2021 45(1): 129-138
DOI:10.32604/biocell.2021.013275

www.techscience.com/journal/biocell
| BIOCELL 2021 45(1): 129-138 DOI:10.32604/biocell.2021.013275 |  www.techscience.com/journal/biocell |
Upregulated IRF9 promotes cell apoptosis of hyperlipidemia acute pancreatitis with heart injury by regulating SIRT1
Department of Intensive Care Unit, The Second Affiliated Hospital of Anhui Medical University, Hefei, 230601, China
*Address correspondence to: Weili Yu, ywl7026@mail.ustc.edu.cn
Received: 31 July 2020; Accepted: 25 September 2020
#These authors contributed equally to this work
Abstract: Hyperlipidemia acute pancreatitis (HLAP) is a significant cause of AP, characterized by recurrent attacks, more complications and high incidence and mortality. HLAP is often accompanied by single or multiple organ damage. Negative regulation of interferon-regulatory factor 9 (IRF9) on sirtuin-1 (SIRT1) contributes to a range of diseases. However, the function of IRF9 and SIRT1, and the relationship of the two in HLAP with heart injury remain to be illustrated so far. Animal models of HLAP were set up by feeding with high-fat chow and subsequently injecting 20% L-arginine intraperitoneally. The degree of pancreas and heart tissue injury was evaluated. Heart cell apoptosis was assayed by the TUNEL technique. IRF9, SIRT1, p53 and acetylated p53 (Ac-p53) expression levels were measured by qRT-PCR and western blot. The correlation between SIRT1 and IRF9 expression levels was analyzed. Results showed that the damage degree in rat pancreas and heart tissues of AP and HLAP group was more distinctly and heart cell apoptosis was elevated. Pancreas, heart injury and cell apoptosis of the HLAP group were more remarkable than that of the AP group. Apparent increases of IRF9 and Ac-p53/p53 expression and a marked drop of SIRT1 expression were observed in the AP and HLAP group rather than NC and HLNC group. IRF9 and Ac-p53/p53 expression levels of the HLAP group were markedly raised compared with the AP group. SIRT1 expression of the HLAP group was obviously lower than that of the AP group. There was an inverse correlation between the decrease of SIRT1 and the increase of IRF9 in AP and HLAP groups. Based on the above findings, we drew a conclusion that in pancreatitis with heart injury, upregulated IRF9 inhibited SIRT1 expression, elevated the acetylation of p53, and increased myocardial cell apoptosis. Hyperlipidemia further exacerbated pancreas and heart injury and accelerated myocardial cell apoptosis. These results would furnish an experimental and theoretical basis to diagnose and therapy diseases.
Keywords: IRF9; SIRT1; HLAP; Cardiac injury; Apoptosis
Abbreviations
| HLAP: | hyperlipidemia acute pancreatitis |
| AP: | acute pancreatitis |
| IRF9: | interferon-regulatory factor 9 |
| SIRT1: | sirtuin-1 |
| ISGs: | interferon-stimulated genes |
| I/R: | ischemia and reperfusion |
| AML: | acute myeloid leukemia |
| NC: | normal control |
| HLNC: | hyperlipidemia normal control |
| SD: | Sprague-Dawley |
| TG: | triglycerides |
| CK-MB: | creatine kinase MB isoenzyme |
| LDH: | lactate dehydrogenase |
| IL-1β: | interleukin-1β |
| TNF-α: | tumor necrosis factor α |
| HE: | hematoxylin and eosin |
| Ac-p53: | acetylated p53 |
| qRT-PCR: | quantitative real-time PCR |
| FFA: | free fatty acids |
Acute pancreatitis (AP) is an acute inflammatory disorder characterized by the excessive activation and self-digestive of trypsin, usually associated with several local and systemic complications (Johnson et al., 2014). The incidence of AP is increasing around the world, and the death rate of AP is about 10%–15%. The major cause of high incidence and death rate of AP is systemic inflammatory response syndrome and multiple organ dysfunctions (Johnson et al., 2014).
Hyperlipidemia is also another noteworthy cause of AP following gallstones and alcohol and accounts for up to 10% of total cases (Valdivielso et al., 2014; Ewald et al., 2009; Papachristou et al., 2017). Especially in China, the incidence rate has reached 15%–20% (Forsmark et al., 2016). Previous reports have suggested that up to 26% of patients with hyperlipidemia recurrent attack AP in their lifetime (Lloret Linares et al., 2008; Carr et al., 2016). Some studies have shown that hyperlipidemia can cause a more severe systemic inflammatory response and multiple organ dysfunctions in AP patients (Zhou et al., 2015). Compared with other types of AP, hyperlipidemia acute pancreatitis (HLAP) is characterized by more complications and a higher recurrence rate.
At all stages of AP, the occurrence of heart injury may be alone or along with other organ systems. The common cardiac manifestations, including arrhythmia, cardiogenic shock, toxic myocarditis, myocardial infarction, pericarditis, and hydropericardium are frequent among the multi-organ system dysfunctions in AP. Electrocardiographic and echocardiographic changes have been reported in approximately 50% of AP patients (Buch et al., 1980; Khairy and Marsolais, 2001; Bauerlein and Stobbe, 1954; Ates et al., 2005; Pezilli et al., 1999; Rubio-Tapia et al., 2005; Easwaran and Shah, 1987; Pezzilli et al., 1996; Yegneswaran et al., 2011; Meuleman et al., 2011; Albrecht and Laws, 2003; Makaryus et al., 2008, Mautner et al., 1982). Pericardial effusion has also been seen in 14%–48% AP patients (Pezilli et al., 1999; Mautner et al., 1982; Variyam and Shah, 1987; Maringhini et al., 1996). The early onset of a persistent single-organ dysfunction or multiple organ dysfunctions in AP is related to poor outcomes (Yegneswaran et al., 2011).
As a member of the interferon regulatory factor (IRF) family, IRF9 is first discovered as a part of the component named interferon-stimulated gene factor 3 (ISGF3) (Fu et al., 1990). IRF9 is best recognized as a transcription factor that participates in the regulation of downstream ISGs expressions (Veals et al., 1992; Improta et al., 1994). IRF9 also performs a major function in the modulation of cell differentiation, proliferation and apoptosis, inflammation process, immune cell regulation, tumor formation and ischemia and reperfusion (I/R) (Wan et al., 2018; Smith et al., 2017; Huber et al., 2017; Luker et al., 2001; Wang et al., 2015; Zhang et al., 2014; Chen et al., 2014; Zhang et al., 2014; Tian et al., 2018; Jiang et al., 2014; Rauch et al., 2015).
Sirtuin-1 (SIRT1), also known as an NAD+-dependent histone deacetylase, plays an important role in regulating glucose-lipid metabolism, oxidative stress, cellular senescence/aging and apoptosis, DNA damage, and tumor formation (Chalkiadaki and Guarente, 2015). Previous reports have shown that the expression of SIRT1 is down-regulated by IRF9 in the process of I/R injury, vascular injury, subarachnoid hemorrhage, and acute myeloid leukemia (AML) (Wan et al., 2018; Wang et al., 2015; Zhang et al., 2014; Chen et al., 2014; Tian et al., 2018; Zhang et al., 2014). IRF9 inhibits the activity of SIRT1, elevates the expression of acetylated proteins (such as p53, NF-κB) in the downstream, thus mediating cell apoptosis and proliferation in response to I/R injury (Wan et al., 2018; Wang et al., 2015; Zhang et al., 2014; Chen et al., 2014). And our previous researches have shown that IRF9 functions as an inhibitor to suppress SIRT1 in AP and HLAP with kidney injury (Liu et al., 2020). Nevertheless, the function of IRF9 and SIRT1 as well as the relationship of the two in HLAP with heart injury remain to be elucidated until now. This research is aimed to expound on the function of IRF9 and SIRT1, and whether the negative connection of IRF9 and SIRT1 also exists in HLAP with heart injury.
Male Sprague-Dawley (SD) rats used in this study, weighing approximately 300–350 g, were purchased from the experimental animal center of Anhui medical university. Animals were raised under the laboratory condition (a standard circadian rhythm at 22 ± 2°C, given water and standard laboratory chow ad libitum) for a minimum of one week to acclimate the condition. The experimental protocol was approved by the Animal Ethics Committee of Anhui Medical University.
Experimental design and procedures
A hyperlipidemia rat model was developed by high-fat diet feeding. SD rats were randomly assigned to 4 groups: normal control (NC, n = 10), acute pancreatitis (AP, n = 10), hyperlipidemia normal control (HLNC, n = 10) and hyperlipidemia acute pancreatitis (HLAP, n = 10). As described in Tab. 1, HLNC and HLAP group were feed with a high-fat chow. NC and AP groups were given a normal chow. After 12 weeks, all rats were fasted for 12 h feeding before the AP model was established. Then animals of the AP and HLAP groups were treated with an intraperitoneal administration of 20% L-arginine (2000 mg/kg) (Solarbio, Beijing, China), and animals of the NC and HLNC group were treated with normal saline (Nanjing JianCheng Biotechnology Co. Ltd., Nanjing, Jiangsu, China) in the same way. Blood samples were obtained from the tail veins after 24 h injection. Sodium pentobarbital (40 mg/kg) (Sigma, USA) was injected intraperitoneally to anesthetize the animals. Subsequently, all rats were sacrificed, pancreas tissues and heart tissues were collected and fixed with 4% paraformaldehyde in PBS and embedded by paraffin.
Table 1: Compositions of the experimental chow
Rat blood was centrifuged at 8000×g for 20 min at 4°C to obtain supernatants for following analysis. The levels of triglycerides (TG), lipase, amylase, creatine kinase MB isoenzyme (CK-MB), and lactate dehydrogenase (LDH) were determined with commercial kits (Nanjing JianCheng Biotechnology Co. Ltd., Nanjing, Jiangsu, China) according to the manufacturer’s instruction.
Measurement of serum inflammatory cytokines levels
Inflammatory cytokines levels, including interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), were detected respectively with ELISA Kits (Nanjing JianCheng Biotechnology Co. Ltd., Nanjing, Jiangsu, China) according to the manufacturer’s instructions.
Paraffin blocks of the pancreas and heart tissues were sectioned and stained with hematoxylin and eosin (HE). The injury degree of the pancreas and heart tissues was observed by light microscopes. Two blinded investigators with expertise scored the pathological change of pancreas and heart tissues for evaluating the severity of inflammation, edema, and necrosis, as described previously in Tabs. 2 and 3 (Schmidt et al., 1992; Tanwar et al., 2010).
Table 2: Histopathological criteria and grading severity score of AP

Table 3: Histopathological criteria and grading severity score of heart injury
Total proteins were extracted from rat heart tissues with a protein extraction kit (Beyotime, Shanghai, China). The concentration of proteins was determined using BCA Protein Assay Kit (Beyotime, Shanghai, China). Equal amounts of protein samples were separated by 10% SDS-PAGE gel electrophoresis (Beyotime, Shanghai, China) and then transferred onto a 0.45 μm PVDF membrane (Millipore, USA). Subsequently, membranes were blocked with 5% milk for 2 h and then incubated overnight at 4°C with anti-rat antibodies (anti-IRF9, anti-SIRT1, anti-p53, anti- Ac-p53, anti-β-actin) (Abcam, USA). Next, membranes were incubated with anti-rabbit antibodies (Abcam, USA). After TBST washing, immunoreactive proteins were detected by the ECL chemiluminescent substrate (Beyotime, Shanghai, China).
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA samples from heart tissues were isolated with TRIzol reagent (Beyotime, Shanghai, China), and cDNA strands were synthesized using the reverse transcription system (Sangon Biotech, Shanghai, China) according to the manufacturer’s instructions. Then qRT-PCR amplifications were quantified using SYBR Green reagent (Vazyme, Nanjing, Jiangsu, China) under the following conditions: 95°C for 3 min, followed by 40 cycles of 95°C for 5 s, 60°C for 15 s, 72°C for 25 s. The primer pairs used in this study were: IRF9 (F: 5’-TTAAGGAAAAGCACAAAGACGG-3’, R: 5’-CCTGCTGGCAGTATTCGATATA-3’), SIRT1 (F: 5’-CAGTTCCAGCCATCTCTGTGTCAC-3’, R: 5’-GATTCCT GCAACCTGCTCCAAGG-3’), GAPDH (F: 5’-GCTGGTG CCGAGTATGTT-3’, R: 5’-CAGAAGGTGCGGAGATGA-3’). The mRNA expression of target genes was normalized to GAPDH gene expression using the 2−ΔΔCt method (Taylor et al., 2019).
The TUNEL staining was performed to detect the apoptosis of heart tissues by using DeadEnd™ Fluorometric TUNEL System (Promega, USA) according to the manufacturer’s protocols as described previously (Liu et al., 2020).
All data analyses were performed using SPSS software (version 18.0), and results were shown as mean ± standard deviation. Statistical differences in various groups were assessed by the t- test. Three biological replicates were performed. P < 0.05 represented a statistically significant difference.
Serum TG, lipase, amylase, CK-MB, LDH, and inflammatory cytokines levels of various groups
As shown by the result of the biochemistry assay in Fig. 1A, serum TG levels were substantially elevated in the HLNC and HLAP group compared with those in NC and AP group (P < 0.05). High-fat diet feeding induced hyperlipidemia model establishment successfully. Serum lipase, amylase, CK-MB, and LDH levels were higher in the rats of AP and HLAP groups than those in NC and HLNC groups, especially highest in the HLAP group (P < 0.05; Figs. 1B, 1C, 1D, and 1E). Results of the ELISA analysis exhibited that serum IL-1β and TNF-α levels were obviously raised in AP and HLAP groups compared with NC and HLNC groups, particularly highest in the HLAP group (P < 0.05, Fig. 1F). 20% L-arginine injection led to significant increases in serum lipase, amylase, CK-MB, LDH, IL-1β, and TNF-α levels of AP and HLAP group (P < 0.05; Figs. 1B–1F).
Pancreatic and cardiac damage evaluation of various groups
Rat tissue samples were stained with hematoxylin and eosin (HE) to detect histopathology features of the pancreas and the heart under an optical microscope. Inflammatory cell infiltration, interstitial tissue edema, and necrosis were evaluated in the tissues of each group of rats. There were almost no obvious pathological changes in the pancreas and heart tissues of the NC group. The HLNC group exhibited a small amount of inflammatory cell infiltration, edema, and necrosis in the pancreas tissues and heart tissues. The pancreas tissues of AP and HLAP groups presented inflammatory cell infiltration, interstitial tissue edema, and necrosis with varying degrees (Fig. 2A). The heart tissues of AP and HLAP groups presented swelling and fracturing of the myocardial fiber and inflammatory cell infiltration (Fig. 2A). The scores of individual histopathological parameters and total pathological scores showed that the damage of the pancreas and heart tissues in the HLNC group was elevated compared with the NC group (Figs. 2B, 2C, 2D, and 2E). The injury degree of the pancreas and heart tissues in AP and HLAP groups was more obvious compared with NC and HLNC groups, especially most obvious in the HLAP group (Figs. 2B–2E).
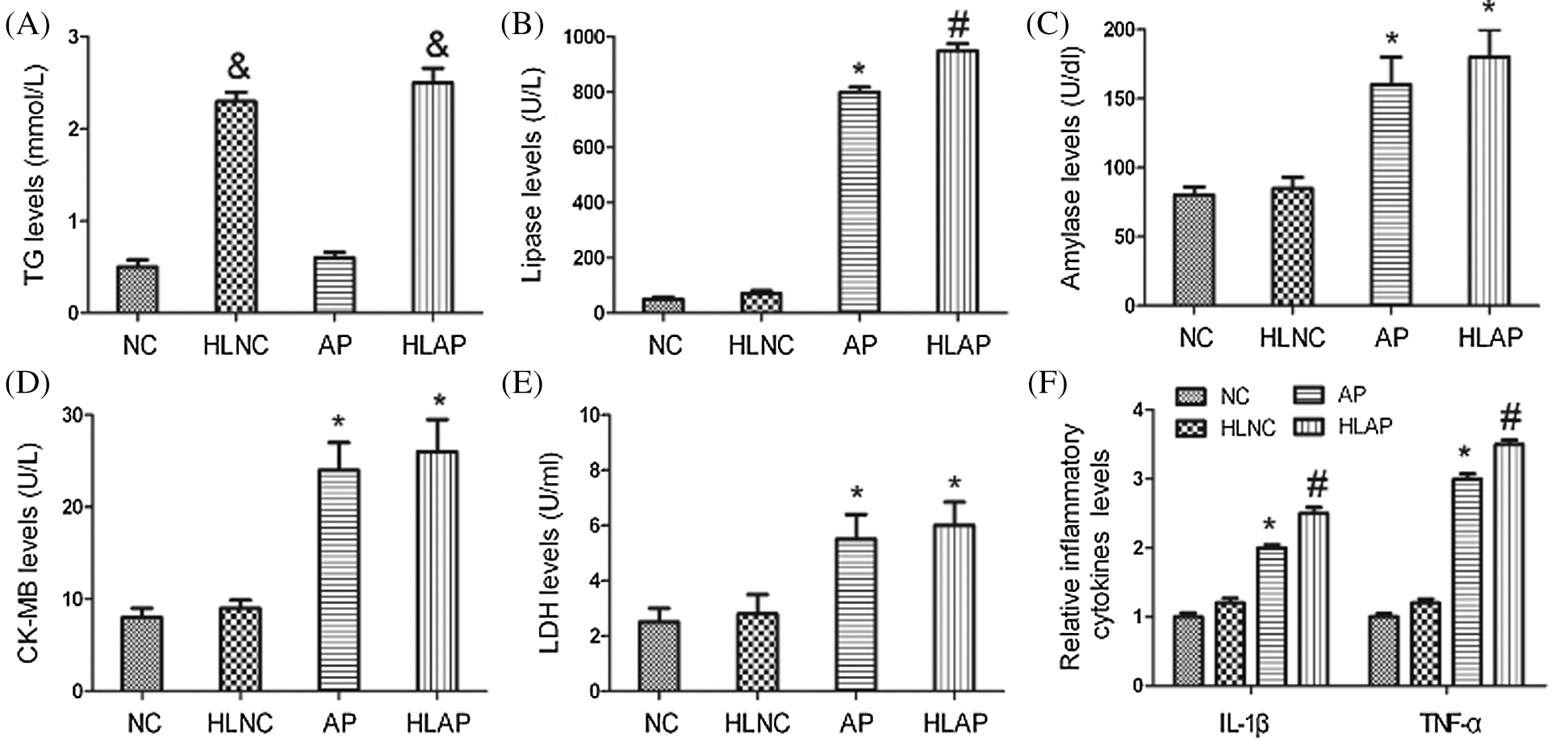
Figure 1: Serum TG, lipase, amylase, CK-MB, LDH, and inflammatory cytokines levels of the rats. TG (A), Lipase (B), Amylase (C), CK-MB (D), LDH (E), and Relative inflammatory cytokines (IL-1β, and TNF-α) (F) levels of various groups. &P < 0.05 vs. NC and AP group. *P < 0.05 vs. NC and HLNC group. #P < 0.05 vs. NC, HLNC, and AP groups.
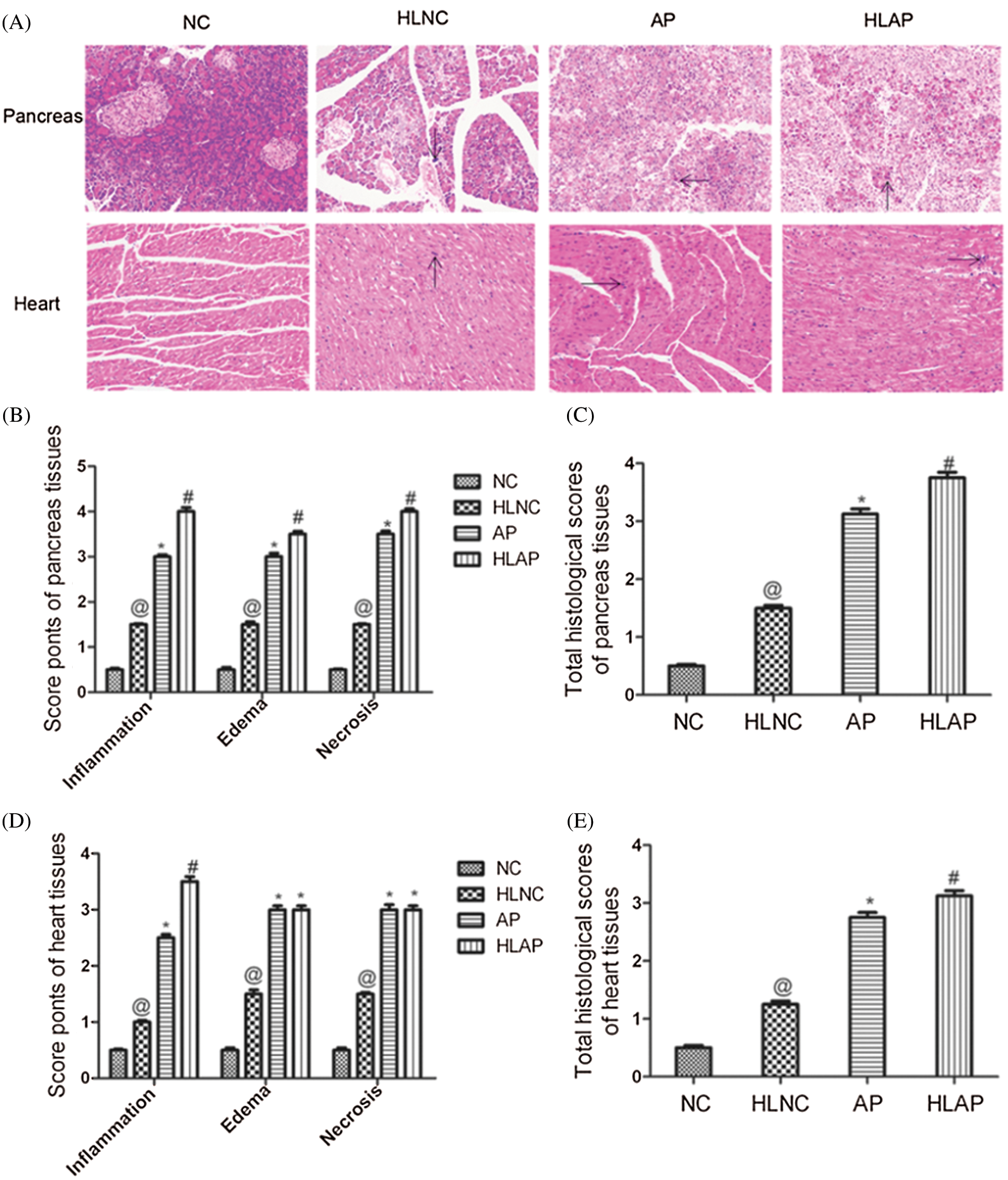
Figure 2: Histopathology features and scores of pancreas and heart tissues of various groups. (A) Histopathology changes of pancreas and heart of various groups (Inflammation was indicated by black arrow signs) scale as 200 μm, (B) Scores of individual histopathological parameters of pancreas tissues in various groups, (C) Total histological scores of pancreas tissues in various groups, (D) Scores of individual histopathological parameters of heart tissues in various groups, (E) Total histological scores of heart tissues in various groups. @P < 0.05 vs. NC group. *P < 0.05 vs. NC and HLNC group. #P < 0.05 vs. NC, HLNC and AP group.
We next analyzed the level of apoptosis in several groups (i.e., NC, HLNC, AP, and HLAP groups). Fig. 3 exhibited that the myocardial cells of NC and HLNC groups were almost normal. Some myocardial cell apoptosis was presented in the AP group. Amounts of myocardial cell apoptosis were also observed in the HLAP group. The myocardial cell apoptosis of the HLAP group was more starkly compared with the AP group (Fig. 3).
IRF9, SIRT1, p53 and Ac-p53 protein expression of heart tissues of various groups
Results in Fig. 4 demonstrated that substantial increases of IRF9, p53, and Ac-p53 expression in AP and HLAP groups were observed, while marked decrease of SIRT1 expression in AP and HLAP groups was discovered compared with NC and HLNC groups (P < 0.05). IRF9, p53 and Ac-p53 expressions of the HLAP group were starkly enhanced than the AP group, while SIRT1 expression of the HLAP group was obviously reduced than the AP group (P < 0.05).
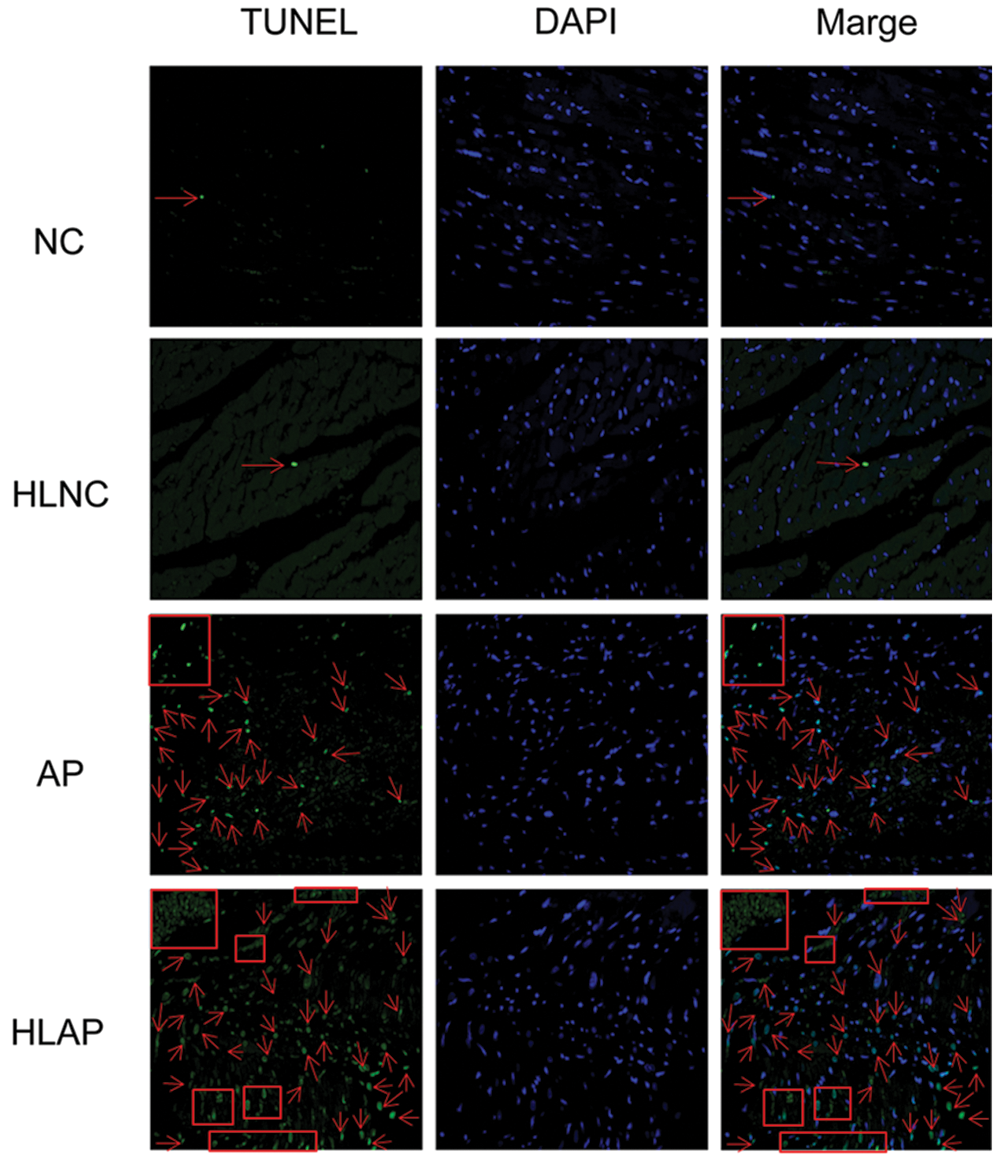
Figure 3: Myocardial cell apoptosis of heart tissues was stained with TUNEL and DAPI (blue). Scale as 200 μm. Cell apoptosis was indicated by red arrow and box signs.
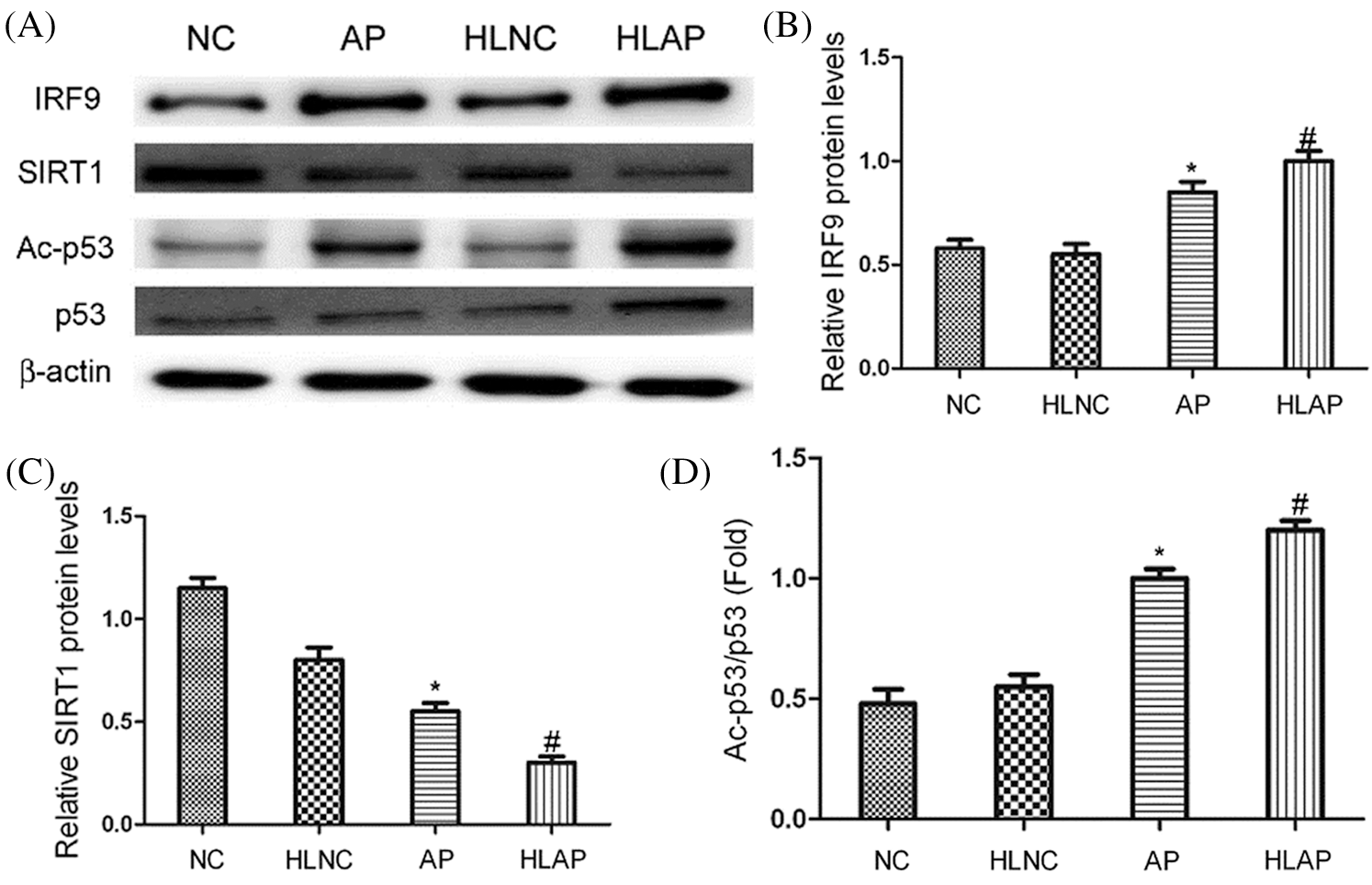
Figure 4: The levels of IRF9, SIRT1, p53 and Ac-p53 expression of heart tissues in various groups. (A) Representative results exhibited that substantial increases of IRF9, p53 and Ac-p53 expression and marked decreases of SIRT1 expression were observed in heart tissues of AP and HLAP group rather than NC and HLNC group. (B–D) The relative protein expression levels of IRF9, SIRT1 and Ac-p53/p53 were showed in Figs. 4B–4D. *P < 0.05 vs. NC and HLNC group. #P < 0.05 vs. NC, HLNC and AP group.
IRF9 and SIRT1 mRNA expression of heart tissues of various groups
As shown by the results of the qRT-PCR assay in Fig. 5, it demonstrated that SIRT1 mRNA levels of AP and HLAP groups were substantially attenuated, while the IRF9 mRNA levels of AP and HLAP groups were markedly enhanced compared with NC and HLNC groups (P < 0.05). The SIRT1 mRNA levels of HLAP group were obviously reduced than AP group, while the IRF9 mRNA level of HLAP group was starkly raised than AP group (P < 0.05).
Analysis on the correlativity between SIRT1 and IRF9
Results of correlation analysis in Fig. 6 indicated that IRF9 could affect the level of SIRT1 expression. There was an inverse correlation between their levels of expression. The level of SIRT1 expression was decreased as the increase of IRF9 (P < 0.05).
Analysis on the correlativity between hyperlipidemia and damage of pancreatic and cardiac tissues
As shown in Fig. 7, correlation analysis exhibited that TG levels could affect the damage degree of pancreas and heart tissues. There was a positive correlation between TG levels and total histological scores of pancreas and heart tissues. The injury degree of pancreas and heart tissues was aggravated as the increase of TG levels (P < 0.05).
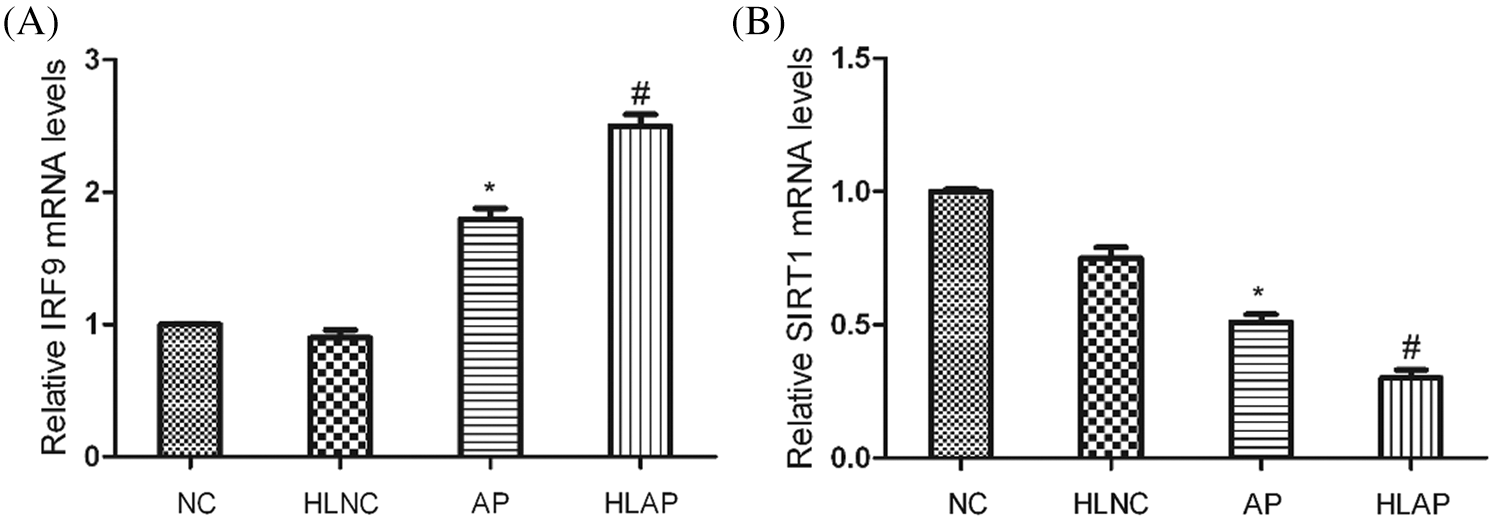
Figure 5: IRF9 mRNA levels were enhanced and SIRT1 mRNA levels were decreased in heart tissues of AP and HLAP group rather than that of NC and HLNC group. Relative mRNA expression levels of IRF9 and SIRT1 were shown as Figs. 5A and 5B. *P < 0.05 vs. NC and HLNC group. #P < 0.05 vs. NC, HLNC and AP group.
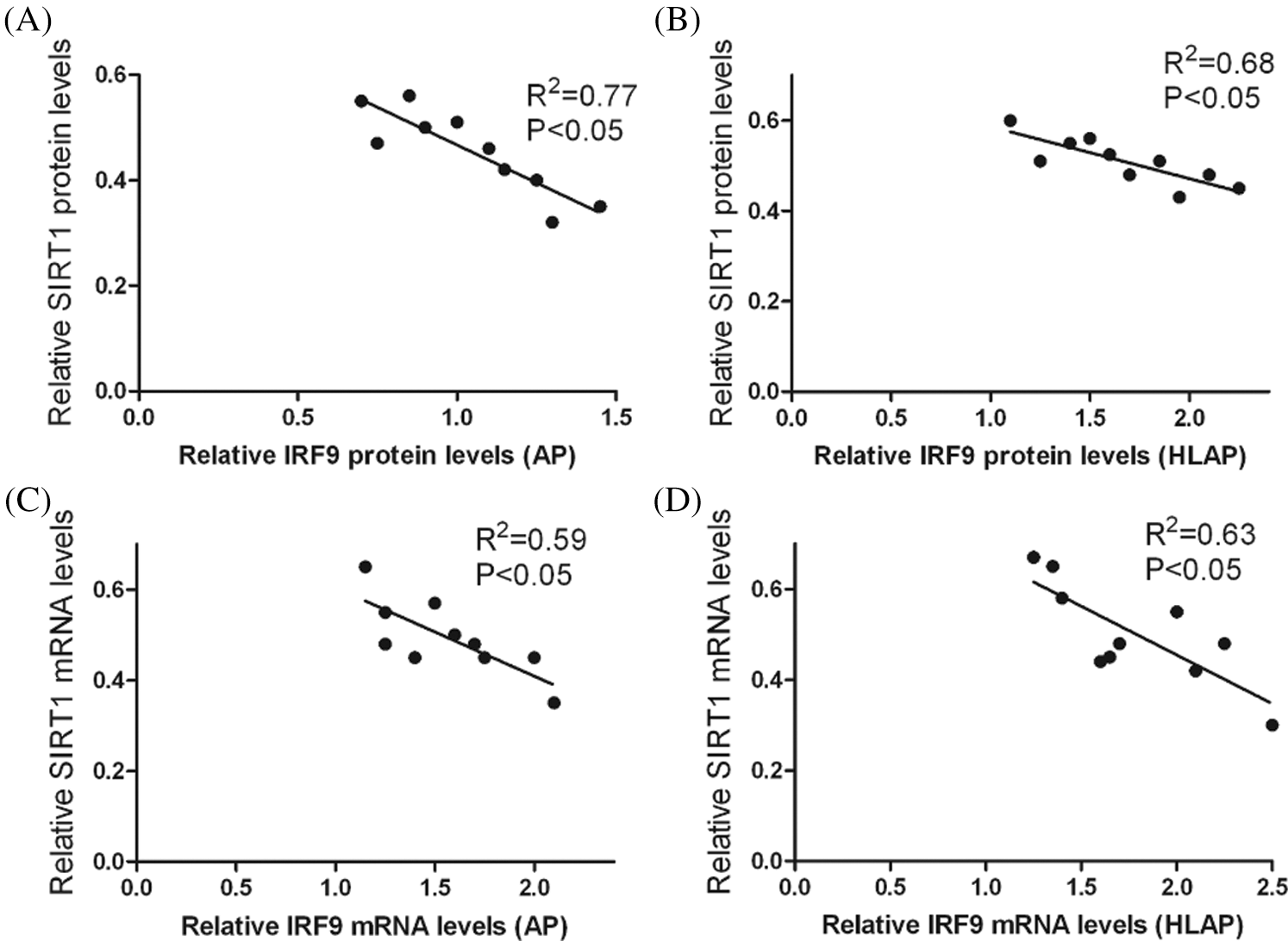
Figure 6: Correlation analysis indicated that there was an inverse correlation between the levels of SIRT1 expression and IRF9 expression in heart tissues of AP and HLAP. Relative protein expression levels of SIRT1 and IRF9 were inversely connected in heart tissues of AP (A) and HLAP (B). Relative mRNA expression levels of SIRT1 and IRF9 were inversely connected in heart tissues of AP (C) and HLAP (D).
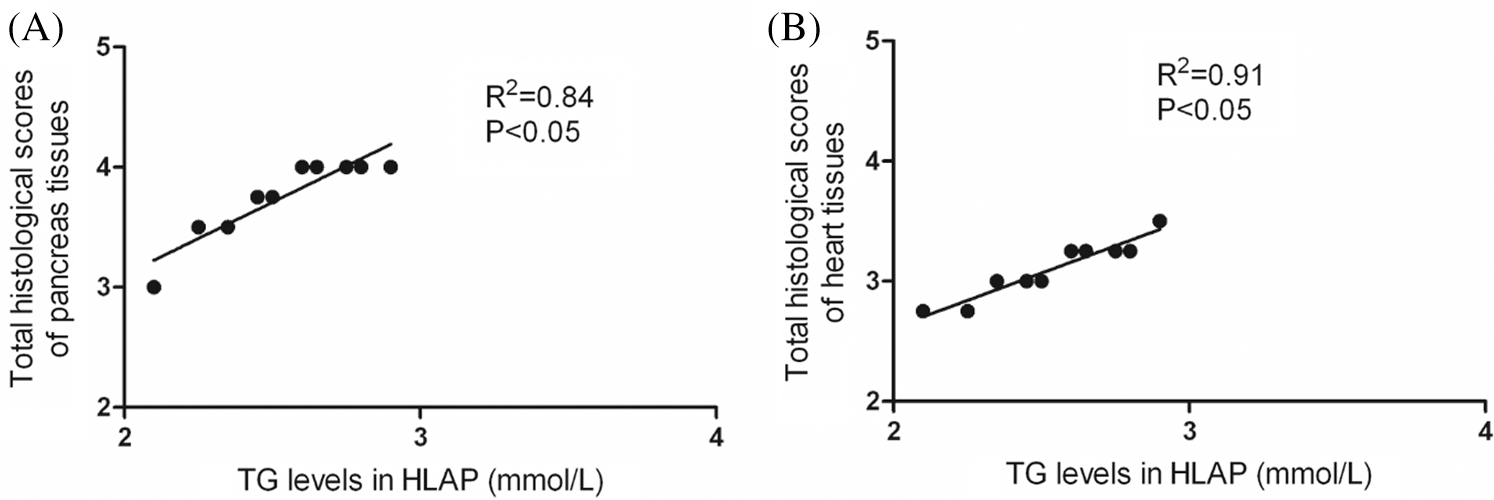
Figure 7: Correlation analysis exhibited that there was a positive correlation between hyperlipidemia and damage of pancreatic and cardiac tissues. TG levels and total histological scores of pancreas (A) and heart tissues (B) were positively connected in HLAP group.
In this study, we focused on investigating the function of IRF9 and SIRT1 in AP and HLAP with heart injury. As shown in Fig. 1A, TG levels were increased in HLNC and HLAP groups, suggesting that the hyperlipidemia model was established successfully. Pancreatic injury in AP and HLAP rats manifested as elevated serum lipase and amylase levels, increased inflammatory cytokines as reflected by serum IL-1β and TNF-α levels, and varying degrees of inflammatory cell infiltration, interstitial tissue edema, and necrosis (Figs. 1B, 1C, 1F, and 2A). Cardiac injury in AP and HLAP rats showed elevated serum CK-MB, LDH levels, various degrees of myocardial fiber swelling and fracturing, and inflammatory cell infiltration (Figs. 1D, 1E, and 2A). Histopathological injury degree of pancreatic and cardiac tissues indicated that the tissue damage in the HLNC group was more distinct than the NC group. And injury degree of pancreatic in AP and HLAP groups was more remarkable than NC and HLNC groups. Additionally, the injury degree of pancreas and heart damage of the HLAP group was much severer compared with AP group (Figs. 2A–2E). A large number of myocardial cell apoptosis was observed in AP and HLAP groups. The apoptosis degree of myocardial cells of the HLAP group was more distinct compared with the AP group (Fig. 3).
Moreover, our results showed that the levels of IRF9 and Ac-p53/p53 expression in AP and HLAP groups were all enhanced rather than in NC and HLNC groups. Oppositely, SIRT1 expression of AP and HLAP groups were attenuated compared with that of NC and HLNC groups (P < 0.05). In addition, the levels of IRF9, Ac-p53/p53 expression in the HLAP group were markedly elevated compared with the AP group. SIRT1 expression in the HLAP group was substantially reduced compared with the AP group (P < 0.05; Figs. 4 and 5). And inverse connection existed between SIRT1 and IRF9. SIRT1 expression was decreased as the increase of IRF9 in AP and HLAP groups (P < 0.05; Fig. 6). Furthermore, there was a positive correlation between hyperlipidemia and the damage of pancreatic and cardiac tissues. The injury of the pancreas and heart tissues was increased as the increase of TG levels.
AP is very common in the clinic, and the incidence of AP was increased year by year globally. The death rate of AP has been as high as 10%–15%. Up to 20%–30% of patients with AP may become severe acute pancreatitis (SAP) (Frossard et al., 2008; Bollen, 2016; Trikudanathan et al., 2019). The mortality of SAP is up to 25%–30% (Banks et al., 2013; Koizumi et al., 2006). In the case of several local and systemic complications, the death rate of SAP will increase to more than 55% (Banks et al., 2013; Koizumi et al., 2006). Hyperlipidemia has become one of the most common causes of AP, and the clinical severity, the rate of complications, and recurrence of HLAP are higher compared with other types of AP. The prevention and therapy of HLAP develop slowly worldwide because of the lack of full recognition of the pathogenesis of HLAP. Hyperlipidemia, especially increased TG levels, could expose patients to a high risk of AP and be associated with recurrent AP episodes (Guo et al., 2019; Wang et al., 2016). Free fatty acids (FFA) resulting from pancreatic lipase-induced hydrolysis of TG was substantially increased and abundantly accumulated in the pancreas (Wang et al., 2016; Backes et al., 2016). Subsequently, excess FFA caused pancreatic inflammation (Backes et al., 2016). Havel (1969) had proposed that high concentrations of FFA could self-aggregate to form micelles and initially activated the attack on platelets and the vascular endothelium. Finally, the acinar cells were attacked, thus leading to ischemia and pancreatic injury. Our observations indicated that hyperlipidemia might increase the injury of pancreatic and heart tissues, elevated myocardial cell apoptosis.
Previous literature had reported that IRF9 functioned as a transcription suppressor to inhibit the expression of SIRT1 in many diseases (Wan et al., 2018; Wang et al., 2015; Zhang et al., 2014; Chen et al., 2014; Zhang et al., 2014). SIRT1 knockdown led to the activation of inflammatory pathways through enhancing inflammatory gene expression, whereas activated SIRT1 could generate anti-inflammatory action (Shen et al., 2017). Down-regulated SIRT1 suppressed the deacetylation of target genes (including NF-κB and p53), increased acetylation of target genes, caused inflammation and apoptosis, finally exacerbated the severity of AP (Shen et al., 2017). Increased acetylation of p53 activated its transcriptional activity and promoted p53-mediated cell apoptosis (Brooks and Gu, 2011). And our previous study had proved that IRF9 could combine with the promoter region of SIRT1 to inhibit its expression. IRF9 was a suppressor of SIRT1 (Liu et al., 2020). In the present study, the results also manifested that IRF9 might function as an inhibitor of SIRT1, elevate the acetylation level of p53, exacerbate the injury of pancreatic and cardiac tissues, accelerate myocardial cell apoptosis in AP and HLAP with heart injury.
In conclusion, these observations implied that increased IRF9 in pancreatitis with heart injury led to the decrease of SIRT1 expression, elevated the levels of Ac-p53, and caused the activation of inflammation and apoptosis signaling pathways in the downstream. What we had found might be a vital basis to explain the disease mechanism of HLAP with heart injury. Therefore, further research with a focus on exploring the function and mechanism of IRF9 and SIRT1 in other organ dysfunctions of HLAP and investigating their clinical values would provide us with novel direction, thinking, and prospect for the treatment of HLAP.
Availability of Data and Materials: The data is available upon the request from the corresponding author
Funding Statement: This work was supported by the National Natural Science Foundation of China (Grant No. 32000099), National Natural Science Foundation Incubation plan (Grant No. 2019GQFY03), Anhui Provincial Natural Science Foundation (Grant No. 1508085QC49), Natural Science Foundation of Anhui Provincial Education Department (KJ2017A183), and the doctoral research fund project of the Second Affiliated Hospital of Anhui Medical University (Grant Nos. 2014BKJ034, 2018BSJJ005).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Albrecht CA, Laws FA. (2003). ST segment elevation pattern of acute myocardial infarction induced by acute pancreatitis. Cardiology Reviews 11: 147–151. DOI 10.1097/01.CRD.0000051401.00517.20. [Google Scholar] [CrossRef]
Ates F, Kosar F, Aksoy Y, Yildirim B, Sahin I, Hilmioglu F. (2005). QT interval analysis in patients with acute biliary pancreatitis. Pancreas 31: 238–241. DOI 10.1097/01.mpa.0000178279.73405.25. [Google Scholar] [CrossRef]
Backes J, Anzalone D, Hilleman D, Catini J. (2016). The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids in Health and Disease 15: 118. DOI 10.1186/s12944-016-0286-4. [Google Scholar] [CrossRef]
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS, Acute Pancreatitis Classification Working Group. (2013). Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 62: 102–111. DOI 10.1136/gutjnl-2012-302779. [Google Scholar] [CrossRef]
Bauerlein T, Stobbe LH. (1954). Acute pancreatitis simulating myocardial infarction with characteristic ECG changes. Gastroenterology 27: 861–864. DOI 10.1016/S0016-5085(19)36084-6. [Google Scholar] [CrossRef]
Bollen TL. (2016). Acute pancreatitis: international classification and nomenclature. Clinical Radiology 71: 121–133. DOI 10.1016/j.crad.2015.09.013. [Google Scholar] [CrossRef]
Brooks CL, Gu W. (2011). The impact of acetylation and deacetylation on the p53 pathway. Protein & Cell 2: 456–462. DOI 10.1007/s13238-011-1063-9. [Google Scholar] [CrossRef]
Buch J, Buch A, Schmidt A. (1980). Transient ECG changes during acute attacks of pancreatitis. Acta Cardiologica 35: 381–390. [Google Scholar]
Carr RA, Rejowski BJ, Cote GA, Pitt HA, Zyromski NJ. (2016). Systematic review of hypertriglyceridemia-induced acute pancreatitis: A more virulent etiology? Pancreatology 16: 469–476. DOI 10.1016/j.pan.2016.02.011. [Google Scholar] [CrossRef]
Chalkiadaki A, Guarente L. (2015). The multifaceted functions of sirtuins in cancer. Nature Reviews Cancer 15: 608–624. DOI 10.1038/nrc3985. [Google Scholar] [CrossRef]
Chen HZ, Guo S, Li ZZ, Lu Y, Jiang DS, Zhang R, Lei Hao, Gao L, Zhang XF, Zhang Y, Wang L, Zhu LH, Xiang M, Zhou Y, Wan Q, Dong HL, Liu DP, Li HL. (2014). A critical role for interferon regulatory factor 9 in cerebral ischemic stroke. Journal of Neuroscience 34: 11897–11912. DOI 10.1523/JNEUROSCI.1545-14.2014. [Google Scholar] [CrossRef]
Easwaran P, Shah A. (1987). Pericardial effusion and left ventricular function in acute alcoholic pancreatitis. Archives of Internal Medicine 147: 923–925. DOI 10.1001/archinte.1987.00370050119020. [Google Scholar] [CrossRef]
Ewald N, Hardt PD, Kloer HU. (2009). Severe hypertriglyceridemia and pancreatitis: presentation and management. Current Opinion in Lipidology 20: 497–504. DOI 10.1097/MOL.0b013e3283319a1d. [Google Scholar] [CrossRef]
Forsmark CE, Swaroop VS, Wilcox CM. (2016). Acute pancreatitis. New England Journal of Medicine 375: 1972–1981. DOI 10.1056/NEJMra1505202. [Google Scholar] [CrossRef]
Frossard JL, Steer ML, Pastor CM. (2008). Acute pancreatitis. The Lancet 371: 143–152. DOI 10.1016/S0140-6736(08)60107-5. [Google Scholar] [CrossRef]
Fu XY, Kessler DS, Veals SA, Levy DE, Darnell JE. (1990). ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting poly-peptide chains. Proceedings of the National Academy of Sciences of the United States of America 87: 8555–8559. DOI 10.1073/pnas.87.21.8555. [Google Scholar] [CrossRef]
Guo YY, Li HX, Zhang Y. (2019). Hypertriglyceridemia-induced acute pancreatitis: progress on disease mechanisms and treatment modalities. Discovery Medicine 27: 101–109. [Google Scholar]
Havel RJ. (1969). Pathogenesis, differentiation and management of hypertriglyceridemia. Advances in Internal Medicine 15: 117–145. [Google Scholar]
Huber M, Suprunenko T, Ashhurst T, Marbach F, Raifer H, Wolff S, Strecker T, Viengkhou B, Jung SR, Obermann HL, Bauer S, Xu HFC, Lang PA, Tom A, Lang KS, King NJC, Campbell IL, Hofer MJ. (2017). IRF9 prevents CD8+ T cell exhaustion in an extrinsic manner during acute LCMV infection. Journal of Virology 91: e01219. DOI 10.1128/JVI.01219-17. [Google Scholar] [CrossRef]
Improta T, Schindler C, Horvath CM, Kerr IM, Stark GR, Darnell JE. (1994). Transcription factor ISGF-3 formation requires phosphorylated Stat91 protein, but Stat113 protein is phosphorylated independently of Stat91 protein. Proceedings of the National Academy of Sciences of the United States of America 91: 4776–4780. DOI 10.1073/pnas.91.11.4776. [Google Scholar] [CrossRef]
Jiang DS, Luo YX, Zhang R, Zhang XD, Chen HZ, Zhang Y, Chen K, Zhang SM, Fan GC, Liu PP, Liu DP, Li H. (2014). Interferon regulatory factor 9 protects against cardiac hypertrophy by targeting myocardin. Hypertension 63: 119–127. DOI 10.1161/HYPERTENSIONAHA.113.02083. [Google Scholar] [CrossRef]
Johnson CD, Besselink MG, Carter R. (2014). Acute pancreatitis. BMJ 349: g4859. DOI 10.1136/bmj.g4859. [Google Scholar] [CrossRef]
Khairy P, Marsolais P. (2001). Pancreatitis with electrocardiographic changes mimicking acute myocardial infarction. Canadian Journal of Gastroenterology 15: 522526–526. DOI 10.1155/2001/604386. [Google Scholar] [CrossRef]
Koizumi M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Sekimoto M, Hirota M, Kimura Y, Takeda K, Isaji S, Otsuki M, Matsuno S. (2006). JPN Guidelines for the management of acute pancreatitis: Diagnostic criteria for acute pancreatitis. Journal of Hepatobiliary Surgery 13: 25–32. DOI 10.1007/s00534-005-1048-2. [Google Scholar] [CrossRef]
Liu Y, Sun Y, Xue BH, Wang XD, Yu WL. (2020). Negative regulation of SIRT1 by IRF9 involved in hyperlipidemia acute pancreatitis associated with kidney injury. Digestive Diseases and Sciences, 109. DOI 10.1007/s10620-020-06331-1. [Google Scholar] [CrossRef]
Lloret Linares C, Pelletier AL, Czernichow S, Vergnaud AC, Rousselot DB, Levy Philippe, Ruszniewski P, Bruckert E. (2008). Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia. Pancreas 37: 13–22. DOI 10.1097/MPA.0b013e31816074a1. [Google Scholar] [CrossRef]
Luker KE, Pica CM, Schreiber RD, Piwnica-Worms D. (2001). Overexpression of IRF9 confers resistance to antimicrotubule agents in breast cancer cells. Cancer Research 61: 6540–6547. [Google Scholar]
Makaryus AN, Adedeji O, Ali SK. (2008). Acute pancreatitis presenting as acute inferior wall ST-segment elevations on electrocardiography. The American Journal of Emergency Medicine 26: 734.e1–734.e4. [Google Scholar]
Maringhini A, Ciambra M, Patti RMA, Dardanoni G, Mancuso L, Termini A, Pagliaro L. (1996). Ascites, pleural and pericardial effusions in acute pancreatitis: A prospective study of incidence, natural history and prognostic role. Digestive Diseases and Sciences 41: 848–852. DOI 10.1007/BF02091521. [Google Scholar] [CrossRef]
Mautner RK, Siegel LA, Giles TD, Kayser J. (1982). Electrocardiographic changes in acute pancreatitis. Southern Medical Journal 75: 317–320. DOI 10.1097/00007611-198203000-00019. [Google Scholar] [CrossRef]
Meuleman VG, Schinkel AF, Vos J. (2011). Electrocardiographic abnormalities caused by acute pancreatitis. Netherlands Heart Journal 19: 137–139. DOI 10.1007/s12471-011-0072-x. [Google Scholar] [CrossRef]
Papachristou GI, Machicado JD, Stevens T, Goenka MK, Ferreira M, Gutierrez SC, Singh VK, Kamal A, Gonzalez-Gonzalez JA, PelaezLuna M, Gulla A, Zarnescu NO, Triantafyllou K, Barbu ST, Easler J, Ocampo C, Capurso G, Archibugi L, Cote GA, Lambiase L, Kochhar R, Chua T, Tiwari SC, Nawaz H, Park WG, deMadaria E, Lee PJ, Wu BU, Greer PJ, Dugum M, Koutroumpakis E, Akshintala V, Gougol A. (2017). Acute Pancreatitis Patient Registry to Examine Novel Therapies in Clinical Experience (APPRENTICEan international, multicenter consortium for the study of acute pancreatitis. Annals of Gastroenterology 30: 106–113. [Google Scholar]
Pezilli R, Barakat B, Billi P, Bertaccini B. (1999). Electrocardiographic abnormalities in acute pancreatitis. European Journal of Emergency Medicine 6: 27–29. [Google Scholar]
Pezzilli R, Billi P, Bertaccini B, Gullo L. (1996). Pericardial effusion and left ventricular function in acute pancreatitis. American Journal of Gastroenterology 91: 997–1000. [Google Scholar]
Rauch I, Rosebrock F, Hainzl E, Heider S, Majoros A, Wienerroither S, Strobl B, Stockinger S, Kenner L, Mathias Müller, Thomas Decker. (2015). Noncanonical effects of IRF9 in intestinal inflammation: More than type I and type III interferons. Molecular and Cellular Biology 35: 2332–2343. DOI 10.1128/MCB.01498-14. [Google Scholar] [CrossRef]
Rubio-Tapia A, García-Leiva J, Asensio-Lafuente E, Robles-Díaz G, Vargas-Vorácková F. (2005). Electrocardiographic abnormalities in patients with acute pancreatitis. Journal of Clinical Gastroenterology 39: 815–828. DOI 10.1097/01.mcg.0000177241.74838.57. [Google Scholar] [CrossRef]
Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. (1992). A better model of acute pancreatitis for evaluating therapy. Annals of Surgery 215: 44–56. DOI 10.1097/00000658-199201000-00007. [Google Scholar] [CrossRef]
Shen AH, Kim HJ, Oh GS, Lee SB, Lee SH, Pandit A, Khadka D, Choe SK, Kwak SC, Yang SH, Cho EY, Kim HS, Kim H, Park R, Kwak TH, So HS. (2017). NAD+ augmentation ameliorates acute pancreatitis through regulation of inflammasome signalling. Scientific Reports 7: 3006. DOI 10.1038/s41598-017-03418-0. [Google Scholar] [CrossRef]
Smith S, Fernando T, Wu PW, Seo J, Ní Gabhann J, Piskareva O, McCarthy E, Howard D, O'Connell P, Conway R, Gallagher P, Molloy E, Stallings RL, Kearns G, Forbess L, Ishimori M, Venuturupalli S, Wallace D, Weisman M, Jefferies CA. (2017). MicroRNA-302d targets IRF9 to regulate the IFN-induced gene expression in SLE. Journal of Autoimmunity 79: 105–111. DOI 10.1016/j.jaut.2017.03.003. [Google Scholar] [CrossRef]
Tanwar V, Sachdeva J, Kishore K, Mittal R, Nag TC, Ray R, Kumari S, Arya DS. (2010). Dose-dependent actions of curcumin in experimentally induced myocardial necrosis: A biochemical, histopathological, and electron microscopic evidence. Cell Biochemistry and Function 28: 74–82. DOI 10.1002/cbf.1623. [Google Scholar] [CrossRef]
Taylor SC, Nadeau K, Abbasi M, Lachance C, Nguyen M, Fenrich J. (2019). The ultimate qPCR experiment: Producing publication quality, reproducible data the first time. Trends in Biotechnology 37: 761–774. DOI 10.1016/j.tibtech.2018.12.002. [Google Scholar] [CrossRef]
Tian WL, Guo R, Wang F, Jiang ZX, Tang P, Huang YM, Sun L. (2018). The IRF9-SIRT1-P53 axis is involved in the growth of human acute myeloid leukemia. Experimental Cell Research 365: 185–193. DOI 10.1016/j.yexcr.2018.02.036. [Google Scholar] [CrossRef]
Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. (2019). Current concepts in severe acute and necrotizing pancreatitis: An evidence-based approach. Gastroenterology 156: 1994–2007. DOI 10.1053/j.gastro.2019.01.269. [Google Scholar] [CrossRef]
Valdivielso P, Ramírez-Bueno A, Ewald N. (2014). Current knowledge of hypertriglyceridemic pancreatitis. European Journal of Internal Medicine 25: 689–694. DOI 10.1016/j.ejim.2014.08.008. [Google Scholar] [CrossRef]
Variyam EP, Shah A. (1987). Pericardial effusion and left ventricular function in patients with acute alcoholic pancreatitis. Archives of Internal Medicine 147: 923–925. DOI 10.1001/archinte.1987.00370050119020. [Google Scholar] [CrossRef]
Veals SA, Schindler C, Leonard D, Fu XY, Aebersold R, Darnell JEJr, Levy DE. (1992). Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Molecular and Cellular Biology 12: 3315–3324. DOI 10.1128/MCB.12.8.3315. [Google Scholar] [CrossRef]
Wan W, Ding Y, Xie Z, Li Q, Yan F, Budbazar E, Pearce WJ, Hartman R, Obenaus A, Zhang JH, Jiang Y, Tang JP. (2018). PDGFR-β modulates vascular smooth muscle cell phenotype via IRF-9/SIRT-1/NF-κB pathway in subarachnoid hemorrhage rats. Journal of Cerebral Blood Flow & Metabolism 39: 1369–1380. DOI 10.1177/0271678X18760954. [Google Scholar] [CrossRef]
Wang PX, Zhang R, Huang L, Zhu LH, Jiang DS, Chen HZ, Zhang Y, Tian S, Zhang XF, Zhang XD, Liu DP, Li H. (2015). Interferon regulatory factor 9 is a key mediator of hepatic ischemia/reperfusion injury. Journal of Hepatology 62: 111–120. DOI 10.1016/j.jhep.2014.08.022. [Google Scholar] [CrossRef]
Wang SH, Chou YC, Shangkuan WC, Wei KY, Pan YH, Lin HC. (2016). Relationship between plasma triglyceride level and severity of hypertriglyceridemic pancreatitis. PLoS One 11: e0163984. DOI 10.1371/journal.pone.0163984. [Google Scholar] [CrossRef]
Yegneswaran B, Kostis JB, Pitchumoni CS. (2011). Cardiovascular manifestations of acute pancreatitis. Journal of Critical Care 26: 225.e11–225.e18. DOI 10.1016/j.jcrc.2010.10.013. [Google Scholar] [CrossRef]
Zhang SM, Zhu LH, Chen HZ, Zhang R, Zhang P, Jiang DS, Gao L, Tian S, Wang L, Zhang Y, Wang PX, Zhang XF, Zhang XD, Liu DP, Li H (2014a). Interferon regulatory factor 9 is critical for neointima formation following vascular injury. Nature Communications 5: 5160. DOI 10.1038/ncomms6160. [Google Scholar] [CrossRef]
Zhang Y, Liu X, She ZG, Jiang DS, Wan N, Xia H, Zhu XH, Wei X, Zhang XD, Li H (2014b). Interferon regulatory factor 9 is an essential mediator of heart dysfunction and cell death following myocardial ischemia/reperfusion injury. Basic Research in Cardiology 109: 434. DOI 10.1007/s00395-014-0434-9. [Google Scholar] [CrossRef]
Zhou J, Li Y, Tang Y, Liu F, Yu S, Zhang L, Zeng X, Zhao Y, Fu P. (2015). Effect of acute kidney injury on mortality and hospital stay in patient with severe acute pancreatitis. Nephrology 20: 485–491. DOI 10.1111/nep.12439. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |