2021 45(1): 157-165
DOI:10.32604/biocell.2021.010261

www.techscience.com/journal/biocell
| BIOCELL 2021 45(1): 157-165 DOI:10.32604/biocell.2021.010261 |  www.techscience.com/journal/biocell |
Pomalidomide improves the function of CD133- or HER2-specific CAR T cells
1Ma’anshan University, Ma’anshan, 243100, China
2Division of Health Science, Graduate School of Medicine, Osaka University, Osaka, 565-0871, Japan
3School of Medical Instrument and Food Engineering, University of Shanghai for Science and Technology, Shanghai, 200093, China
4Shanghai Institute for Advanced Immunochemical Studies (SIAIS), ShanghaiTech University, Shanghai, 201210, China
*Address correspondence to: Xuekai Zhu, zhuxk@shanghaitech.edu.cn
Received: 21 February 2020; Accepted: 30 July 2020
Abstract: Chimeric antigen receptor (CAR) T-cell therapy is mostly limited to hematological malignancies and has a poor effect on solid tumors. CAR T cells as a kind of immune cell may be affected by some immunomodulatory drugs such as pomalidomide, so the use of pomalidomide may improve the effect of CAR T cells on solid tumors. In this study, CD133- or HER2-specific CAR T cells were chosen to investigate whether pomalidomide can regulate the function of CAR T cells in vitro. We found that pomalidomide can significantly enhance the ability of CD133-CAR T cells and HER2-CAR T cells to kill tumor cells and increase the cytokine secretion of CD133-CAR T cells and HER2-CAR T cells. Also, pomalidomide was shown to induce down-regulation of protein levels of IL-2 transcriptional repressors Aiolos and Ikaros in CAR T cells. This study suggests that the combination of pomalidomide and CAR T cells may be a new strategy for the treatment of solid tumors.
Keywords: Chimeric antigen receptor; Actimid; Solid tumor; Immunotherapy
Chimeric antigen receptor (CAR) T cell therapy is mainly performed by genetically engineering T cells of patients or donors in vitro so that they can specifically recognize and destroy tumor cells when transferred into patients (Sadelain et al., 2013). In 2017, the US Food and Drug Administration (FDA) approved the first CAR T cell therapy for the treatment of CD19-positive relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL) (June et al., 2018). However, there are few reports on CAR T cell therapy successfully treating solid tumors, which may be caused by a variety of factors, including very less tumor-specific targets available for designing CARs, the depletion of T cells during trafficking in vivo, CAR T cell functional impairment, and inhibition of tumor microenvironment. Among them, the tumor microenvironment has an essential effect on the activity, persistence, and migration of T cells (Kershaw et al., 2013). Therefore, improving tumor microenvironment and maintaining T cell activity may promote the antitumor effects of CAR T cells.
Immunomodulatory drugs (IMiDs) such as thalidomide and its analogs have shown antitumor activity in hematological and solid tumors (Engelhardt et al., 2014; Holstein and McCarthy, 2017). These compounds function in part by inhibiting tumor angiogenesis (Dredge et al., 2002; Dredge et al., 2005). Moreover, thalidomide and its analogs are effective co-stimulators of T cell activation, which can increase cytokine secretion (Payvandi et al., 2005).
Pomalidomide (CC-4047, Actimid; Celgene Corporation, Warren, NJ, USA) is a third-generation immunomodulatory drug. It can not only regulate T cell function but also improve the tumor microenvironment (Li et al., 2013; Gandhi et al., 2014). However, there are few reports about the combination of pomalidomide and CAR T cells in the treatment of solid tumors. In this study, CD133, which is a defined cancer stem cell marker, and HER2, which is over-expressed on many solid tumors, were chosen as the targets on tumor cells. Two third-generation CAR T cells (Zhu et al., 2015), which were specific to CD133 or HER2, were used as effector cells. U251 CD133-OE luc (human glioma cell transfected with firefly luciferase gene and overexpressing CD133) or MDA-MB-453 luc (human breast cancer cell expressing firefly luciferase) was chosen as the corresponding target cell. We investigated the regulatory effect of pomalidomide on the functions of CD133- and HER2-CAR T cells against solid tumor cells in vitro. Pomalidomide was found to significantly enhance the cytotoxicity and cytokine secretion of both CAR T cells.
Frozen human peripheral blood mononuclear cells (PBMCs) from healthy donors were purchased from ALLCELLS (PB005F, Alameda, CA, USA). The glioma cell line U251 (named as U251-WT) and the human breast cancer cell lines MDA-MB-453 and MDA-MB-468 were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). U251-WT was nucleofected with a piggyBac transposon plasmid encoding human CD133 protein and a piggyBac transposase vector to obtain U251 CD133-OE that overexpressed CD133 through drug selection. U251 CD133-OE was nucleofected again with a transposon plasmid encoding firefly luciferase and a piggyBac transposase vector to acquire U251 CD133-OE luc that express luciferase. Similarly, U251-WT, MDA-MB-453, and MDA-MB-468 were nucleofected with a transposon plasmid encoding the firefly luciferase and a piggyBac transposase vector to obtain U251-WT luc, MDA-MB-453 luc, and MDA-MB-468 luc (Hu et al., 2019). The method for preparing CD133-CAR or HER2-CAR T cells has been described in detail in our previous work (Zhu et al., 2015). Briefly, the piggyBac transposon plasmid expressing CD133-CAR or HER2-CAR and the piggyBac transposase plasmid were co-delivered into PBMC, and CD133-CAR T cells or HER2-CAR T cells were obtained through drug selection and T cell expansion. The HER2-CAR expression vector was constructed based on the CD133-CAR piggyBac transposon plasmid. Through gene synthesis and conventional molecular cloning methods, the anti-CD133 scFv in CD133-CAR was replaced with the scFv derived from the anti-HER2 (clone 4D5) antibody (Morgan et al., 2010). The control cells were nontransfected T (NT) cells, and their preparation methods were similar to CAR T cells, but the steps of nucleofection and drug selection were avoided. Pomalidomide was purchased from MCE (HY-10984, Monmouth Junction, NJ, USA) and dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mM and stored at −80°C.
U251 CD133-OE luc (or U251-WT luc) tumor cells were seeded into 96-well white microplates (PerkinElmer, Waltham, MA, USA) at 104 cells per well. According to different effector to target ratios, CD133-CAR T cells were added. At the same time, pomalidomide was added (final concentrations were 0.1 μM, 1 μM, and 10 μM, respectively) into the wells, and the co-culture volume was 200 μL per well. After co-culture in T cell culture medium for 72 h, 0.75 mg/mL D-luciferin K+ salt (PerkinElmer, 122799) was added, and the signal was detected using an EnSpire Multimode plate reader (PerkinElmer). The percentage of target cell death was calculated according to the following formula: Lysis (%) = (1 − fluorescence value of co-culture well/fluorescence value of tumor cell alone well) × 100. Similarly, MDA-MB-453 luc (or MDA-MB-468 luc) tumor cells were seeded into plates at 104 cells/well, and pomalidomide and HER2-CAR T cells were added. After 24 h of incubation, the luciferase substrate was added to detect tumor signals, and then the killing rate was calculated.
105 tumor cells (U251 CD133-OE luc or MDA-MB-453 luc) were seeded in a 96-well plate, and CD133- or HER2-CAR T cells were added at an effector-to-target ratio of 2:1. Pomalidomide was further added at a final concentration of 1 μM and with a co-culture volume of 200 μL for 24 h. The supernatant was collected, and the cytokine concentration was measured according to the instructions of AlphaLISA kits from PerkinElmer (IL-2, AL221C; IFN-γ, AL217C; TNF-α, AL208C; GM-CSF, AL216C). For the cytokine secretion assay of NT cells, 2 × 105 NT cells were seeded into a 96-well plate, and T Cell TransAct™ human (Miltenyi Biotec GmbH, Cologne, NRW, Germany) was used as an activator according to the instructions from the manufacturer and pomalidomide was added. After 24 h of incubation, the supernatant was collected and tested. The concentration of each cytokine was expressed as the mean of three replicates.
T cells (HER2-CAR T cells, CD133-CAR T cells, or NT cells) were labeled with 0.5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; 65-0850-84, Thermo Fisher Scientific, Waltham, MA, USA) following the instruction for the use of CFSE. In the CAR T cell proliferation experiments, target cells (MDA-MB-453 luc or U251 CD133-OE luc) were irradiated with 70 Gy X-ray (RS2000PRO, Rad Source Technologies, Buford, GA, USA). The CFSE-labeled CAR-T cells (HER2-CAR or CD133-CAR) and the corresponding irradiated tumor cells (5 × 105 cells/well) were plated according to an effector to target ratio of 2:1 with a total volume of 1 mL, and pomalidomide (1 μM) was added. After four days, cells were washed with FACS buffer (PBS containing 0.5% BSA and 2 mM EDTA), centrifuged, and resuspended in FACS buffer. Finally, CFSE signal intensity was analyzed on a CytoFLEX instrument (Beckman Coulter, Fullerton, CA, USA) by flow cytometry. NT cells were labeled with CFSE and seeded into a 24-well plate at 1 × 106 cells/well. T Cell TransAct™ human as the activator, and pomalidomide, were added. Four days later, NT cell proliferation was analyzed by flow cytometry.
NT cells, HER2-CAR T cells, and CD133-CAR T cells were seeded into a 24-well plate at 2 × 106 cells per well, and different concentration of pomalidomide (1 μM or 10 μM) was added. NT cells were divided into unstimulated and activated groups. In the activated group, NT cells were activated by T Cell TransAct™ human. After 48 h, all the cells were collected, washed once with PBS, and then sonicated three times (5 seconds per time). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate the proteins in the samples. Then the proteins were transferred onto a membrane using the semi-dry method (15 V, 30 min). After blocking with 5% BSA for 2 h, the membranes were incubated with anti-Aiolos (NBP2-24495SS, Novus, USA), anti-Ikaros (sc-398265, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-GAPDH (sc-32233, Santa Cruz Biotechnology) antibodies at 4°C overnight. Finally, the membranes were incubated with peroxidase-conjugated AffiniPure Donkey Anti-Rabbit IgG (H + L) (JAC-711-035-152, Jackson, USA) or peroxidase-conjugated AffiniPure Donkey anti-mouse IgG (H + L) (JAC-715-035-150, Jackson, USA) at room temperature for 1 h. The target protein signal was detected on Biorad Imager (Bio-Rad, Hercules, CA, USA).
The calculation of the killing efficiency and the mean value of cytokine concentration was done using Excel. GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA) was used to do statistical analysis. Multiple t-tests -one per row was used to analyze the data from cytotoxicity and cytokine secretion assay, and p < 0.05 was considered statistically significant. Cell proliferation results were analyzed using FlowJo V-10 (BD Biosciences, Bedford, MA, USA). All experiments were repeated at least three times.
Pomalidomide enhanced the functions of CD133-CAR T cells against glioma cells
To investigate the effect of pomalidomide on the cytotoxicity of CD133-CAR T cells against tumor cells, CD133-CAR T cells were co-cultured with U251 CD133-OE luc tumor cells according to different effector to target ratios, and pomalidomide (0.1 μM, 1 μM, 10 μM) was added. After 72 h, the killing efficiency was determined by measuring the fluorescence intensity of tumor cells in each group. At the same pomalidomide concentration, the killing rate of CD133-CAR T cells increased with the increase of the effector to target ratio (E:T). When E:T was 2:1, the killing efficiency of each concentration of the pomalidomide group was significantly improved (Fig. 1a) compared with the DMSO group. When E:T was 1:1 or 1:2, only a lower concentration of pomalidomide (0.1 μM or 1 μM) increased the killing efficiency of CD133-CAR T cells. When E:T was constant, 1 μM pomalidomide had the strongest regulatory effect on the cytotoxicity of CD133-CAR T cells. In addition, if U251-WT luc (glioma cells with low expression of CD133 antigen) as target cells were co-cultured with CD133-CAR T cells, pomalidomide did not affect the killing function of CAR T cells, which means that pomalidomide does not induce non-specific killing activities from CD133-CAR T cells in the case that T cells are not activated.
To further study the effect of pomalidomide on the cytokine secretion of CD133-CAR T cells, U251 CD133-OE luc tumor cells and CD133-CAR T cells were co-cultured in a plate. And 1 μM pomalidomide was added. After 24 h, the supernatant was taken to detect the concentration of cytokines (IFN-γ, IL-2, TNF-α, and GM-CSF) secreted by CD133-CAR T cells according to the method provided by the AlphaLISA kits. There was no significant difference between the DMSO group and the R10 group (culture medium control). However, pomalidomide can significantly promote the secretion of four cytokines from CD133-CAR T cells (Fig. 1b).
To determine whether pomalidomide affected the proliferation of CD133-CAR T cells, CFSE-labeled CD133-CAR T cells and irradiated U251 CD133-OE luc cells were seeded into a plate at an effector to target ratio of 2:1, and 1 μM pomalidomide was added. After four days of co-culture, the signal intensity of CFSE in CD133-CAR T cells was detected by flow cytometry. As shown in Fig. 1c, the peak of CFSE in the DMSO group shifted slower than the R10 group to the left, and the median value was higher, which means DMSO may inhibit the proliferation of CD133-CAR T cells. Compared with the DMSO group, the peak value of the CFSE signal in the pomalidomide group shifted significantly to the left, and the median decreased. Therefore, pomalidomide could promote the proliferation of CD133-CAR T cells.
Pomalidomide also increased the activity of HER2-CAR T cells
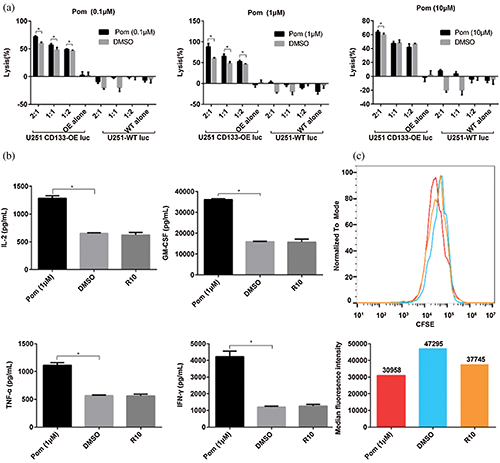
Figure 1: Pomalidomide enhanced CD133-CAR T cell function. (a) CD133-CAR T cells were co-cultured with U251 CD133-OE luc tumor cells or U251-WT luc tumor cells according to different effector to target ratio, and pomalidomide (0.1 μM, 1 μM, or 10 μM) was added. After 72 h, the tumor signal was detected, and the killing rate of CD133-CAR T cells was calculated. Pom, pomalidomide; OE alone, U251 CD133-OE luc alone; WT alone, U251-WT luc alone; *p < 0.05 by multiple t-tests-one per row. (b) CD133-CAR T cells were co-culture with U251 CD133-OE luc tumor cells, and 1 μM pomalidomide or DMSO at equal dilution was added. After 24 h, the supernatant was taken, and the concentrations of four cytokines, including IL-2, TNF-α, GM-CSF, and IFN-γ, were measured according to the instructions of the AlphaLISA kits. *p < 0.05. (c) CFSE-labeled CD133-CAR T cells were co-cultured with irradiated U251 CD133-OE luc tumor cells for four days, then the CFSE signal intensity was detected by flow cytometry. The histogram below shows the median of CFSE in the pomalidomide group (red), DMSO group (blue), or R10 group (orange).
To investigate the effect of pomalidomide on the function of different CAR T cells, we continued to study HER2-CAR T cells.
First, the effect of pomalidomide on the cytotoxicity of HER2-CAR T cells was analyzed. HER2-CAR T cells and MDA-MB-453 luc cells were co-cultured according to two different effector to target ratios
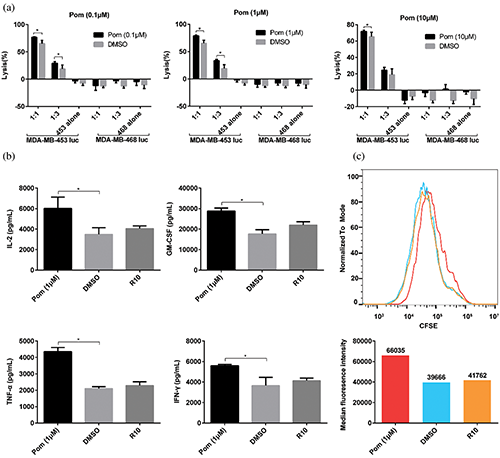
Figure 2: Effect of pomalidomide on HER2-CAR T cell function. (a) HER2-CAR T cells and MDA-MB-453 luc (or MDA-MB-468 luc) tumor cells were co-cultured according to different effector to target ratio, and pomalidomide was added. After 24 h, the killing rate of HER2-CAR T cells was analyzed. 453 alone, MDA-MB-453 luc alone; 468 alone, MDA-MB-468 luc alone; *P < 0.05. (b) HER2-CAR T cells were co-cultured with MDA-MB-453 luc tumor cells for 24 h, and the supernatant was taken to detect the concentrations of four cytokines secreted by HER2-CAR T. *p < 0.05. (c) CFSE-labeled HER2-CAR T cells and irradiated MDA-MB-453 luc tumor cells were co-cultured. After four days, the CFSE signal intensity in the HER2-CAR T cells was detected by flow cytometry. The lower panel shows the median of CFSE intensity from each group, and red, blue, and orange histograms indicate pomalidomide, DMSO, and R10 groups, respectively.
(E:T), and pomalidomide was added. The fluorescence value of tumor cells was detected 24 h later. As shown in Fig. 2a, under the same pomalidomide concentration, the killing efficiency of HER2-CAR T cells increased with the increase of the E:T ratio. At the same E:T ratio, when the compound concentration is 0.1 μM or 1 μM, the killing efficiency of the pomalidomide group is significantly improved compared with the DMSO group; and the killing rate is highest when the pomalidomide concentration is 1 μM. When MDA-MB-468 luc (breast cancer cell with low HER2 antigen expression) was targeted, pomalidomide did not affect the killing function of HER2-CAR T cells.
To know whether pomalidomide affected the cytokine secretion of HER2-CAR T cells, pomalidomide was added to the co-culture of HER2-CAR T cells and MDA-MB-453 luc cells, and the cytokine concentration was measured after 24 h. DMSO did not affect the secretion of IL-2, TNF-α, GM-CSF, and IFN-γ by HER2-CAR T cells. However, when the pomalidomide group was compared with the DMSO group, the levels of all four cytokines secreted by HER2-CAR T cells were significantly increased (Fig. 2b).
In addition, we also analyzed the effect of pomalidomide on the proliferation of HER2-CAR T cells. CFSE-labeled HER2-CAR T cells were co-cultured with irradiated MDA-MB-453 luc cells in a 24-well plate with pomalidomide added. After four days, changes in CFSE signal intensity were detected by flow cytometry. DMSO did not affect the proliferation of HER2-CAR T cells. However, compared with the DMSO group, the pomalidomide group had an inhibitory effect on the proliferation of HER2-CAR T cells (Fig. 2c).
Pomalidomide regulating the function of activated T cells without CAR
To further explore whether the activation way would affect the regulation of pomalidomide on T cells, we also investigated nontransfected T (NT) cells, which did not express CAR on the surface.
First, whether pomalidomide affected cytokine secretion of NT cells was studied. NT cells and T Cell TransAct™ human (Miltenyi Biotec GmbH) as activator were co-cultured in a plate, and pomalidomide was added. After 24 h, cytokine secretion was detected. DMSO still did not affect the cytokine secretion of NT cells, but pomalidomide significantly promoted the secretion of IL-2, TNF-α, GM-CSF, and IFN-γ by NT cells (Fig. 3a).
We then explored the effect of pomalidomide on NT cell proliferation. CFSE-labeled NT cells were cultured in 24-well plates, and the activator and pomalidomide were added. After four days, the signal intensity of CFSE was measured using a flow cytometer. DMSO did not affect the proliferation of NT cells, while pomalidomide inhibited the proliferation of NT cells (Fig. 3b).
Pomalidomide could induce the degradation of Aiolos and Ikaros in CAR T cells
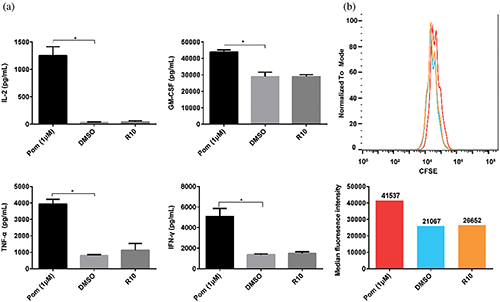
Figure 3: Effect of pomalidomide on the function of nontransfected T (NT) cells, which did not express CAR. (a) NT cells were activated, and pomalidomide was added. After 24 h of culture, the supernatant was taken to detect the concentrations of four cytokines, including IL-2, TNF-α, GM-CSF, and IFN-γ. *p < 0.05. (b) CFSE-labeled NT cells were cultured with pomalidomide for four days after activation. CFSE signals were detected by flow cytometry. The histogram below shows the median CFSE intensity of each group.
Previous mechanism studies have shown that the protease cereblon is one of the targets of pomalidomide. Within T cells, pomalidomide promotes the binding of Aiolos and Ikaros to cereblon, which makes Aiolos and Ikaros ubiquitinated and degraded by the proteasome (Gandhi et al., 2014). To confirm the effect of pomalidomide on the degradation of Aiolos and Ikaros in CAR T cells, NT, CD133-CAR T, or HER2-CAR T cells were cultured with pomalidomide or DMSO for 48 h and then lysed using ultrasound, and Aiolos and Ikaros were detected by western blotting. As shown in Fig. 4, pomalidomide significantly induced Aiolos and Ikaros degradation within the three types of T cells.
In this study, we found that pomalidomide could promote the cytotoxicity of HER2-CAR T cells and CD133-CAR T cells, and the enhancement of the killing was most significant when the concentration of pomalidomide was 1 μM. However, pomalidomide did not affect the killing function of HER2-CAR T cells and CD133-CAR T cells when they were not activated. Pomalidomide also significantly promoted the secretion of IL-2, TNF-α, GM-CSF, and IFN-γ by CD133-CAR T cells, HER2-CAR T cells, and NT cells. Pomalidomide only promoted the proliferation of CD133-CAR T cells, but it inhibited the proliferation of HER2-CAR T cells and NT cells. The reason for this is still to be discovered. Furthermore, we also confirmed that pomalidomide could indeed induce the degradation of Aiolos and Ikaros proteins in NT cells, HER2-CAR T cells, and CD133-CAR T cells.
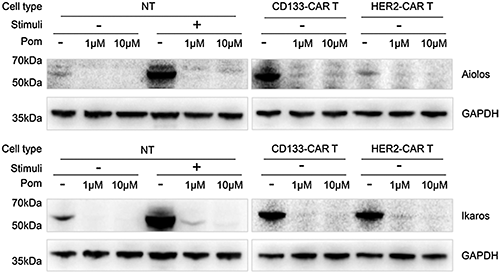
Figure 4: Pomalidomide induced degradation of Aiolos and Ikaros proteins in CAR-T cells. NT cells, CD133-CAR T cells, and HER2-CAR T cells, were treated with 1 μM or 10 μM pomalidomide and cultured for 48 h, respectively. Cells were collected, and the expression of Aiolos, Ikaros, and GAPDH was detected by western blotting. Pom, pomalidomide; Pom−, DMSO; Stimuli +, TransAct-activated cells; Stimuli -, unstimulated cells.
CAR T cells still face many challenges in the treatment of solid tumors, one of which is the inhibitory effect of tumor microenvironment on T cells. The small molecule compound pomalidomide used in our study may improve the tumor microenvironment in many ways. First, pomalidomide may inhibit tumor angiogenesis, affect the polarization status of macrophages, and increase the number of NK cells, which have been reported in lymphoma mouse models (Reddy et al., 2008; Li et al., 2013). Second, previous studies have shown that pomalidomide can directly or indirectly regulate T cells and enhance their functions. For example, increased levels of IL-2 and TNF-α secretion were detected in T cells when human PBMCs (peripheral blood mononuclear cells) were activated by CD3 antibody and treated with pomalidomide in vitro (Marriott et al., 2002). This is consistent with our findings on cytokine secretion. Furthermore, pomalidomide inhibits PD-L1 expression on cancer cells so as to enhance T cell-mediated tumor killing (Fujiwara et al., 2018). Our in vitro results also show that pomalidomide can indeed significantly improve the killing efficiency of CAR T cells to tumor cells. However, pomalidomide was not found to regulate either the PD-L1 expression on the cell lines we used or PD-1 on CAR T cells supplementary data, which meant PD-1/PD-L1 signaling was not the main mechanism in our findings. In addition, pomalidomide promoted the proliferation of CD133-CAR T cells in our study. Therefore, pomalidomide has the potential to improve CAR T cell treatment of solid tumors by restoring the loss of CAR T cell functions, but some questions remain to be answered, such as direct molecular mechanisms related to pomalidomide regulating CAR T cell function and whether pomalidomide will enhance the efficacy of CAR T cells in vivo.
In terms of target research, the protease cereblon has been confirmed as a direct target of pomalidomide by several studies. The combination of pomalidomide and cereblon can promote the recruitment of IL-2 transcriptional repressors Ikaros and Aiolos, resulting in their ubiquitination, further degradation within hours, and increased secretion of IL-2 (Gandhi et al., 2014; Kronke et al., 2014). The effect of pomalidomide on the degradation of Ikaros and Aiolos has also been demonstrated in our western blot experiments.
Pomalidomide’s inhibitory effect on solid tumor cells may also confer CAR T cells treating solid tumors with better results. Pomalidomide can be used in combination with other drugs to enhance the inhibition of solid tumor cells. In vitro studies showed that the combined use of pomalidomide and docetaxel could enhance docetaxel’s killing effect on prostate tumor cell lines (Zhu et al., 2008). The combination therapy of pomalidomide and other drugs has been validated in mouse models. Pomalidomide could enhance the activity of gemcitabine/nab-paclitaxel against pancreatic cancer cells in vitro and in vivo (Saito et al., 2018). In the mice with orthotopic pancreatic cancer xenograft, the combination of Gem/S1 (gemcitabine and S1) and pomalidomide could also improve the antitumor effect compared with monotherapy (Shirai et al., 2018). That provides directions and possibilities for adding additional drugs in our future research.
More importantly, pomalidomide has been used in many clinical trials. In phase 1 clinical trial, pomalidomide in combination with gemcitabine was given to patients with metastatic pancreatic cancer, and serum CA 19-9 levels in 10 patients were reduced by at least 50% (Infante et al., 2011). In terms of hematological malignancies, the combination of pomalidomide and low-dose dexamethasone in clinical studies could extend the average overall survival (OS) of patients with relapsed/refractory multiple myeloma (MM), and the combination is generally well tolerated (Richardson et al., 2014; Dimopoulos et al., 2016; Dimopoulos et al., 2018).
The above research shows that pomalidomide can not only directly inhibit tumor growth but also regulate T cell function. Further, it has been used in treating solid tumors in vitro, in mice, and in clinical studies with certain safety. Moreover, our results also show that when pomalidomide is used in combination with CD133-CAR T and HER2-CAR T cells, respectively, it can increase the secretion of cytokines in both CAR T cells and significantly improve their cytotoxicity against tumor cells. Therefore, the combination of pomalidomide and CAR T cells to improve the efficacy of CAR T cells against solid tumors is expected to be applied to mice experiments and clinical trials in future studies.
Acknowledgement: We would like to thank the HTS Platform in SIAIS for the technical assistance.
Availability of Data and Materials: The data used to support the findings of this study are available from the corresponding author upon request.
Funding Statement: This work was supported by the National Key R&D Program (2019YFA0111001) of China. URL: http://www.cncbd.org.cn/Index/. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Dimopoulos M, Weisel K, van de Donk N, Ramasamy K, Gamberi B, Streetly M, Offidani M, Bridoux F, de la Rubia J, Mateos MV, Ardizzoia A, Kueenburg E, Collins S, Di Micco A, Rosettani B, Li Y, Bacon P, Sonneveld P. (2018). Pomalidomide plus low-dose dexamethasone in patients with relapsed/refractory multiple myeloma and renal impairment: results from a phase II trial. Journal of Clinical Oncology 36: 2035–2043. DOI 10.1200/JCO.2017.76.1742. [Google Scholar] [CrossRef]
Dimopoulos MA, Palumbo A, Corradini P, Cavo M, Delforge M, Di Raimondo F, Weisel KC, Oriol A, Hansson M, Vacca A, Blanchard MJ, Goldschmidt H, Doyen C, Kaiser M, Petrini M, Anttila P, Cafro AM, Raymakers R, San-Miguel J, de Arriba F, Knop S, Rollig C, Ocio EM, Morgan G, Miller N, Simcock M, Peluso T, Herring J, Sternas L, Zaki MH, Moreau P. (2016). Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010A phase 3b study in refractory multiple myeloma. Blood 128: 497–503. DOI 10.1182/blood-2016-02-700872. [Google Scholar] [CrossRef]
Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, Shirley MA, Muller G, Schafer P, Stirling D, Dalgleish AG, Bartlett JB. (2005). Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvascular Research 69: 56–63. DOI 10.1016/j.mvr.2005.01.002. [Google Scholar] [CrossRef]
Dredge K, Marriott JB, Macdonald CD, Man HW, Chen R, Muller GW, Stirling D, Dalgleish AG. (2002). Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. British Journal of Cancer 87: 1166–1172. DOI 10.1038/sj.bjc.6600607. [Google Scholar] [CrossRef]
Engelhardt M, Wäsch R, Reinhardt H, Kleber M. (2014). Pomalidomide. In: Martens U. eds., Small Molecules in Oncology. Recent Results in Cancer Research. vol. 201. Berlin, Heidelberg: Springer, 359–372. [Google Scholar]
Fujiwara Y, Sun Y, Torphy TJ, He J, Yanaga K, Edil BH, Schulick RD, Zhu Y. (2018). Pomalidomide inhibits PD-L1 induction to promote antitumor immunity. Cancer Research 78: 6655. DOI 10.1158/0008-5472.CAN-18-1781. [Google Scholar] [CrossRef]
Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, Ito T, Ando H, Waldman MF, Thakurta A, Klippel A, Handa H, Daniel TO, Schafer PH, Chopra R. (2014). Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4CRBN. British Journal of Haematology 164: 811–821. DOI 10.1111/bjh.12708. [Google Scholar] [CrossRef]
Holstein SA, McCarthy PL. (2017). Immunomodulatory drugs in multiple myeloma: Mechanisms of action and clinical experience. Drugs 77: 505–520. DOI 10.1007/s40265-017-0689-1. [Google Scholar] [CrossRef]
Hu B, Zou Y, Zhang L, Tang J, Niedermann G, Firat E, Huang X, Zhu X. (2019). Nucleofection with plasmid DNA for CRISPR/Cas9-mediated inactivation of programmed cell death protein 1 in CD133-specific CAR T cells. Human Gene Therapy 30: 446–458. DOI 10.1089/hum.2017.234. [Google Scholar] [CrossRef]
Infante J R, Jones S F, Bendell J C, Spigel D R, Yardley D A, Weekes C D, Messersmith W A, Hainsworth J D, Burris H AIII. (2011). A phase I, dose-escalation study of pomalidomide (CC-4047) in combination with gemcitabine in metastatic pancreas cancer. European Journal of Cancer 47: 199–205. DOI 10.1016/j.ejca.2010.09.002. [Google Scholar] [CrossRef]
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. (2018). CAR T cell immunotherapy for human cancer. Science 359: 1361–1365. DOI 10.1126/science.aar6711. [Google Scholar] [CrossRef]
Kershaw MH, Westwood JA, Darcy PK. (2013). Gene-engineered T cells for cancer therapy. Nature Reviews Cancer 13: 525–541. DOI 10.1038/nrc3565. [Google Scholar] [CrossRef]
Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, Hartman E, Munshi N, Schenone M, Schreiber SL, Carr SA, Ebert BL. (2014). Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343: 301–305. DOI 10.1126/science.1244851. [Google Scholar] [CrossRef]
Li Z, Qiu Y, Personett D, Huang P, Edenfield B, Katz J, Babusis D, Tang Y, Shirely MA, Moghaddam MF, Copland JA, Tun HW. (2013). Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PLoS One 8: e71754. DOI 10.1371/journal.pone.0071754. [Google Scholar] [CrossRef]
Marriott JB, Clarke IA, Dredge K, Muller G, Stirling D, Dalgleish AG. (2002). Thalidomide and its analogues have distinct and opposing effects on TNF-α and TNFR2 during co-stimulation of both CD4+ and CD8+ T cells. Clinical & Experimental Immunology 130: 75–84. DOI 10.1046/j.1365-2249.2002.01954.x. [Google Scholar] [CrossRef]
Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy 18: 843–851. DOI 10.1038/mt.2010.24. [Google Scholar] [CrossRef]
Payvandi F, Wu L, Naziruddin SD, Haley M, Parton A, Schafer PH, Chen RS, Muller GW, Hughes CC, Stirling DI. (2005). Immunomodulatory drugs (IMiDs) increase the production of IL-2 from stimulated T cells by increasing PKC-θ activation and enhancing the DNA-binding activity of AP-1 but not NF-κB, OCT-1, or NF-AT. Journal of Interferon & Cytokine Research 25: 604–616. DOI 10.1089/jir.2005.25.604. [Google Scholar] [CrossRef]
Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, Roth M, Vaughn M, Knight J, Wallace P, Czuczman MS. (2008). Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. British Journal of Haematology 140: 36–45. [Google Scholar]
Richardson PG, Siegel DS, Vij R, Hofmeister CC, Baz R, Jagannath S, Chen C, Lonial S, Jakubowiak A, Bahlis N, Song K, Belch A, Raje N, Shustik C, Lentzsch S, Lacy M, Mikhael J, Matous J, Vesole D, Chen M, Zaki MH, Jacques C, Yu Z, Anderson KC. (2014). Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: A randomized phase 2 study. Blood 123: 1826–1832. DOI 10.1182/blood-2013-11-538835. [Google Scholar] [CrossRef]
Sadelain M, Brentjens R, Riviere I. (2013). The basic principles of chimeric antigen receptor design. Cancer Discovery 3: 388–398. DOI 10.1158/2159-8290.CD-12-0548. [Google Scholar] [CrossRef]
Saito N, Shirai Y, Uwagawa T, Horiuchi T, Sugano H, Haruki K, Shiba H, Ohashi T, Yanaga K. (2018). Pomalidomide enhanced gemcitabine and nab-paclitaxel on pancreatic cancer both in vitro and in vivo. Oncotarget 9: 15780–15791. DOI 10.18632/oncotarget.24608. [Google Scholar] [CrossRef]
Shirai Y, Saito N, Uwagawa T, Shiba H, Horiuchi T, Iwase R, Haruki K, Ohashi T, Yanaga K. (2018). Pomalidomide promotes chemosensitization of pancreatic cancer by inhibition of NF-κB. Oncotarget 9: 15292–15301. DOI 10.18632/oncotarget.24577. [Google Scholar] [CrossRef]
Zhu D, Corral LG, Fleming YW, Stein B. (2008). Immunomodulatory drugs Revlimid® (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunology, Immunotherapy 57: 1849–1859. DOI 10.1007/s00262-008-0512-7. [Google Scholar] [CrossRef]
Zhu X, Prasad S, Gaedicke S, Hettich M, Firat E, Niedermann G. (2015). Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget 6: 171–184. DOI 10.18632/oncotarget.2767. [Google Scholar] [CrossRef]
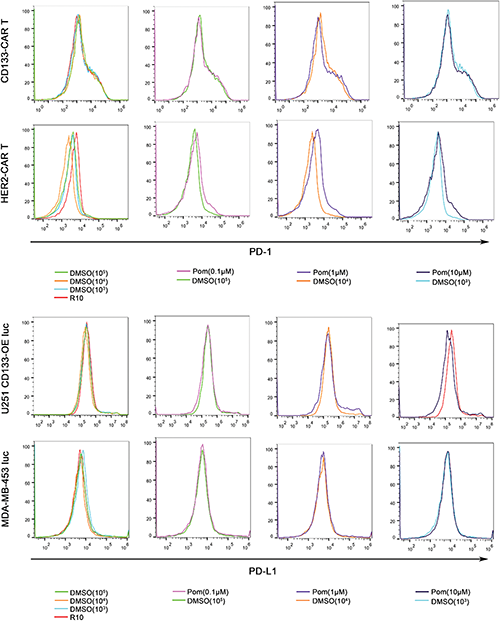
SUPPLEMENTARY FIGURE: Pomalidomide has no significant effect on the expression of PD-1 on CAR T cells and PD-L1 on tumor cells. (Top) CD133 CAR T cells and HER2-CAR T cells were activated with U251 CD133-OE luc and MDA-MB-453 luc cells, respectively. Meanwhile, pomalidomide was added, and DMSO or culture medium (R-10) was used as control. After two days, PD-1 on T cells was detected by flow cytometry using an anti-PD-1 antibody (PE Mouse Anti-Human CD279, BD Pharmingen). (Bottom) U251 CD133-OEluc or MDA-MB-453 luc was treated with pomalidomide, DMSO, or culture medium (D-10) for two days. Cells were stained with an anti-PD-L1 antibody (PE Mouse Anti-Human CD274, BD Pharmingen), and then detected by flow cytometry.
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |