2021 45(1): 57-64
DOI:10.32604/biocell.2021.011756

www.techscience.com/journal/biocell
| BIOCELL 2021 45(1): 57-64 DOI:10.32604/biocell.2021.011756 |  www.techscience.com/journal/biocell |
MiR-494-3p regulates cell proliferation and apoptosis via KLF7 in Schwann cells
1Department of Neurosurgery, The Affiliated Mindong Hospital of Fujian Medical University, Fuan, 355000, China
2Department of Neurosurgery, Hangzhou Hospital of Traditional Chinese Medcine, Hangzhou, 310007, China
*Address correspondence to: Zhanfang Shang, shangzhanfang1@sina.com
Received: 28 May 2020; Accepted: 19 August 2020
#They contributed equally to the writing of this article.
Abstract: Peripheral nerve injury is a common neurodegenerative disease, which causes disability and a huge economic burden for patients. MicroRNAs (miRNAs) have been acknowledged as major regulators and therapeutic targets of neurological disease. Thus, the functional studies of miRNAs in neurological disease will contribute to discover new therapeutic targets for peripheral nerve injury. Sprague Dawley rats treated sciatic nerve surgical injury were regarded as peripheral nerve injury model in vivo. The expression of miR-494-3p and Kruppel like factor7 (KLF7) were measured by Real-time quantitative polymerase chain reaction (RT-qPCR) assay. In addition, western blot analysis was conducted to measure the protein levels of KLF7, Bax, Bcl-2, and C-caspase 3. Cell viability and apoptosis were detected in Schwann cells by EdU stain and flow cytometry, respectively. The interaction between miR-494-3p and KLF7 was investigated by dual-luciferase reporter assay. The expression of miR-494-3p was reduced at the beginning, but KLF7 was enhanced in Sprague Dawley rats with peripheral nerve injury. Knockdown of miR-494-3p promoted cell proliferation and suppressed apoptosis, while overexpression of miR-494-3p or silencing KLF7 led to opposite results. Moreover, the upregulation of KLF7 attenuated miR-494-3p overexpression-induced suppressive effects on viability and promotion of apoptosis in Schwann cells. MiR-494-3p negatively regulates KLF7 in Schwann cells to mediate proliferation and apoptosis.
Keywords: Peripheral nerve injury; miR-494-3p; KLF7; Schwann cells
Previous reports indicated that mechanical compression, ischemia, penetrating injury, and stretch injury could result in nerve injury that is a worldwide trouble and largely affect patients’ life quality (Gu et al., 2011). The peripheral nervous system has discrimination from the central nervous system is its intrinsic regenerative power and the repair function of spontaneous peripheral nerve repair after injury, although the regeneration rate is slow and usually far from satisfactory (Chen et al., 2007; Battiston et al., 2009). With the advance of neurological disease pathogenesis in recent decades, multiple targets have been regarded as promising avenues, such as brain-derived neurotrophic factor (BDNF) (Yi et al., 2016), methyl-CpG binding protein 2 (Chahrour et al., 2008), and L-carnitine (Wang et al., 2007). Additionally, microRNAs (miRNAs or miRs) may be key therapeutic targets.
MiRNAs, a novel class of non-coding RNAs, play crucial roles in cancer development (Liz and Esteller, 2016; Zhao et al., 2010). Moreover, deep sequencing profile of miRNAs following sciatic nerve injury had revealed that differential expression of miRNAs was associated with proliferation and differentiation of neural stem cells (Yu et al., 2011; Li et al., 2011). For instance, miR-182 directly targeted fibroblast growth factor 9 to modulate the phenotype of Schwann cells, suggesting that miR-182 is involved in nerve injury (Yu et al., 2012). MicroRNA-338 and microRNA-21 had been indicated to associate with the development of neural tissues after injury (Wang et al. 2016). In addition, miR-137 and miR-491 regulated dopamine transporter to mediate aberrant regulation of dopaminergic neurotransmission (Jia et al., 2016). A recent work conducted by (Bremer et al., 2010) demonstrated that 16 high expression of miRNAs may participate in myelination; additionally, some miRNAs were required for Schwann cell ablation of the enzyme Dicer1 (Bremer et al., 2010). Li et al. reported that inhibition of let-7 enhanced Schwann cell migration and axon growth by targeting nerve growth factor in vivo (Li et al., 2015). Analogously, the overexpression miR-221/miR-222 was found Schwann cell in answer to peripheral nerve injury, while miR-221/222 silencing decreased proliferation and migration of Schwann cell in vitro (Yu et al., 2012). In summary, emerging evidence suggests that dysregulation of miRNAs may associate with neurological disorders, which was involved in the inflammation, oxidation, and apoptosis (Wang et al., 2012; Liu et al., 2009). Above these studies have implied that miRNAs are one of the important regulators in gene expression for appropriate neural functions. However, the mechanisms of miR-494-3p remain unclear under nerve injury conditions.
In this study, we established the Sprague Dawley rats model treated surgical injury to inspect the expression of Kruppel like factor7 (KLF7) mRNA and miR-494-3p in vivo and investigate their association in vitro. This study may contribute to providing potential treatment targets for peripheral nerve injury therapeutic in the future.
For the establishment of the peripheral nerve injury model in vivo, all animals (Sprague Dawley, SD) used in this study were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). SD rats fed on standard pellet chow and water in an incubator with a 12 h light/dark cycle. SD rats were treated sciatic nerve crush injury by following previous practices (Pepper et al., 2017). Briefly, under sterile surgical conditions, rats were deeply sedated using inhaled isoflurane anesthesia; subsequently, sharp transection at 5 mm above the sciatic notch was performed with forceps to form nerve crush injury. Rats were sacrificed (on 14 d) for measuring the expression of miR-494-3p and KLF7. The animal experiment was performed according to the protocol, which was approved by the Animal Care Committee of The Affiliated Mindong Hospital of Fujian Medical University.
Schwann cells RSC96 (ATCC, Manassas, VA, USA) were maintained in F-12 (GIBCO BRL, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS: GIBCO BRL) in an environment with 5% CO2 and 95% air at 37°C.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA isolated from RSC96 cells with TRIzol (Thermo Fisher Scientific, Waltham, MA, USA) referring to instructions. RNA reverse transcription was exercised by using All-in-One miRNA cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA) and Prime Script RT Reagent kit (Thermo Fisher Scientific). After that, the SYBR Green PCR Kit (Thermo Fisher Scientific) was used to test the levels of miR-494-3p and KLF7 under the ABI Step One Real-time PCR System (Thermo Fisher Scientific). The relative expression of miR-494-3p and KLF7 were analyzed normalized to endogenous small nuclear RNA U6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCt method, respectively.
The special primers were purchased from Sangon (Shanghai, China) and listed: miR-494-3p (F, 5’-GAAACATACACGGGAAACC-3’); R, 5’-AAAGAGGTTTCCCGTGTATG -3’);
KLF7 (F, 5’-TTTCCTGGCAGTCATCTGCAC-3’; R, 5’-GGGTCTGTTTGTTTGTCAGTCTGTC-3’);
GAPDH (F, 5’-CCTCTCTCTAATCAGCCCTCTG-3’; R, 5’- AGAAGGCTGGGGCTCATTTG-3’);
U6 (F, 5’-CTCGCTTCGGCAGCACA-3’; R, 5’-AACGCTTCA CGAATTTGCGT-3’).
RSC96 cells or tissue samples were lysed with RIPA lysis buffer (Thermo Fisher Scientific) on ice for 30 min. The protein in the supernatant was quantified by using a BCA protein assay kit (Beyotime, Shanghai, China). After denaturation, equal proteins were segregated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then delivered onto polyvinylidene difluoride membranes (Beyotime). Then the blots were incubated with primary antibodies Bax (1:800 dilution; Ambion, Foster City, CA, USA), Bcl-2 (1:800 dilution; Ambion), C-caspase 3 (1:800 dilution; Ambion), KLF7 (1:800 dilution; Millipore, Billerica, MA, USA), ERK (1:800 dilution; Ambion), p-ERK (1:800 dilution; Ambion), JNK (1:800 dilution; Ambion), p-JNK(1:800 dilution; Ambion), or GAPDH (1:1200 dilution; Millipore) at 4°C. After 24 h, and the membranes were interacted with HRP-conjugated secondary antibody (1:2000 dilution; Ambion) for 2 h after washed twice times. Blots were visualized via commercial enhanced chemiluminescence chromogenic substrate (Beyotime).
Vector construction and transfection
MiR-494-3p mimic (miR-494-3p) and control miR-NC, miR-494-3p inhibitor (anti-miR-494-3p) and control (anti-NC), specific small interfering RNA (siRNA) against KLF7 (si-KLF7) and negative control (si-RNA), KLF7 overexpression vector (KLF7) and empty vector (vector) were obtained from Beyotime. Lipofectamine 2000 reagent (Thermo Fisher Scientific) was used to infect RSC96 cells with 50 nM of synthetic oligonucleotides or 1 μg of vectors by referring instructions. After transfection for 48 h, cells were collected for further analysis.
Cell-LightTM EdU DNA Cell Proliferation Kit (Beyotime) was applied for measuring cell viability value. RSC96 were placed into 96-well plates (5 × 103 cells/well) and grew at 37°C with 5% CO2 overnight, and then incubated with 50 μM of EdU. After fixation, cells interacted with a Hoechst33342 reaction cocktail for another 30 min. All steps were executed at room temperature. We counted the number of EdU-labeled nuclei under the fluorescence microscope (Bio-Rad, Hercules, CA, USA).
The cell apoptosis rate was measured with Annexin V-FITC/PI kit (Solarbio, Beijing, China) under flow cytometry. Briefly, post-transfected for 24 h, RSC96 cells were harvested and stained with 5 μL Annexin V-FITC and PI for 10min in the dark. Next, flow cytometry was performed to test the apoptotic rates of RSC96.
Dual-luciferase reporter assay
The putative relationship between miR-494-3p and KLF7 was predicted by TargetScan. The 3’UTR sequences of KLF7 containing binding sites of miR-494-3p were amplified by PCR inserted into pGL3-basic vectors (Realgene, Nanjing, China), named as KLF7 WT 3’-UTR. KLF7 MUT 3’-UTR was constructed by Quik-Change II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). RSC96 cells co-transfected with 40 nM miR-494-3p or miR-NC and 100 ng corresponding luciferase reporter vector using Lipofectamine2000 (Thermo Fisher Scientific). After the post-transfection 48 h, luciferase activity was examined.
In this study, we used SPSS (IBM, Chicago, IL, USA) for statistical analysis. The data were shown as mean ± SD from three independent experiments. The comparisons between the two groups were conducted using Student’s t-test, while multiple group comparisons were assessed using a one-way analysis of variance (ANOVA). If p < 0.05, the difference was regarded as statistically significant.
MiR-494-3p was downregulated while KLF7 was upregulated after sciatic nerve injury
To begin with, Sprague Dawley rats with sciatic nerve injury model was established to explore the potential roles of miR-494-3p and KLF7 in vivo. The time-dependent differential expression of miR-494-3p was measured by RT-qPCR assay. The results implied that the abundance of miR-494-3p was decreased first and then rose, reaching a minimum at 4 d (Fig. 1A). Conversely, KLF7 mRNA levels were gradually increased until 7 d but downregulated at 14 d (Fig. 1B). In addition, the KLF7 protein level change result was in parallel with the mRNA level (Fig. 1C). These data suggest that miR-494-3p and KLF7 were involved with peripheral nerve injury in Sprague Dawley rats.
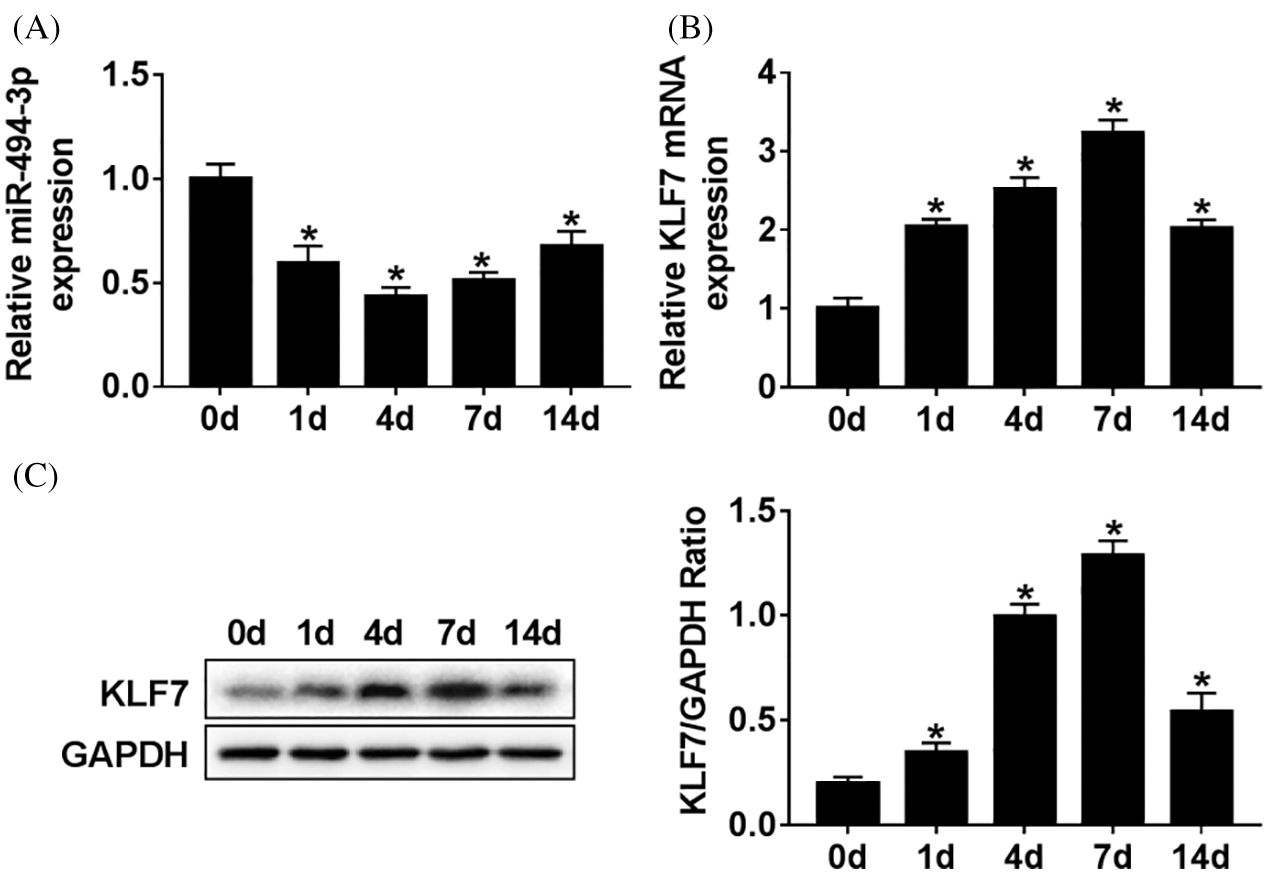
Figure 1: The expressions level of miR-494-3p and KLF7 in vivo.
Inhibition of miR-494-3p promoted cell proliferation and suppressed apoptosis of Schwann cells
As shown in Figs. 2A and 2B, transfection experiences were successful and confirmed that the expression of miR-494-3p was effectively increased in the miR-494-3p group when compared with that in the miR-NC group. In turn, anti-miR-494-3p led to a decrease of miR-494-3p in Schwann cells. To assess the biological effects of miR-494-3p on the Schwann cells, EdU based proliferation assays were conducted. The results suggested that the proliferation of Schwann cells transfected with miR-494-3p was inhibited compared to that in the miR-NC controls, while a higher level of cell viability was observed in anti-miR-494-3p cells than in the anti-NC group (Figs. 2C and 2D). Moreover, overexpression of miR-494-3p facilitated Schwann cells apoptosis, while miR-494-3p knockdown inhibited apoptosis by flow cytometry assays (Figs. 2E and 2F). Finally, western blot analysis was used to assess protein levels of Bcl-2, Bax, and C-caspase 3 in Schwann cells. Consistent with apoptosis results, overexpression of miR-494-3p repressed Bcl-2, but enhanced Bax and C-caspase 3 expression in Schwann cells, while miR-494-3p silencing led to opposite effect on the expression of Bcl-2, Bax, and C-caspase 3 (Figs. 2G–2J). Collectively, miR-494-3p knockdown promoted cell proliferation and suppressed apoptosis of Schwann cells.
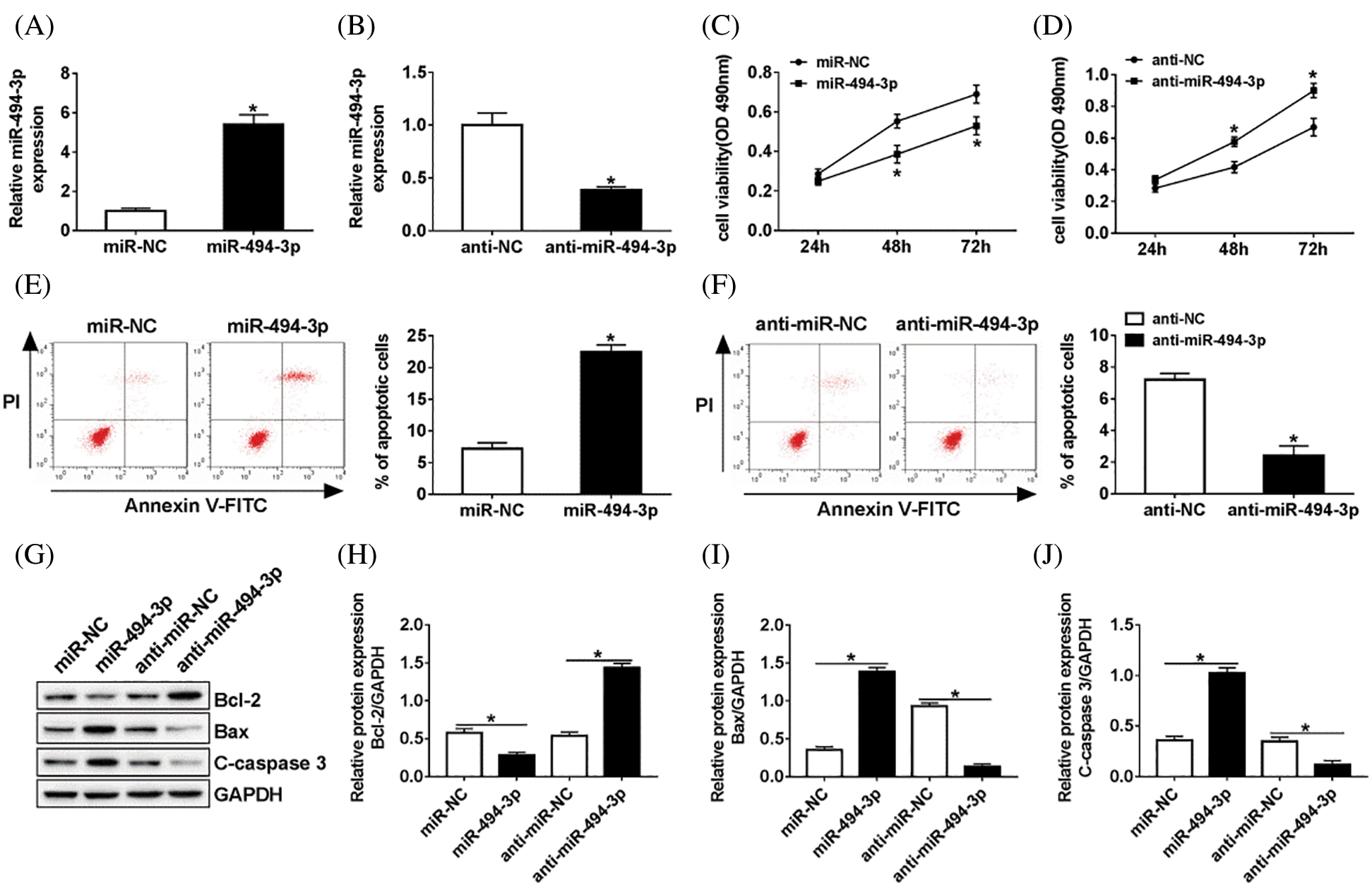
Figure 2: Efficacy of miR-494-3p in Schwann cells in vitro.
Since KLF7 and miR-494-3p were aberrantly expressed in vivo, we hypothesized that KLF7 might be targeted by miR-494-3p. As shown in Fig. 3A, TargetScan online provided the binding sites between KLF7 and miR-494-3p. To validate this prediction, we constructed the KLF7-WT/MUT luciferase reporter vector and then insured them into Schwann cells with miR-494-3p or miR-NC. Luciferase reporter analysis revealed that the luciferase activity was remarkably inhibited in Schwann cells transfected with KLF7-WT and miR-494-3p compared with that in the miR-NC group, while similar results were not found in the KLF7-MUT group (Fig. 3B). Moreover, overexpression of miR-494-3p resulted in an obvious reduced of KLF7 expression, no matter mRNA or protein (Figs. 3C–3E). All data indicated that miR-494-3p suppressed KLF7 expression in Schwann cells.
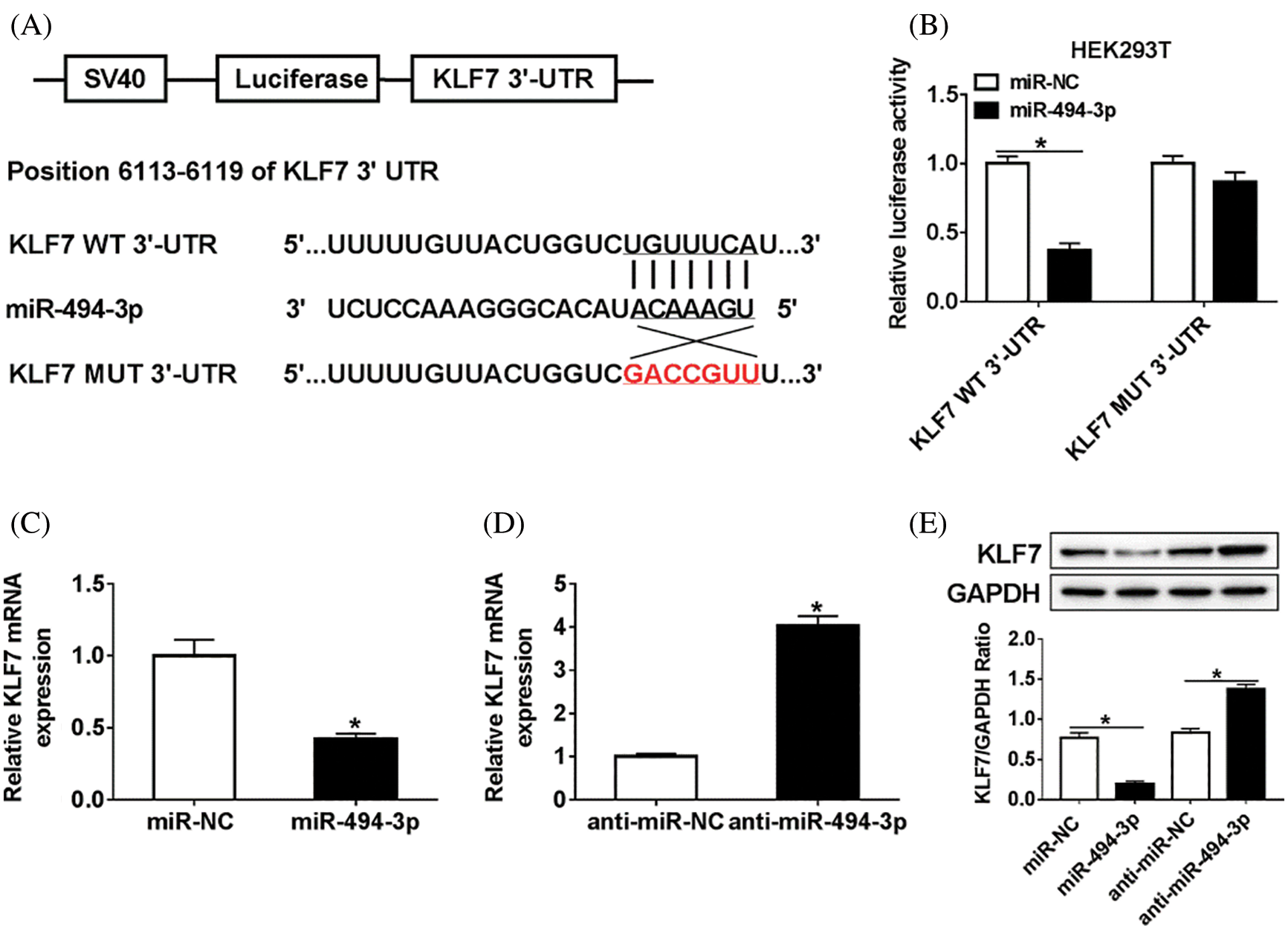
Figure 3: The association between miR-494-3p and KLF7 in Schwann cells.
KLF7 silencing inhibited proliferation and induced apoptosis of Schwann cells
To further explore the molecular mechanism of KLF7 regulating the biological processes, the loss functional experience was established. As present in Figs. 4A and 4B, KLF7 was decreased in Schwann cells treated with si-KLF7#1 or si-KLF7#2 when compared with the si-NC group. Meanwhile, cell viability was measured by Cell-LightTM EdU DNA Cell Proliferation Kit, and the results showed that the KLF7 knockdown impeded the proliferation of Schwann cells (Fig. 4C). In addition, flow cytometry analysis implied that the apoptosis rate of Schwann cells transfected with si-KLF7#1 and si-KLF7#2 was dramatically increased compared with control groups (Fig. 4D). The protein levels of Bcl-2, Bax, and C-caspase 3 were measured in cells transfected with si-KLF7#1, si-KLF7#2, or si-NC. The results of western blot analysis displayed that low-expression of KLF7 triggered a remark promotion in Bax and C-caspase 3 expression, while induced a reduction in Bcl-2 expression, suggesting that silencing of KLF7downregulated Bcl-2 and promoted Bax and C-caspase 3 expression to anti-apoptosis (Figs. 4E–4H). These data suggested that KLF7 has a vital role in Schwann cell growth and apoptosis.
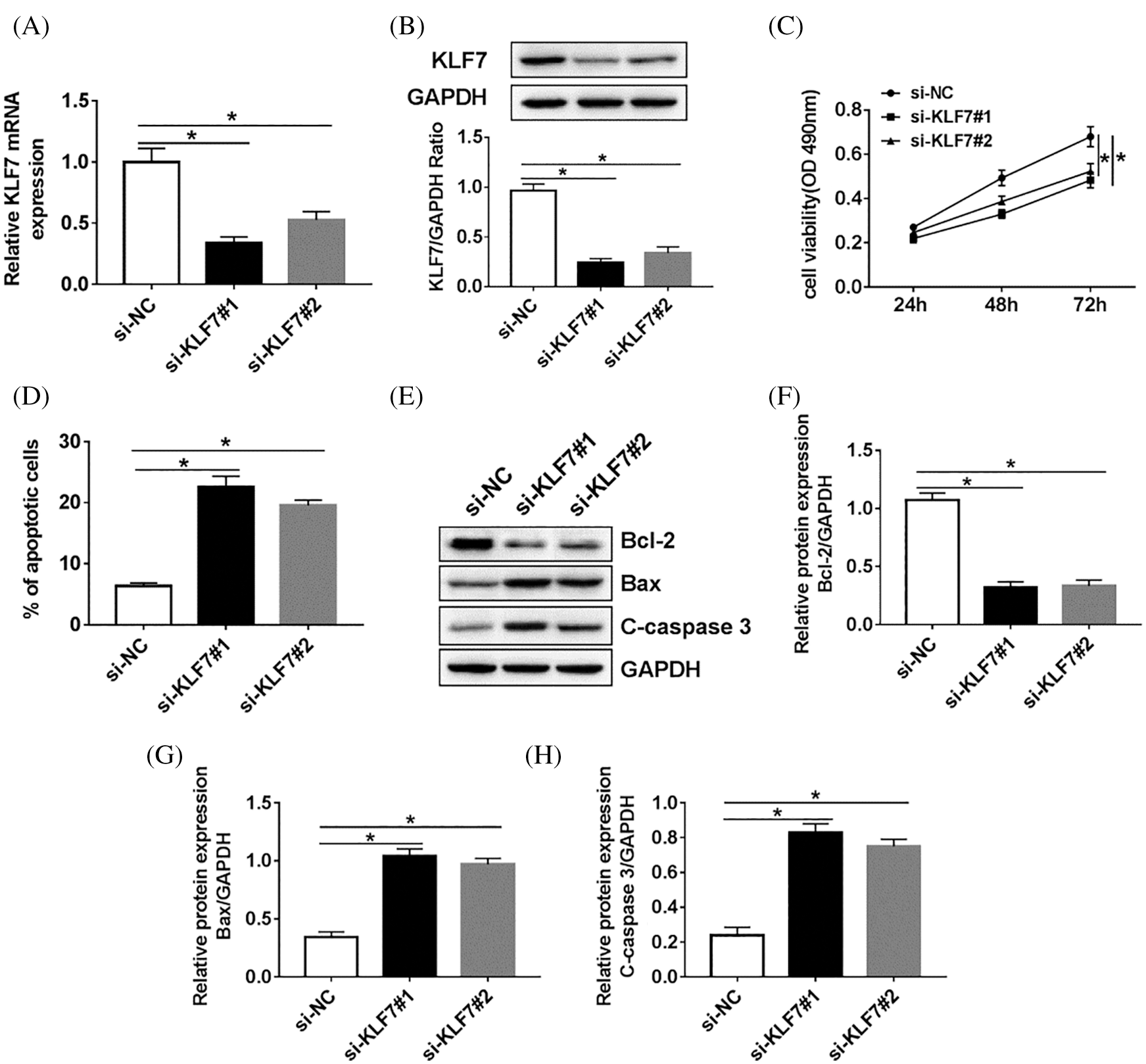
Figure 4: The effect of KLF7 silencing on proliferation and apoptosis of Schwann cells.
Overexpression of KLF7 reversed effects of miR-494-3p upregulation on proliferation and apoptosis in Schwann cells
Subsequently, the relationship between miR-494-3p and KLF7 in Schwann cells was analyzed. RT-qPCR and western blot assay were performed to assess the KLF7 level in Schwann cells transfected with miR-NC, miR-494-3p, miR-494-3p+Vector, or miR-494-3p+KLF7. The data showed that high expression of miR-494-3p induced the inhibitory effects on KLF7 expression, whereas KLF7 overexpression reversed these effects (Figs. 5A and 5B). Furthermore, the upregulation of KLF7 abolished the reduction of cell viability and the increase of apoptosis in Schwann cells caused by miR-494-3p overexpression (Figs. 5C and 5D). Similarly, apoptosis-related protein was examined by western blot and implied that miR-494-3p overexpression repressed Bcl-2 and promoted the expression of Bax and C-caspase 3, while the gain of KLF7 overturned upregulation of miR-142-5p mediated-effects on protein levels of Bcl-2, Bax, and C-caspase 3 (Figs. 5E–5H). Given the critical role of the ERK pathway during the sciatic nerve injury, we measured the expression levels of ERK pathway-related proteins in Schwann cells. As displayed in Supplementary Fig. 1, the ratio of ERK/p-ERK and JNK/p-JNK was increased in Schwann cells after transfection with KLF7, suggesting overexpression of KLF7 activating ERK pathway. Taken together, miR-142-5p directly targeted KLF7 to regulate proliferation and apoptosis of Schwann cells.
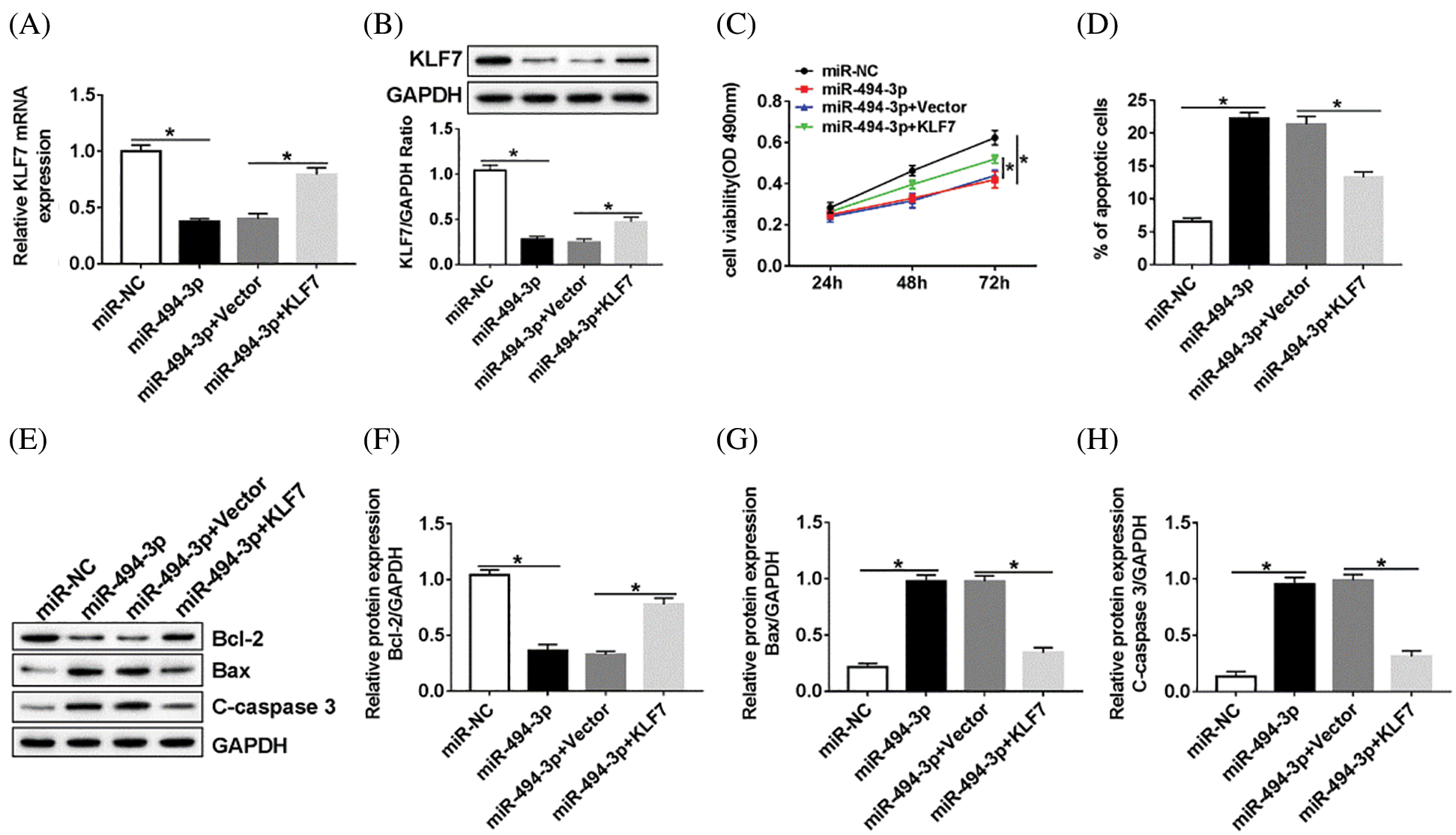
Figure 5: MiR-494-3p regulated cell proliferation and apoptosis through targeting KLF7.
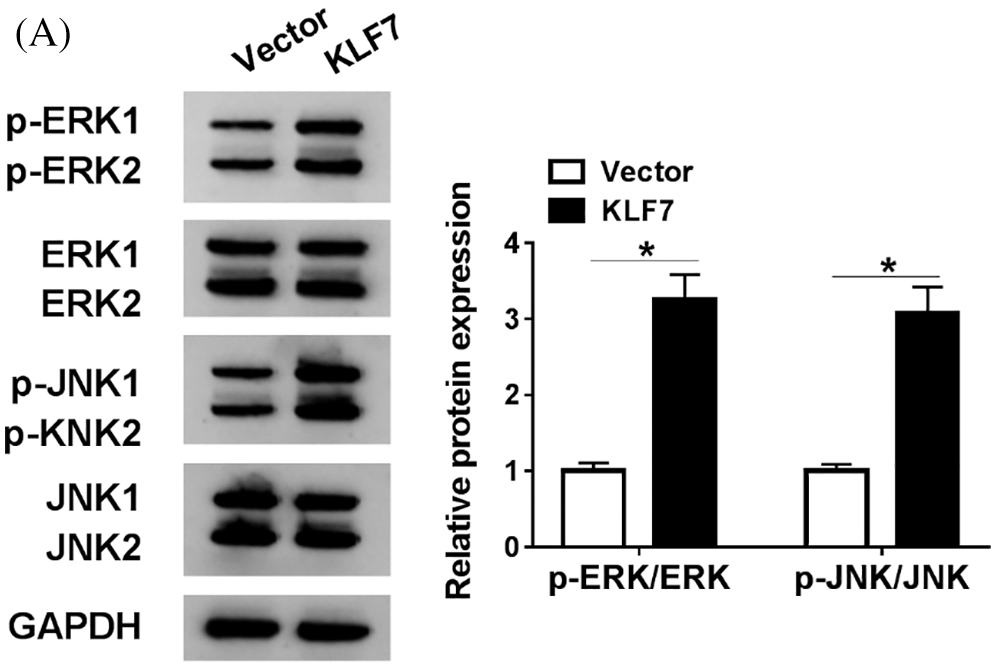
SuPPLEMENTARY figure 1: The expression of ERK pathway-related protein in Schwann cells.
A previous study has shown that miR-494-3p was downregulated in rat with sciatic nerve injury (Qian et al., 2018), but whether miR-494-3p plays a regulator role in the repair of peripheral nerve injury has not been defined. (Yi et al., 2016) tested miR-30c expression in Sprague Dawley rats that underwent sciatic nerve transection within 28 d (Yi et al., 2016); similarly, in the present study, we also established the Sprague Dawley model with peripheral nerve injury. Following sciatic nerve injury (within 14 d), miR-494-3p was downregulated, reaching a minimum on day 4, and was then upregulated to near normal level, while the KLF7 level was gradually increased until 7 d, but downregulated at 14 d. These results confirmed that the abnormal expression of miR-494-3p and KLF7 might play essential roles in peripheral nerve injury progression. However, the molecular events during peripheral nerve injury and regeneration remain not fully clear. (Zhan et al., 2017) firstly implied that miR-494 was upregulated in retinoblastoma, and miR-494 might contribute to the development of various cancer types (Zhan et al., 2017). Recent work illustrated that overexpression of miR-494 aggravated of oxidative stress in vivo and induced neuronal death by inhibiting DJ-1 (Xiong et al., 2014). Furthermore, miR-494 led to A549 lung cancer cells’ premature aging (Ohdaira et al., 2012). Not surprisingly, Li et al. further implied that miR-494 impeded cell proliferation and invasion via targeting SRY-related high mobility group-Box gene 9 (Li et al., 2015). These findings indicated that miR-494 might exert a suppression effect on cell proliferation by targeting different genes. Consistent with (Ventura and Jacks 2009) results, miRNAs functioning was depending on particular targets in different tissues (Ventura and Jacks, 2009). Our results showed that overexpression of miR-494 decreased cell viability and induced a significant increase of apoptosis; additionally, miR-494-3p knockdown obviously enhanced cell proliferation and suppressed death. Moreover, functional analysis implied that miR-494 triggered an obvious arrest in gap 2/mitosis in cholangiocarcinoma cells to modulate the cell cycle progression (Yamanaka et al., 2012). Moreover, miR-494-3p promoted tumor survival and metastasis in human glioblastoma cells by regulating proliferation and apoptosis through the PTEN/AKT signaling pathway (Li et al., 2015). However, it is unknown about the role of miR-494 during peripheral nerve injury. It has been demonstrated that KLF7 is principally involved in numerous processes of the nervous systems and can promote axon regeneration in the corticospinal tract (Blackmore et al., 2012; Laub et al., 2006). The association between miR-494 and KLF7 is worth further studied. KLF7 has been recognized as a regulator of axon outgrowth and regeneration (Lei et al., 2006). KLF7 was suggested to facilitate the recovery after injured nerves through the formation of synapses with motor neurons and axonal plasticity (Li et al., 2017). Furthermore, previous research revealed the important role of the ERK pathway in myelination (Veldman et al., 2007; Napoli et al., 2012). The activation of the ERK pathway contributed to the dedifferentiation of Schwann cells and nerve repair (Cervellini et al., 2018). Not surprisingly, the ERK pathway was activated in Schwann by KLF7 overexpression through phosphorylation of ERK and JNK, implying the neuroprotective properties of KLF7. Overexpression of KLF7 protected nerve cells from sciatic nerve injury by improving Schwann cells survival and axonal regeneration of the peripheral nerve and induced myelination fiber’s regeneration function (Wang et al., 2007).
Collectively, our data suggested that KLF7 was regulated by miR-494-3p in Schwann cells. Besides, restoration of KLF7 reversed the effects of miR-494-3p overexpression in Schwann cells, suggesting that miR-494-3p has a key role in the proliferation and apoptosis of Schwann cells via targeting KLF7. Collectively, our results clarified that miR-494 played a protective role in peripheral nerve injury, which will contribute to finding novel potential therapeutic targets for peripheral nerve injury in the future.
Overall, our study suggested that miR-494-3p silencing promoted cell proliferation and inhibited apoptosis of Schwann cells by negatively targeting KLF7 in vitro. In addition, the upregulation of KLF7 abolished the effects of miR-494-3p overexpression on proliferation and apoptosis of Schwann cells.
Availability of Data and Materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding Statement: The author(s) received no specific funding for this study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Battiston B, Papalia I, Tos P, Geuna S. (2009). Chapter 1 peripheral nerve repair and regeneration research: A historical note. International Review of Neurobiology 87: 1–7. [Google Scholar]
Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL. (2012). Krüppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proceedings of the National Academy of Sciences of the United States of America 109: 7517–7522. DOI 10.1073/pnas.1120684109. [Google Scholar] [CrossRef]
Bremer J, O’Connor T, Tiberi C, Rehrauer H, Weis J, Aguzzi A. (2010). Ablation of Dicer from murine Schwann cells increases their proliferation while blocking myelination. PLoS One 5: e12450. DOI 10.1371/journal.pone.0012450. [Google Scholar] [CrossRef]
Cervellini I, Galino J, Zhu N, Allen S, Birchmeier C, Bennett DL. (2018). Sustained MAPK/ERK activation in adult Schwann cells impairs nerve repair. Journal of Neuroscience 38: 679–690. DOI 10.1523/JNEUROSCI.2255-17.2017. [Google Scholar] [CrossRef]
Chahrour M, Jung SY, Shaw C, Zhou X, Wong STC, Qin J, Zoghbi HY. (2008). MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320: 1224–1229. DOI 10.1126/science.1153252. [Google Scholar] [CrossRef]
Chen ZL, Yu WM, Strickland S. (2007). Peripheral regeneration. Annual Review of Neuroscience 30: 209–233. DOI 10.1146/annurev.neuro.30.051606.094337. [Google Scholar] [CrossRef]
Gu X, Ding F, Yang Y, Liu J. (2011). Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Progress in Neurobiology 93: 204–230. DOI 10.1016/j.pneurobio.2010.11.002. [Google Scholar] [CrossRef]
He W, Li Y, Chen X, Lu L, Tang B, Wang Z, Pan Y, Cai S, He Y, Ke Z. (2014). miR-494 acts as an anti-oncogene in gastric carcinoma by targeting c-myc. Journal of Gastroenterology and Hepatology 29: 1427–1434. DOI 10.1111/jgh.12558. [Google Scholar] [CrossRef]
Jia X, Wang F, Han Y, Geng X, Li M, Shi Y, Lu L, Chen Y. (2016). miR-137 and miR-491 negatively regulate dopamine transporter expression and function in neural cells. Neuroscience Bulletin 32: 512–522. DOI 10.1007/s12264-016-0061-6. [Google Scholar] [CrossRef]
Laub F, Dragomir C, Ramirez F. (2006). Mice without transcription factor KLF7 provide new insight into olfactory bulb development. Brain Research 1103: 108–113. DOI 10.1016/j.brainres.2006.05.065. [Google Scholar] [CrossRef]
Lei L, Zhou J, Lin L, Parada LF. (2006). Brn3a and Klf7 cooperate to control TrkA expression in sensory neurons. Developmental Biology 300: 758–769. DOI 10.1016/j.ydbio.2006.08.062. [Google Scholar] [CrossRef]
Li J, Wang L, Liu Z, Zu C, Xing F, Yang P, Yang Y, Dang X, Wang K. (2015). MicroRNA-494 inhibits cell proliferation and invasion of chondrosarcoma cells in vivo and in vitro by directly targeting SOX9. Oncotarget 6: 26216–26229. DOI 10.18632/oncotarget.4460. [Google Scholar] [CrossRef]
Li S, Wang X, Gu Y, Chen C, Wang Y, Liu J, Hu W, Yu B, Wang Y, Ding F, Liu Y, Gu X. (2015). Let-7 microRNAs regenerate peripheral nerve regeneration by targeting nerve growth factor. Molecular Therapy 23: 423–433. DOI 10.1038/mt.2014.220. [Google Scholar] [CrossRef]
Li S, Yu B, Wang Y, Yao D, Zhang Z, Gu X. (2011). Identification and functional annotation of novel microRNAs in the proximal sciatic nerve after sciatic nerve transection. Science China Life Sciences 54: 806–812. DOI 10.1007/s11427-011-4213-7. [Google Scholar] [CrossRef]
Li WY, Wang Y, Zhai FG, Sun P, Cheng YX, Deng LX, Wang ZY. (2017). AAV-KLF7 promotes descending propriospinal neuron axonal plasticity after spinal cord injury. Neural Plasticity 2017: 22–22. DOI 10.1155/2017/1621629. [Google Scholar] [CrossRef]
Li XT, Wang HZ, Wu ZW, Yang TQ, Zhao ZH, Chen GL, Xie XS, Li B, Wei YX, Huang YL, Zhou YX, Du ZW. (2015). miR-494-3p regulates cellular proliferation, invasion, migration, and apoptosis by PTEN/AKT signaling in human glioblastoma cells. Cellular and Molecular Neurobiology 35: 679–687. DOI 10.1007/s10571-015-0163-0. [Google Scholar] [CrossRef]
Liu NK, Wang XF, Lu QB, Xu XM. (2009). Altered microRNA expression following traumatic spinal cord injury. Experimental Neurology 219: 424–429. DOI 10.1016/j.expneurol.2009.06.015. [Google Scholar] [CrossRef]
Liz J, Esteller M. (2016). lncRNAs and microRNAs with a role in cancer development. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 1859: 169–176. DOI 10.1016/j.bbagrm.2015.06.015. [Google Scholar] [CrossRef]
Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. (2012). A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 73: 729–742. DOI 10.1016/j.neuron.2011.11.031. [Google Scholar] [CrossRef]
Ohdaira H, Sekiguchi M, Miyata K, Yoshida K. (2012). MicroRNA-494 suppresses cell proliferation and induces senescence in A549 lung cancer cells. Cell Proliferation 45: 32–38. DOI 10.1111/j.1365-2184.2011.00798.x. [Google Scholar] [CrossRef]
Pepper JP, Wang TV, Hennes V, Sun SY, Ichida JK. (2017). Human induced pluripotent stem cell-derived motor neuron transplant for neuromuscular atrophy in a mouse model of sciatic nerve injury. JAMA Facial Plastic Surgery 19: 197–205. DOI 10.1001/jamafacial.2016.1544. [Google Scholar] [CrossRef]
Qian T, Fan C, Liu Q, Yi S. (2018). Systemic functional enrichment and ceRNA network identification following peripheral nerve injury. Molecular Brain 11: 73. DOI 10.1186/s13041-018-0421-4. [Google Scholar] [CrossRef]
Veldman MB, Bemben MA, Thompson RC, Goldman D. (2007). Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Developmental Biology 312: 596–612. DOI 10.1016/j.ydbio.2007.09.019. [Google Scholar] [CrossRef]
Ventura A, Jacks T. (2009). MicroRNAs and cancer: Short RNAs go a long way. Cell 136: 586–591. DOI 10.1016/j.cell.2009.02.005. [Google Scholar] [CrossRef]
Wang C, Sadovova N, Ali HK, Duhart HM, Fu X, Zou X, Patterson TA, Binienda ZK, Virmani A, Paule MG, Slikker W, Ali SF. (2007). l-Carnitine protects neurons from 1-methyl-4-phenylpyridinium-induced neuronal apoptosis in rat forebrain culture. Neuroscience 144: 46–55. DOI 10.1016/j.neuroscience.2006.08.083. [Google Scholar] [CrossRef]
Wang J, Muheremu A, Zhang M, Gong K, Huang C, Ji Y, Wei Y, Ao Q. (2016). MicroRNA-338 and microRNA-21 co-transfection for the treatment of rat sciatic nerve injury. Neurological Sciences 37: 883–890. DOI 10.1007/s10072-016-2500-6. [Google Scholar] [CrossRef]
Wang W, Kwon EJ, Tsai LH. (2012). MicroRNAs in learning, memory, and neurological diseases. Learning & Memory 19: 359–368. DOI 10.1101/lm.026492.112. [Google Scholar] [CrossRef]
Wang Y, Li WY, Jia H, Zhai FG, Qu WR, Cheng YX, Liu YC, Deng LX, Guo SF, Jin ZS. (2017). KLF7-transfected Schwann cell graft transplantation promotes sciatic nerve regeneration. Neuroscience 340: 319–332. DOI 10.1016/j.neuroscience.2016.10.069. [Google Scholar] [CrossRef]
Xiong R, Wang Z, Zhao Z, Li H, Chen W, Zhang B, Wang L, Wu L, Li W, Ding J, Chen S. (2014). MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiology of Aging 35: 705–714. DOI 10.1016/j.neurobiolaging.2013.09.027. [Google Scholar] [CrossRef]
Yamanaka S, Campbell NR, An F, Kuo SC, Potter JJ, Mezey E, Maitra A, Selaru FM. (2012). Coordinated effects of microRNA-494 induce G2/M arrest in human cholangiocarcinoma. Cell Cycle 11: 2729–2738. DOI 10.4161/cc.21105. [Google Scholar] [CrossRef]
Yi S, Yuan Y, Chen Q, Wang X, Gong L, Liu J, Gu X, Li S. (2016). Regulation of Schwann cell proliferation and migration by miR-1 targeting brain-derived neurotrophic factor after peripheral nerve injury. Scientific Reports 6: 29121. DOI 10.1038/srep29121. [Google Scholar] [CrossRef]
Yu B, Qian T, Wang Y, Zhou S, Ding G, Ding F, Gu X. (2012). miR-182 inhibits Schwann cell proliferation and migration by targeting FGF9 and NTM, respectively at an early stage following sciatic nerve injury. Nucleic Acids Research 40: 10356–10365. DOI 10.1093/nar/gks750. [Google Scholar] [CrossRef]
Yu B, Zhou S, Wang Y, Ding G, Ding F, Gu X. (2011). Profile of microRNAs following rat sciatic nerve injury by deep sequencing: Implication for mechanisms of nerve regeneration. PLoS One 6: e24612. DOI 10.1371/journal.pone.0024612. [Google Scholar] [CrossRef]
Yu B, Zhou S, Wang Y, Qian T, Ding G, Ding F, Gu X. (2012). miR-221 and miR-222 promote Schwann cell proliferation and migration by targeting LASS2 after sciatic nerve injury. Journal of Cell Science 125: 2675–2683. DOI 10.1242/jcs.098996. [Google Scholar] [CrossRef]
Zhan MN, Yu XT, Tang J, Zhou CX, Wang CL, Yin QQ, Gong XF, He M, He JR, Chen GQ, Zhao Q. (2017). MicroRNA-494 inhibits breast cancer progression by directly targeting PAK1. Cell Death & Disease 8: e2529. DOI 10.1038/cddis.2016.440. [Google Scholar] [CrossRef]
Zhao C, Sun G, Li S, Lang MF, Yang S, Li W, Shi Y. (2010). MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proceedings of the National Academy of Sciences of the United States of America 107: 1876–1881. DOI 10.1073/pnas.0908750107. [Google Scholar] [CrossRef]
 | This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |