 Open Access
Open Access
ARTICLE
IMPACT de la déprivation sociale sur les difficultés psychosociales au décours d’un cancer pédiatrique : une étude prospective
IMPACT of the Social Deprivation on Psychosocial Difficulties of Pediatric Cancer Survivors: A Prospective Study
1 U1086 INSERM “Anticipe”, Caen, 14000, France
2 Department of Pediatric Hematology and Oncology, University Hospital of Caen, Caen, 14000, France
3 Research Department, University Hospital of Caen, Caen cedex, 14000, France
4 Department of Pediatric Hematology and Oncology, University Hospital of Angers, Angers, 49000, France
5 Centre François Baclesse, Caen, 14000, France
6 CIC 1414 (Centre d’Investigation Clinique de Rennes), Rennes University, CHU Rennes, Inserm, Rennes, 35000, France
7 Department of Pediatric Hematology and Oncology, University Hospital of Brest, Brest, 29200, France
8 Department of Pediatric Hematology-Oncology, Clocheville Hospital, University Hospital of Tours, Tours, 37000, France
9 Pediatric Oncology Unit, CIC 802 INSERM, University Hospital of Poitiers, Poitiers, 86000, France
10 Department of Pediatric Hematology-Oncology, University Hospital of Nantes, Nantes, 44000, France
11 Department of Pediatric Hematology and Oncology, University Hospital of Rennes, Rennes, 35000, France
12 Service de Chirurgie Digestive, CHU Caen, Université de Caen-Normandie, Caen, 14000, France
13 Department of Pediatric Surgery, Caen University Hospital, Avenue de la Côte de Nacre, Caen, 14000, France
* Corresponding Author: Fanny Delehaye. Email:
Psycho-Oncologie 2024, 18(2), 117-126. https://doi.org/10.32604/po.2024.043073
Received 20 June 2023; Accepted 20 November 2023; Issue published 06 August 2024
RÉSUMÉ
La période post-traitement une part fondamental de la prise en charge du cancer pédiatrique. Durant cette période, les difficultés scolaires et psychologiques chez les survivants d’un cancer pédiatriques (SCP) sont connues et peuvent être pronostic sur la bonne réintégration sociale. Cette étude estime l’influence de la déprivation sociale du foyer de l’enfant sur ces difficultés. Notre étude se base sur une base de données multicentrique, et se concentre sur les SCP avant reçu une évaluation psychosociale au cours de leur suivi, de 2013 à 2020. Nous rapportons les données des difficultés scolaires et psychologiques. Le statut socio-économique du foyer de l’enfant a été estimé par un score de déprivation sociale. Nous rapportons les données de 1003 patients. Les difficultés scolaires ont été rapportés chez 22% d’entre eux. Une déprivation sociale plus importante était associée à la survenue de difficultés scolaires. La rechute tumorale, le traitement par greffe de cellules souches hématopoïétiques, et les tumeurs du système nerveux central étaient d’autres facteurs de risque. Dans le groupe des patients avec tumeurs du système nerveux central, la déprivation sociale était également un facteur associé à la survenue de difficultés scolaires. Les difficultés psychologiques n’étaient quant à elles pas associées avec le score de déprivation. Il existe un lien entre statut socio-économique et les difficultés scolaires chez les SCP. Des analyses complémentaires doivent être réalisées, notamment chez les enfants avec tumeurs du système nerveux central, qui est la population la plus concernée.Abstract
The posttreatment period is a key part of the management of pediatric cancer. During this time, school and psychological difficulties have been described in childhood cancer survivors (CCS) and can be prognostic for the success of social reintegration. This study estimated the influence of the household’s socioeconomic status (SES) on these psychosocial difficulties. This study is based on a prospective multicentric database and focused on children who received a psychosocial evaluation during their follow-up from 2013 to 2020. We retrieved data on school and psychological difficulties. Household SES was estimated by a social deprivation score. Data from1003 patients were analyzed. School difficulties were noted in 22% of CCS. A greater social deprivation was significantly associated with school difficulty. Tumor relapse, treatment with hematopoietic stem cell transplantation, and central nervous system (CNS) tumors remained significant risk factors. In the subgroup of CNS tumors, school difficulties were increased and associated with greater social deprivation. Psychological difficulties were not associated with the deprivation score. There is a link between SES and school difficulties in CCS. Further investigations should be carried out for children with CNS tumors, which is the population of the greatest concern.MOTS CLÉS
Keywords
In France, the incidence of pediatric cancer is reported to be 156.6 per million children aged 0–14 years per year and increases to 231.9 cases per million person-years in children aged 15–19 years [1,2]. Over time, a significant improvement in long-term survival rates of pediatric cancers has been achieved. Currently, the 5-year survival rate is reported at 75% in Europe [3]. Therefore, the post-treatment period is a key part of the management. At this period, the main goal is to help the patient return to a normal life. Childhood cancer and its treatments may induce sequalae. Among them, psychosocial affections, i.e., difficulties in educational achievement, and psychological well-being can be a prognosis for the success of the patients’ post-cancer reintegration [4,5]. These consequences are mostly described for children with central nervous system (CNS) tumors, with cranial radiotherapy, and with a younger age at diagnosis [4,6–9]. Some of these consequences may be potentiated by the household’s socio-economic status (SES). Therefore, SES can be associated with the probability of having a psychosocial difficulty as well as the ability of the family to be able to deal with it. Associations between school difficulties, psychological well-being, and household SES are already known in cancer survivors [10–13]. However, these results mainly focus on the evaluation of these difficulties with important hindsight, when the children’s social reintegration is effective [6–8,11,12].
The aim of our study was to estimate the association between SES and psychosocial difficulties in childhood cancer survivors (CCS) during the posttreatment period based on a social deprivation score. We evaluated school and psychological difficulties immediately after the hospital care period, a time when interventions could be possible to avoid the long-term consequences and improve social reintegration.
We carried out a retrospective cross-sectional study based on a multicentric database.
This multicentric study focused on the children from the RECAPGO database (REcueil des CAncers Pédiatriques du Grand Ouest) who received a short psychosocial evaluation (described in the variables paragraph). This evaluation was carried out during a dedicated time allowed in a consultation interviewing CCS and their parents. These consultations occurred during years of follow-up (FU) after the end of the child’s intense treatment requiring hospital care when the social reintegration is effective. The interviews did not respect a specific structure, all pediatricians were free in how they conducted this evaluation. However, the structure of the interview was suggested by a global questionnaire, which included the evaluation of clinical variables, but also two open questions about the presence of psychological and school difficulties. Each SE difficulty was reported independently. All the children included in the database were eligible for this evaluation, without difference between children who received the evaluation or not according to their SES (Table 1). We defined the psychosocial FU as completing at least one assessment for these two difficulties at least once during this FU period.
The RECAPGO database results from a collaboration among seven French pediatric oncology hospitals, reported as the French children’s oncology study Group GOCE (Grand Ouest pour les Cancers de l’Enfant). This prospective database has been open since 01/01/2013 and aims to include all patients aged under 25 years diagnosed with cancer, a hematological malignancy, aplastic anemia, or a Langerhans cell histiocytosis at these participating hospitals. We included patients recorded at diagnosis with a single tumor experience from 01/01/2013 to 08/01/2020. Patients with no known address at diagnosis, those living outside of the GOCE departments, those with no estimated social deprivation score, those aged over 18 years, and those with missing tumor-type data were excluded. One hospital did not complete the psychosocial questionnaire and was excluded from the analysis (Fig. 1).
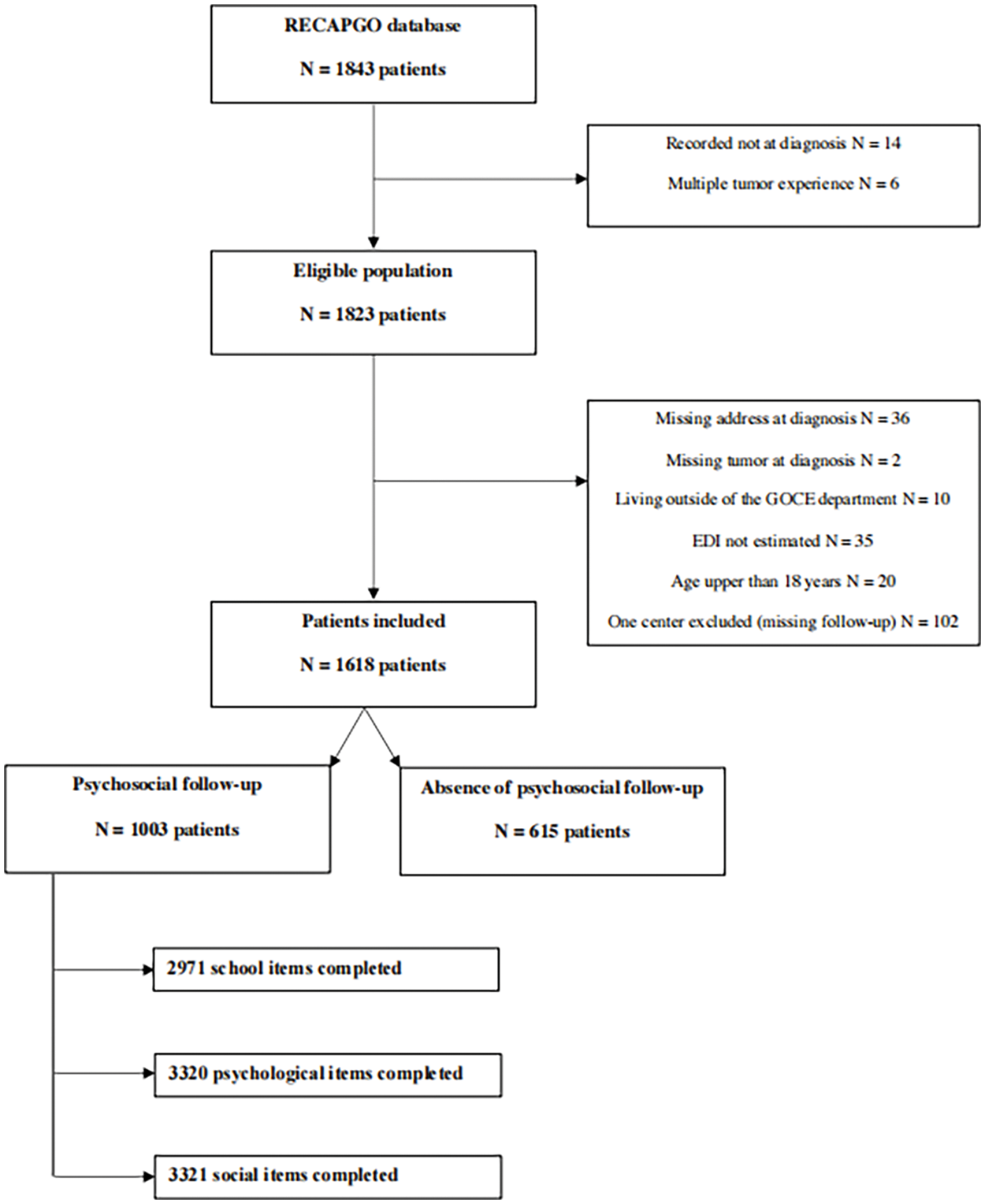
Figure 1: Flow chart of the study.
We defined three variables (i) At each consultation during the FU, oncologist pediatricians focused on some items: psychological (global well-being, anxiety, depression,…) and scholarly (marks, attitude at school, socialization,…), collected during the patient interviews without following a specific questionnaire. For some patients already known to be in socio-economic difficulties, the hospital management of the psychosocial FU could be done by a multidisciplinary team involving psychologists and teachers dedicated to social reintegration. Association with scholar and psychological assistance presented by the patient let clinicians synthesize psychological and scholarly difficulties by a binary response (yes/no) if the difficulty was present or not (for example repeated grades, access to special education, anxiety in socialization, sadness, …). (ii) The clinical variables were the patients and the characteristics of their tumors. We merged the tumor types into five categories; i.e., blood disorders (leukemia, aplastic anemia, lymphoma), CNS tumors, solid tumors, bone tumors, and other tumors (iii) Two variables evaluated the SES of CCS and their household. First, the European Deprivation Index (EDI) assessed the socioeconomic environment of each patient. This deprivation indicator is constructed by selecting fundamental needs associated both with objective and subjective poverty [14]. This score is determined by an ecological measure using the IRIS scale (Ilôts Regroupés pour l’Information Statistique), which represents the smallest French geographical area for which there is a statistical evaluation to estimate social deprivation. Based on their addresses at diagnosis, each patient can be associated with an IRIS, and thus, their EDI can be established [14,15]. For interpretation, the highest EDI is associated with greater social deprivation. Second, we used the travel time defined as the shortest time to travel by car from the patient’s address to the referring hospital to assess geographical disparities. A geographical information system (ArcGIS 10.5®—Esri France) associated with a road map database (Navstreets®, provided by HERE and Esri France) was used for this estimation. For our database, each patient’s EDI and travel time were estimated from the address at diagnosis.
Baseline characteristics of the population were described as the mean (+/− standard deviation) for quantitative variables and numbers (percentages) for qualitative variables. Quantitative and qualitative variables were analyzed by t-tests and chi2 tests, respectively. Two types of analysis were carried out (i) A multivariate analysis by logistic regression model was conducted to estimate factors associated with the probability of declaring a school or psychological difficulty, considering mixed effects for longitudinal data. We defined at the first level each consultation during which the psychosocial evaluation was completed. We defined at the second level the children for which the consultations were conducted. Factors associated with a p-value < 0.10 in the univariate analysis were considered in the multivariate logistic model. Subgroup analyses were carried out according to the tumor type (ii) We evaluated the probability of school and psychological difficulty over time by using Kaplan-Meier failure function curves. We completed a parametric survival regression model based on the same longitudinal data structure with two levels. All statistical analyses were performed with STATA software V14 and a p-value < 0.05 was considered to denote statistical significance.
We defined the primary endpoint as the probability of presenting a learning difficulty at school. The secondary endpoint was to assess these difficulties over time. Psychological difficulties were also analyzed for further explorations.
This study was based on a database supported by the Commission Nationale de l’Informatique et des Libertés (CNIL) registered under the N° 912302.
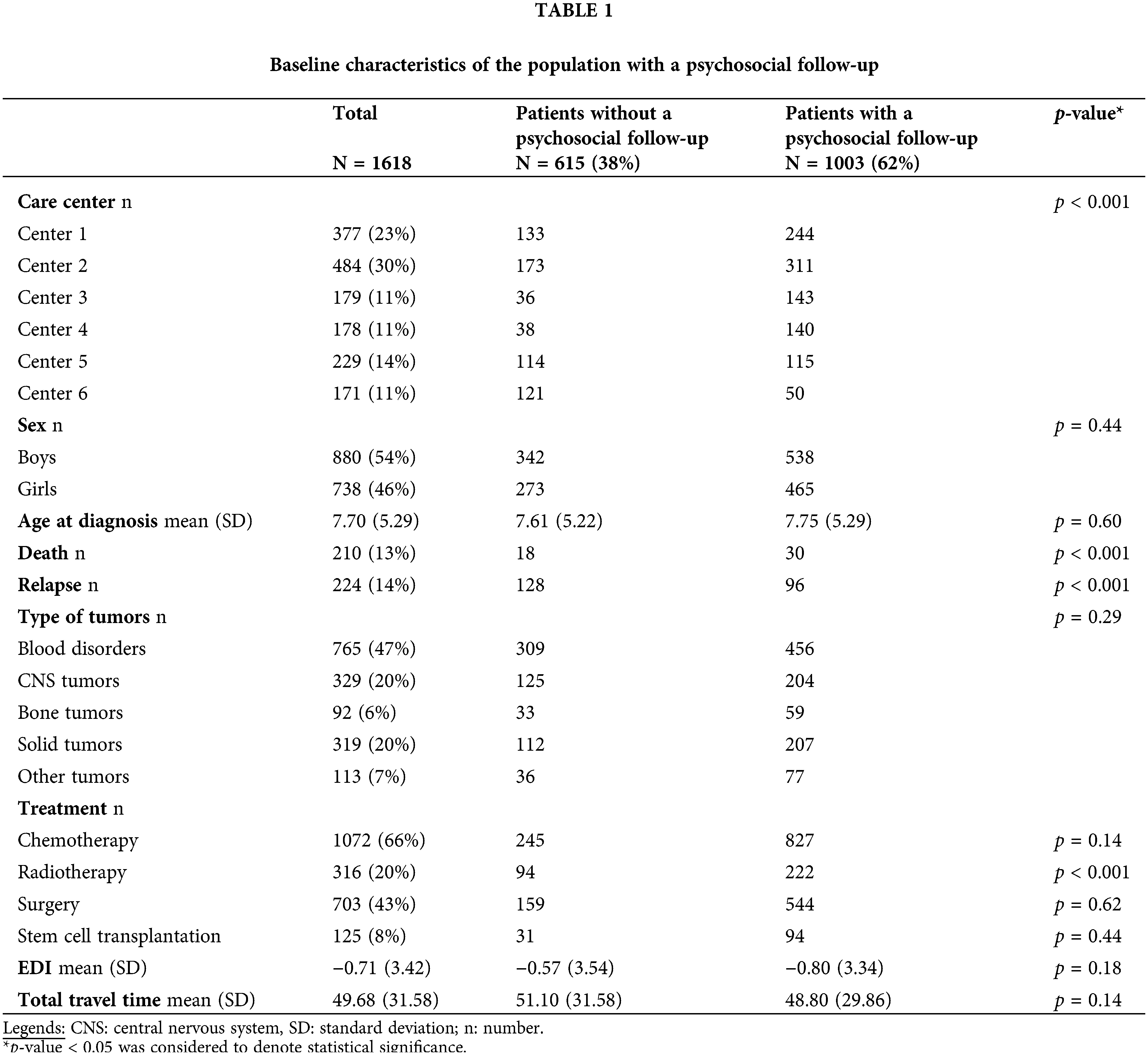
Overall, 1618 patients were included from six hospitals, and 1003 patients (62%) received a psychosocial functioning FU. School and psychological difficulties were evaluated in 2971 and 3320 consultations, respectively. Academic and psychological supports were evaluated in 2625 and 3320 consultations, respectively (Fig. 1). Since the first consultation at FU and the social reintegration, the mean duration of the FU was 34.6 months (+/−20.6). The population who received a psychosocial FU did not differ from the population who did not receive a psychosocial assessment (Table 1). However, children with a psychosocial FU were less frequently treated with radiotherapy. The rate of children followed differed significantly between hospitals, and also according to vital status and relapse.
Learning difficulties at school
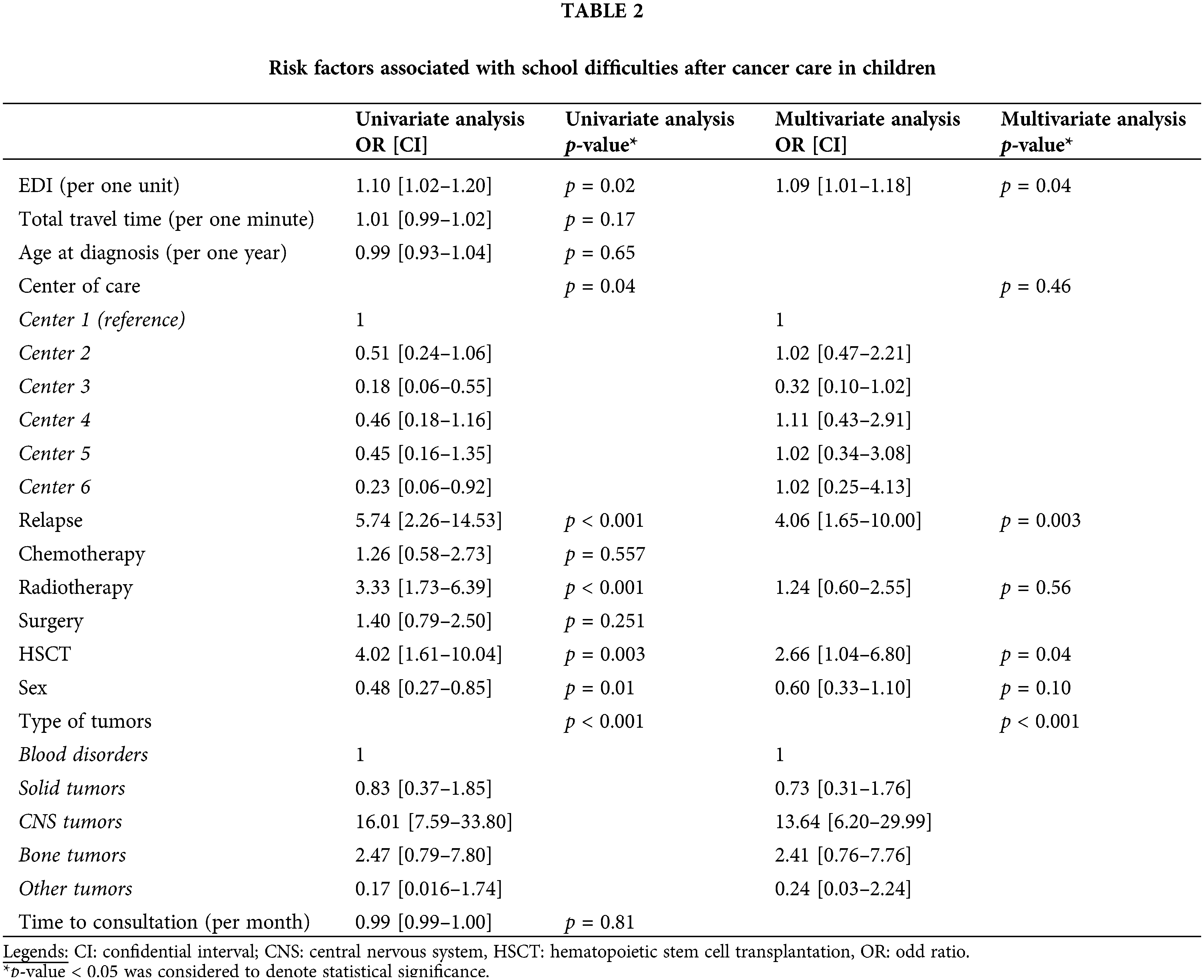
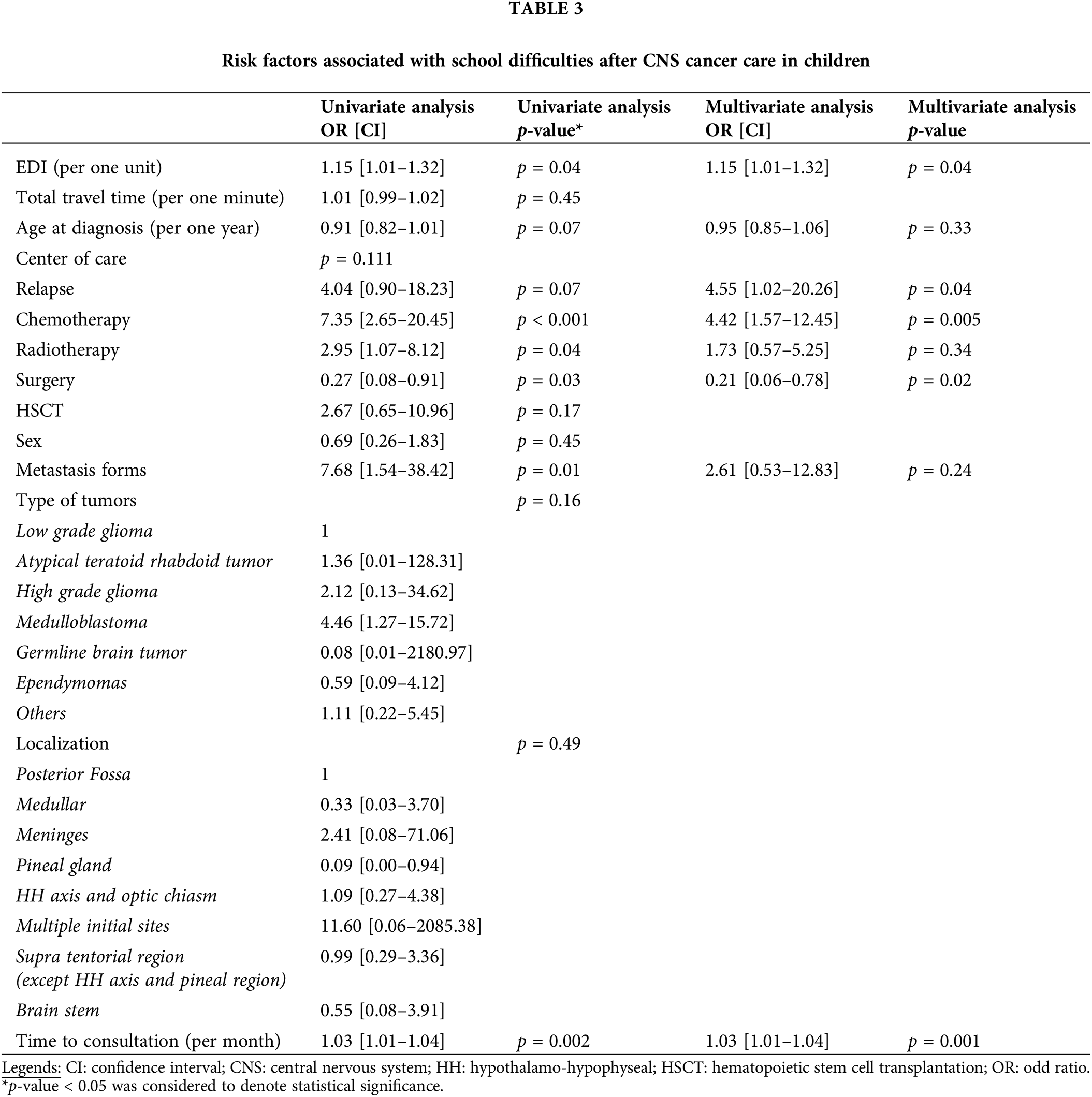
School difficulties were reported by 224 children (22%) (Table 2). Greater social deprivation was significantly associated with learning difficulty at school; however, the increased geographic distance was not. Tumor relapse, treatment with hematopoietic stem cell transplantation (HSCT), and diagnosis of a CNS tumor remained also significant risk factors. Age expressed continuously (Table 2) or by categories (preschoolers under six years old, school-aged from six to 15 years old, and adolescents up to 16 years old) was not a significant risk factor. Three-hundred seventy-one children received help with academic support, of which 74% reported difficulties at school. Academic support was mainly provided for children with CNS tumors (p < 0.001). Although learning difficulties were significantly associated with greater social deprivation, the probability of benefitting from support was not. However, there was an important correlation between the learning disabilities and academic support (R2 = 0.79).
Two factors were significantly associated with the probability of having a school difficulty over time. CNS tumors were a significant risk factor in comparison with the other types, with a hazard ratio of 4.48 (CI: 2.79–7.21) (Fig. 2A). Tumor relapse was also a significant risk factor, with a hazard ratio of 1.80 (CI: 1.05–3.09) (Fig. 2B).
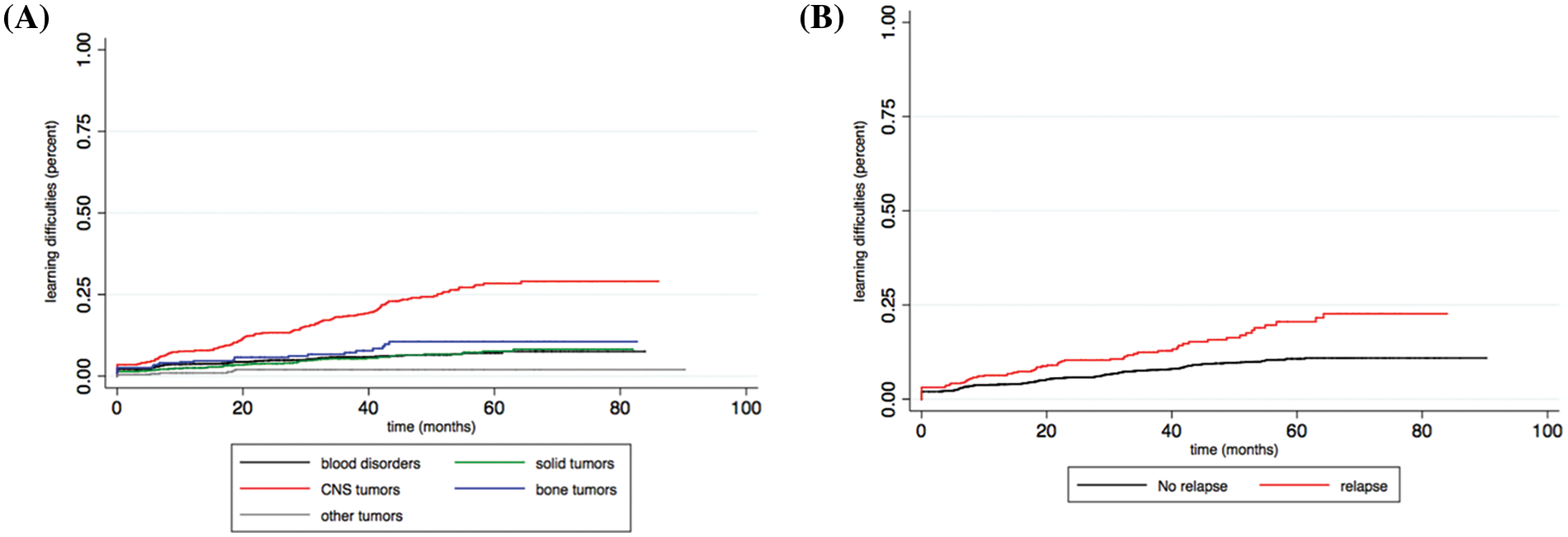
Figure 2: (A) Probability of acquiring a learning disability at school over time according to tumor type. (B) Probability of acquiring a learning disability at school over time according to tumor relapse.
Ninety-two children with a CNS tumor reported a learning difficulty at school (46%). In this CNS group, greater social deprivation, tumor relapse, treatment by chemotherapy, and time were significant risk factors. In contrast, surgical management, alone or in combination with other therapies, remained a protective factor (Table 3). Neither the type of CNS tumor nor its localization had a significant impact on the risk of having a school difficulty. Of these children with CNS tumors who declared learning disabilities, 19% of them did not receive academic support.
Seventy-eight children with blood disorders reported learning difficulty at school (17%). Tumor relapse and management by HSCT were significant risk factors. Time and the female sex were protective.
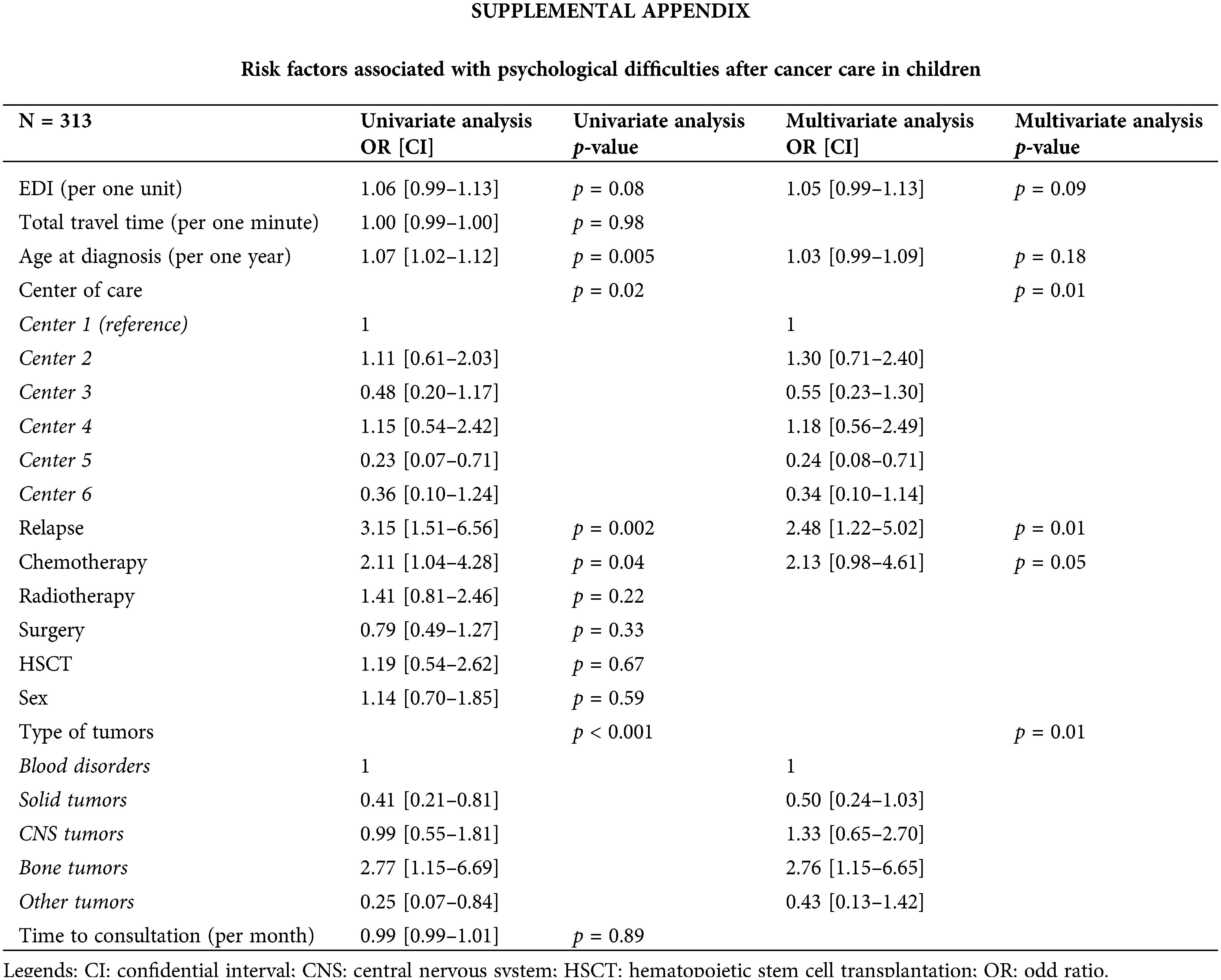
Greater social deprivation was not significantly associated with the report of psychological difficulties. However, significant differences were noted between hospitals. A psychological difficulty was reported in 196 children (19.5%), but we did not have precisions about these difficulties. Risk factors associated with psychological difficulty were the relapse and the diagnosis of bone tumors (Suppl. Appendix). Psychological support was reported for 70% of all patients included. Over time, diagnosis of bone tumors was a risk factor for psychological difficulty (HR = 2.04, CI: 1.05–3.96), whereas a solid tumor was a protective factor (HR = 0.53, CI: 0.30–0.93). Greater social deprivation was not a risk factor as such. However, a trend between greater social deprivation and the presence of psychological difficulties could be noted (p = 0.064).
The results of this multicentric study show that greater social deprivation, and therefore poverty, is associated with school difficulties in CCSs.
Learning difficulties were reported in 22% of the children in our study, but higher rates have been noted in the literature [16,17]. CCSs are at risk for learning difficulties with scholarly consequences [7,11,16,17], ultimately leading to a lower overall quality of life (QOL) [18]. School absenteeism induced by cancer is the most common cause of school difficulties [6,19]. Even if we did not evaluate the rate of absenteeism in our cohort, we found that tumor relapse was a risk factor for school difficulties. Indeed, relapse frequently requires extended hospital stays and therefore, induces school absenteeism.
In our study, the probability of declaring a learning difficulty at school was associated with greater social deprivation. Irrespective of the disease status, there is a strong relationship between SES and scholarly achievement [20–22]. This supports our results and those of the literature, even though different methods were used to estimate the SES across studies [10,11,15]. School difficulties may be due to a lack of socialization in the school environment, this parameter being important for academic success [22]. However, our data collection precludes this analysis; but Duan et al. demonstrated that greater social deprivation negatively influences the relationship between academic socialization and achievement [22]. Therefore, CCSs suffer from poor social integration, potentiated by absenteeism [16,19,19,23]. Among CCSs, children with CNS tumors are the population of greatest concern. Indeed, CNS tumors and their therapies (i.e., cranial radiotherapy, the intrathecal chemotherapy) increase the risk of adverse psychosocial and neurocognitive consequences [5,6,16,17,24]. We did not confirm the inherent risk of radiotherapy in our CNS subgroup analysis, probably due to the low number of children receiving irradiation followed in our cohort. In this CNS subgroup, social deprivation remained a prognostic factor for school difficulties, an observation also noted by Ach et al. [25].
Due to the risk of school difficulties in CCSs, academic support remains necessary. Thirty-six percent of children received such support in our study, a higher rate in comparison to the literature from 20% to 32.5% [19,24,26]. Although greater deprivation was associated with school disabilities, it was not associated with the probability of benefiting from academic support. Academic support seems to be equally shared between children according to the EDI, whereas we showed a higher need in deprived areas.
Our study did not show an association between, psychological difficulties and SES. However, it is well described in the literature that individual SES may participate in causal mechanisms for psychological affections, i.e., low levels of income, education, and employment in adulthood [12]. Indeed, children with high SES may benefit from privileged cultural, social, and economic resources which contribute to good mental health. However, these individual socio-economic parameters were not evaluated in our study, focused on ecological parameters, and may explain the difference in our results. In the literature, children with CNS tumors were reported to be at risk of psychological affections, whereas children with bone tumors were the population of greatest concern in our study [8,14]. Indeed, these last children presented frequently with physical damages induced by the surgery. Therefore, they may suffer from physical performance limitations, diminished ability to attend school, a decreased QOL [27,28]. In our results, differences between hospitals according to the presence of psychological difficulties may be explained by different factors. Indeed, hospitals did not present similar psychological support during the time of intensive treatment, and some units suffered from a lack of financial and human resources to properly carry out this mission. At the time of the reinsertion, psychological support can be continued with the hospital staff. However, new events are usually managed by private health professionals, and some regions suffer from a lack of private psychologists. Plus, these managements “outside the hospital” may not be reported to the pediatrician at the time of psychological evaluation.
This study is based on a large multicentric prospective cohort in France to evaluate the psychosocial status of survivors after hospital treatment and its evolution over time. However, this study presents some limitations. Our population was based on a database that aims to represent all the children treated for cancer in the GOCE departments. However, one hospital was excluded from the study because it did not participate in patients’ psychosocial evaluation, and may expose us to a selection bias (Fig. 1). Each outcome was evaluated during a consultation with the oncologist pediatrician at a time when the clinical evaluation remained the main objective to ensure the remission persistence, monitoring and screening for physical signs of disease. The outcomes were self-reported by children’s families, and a subjective part of these reports cannot be ruled out. Additionally, pediatric oncologists did not present a specific background to conduct such interviews, and may not distinguish psychological issues. Indeed, clinicians, patients but also psychologists may have different definitions of well-being, and the subjective part of this evaluation may be shared between these actors. Besides, we did not have precisions about the type of psychological and scholarly difficulties, as well as their intensity. All of these questions the validity and reliability of the data collected, and the use of detailed and standardized questionnaires could have been helpful in clarifying these points.
The data was collected during the posttreatment period with no baseline at diagnosis available. This precludes any analysis to assess the evolution of the difficulties before and after cancer management. This limits our results to an observation of post-treatment difficulties without taking account of some potential predisposed conditions. Indeed, children with CNS tumors can present preexisting predisposition syndromes which can already induce psychosocial effects, as well as the tumor symptoms themselves. However, the evaluation of psychosocial status at diagnosis may be difficult. This time is a period when clinical management remains the absolute priority. This can explain the choice of clinicians to defer the psychosocial evaluation to after treatment.
The measurement of social deprivation using the EDI assesses the environment SES of each patient and can induce an ecological bias. This measurement considers homogeneity between children living in the same IRIS and could induce misclassification and underestimate the effect of the SES. Data on individual deprivation such as the education level of the parents could be a complementary method to precise the SES of children but were not available in the database.
Ecological deprivation is a prognosis factor for learning difficulties, particularly for children with CNS tumors. Therefore, our results should alert the professionals involved in children’s cancer management, to be attentive to deprived children at risk of learning difficulties. Success at school is important for every child and has an impact on their current and future QOL. Although deprivation did not appear to be a risk factor for psychological difficulties, our rate of reporting poor well-being was high and probably underestimated due to the declarative bias.
Our study suggests further investigations. By focusing on CNS tumor patients, the difficulties of these patients could be detailed to propose adequate support. Plus, evaluation of the SES may integrate individual SES (family structure). Plus, evaluation of the SES may integrate individual SES (family structure, parental profession…) to complete investigations. Therefore, further analysis for attaining solutions to reduce SES-related social inequalities may be carried out.
Acknowledgement: Remerciements/Acknowledgment: Authors gratefully acknowledge patients and their families. Additionally, the authors acknowledge Dr. Bouvier Véronique (U1086 INSERM UCBN, Caen, France) for her valued help and advices regarding this paper. We gratefully acknowledge the AJE editing services for help in manuscript edition.
Financements/Funding Statement: No specific funding sources were obtained for this work. The French RECAPGO cancer database is supported by a grant from SFCE INCa (Institut National du Cancer) and GOCE (Grand Ouest Cancer de l’Enfant).
Contributions des auteurs/Author Contributions: Fanny Delehaye, Olivier Dejardin, Julien Rod, and Arnaud Alves conceptualized this study and wrote the original draft. The data curation and formal analyses were carried out by Fanny Delehaye, Olivier Dejardin and Ludivine Launay. Isabelle Pellier, Maxime Esvan were in charge of the RECAPGO database and participated in the review and the editing of this manuscript. Damien Bodet, Liana Carausu, Virginie Gandemer, Frédéric Millot, Julien Lejeune and Caroline Thomas helped in the data collection, the wrinting and reviewing of this manuscript.
Disponibilité des données et du matériel/Availability of Data and Materials: Data available on request.
Avis éthiques/Ethics Approval: This study was based on a database supported by the Commission Nationale de l’Informatique et des Libertés (CNIL) registered under the N° 912302.
Conflits d’intérêt/Conflicts of Interest: Authors have nothing to disclose.
References
1. Lacour B, Guyot-Goubin A, Guissou S, Bellec S, Désandes E, Clavel J. Incidence of childhood cancer in France: national children cancer registries, 2000–2004. Eur J Cancer Prev. 2010;19(3):173–81. [Google Scholar] [PubMed]
2. Raze T, Lacour B, Cowppli-Bony A, Delafosse P, Velten M, Trétarre B, et al. Cancer among adolescents and young adults between 2000 and 2016 in France: incidence and improved survival. J Adolesc Young Adul. 2021;10(1):29–45. [Google Scholar]
3. Gatta G, Capocaccia R, Stiller C, Kaatsch P, Berrino F, Terenziani M, et al. Childhood cancer survival trends in Europe: a EUROCARE working group study. J Clin Oncol. 2005;23(16):3742–51. [Google Scholar] [PubMed]
4. Frederiksen LE, Mader L, Feychting M, Mogensen H, Madanat-Harjuoja L, Malila N, et al. Surviving childhood cancer: a systematic review of studies on risk and determinants of adverse socioeconomic outcomes. Int J Cancer. 2019;144(8):1796–823. [Google Scholar] [PubMed]
5. Brinkman TM, Recklitis CJ, Michel G, Grootenhuis MA, Klosky JL. Psychological symptoms, social outcomes, socioeconomic attainment, and health behaviors among survivors of childhood cancer: current state of the literature. J Clin Oncol. 2018;36(21):2190–7. [Google Scholar] [PubMed]
6. French AE, Tsangaris E, Barrera M, Guger S, Brown R, Urbach S, et al. School attendance in childhood cancer survivors and their siblings. J Pediatr. 2013;162(1):160–5. [Google Scholar] [PubMed]
7. Andersen KK, Duun-Henriksen AK, Frederiksen MH, Winther JF. Ninth grade school performance in Danish childhood cancer survivors. Br J Cancer. 2017;116(3):398–404. [Google Scholar] [PubMed]
8. Lund LW, Winther JF, Dalton SO, Cederkvist L, Jeppesen P, Deltour I, et al. Hospital contact for mental disorders in survivors of childhood cancer and their siblings in Denmark: a population-based cohort study. Lancet Oncol. 2013;14(10):971–80. [Google Scholar] [PubMed]
9. Molcho M, D’Eath M, Alforque Thomas A, Sharp L. Educational attainment of childhood cancer survivors: a systematic review. Cancer Med. 2019;8(6):3182–95. [Google Scholar] [PubMed]
10. Patel SK, Johansen C, Gold AO, Delgado N, Xu S, Dennis J. Social-ecological predictors of school functioning in Hispanic children treated for cancer with central nervous system-directed therapies. Pediatr Blood Cancer. 2020;67(10):e28320. [Google Scholar] [PubMed]
11. Bonneau J, Lebreton J, Taque S, Chappe C, Bayart S, Edan C, et al. School performance of childhood cancer survivors: mind the teenagers! J Pediatr. 2011;158(1):135–41. [Google Scholar] [PubMed]
12. Zebrack BJ, Zevon MA, Turk N, Nagarajan R, Whitton J, Robison LL, et al. Psychological distress in long-term survivors of solid tumors diagnosed in childhood: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2007;49(1):47–51. [Google Scholar] [PubMed]
13. Zebrack BJ, Zeltzer LK, Whitton J, Mertens AC, Odom L, Berkow R, et al. Psychological outcomes in long-term survivors of childhood leukemia, Hodgkin’s disease, and non-Hodgkin’s lymphoma: a report from the childhood cancer survivor study. Pediatr. 2002;110(1):42–52. [Google Scholar]
14. Pornet C, Delpierre C, Dejardin O, Grosclaude P, Launay L, Guittet L, et al. Construction of an adaptable European transnational ecological deprivation index: the French version. J Epidemiol Community Health. 2012;66(11):982–9. doi:10.1136/jech-2011-200311. [Google Scholar] [PubMed] [CrossRef]
15. Guillaume E, Pornet C, Dejardin O, Launay L, Lillini R, Vercelli M, et al. Development of a cross-cultural deprivation index in five European countries. J Epidemiol Community Health. 2016;70(5):493–9. [Google Scholar] [PubMed]
16. Park M, Park HJ, Lee JM, Ju HY, Park BK, Yu ES, et al. School performance of childhood cancer survivors in Korea: a multi-institutional study on behalf of the Korean society of pediatric hematology and oncology. Psycho-Oncol. 2018;27(9):2257–64. [Google Scholar]
17. Barrera M, Shaw AK, Speechley KN, Maunsell E, Pogany L. Educational and social late effects of childhood cancer and related clinical, personal, and familial characteristics. Ann Ny Acad Sci. 2005;104(8):1751–60. [Google Scholar]
18. Litzelman K, Barker E, Catrine K, Puccetti D, Possin P, Witt WP. Socioeconomic disparities in the quality of life in children with cancer or brain tumors: the mediating role of family factors: SES, family functioning, and QOL among children with cancer. Psycho-Oncol. 2013;22(5):1081–8. [Google Scholar]
19. Tsimicalis A, Genest L, Stevens B, Ungar WJ, Barr R. The impact of a childhood cancer diagnosis on the children and siblings’ school attendance, performance, and activities: a qualitative descriptive study. J Pediatr Oncol Nurs. 2018;35(2):118–31. [Google Scholar] [PubMed]
20. Battle J, Lewis M. The increasing significance of class: the relative effects of race and socioeconomic status on academic achievement. J Poverty. 2002;6(2):21–35. [Google Scholar]
21. Sirin SR. Socioeconomic status and academic achievement: a meta-analytic review of research. Rev Educ Res. 2005;75(3):417–53. [Google Scholar]
22. Duan W, Guan Y, Bu H. The effect of parental involvement and socioeconomic status on junior school students’ academic achievement and school behavior in China. Front Psychol. 2018;9:952. [Google Scholar] [PubMed]
23. McLoone JK, Wakefield CE, Cohn RJ. Childhood cancer survivors’ school (re)entry: Australian parents’ perceptions: school (re)entry after childhood cancer. Eur J Cancer Care. 2013;22(4):484–92. [Google Scholar]
24. Mitby PA, Robison LL, Whitton JA, Zevon MA, Gibbs IC, Tersak JM, et al. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Ny Acad Sci. 2003;97(4):1115–2. [Google Scholar]
25. Ach E, Gerhardt CA, Barrera M, Kupst MJ, Meyer EA, Patenaude AF, et al. Family factors associated with academic achievement deficits in pediatric brain tumor survivors: academic achievement in survivors. Psycho-Oncol. 2013;22(8):1731–7. [Google Scholar]
26. Lorenzi M, McMillan AJ, Siegel LS, Zumbo BD, Glickman V, Spinelli JJ, et al. Educational outcomes among survivors of childhood cancer in British Columbia, Canada: report of the childhood/adolescent/young adult Cancer survivors (CAYACS) program. Ann Ny Acad Sci. 2009;115(10):2234–45. [Google Scholar]
27. Ness KK, Gurney JG. Adverse late effects of childhood cancer and its treatment on health and performance. Annu Rev Public Health. 2007;28(1):279–302. [Google Scholar] [PubMed]
28. Ness KK, Mertens AC, Hudson MM, Wall MM, Leisenring WM, Oeffinger KC, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143(9):639–47. doi:10.7326/0003-4819-143-9-200511010-00007. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools