2) levels in lung epithelial (LE) cells (Sharma et al., 2007). Exposure to CNTs causes concentration-dependent cytotoxicity in cultured HEK293 cells by increasing thiobarbituric acid reactive substances (TBARS) and decreasing intracellular glutathione levels (Reddy et al., 2010). MWCNTs induced ROS production significantly reduce the activity of catalase and glutathione in human lung epithelial cell line-A549 (Srivastava et al., 2010).
Investigation on rat lung epithelial (LE) cells showed that the incubation of LE cells with 0.5–10 μg/mL of MWCNTs caused a time-dependent increase in the formation of free radicals, the accumulation of peroxidative products, the loss of cell viability, and antioxidant depletion are the major effects (Ravichandran et al., 2009). Mitochondria are the energy factory of cells and are the primary source of intracellular ROS (Murphy, 2009). They are involved in many cell signaling pathways and play essential roles in redox homeostasis and apoptosis (Finkel, 2012). Cellular mechanisms leading to redox homeostasis involve their interactions with other organelles, like the endoplasmic reticulum (ER), and play an essential role in the folding and trafficking of proteins. If any defects arise in the ER’s protein-folding mechanism, it will induce aggregation of miss-folded proteins that lead to apoptosis due to defected cellular signaling process and redox homeostasis (Cao and Kaufman, 2014; Zhang and Kaufman, 2008). Evidence suggests that ROS mediated oxidative stress profoundly affects mitochondria and ER’s functioning by activating complex adaptive or pro-apoptotic signaling known as the unfolded protein response (UPR) that alter the redox homeostasis of ER, causing cell death (He et al., 2011).
Another study showed that SWCNTs cause a reduction of cytochrome C (Cyt C) and affect the redox activity of Cyt C due to attenuated electron transfer and conformational change of Cyt C. SWCNTs also modulates membrane potential in mitochondrial and cellular respiration in human epithelial cells (Ma et al., 2012). A Recent recent study on C. elegans described that MWCNT causes significant ER stress (Eom et al., 2015). Oxidative stress further modulates lipid homeostasis, inflammatory pathways, autophagy, apoptosis, and cell necrosis. In vitro studies conducted on neuronal PC12 cells at the biochemical, cellular, and gene expressional levels demonstrated that SWCNTs elicited ROS, indicating oxidative stress. CNTs explicitly expressed the gene involved in the dysfunction of oxidoreductase and antioxidant activity, nucleic acid or lipid metabolism, and mitochondria (Zhang et al., 2011). The integrated system toxicology approach in C. elegans provided a comprehensive insight into the toxic mechanism of MWCNTs, Microarray, and proteomic analyses that were conducted along with the pathway analyses. A decreased expression of hsp-4 and increased sensitivity of the hsp-4(gk514) mutant and at both replication and translation level suggests that MWCNTs may affect ER stress response (Eom et al., 2015). A very recent study conducted on THP-1 macrophages documented that MWCNT exposure promoted the expression of ER stress gene DDIT3 as well as ER stress protein p-chop as well as scavenger receptors, namely CD36 and MSR1. The results suggested that MWCNT could promote lipid accumulation, which could be related to the modulation of ER stress leading to upregulation of scavenger receptors (Long et al., 2019).
Inflammation is defined as a defense response induced by cellular damage and stress. The inflammatory process starts with two types of stimuli: Direct damage to cell organelles caused by external invaders and indirect effects caused by an extreme imbalance of cellular homeostasis (Chovatiya and Medzhitov, 2014). Cellular inflammation is characterized as increased gene transcription factor activities known as a nuclear factor-kappaB (NF-κB). CNTs get administered inside the cell via intratracheal instillation or intravenous administration. A high dose of CNTs elicits pulmonary edema, granuloma formation, and inflammatory cell responses. It is reported that Double-walled carbon nanotubes (DWCNTs) could localize in mice lungs can trigger inflammation by the accumulation of cytokines TNF-α, IL-1α, IL-1β, IL-6, IGF-1, leptin, granulocyte colony-stimulating factor (G-CSF), a type of growth factor, and vascular endothelial growth factor (VEGF), a signaling protein (Crouzier et al., 2010). Exposure of MWCNTs to A549 cells led to induction in interleukin-8 (IL-8) gene expression and nuclear factor NF-κB activation (Ye et al., 2009). A study on human keratinocytes suggests that SWCNT activates stress-related kinases due to the activation of NF-κB in a dose-dependent manner (Manna et al., 2005). MWCNTs promote inflammation by activating the NF-κB signaling pathway in macrophages (e.g., RAW264.7) to increase the secretion of cytokines and chemokines (TNFα, IL-1β, IL-6, IL-10, and MCP1). NF-κB activation rapidly degrades IκBα, nuclear accumulation of NF-κBp65, binding of NF-κB to specific DNA-binding sequences, and transactivation of target gene promoters. Moreover, MWCNTs cause fibrosis by inducing the production of profibrogenic growth factors (e.g., transforming growth factor-beta 1 (TGF-β1) and platelet-derived growth factor). The study revealed that MWCNTs provoke a network of interconnected signal pathways resulting in oxidative damage, production of cytokine production, and transformation of myofibroblast that trigger the toxicity and fibrosis in human lungs (He et al., 2011).
Histopathological studies revealed pulmonary fibrosis in male C57BL/6J mice, resulting in adverse health outcomes in the lung upon exposure to MWCNTs (Porter et al., 2010). CNTs evoke an inflammatory response when bind to toll-like receptors (TLRs) and activate the NF-κB signaling pathway in cells that induce an excessive expression of specific cytokines and chemokines (i.e., IL-8 and MCP1) (Meunier et al., 2012; Turabekova et al., 2014). A study compared the toxicity of CNTs with asbestos and reported a similar type of toxicity. Like asbestos CNT also activate the secretion of IL-1β from LPS-primed macrophages but only long needle-like CNT induced IL-1α secretion. siRNA experiments suggested that the NLRP3 inflammasome was essential for long, CNT, and asbestos-induced IL-1β secretion (PalomäKi et al., 2011). In vitro and in vivo effects of SWCNT were assessed on murine epidermal cells (JB6 P+) and immune-competent hairless SKH-1 mice. The findings revealed the generation of free radicals and oxidative stress on the topical exposure of SWCNT. These also activate NF-κB that ultimately causes dermal toxicity besides increasing the number of dermal cells and skin thickening due to the accumulation of polymorphonuclear leukocytes (PMNs) and mast cells (Murray et al., 2009). SWCNTs exposure in mice significantly decreased their brain to body ratio. SWCNTs induce monocytes’ production into the bloodstream and significant hematological changes and induce secretion of Th2-type cytokines (IL-10) in the bronchoalveolar lavage (BAL) fluid. Activation of normal T cells by CNTs increases the secretion of (RANTES), p53, and induces nitric oxide synthase (iNOS). Fibrotic histopathological induce the expression of cyclooxygenase-2 (COX-2) and transcription 3 (pSTAT3) that decrease the histamine secretion in BAL fluid (Park et al., 2011). It was recently reported that CNTs might directly affect the fibroblasts by induction of fibroblast proliferation and differentiation by Smad signaling. Moreover, there was an indirect activation of fibroblasts via the release of pro-fibrotic (PDGF and TGF-β) and pro-inflammatory (IL-1β) mediators by macrophages through the induction of oxidative stress, inflammasome, or NF-kB (Vietti et al., 2015).
Apoptosis, Autophagy, and Necrosis
Apoptosis is defined as the self-destruction of a cell regulated by many genes/proteins through a complex mechanism (Wei et al., 2010). Activation of apoptosis is trigger by either intrinsic cues or activation of the appropriate extrinsic pathways by external stimuli. The extrinsic apoptosis pathway is driven by caspase, whereas the intrinsic apoptosis pathway may transpire by either caspase-dependent or caspase-independent signaling (Fadeel and Orrenius, 2005). Extrinsic apoptosis is mediated through transmembrane receptors of the tumor necrosis factor (TNF) receptor superfamily (Meier and Vousden, 2007). Numerous types of cellular stress like DNA damage, oxidative stress, overload, endoplasmic reticulum ER stress activates the intrinsic pathway of apoptosis (Andón and Fadeel, 2012). ROS-dependent activation of mitogen-activated protein kinase (MAPK) p38 and TGF-β, as well as a vascular endothelial growth factor (VEGF), related signaling pathways, reported in cytotoxic response to CNTs (Azad et al., 2012). MWCNTs-induced apoptotic changes were studied in human lung epithelial cell line-A549. Apoptotic changes were estimated by nuclear condensation, DNA laddering, and expression of associated markers: p53, p21WAF1/CIP1, Bax, Bcl2, and activated caspase-3 (Srivastava et al., 2010). A previous study compares differences in cytotoxicity between the acid-treated and taurine functionalized MWCNTs, using a murine macrophage (RAW 264.7) cell line (Wang et al., 2012).
SWCNTs can inhibit HEK293 cells by inducing cell apoptosis and decrease the expression of cell cycle-associated genes (e.g., p16, bax, p57, hrk, cdc42, and cdc37). Also, a down-regulated expression of cell cycle genes such as cyclin-dependent kinases (cdk2, cdk4, cdk6, and cyclin D3) and signal transduction-associated genes (e.g., mitotic arrest deficient 2, Janus kinase 1, and MAP kinases). Protein associated with cell adhesion (laminin, fibronectin, cadherin, FAK, and collagen IV) were also downregulated. It has been found that ES cells exposed to MWNTs induce apoptosis in mouse ES cells and activate the tumor suppressor protein p53 within two h of exposure (Zhu et al., 2007). Investigation of the adverse effects of MWCNTs in rat lung epithelial (LE) cells showed stimulated apoptosis signaling pathways through caspase activation. Incorporation of deoxyuridine triphosphate (dUTPs) in the nucleus and an increase in the activity of both caspases-3 and caspase-8 in cells justify the hypothesis (Ravichandran et al., 2009). It was reported that MWCNT causes an alteration in mitochondrial membrane integrity, increased the levels of the mitochondrial apoptogenic factor, and nuclear translocation of NF-κB in lung epithelial cells. Induction of phosphorylated IκBα and its degradation and activation of several apoptotic proteins and factors p53, p21, has been reported to MWCNT treatment (Ravichandran et al., 2010).
Autophagy is the process of self-degradation of cellular components and is recently recognized as a non-apoptotic, lysosome-based pathway of cell death (Levine and Klionsky, 2004). Autophagy activation requires autophagosome formation containing Beclin 1, multiple autophagy-related proteins (ATG), microtubule-associated protein light chain 3 (LC3), and p62 (Stern et al., 2012). Exposer of various nanoparticles led to the accumulation of autophagosome, but the exact mechanisms are still unknown because autophagosome accumulation can result in autophagy blockade (Lee et al., 2014; Wan et al., 2013). Some studies have been conducted dealing with the CNTs induced autophagy. Evaluation of SWCNTs toxicity revealed that carbon nanomaterials cause adverse effects in murine peritoneal macrophages. CNTs induced autophagosome accumulation, and degradation of the autophagic p62 protein was also inhibited. A study on CNTs infected lysosomes revealed that the lysosome membrane was destabilized, indicating reduced autophagic degradation (Wan et al., 2013). Another study reported that carboxylated MWCNTs induce a decrease in the viability of cultured human umbilical vein endothelial cells (HUVECs); these cells are associated with the accumulation of autophagosomes.
This autophagosomes accumulation was mTOR kinase-independent and was caused by the blockade of the autophagic flux (Orecna et al., 2014). Moreover, it has been found that CNT-induced NLRP3 inflammasome activation depended on ROS production, cathepsin B activity, tyrosine kinases (PalomäKi et al., 2011). The increasing use of nanoparticles also resulted in the development of a novel class of autophagy activators. In an in vivo study, it was reported that COOH-CNT induces autophagic cell death in A549 cells through the AKT–TSC2–mTOR pathway and causes acute lung injury. Inhibition of autophagy significantly reduces COOH-CNT-induced autophagic cell death and ameliorated acute lung injury in mice (Liu et al., 2011). Necrosis is another mode of cell death induced by cellular damage and inflammatory responses. The exposure of mouse macrophage cell line RAW 264.7 to CNTs induces an inflammatory response, the release of tumor necrosis factor-α (TNF-α), and cell death by necrosis and apoptosis (Di Giorgio et al., 2011). Macrophages treated with a mixture of lipopolysaccharide and SWCNTs induced cell death via necrosis since SWCNTs induced the expression of TNFs (Kim et al., 2014). All these findings suggest that CNTs may influence immune responses and causes apoptosis and necrosis. MWCNT induces injury or necrosis of lung epithelial cells and releases HMGB1 and DNA into the extracellular space (Hiraku et al., 2015).
DNA Damage and Epigenetic Changes
Due to small size and high surface area, CNTs may possess significant genotoxic properties that may cause severe DNA damage and influence epigenetic changes. Epigenetic modifications such as DNA methylation, histone modification, and microRNAs influence gene activity without affecting the DNA sequence (Fabian and Sonenberg, 2012; Nishikura, 2010; Smith and Meissner, 2013). DNA methylation, one of the best-studied epigenetic modifications, can lead to chromatin remodeling, comprising phosphorylation, ubiquitination, and ATP-ribosylation (Portela and Esteller, 2010; Smith and Meissner, 2013). CNTs interact with DNA and chromosome and may cause chromosomal fragmentation, DNA strand breakages, point mutations, and alterations and defects in DNA repair pathways and blockade in cell cycle progression (Catalán et al., 2015; Ghosh et al., 2011; Lan et al., 2014; Møller and Jacobsen, 2017; Sasaki et al., 2016; Siegrist et al., 2014). MWCNTs can induce structural chromosomal aberrations in cultures of isolated human lymphocytes (Catalán et al., 2011; Sasaki et al., 2016). In an in vitro study, the potential genotoxic effects of CNTs on human bronchial epithelial BEAS 2B cells revealed that both CNTs are genotoxic to the cells (Lindberg et al., 2009). Another study conducted on the Allium cepa, mouse bone marrow cells, and pBR322 plasmid DNA suggests that MWCNTs show genotoxic effects on the plant and mammalian cells. Chromosomal aberrations, DNA strand breakages, and apoptosis were reported as the main genotoxic responses to alter genomic activities (Ghosh et al., 2011). The treatment of MWCNTs does not cause significant DNA damage but halts the DNA damage repair mechanism of the cells and ultimately causes oxidative DNA damage (McShan and Yu, 2012). A quantitative toxicogenomics study evaluated the nanotoxicity of SWCNTs on yeast. The results designated that oxidative stress and DNA damage were the principal mechanisms of action for all the selected SWCNTs. Level of toxicity varied with length, surface functionalization, and electronic structure of SWCNTs. Short SWCNT exerts higher toxicity than the long one. Surface functionalization, namely carboxylation (higher) and hydroxylation, led to more overall toxicity, especially genotoxicity, as compared to the non-functionalized counterpart. The nucleus is likely the primary target site for long, short, and carboxylated SWCNTs, and mechanical damage is likely responsible for the DNA damage, specifically related to degradation of the DNA double helix structure. Finally, the metallic SWCNT exerting much higher toxicity than the semiconducting one, which exhibited minimal toxicity among all the SWCNTs (Jiang et al., 2020).
Zhu et al. (2007) reported overexpression of two protein 8-oxoguanine-DNA glycosylase 1 (OGG1), double-strand break repair protein Rad 51, phosphorylation of H2AX histone at serine 139, and SUMO modification of XRCC4 in mouse embryonic stem cells under the stress of MWCNTs. Within the centrosome, CNTs can be integrated with microtubules and DNA strands. They can cause blockage in the cell cycle, indicating a G1/S block in the cell cycle (Siegrist et al., 2014). The effect of MWCNTs was tested on three human leukemia cell lines (HL-60, U-937, and K-562). There was a reduction of cell proliferation linked with an arrest in the G0/G1 phase and the increase of apoptosis. Diminished expression of cyclins D, E, A, B1 levels, and CDK4 likely mediated growth inhibition.
Moreover, the apoptotic effect is presumably mediated by the combined action of the survival and pro-apoptotic AKT and mitogen-activated protein kinase (MAPK) signal transduction pathways (Dinicola et al., 2015). Hiraku and co-workers demonstrated that high mobility group box protein 1 (HMGB1) and dsDNA from A549 cells could be released into culture supernatant after MWCNT exposure. The HMGB1-DNA complex binds to RAGE on neighboring cells, and then CpG DNA is recognized by TLR9 in lysosomes, which leads to the generation of nitric oxide and contributed to carcinogenesis (Hiraku et al., 2015). A study conducted on the R&D workers revealed significant hyper-methylation of DNA-methyltransferase 1 (DNMT1). However, no significant change was observed in the sequence-specific DNA methylation in the promoter region of ATM, SKI, and HDAC4 genes (Ghosh et al., 2016).
Genotoxic and alteration in DNA methylation have been reported in human monocytic cells (THP-1) in response to SWCNTs and MWCNTs. It was observed that the CNTs induced methylation of promoter-specific genes, and about 1127 different genes were identified to be hypomethylated. Several genes for signaling cascade pathways, vascular endothelial growth factor, and platelet activation pathways got methylated and contributed to epigenetic alternations (Öner et al., 2016). A recent study suggests that exposure to MWCNT for a long duration may affect 755 CpG sites, mainly located at low-density CpG regions (Sierra et al., 2017). Altogether, these data suggest that CNTs could significantly alter gene expression programming by remodeling epigenetic changes. However, studies of CNTs-induced epigenetic changes are not many, and the epigenetic mechanism caused by CNTs exposure is not completely understood.
To conclude, many studies have discussed CNTs toxicity and revealed the involvement of four signaling pathways: Toll-like receptors (TLRs), TGF-β, TNF-α, and MAPKs. These four signaling pathways are correlative and start the inflammatory response, autophagy, and apoptosis at the end of cycles, and most importantly, oxidative stress activates these pathways. However, GFNs toxicity investigated in very few papers to date, and the network of signaling pathways need to be explored in detail in the future.
Despite a commercial commodity, CNTs were remained associated with several health issues and had a great concern among researchers worldwide to overcome associated nano-toxicity. First and most important, the CNTs toxicity with biological tissues remains a major challenge for its applications in diagnostics purposes as they are difficult to solubilize in biological milieu, non-bio-degradable, -compatible, and immunogenic. Apart from the biological application, the level of synthesis and fabrication remains a major challenge for CNTs synthesis. The physical properties of the CNTs depreciated due to their higher rate of self-assembling and random distribution. For example, suppose a CNT is used for bio-molecular therapy (Gene Therapy, gene silencing, and enzymes). In that case, biomolecules (DNA, RNA, and proteins) and the strength binds with CNTs will not be adequate to complete delivery. Extrinsic residues such as catalysts (As, Ni, Fe, etc.) may further negatively affect the biological applications of CNTs in the milieu of biological tissues. The determination of physical properties and synthesis, purification, and microfabrication CNTs remains associated with several challenges to end with specific CNTs (Tab. 1). Further, large numbers of CNTs are being synthesized from a chemical-based precursor, which had a negative impact on the environment. Hence, there is an immense need to find novel methods such as green synthesis of CNTs.
Table 1: The table depicts a close association of physiochemical properties of CNTs with toxicity and benefits
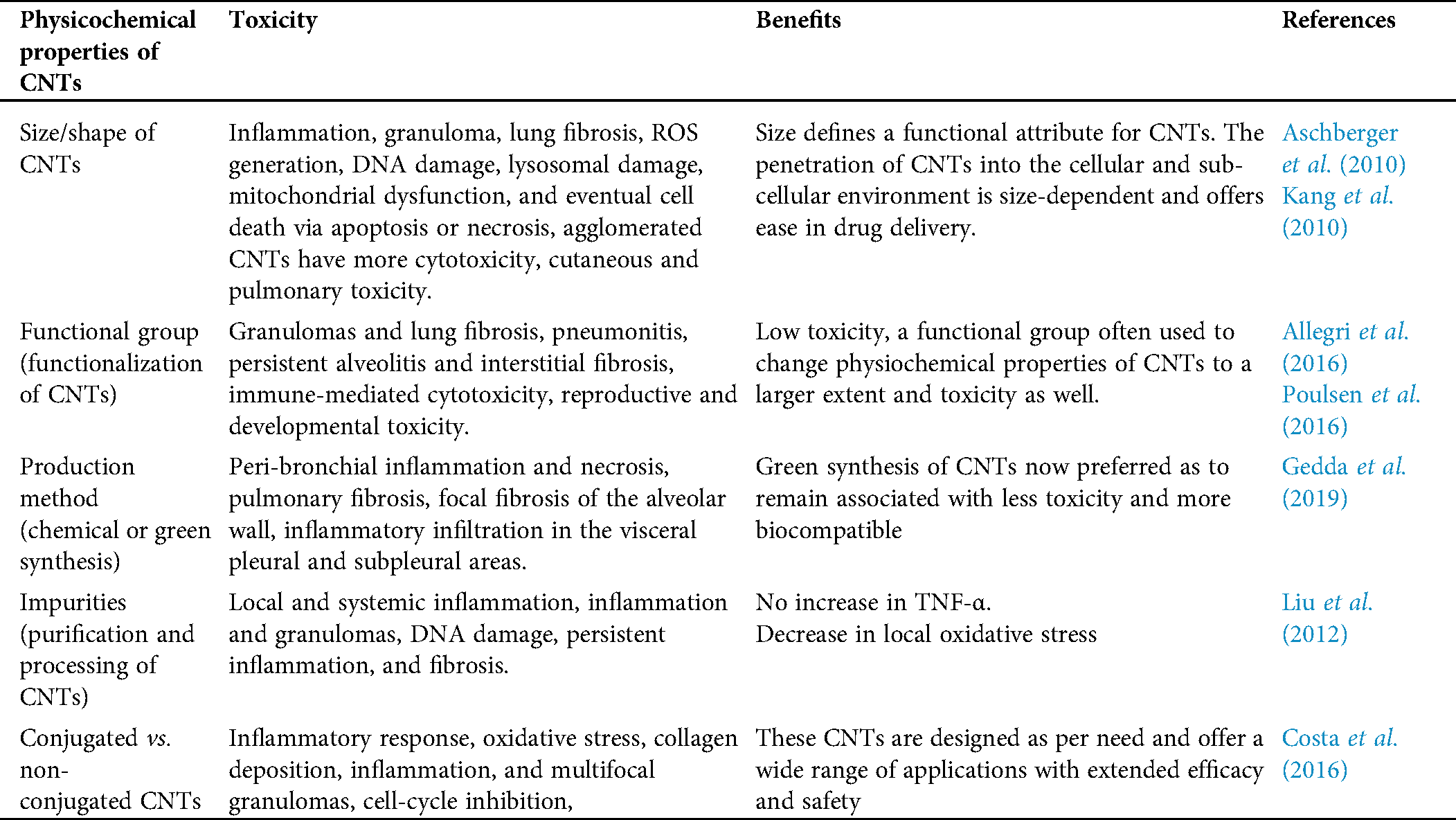
The CNTs and associated toxicity remain a concern in developing approaches and strategies to minimize the negative impact on biological tissues and the environment. Several factors result in tissue-specific toxicity of CNTs, including a functional group, purity of CNTs, and size of the nanoparticle. It is essential to understand why these are associated with CNTs toxicity. Now, optimizing all these parameters will surely minimize CNTs toxicity. Several ways via CNTs get introduced into biological tissue include oral, nasal, transdermal, subcutaneous injection, intraperitoneal, etc. The toxicity of CNTs largely depends on the affinity of CNTs with biological tissue and biomolecules. Hence, optimizing physicochemical properties, mainly in synthesis and purification of CNTs, will minimize affinity with the biomolecule and reduce associated toxicity. Having a centralized database for CNTs toxicity in the context of physicochemical properties of CNTs will be added benefits to minimize associated toxicity from the beginning, i.e., synthesis to purification. Specific studies were carried out in organs, such as the spleen, liver, and kidney, and the injury symptoms, damage index, and level of damage to these internal organs were investigated thoroughly (Reddy et al., 2010; Gedda et al., 2019). Neurotoxicity studies of CNTs are very scarce. Not many studied are conducted on how nerves or brain tissues, how CNTs cause damage, and its effect on the infected individual’s behavior are not well reported. Therefore, these studies are extremely significant and require considerable attention in the future. In conclusion, a well-established in vivo system will be essential in understanding the nature and extent of the toxicity of CNTs. Considering all the given measures, it will be quite useful in minimizing asthma, bronchitis, emphysema, and lung cancer reported as major CNTs toxicity.
Acknowledgement: PKB and MKV are very much thankful to Science and Engineering Research Board, Department of Science and Technology (SERB-DST) New Delhi, India for providing financial support in the form of national postdoctoral fellowship (NPDF). RKB acknowledges the UGC, New Delhi, India for providing opportunity in the form of a postdoctoral fellowship.
Author Contributions: PB conceived the idea and worked on study conception and design. MKV, PKB, and RKB, screened titles for relevance and abstracted the data from the eligible full-text articles. PKB, RKB, and MKV analyzed and interpreted the data and drafted the manuscript. All authors critically revised the manuscript with input from the reviewers. All authors have read and approved the final draft.
Funding Statement: This work was supported by a project grant from the Science and Engineering Research Board, Department of Science and Technology (SERB-DST) New Delhi, India [File No. PDF/2016/000200].
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Al-Jamal KT, Nerl H, Müller KH, Ali-Boucetta H, Li S, Haynes PD, Jinschek JR, Prato M, Bianco A, Kostarelos K. (2011). Cellular uptake mechanisms of functionalised multi-walled carbon nanotubes by 3D electron tomography imaging. Nanoscale 3: 2627–2635. [Google Scholar]
Alidori S, Bowman RL, Yarilin D, Romin Y, Barlas A, Mulvey JJ, Fujisawa S, Xu K, Ruggiero A, Riabov V. (2016). Deconvoluting hepatic processing of carbon nanotubes. Nature Communications 7: 266. [Google Scholar]
Allegri M, Perivoliotis DK, Bianchi MG, Chiu M, Pagliaro A, Koklioti MA, Trompeta AA, Bergamaschi E, Bussolati O, Charitidis CA (2016). Toxicity determinants of multi-walled carbon nanotubes: The relationship between functionalization and agglomeration. Toxicology Reports 3: 230–243. [Google Scholar]
Andón FT, Fadeel B. (2012). Programmed cell death: Molecular mechanisms and implications for safety assessment of nanomaterials. Accounts of Chemical Research 46: 733–742. [Google Scholar]
Arias LR, Yang L. (2009). Inactivation of bacterial pathogens by carbon nanotubes in suspensions. Langmuir 25: 3003–3012. [Google Scholar]
Aschberger K, Johnston HJ, Stone V, Aitken RJ, Hankin SM, Peters SA, Tran CL, Christensen FM. (2010). Review of carbon nanotubes toxicity and exposure—Appraisal of human health risk assessment based on open literature. Critical Reviews in Toxicology 40: 759–790. [Google Scholar]
Azad N, Iyer AKV, Wang L, Liu Y, Lu Y, Rojanasakul Y. (2012). Reactive oxygen species-mediated p38 MAPK regulates carbon nanotube-induced fibrogenic and angiogenic responses. Nanotoxicology 7: 157–168. [Google Scholar]
Babele PK, Thakre PK, Kumawat R, Tomar RS. (2018). Zinc oxide nanoparticles induce toxicity by affecting cell wall integrity pathway, mitochondrial function and lipid homeostasis in Saccharomyces cerevisiae. Chemosphere 213: 65–75. [Google Scholar]
Cao SS, Kaufman RJ. (2014). Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxidants & Redox Signaling 21: 396–413. [Google Scholar]
Catalán J, Järventaus H, Vippola M, Savolainen K, Norppa H. (2011). Induction of chromosomal aberrations by carbon nanotubes and titanium dioxide nanoparticles in human lymphocytes in vitro. Nanotoxicology 6: 825–836. [Google Scholar]
Catalán J, Siivola KM, Nymark P, Lindberg H, Suhonen S, Järventaus H, Koivisto AJ, Moreno C, Vanhala E, Wolff H. (2015). In vitro and in vivo genotoxic effects of straight versus tangled multi-walled carbon nanotubes. Nanotoxicology 10: 794–806. [Google Scholar]
Chovatiya R, Medzhitov R. (2014). Stress, inflammation, and defense of homeostasis. Molecular Cell 54: 281–288. [Google Scholar]
Corredor C, Hou WC, Klein SA, Moghadam BY, Goryll M, Doudrick K, Westerhoff P, Posner JD. (2013). Disruption of model cell membranes by carbon nanotubes. Carbon 60: 67–75. [Google Scholar]
Costa PM, Fadeel B. (2016). Emerging systems biology approaches in nanotoxicology: Towards a mechanism-based understanding of nanomaterial hazard and risk. Toxicology and Applied Pharmacology 299: 101–111. [Google Scholar]
Costa PM, Bourgognon M, Wang JT, Al-Jamal KT (2016). Functionalised carbon nanotubes: From intracellular uptake and cell-related toxicity to systemic brain delivery. Journal of Controlled Release 241: 200–219. [Google Scholar]
Crouzier D, Follot S, Gentilhomme E, Flahaut E, Arnaud R, Dabouis V, Castellarin C, Debouzy JC. (2010). Carbon nanotubes induce inflammation but decrease the production of reactive oxygen species in lung. Toxicology 272: 39–45. [Google Scholar]
Czarny B, Georgin D, Berthon F, Plastow G, Pinault M, Patriarche G, Thuleau A, L’hermite MM, Taran F, Dive V. (2014). Carbon nanotube translocation to distant organs after pulmonary exposure: Insights from in situ 14C-Radiolabeling and tissue radioimaging. ACS Nano 8: 5715–5724. [Google Scholar]
Deng X, Jia G, Wang H, Sun H, Wang X, Yang S, Wang T, Liu Y. (2007). Translocation and fate of multi-walled carbon nanotubes in vivo. Carbon 45: 1419–1424. [Google Scholar]
Di Giorgio ML, Di Bucchianico S, Ragnelli AM, Aimola P, Santucci S, Poma A. (2011). Effects of single and multi walled carbon nanotubes on macrophages: Cyto and genotoxicity and electron microscopy. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 722: 20–31. [Google Scholar]
Dinicola S, Masiello MG, Proietti S, Coluccia P, Fabrizi G, Palombo A, Micciulla F, Bistarelli S, Ricci G, Catizone A. (2015). Multiwalled carbon nanotube buckypaper induces cell cycle arrest and apoptosis in human leukemia cell lines through modulation of AKT and MAPK signaling pathways. Toxicology in Vitro 29: 1298–1308. [Google Scholar]
Eom HJ, Roca CP, Roh JY, Chatterjee N, Jeong JS, Shim I, Kim HM, Kim PJ, Choi K, Giralt F. (2015). A systems toxicology approach on the mechanism of uptake and toxicity of MWCNT in Caenorhabditis elegans. Chemico-Biological Interactions 239: 153–163. [Google Scholar]
Fabian MR, Sonenberg N. (2012). The mechanics of miRNA-mediated gene silencing: A look under the hood of miRISC. Nature Structural & Molecular Biology 19: 586–593. [Google Scholar]
Facciol A, Visalli G, Maestra SL, Ceccarelli M, D’Aleo F, Nunnari G, Pellican GF, Pietro AD (2019). Carbon nanotubes and central nervous system: Environmental risks, toxicological aspects and future perspectives. Environmental Toxicology and Pharmacology 65: 23–30. [Google Scholar]
Fadeel B, Orrenius S. (2005). Apoptosis: A basic biological phenomenon with wide-ranging implications in human disease. Journal of Internal Medicine 258: 479–517. [Google Scholar]
Finkel T. (2012). Signal transduction by mitochondrial oxidants. Journal of Biological Chemistry 287: 4434–4440. [Google Scholar]
Galluzzi L, Kepp O, Kroemer G. (2012). Mitochondria: Master regulators of danger signalling. Nature Reviews Molecular Cell Biology 13: 780–788. [Google Scholar]
Gedda MR, Babele PK, Zahra K, Madhukar P (2019) Epigenetic aspects of engineered nanomaterials: Is the collateral damage inevitable? Front Bioeng Biotechnol 7: 228. DOI 10.3389/fbioe.2019.00228. [Google Scholar] [CrossRef]
Genestra M. (2007). Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cellular Signalling 19: 1807–1819. [Google Scholar]
Gholamine B, Karimi I, Salimi A, Mazdarani P, Becker LA. (2016). Neurobehavioral toxicity of carbon nanotubes in mice: Focus on brain-derived neurotrophic factor messenger RNA and protein. Toxicology and Industrial Health 33: 340–350. [Google Scholar]
Ghosh M, Chakraborty A, Bandyopadhyay M, Mukherjee A. (2011). Multi-walled carbon nanotubes (MWCNTInduction of DNA damage in plant and mammalian cells. Journal of Hazardous Materials 197: 327–336. [Google Scholar]
Ghosh M, Öner D, Tabis A, Poels K, Hoet P, Pronk A, Vermeulen R, Bekaert B, Godderis L. (2016). DNA methylation changes in workers occupational exposed to carbon nanotubes. European Respiratory Journal 48: PA4275. [Google Scholar]
He X, Young SH, Schwegler-Berry D, Chisholm WP, Fernback JE, Ma Q. (2011). Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation. Chemical Research in Toxicology 24: 2237–2248. [Google Scholar]
Hiraku Y, Guo F, Ma N, Yamada T, Wang S, Kawanishi S, Murata M. (2015). Multi-walled carbon nanotube induces nitrative DNA damage in human lung epithelial cells via HMGB1-RAGE interaction and Toll-like receptor 9 activation. Particle and Fibre Toxicology 13: 378. [Google Scholar]
Huang X, Zhang F, Sun X, Choi KY, Niu G, Zhang G, Guo J, Lee S, Chen X. (2014). The genotype-dependent influence of functionalized multiwalled carbon nanotubes on fetal development. Biomaterials 35: 856–865. [Google Scholar]
Jacobsen NR, Pojana G, White P, Møller P, Cohn CA, Smith Korsholm K, Vogel U, Marcomini A, Loft S, Wallin H. (2008). Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C60 fullerenes in the FE1-MutaTM Mouse lung epithelial cells. Environmental and Molecular Mutagenesis 49: 476–487. [Google Scholar]
Jain KK. (2012). Nanobiotechnology-based strategies for crossing the blood–brain barrier. Nanomedicine 7: 1225–1233. [Google Scholar]
Jiang T, Amadei CA, Gou N, Lin Y, Lan J, Vecitis CD, Gu AZ (2020). Toxicity of single-walled carbon nanotubes (SWCNTsEffect of lengths, functional groups and electronic structures revealed by a quantitative toxicogenomics assay. Environmental Science: Nano 7: 1348–1364. [Google Scholar]
Kafa H, Wang JTW, Rubio N, Venner K, Anderson G, Pach E, Ballesteros B, Preston JE, Abbott NJ, Al-Jamal KT. (2015). The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo. Biomaterials 53: 437–452. [Google Scholar]
Kagan VE, Kapralov AA, St. Croix CM, Watkins SC, Kisin ER, Kotchey GP, Balasubramanian K, Vlasova II Yu, Yu J, Kim K. (2014). Lung macrophages digest carbon nanotubes using a superoxide/peroxynitrite oxidative pathway. ACS Nano 8: 5610–5621. [Google Scholar]
Kang B, Chang S, Dai Y, Yu D, Chen D. (2010). Cell response to carbon nanotubes: Size-dependent intracellular uptake mechanism and subcellular fate. Small 6: 2362–2366. [Google Scholar]
Kang S, Pinault M, Pfefferle LD, Elimelech M. (2007). Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir 23: 8670–8673. [Google Scholar]
Kavosi A, Hosseini Ghale Noei S, Madani S, Khalighfard S, Khodayari S, Khodayari H, Mirzaei M, Kalhori MR, Yavarian M, Alizadeh AM, Falahati M (2018). The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Scientific Reports 8: 8375. [Google Scholar]
Kim KH, Yeon SM, Kim HG, Lee H, Kim SK, Han SH, Min KJ, Byun Y, Lee EH, Lee KS. (2014). Single-walled carbon nanotubes induce cell death and transcription of TNF-α in macrophages without affecting nitric oxide production. Inflammation 37: 44–54. DOI 10.1007/s10753-013-9710-3. [Google Scholar] [CrossRef]
Kota S, Dumpala P, Anantha RK, Verma MK, Kandepu S. (2017). Evaluation of therapeutic potential of the silver/silver chloride nanoparticles synthesized with the aqueous leaf extract of Rumex acetosa. Scientific Reports 7: 1587. [Google Scholar]
Labib S, Williams A, Yauk CL, Nikota JK, Wallin H, Vogel U, Halappanavar S. (2015). Nano-risk Science: Application of toxicogenomics in an adverse outcome pathway framework for risk assessment of multi-walled carbon nanotubes. Particle and Fibre Toxicology 13: 1345. [Google Scholar]
Lan J, Gou N, Gao C, He M, Gu AZ. (2014). Comparative and mechanistic genotoxicity assessment of nanomaterials via a quantitative toxicogenomics approach across multiple species. Environmental Science & Technology 48: 12937–12945. [Google Scholar]
Lee YH, Cheng FY, Chiu HW, Tsai JC, Fang CY, Chen CW, Wang YJ. (2014). Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials 35: 4706–4715. [Google Scholar]
Levine B, Klionsky DJ. (2004). Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Developmental Cell 6: 463–477. [Google Scholar]
Li X, Chen N, Su Y, He Y, Yin M, Wei M, Wang L, Huang W, Fan C, Huang Q. (2014). Autophagy-sensitized cytotoxicity of quantum dots in PC12 cells. Advanced Healthcare Materials 3: 354–359. [Google Scholar]
Lindberg HK, Falck GCM, Suhonen S, Vippola M, Vanhala E, Catalán J, Savolainen K, Norppa H. (2009). Genotoxicity of nanomaterials: DNA damage and micronuclei induced by carbon nanotubes and graphite nanofibres in human bronchial epithelial cells in vitro. Toxicology Letters 186: 166–173. [Google Scholar]
Liu H, Zhang Y, Yang N, Zhang Y, Liu X, Li C, Zhao Y, Wang Y, Zhang G, Yang P. (2011). A functionalized single-walled carbon nanotube-induced autophagic cell death in human lung cells through Akt-TSC2-mTOR signaling. Cell Death & Disease 2: e159. [Google Scholar]
Liu Y, Zhao Y, Sun B, Chen C. (2012). Understanding the toxicity of carbon nanotubes. Accounts of Chemical Research 46: 702–713. [Google Scholar]
Long J, Ma W, Yu Z, Liu H, Cao Y. (2019). Multi-walled carbon nanotubes (MWCNTs) promoted lipid accumulation in THP-1 macrophages through modulation of endoplasmic reticulum (ER) stress. Nanotoxicology 13: 938–951. [Google Scholar]
Ma X, Zhang LH, Wang LR, Xue X, Sun JH, Wu Y, Zou G, Wu X, Wang PC, Wamer WG. (2012). Single-walled carbon nanotubes alter cytochrome c electron transfer and modulate mitochondrial function. ACS Nano 6: 10486–10496. [Google Scholar]
Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, Rice-Ficht AC, Ramesh GT. (2005). Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-κB in human keratinocytes. Nano Letters 5: 1676–1684. [Google Scholar]
Maruyama K, Haniu H, Saito N, Matsuda Y, Tsukahara T, Kobayashi S, Tanaka M, Aoki K, Takanashi S, Okamoto M, Kato H (2015). Endocytosis of multiwalled carbon nanotubes in bronchial epithelial and mesothelial cells. BioMed research international 2015: 793186. DOI 10.1155/2015/793186. [Google Scholar] [CrossRef]
McShan D, Yu H. (2012). DNA damage in human skin keratinocytes caused by multiwalled carbon nanotubes with carboxylate functionalization. Toxicology and Industrial Health 30: 489–498. [Google Scholar]
Meier P, Vousden KH. (2007). Lucifer’s labyrinth—Ten years of path finding in cell death. Molecular Cell 28: 746–754. [Google Scholar]
Meunier E, Coste A, Olagnier D, Authier H, Lefèvre L, Dardenne C, Bernad J, Béraud M, Flahaut E, Pipy B. (2012). Double-walled carbon nanotubes trigger IL-1β release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine: Nanotechnology, Biology and Medicine 8: 987–995. [Google Scholar]
Møller P, Jacobsen NR. (2017). Weight of evidence analysis for assessing the genotoxic potential of carbon nanotubes. Critical Reviews in Toxicology 47: 1–18. [Google Scholar]
Morimoto Y, Hirohashi M, Ogami A, Oyabu T, Myojo T, Todoroki M, Yamamoto M, Hashiba M, Mizuguchi Y, Lee BW. (2011). Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology 6: 587–599. [Google Scholar]
Murphy MP. (2009). How mitochondria produce reactive oxygen species. Biochemical Journal 417: 1–13. [Google Scholar]
Murray A, Kisin E, Leonard S, Young S, Kommineni C, Kagan V, Castranova V, Shvedova A. (2009). Oxidative stress and inflammatory response in dermal toxicity of single-walled carbon nanotubes. Toxicology 257: 161–171. [Google Scholar]
Nel A, Xia T, Mädler L, Li N. (2006). Toxic potential of materials at the nanolevel. Science 311: 622–627. [Google Scholar]
Nepal D, Balasubramanian S, Simonian AL, Davis VA. (2008). Strong antimicrobial coatings: Single-walled carbon nanotubes armored with biopolymers. Nano Letters 8: 1896–1901. [Google Scholar]
Nishikura K. (2010). Functions and regulation of RNA editing by ADAR deaminases. Annual Review of Biochemistry 79: 321–349. [Google Scholar]
Öner D, Moisse M, Ghosh M, Duca RC, Poels K, Luyts K, Putzeys E, Cokic SM, Van Landuyt K, Vanoirbeek J. (2016). Epigenetic effects of carbon nanotubes in human monocytic cells. Mutagenesis 32: 181–191. [Google Scholar]
Orecna M, De Paoli SH, Janouskova O, Tegegn TZ, Filipova M, Bonevich JE, Holada K, Simak J. (2014). Toxicity of carboxylated carbon nanotubes in endothelial cells is attenuated by stimulation of the autophagic flux with the release of nanomaterial in autophagic vesicles. Nanomedicine: Nanotechnology, Biology and Medicine 10: e939–e948. [Google Scholar]
Pacurari M, Yin XJ, Zhao J, Ding M, Leonard SS, Schwegler-Berry D, Ducatman BS, Sbarra D, Hoover MD, Castranova V. (2008). Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-κB, and Akt in normal and malignant human mesothelial cells. Environmental Health Perspectives 116: 1211–1217. [Google Scholar]
PalomäKi J, VäLimäKi E, Sund J, Vippola M, Clausen PA, Jensen KA, Savolainen K, Matikainen S, Alenius H. (2011). Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano 5: 6861–6870. [Google Scholar]
Park EJ, Roh J, Kim SN, Kang MS, Lee BS, Kim Y, Choi S. (2011). Biological toxicity and inflammatory response of semi-single-walled carbon nanotubes. PLoS One 6: e25892. [Google Scholar]
Portela A, Esteller M. (2010). Epigenetic modifications and human disease. Nature Biotechnology 28: 1057–1068. [Google Scholar]
Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S. (2010). Mouse pulmonary dose-and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology 269: 136–147. [Google Scholar]
Poulsen SS, Jackson P, Kling K, Knudsen KB, Skaug V, Kyjovska ZO, Thomsen BL, Clausen PA, Atluri R, Berthing T, Bengtson S, Wolff H, Jensen KA, Wallin H, Vogel U (2016). Multi-walled carbon nanotube physicochemical properties predict pulmonary inflammation and genotoxicity. Nanotoxicology 10: 1263–1275. [Google Scholar]
Pulskamp K, Diabaté S, Krug HF. (2007). Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicology Letters 168: 58–74. [Google Scholar]
Qi W, Bi J, Zhang X. et al. (2014). Damaging effects of multi-walled carbon nanotubes on pregnant mice with different pregnancy times. Scientific Reports 4: 4352. DOI 10.1038/srep04352. [Google Scholar] [CrossRef]
Qu G, Bai Y, Zhang Y, Jia Q, Zhang W, Yan B. (2009). The effect of multiwalled carbon nanotube agglomeration on their accumulation in and damage to organs in mice. Carbon 47: 2060–2069. [Google Scholar]
Raffa V, Ciofani G, Vittorio O, Riggio C, Cuschieri A. (2010). Physicochemical properties affecting cellular uptake of carbon nanotubes. Nanomedicine 5: 89–97. [Google Scholar]
Rajavel K, Gomathi R, Manian S, Rajendra Kumar RT. (2014). In vitro bacterial cytotoxicity of CNTs: Reactive oxygen species mediate cell damage edges over direct physical puncturing. Langmuir 30: 592–601. [Google Scholar]
Ravichandran P, Baluchamy S, Sadanandan B, Gopikrishnan R, Biradar S, Ramesh V, Hall JC, Ramesh GT. (2010). Multiwalled carbon nanotubes activate NF-κB and AP-1 signaling pathways to induce apoptosis in rat lung epithelial cells. Apoptosis 15: 1507–1516. [Google Scholar]
Ravichandran P, Periyakaruppan A, Sadanandan B, Ramesh V, Hall JC, Jejelowo O, Ramesh GT. (2009). Induction of apoptosis in rat lung epithelial cells by multiwalled carbon nanotubes. Journal of Biochemical and Molecular Toxicology 23: 333–344. [Google Scholar]
Reddy ARN, Reddy YN, Krishna DR, Himabindu V. (2010). Multi wall carbon nanotubes induce oxidative stress and cytotoxicity in human embryonic kidney (HEK293) cells. Toxicology 272: 11–16. [Google Scholar]
Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME. (2009). Inhaled carbon nanotubes reach the subpleural tissue in mice. Nature Nanotechnology 4: 747–751. [Google Scholar]
Sasaki T, Asakura M, Ishioka C, Kasai T, Katagiri T, Fukushima S. (2016). In vitro chromosomal aberrations induced by various shapes of multi-walled carbon nanotubes (MWCNTs). Journal of Occupational Health 58: 622–631. [Google Scholar]
Sasrimuang S, Chuchuen O, Artnaseaw A (2020). Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth. Green Processing and Synthesis 9: 340–348 [Google Scholar]
Sharma CS, Sarkar S, Periyakaruppan A, Barr J, Wise K, Thomas R, Wilson BL, Ramesh GT. (2007). Single-walled carbon nanotubes induces oxidative stress in rat lung epithelial cells. Journal of Nanoscience and Nanotechnology 7: 2466–2472. [Google Scholar]
Shah KA, Tali BA (2016). Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Materials Science in Semiconductor Processing 41: 67–82. [Google Scholar]
Shimizu K, Uchiyama A, Yamashita M, Hirose A, Nishimura T, Oku N. (2013). Biomembrane damage caused by exposure to multi-walled carbon nanotubes. Journal of Toxicological Sciences 38: 7–12. [Google Scholar]
Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE. (2012). Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicology and Applied Pharmacology 261: 121–133. [Google Scholar]
Siddiqi KS, Husen A, Sohrab SS, Yassin MO (2018). Recent status of nanomaterial fabrication and their potential applications in neurological disease management. Nanoscale Research Letters 13: 231. [Google Scholar]
Siegrist KJ, Reynolds SH, Kashon ML, Lowry DT, Dong C, Hubbs AF, Young SH, Salisbury JL, Porter DW, Benkovic SA. (2014). Genotoxicity of multi-walled carbon nanotubes at occupationally relevant doses. Particle and Fibre Toxicology 11: 6. [Google Scholar]
Sierra MI, Rubio L, Bayón GF, Cobo I, Menendez P, Morales P, Mangas C, Urdinguio RG, Lopez V, Valdes A. (2017). DNA methylation changes in human lung epithelia cells exposed to multi-walled carbon nanotubes. Nanotoxicology 11: 857–870. [Google Scholar]
Simkó M, Mattsson MO. (2010). Risks from accidental exposures to engineered nanoparticles and neurological health effects: A critical review. Particle and Fibre Toxicology 7: 42. [Google Scholar]
Singh G, Babele PK, Kumar A, Srivastava A, Sinha RP, Tyagi MB. (2014). Synthesis of ZnO nanoparticles using the cell extract of the cyanobacterium, Anabaena strain L31 and its conjugation with UV-B absorbing compound shinorine. Journal of Photochemistry and Photobiology B: Biology 138: 55–62. [Google Scholar]
Smith ZD, Meissner A. (2013). DNA methylation: Roles in mammalian development. Nature Reviews Genetics 14: 204–220. [Google Scholar]
Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. (2011). Mitogen-activated protein kinases and reactive oxygen species: How can ROS activate MAPK pathways? Journal of Signal Transduction 2011: 792639. [Google Scholar]
Srivastava RK, Pant AB, Kashyap MP, Kumar V, Lohani M, Jonas L, Rahman Q. (2010). Multi-walled carbon nanotubes induce oxidative stress and apoptosis in human lung cancer cell line-A549. Nanotoxicology 5: 195–207. [Google Scholar]
Stern ST, Adiseshaiah PP, Crist RM. (2012). Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Particle and Fibre Toxicology 9: 20. [Google Scholar]
Sturm R. (2014). Clearance of carbon nanotubes in the human respiratory tract—A theoretical approach. Annals of Translational Medicine 2: 46. [Google Scholar]
Turabekova M, Rasulev B, Theodore M, Jackman J, Leszczynska D, Leszczynski J. (2014). Immunotoxicity of nanoparticles: A computational study suggests that CNTs and C60 fullerenes might be recognized as pathogens by Toll-like receptors. Nanoscale 6: 3488–3495. [Google Scholar]
Valle RP, Wu T, Zuo YY. (2015). Biophysical influence of airborne carbon nanomaterials on natural pulmonary surfactant. ACS Nano 9: 5413–5421. [Google Scholar]
Vandivort TC, Birkland TP, Domiciano TP, Mitra S, Kavanagh TJ, Parks WC. (2017). Stromelysin-2 (MMP-10) facilitates clearance and moderates inflammation and cell death following lung exposure to long multiwalled carbon nanotubes. International Journal of Nanomedicine 12: 1019–1031. [Google Scholar]
Vietti G, Lison D, Van Den Brule S. (2015). Mechanisms of lung fibrosis induced by carbon nanotubes: Towards an Adverse Outcome Pathway (AOP). Particle and Fibre Toxicology 13: 2078. [Google Scholar]
Visalli G, Curr M, Iannazzo D, Pistone A, Ciarello MP, Acri G, Testagrossa B, Bertuccio MP, Squeri R, Pietro AD, (2017). In vitro assessment of neurotoxicity and neuroinflammation of homemade. MWCNTs, Environmental Toxicology and Pharmacology 56: 121–128. [Google Scholar]
Wan B, Wang ZX, Lv QY, Dong PX, Zhao LX, Yang Y, Guo LH. (2013). Single-walled carbon nanotubes and graphene oxides induce autophagosome accumulation and lysosome impairment in primarily cultured murine peritoneal macrophages. Toxicology Letters 221: 118–127. [Google Scholar]
Wang X, Guo J, Chen T, Nie H, Wang H, Zang J, Cui X, Jia G (2012) Multi-walled carbon nanotubes induce apoptosis via mitochondrial pathway and scavenger receptor. Toxicol in Vitro 26: 799–806. [Google Scholar]
Wei H, Li Z, Hu S, Chen X, Cong X. (2010). Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. Journal of Cellular Biochemistry 111: 967–978. [Google Scholar]
Wiemann M, Vennemann A, Sauer UG, Wiench K, Ma-Hock L, Landsiedel R. (2016). An in vitro alveolar macrophage assay for predicting the short-term inhalation toxicity of nanomaterials. Journal of Nanobiotechnology 14: 492. [Google Scholar]
Wu YL, Putcha N, Ng KW, Leong DT, Lim CT, Loo SCJ, Chen X. (2012). Biophysical responses upon the interaction of nanomaterials with cellular interfaces. Accounts of Chemical Research 46: 782–791. [Google Scholar]
Yaari Z, Da Silva D, Zinger A, Goldman E, Kajal A, Tshuva R, Barak E, Dahan N, Hershkovitz D, Goldfeder M. (2016). Theranostic barcoded nanoparticles for personalized cancer medicine. Nature Communications 7: 4414. [Google Scholar]
Ye SF, Wu YH, Hou ZQ, Zhang QQ. (2009). ROS and NF-κB are involved in upregulation of IL-8 in A549 cells exposed to multi-walled carbon nanotubes. Biochemical and Biophysical Research Communications 379: 643–648. [Google Scholar]
Zhang K, Kaufman RJ. (2008). From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462. [Google Scholar]
Zhang Y, Xu Y, Li Z, Chen T, Lantz SM, Howard PC, Paule MG, Slikker W, Jr, Watanabe F, Mustafa T. (2011). Mechanistic toxicity evaluation of uncoated and PEGylated single-walled carbon nanotubes in neuronal PC12 cells. ACS Nano 5: 7020–7033. [Google Scholar]
Zhou Y, Fang Y, Ramasamy RP (2019). Non-Covalent functionalization of carbon nanotubes for electrochemical biosensor development. Sensors (Basel, Switzerland) 19: 392. DOI 10.3390/s19020392. [Google Scholar] [CrossRef]
Zhu L, Chang DW, Dai L, Hong Y. (2007). DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Letters 7: 3592–3597. [Google Scholar]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |