 Open Access
Open Access
ARTICLE
Evaluation de l’expression de la fatigue liée au cancer : comparant l’expression de la fatigue chez les patients atteints de cancer, chez les patients touchés par d’autres maladies chroniques et chez les individus en bonne santé
Assessment of Cancer-Related Fatigue Expression: Comparing the Expression of Fatigue in Patients with a History of Cancer, Patients with Other Chronic Diseases, and Healthy Individuals
1 Chronopsychology and Cognitive Systems Lab (ChronCog), University of Coimbra, Coimbra, 3000-115, Portugal
2 Center for Research in Neuropsychology and Cognitive and Behavioral Intervention (CINEICC), University of Coimbra, Coimbra, 3000-115, Portugal
3 Faculty of Psychology and Educational Sciences, University of Coimbra, Coimbra, 3000-115, Portugal
4 Oncology Department, Centro Hospitalar do Médio Tejo (CHMT), Torres Novas, 2350-754, Portugal
* Corresponding Author: Maria Inês Clara. Email:
Psycho-Oncologie 2024, 18(1), 49-57. https://doi.org/10.32604/po.2023.044320
Received 06 April 2023; Accepted 21 July 2023; Issue published 25 March 2024
RÉSUMÉ
Objectif: L’objectif de l’étude fut de comparer la fatigue ressentie par les patients du cancer par rapport à celle de la population en générale, ainsi que d’examiner les facteurs de risque psychobiologiques associés à la fatigue. Matériel et méthodes: Dans cette étude quantitative et transversale, nous avons analysé les indicateurs cliniques et sociodémographiques de 389 participants (68.38% de femmes) : 148 patients du cancer sous traitement actif, 55 patients dans l’après-traitement d’un cancer, 75 patients atteints d’une autre maladie chronique et 111 personnes en bonne santé. Résultats: La fatigue s’exprimait de manière différente chez les patients ayant des antécédents de cancer et chez les participants sans antécédent de cancer. Les patients sous traitement actif ont signalé des niveaux de fatigue significativement plus élevés que les autres groupes. Néanmoins, une certaine fatigue associée au cancer a persisté, dans un cadre similaire, après un traitement actif et jusqu’à la phase de survie. Les patients dans l’après-traitement d’un cancer ont montré des niveaux de vigueur significativement inférieurs à ceux des patients atteints de maladies chroniques. La détresse psychologique et la somnolence diurne sont apparues comme des facteurs transdiagnostiques associés à la fatigue. Conclusion: La fatigue liée au cancer peut avoir un cadre unique, caractérisé par une endurance réduite et une faiblesse musculaire. Dans la présente étude, la détresse psychologique et la somnolence diurne sont associées à la fatigue liée au cancer. Ces résultats suggèrent la pertinence d’études futures examinant si des interventions ciblant ces facteurs peuvent aider à gérer cette plainte pesante.Abstract
Aims: We aimed to compare cancer survivors’ fatigue expression with that of the general population and examine psychobiological factors associated with fatigue. Procedure: In this quantitative, transversal study, we analyzed clinical and sociodemographic indicators of 389 participants (68.38% females): 148 cancer survivors on active treatment, 55 disease-free survivors, 75 patients with another chronic disease, and 111 healthy individuals. Results: Fatigue was expressed dissimilarly in patients with a previous history of cancer and participants without a history of cancer. Survivors on active treatment reported significantly higher levels of fatigue than the other clinical status groups. Nonetheless, some level of cancer-related fatigue persisted, in a similar pattern, after active treatment into the survivorship phase. Disease-free survivors showed significantly lower vigor levels when compared to patients with other chronic diseases. Psychological distress and daytime sleepiness emerged as transdiagnostic factors associated with fatigue. Conclusion: Cancer-related fatigue may have a unique pattern, characterized by reduced endurance and muscle weakness. In the present study, psychological distress and daytime sleepiness are associated with cancer-related fatigue. These findings suggest the pertinence of future studies examining whether interventions targeting those factors may help manage this burdensome complaint.MOTS CLÉS
Keywords
Fatigue is a widely used term referring to different domains, meanings, and causalities [1]. As a subjective experience, it can manifest in multiple domains: as decreased levels of concentration (mental fatigue), pain and muscle weakness (physical fatigue), increased negative affect (emotional fatigue), or as a mismatch between expended effort and actual performance or exhaustion and reduced endurance (general fatigue). Fatigue experienced by healthy individuals may be defined as a predictable, transient sense of exhaustion related to prolonged, intense activity that is eventually relieved by sleep and rest [2]. Healthy fatigue usually does not interfere with daily activities, albeit it can impact social, emotional, or occupational functioning and quality of life [3]. Contrariwise, patients with a chronic illness diagnosis describe fatigue as an overwhelming sense of tiredness at rest, exhaustion with activity, lack of energy that precludes daily tasks, inertia or lack of endurance, or as loss of vigor [4].
Cancer-related fatigue (CRF) has been conceptualized as a persistent subjective sense of physical, emotional, and/or cognitive exhaustion related to cancer or cancer treatment that is not proportional to recent activity, is not alleviated by usual strategies of energy reparation and interferes with daily functioning [5]. Cancer survivors (i.e., individuals with a cancer experience [6]) describe fatigue as one of the most distressing side effects associated with cancer and cancer treatment. CRF impairs quality of life and all areas of functioning, including mood, work, cognitive and physical performance, as well as interpersonal relations and family care. This cancer behavioral comorbidity may even lead to the discontinuation of cancer treatment and reduce survival. CRF usually increases during cancer treatment, reaching its peak towards the treatment end and diminishing thereafter [7–9]. Nonetheless, CRF often persists for months, years, or even decades after treatment completion [10]. Fatigue affects 39% to 99% of patients undergoing active cancer treatment [11–15] and 19% to 82% of disease-free posttreatment survivors [16,17]. Survivors have been reported to experience constant levels of fatigue from 5 to 15 years post-diagnosis [18]. Regardless of being reported more frequently than any other side-effect and described as causing the most suffering both during and after cancer treatment, fatigue remains under-assessed and undertreated in oncology care [19].
It is accepted CRF is different from healthy fatigue. Survivors often describe CRF as more severe and debilitating than healthy fatigue. However, focusing on fatigue severity only hinders the understanding of the full spectrum of the fatigue symptom profile. The idiosyncrasy of CRF has not yet been the subject of investigation. To understand the expression of healthy fatigue and CRF, our first aim was to explore fatigue patterns in different clinical groups: cancer survivors during active treatment, disease-free survivors, participants with other chronic diseases, and healthy controls. Moreover, despite its prevalence and consequences, the pathophysiology mechanisms underlying fatigue are not well-understood and consistent correlates of this condition have been difficult to identify. Fatigue is influenced by the complex interaction of demographic, biological, medical, and psychological aspects, including the stress associated with the cancer experience. To reduce the burden associated with fatigue, it is important to understand its trajectory, its psychobiological processes, and determine the best strategies to prevent and treat this condition. Hence, our second aim was to examine psychobiological factors that may explain the variability of fatigue in people with and without a history of cancer to identify factors that may signalize vulnerable patients at risk for fatigue or factors that may be a target of intervention.
This study is part of a broader investigation project in which all procedures were approved by the Ethics and Deontology Committee for Investigation of the Faculty of Psychology and Educational Sciences of the University of Coimbra. The project was also approved by the Administration Board of the Médio Tejo Hospital Center, based on the reports of that Medical Center’s Ethics Committee and Legal Support Unit. All procedures were in accordance with the Helsinki Declaration.
For this cross-sectional study, we recruited a clinical and a community sample with a total of 389 Portuguese participants. Table 1 displays participants’ sociodemographic and clinical data. Inclusion criteria were: (1) 18+ years and (2) having provided informed consent. The clinical sample was composed of cancer survivors receiving treatment (CAN, n = 148, mean age = 60.82 ± 10.19 years old) and disease-free cancer survivors (i.e., after treatment completion; SUR, n = 55, mean age = 59.24 ± 14.09) receiving follow-up care in the Oncology Unit of the Médio Tejo Hospital Center, EPE (Portugal). Following their medical appointments, patients were asked to complete a survey on their experiences of fatigue. The community sample was derived from a general population anonymous online survey and comprised patients diagnosed with a chronic disease other than cancer (CD, n = 75, mean age = 59.08 ± 11.76) and healthy participants (H, n = 111, mean age = 56.89 ± 11.67 years). The most prevalent diagnoses in the chronic disease group were respiratory disease (including asthma, chronic obstructive pulmonary disorder, and cystic fibrosis), hypertension, thyroid disease, chronic pain, heart disease (i.e., angina and heart failure), diabetes, and gastrointestinal disease (i.e., irritable bowel syndrome and Chron’s disease). The total sample mean age was 59.14 ± 11.60 (28–87) years. The groups did not differ significantly in terms of age [F(3, 388) = 2.45, p = 0.06].
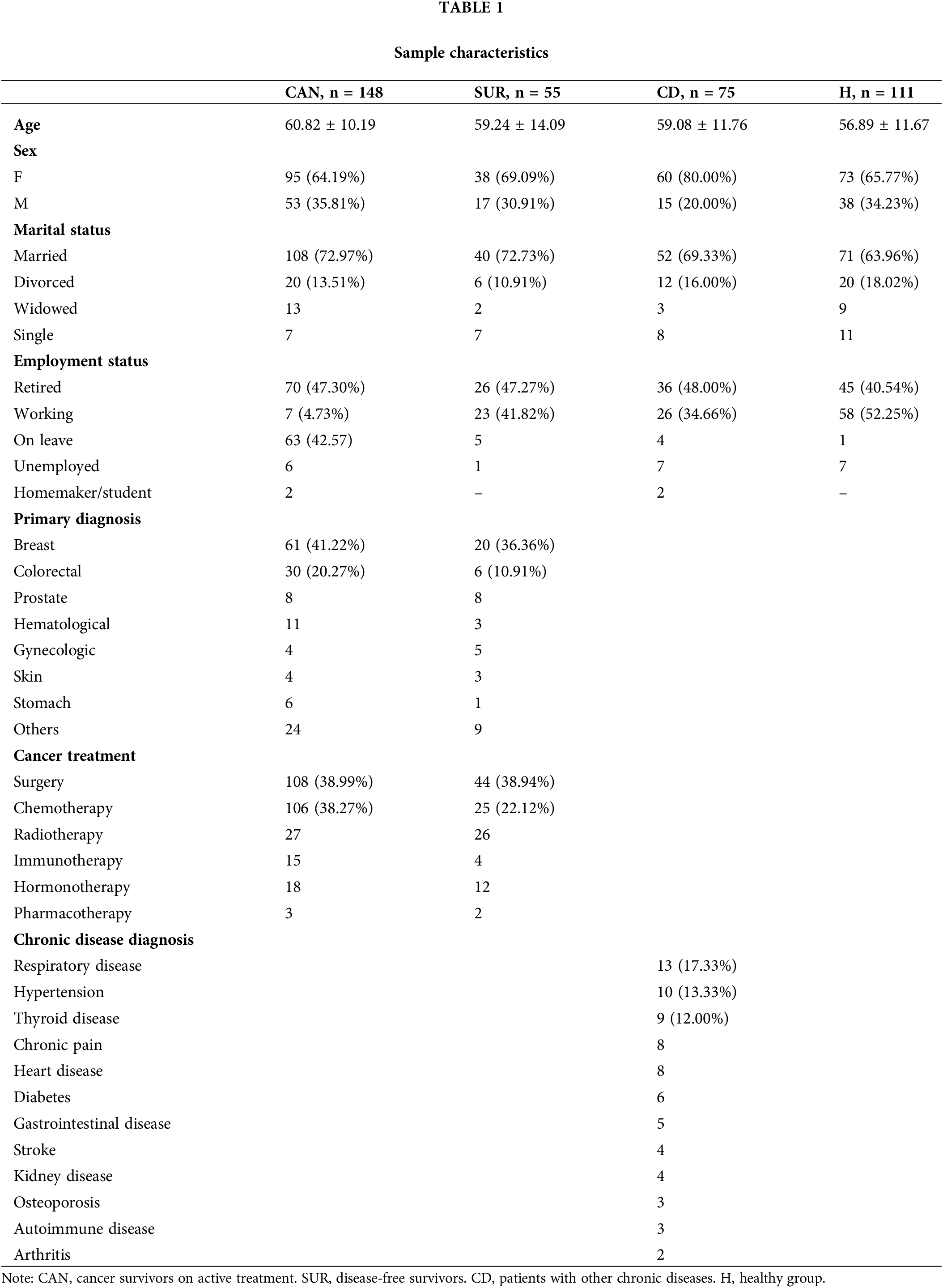
All patients completed a self-report set of questions concerning sociodemographic and medical questions, as well as the following questionnaires:
Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF [20,21])
Being a subjective experience, patient self-report is the gold standard for assessing CRF [7]. The MFSI-SF is a valid, reliable self-report questionnaire to assess fatigue in clinical and nonclinical populations that characterize fatigue through five empirically derived scales: general fatigue, physical fatigue, emotional fatigue, mental fatigue, and vigor. Each of these subscales includes 6 items in which participants indicate to what extent they have experienced, in the previous week, each symptom in a Likert-type scale with 5 points (0 = did not experience at all to 4 = extremely). Total fatigue is computed based on the subtraction of the vigor subscale from the sum of the four fatigue subscales, with higher scores denoting higher levels of fatigue. The MFSI-SF revealed high internal consistency in this study (Cronbach’s alpha coefficient [α] = 0.97; McDonald’s omega [ω] = 0.95).
Hospital Anxiety and Depression Scale (HADS [22,23])
Psychological distress was assessed via this 14-question instrument, with 7 items each for the two subscales of depression and anxiety. Each item is scored between 0 (no impairment) and 3 (severe impairment). Scores below 7 indicate non-significant cases, scores of 8–14 denote light symptomology and scores above 15 denote considerable anxiety or depression. We found a high internal consistency (α and ω = 0.89) for the HADS in this study.
Epworth Sleepiness Scale (SES [24,25])
To assess the propensity to daytime sleepiness, participants indicated the probability of falling asleep in 8 distinct scenarios on a 4-point scale (0 = no probability to 3 = high probability of dozing). The composite score ranges between 0 and 24 (11–12 denotes light sleepiness, 13–15 moderate sleepiness, e 16–24 severe sleepiness). In the current study, the ESS showed high internal consistency, with α = 0.83 and ω = 0.86.
Daytime Sleepiness Perception Scale (DSPS-4 [26])
The subjective perception of sleepiness was assessed via this 4-item questionnaire, with scores ranging from 0 to 16 (higher scores denote a greater sleepiness perception). In the current study, α = 0.82 and ω = 0.83.
Patients with a history of cancer were also assessed via the Eastern Cooperative Oncology Group Performance Status Rating (ECOG PSR [27]), a single-item instrument examining patients’ levels of functioning and physical ability. In this study, patients were assessed from 0 = totally active to 3 = in bed at least 50% of the time.
All data analyses were conducted using the 22nd version of IBM SPSS. To characterize sociodemographic and clinical parameters, we used descriptive statistics. Cronbach’s alpha (α) and McDonald’s omega (ω) coefficients were used as indicators of the scales’ internal consistency. To compare cancer survivors on active treatment, disease-free survivors, patients with other chronic diseases, and healthy individuals on fatigue (assessed by the MFSI-SF emotional, general, mental, physical, and vigor subscales), we performed a one-way between-groups analysis of variance (ANOVA), followed by Games-Howell (GH) post-hoc comparisons. An independent-samples t-test was used to examine whether males and females differed in terms of fatigue. Effect sizes were computed as the eta squared (η2) and considered small from 0.01, moderate from 0.06, and large when 0.14 or above [28]. To examine the potential risk factors of total fatigue as measured by the MFSI-SF, we performed a standard analysis of multiple regression separately for patients with a history of cancer (CAN and SUR) and participants without a history of cancer (H and CD). Our models included the following variables: sex (0 = females/1 = males), marital status (0 = in a relationship/1 = single/widowed), occupation (0 = working/1 = not working), undergone chemotherapy (0 = no/1 = yes), chronic disease diagnosis (0 = no/1 = yes).
Patients undergoing active cancer treatment exhibited significantly lower vigor levels than the other groups, as well as greater fatigue levels in all its dimensions (Table 2), associated with large effect sizes (η2 = 0.14 to 0.31), except for mental fatigue (where they differed only from healthy individuals). Posttreatment survivors did not differ significantly from patients with other chronic diseases in terms of total [F(3, 388) = 43.62, p < 0.001, CAN > CD, SUR > H], emotional [F(3, 388) = 21.20, p < 0.001, GH: ONC > SUR, CD, H; CD > H], general [F(3, 388) = 58.87, p < 0.001, GH: CAN > CD, SUR > H], mental [F(3, 388) = 10.21, p < 0.001, GH: CAN, CD > H], or physical fatigue [F(3, 388) = 53.22, p < 0.001, GH: CAN > CD, SUR > H], but reported significantly lower levels of vigor [F(3, 388) = 21.24, p < 0.001, GH: CAN < SUR < CD, H]. Posttreatment survivors and patients with other chronic diseases reported significantly higher fatigue levels than healthy participants.
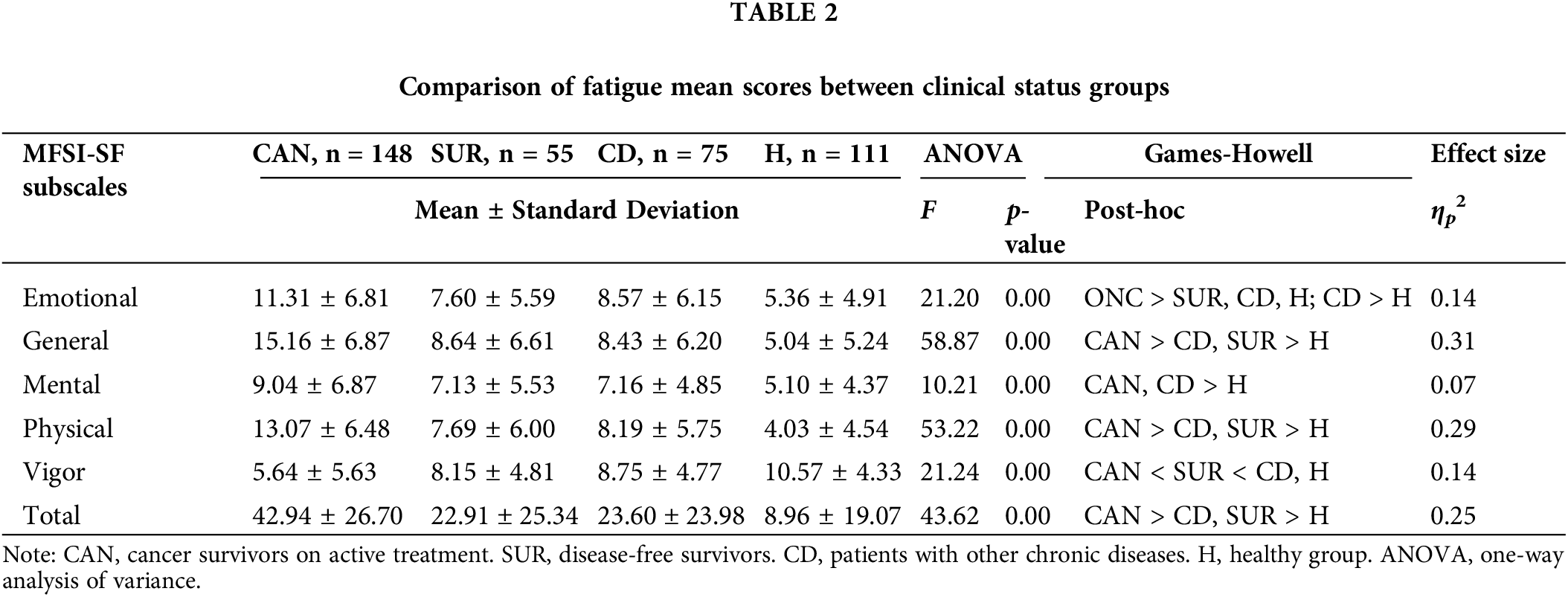
The inspection of mean scores indicated that, for patients with a history of cancer (i.e., patients undergoing active cancer treatment and posttreatment survivors), general fatigue had the greatest expression. Conversely, for participants without a history of cancer (i.e., healthy individuals and patients with other chronic diseases), emotional fatigue was the prevailing fatigue manifestation. Patients with a history of cancer reported more general fatigue, followed by physical fatigue, emotional fatigue, and, finally, mental fatigue. Participants with other chronic diseases reported predominantly emotional fatigue, followed by general fatigue, physical fatigue, and mental fatigue. Healthy participants expressed more emotional fatigue, followed by mental fatigue, general fatigue, and, finally, physical fatigue.
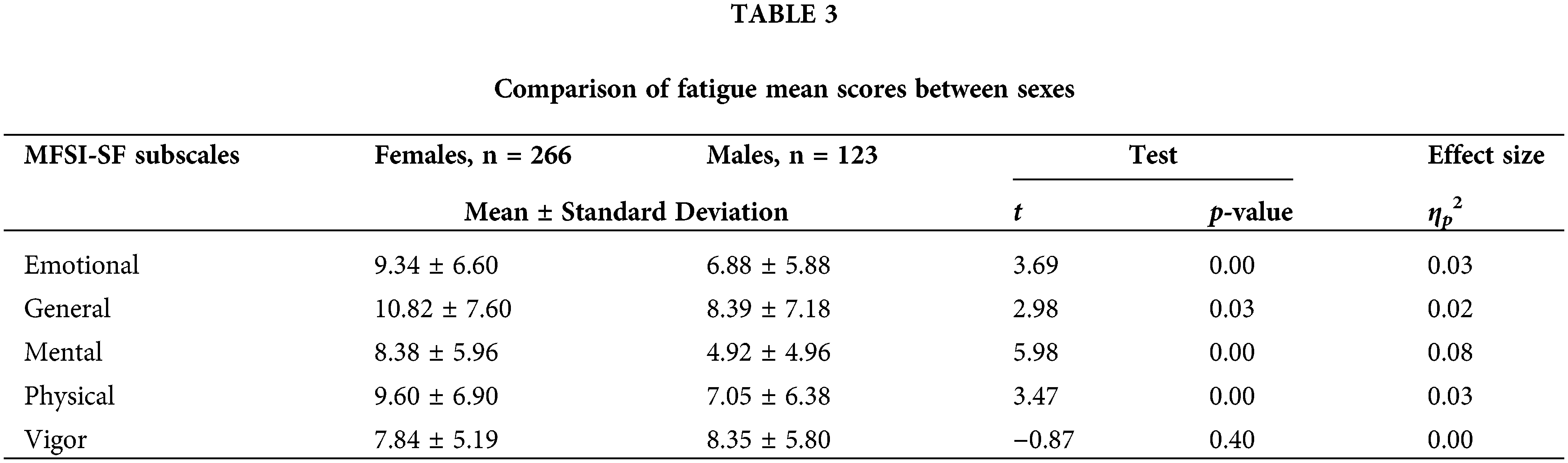
Females reported higher levels of emotional [t(387) = 3.69, p < 0.001], general [t(387) = 2.98, p = 0.03], physical [t(387) = 3.47, p = 0.03], and mental fatigue [t(387) = 5.98, p < 0.001] than males, but the sexes did not differ in terms of vigor (Table 3).
Profiling potential risk factors for fatigue
For patients with a history of cancer, our model, which included sex, age, marital status, performance status, psychological distress, sleepiness propension, sleepiness perception, and having undergone chemotherapy, explained 65.00% of the variance in total fatigue [F(8, 164) = 36.21, p < 0.001]. Assumptions of multicollinearity and homo-scedasticity were met: the correlation between each of the variables was less than 0.7; Tolerance values ranged between 0.42 and 0.91; Variance Inflation Factor (VIF) values ranged between 1.08 and 2.37; the Durbin-Watson statistic was 2.08 and the scatterplot analysis indicated the plots were randomly scattered around zero. Psychological distress, sex, sleepiness propension, sleepiness perception, and having undergone chemotherapy made statistically significant unique contributions to explaining fatigue (Table 4). Based on beta standardized coefficients (β), psychological distress made the strongest unique contribution to explaining fatigue. The negative β for sex suggests females are more likely to report fatigue than males.
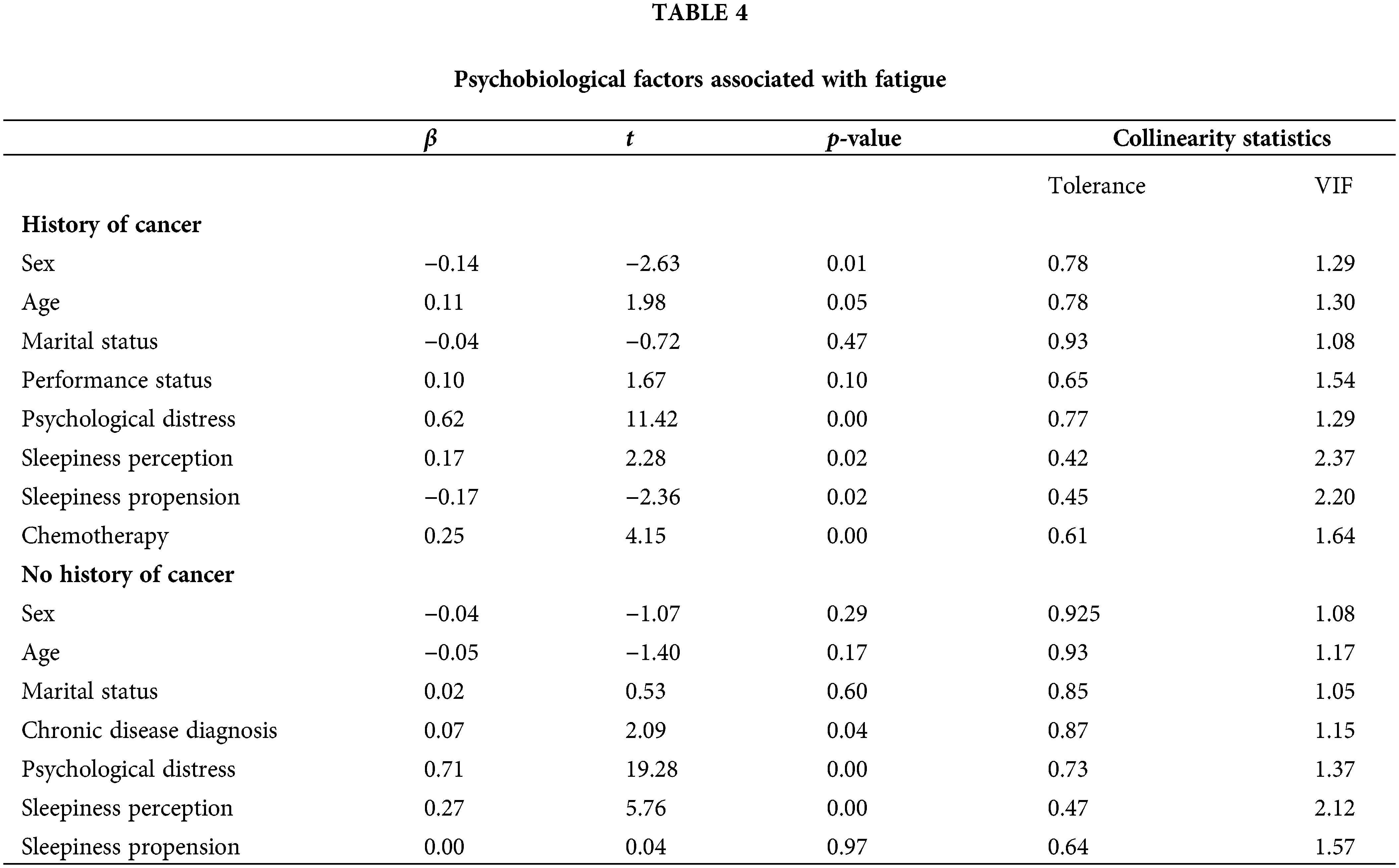
For participants without a history of cancer, our model, which included sex, age, marital status, diagnosis of chronic disease, psychological distress, propension to sleepiness, and perception of sleepiness explained 82.3% of total fatigue variance [F(7, 185) = 100.19, p < 0.001]. The correlation between variables was less than 0.7; Tolerance and VIF values ranged between 0.47–0.92 and 1.05–2.12, respectively. The Durbin-Watson Statistic was 2.03 and residuals were randomly distributed along the zero point. Psychological distress, daytime sleepiness, and the diagnosis of a chronic disease made statistically significant unique contributions to the prediction of fatigue.
Fatigue is described as the most prevailing and one of the most burdensome conditions associated with cancer, but its complex etiology renders it hard to mitigate. Survivors describe CRF as more severe and debilitating than healthy fatigue, as it cannot be relieved by adequate rest. In this study, we compared the intensity and pattern expression of fatigue across four groups: survivors on active cancer treatment, disease-free cancer survivors, patients with other chronic diseases, and participants without a health condition. We found that cancer survivors report a specific pattern in the expression of dimensions of fatigue, albeit fatigue levels are more intense for survivors during active treatment than disease-free survivors. Additionally, we found psychological distress and daytime sleepiness perception to be potential transdiagnostic risk factors for fatigue.
We found a similar pattern in the multidimensional expression of fatigue for survivors undergoing active cancer treatment and disease-free survivors, different from the pattern exhibited by patients with other chronic diseases and healthy individuals. These results seem to indicate that cancer-related fatigue has a unique pattern, distinct from healthy fatigue: while healthy fatigue expression is predominantly emotional and mental, cancer-related fatigue expression is predominantly general and physical. Albeit lower in intensity, it seems like this pattern of fatigue endures from the active phase of treatment into the posttreatment survivorship phase. Hence, besides evincing fatigue should be routinely screened for among posttreatment survivors who may be dealing with prolonged states of cancer-related fatigue, these results have potential implications for psychoeducational interventions.
Evidence-based recommendations for improving fatigue in adults by the four premier cancer organizations–the National Comprehensive Cancer Network [5], the Oncology Nursing Society [29], the Canadian Partnership Against Cancer/Canadian Association of Psychosocial Oncology, and the American Society of Clinical Oncology [30,31]–include psychoeducation, addressing treatable contributors to fatigue and managing concurrent symptoms, physical activity/exercise, and cognitive-behavioral interventions for fatigue, depression, and sleep. Psychoeducational interventions have been shown to be efficacious for fatigue management and involve providing patients with information about the experience of fatigue, its anticipated characteristics, patterns, and consequences [32–34]. Considering our results, patients can be provided with anticipatory guidance to expect reduced endurance and muscle weakness and this pattern may persist post treatment, albeit probably lower in severity. Both patients beginning fatigue-inducing treatments and patients transitioning to long-term survivorship should be provided with information concerning anticipated patterns of fatigue, as well as about evidenced-based interventions effective in limiting its severity [19]. Our results have further clinical implications. Since CRF appears to express predominantly through reduced endurance and muscle weakness, prescribing physical activity and promoting behavioral activation may be particularly relevant to cancer survivors. Several meta-analyses and systematic reviews have confirmed the effectiveness of physical activity/exercise (e.g., [35,36]).
We also examined psychobiological factors associated with fatigue in participants with and without a history of cancer. Among patients with a history of cancer, being a female and having undergone chemotherapy were significantly associated with fatigue. Psychological distress made the strongest unique contribution to explaining the variance in fatigue. While the transversal design precludes the assumption of causality, we may consider these potential risk factors for cancer-related fatigue. Albeit the specific mechanisms that underlie common pathophysiology for cancer-related fatigue have not been fully elucidated, a leading process by which cancer, cancer treatments, and stress associated with the cancer experience may contribute to fatigue is elevations in levels of pro-inflammatory cytokines. Cancer-related fatigue is often the end result of a complex interplay between causal, contributing, and modulating factors; and it is possible psychological distress maintains fatigue originated primarily by biological factors such as inflammatory activity [37]. A higher daytime sleepiness perception was also identified as a potential risk factor for cancer-related fatigue, along with a lower sleepiness propension. We hypothesize these results may occur due to mediation by insomnia symptoms (both diurnal and nocturnal). Survivors may report an increased sleepiness perception due to poor sleep while showing a decreased sleepiness propension due to hyperarousal (i.e., increased levels of physiological, cognitive, and emotional levels in insomnia). Insomnia hyperarousal may result in a state of chronic inflammation with the hyperactivation of stress and pro-inflammatory systems, which may, in turn, elevate cancer-related fatigue by aggravating psychological distress and cytokine and hypothalamic-pituitary-adrenal axis dysregulations [38]. It has been hypothesized that altered diurnal levels of cortisol and circadian rhythm disturbances caused by elevated levels of tumor necrosis factor-alpha induced by chemotherapy lead to sleep disturbance, increased release of peripheral 5-HT, activation of afferent vagal nerves and decreased skeletal muscle tone, causing general weakness, which may might then result in wasting [19].
There is expert consensus that patients with fatigue should be evaluated, and managed as indicated, for potential etiologic factors and concurrent symptoms (e.g., impaired sleep quality, depression [19]). Thus, our results suggest psychological distress and impaired sleep should be routinely screened for and addressed in clinical practice. Psychological interventions alleviating anxiety and depression symptoms and promoting adaptative coping mechanisms, such as cognitive-behavioral therapy (CBT), may be promising approaches to both healthy fatigue and cancer-related fatigue. There is strong, consistent evidence that cognitive-behavioral therapies are effective for CRF: not only CBT for fatigue and depression, but also CBT for insomnia (CBT-I), [e.g., 39–41] a multicomponent intervention encompassing sleep consolation, relaxation training, stimulus control, and reducing cognitive-emotional arousal. It is possible that by interrupting the vicious cycle of insomnia, CBT-I alleviates hyperarousal and psychological distress, thereby mitigating cancer-related fatigue [38].
Our study would have been enriched with information about insomnia and sleep; thus, future studies should include these variables. Additionally, the results of our quantitative, transversal study should be complemented by studies with longitudinal and qualitative designs to explore the trajectory and predictors of fatigue and the experience of fatigue across cancer survivorship, respectively. Other limitations of this study include sample size, which prevented us from comparing fatigue across different cancer diagnoses and treatments other than chemotherapy. Nonetheless, psychological distress predicted fatigue much more substantially than performance status, assessed through ECOG PSR. Notwithstanding these limitations, our study allowed us to shed light on the unique expression pattern of CRF.
Cancer-related fatigue, expressed mainly through reduced endurance and muscle weakness, was associated with psychological distress and daytime sleepiness perception in our study. We hypothesize that due to their sleepiness perception and low mood, survivors may inhibit their daytime activity, which, in turn, may decrease their energy and make them feel more fatigued. In fact, evidence suggests cognitive and behavioral mechanisms, including catastrophic coping and physical inactivity, contribute to the exacerbation and persistence of CRF [32]. Hence, we posit sleep-promoting (e.g., CBT-I) and behavioral activation techniques (i.e., helping patients get more active through the progressive prescription of physical and social activities to boost experiences of pleasure and mastery and, consequently, improve mood) may be promising additions to multicomponent CBT interventions to reduce cancer-related fatigue.
Despite being one of the most pervasive and debilitating complaints associated with cancer, the recognition of and access to evidence-based psychological interventions for fatigue is limited in cancer care. Obstacles hindering its assessment and the implementation and dissemination of such interventions in clinical practice include clinicians’ lack of resources (time and expertise) and healthcare systems’ lack of access to integrated supportive care services. To overcome these barriers, collaborations between clinicians and researchers are critical, with an emphasis on capitalizing on technology to improve the capacity to screen and deliver evidence-based interventions to reduce fatigue severity and improve the general, physical, mental, and emotional functioning of cancer survivors [19]. Future research should focus on testing telehealth delivery of interventions recommended for cancer-related fatigue based on current evidence, such as CBT-I, to widespread access to guideline treatment and reduce the cancer burden [19,42].
Despite reducing intensity, fatigue seems to express a similar pattern during and after cancer treatment cessation. The identical pattern in the array of fatigue manifestations among posttreatment survivors and survivors undergoing active treatment, differentiated from fatigue experienced by non-cancer individuals, suggests that cancer-related fatigue has a unique expression, characterized by reduced endurance and muscle weakness. Psychological distress and daytime sleepiness were identified as potential risk factors for fatigue, warranting future research testing interventions, such as behavioral activation and sleep techniques, that may hold great potential to target these factors and survivors’ characteristic symptoms, thus improving cancer-related fatigue.
Remerciements/Acknowledgment: The authors are grateful to the Oncology Care team and to the Administration Board of the Médio Tejo Hospital Center (CHMT).
Financements/Funding Statement: This work is part of an ongoing Ph.D. research supported by the Portuguese Foundation for Science and Technology (FCT) through a doctoral scholarship (Grant Number 2020.05728.BD) awarded to Maria Inês Clara.
Contributions des auteurs/Author Contributions: The authors confirm their contribution to the paper as follows: conceptualization and design: Maria Inês Clara; data collection: Maria Inês Clara, Ana Severina, Susana Ramos, Carla Rafael; analysis and interpretation of results: Maria Inês Clara, Ana Allen Gomes; draft manuscript preparation: Maria Inês Clara; review and editing: Maria Cristina Canavarro, Ana Severina, Susana Ramos, Carla Rafael, Ana Allen Gomes; supervision: Ana Allen Gomes, Maria Cristina Canavarro. All authors reviewed the results and approved the final version of the manuscript.
Disponibilité des données et du matériel/Availability of Data and Materials: The sensitive nature of the research prevents the release of underlying data.
Avis éthqiues/Ethics Approval: Not applicable.
Conflits d’intérêt/Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care. 2014 Aug;31(5):562–75. doi:10.1177/1049909113494748 [Google Scholar] [PubMed] [CrossRef]
2. Berger AM, Abernethy AP, Atkinson A, Barsevick AM, Breitbart WS, Cimprich B, et al. Cancer-related fatigue. J Natl Compr Canc Netw. 2010;8(8):904–31. doi:10.6004/jnccn.2010.0067 [Google Scholar] [PubMed] [CrossRef]
3. Bower JE. Fatigue, brain, behavior, and immunity: summary of the 2012 named series on fatigue. Brain Behav Immun. 2012 Nov;26(8):1220–3. doi:10.1016/j.bbi.2012.08.009 [Google Scholar] [PubMed] [CrossRef]
4. Davis MP, Walsh D. Mechanisms of fatigue. J Support Oncol. 2010 Jul-Aug;8(4):164–74 [Google Scholar] [PubMed]
5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: cancer-related fatigue. version 2.2022. Plymouth Meeting, Pa: National Comprehensive Cancer Network; 2022. [Google Scholar]
6. Available from: www.canceradvocacy.org/. [Accessed 2023 January 27]. [Google Scholar]
7. Bower JE. Cancer-related fatigue: mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi:10.1038/nrclinonc.2014.127 [Google Scholar] [PubMed] [CrossRef]
8. Mo J, Darke AK, Guthrie KA, Sloan JA, Unger JM, Hershman DL, et al. Association of fatigue and outcomes in advanced cancer: an analysis of four SWOG treatment trials. JCO Oncol Pract. 2021;17(8):e1246–57. doi:10.1200/OP.20.01096 [Google Scholar] [PubMed] [CrossRef]
9. Thong MSY, van Noorden CJF, Steindorf K, Arndt V. Cancer-related fatigue: causes and current treatment options. Curr Treat Options Oncol. 2022;23(3):450–1. doi:10.1007/s11864-021-00916-2 [Google Scholar] [PubMed] [CrossRef]
10. Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16(7):791–801. doi:10.1007/s00520-007-0380-2 [Google Scholar] [PubMed] [CrossRef]
11. Fosså SD, Dahl AA, Loge JH. Fatigue, anxiety, and depression in long-term survivors of testicular cancer. J Clin Oncol. 2003;21(7):1249–54. doi:10.1200/JCO.2003.08.163 [Google Scholar] [PubMed] [CrossRef]
12. Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Sriram Y. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23(8):2461–78. doi:10.1007/s00520-015-2763-0 [Google Scholar] [PubMed] [CrossRef]
13. Detmar SB, Aaronson NK, Wever LD, Muller M, Schornagel JH. How are you feeling? Who wants to know? Patients’ and oncologists’ preferences for discussing health-related quality-of-life issues. J Clin Oncol. 2000;18(18):3295–301. doi:10.1200/JCO.2000.18.18.3295 [Google Scholar] [PubMed] [CrossRef]
14. Costantini M, Mencaglia E, Giulio PD, Cortesi E, Roila F, Ballatori E, et al. Cancer patients as ‘experts’ in defining quality of life domains. A multicentre survey by the Italian group for the evaluation of outcomes in oncology (IGEO). Qual Life Res. 2000;9(2):151–9. doi:10.1023/A:1008967104082 [Google Scholar] [PubMed] [CrossRef]
15. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Ann Ny Acad Sci. 2002;94:528–38. doi:10.1002/(ISSN)1097-0142. [Google Scholar] [CrossRef]
16. Aapro M, Scotte F, Bouillet T, Vigano D, Currow A. A practical approach to fatigue management in colorectal cancer. Clin Colorectal Cancer. 2018;16(4):275–85. doi:10.1016/j.clcc.2016.04.010 [Google Scholar] [PubMed] [CrossRef]
17. Stone PC, Minton O. Cancer-related fatigue. Eur J Cancer. 2008;44(8):1097–104. doi:10.1016/j.ejca.2008.02.037 [Google Scholar] [PubMed] [CrossRef]
18. Arndt V, Koch-Gallenkamp L, Jansen L, Bertram H, Eberle A, Holleczek B, et al. Quality of life in long-term and very long-term cancer survivors versus population controls in Germany. Acta Oncol. 2017;56(2):190–7. doi:10.1080/0284186X.2016.1266089 [Google Scholar] [PubMed] [CrossRef]
19. Berger AM, Mitchell SA, Jacobsen PB, Pirl WF. Screening, evaluation, and management of cancer-related fatigue: ready for implementation to practice? CA Cancer J Clin. 2015 May–Jun;65(3):190–211. doi:10.3322/caac.21268 Epub 2015 Mar 11 [Google Scholar] [PubMed] [CrossRef]
20. Stein KD, Jacobsen PB, Blanchard CM, Thors CT. Further validation of the multidimensional fatigue symptom inventory-short form (MFSI-SF). J Pain Symptom Manag. 2004;27(1):14–23. doi:10.1016/j.jpainsymman.2003.06.003 [Google Scholar] [PubMed] [CrossRef]
21. Clara MI, Stein K, Canavarro MC, Allen Gomes A. European Portuguese version of the multidimensional fatigue symptom inventory-short form: validation study. Acta Medica Port. 2023; doi:10.20344/amp.18797 [Google Scholar] [PubMed] [CrossRef]
22. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1982;67(6):361–70. doi:10.1111/j.1600-0447.1983.tb09716.x [Google Scholar] [PubMed] [CrossRef]
23. Pais-Ribeiro J, Silva I, Ferreira T, Martins A, Meneses R, Baltar M. Validation study of a Portuguese version of the hospital anxiety and depression scale. Psychol Health Med. 2007;12(2):225–35. doi:10.1080/13548500500524088 [Google Scholar] [PubMed] [CrossRef]
24. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep: J Sleep Res Sleep Med. 1991;14:54054–5. [Google Scholar]
25. Santos CR. Avaliação da sonolência diurna excessiva : adaptação cultural e linguística da escala de sonolência de Epworth para a população portuguesa. Monografia de licenciatura em neurofisiologia. Porto: Escola Superior de Tecnologia do Porto; 2001. [Google Scholar]
26. Marques D, Gomes A, Azevedo MH. DSPS-4: a brief measure of perceived daytime sleepiness. Curr Psychol. 2017;38:3. [Google Scholar]
27. Oken MM, Creech RH, Tormey DC, John H, Thomas D, Eleanor M, et al. Toxicity and response criteria for the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–55. doi:10.1097/00000421-198212000-00014. [Google Scholar] [CrossRef]
28. Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
29. Oncology Nursing Society. Putting Evidence into Practice (PEPfatigue. ons.org/practice-resources/pep. [Accessed 2023]. [Google Scholar]
30. Howell D, Keshavarz H, Broadfield L, Hack T, Hamel M, Harth T, et al. A pan-Canadian practice guideline for screening, assessment, and management of cancer-related fatigue in adults (version 2). 10.7939/R33R6N. [Accessed 2015]. [Google Scholar] [CrossRef]
31. Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. American society of clinical oncology. Screening, assessment, and management of fatigue in adult survivors of cancer: an American society of clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014 Jun 10;32(17):1840–50. doi:10.1200/JCO.2013.53.4495 [Google Scholar] [PubMed] [CrossRef]
32. Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007 Nov;26(6):660–7. doi:10.1037/0278-6133.26.6.660 Erratum in: Health Psychol [Internet]. 2008 Jan;27(1):42 [Google Scholar] [PubMed] [CrossRef]
33. Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008 Sep;134(5):700–41. doi:10.1037/a0012825 Erratum in: Psychol Bull. 2009 Jan;135(1):172 [Google Scholar] [PubMed] [CrossRef]
34. Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors—a meta-analysis. Psychooncology. 2011 Feb;20(2):115–26. doi:10.1002/pon.1728 [Google Scholar] [PubMed] [CrossRef]
35. Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc. 2013 Nov;45(11):2080–90. doi:10.1249/MSS.0b013e31829a3b63 [Google Scholar] [PubMed] [CrossRef]
36. Kessels E, Husson O, van der Feltz-Cornelis CM. The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2018 Feb 9;14:479–94. doi:10.2147/NDT.S150464 [Google Scholar] [PubMed] [CrossRef]
37. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comor-bidities in patients with cancer. J Clin Oncol. 2008;26(6):971–82. doi:10.1200/JCO.2007.10.7805 [Google Scholar] [PubMed] [CrossRef]
38. Palagini L, Miniati M, Riemann D, Zerbinati L. Insomnia, fatigue, and depression: theoretical and clinical implications of a self-reinforcing feedback loop in cancer. Clin Pract Epidemiol Ment Health: CP & EMH. 2021;17:257–63. doi:10.2174/1745017902117010257 [Google Scholar] [PubMed] [CrossRef]
39. Heckler CE, Garland SN, Peoples AR, Perlis ML, Shayne M, Morrow GR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer. 2016 May;24(5):2059–66. doi:10.1007/s00520-015-2996-y [Google Scholar] [PubMed] [CrossRef]
40. Fleming L, Randell K, Harvey CJ, Espie CA. Does cognitive behaviour therapy for insomnia reduce clinical levels of fatigue, anxiety and depression in cancer patients? Psychooncology. 2014 Jun;23(6):679–84. doi:10.1002/pon.3468 [Google Scholar] [PubMed] [CrossRef]
41. Li X, Liou KT, Chimonas S, Bryl K, Wong G, Spiguel E, et al. Addressing cancer-related fatigue through sleep: a secondary analysis of a randomized trial comparing acupuncture and cognitive behavioral therapy for insomnia. Integr Med Res. 2023 Mar;12(1):100922. doi:10.1016/j.imr.2023.100922 [Google Scholar] [PubMed] [CrossRef]
42. Clara MI, Canavarro MC, Miller-Mendes M, Allen Gomes A. Insomnia in cancer survivors: a precision behavioral sleep medicine approach. Eur Psychol. 2023;28(2):110–21. doi:10.1027/1016-9040/a000506. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools