2021 45(1): 167-175
DOI:10.32604/biocell.2021.013636

www.techscience.com/journal/biocell
| BIOCELL 2021 45(1): 167-175 DOI:10.32604/biocell.2021.013636 |  www.techscience.com/journal/biocell |
Melittin inhibited glycolysis and induced cell apoptosis in cisplatin-resistant lung adenocarcinoma cells via TRIM8
1Department of Oncology, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 200071, China
2Department of Traditional Chinese and Western Medicine, Shanghai pulmonary Hospital, Tongji University School of Medicine, Shanghai, 200433, China
3Department of Respiratory Disease, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 201999, China
*Address correspondence to: Lixin Wang, Wlx1126@hotmail.com; Yan Li, yan.xiaotian@shutcm.edu.cn
Received: 14 August 2020; Accepted: 25 September 2020
#These authors contributed equally to this study
Abstract: Chemotherapy is widely used for non-small cell lung cancer (NSCLC) patients at a late stage; however, NSCLC patients often acquire resistance to chemotherapeutic drugs, thus limiting the therapy efficacy. Melittin, a major component of bee venom, possesses anti-tumor activity in various cancer cells. Here, we examined the effects of melittin on A549/DDP cisplatin-resistant lung adenocarcinoma cells and xenografts formed from this cell line and investigated the possible target of melittin. Treatment with melittin resulted in the induction of cell apoptosis, glycolysis inhibition, and reduction of phosphorylated AKT (p-AKT) in A549/DDP cells. We also identified that tripartite motif-containing 8 (TRIM8) was a potential target of melittin. Moreover, we found that TRIM8 mRNA expression was elevated in NSCLC specimens as compared to adjacent normal tissues (N = 25) and that patients with high expression of TRIM8 had a poor prognosis for lung adenocarcinoma. The knockdown of TRIM8 had a similar effect of melittin, while overexpression of TRIM8 reversed the effects of melittin in A549/DDP cells. More importantly, we revealed that melittin enhanced cisplatin sensitivity in A549/DDP cells and tumor growth in vivo using a xenograft model of A549/DDP cells. In conclusion, melittin appears to be a potential chemotherapy sensitization agent in NSCLC.
Keywords: Glycolysis; Apoptosis; TRIM8; Cisplatin
Globally, lung cancer is the most comment cause of cancer-associated deaths (Bray et al., 2018). Non-small cell lung cancer (NSCLC) accounts for approximately 80% of lung cancer (Tsim et al., 2010). Lung adenocarcinoma is the main histological type of NSCLC. The platinum-based drug, cisplatin, is widely applied in NSCLC patients at a late stage. However, chemoresistance limits the therapy efficacy and emerges as a serious problem for these patients (Chen et al., 2014; Kuribayashi et al., 2016). Currently, there is no method to effectively reverse cisplatin resistance in patients with NSCLC. The 5-year survival rate for stage III and stage IV NSCLC patients is about 10% and 2%, respectively (Chen et al., 2014; Kuribayashi et al., 2016).
It is well known that cancer cells display increased glucose uptake, glycolysis, and lactic acid fermentation. This phenomenon terms the Warburg effect, which is regarded as a feature of tumors. The Warburg effect provides energy and materials for cancer cell growth and invasion, hence promotes tumorigenesis (Potter et al., 2016). Recently, several key regulators of the Warburg effect have been implicated in chemoresistance (Bhattacharya et al., 2016). For instance, the PI3K/AKT pathway can active hexokinase 2, a key enzyme of the glycolytic pathway, and induce drug resistance in laryngeal cancer cells (Min et al., 2013).
Melittin, a major active component of bee venom, is a water-soluble peptide containing 26 amino acids. Evidence has suggested the pro-apoptosis ability of melittin in hepatoma (Hu et al., 2006), ovarian cancer (Jo et al., 2012), gastric cancer (Kong et al., 2016), and colon cancer cells (Nikodijević et al., 2019). Several cancer-related signaling pathways, such as the mitochondria pathway (Kong et al., 2016), Janus kinase (JAK2)/signal transducers and activators of transcription (STAT3) (Jo et al., 2012), have been linked to the anti-tumor activity of melittin. In our previous study, we reported that melittin was able to inhibit cell growth, migration and invasion, and induce apoptosis in NSCLC cells (Zhang and Chen, 2017). However, little is known about the effects of melittin on cisplatin-resistant lung adenocarcinoma cell line (A549/DDP) and whether the Warburg effect is involved in this process.
Tripartite motif-containing (TRIM) proteins are characterized by a TRIM motif consisting of a RING-finger domain, one or two B boxes, and a coiled-coil region. More than 80 members of TRIM proteins have been identified in humans (Hatakeyama, 2011). Abundant evidence has been obtained regarding the functions of TRIM proteins in the regulation of carcinogenesis (Hatakeyama, 2011). Several researchers have reported the functions of TRIM proteins in the chemoresistance of various solid tumors, including lung cancer (Liu et al., 2015; Liu et al., 2017; Ni et al., 2016; Qin et al., 2017; Tan et al., 2018; Yu et al., 2018; Zhang et al., 2015; Zhao et al., 2018). As reported, knockdown of TRIM29 (Liu et al., 2015) and TRIM25 (Qin et al., 2017), respectively, made lung squamous cancer NCI-H520 cells and cisplatin-resistant lung adenocarcinoma cells (A549/DDP) more sensitive to cisplatin therapy. Besides, TRIM proteins may display controversial functions in glucose metabolism (Chen et al., 2015; Jin et al., 2017; Pathiraja et al., 2015). For example, increased glucose uptake and aerobic glycolysis were observed in immortalized human mammary epithelial cells with ectopic expression of TRIM24 (Pathiraja et al., 2015). TRIM35 suppressed the Warburg effect (Chen et al., 2015), while TRIM28 promotes the Warburg effect in hepatocellular carcinoma (Jin et al., 2017).
In the current study, we explore the effects of melittin on cell apoptosis and the Warburg effect in A549/DDP cisplatin-resistant lung adenocarcinoma cells and to identify the potential target of melittin. Moreover, we investigated the effects of melittin on cisplatin sensitivity in vitro and in vivo systems.
A549/DDP cells obtained from JRDUN Biotech (Shanghai, China) were grown in RPMI 1640 medium (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA) at 37°C under a humidified atmosphere containing 5% CO2.
To explore the effects of melittin on A549/DDP cell proliferation, A549/DDP cells were incubated with 0, 1, 2, 4, 8 or 16 μg/mL melittin (Sigma-Aldrich, St. Louis, MO, USA), and cell proliferation inhibition was assessed by Cell Counting Kit-8 (CCK-8) assay for 0, 24, 48, or 72 h. At the end of incubation, CCK-8 solution (Beyotime, Shanghai, China) was added to the palates. After 2 h of incubation, cell proliferation was determined by measuring optical density at 450 nm.
The study was approved by the Institute Research Ethics Committee of Shanghai Municipal Hospital of Traditional Chinese Medicine (Shanghai, China) and conducted in accordance with the Declaration of Helsinki. A total of 25 NSCLC patients were enrolled at Shanghai Municipal Hospital after all patients provided written informed consent. Paired NSCLC specimens and adjacent normal tissues were collected, snap-frozen, and stored at −80°C until use.
Prognostic analysis of TRIM8 mRNA
The relevance of TRIM8 mRNA expression to overall survival was determined by using www.kmplot.com (Győrffy et al., 2013). The tool is based on public NSCLC microarray data from Gene Expression Omnibus (GEO). The patients were divided into high and low expression groups, HR (95% CIs), log-rank P, and Kaplan–Meier survival plots were then displayed on the webpage.
Knocking down and overexpression of TRIM8
Three short hairpin RNAs targeting TRIM8 were designed, synthesized, and cloned into pLKO.1 lentiviral vector (Addgene, Cambridge, MA, USA). The target sites were as follows: siTRIM8-1, 5’-CCAACATCGTGGAGAAGTT-3’; siTRIM8-2, 5’-CCAGCTGTACAAACTCGAG-3’.
Human TRIM8 full-length cDNA was amplified and cloned into the pLVX-puro vector (Clontech, Palo Alto, CA, USA). The primers were as follows: forward primer, 5’-CGGAATTCATGGCGGAGAATTGGAAGAAC-3’ and reverse primer, 5’-CGGGATCCTTAGCTCGTCACGTAGTGTTTGG-3’.
To package the lentivirus, the constructs were co-transfected with packaging plasmids into 293 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). The supernatant was harvested 48–72 h later and used to infect A549/DDP cells.
Total RNA was isolated with TRIzol Reagent (Invitrogen) and reverse-transcribed with MMLV reverse transcriptase (Promega, Madison, WI, USA) in accordance with the manufacturers’ instruction. To determine the relative gene expression, real-time PCR was conducted with an ABI 7500 instrument (Applied Biosystems, Foster City, CA, USA). β-actin was applied as an internal control. All the primers are listed in Tab. S1.
The cells were lysed with ice-cold radioimmunoprecipitation assay (RIPA) buffer containing proteinase and phosphatase inhibitor cocktails (Sigma-Aldrich) at 4°C. Following centrifugation at 12,000 rpm for 15 min at 4°C, the supernatant was collected, and protein concentration was determined by BCA assay (Walker, 2009). Equal protein concentrations from the samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes (Millipore, Bredford, MA, USA). The membranes were blocked with 5% skim milk and then probed with primary antibodies against TRIM8 (Abcam, Cambridge, MA, USA), p-AKT (Cell Signaling Technology, Danvers, MA, USA), AKT (Cell Signaling Technology) or β-actin (Abcam). Following incubation with an HRP-conjugated secondary antibody (Beyotime), immunoreactive signals were detected using by Enhanced chemiluminescence (ECL) technique (Millipore). β-actin was detected for equal loading control.
Annexin V-FITC/propidium iodide (PI) staining kit (Beyotime) was used to stained cells undergoing apoptosis following the manufacturer’s protocol. In brief, A549/DDP cells in 6-well plates were harvest by trypsinization. Following washing, once with PBS, the cells were collected by centrifugation and re-suspended in 195 μL Annexin V-FITC binding buffer. Subsequently, the cells were mixed with 5 μL Annexin V-FITC and 5 μL PI, and then incubation at dark for 20 min at room temperature. The apoptotic rate (Annexin V positive and PI negative cells) was analyzed by a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
A549/DDP cells were cultured with glucose-free medium for 3 h and then incubated with 100 μM 2-NBDG (2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose; Cayman, Ann Arbor, MI, USA) in glucose-free medium for 45 min. After washing with glucose-free Krebs-Ringer buffer (KRB), cells were collected by trypsinization and the fluorescent density was measured with a flow cytometer (BD Biosciences). The relative fluorescent density of treated samples was normalized to the control group, which was set as 100%.
Measurement of lactate production
Lactate production in the culture medium was measured with a lactic acid detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s protocol.
The experimental procedures were approved by the Committee on Animal Care and Use of Shanghai Municipal Hospital of Traditional Chinese Medicine (Shanghai, China) and performed in accordance with the Guidelines for Animal Experiments of Shanghai Municipal Hospital of Traditional Chinese Medicine. Subcutaneous xenografts were established in BALB/c athymic nude mice (4–5 weeks old) by subcutaneous injection of A549/DDP cells (5 × 106 cells per mouse). After 7 days, the mice were randomly divided into 4 groups and there were 5 mice in each group: Control, DDP, melittin (MEL) and MEL + DDP. The mice in DDP and MEL group were intraperitoneally injected with cisplatin (5 mg/kg, Chinese medicine reagent, Beijing, China) and melittin (2 mg/kg) every 7 days for 3 weeks, respectively. The mice in the MEL + DDP group were administrated with cisplatin (5 mg/kg) and melittin (2 mg/kg) every 7 days for 3 weeks. The tumor volume was estimated every 3 days and calculated using the following formula: volume = (width2 × length) / 2. After 3 weeks, the mice were euthanized, and the tumor xenografts were harvested for TUNEL (Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling) analyses (Roche, Indianapolis, IN, USA).
All data are presented as the mean ± SD. Statistical analysis was performed using Graphpad Prism Software (Graphpad Prism, San Diego, CA, USA). One-way analysis of variance (ANOVA) was performed for the in vitro experiments and xenograft experiments. A two-tailed Student’s t-test was conducted for comparing TIMIM8 expression in clinical samples. Differences were considered statistically significant at p < 0.05.
Melittin induced cell apoptosis and inhibited the Warburg effect in A549/DDP cells
First, we confirmed that A549/DDP cells were resistant to cisplatin comparing to A549 cells (Fig. S1). Then, A549/DDP cells were incubated with 0–16 μg/mL melittin (MEL) and cell proliferation inhibition was assessed by CCK-8 assay for 24, 48, or 72 h. As shown in Fig. 1A, the inhibitory rate of proliferation was dose- and time-dependently increased by melittin exposure. The inhibitory rate of proliferation was not significant at a concentration of 1 μg/mL at 24 h post-treatment, while 16 μg/mL caused more than 50% inhibition at 72 h post-treatment. Therefore, 2, 4, and 8 μg/mL were chosen for the following experiments.
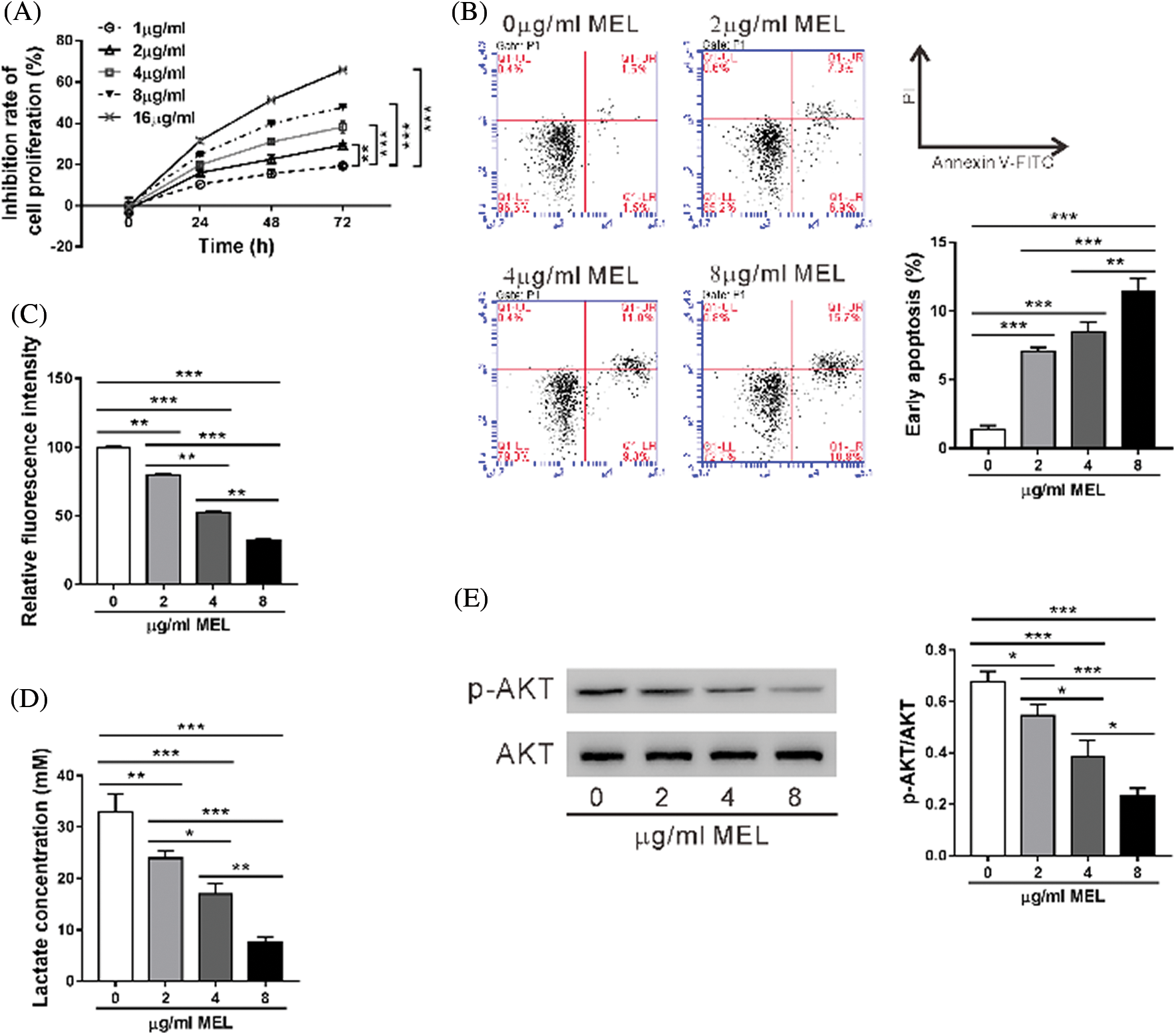
Figure 1: Melittin suppressed cell proliferation, induced cell apoptosis, and inhibited the Warburg effect in A549/DDP cisplatin-resistant lung adenocarcinoma cells.
We used flow cytometry to determine the effect of melittin on A549/DDP cell apoptosis (Fig. 1B). Apoptosis rate of the A549/DDP cells was significantly increased at 48 h following melittin treatment (0 μg/mL, 1.40 ± 0.26%; 2 μg/mL, 7.07 ± 0.29%; 4 μg/mL, 8.47 ± 0.72%; 8 μg/mL, 11.40 ± 0.95%) in comparison with the control.
The Warburg effect, a key feature of tumors, has been implicated in chemoresistance (Bhattacharya et al., 2016); thus, we detected the effects of melittin on glucose uptake and lactate production. A549/DDP cells showed a dose-dependent decrease in 2-NBDG uptake (Fig. 1C) and lactate production (Fig. 1D) following melittin treatment.
AKT pathway plays an important role in regulating cell apoptosis and glycolysis (Cairns et al., 2011). As expected, the Western blotting analysis indicated that the level of phosphorylated AKT (p-AKT) was also reduced with melittin treatment (Fig. 1E). These results suggest that melittin induced cell apoptosis and inhibited AKT signaling and Warburg effect in A549/DDP cells.
TRIM8 was a potential target of melittin
To identify TRIM proteins possibly involving in the effects of melittin, mRNA levels of several TRIM proteins were assessed in A549 cells, and A549/DDP cells treated with melittin or not. As shown in Fig. 2, significant differences were observed in 9 TRIM proteins between A549 cells and A549/DDP cells, while only 3 TRIM proteins, TRIM8, TRIM31, and TRIM40, showed significant differences between melittin-treated and -untreated A549/DDP cells. The most significant change was observed in TRIM8, which may be a selective target of melittin, and further study was focused on TRIM8.
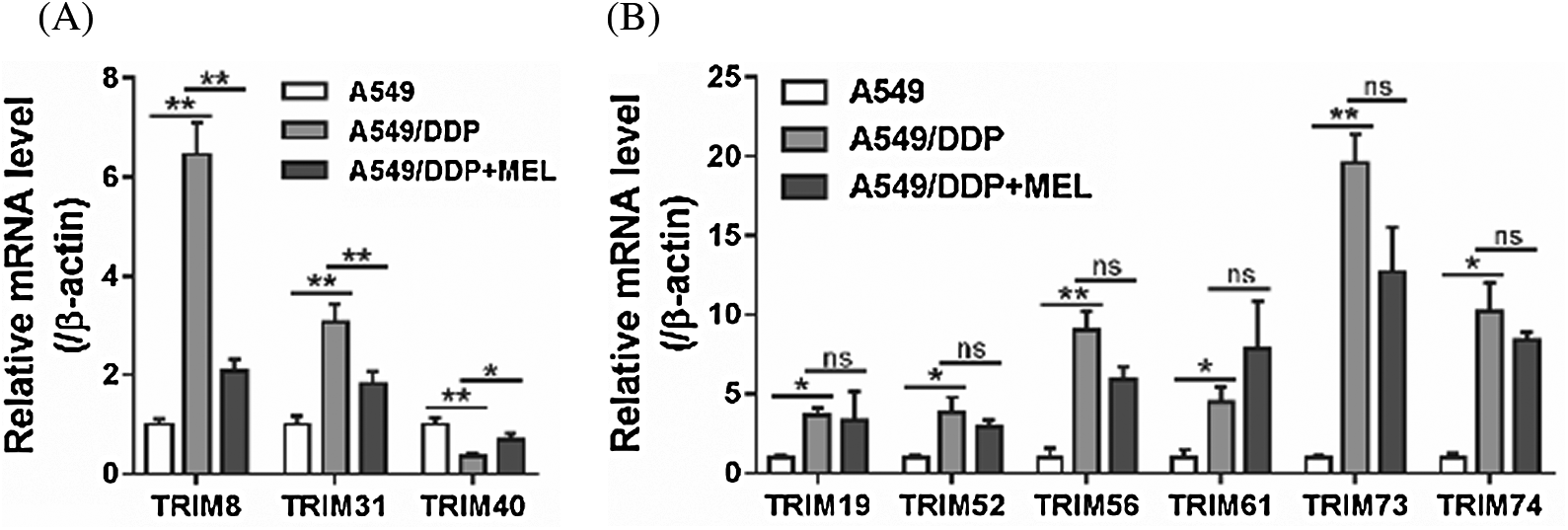
Figure 2: TRIM8 was a potential target of melittin.
TRIM8 overexpression reversed the effects of melittin
Western blotting results showed that TRIM8 protein was reduced with the treatment of melittin in A549/DDP cells in a dose-dependent manner (Fig. 3A). To determine whether TRIM8 is involved in the effect of melittin, A549/DDP cells were transduced with a virus overexpressing TRIM8 and then treated with 4 μg/mL melittin. As shown in Fig. 3B, TRIM8 expression was significantly increased in A549/DDP cells by lentivirus transduction. Consequently, TRIM8 overexpression reduced the induction of cell apoptosis (Fig. 3C) and the inhibition of 2-NBDG uptake (Fig. 3D), lactate production (Fig. 3E), and p-AKT level (Fig. 3F) caused by melittin exposure.
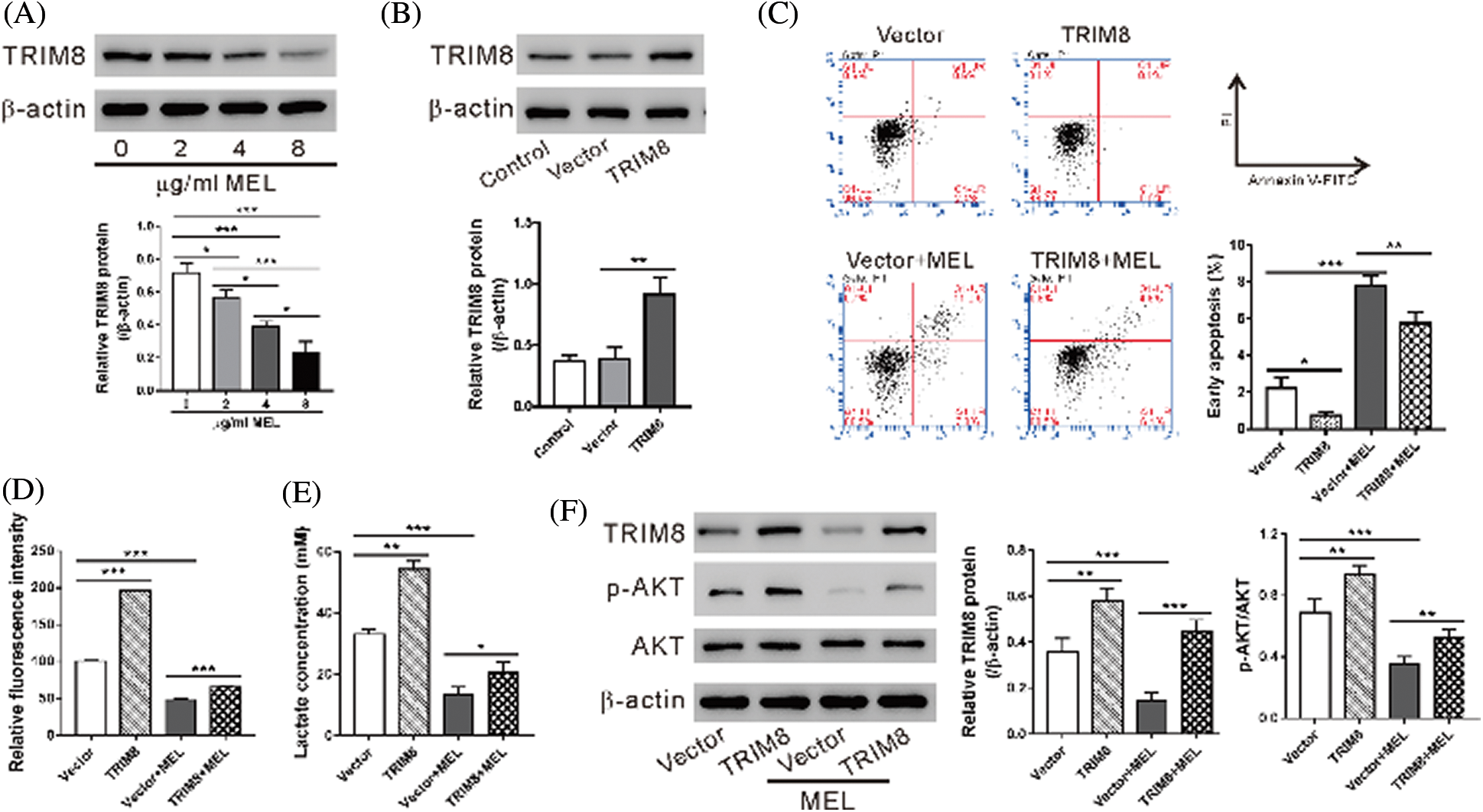
Figure 3: TRIM8 overexpression reversed the effects of melittin.
TRIM8 knockdown induced apoptosis and inhibited glycolysis in A549/DDP cells
TRIM8 mRNA expression was found elevated in NSCLC specimens as compared to adjacent normal tissues (N = 25, Fig. 4A). The prognostic value of TRIM8 mRNA expression was then examined by using www.kmplot.com. Survival curves were plotted for all patients (N = 1,926) and adenocarcinoma (N = 720) with probe 22131_s_at. High expression of TRIM8 mRNA was correlated with poor overall survival for all patients and adenocarcinoma (Fig. 4B).
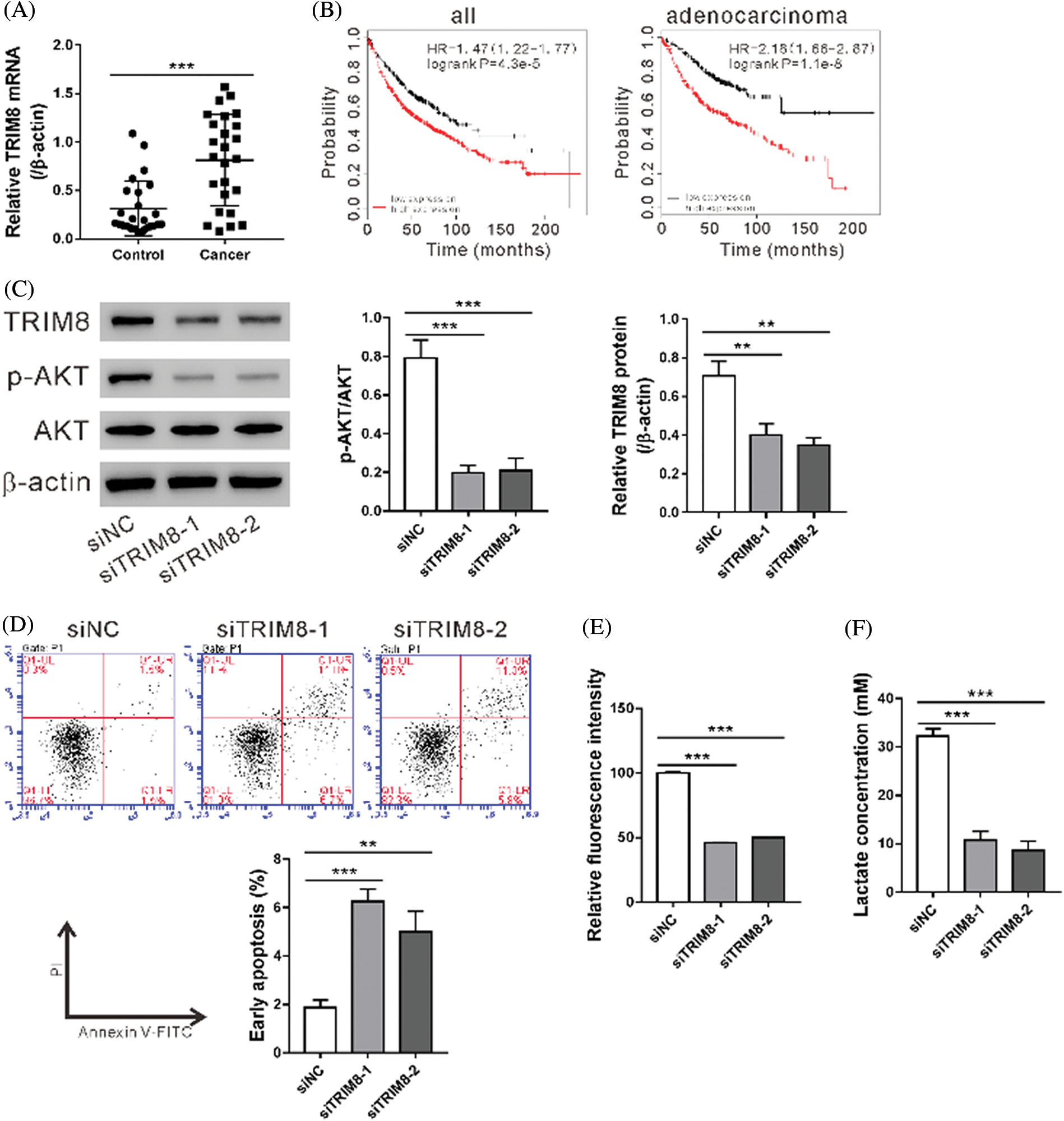
Figure 4: TRIM8 knockdown induced cell apoptosis and inhibited glycolysis in A549/DDP cells.
To explore the biological function of TRIM8, TRIM8 expression was suppressed in A549/DDP adenocarcinoma cells by RNAi. As shown in Fig. 4C, TRIM8 siRNAs obviously reduced TRIM8 expression, and siTRIM8-1 and siTRIM8-2 were then used in the following experiments. A549/DDP cells showed a notable increase in apoptosis (Fig. 4D) and a significant decrease in 2-NBDG uptake (Fig. 4E), lactate production (Fig. 4F), and p-AKT level (Fig. 4C) when TRIM8 expression was down-regulated. These data demonstrated the functions of TRIM8 in apoptosis and glycolysis of A549/DDP cells.
Effect of melittin on cisplatin sensitivity in vitro and in vivo systems
To investigate the function of melittin on cisplatin resistance in vitro, A549/DDP cells were incubated with melittin (MEL, 4 μg/mL), cisplatin (DDP, 50 μM), or MEL plus DDP, and then cell proliferation was assessed. Following incubation with melittin in combination with cisplatin, cell proliferation was significantly inhibited in the co-treatment group as compared to that in the DDP-treated group (Fig. 5A). These data indicate that A549/DDP cells presented enhanced cisplatin sensitivity in the presence of melittin.
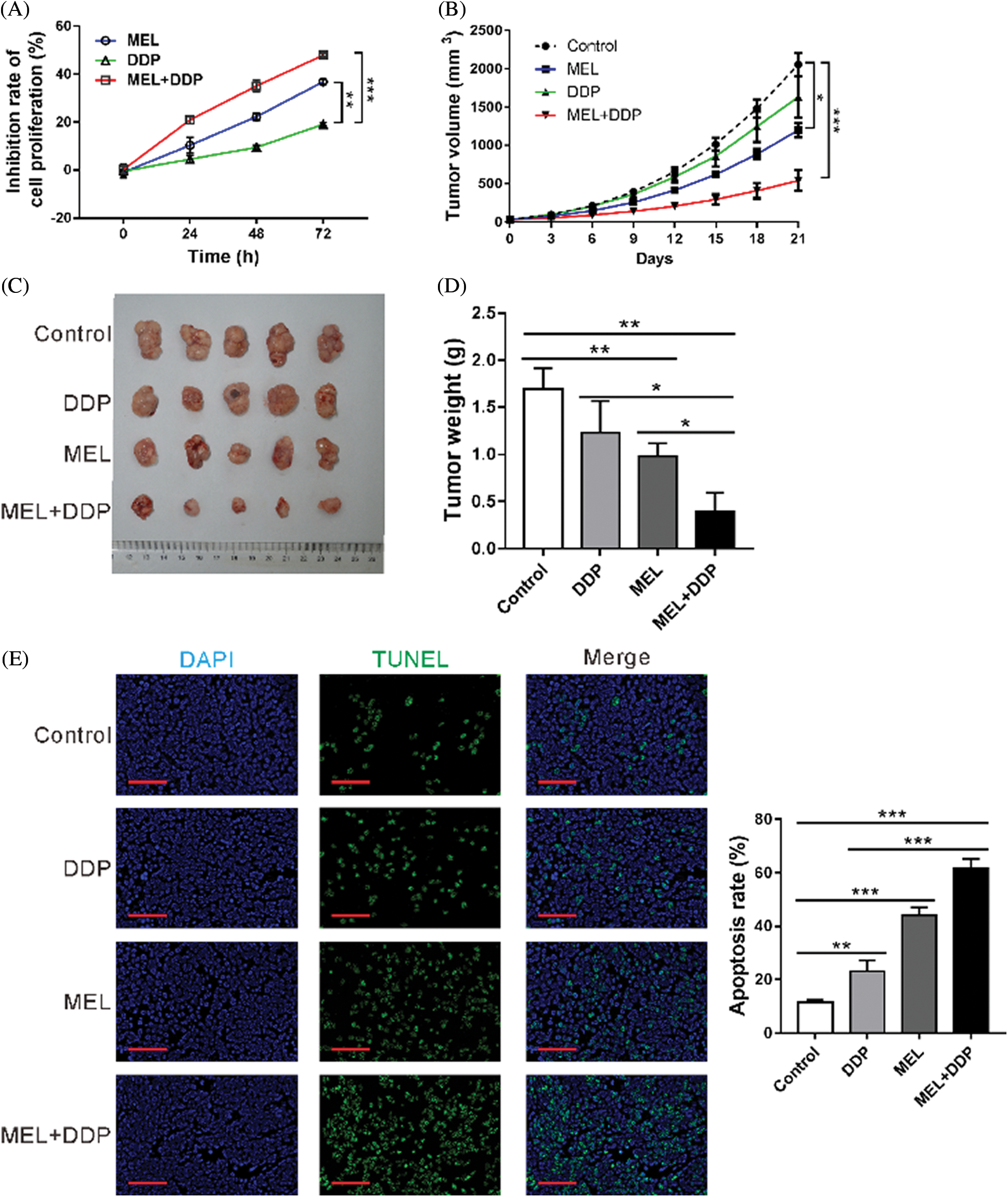
Figure 5: Effect of melittin on cisplatin sensitivity in vitro and in vivo.
To investigate the function of melittin in vivo, a mouse xenograft model was constructed by using A549/DDP cells. Tumor growth was significantly decreased by melittin, cisplatin, or melittin plus cisplatin at 12–21 days following treatment, when compared with the control group (p < 0.05, Fig. 5B). At 21 days after treatment, tumor size (Fig. 5C) and tumor weight (Fig. 5D) were decreased in the melittin group, cisplatin group, or melittin plus cisplatin group as compared with the control group (p < 0.01). Co-treatment with melittin plus cisplatin exhibited the best inhibitory effects. Cell apoptosis rate was increased by treatment, and co-treatment also displayed the highest apoptotic rate (Fig. 5E). These results indicate that melittin enhanced cisplatin sensitivity of tumors derived from A549/DDP cells.
We previously reported that melittin inhibited cell growth and induce apoptosis in NSCLC cells (Zhang and Chen, 2017), and the present study revealed the similar effects of melittin in cisplatin-resistant lung adenocarcinoma cell line (A549/DDP) (Fig. 1A, 1B). Enhanced Warburg effect is considered as a feature of tumors (Potter et al., 2016). The PI3K/AKT pathway, a key regulator of the Warburg effect (Vander Heiden et al., 2009) has been linked to the anti-tumor activity of melittin (Jeong et al., 2014). Here, melittin inhibited glucose uptake, lactate production, and AKT phosphorylation in A549/DDP cells (Figs. 1C, 1E), demonstrating the inhibitory effect of melittin on the Warburg effect. Although melittin is toxic to normal cells (Gajski and Garaj-Vrhovac, 2013), Zhu et al. have reported that lung cancer cells are more susceptible to melittin than normal cells (Zhu et al., 1991). Whether melittin inhibited glycolysis of normal cells is to be determined. The development of suitable targeted delivery carriers that could specifically and efficiently deliver melittin to cisplatin-resistant tumor cells may significantly improve the therapeutic effects of melittin in the future.
In addition, we identified that TRIM8 may be a selective target of melittin (Fig. 2). TRIM8 encodes a member of TRIM proteins, which are widely involved in carcinogenesis (Hatakeyama, 2011) and chemoresistance (Liu et al., 2015; Liu et al., 2017; Ni et al., 2016; Qin et al., 2017; Tan et al., 2018; Yu et al., 2018; Zhang et al., 2015; Zhao et al., 2018). Recent studies have linked TRIM8 to cancer cell growth and chemosensitivity. TRIM8 expression was decreased in glioma and TRIM8 expression recovery significantly reduced the clonogenic potential of glioblastoma cells (Micale et al., 2015). Restored expression of TRIM8 in renal cell carcinoma (RCC) cells (Caratozzolo et al., 2014) or colorectal cancer cells (Ni et al., 2016) sensitized the cells to chemotherapeutic drugs. In the present study, we found that TRIM8 mRNA expression was elevated in NSCLC specimens (Fig. 4A). Kaplan–Meier Plotter online survival analysis revealed that patients with high expression of TRIM8 had a poor prognosis for all NSCLC patients and lung adenocarcinoma patients (Fig. 4B). These data were inconsistent with the findings in glioma (Micale et al., 2015), RCC (Caratozzolo et al., 2014), and colorectal cancer cells (Ni et al., 2016), which may ascribe to the different cancer types. The knockdown of TRIM8 had a similar effect of melittin (Fig. 4), while overexpression of TRIM8 reversed the effects of melittin (Fig. 3). These data further demonstrate that TRIM8 mediates the effects of melittin in A549/DDP cells.
Resistance to cisplatin-based chemotherapy promotes tumor progression and relapse of NSCLC, thus limiting the therapeutic efficacy (Chen et al., 2014; Kuribayashi et al., 2016). In order to increase the 5-year survival rate, it is necessary to improve the cisplatin sensitivity of patients. A previous study has suggested that cisplatin-resistant ovarian cancer cells were slightly more sensitive to melittin than those cisplatin sensitive cells (Alonezi et al., 2016), while the combined effects of melittin and cisplatin in cancer cells were not clear. Currently, we observed that co-treatment with melittin and cisplatin significantly inhibited the proliferation of A549/DDP cells, and the growth of tumors derived from A549/DDP cells (Fig. 5) as compared to treatment with cisplatin. These data suggest that melittin may enhance the cisplatin sensitivity of NSCLC cells in vitro and in vivo. Enhanced Warburg effect is implicated in chemoresistance (Bhattacharya et al., 2016). By metabolomic profiling, Alonezi et al. (2016) found that cisplatin-resistant ovarian cancer cells have increased levels of ATP both before and after melittin compared with those cisplatin sensitive cells, suggesting that melittin may target glycolysis to function as an anticancer agent (Alonezi et al., 2016). Here, our data showed that melittin inhibited glycolysis in A549/DDP cells (Figs. 1C, 1D). Further experiments are required to clarify whether the Warburg effect mediated the effects of melittin on chemoresistance. Nevertheless, our data provided the first evidence that melittin may be used to improve the cisplatin-based chemotherapy sensitivity of NSCLC.
In summary, we found that melittin induced apoptosis and reduced glycolysis in cisplatin-resistant lung adenocarcinoma cells via regulating TRIM8. Interestingly, we showed that melittin sensitizes lung adenocarcinoma cells to chemotherapeutic drugs in vitro and in vivo. Therefore, melittin might be helpful for treating lung adenocarcinoma and reversing cisplatin resistance.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding Statement: This research was funded by the Natural Science Foundation of China (81673947), Natural Science Foundation of Shanghai (1428000), the Program of Science and Technology Commission of Shanghai Municipality (16401932900 and 17401933500) and the Fundamental Research Funds for the Central Universities (22120180373).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Alonezi S, Tusiimire J, Wallace J, Dufton MJ, Parkinson JA, Young LC, Clements CJ, Park JK, Jeon JW, Ferro VA. (2016). Metabolomic profiling of the effects of melittin on cisplatin resistant and cisplatin sensitive ovarian cancer cells using mass spectrometry and biolog microarray technology. Metabolites 6: 35. DOI 10.3390/metabo6040035. [Google Scholar] [CrossRef]
Bhattacharya B, Mohd Omar MF, Soong R. (2016). The Warburg effect and drug resistance. British Journal of Pharmacology 173: 970–979. DOI 10.1111/bph.13422. [Google Scholar] [CrossRef]
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68: 394–424. DOI 10.3322/caac.21492. [Google Scholar] [CrossRef]
Cairns RA, Harris IS, Mak TW. (2011). Regulation of cancer cell metabolism. Nature Reviews Cancer 11: 85–95. DOI 10.1038/nrc2981. [Google Scholar] [CrossRef]
Caratozzolo MF, Valletti A, Gigante M, Aiello I, Mastropasqua F, Marzano F, Ditonno P, Carrieri G, Simonnet H, D’Erchia AM, Ranieri E, Pesole G, Sbisà E, Tullo A. (2014). TRIM8 anti-proliferative action against chemo-resistant renal cell carcinoma. Oncotarget 5: 7446–7457. DOI 10.18632/oncotarget.2081. [Google Scholar] [CrossRef]
Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong K. (2014). Non-small-cell lung cancers: A heterogeneous set of diseases. Nature Reviews Cancer 14: 535–546. DOI 10.1038/nrc3775. [Google Scholar] [CrossRef]
Chen Z, Wang Z, Guo W, Zhang Z, Zhao F, Zhao Y, Jia D, Ding J, Wang H, Yao M, He X. (2015). TRIM35 Interacts with pyruvate kinase isoform M2 to suppress the Warburg effect and tumorigenicity in hepatocellular carcinoma. Oncogene 34: 3946–3956. DOI 10.1038/onc.2014.325. [Google Scholar] [CrossRef]
Gajski G, Garaj-Vrhovac V. (2013). Melittin: a lytic peptide with anticancer properties. Environmental Toxicology and Pharmacology 36: 697–705. DOI 10.1016/j.etap.2013.06.009. [Google Scholar] [CrossRef]
Győrffy B, Surowiak P, Budczies J, Lánczky A, Chellappan SP. (2013). Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One 8: e82241. DOI 10.1371/journal.pone.0082241. [Google Scholar] [CrossRef]
Hatakeyama S. (2011). TRIM proteins and cancer. Nature Reviews Cancer 11: 792–804. DOI 10.1038/nrc3139. [Google Scholar] [CrossRef]
Hu H, Chen D, Li Y, Zhang X. (2006). Effect of polypeptides in bee venom on growth inhibition and apoptosis induction of the human hepatoma cell line SMMC-7721 in-vitro and Balb/c nude mice in-vivo. Journal of Pharmacy and Pharmacology 58: 83–89. DOI 10.1211/jpp.58.1.0010. [Google Scholar] [CrossRef]
Jeong Y, Choi Y, Shin J, Cho H, Kang JH, Park K, Choe J, Bae Y, Han S, Kim C. (2014). Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food and Chemical Toxicology 68: 218–225. DOI 10.1016/j.fct.2014.03.022. [Google Scholar] [CrossRef]
Jin X, Pan Y, Wang L, Zhang L, Ravichandran R, Potts PR, Jiang J, Wu H, Huang H. (2017). MAGE-TRIM28 complex promotes the Warburg effect and hepatocellular carcinoma progression by targeting FBP1 for degradation. Oncogenesis 6: e312–e312. DOI 10.1038/oncsis.2017.21. [Google Scholar] [CrossRef]
Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han S, Kim JH, Song MJ, Hong JT. (2012). Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicology and Applied Pharmacology 258: 72–81. DOI 10.1016/j.taap.2011.10.009. [Google Scholar] [CrossRef]
Kong GM, Tao WH, Diao YL, Fang PH, Wang JJ, Bo P, Qian F. (2016). Melittin induces human gastric cancer cell apoptosis via activation of mitochondrial pathway. World Journal of Gastroenterology 22: 3186. DOI 10.3748/wjg.v22.i11.3186. [Google Scholar] [CrossRef]
Kuribayashi K, Funaguchi N, Nakano T. (2016). Chemotherapy for advanced non-small cell lung cancer with a focus on squamous cell carcinoma. Journal of Cancer Research and Therapeutics 12: 528–534. DOI 10.4103/0973-1482.174185. [Google Scholar] [CrossRef]
Liu C, Huang X, Hou S, Hu B, Li H. (2015). Silencing of tripartite motif (TRIM) 29 inhibits proliferation and invasion and increases chemosensitivity to cisplatin in human lung squamous cancer NCI-H520 cells. Thoracic Cancer 6: 31–37. DOI 10.1111/1759-7714.12130. [Google Scholar] [CrossRef]
Liu Y, Zhang B, Shi T, Qin H. (2017). miR-182 promotes tumor growth and increases chemoresistance of human anaplastic thyroid cancer by targeting tripartite motif 8. OncoTargets and Therapy 10: 1115–1122. DOI 10.2147/OTT.S110468. [Google Scholar] [CrossRef]
Micale L, Fusco C, Fontana A, Barbano R, Augello B, De Nittis P, Copetti M, Pellico MT, Mandriani B, Cocciadiferro D, Parrella P, Fazio VM, Dimitri LMC, D’Angelo V, Novielli C, Larizza L, Daga A, Merla G. (2015). TRIM8 downregulation in glioma affects cell proliferation and it is associated with patients survival. BMC Cancer 15: 2. DOI 10.1186/s12885-015-1449-9. [Google Scholar] [CrossRef]
Min JW, Kim KI, Kim HA, Kim EK, Noh WC, Jeon HB, Cho DH, Oh JS, Park IC, Hwang SG, Kim JS. (2013). INPP4B-mediated tumor resistance is associated with modulation of glucose metabolism via hexokinase 2 regulation in laryngeal cancer cells. Biochemical and Biophysical Research Communications 440: 137–142. DOI 10.1016/j.bbrc.2013.09.041. [Google Scholar] [CrossRef]
Ni B, Hu J, Chen D, Li L, Chen D, Wang J, Wang L. (2016). Alternative splicing of spleen tyrosine kinase differentially regulates colorectal cancer progression. Oncology Letters 12: 1737–1744. DOI 10.3892/ol.2016.4858. [Google Scholar] [CrossRef]
Nikodijević DD, Milutinović MG, Cvetković DM, Ćupurdija MĐ, Jovanović MM, Mrkić IV, Jankulović-Gavrović MĐ, Marković SD. (2019). Impact of bee venom and melittin on apoptosis and biotransformation in colorectal carcinoma cell lines. Toxin Reviews 48: 1–8. DOI 10.1080/15569543.2019.1680564. [Google Scholar] [CrossRef]
Pathiraja TN, Thakkar KN, Jiang S, Stratton S, Liu Z, Gagea M, Shi X, Shah PK, Phan L, Lee MH, Andersen J, Stampfer M, Barton MC. (2015). TRIM24 links glucose metabolism with transformation of human mammary epithelial cells. Oncogene 34: 2836–2845. DOI 10.1038/onc.2014.220. [Google Scholar] [CrossRef]
Potter M, Newport E, Morten KJ. (2016). The Warburg effect: 80 years on. Biochemical Society Transactions 44: 1499–1505. DOI 10.1042/BST20160094. [Google Scholar] [CrossRef]
Qin X, Qiu F, Zou ZJB, Communications BR. (2017). TRIM25 is associated with cisplatin resistance in non-small-cell lung carcinoma A549 cell line via downregulation of 14-3-3σ. Biochemical and Biophysical Research Communications 493: 568–572. DOI 10.1016/j.bbrc.2017.08.151. [Google Scholar] [CrossRef]
Tan Z, Song L, Wu W, Zhou Y, Zhu J, Wu G, Cao L, Song J, Li J, Zhang W. (2018). TRIM14 promotes chemoresistance in gliomas by activating Wnt/β-catenin signaling via stabilizing Dvl2. Oncogene 37: 5403–5415. DOI 10.1038/s41388-018-0344-7. [Google Scholar] [CrossRef]
Tsim S, O’Dowd CA, Milroy R, Davidson S. (2010). Staging of non-small cell lung cancer (NSCLCA review. Respiratory Medicine 104: 1767–1774. DOI 10.1016/j.rmed.2010.08.005. [Google Scholar] [CrossRef]
Vander Heiden MG, Cantley LC, Thompson CB. (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 324: 1029–1033. DOI 10.1126/science.1160809. [Google Scholar] [CrossRef]
Walker JM. (2009). The bicinchoninic acid (BCA) assay for protein quantitation. The protein Protocols Handbook. Totowa, NJ: Humana Press, 11–15. [Google Scholar]
Yu C, Chen S, Guo Y, Sun C. (2018). Oncogenic TRIM31 confers gemcitabine resistance in pancreatic cancer via activating the NF-κB signaling pathway. Theranostics 8: 3224–3236. DOI 10.7150/thno.23259. [Google Scholar] [CrossRef]
Zhang L, Yin A, Cheng J, Huang H, Li X, Zhang Y, Han N, Zhang X. (2015). TRIM24 promotes glioma progression and enhances chemoresistance through activation of the PI3K/Akt signaling pathway. Oncogene 34: 600–610. DOI 10.1038/onc.2013.593. [Google Scholar] [CrossRef]
Zhang SF, Chen Z. (2017). Melittin exerts an antitumor effect on non-small cell lung cancer cells. Molecular Medicine Reports 16: 3581–3586. DOI 10.3892/mmr.2017.6970. [Google Scholar] [CrossRef]
Zhao TT, Jin F, Li JG, Xu YY, Dong HT, Liu Q, Xing P, Zhu GL, Xu H, Yin SC, Miao ZF. (2018). TRIM32 promotes proliferation and confers chemoresistance to breast cancer cells through activation of the NF-κB pathway. Journal of Cancer 9: 1349–1356. DOI 10.7150/jca.22390. [Google Scholar] [CrossRef]
Zhu H, Tayeh I, Israel L, Castagna M. (1991). Different susceptibility of lung cell lines to inhibitors of tumor promotion and inducers of differentiation. Journal of Biological Regulators Homeostatic Agents 5: 52–58. [Google Scholar]
Table S1: Primers for real-time PCR
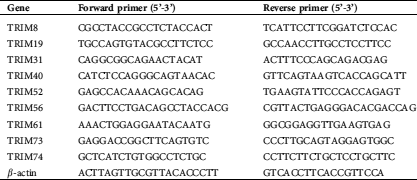
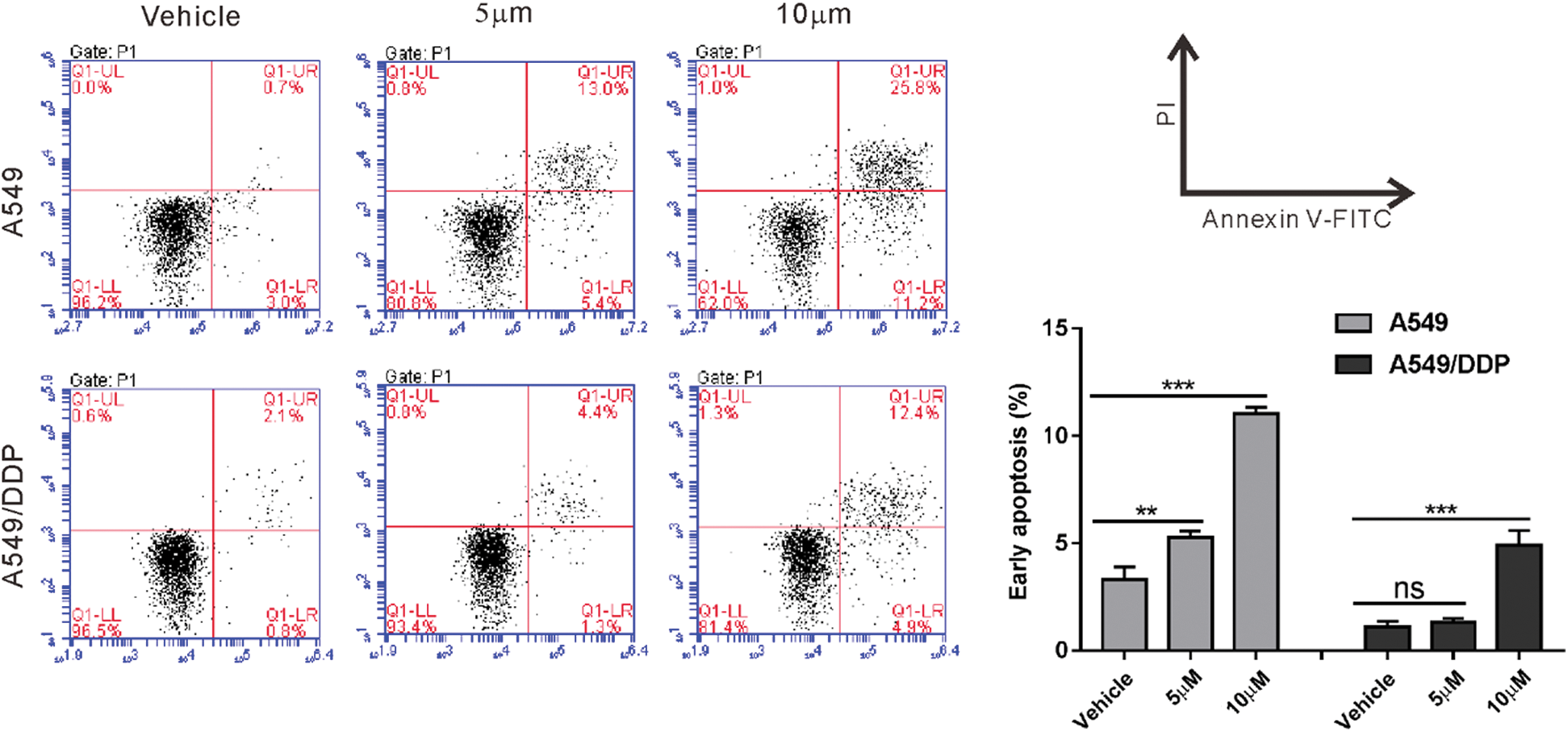
Figure S1: A549/DDP cells were resistant to cisplatin comparing to A549 cells. A549 and A549/DDP cells were treated with 10 and 20 mM cisplatin or vehicle for 24 h. Annexin V-PI staining was performed to assess early apoptosis. ns, nonsignificant, **P < 0.01, ***P < 0.001
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |