 Open Access
Open Access
ARTICLE
Associated Factors of Anxiety Symptoms in Patients with Keratinocyte Carcinoma: A Cross-Sectional Study
1 No. 4 Ward, Hospital for Skin Diseases and Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, 210042, China
2 Department of One-Stop Service Center, Hospital for Skin Diseases and Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, 210042, China
3 Department of Clinical Laboratory, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, 210042, China
4 Pathology Department, Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, 210042, China
* Corresponding Authors: Xianfeng Cheng. Email: ; Hao Chen. Email:
# These authors contributed equally to this work
Psycho-Oncologie 2024, 18(3), 213-221. https://doi.org/10.32604/po.2024.052607
Received 28 April 2024; Accepted 27 May 2024; Issue published 12 September 2024
Abstract
Background: Keratinocyte carcinoma (KC) is a common malignancy characterized by a high recurrence rate and considerable psychological distress. The incidence of KC is increasing in China, raising concerns about its psychological consequences and adverse effects on quality of life. Demographic and clinical factors are thought to influence mental health outcomes in these patients. Nonetheless, data on the prevalence of anxiety in Chinese patients with KC and the factors associated with this anxiety are notably lacking. Therefore, a comprehensive investigation into the anxiety of patients with KC is imperative. Objective: This study aimed to investigate the prevalence of anxiety in patients with KC, a disease that can significantly affect a patient’s appearance and overall quality of life. Understanding the level of anxiety in this population is critical to developing targeted interventions, improving treatment outcomes, supporting mental health, and improving patient care practices. Methods: This cross-sectional study was conducted at China’s largest dermatology hospital from November 2017 to September 2022. A consecutive sampling method was used to recruit participants. Anxiety status was surveyed by the Self-rating Anxiety Scale (SAS). Explanatory variables were surveyed by demographic data questionnaires. Non-parametric test and Chi-square test analyses were used to compare the differences between groups. Multiple linear regression analysis was performed to identify factors associated with anxiety. Results: A total of 192 patients with KC were included. The median score of SAS was 35 (IQR 16.25). The prevalence of anxiety in patients with KC was 20.8%. Females (p = 0.008), under 60 years old (p = 0.011), living in rural (p = 0.010) or urban areas (p = 0.029), having fewer than three children (p = 0.016), with a history of skin diseases (p < 0.001), with a history of long-term oral medication (p = 0.001), and experiencing pain or itching (p = 0.001) had SAS scores that were significantly higher than their counterparts. Conclusion: This study showed that the prevalence of anxiety was very high among Chinese patients with KC, especially among women, young patients, rural residents, patients with fewer than three children, and individuals with a history of skin disease, long-term oral medications, or symptoms of pain or itching. Targeted psychological interventions for these specific populations should be implemented to effectively alleviate anxiety and improve quality of life in these at-risk groups.Keywords
Keratinocyte carcinoma (KC) is the most common malignant tumor in the world. It is a type of skin cancer that shares a common lineage with keratinocytes and is histologically similar to epidermal keratinocytes [1]. KC is also known as non-melanoma skin cancer, which includes basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) [1]. Among them, BCC accounts for 75%–80%, and SCC accounts for 20%–25% [2,3]. In 2018, an estimated number of 83,000 new cases of KC were reported in Italy [2]. A survey conducted in China revealed that in 2013, the prevalence of KC among individuals aged 60 and above was 0.59% [4]. The incidence of KC is high in older people over 65 years of age [5]. With population aging, the cases of KC have been steadily increasing globally in recent decades [6–8].
KC usually occurs in exposed parts of the body, such as the face and neck, so patients with KC often feel unpleasant and awkward in social situations [9–11]. KC typically progresses with significant physical symptoms, including pain, discharge, and bleeding, exacerbating the suffering of patients [12]. Furthermore, KC often presents as a local destructive growth pattern [13]. Treatment following diagnosis might result in scarring, physical anomalies, and functional limitations, potentially causing aesthetic discontent and daily life inconveniences [13]. A study showed that the development of KC is considered a chronic and disabling process. The likelihood of recurrence for KC is notably high, with around 40% of patients developing new tumors within a two-year period [14]. Thus, patients with KC are highly concerned about the prognosis of the disease [15]. The negative psychological impact of these diseases significantly affects the quality of life of patients and has attracted considerable research attention [11–13]. In the West, tumor location, age, gender, and education level are considered important factors influencing the psychological impact of patients with KC [16–20].
The rapid aging of the Chinese population, coupled with the rising prevalence of KC among the elderly, has heightened concerns about disease management and its impact on mental health [21–24]. In Chinese hospitals, patients with KC often express concerns about the disease affecting their eating and sleeping habits. Even though studies have proved that psychological distress is common in patients with KC, the prevalence of anxiety status and related risk factors in Chinese patients with KC remain unclear. The aim of this study was to estimate the prevalence and explore the risk factors of anxiety among Chinese patients with KC.
Research design and participants
A cross-sectional study was designed to investigate anxiety status and possible risk factors among patients with KC. A total of 212 participants were conveniently recruited between November 2017 to October 2022 in the Dermatology Hospital and Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College, the largest tertiary skin disease hospital in China. The inclusion criteria were as follows: (a) age over 18 years, (b) diagnosis of KC by pathological examination, and (c) with capacity to understand and complete the questionnaires. The exclusion criteria were as follows: (a) with the presence of psychiatric disorders, (b) with cognitive impairment, (c) with hyperthyroidism or other tumors or concomitant other serious systemic diseases, or (d) with history of alcohol and/or drug abuse. The calculation of the sample size was guided by the requirements for multivariate analysis, which generally recommends a minimum of 5–10 times the number of explanatory variables included in the model to ensure statistical validity. In this study, 13 explanatory variables were selected based on their relevance and prior research indicating their potential influence on anxiety outcomes in similar patient populations. Accordingly, the initial sample size needed ranged from 65 (13 variables × 5) to 130 (13 variables × 10). To account for potential sample attrition, such as non-response or incomplete data, which was anticipated at a rate of 20%, we adjusted the total number of survey samples required to between 78 and 156 (adding 20% to the initial estimate). This approach ensures adequate power to detect significant relationships between the explanatory variables and the outcome of interest, facilitating robust multivariate analysis.
Demographic data (gender, age, marital status, education level, place of residence, source of income, and number of children) and clinical information (type of carcinoma, tumor location, long history of UV exposure, history of long-term oral medication, original skin disease, and feeling of pain or itching) were collected using self-reported questionnaires.
Self-rating anxiety scale (SAS)
Anxiety status was assessed using the Self-rating Anxiety Scale. This scale was developed by Zung to evaluate affective and somatic aspects of anxiety [25]. Each item of this scale indicates the extent to which the patient felt about the problem with four response options and grading. The four response options are as follows: “none or a little of the time,” “some of the time,” “good part of the time,” or “most or all of the time” [25]. Every item’s score was determined as the raw score multiplied by 1.25. Utilizing a 20-item self-assessment scale, overall scores range from 25 to 100, where high scores correspond to increased anxiety levels [26]. A score above 45 is regarded as a strong predictor of anxiety [27]. Its concurrent validity is indicated by a correlation coefficient of 0.30 with the Taylor Manifest Anxiety Scale [25]. The Cronbach’s alpha coefficient of SAS was 0.865 [28]. In this study, the Cronbach’s alpha coefficient of SAS was 0.860.
The investigators were four registered nurses with bachelor’s degrees who had been working in the unit for more than 5 years, who had received standardized training in presenting the study and collecting data, and who were equipped to conduct questionnaires and respond to unexpected clinical situations. Before data collection, the aim of the study was explained to the participants. The study protocol was approved by the ethical review committee of Dermatology Hospital and Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College (2017-K-Y-006). Written informed consent for participation in the study was signed by each participant. The survey was conducted anonymously to ensure the confidentiality of participants. Prior to the formal study, a pilot study was conducted on 20 patients with KC who were subsequently excluded from the formal study. Participants’ understanding of the questionnaire items was ensured through face-to-face interviews in quiet areas of the ward during patients’ non-treatment and rest times.
We used the software IBM SPSS version 21.0 for statistical analyses. The explanatory variables (demographic and clinical data) of this study were presented in the form of categorical variables. Shapiro–Wilk Z test was used to evaluate the normality of continuous data. The continuous data with normal distribution were described by mean ± standard deviation (SD), and the continuous data with skewed distribution were represented by the median and interquartile range (IQR: Q3–Q1). For disordered categorical data, comparisons between groups were conducted using either Chi-square or Fisher’s exact tests. Continuous variables with a normal distribution were analyzed using t-tests, whereas those with a skewed distribution were examined through non-parametric tests (the Wilcoxon rank sum test for two-group comparisons and the Kruskal–Wallis H test for comparisons among multiple groups). Multiple linear regression analysis identified factors linked to SAS scores. p-values were bidirectional, and values below 0.05 were deemed significant. To identify and control confounding variables and to improve the accuracy and reliability of the findings, we first screened for factors that were statistically significantly associated with anxiety status through nonparametric tests. Subsequently, these factors were included in multiple linear regression analyses, and backward elimination by stepwise regression was used to further pinpoint variables with significant effects on anxiety.
A total of 192 patients out of 212 patients with KC were eventually investigated, with a response rate at 90.57%. The demographic and clinical data of participants are shown in Table 1. In this study, the average age of patients was 67 years (IQR 21). The participants of this study were predominantly over 59 years old (72.4%), male (52.6%), and married (83.9%). Rural patients accounted for 36.5% of the total participants, whereas patients living in town and in urban areas accounted for 23.4% and 40.1% of the total number, respectively. About 42.7% of participants had 3 or more children. BCC was more frequent (54.2%) than SCC (45.8%). Among the study participants, 72.4% presented with facial tumors, whereas 26% reported a history of prolonged exposure to UV radiation. Participants with a history of long-term oral medication, with previous primary skin diseases, and having a feeling of pain or itching accounted for 26%, 41.7%, and 56.3% of the total participants, respectively.
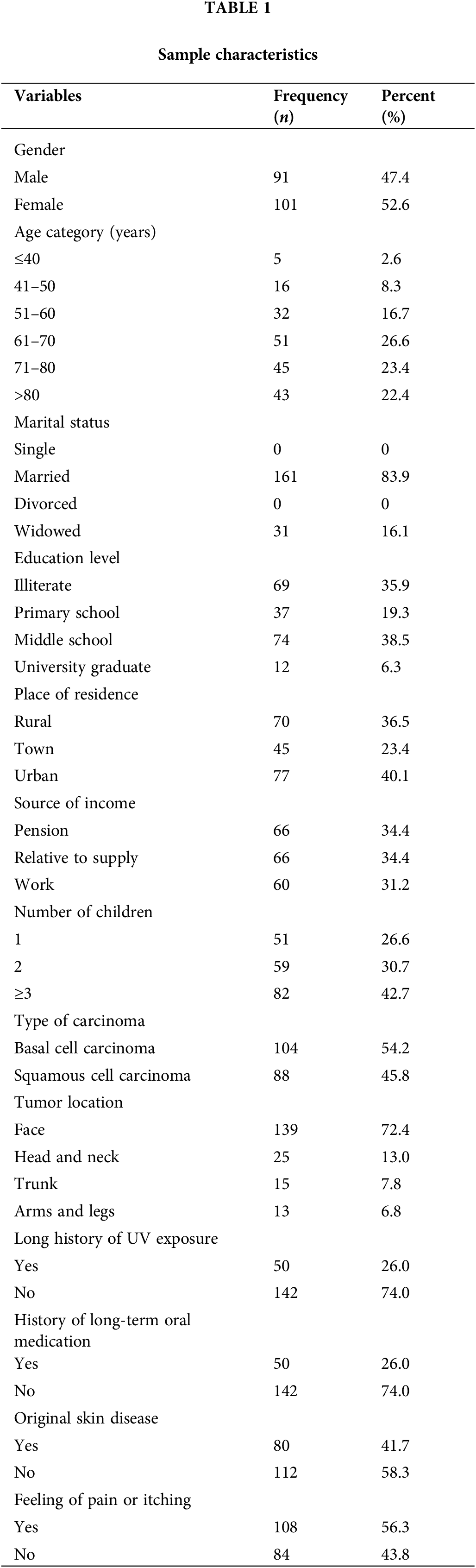
The median score on the SAS was 35 (IQR 16.25). The minimum value obtained was 25, and the maximum value was 75. Forty (20.8%) patients with KC in this study were categorized into the anxiety group according to the recommended cut-off points of SAS.
SAS scores were not significantly different among groups of marital status (p = 0.502), educational level (p = 0.679), source of income (p = 0.192), type of carcinoma (p = 0.466), tumor location (p = 0.084), and long history of UV exposure (p = 0.565). Nevertheless, we observed significant differences in SAS scores among groups with different gender (p = 0.016), age (p = 0.008), place of residence (p = 0.003), number of children (p = 0.006), history of long-term oral medication (p = 0.029), original skin disease (p = 0.001), and feeling of pain or itching (p = 0.001). The SAS scores were significantly higher in female and young patients than in males and older patients. Meanwhile, patients with 3 or more children had significantly lower SAS scores than those below 3 children. Compared with participants without a history of long-term oral medication, patients with long-term oral medication had higher SAS scores. Patients with original skin disease showed a statistically significant increase than the others. The SAS score was significantly higher in patients with a feeling of pain or itching than in those without a feeling (Table 2). We also observed a significant difference regarding place of residence. Following the Rank Cases operation, ranked SAS scores were obtained. Bonferroni’s post-hoc test indicated significantly lower SAS scores in patients residing in towns compared with those in rural (M = −4.907, SD = 1.841, p = 0.025) and urban areas (M = −6035, SD = 1.808, p = 0.003).
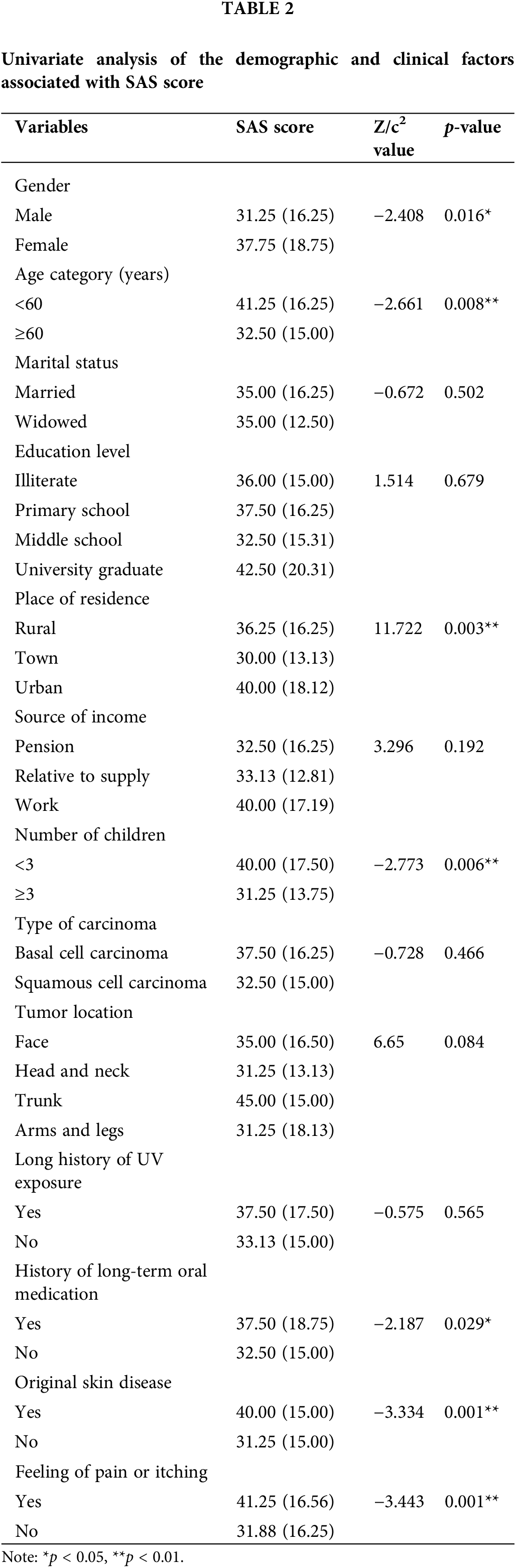
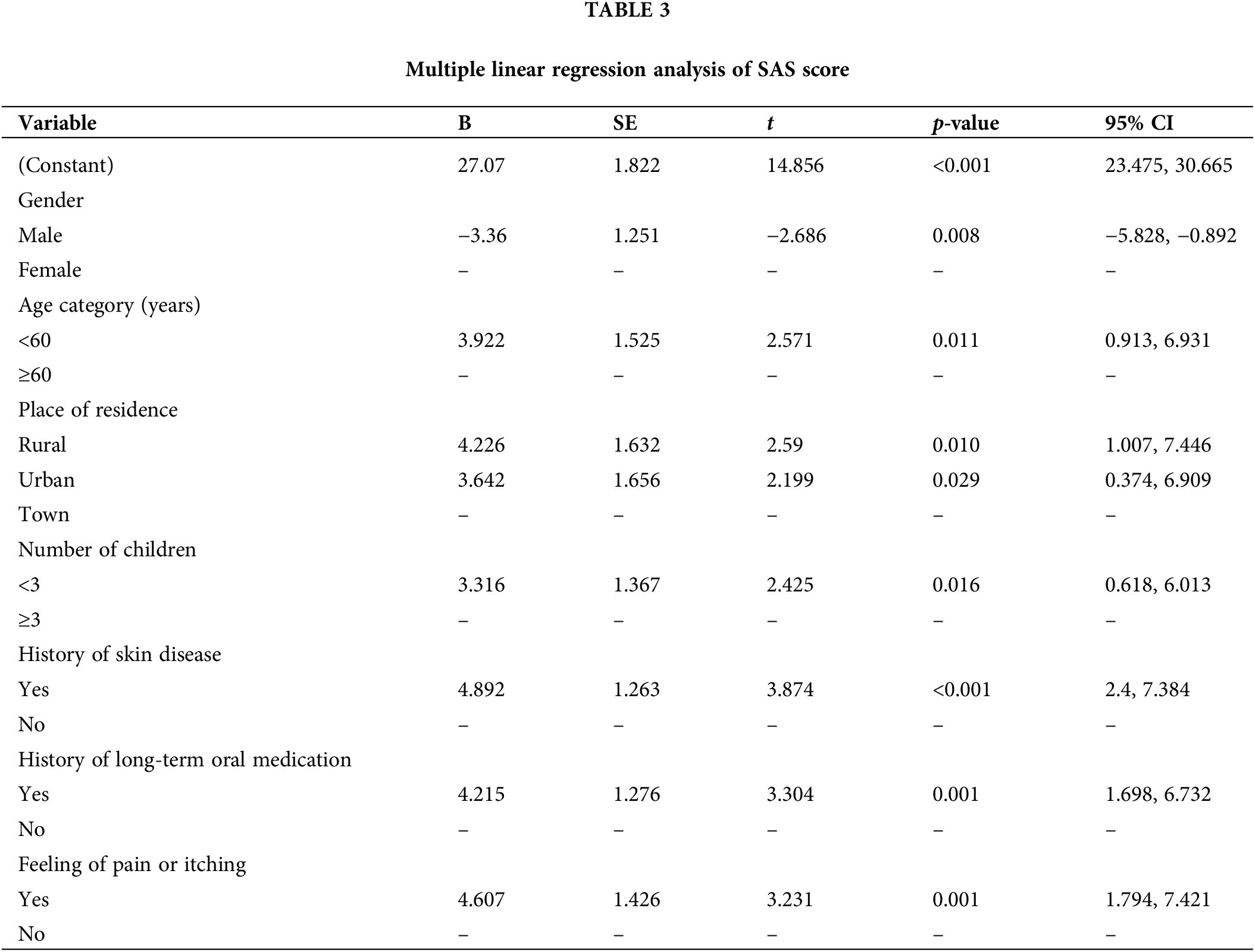
Multiple linear regression showed that gender (p = 0.008) was negatively associated with the SAS score, whereas factors of age (p = 0.011), place of residence (p = 0.010, p = 0.029), number of children (p = 0.016), history of skin disease (p < 0.001), history of long-term oral medication (p = 0.001), and feeling of pain or itching (p = 0.001) were positively associated with the SAS score, explaining 29.4% of the overall variance in anxiety after accounting for combined contribution (Table 3).
Subgroup analyses of anxiety scores
On the basis of the results of the multiple linear regression analyses, further subgroup analyses of the data were conducted to explore the potential impact of different factors on the anxiety state of specific patients with KC.
In the analyses grouped by gender, the number of significantly different results was much higher than that of other factors. This finding implied that gender may be an important dimension influencing the mental health of patients with KC, especially when assessing anxiety status. Specifically, the SAS score in women (37.75 [IQR 18.75]) was significantly higher than that in men (31.25 [IQR 16.25]; c2 = 6.133, p = 0.020). The prevalence rates of anxiety were 13.19% and 27.72% in male and female participants, respectively, in our study (X2 = 6.133, p =0.013; Fig. 1).
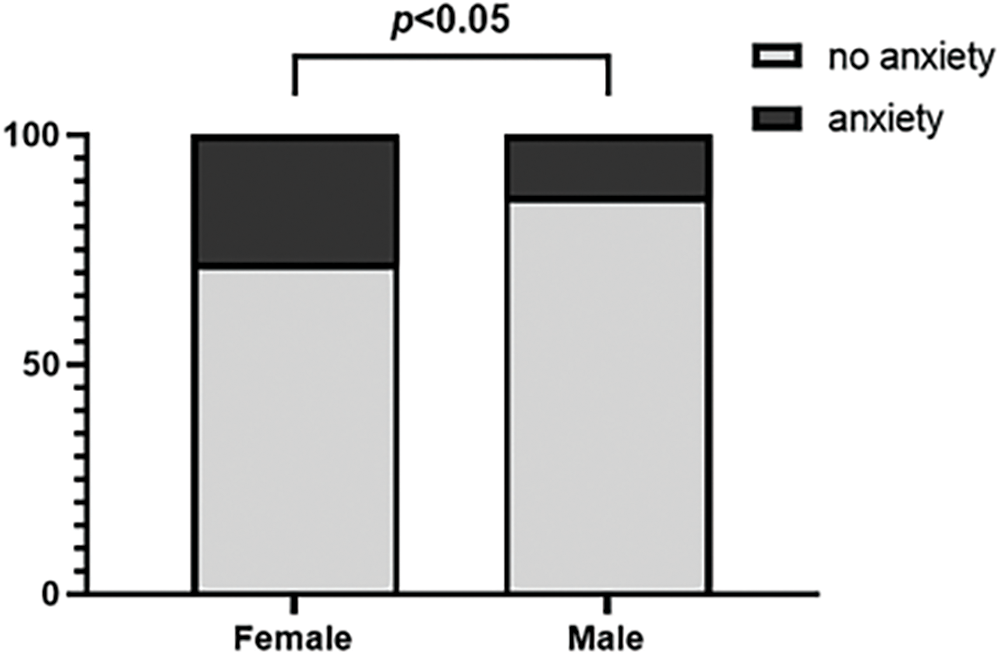
Figure 1: Prevalence of anxiety stratified by gender.
For women, anxiety level was not significantly different in groups of places of residence and history of long-term oral medication. Regarding age, women below 60 (44.38 [IQR 15.31]) had significantly higher anxiety scores than women aged 60 years or above (35.00 [IQR 16.25]; Z = −2.139, p = 0.032). In terms of number of children, women having few children (42.50 [IQR 16.25]) reported significantly higher anxiety scores than women having 3 or more children (35.00 [IQR 12.51]; Z = −2.401, p = 0.016). Regarding original skin disease, women with it (41.25 [IQR 12.19]) had significantly higher anxiety scores than women without it (32.50 [IQR 18.13]; Z = −2.872, p = 0.004). In terms of feeling of pain or itching, women with it (44.38 [IQR 13.44]) reported significantly higher anxiety scores than women without it (35.00 [IQR 15.63]; Z = −3.279, p = 0.001).
For men, of all the explanatory variables explored in this study, significant differences in anxiety levels were found only in groups of places of residence (c2 = 6.971, p = 0.031). However, further Bonferroni post-hoc test revealed no significant differences among patients who lived in town, rural, and urban areas.
This study estimated the prevalence and explored the risk factors of anxiety in Chinese patients with KC.
Anxiety status of patients with KC in China
We estimated that the prevalence of anxiety is 20.8% in patients with KC. However, the results of this study showed that its psychological impact status in patients with KC should not be ignored. Anxiety disorder is one of the most common comorbid mental disorders of cancer [29].
When dermatologists diagnose a skin disease as “cancer,” this label can provoke a high level of psychological distress in patients [30,31]. In particular, KC often occurs in areas critical for function and aesthetics, such as the face and neck, where treatment may result in disfigurement, leading to social and psychological functional impairments [32,33]. Additionally, the high recurrence rate of KC exacerbates the anxiety experienced by patients [34]. Unsurprisingly, patients exhibit a high prevalence of anxiety, reflecting the complex impact of this disease on psychological well-being.
Compared with the results of Radiotis, 18% of Canadian patients with KC were reported to experience anxiety, and the prevalence of anxiety in our patients was slightly higher [18]. KC has a high incidence but low mortality. For example, in the United States, the mortality rate of KC is 0.1%–0.3% [35]. Studies have shown that patients with higher education generally have a better understanding of the illness than those with low educational attainment [36]. In the past, higher education was not widely available in China.
The average age of our participants with KC was 67 years old. Only 6.3% of them received a university education, which was much lower than that in Canada (74.6%) [16]. Thus, when Chinese patients with lower education are diagnosed with cancer, they often link their disease to high mortality and severe complications, leading to anxiety.
When disclosing a cancer diagnosis to patients, healthcare professionals should be careful in choosing the appropriate time and place. In addition, health education must be strengthened, and healthcare professionals should patiently explain to patients the differences between KC and other types of cancer, particularly its low mortality rate, to alleviate their undue worries.
Factors influencing the anxiety of patients with KC in China
Several risk factors of anxiety were identified in this study. Factors of gender, age, place of residence, number of children, history of skin disease, history of long-term oral medication, and feeling of pain or itching were significantly associated with anxiety in patients with KC.
In this study, the prevalence of anxiety was 27.72% in female patients with KC and 13.19% male patients with KC. The number of women who suffered from negative psychological effects was double than that of men. Female patients with KC were significantly more anxious than male patients with KC. This result was in line with an Italian study [19]. Other studies also found that women with skin disease generally report greater impairment in psychology than men [37]. Women might face more severe and more constant pressure than men in real life [38]. Meanwhile, women might lack adequate and effective resources or strategies to cope with such stressors [39]. Compared with men, female patients with cancer are more sensitive, emotional, and prone to wallow in stress, with an increased risk of turning temporary negative emotions into anxiety [40]. In a study conducted by Buchhold et al. involving German patients with skin cancer, approximately 16.15% women and 13% men were found to experience psychological distress [41]. The high prevalence of anxiety in Chinese female patients with KC may be related to Chinese women’s social roles and role conflicts. In Chinese families, women not only take on most of the housework but also bear the main responsibility for educating children, which seriously affects their self-concept and individual identity. They desire more help, care, and support than men; when these needs are not adequately met in real life, severe anxiety may occur. In addition, KC challenges their self-confidence and mental image as they struggle with the visibility of their condition and its impact on their ability to fulfill expected social and family roles. This dynamic can exacerbate psychological distress and complicate their role-playing in social situations, further increasing their anxiety levels.
Our study found that younger patients (<60 years) were significantly more anxious than older patients (≥60 years). This finding was consistent with the result of a study conducted by Sobanko et al., who found that young US patients with skin cancer (40–60 years) reported higher anxiety levels than older patients (>60 years) [18]. A single-blinded prospective study showed that young age is a predictor of anxiety in patients with KC undergoing Mohs surgery [42]. KC is prone to occur in cosmetically important areas, and its treatment may cause disfigurement [33], which may commonly affect young patients’ social issues, such as attending meetings and returning to work [37]. Young patients with cancer often have multiple responsibilities with childcare, work, and other social roles, so they may not be able to receive adequate social support if their spouses have a full-time job [38]. Young patients may experience more competing demands during the disease phase, so their daily lives may be more disturbed by the disease than older patients [43]. Furthermore, young female patients are more anxious than those at an older age. Young women are more concerned about physical appearance [18]. The occurrence of KC at the exposed site and subsequent treatment at the visible site may exacerbate negative psychological effects, particularly affecting their self-confidence and individual identity. This may lead to a distorted mental image. Together, these factors may exacerbate the development of anxiety in this population.
This study found that patients having 3 or more children had lower anxiety levels than those with few children, especially for women. KC increases the financial burden on the family and the burden of care. Families with few children are unable to take turns caring for patients during hospital days in the same way as families with many children. The relatively high caring pressure can be passed on to patients and affect how they feel. For parents, especially mothers, having more children means having more family support and strength to rely on. This perceived family support may reduce patients’ anxiety [44].
In terms of patients with original skin disease, they had higher anxiety scores than those without it. In this study, original skin diseases mainly included actinic keratosis, chronic ulcers, and naevus. Skin abnormalities present before skin cancer is diagnosed may cause constant psychological stress and increase anxiety among patients.
Patients with long-term oral medication were more anxious than patients without it, especially women. To some extent, the long history of oral medication indicated that patients had other diseases requiring long-term medication, which could increase patients’ psychological burden. Female patients might adopt the sick role more easily than men [39], and a long medical history makes women more worried about their future compared with men.
This study also found that the feeling of pain or itching increased patients’ anxiety level, especially for women. The tumors may cause pain and pruritis and lead to functional limitations and cosmetic concerns [13]. These symptoms can increase patients’ perception and distress of the disease [13]. Women are more likely to communicate their symptoms and pain than men [39], and such repeated discussions might make women feel worse.
Moreover, the study revealed that patients living in town had lower anxiety than those living in rural and urban areas. On the one hand, people living in Chinese towns experience more convenient infrastructure (i.e., hospitals and supermarkets) and better medical insurance than those living in rural areas. On the other hand, patients living in towns may have less social and life stress than urban patients. These differences may be the reason why the anxiety level of the town patients was lower than that of the rural and urban patients.
Interestingly, other studies reported that the location of the tumor, particularly in the nasal cavity, is a significant factor affecting psychological distress [38], but we did not find this association in our study. This result indicated that the diagnosis of cancer may contribute to high levels of anxiety in Chinese patients with KC, regardless of the location of the tumors.
The findings emphasized the negative impact of KC on patients’ psychological health and the need for timely assessment of anxiety. To reduce public concerns and misconceptions about KC, especially the lower mortality rate of KC, the government should utilize the media and public education campaigns to raise public awareness of KC and its psychological impacts. They should also allocate special funds to support mental health services and related research to advance psychological intervention strategies. Healthcare facilities are urged to conduct training programs for medical staff, especially those who communicate diagnoses, so that cancer diagnoses are communicated in a supportive environment and in a compassionate and informative manner. Hospitals should create and distribute educational materials detailing the characteristics of KC, low risk of death, and treatment options to reduce patient anxiety. In addition, an interdisciplinary team of dermatologists, psychologists, nurses, and social workers should be formed to provide comprehensive mental health support to patients. Medical staff should raise awareness of mental health and focus on common psychological reactions after a cancer diagnosis, such as anxiety and depression, to effectively guide patients. Medical staff should utilize educational resources provided by the hospital to explain the nature, treatment, and prognosis of KC to patients, thereby reducing unnecessary panic. As advocates for patient well-being, nurses should play a key role in managing patients with KC. Nurses can provide vital emotional support, actively listen, show empathy, and create an environment where patients feel safe to express their fears and concerns. Additionally, spiritual care tailored to a patient’s beliefs can profoundly impact their ability to cope with their illness, providing comfort and strength in times of distress. Furthermore, providing patients with self-care skills enables them to effectively manage their condition, fosters independence, and improves overall quality of life.
This investigation is the first to determine the prevalence and identifying risk factors of anxiety in Chinese patients with KC. The insights from this research lay the groundwork for future interventions aimed at mitigating the negative psychological impacts on these patients. Notably, the study’s consecutive sampling method may introduce selection bias, potentially affecting the generalizability of the findings. Thus, further studies should aim for broad and representative samples. Additionally, the cross-sectional design precludes the establishment of causality among variables, highlighting the need for subsequent longitudinal studies. Lastly, the reliance on self-reported data could impact response accuracy, suggesting a need for objective measurement methods in future research.
This study demonstrated that the anxiety prevalence among patients with KC in China, particularly among women, is high. It highlighted the critical importance for healthcare professionals to identify and manage the anxiety symptoms in these patients, especially those at high risk for psychological distress, such as women, young patients, rural residents, having less than three children, history of skin disease, long-term history of oral medication, and feeling pain or itching. By providing targeted psychological support and therapy to alleviate anxiety, an improvement in their quality of life is anticipated. The identification of risk factors for anxiety in this study lays a foundation for devising intervention strategies, suggesting a future need for personalized approaches to intervention.
Acknowledgement: The researchers are very grateful to the Hospital for Skin Diseases and the Institute of Dermatology for allowing us to conduct this study. We also sincerely thank the data collectors and respondents. Without their persistent help, the study would not have been possible.
Funding Statement: This work was supported by the National Key R&D Program of China (2022YFC3601800); Natural Science Foundation of Jiangsu Province, Grant/Award Number: BK20211026; Natural Science Foundation of Jiangsu Province, Grant/Award Number: BK20231115; and Nursing Research Fund, Hospital for Skin Diseases and Institute of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College, Grant/Award Number: PYSHL2022001.
Author Contributions: (I) Conception and design: Qian Liu, Xianfeng Cheng, Hao Chen; (II) Administrative support: Qian Liu, Xianfeng Cheng, Hao Chen; (III) Provision of study materials or patients: Qian Liu, Hui Zhang; (IV) Collection and assembly of data: Qian Liu, Hui Zhang, Lijun Shen; (V) Data analysis and interpretation: Qian Liu; (VI) Manuscript writing: All authors; (VII) All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data will be provided upon request to the corresponding author.
Ethics Approval: The study protocol was approved by the Ethical Review Committee of Dermatology Hospital and Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College (2017-K-Y-006). Written informed consent for participation in the study was signed by each participant.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Karimkhani C, Boyers LN, Dellavalle RP, Weinstock MA. It’s time for “keratinocyte carcinoma” to replace the term “nonmelanoma skin cancer”. J Am Acad Dermatol. 2015;72(1):186–7. doi:10.1016/j.jaad.2014.09.036. [Google Scholar] [PubMed] [CrossRef]
2. Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–80. doi:10.1111/bjd.2012.166.issue-5. [Google Scholar] [CrossRef]
3. Cives M, Mannavola F, Lospalluti L, Sergi MC, Cazzato G, Filoni E, et al. Non-melanoma skin cancers: biological and clinical features. Int J Mol Sci. 2020;21(15):5394. doi:10.3390/ijms21155394. [Google Scholar] [PubMed] [CrossRef]
4. Tu QF, Lv T, Lai YX, Zhang LL, Shi L, Li JJ, et al. Epidemiological study of elderly skin tumors in a community in Shanghai. Chin J Geriatr. 2013;19(3):142–5 (In Chinese). [Google Scholar]
5. Ciążyńska M, Kamińska-Winciorek G, Lange D, Lewandowski B, Reich A, Sławińska M, et al. The incidence and clinical analysis of non-melanoma skin cancer [published correction appears in Sci Rep. 2021 Jul 28;11(1):15705]. Sci Rep. 2021;11(1):4337. [Google Scholar] [PubMed]
6. Tiyawatanaroj A, Sudtikoonaseth P, Chayangsu O. Basal cell carcinoma trends in Thailand: a 10-year retrospective study of demographic, clinical and histopathological features. Dermatol Reports. 2021;14(1):9413. [Google Scholar] [PubMed]
7. Hu W, Fang L, Ni R, Zhang H, Pan G. Changing trends in the disease burden of non-melanoma skin cancer globally from 1990 to 2019 and its predicted level in 25 years. BMC Cancer. 2022;22(1):836. doi:10.1186/s12885-022-09940-3. [Google Scholar] [PubMed] [CrossRef]
8. Jia MX, Li DX, Liu YL. Clinical retrospective analysis of 340 inpatients with malignant skin tumors in western inner mongolia. Int J Dermatol Venereol. 2021;4(1):36–9. doi:10.1097/JD9.0000000000000123. [Google Scholar] [CrossRef]
9. Philipp-Dormston WG, Müller K, Novak B, Strömer K, Termeer C, Hammann U, et al. Patient-reported health outcomes in patients with non-melanoma skin cancer and actinic keratosis: results from a large-scale observational study analysing effects of diagnoses and disease progression. J Eur Acad Dermatol Venereol. 2018;32(7):1138–46. doi:10.1111/jdv.2018.32.issue-7. [Google Scholar] [CrossRef]
10. Liu Q, Sha M, Xue B, Shen L, Li G, Cheng X. Health-related quality of life and associated factors among non-melanoma skin cancer patients: a cross-sectional study. Ann Transl Med. 2023;11(3):150. doi:10.21037/atm. [Google Scholar] [CrossRef]
11. Gaulin C, Sebaratnam DF, Fernández-Peñas P. Quality of life in non-melanoma skin cancer. Australas J Dermatol. 2015;56(1):70–6. doi:10.1111/ajd.2015.56.issue-1. [Google Scholar] [CrossRef]
12. Barazzetti DO, Barazzetti PHO, Cavalheiro BT, Ely JB, Nunes DH, Stamm AMNF. Quality of life and clinical and demographic characteristics of patients with cutaneous squamous cell carcinoma submitted to tumor resection by double-bladed scalpel. An Bras Dermatol. 2019;94(3):304–12. doi:10.1590/abd1806-4841.20197842. [Google Scholar] [PubMed] [CrossRef]
13. Ulrich C, Salavastru C, Agner T, Bauer A, Brans R, Crepy MN, et al. The European Status Quo in legal recognition and patient-care services of occupational skin cancer. J Eur Acad Dermatol Venereol. 2016;30(Suppl 3):46–51. [Google Scholar] [PubMed]
14. Black N. Patient-reported outcome measures in skin cancer. Br J Dermatol. 2013;168(6):1151. doi:10.1111/bjd.2013.168.issue-6. [Google Scholar] [CrossRef]
15. Siegel JA, Chren MM, Weinstock MA. Department of Veterans affairs keratinocyte carcinoma chemoprevention trial group. Correlates of skin-related quality of life (QoL) in those with multiple keratinocyte carcinomas (KCsa cross-sectional study. J Am Acad Dermatol. 2016;75(3):639–42. doi:10.1016/j.jaad.2016.05.008. [Google Scholar] [PubMed] [CrossRef]
16. Radiotis G, Roberts N, Czajkowska Z, Khanna M, Körner A. Nonmelanoma skin cancer: disease-specific quality-of-life concerns and distress. Oncol Nurs Forum. 2014;41(1):57–65. doi:10.1188/14.ONF.57-65. [Google Scholar] [PubMed] [CrossRef]
17. El Abbadi S, Susok L, Stockfleth E, Bechara FG, Gambichler T, Herbrandt S, et al. Comparison of the skin cancer quality of life impact tool and the skin cancer index questionnaire in measurement of health-related quality of life and the effect of patient education brochures in patients with actinic keratosis, non-melanoma skin cancer, and cutaneous melanoma [published correction appears in Dermatol Ther. 2021 May 6]. Dermatol Ther. 2021;11(3):929–40. [Google Scholar]
18. Sobanko JF, Zhang J, Margolis DJ, Etzkorn JR, Shin TM, Sarwer DB, et al. Patient-reported quality of life and psychosocial health prior to skin cancer treatment–A cross-sectional study. J Am Acad Dermatol. 2016;75(1):217–8.e2. doi:10.1016/j.jaad.2016.01.033. [Google Scholar] [PubMed] [CrossRef]
19. Sampogna F, Paradisi A, Iemboli ML, Fania L, Ricci F, Napolitano M, et al. Sex differences in health-related quality of life in patients with keratinocyte carcinomas. Acta Derm Venereol. 2021;101(4):adv00439. doi:10.2340/00015555-3736. [Google Scholar] [PubMed] [CrossRef]
20. Aggarwal P, Knabel P, Fleischer Jr AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J Am Acad Dermatol. 2021;85(2):388–95. doi:10.1016/j.jaad.2021.03.109. [Google Scholar] [PubMed] [CrossRef]
21. Asgari MM, Moffet HH, Ray GT, Quesenberry CP. Trends in basal cell carcinoma incidence and identification of high-risk subgroups, 1998–2012. JAMA Dermatol. 2015;151(9):976–81. doi:10.1001/jamadermatol.2015.1188. [Google Scholar] [PubMed] [CrossRef]
22. Garcovich S, Colloca G, Sollena P, Andrea B, Balducci L, Cho WC, et al. Skin cancer epidemics in the elderly as an emerging issue in geriatric oncology. Aging Dis. 2017;8(5):643–61. doi:10.14336/AD.2017.0503. [Google Scholar] [PubMed] [CrossRef]
23. Meng YH, Zhang Q, Zhang YB, Zhang CL. Retrospective analysis of 4695 cases of single skin tumor. Chin J Peking Univ (Health Sci). 2016;48(2):195–7 (In Chinese). [Google Scholar]
24. Guo HX, Liu G, Liu F, Xiao Y, Li PY. A retrospective analysis of 1293 cases of cutaneous malignant tumors. Chin J Dermatovenereol Integr Tradit West Med. 2019;18(2):107–10 (In Chinese). [Google Scholar]
25. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–9. doi:10.1016/S0033-3182(71)71479-0. [Google Scholar] [PubMed] [CrossRef]
26. Zung WW. The measurement of affects: depression and anxiety. Mod Probl Pharmacopsychiat. 1974;7:170–88. doi:10.1159/issn.0077-0094. [Google Scholar] [CrossRef]
27. Lindsay WR, Michie AM. Adaptation of the Zung self-rating anxiety scale for people with a mental handicap. J Ment Defic Res. 1988;32(6):485–90. [Google Scholar] [PubMed]
28. Ramirez SZ, Lukenbill J. Psychometric properties of the Zung self-rating anxiety scale for adults with intellectual disabilities (SAS-ID). J Dev Phys Disabil. 2008;20:573–80. doi:10.1007/s10882-008-9120-x. [Google Scholar] [CrossRef]
29. Mehnert A, Brähler E, Faller H, Härter M, Keller M, Schulz H, et al. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol. 2014;32(31):3540–6. doi:10.1200/JCO.2014.56.0086. [Google Scholar] [PubMed] [CrossRef]
30. Meggiolaro E, Berardi MA, Andritsch E, Nanni MG, Sirgo A, Samorì E, et al. Cancer patients’ emotional distress, coping styles and perception of doctor-patient interaction in European cancer settings. Palliat Support Care. 2016;14(3):204–11. doi:10.1017/S1478951515000760. [Google Scholar] [PubMed] [CrossRef]
31. Aymonier M, Taieb C, Corgibet F, Joly P, Sei JF, Chaussade V, et al. Patient perception of the diagnosis announcement and its impact on quality of life of patients with primary melanoma or basal cell carcinoma. Acta Derm Venereol. 2022;102:adv00717. doi:10.2340/actadv.v102.2217. [Google Scholar] [PubMed] [CrossRef]
32. Sampogna F, Paradisi A, Iemboli ML, Ricci F, Sonego G, Abeni D. Comparison of quality of life between melanoma and non-melanoma skin cancer patients. Eur J Dermatol. 2019;29(2):185–91. doi:10.1684/ejd.2019.3523. [Google Scholar] [PubMed] [CrossRef]
33. Sanchez N, Griggs J, Nanda S, Fayne R, Castillo D, De Bedout V, et al. The skin cancer index: quality-of-life outcomes of treatments for nonmelanoma skin cancer. J Dermatolog Treat. 2020;31(5):491–3. doi:10.1080/09546634.2019.1674772. [Google Scholar] [PubMed] [CrossRef]
34. Rhee JS, Matthews BA, Neuburg M, Logan BR, Burzynski M, Nattinger AB. Validation of a quality-of-life instrument for patients with nonmelanoma skin cancer. Arch Facial Plast Surg. 2006;8(5):314–8. doi:10.1001/archfaci.8.5.314. [Google Scholar] [PubMed] [CrossRef]
35. Lai SY, Weber RS. High-risk non-melanoma skin cancer of the head and neck. Curr Oncol Rep. 2005;7(2):154–8. doi:10.1007/s11912-005-0042-9. [Google Scholar] [PubMed] [CrossRef]
36. Raghupathi V, Raghupathi W. The influence of education on health: an empirical assessment of OECD countries for the period 1995–2015. Arch Public Health. 2020;78:1–18. [Google Scholar]
37. Vaidya TS, Mori S, Dusza SW, Rossi AM, Nehal KS, Lee EH. Appearance-related psychosocial distress following facial skin cancer surgery using the FACE-Q Skin Cancer. Arch Dermatol Res. 2019;311(9):691–6. doi:10.1007/s00403-019-01957-2. [Google Scholar] [PubMed] [CrossRef]
38. Peters L, Brederecke J, Franzke A, de Zwaan M, Zimmermann T. Psychological distress in a sample of inpatients with mixed cancer-a cross-sectional study of routine clinical data. Front Psychol. 2020;11:591771. doi:10.3389/fpsyg.2020.591771. [Google Scholar] [PubMed] [CrossRef]
39. Matud MP, Bethencourt JM, Ibáñez I. Gender differences in psychological distress in Spain. Int J Soc Psychiatry. 2015;61(6):560–8. doi:10.1177/0020764014564801. [Google Scholar] [PubMed] [CrossRef]
40. Bektas DK, Demir S. Anxiety, depression levels and quality of life in patients with gastrointestinal cancer in Turkey. Asian Pac J Cancer Prev. 2016;17(2):723–31. doi:10.7314/APJCP.2016.17.2.723. [Google Scholar] [PubMed] [CrossRef]
41. Buchhold B, Lutze S, Arnold A, Jülich A, Daeschlein G, Wendler M, et al. Psychosocial distress and desire for support among skin cancer patients-impact of treatment setting. J Dtsch Dermatol Ges. 2018;16(7):861–71. doi:10.1111/ddg.2018.16.issue-7. [Google Scholar] [CrossRef]
42. Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in mohs surgery. Dermatol Surg. 2017;43(8):1029–35. doi:10.1097/DSS.0000000000001152. [Google Scholar] [PubMed] [CrossRef]
43. Mor V, Allen S, Malin M. The psychosocial impact of cancer on older versus younger patients and their families. Cancer. 1994;74(7 Suppl):2118–27. doi:10.1002/(ISSN)1097-0142. [Google Scholar] [CrossRef]
44. Wang L, Luo J, Li Y, Zhou Y, Wang W. Social support, anxiety, and depression in patients with prostate cancer: complete mediation of self-efficacy. Support Care Cancer. 2022;30(8):6851–6. doi:10.1007/s00520-022-07065-8. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools