 Open Access
Open Access
REVIEW
The Effects of Mindfulness-Based Interventions on Symptoms of Mild Traumatic Brain Injury: A Systematic Review
1 School of Physical Education and Sports, Central China Normal University, Wuhan, 430079, China
2 School of Physical Education, Hubei University, Wuhan, 430062, China
* Corresponding Author: Bin Wang. Email:
International Journal of Mental Health Promotion 2024, 26(6), 417-428. https://doi.org/10.32604/ijmhp.2024.049010
Received 25 December 2023; Accepted 17 April 2024; Issue published 28 June 2024
Abstract
Mindfulness-based interventions (MBIs) are emerging non-pharmacological treatments for mild traumatic brain injury (mTBI). In this systematic review, the authors aimed to evaluate the potential efficacy of MBIs to provide recommendations for treating patients with mTBI. We searched of the English literature on MBIs for patients with mTBI as of 01 September, 2023, using the PubMed, Web of Science, PsycINFO, and Scopus databases. One author performed data extraction and quality scoring of the included literature according to the proposed protocol, and another conducted the review. The review was not registered. A total of 11 studies met the final inclusion criteria, 5 of which involved military personnel (veterans). MBIs covered in this review include goal-oriented attention self-regulation (GOALS), mindfulness-based stress reduction (MBSR), acceptance and commitment therapy (ACT), and so on. Research shows that MBSR mainly reduces mental fatigue symptoms in mTBI patients, and GOALS tend to improve their cognitive function. The effect of MBIs on psychological symptoms needs further exploration. Other studies, such as mindfulness-based group therapy and intervention studies targeting mTBI military personnel, are relatively sparse. MBIs have specific effects on mental fatigue and cognitive dysfunction in patients with mTBI. However, the effect on psychological distress and the sustained effectiveness across all symptoms still need further exploration. Considering the particularity of military personnel suffering from mTBI, researchers need to do more intervention studies targeting mTBI military personnel. Therefore, the design of future MBIs trials for mTBI patients’ needs to take into account all the factors, such as different populations and severity of traumatic brain injury, to verify the effectiveness of MBIs in alleviating mTBI symptoms and explore the mechanism of intervention.Keywords
Supplementary Material
Supplementary Material FileTraumatic brain injury (TBI) causes more deaths and disability worldwide than any other traumatic injury, of which mild traumatic brain injury (mTBI) accounts for 81.02% [1]. The American Congress of Rehabilitation Medicine (ACRM) [2] defines mTBI as follows: A traumatically induced physiological disruption of brain function, as manifested by at least one of the following: 1. any loss of consciousness; 2. any loss of memory for events immediately before or after the accident; 3. any alteration in mental state at the time of the accident (e.g., feeling dazed, disoriented, or confused); and 4. focal neurological deficit (s) that may or may not be transient; but where the severity of the injury does not exceed the following: (1) loss of consciousness of approximately 30 min or less; (2) after 30 min, an initial Glasgow Coma Scale (GCS) of 13–15; (3) and posttraumatic amnesia (PTA) not greater than 24 h”. In 2019, the ACRM Brain Injury Special Interest Group Mild TBI Task Force began to undertake an update of the 1993 ACRM definition of mTBI. It is undeniable that mTBI is also a prevalent injury in war [3]. It is common among soldiers after being exposed to blast [4], as evidenced by data from the Defense and Veterans Brain Injury Center (DVBIC), which showed that more than 80% of the approximately 379,519 TBI service members from 2000 to 2018 were classified as mTBI [5,6]. One of the TBI symptoms is cognitive impairment. In a study of people after brain injury in the chronic phase, the most common cognitive symptoms were memory impairment, attention disorder, and executive function impairment [7,8]. During the acute phase, mental fatigue is one of the complaints after mTBI [9]. Cognitive impairment and mental fatigue are both main complaints of patients with mTBI. In addition, mTBI patients also suffer from depression and anxiety [10,11]. Moreover, the rates of depression and anxiety remain high beyond the first year after injury [12–14]. One study found that five years post-deployment, veterans with a history of combat-related blast mTBI had significantly worse neurobehavioral and psychiatric symptom severity, overall disability, and sleep [15]. The symptoms of mTBI will prevent soldiers from concentrating highly during battle and make them unable to perform tasks quickly, significantly affecting the troops’ combat effectiveness.
Mindfulness-based interventions (MBIs) mainly include mindfulness-based stress reduction training (MBSR), mindfulness-based cognitive training (MBCT), acceptance and commitment therapy (ACT), and goal-oriented attention self-regulation (GOALS). MBIs were first used in medical clinical treatment. Kabat-Zinn applied MBSR to medical patients with chronic pain to relieve patients’ pain and improve their quality of life [16]. MBIs can be applied across multiple symptom and functional domains [17,18] and may be beneficial in addressing mTBI patients’ clinical presentation [19,20]. Several studies have demonstrated the effectiveness of MBIs on mental fatigue, quality of life, and aggression in various medical and psychological conditions [21,22], with the potential to improve attention, memory, and other cognitive functions [23]. After most studies focused on evaluating the effectiveness of MBIs, the structure of mindfulness in psychology has received attention. The psychological training of mindfulness consists of three elements: intention, attention, and attitude. It cultivates the ability of recognition or re-perception of personal experiences [24].
The purpose of this article is to systematically review randomized controlled trials (RCTs) of MBIs for patients with mTBI and objectively evaluate the effects of MBIs on cognitive deficits, memory, attention, executive function, mental fatigue, and psychological symptoms in mTBI patients, providing support for their recovery.
We conducted online searches in English literature until 01 September, 2023, utilizing multiple databases, including PubMed, Web of Science, PsycINFO, and Scopus. We used the following search string: (“mindfulness*” OR “goal-oriented attention self-regulation” OR “GOALS” OR “acceptance and commitment therapy” OR “ACT” OR “dialectical behavior therapy” OR “DBT” OR “MBSR” OR “MBCT”) AND (“traumatic brain injur*” OR “brain injur*” OR “TBI”) AND (“RCT” OR “randomized*” OR “controlled trial” OR “clinical trial”). We used varied search element settings based on the database’s specific retrieval requirements and features. The retrieved literature was screened by title and abstract, and then the complete text was extracted for evaluation. Following screening, we assessed the full-text articles for eligibility. The systematic review was conducted according to PRISMA guidelines (Supplementary Checklist). The literature search and inclusion process are shown in Fig. 1.
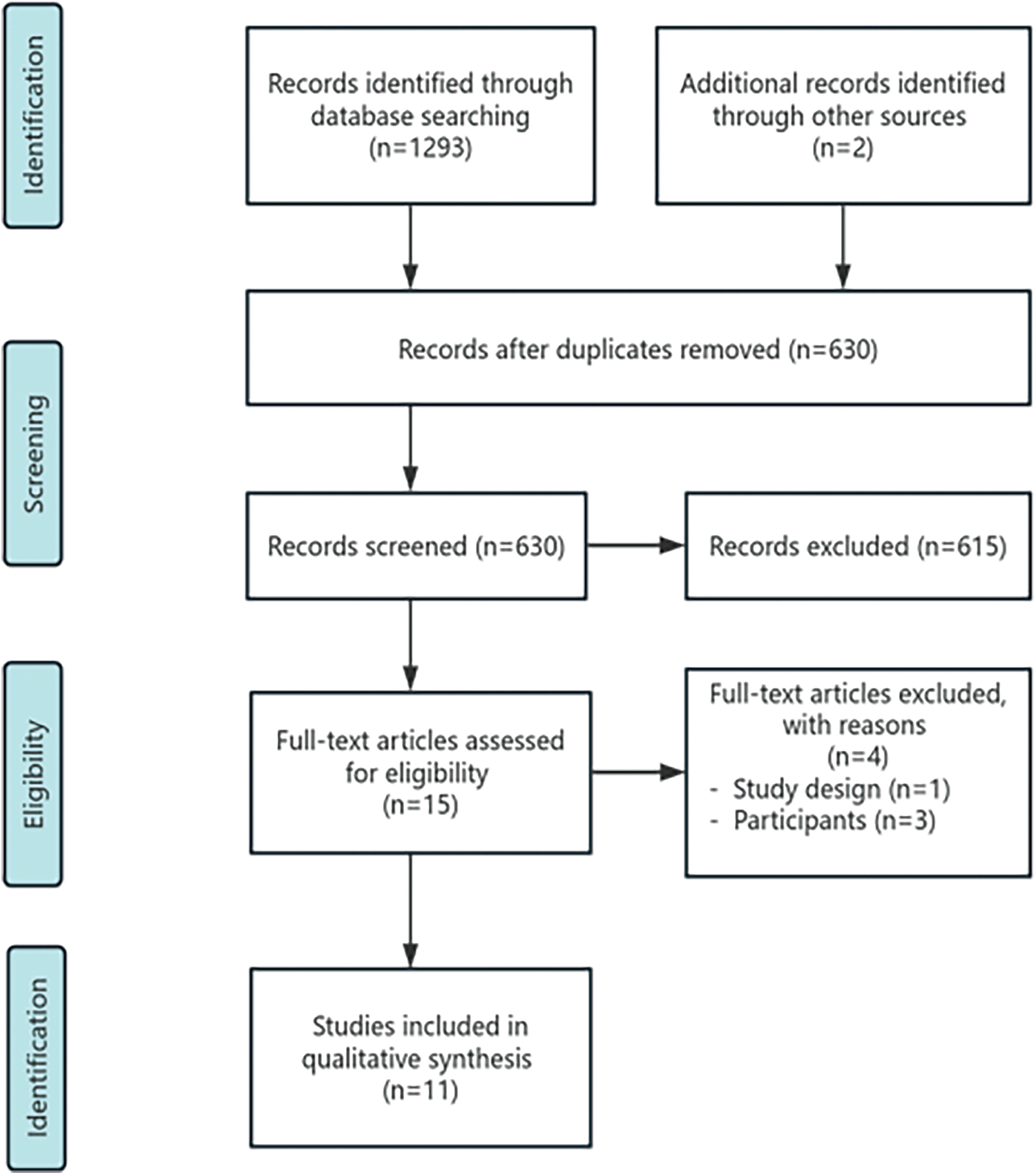
Figure 1: Study flow diagram.
Two authors independently reviewed all potential studies for inclusion criteria. We first screened the titles and abstracts of the articles and finally reviewed the full text of the studies to assess whether they met the inclusion criteria. If there was a dispute about study inclusion, the third author made the final decision on eligibility. We included RCTs of MBIs for mTBI rehabilitation or training. Included studies were required to reflect primary outcomes: cognitive function (e.g., attention, executive function and memory) [25,26], level of mental fatigue [27,28], and psychological symptoms (e.g., depression, anxiety, somatization) [29]. Secondary outcomes are also considered: stress, aggression, and pain.
Since MBIs are emerging non-pharmacological rehabilitation treatments for mTBI, the included research subjects only need to include mTBI patients. The time from the subject’s brain injury to the experiment and the duration of the trials are not limited. Regarding the exclusion criteria, we decided not to include (1) studies with inconsistent topics; (2) studies in which the severity of brain injury was not assessed or unspecified whether the research subjects included mTBI patients; (3) studies that lacked pre-specified primary and/or secondary outcomes; (4) animal or cytology experiments; (5) trial protocols, retrospective studies, case reports, conference abstracts, reviews, thesis and books.
Two authors independently used standard forms to extract study characteristics and outcome data from the studies and discussed the inconsistencies. The third author made the final decision if agreement could not be reached. Data extracted from each study included the first author, year of publication, sample size, participant information, time between post-injury and treatment, study design, intervention modality, outcome measures, and results. We contacted an author of a study via email to verify the number of participants in their study, but we are still waiting for a reply.
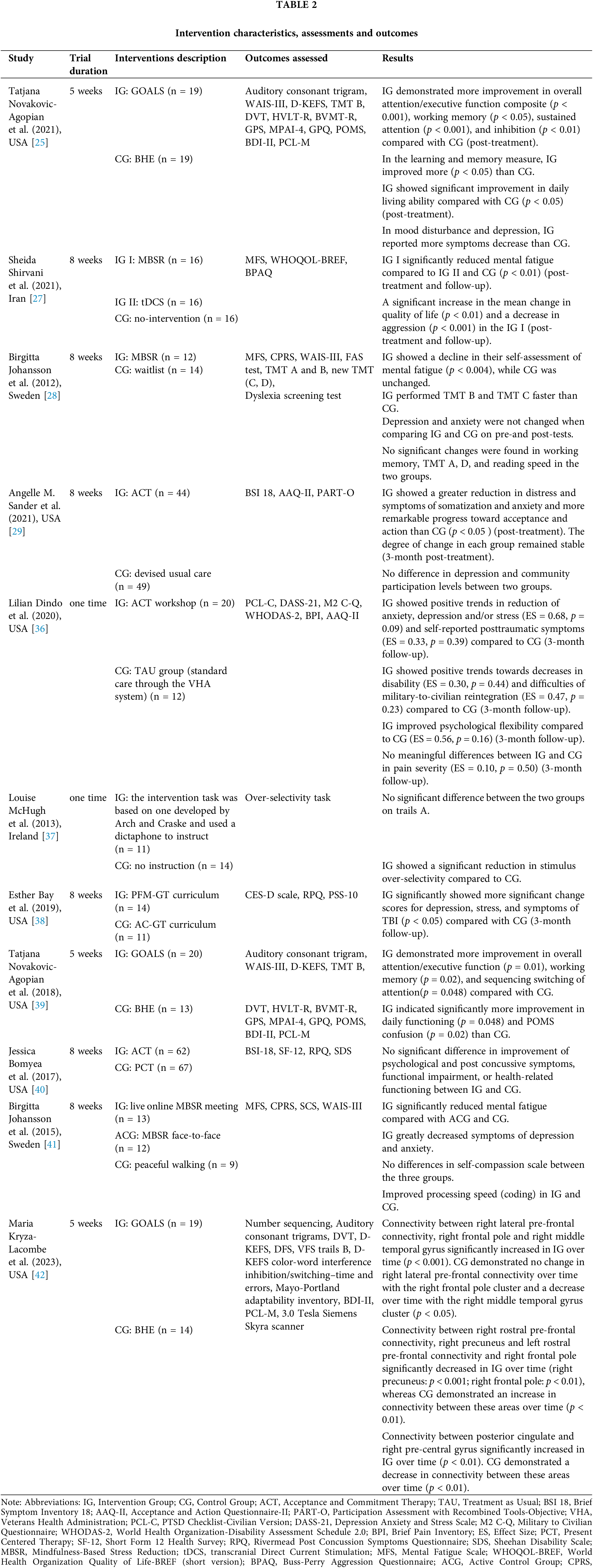
Based on the topic of this review and the description of randomization, blinding, and subject loss, we chose the Physiotherapy Evidence Database (PEDro) scoring system [30] to assess the methodological quality of our selected studies. PEDro is widely used in systematic evaluations in the field of rehabilitation. Research shows that PEDro has been used in more than 46,000 RCTs in 14 fields of physical therapy, including quite a few in neurological rehabilitation [31]. The PEDro scale evaluates the quality of RCTs through 11 scales. The first item is a measure of external validity, and its score is not included in the total score, so the full score is ten points. Randomized controlled trials rated 5 on the PEDro scale were of moderate quality, and more than 5 were of high quality. The literature quality evaluation was completed independently by two authors. After completion, two authors compared the results of evaluation and discussed inconsistencies. If the results could not reach an agreement, the authors negotiated with a third author. The average quality score of the studies that were finally included was about 8. The quality of this evidence was judged to be high overall. See Table 1.
After removing duplicates, we identified 630 records. We excluded 615 records after screening titles and abstracts for studies that did not choose MBIs, and the studies were animal and cytology experiments, experimental protocols, retrospective studies, case reports, conference abstracts, reviews, and completely unrelated articles. The remaining studies (n = 15) were reviewed in full text. One study did not use RCTs design [32]. The severity of brain injury was unclear or unspecified whether the participants included mTBI patients in three studies [33–35]. The number of experiments in the two studies [36,37] was once. In one study, the researchers identified practical barriers to conducting multiple, long-term rehabilitation interventions, including time constraints and things that the participants needed to take priority over the experiment [36]. In the other study, the researchers wanted to explore whether a short period of mindfulness training before completing a decision-making task could produce positive changes [37]. Although the number and the time of these two experiments were small, we still decided to include them for review together with the remaining night studies [25,27–29,38–42]. Therefore, 11 RCTs of MBIs for mTBI patients’ rehabilitation were finally included, and 5 studies were conducted on veterans [25,36,39,40,42].
Most of the ten studies included in this article were conducted in the United States or some European countries, and only one study was conducted in an Asian country [27]. Five studies used veterans as participants, while six used civilians as subjects [27–29,37,38,41], and no studies chose active military personnel as the research subjects. A total of 517 participants were involved, 265 of whom were veterans, and the sample size in each study ranged from 25 to 129. Regarding the severity of TBI, only four studies focused on mTBI [25,27,36,42], and seven studies paid attention to TBI patients with different severities, including mTBI [28,29,37–41]. All literature related to the topic is RCTs. Two studies did not report the average age of the participants [27,38] but gave the age range criteria for participant selection. In the other nine studies [25,28,29,36,37,39–42], the average age of participants ranged from 29 to 60 years. The average time from mTBI to intervention ranged from 9 months to 17 years in seven studies [25,28,29,37,38,41,42]. One study only reported a median time of 6 years after injury [39], and three studies did not disclose [27,36,40] (Table 1).
Table 2 shows the included studies’ intervention characteristics, outcome measures, and results. There are three studies on ACT [29,36,40] and MBSR [27,28,41], respectively, and three studies on GOALS [25,39,42]. There was one each from positive focused mindfulness group therapy (PFM-GT) [38] and an intervention [37] developed based on related research by Arch and Craske [43]. The majority of studies (9 studies) conducted continuous interventions [25,27–29,38–42] with a total duration of 5 to 12 weeks, and two were one-time brief trials [36,37]. Three RCTs were blank controls [27,28,37]. Two RCTs used conventional treatment [29,36], and three used brain health education (BHE) intervention [25,39,42]. The control groups of the three studies included arranging patients to walk in a natural environment and implementing face-to-face MBSR [41], present centered therapy (PCT) [40] and active control group therapy (AG-CT) [38]. Among the control groups in these studies, five groups were active [25,38–41].
Nine studies [25,29,36–42] assessed cognitive functions (e.g., attention, memory, working memory, executive function, acceptance, and psychological flexibility), and eight [25,29,36,38–42] assessed psychological symptoms (e.g., depression, anxiety). There were two studies on the assessment of mental fatigue [27,41], aggression [27,41], stress [36,38], and pain [29,36].
In a study of ACT intervention for mTBI patients, Sander et al. [29] used mTBI civilians as trial subjects. They found that ACT can significantly reduce pain, somatization, and anxiety symptoms and have significant improvements in acceptance and psychological flexibility. Symptoms improved and stabilized in the follow-up after three months. However, there was no significant improvement in the level of depression. There are two ACT studies on veterans. Dindo et al. [36] found that ACT showed a positive trend in reducing anxiety, depression, and stress and improving psychological flexibility. However, they found that ACT could not effectively reduce pain. A 12-week trial conducted by Bomyea et al. [40] concluded that there was no significant difference in improvement for veterans on psychological and post-concussion symptoms after brain injury after ACT.
In the included MBSR studies, the intervention program developed by Kabat-Zinn [27,28,41], and all conducted 8-week interventions in civilian patients with mTBI. Shirvani et al. [27] found that compared with transcranial direct current stimulation (tDCS) and the control group, MBSR showed significantly reduced mental fatigue after treatment and follow-up. Patients treated with MBSR reported a significant increase in average quality of life and a decrease in aggressive behavior after treatment and at follow-up. Johansson et al. [28] demonstrated that participants who completed MBSR had significantly reduced mental fatigue and improved cognitive executive functions. However, there were no significant changes in depression, anxiety, working memory, visual scanning and movement speed, and reading speed. Then, Johansson et al. [41] further studied the effects of face-to-face MBSR and live online MBSR. They found that live online MBSR can reduce patients’ mental fatigue, especially depression and anxiety. Also, it can speed up patients’ information processing (encoding) and improve their attention. But there was no difference in score changes on the self-compassion scale between face-to-face MBSR and live online MBSR.
All GOALS intervention trials were conducted on mTBI veterans, and the intervention periods were five weeks. Novakovic-Agopian et al. [39] conducted a study on veterans with chronic TBI. Participants in the GOALS group significantly improved overall attention or executive function, working memory and attention, daily functioning, and psychological distress. Nevertheless, GOALS did not significantly reduce symptoms of depression and posttraumatic stress disorder (PTSD) in mTBI veterans. Their team [25] also conducted a study on veterans with PTSD and mTBI and found that veterans who completed the GOALS intervention had more significant improvements in overall attention/executive function composite, working memory, sustained attention and cognitive inhibition, learning, memory, and daily living abilities, better overcoming psychological distress. Kryza-Lacombe et al. [42] conducted a GOALS intervention for veterans with a history of mTBI injuries of more than six months. They found that GOALS is effective for patients probably because it works through synchronization of communication between task-negative and-positive networks underlying attention and executive processes [42].
After eight weeks of PFM-GT, researchers found that it significantly impacted the scores of depression, stress, and TBI symptoms in civilian patients with TBI (including mTBI) at post-treatment and follow-up three months [38]. Louise McHugh et al. [37] used an intervention task based on the development of Arch and Craske. They found that attention in civilian patients with TBI (including mTBI) was significantly improved, but there was no remarkable change in visual scanning and movement speed.
We conducted a systematic review of the effects on MBIs for mTBI patients. There are three main MBIs for mTBI: ACT, MBSR, and GOALS. Fewer studies exist on interventions based on tasks developed by Arch and Craske and mindfulness-based group therapy.
ACT is efficacious in improving the anxiety symptoms of mTBI patients. However, researchers had inconsistent results in the improvement of post-brain injury pain and depression. The ACT studies only measured psychological flexibility and lacked measurement of overall cognitive function, which cannot sufficiently reflect whether the cognitive deficits of mTBI patients have changed. Although a single session of ACT can improve the emotional function and reintegration into civilian society of veterans suffering from mTBI, the effect is poor. ACT intervention has not been performed in mTBI civilians, so the effect on this population is unknown.
Regarding the included studies on the effect of MBSR on TBI (including mTBI), we all found that the mental fatigue of civilian patients with mTBI can be reduced [27,28,41]. In terms of mental fatigue relief, a study has found that MBSR is even better than tDCS [27], while live online MBSR is more effective than face-to-face MBSR [41]. A study performed MBSR on patients with chronic fatigue syndrome. After intervention, researchers found that patients’ fatigue was reduced [44]. It confirmed that MBSR is effective in treating mental fatigue in mTBI civilians. After MBSR treatment, the aggression of mTBI civilians can be lowered to a certain extent [27], and some cognitive functions are improved [28,41], but there was no noticeable effect on working memory, visual scanning and movement speed, and reading speed [28,41]. The effectiveness of MBSR in treating depression and anxiety in mTBI civilians is still controversial. The study by Johansson’s team in 2012 showed that MBSR did not significantly reduce patients’ anxiety and depression [28]. However, in 2015, their team found a significant effect of live online MBSR on the above two symptoms [41]. Therefore, it is necessary to increase the sample size and repeat the trial to clarify whether the change in the live online and offline forms of MBSR leads to improving symptoms. Regarding the included MBSR studies, there is a lack of targeting of mTBI military personnel. The effect of face-to-face MBSR or live online MBSR on the rehabilitation of military personnel with mTBI is unclear.
GOALS can effectively improve the cognitive functions (e.g., overall attention or executive function, working memory) and psychological symptoms (e.g., depression) of mTBI veterans. However, GOALS has no significant effect on improving the anxiety and fatigue symptoms of mTBI veterans. The effect of GOALS on cognitive function in mTBI civilians is unknown.
Mindfulness-based group therapy [38] is effective for mTBI civilians in terms of psychological symptoms (e.g., depression, stress) and TBI symptoms. Conducting a single-session intervention based on the development of Arch and Craske can improve the attention of TBI (including mTBI) civilian patients [37]. However, it only has minor improvements in visual scanning and movement speed, and improvements in attention may last only a short time. Neither PFM-GT nor the intervention developed by Arch and Craske involve research on mTBI military personnel. The efficacy of these two therapies on mTBI military personnel in terms of psychological symptoms, TBI symptoms, and attention needs further exploration.
Overall, research shows that MBSR is more effective in relieving mental fatigue symptoms in mTBI civilians, but it has little effect on improving psychological symptoms. Its effect on military personnel with mTBI is unclear. The effectiveness of different forms of MBSR on post-mTBI symptoms in military personnel still needs to be proven experimentally. GOALS is efficacious in improving the cognitive functions and depressive symptoms of veterans with TBI (including mTBI), but its effect on mental fatigue and some psychological symptoms (such as anxiety) is weak, and further research on mTBI military personnel is still needed. ACT has potential effects on psychological symptoms, but there are no relevant studies on its improvement in mental fatigue and cognitive deficits. Regarding the poor effectiveness of PFM-GT as a single intervention in patients with mTBI, more relevant trials are needed to verify its effectiveness in treating other symptoms.
While the findings suggest that MBIs have some promising clinical benefits for mTBI symptoms, we must also acknowledge that there are certain limitations to the included studies. In terms of study subjects, the number of subjects in three studies was less than 30, indicating a small sample size. Studies should recruit a large enough number of mTBI participants to test statistical differences in results. Research on MBIs has yet to cover the population comprehensively. The researchers of the seven trials mixed patients with different TBI severities into the study. There needed to be more comparison of outcome measures among patients with different TBI severities to show whether the therapy was more effective in patients with mTBI. The average time between patients’ injury and the start of intervention is more than nine months. We have not found research implementing MBIs in the short term (e.g., several weeks or less than three months) after mTBI. It is impossible to provide recommendations for patients to implement MBIs in that phase. As for the follow-up time after the intervention, some studies lacked follow-up, or the time was not clear, resulting in unclear persistence of the intervention effect. MBIs may have a positive trend in improving anxiety, depression, and pain, but the effect size of the evidence is relatively small or medium. The existing evidence has inconsistent results on anxiety, depression, and pain. Further research is needed. The included studies lack comparative trials between different interventions (e.g., RCTs between MBSR and ACT), as well as the application of some potential MBIs, such as the Mantram Repetition Program (MRP) [45] to mTBI.
This systematic review may have been limited by higher literature inclusion criteria, which resulted in a smaller number of articles being included. In addition, some of the literature has been studied in patients with TBI of other severities in addition to mTBI. There may be a lack of relevance to the evidence-based results, but we still included this part of the literature because we considered that the application of MBIs in patients with mTBI is still in a novel stage.
Implications and future directions
This review points out the potential effectiveness of MBIs in improving symptoms of mTBI. However, some key factors still need to be considered in future work. In terms of subject selection, trials should focus solely on mTBI patients and classify them as civilians or military personnel to explore the effects of MBIs on different mTBI populations. If the included participants encompass TBI patients of different severities, the patients should be divided according to the severity of TBI. Then, the researchers should compare the effect of MBIs on TBI patients of different severities to explore the effect of MBIs on mTBI patients and whether the intervention has the best effect in the mTBI stage. Research should also consider the greater need for MBIs for mTBI rehabilitation among active-duty service members than among veterans. Because active-duty soldiers are prone to mental fatigue and cognitive deficits after mTBI, which affects their decision-making and execution during combat, and they need to recover in time to return to the battlefield and reduce errors in the field. It would have been a long time if MBIs had been performed after the military had been discharged and mTBI has changed from acute to chronic at that time. Based on this, subsequent pilot studies of MBIs in the acute phase of mTBI should be increased.
Regarding the intervention time, further research can be conducted on continuous intervention of MBSR, ACT, and PFM-GT for more than eight weeks or GOALS for more than five weeks to verify whether the effect will be better [46]. Follow-up between 1 and 3 years after the 8-week MBSR showed potential long-term benefits of mindfulness interventions in diverse patient populations [47–52]. We suggest that researchers follow up with participants for a more extended period and increase the follow-up time to 6 or 12 months to evaluate the long-term impact of the intervention on mTBI patients and explore the duration of the effect. In outcome measures, patient complaints should be considered (e.g., cognitive deficits, mental fatigue). Researchers can consider conducting trials comparing various MBIs (e.g., MBSR and ACT) and validating the effects of some potential interventions (e.g., MBCT, MRP, BrainACT [53,54]). The included studies mainly conducted face-to-face intervention. Future studies can implement different forms of intervention like teleintervention [55] (e.g., telephone and Internet intervention) or virtual reality (VR) for mTBI patients to explore which form is more effective, as well as to make the interventions easier to carry out and reduce subject shedding. In addition, it is also essential to explore the mechanism of the effect of MBIs on mTBI symptoms. Research shows mindfulness intervention can increase telomerase activity [56], thereby reducing cortisol levels [57,58]. Lower cortisol levels may be associated with less stressful experiences and fewer symptoms of anxiety and depression [58–60]. Some researchers believe that mindfulness interventions can affect telomere length [61], and shorter telomere length is associated with conditions such as psychiatric disorders, experience of stress, and poor immune functioning [58,60]. Researchers can also focus on neuroimaging outcomes, using magnetic resonance imaging (MRI) to explore the impact of MBIs on patients’ neural networks [62]. Mindfulness-based intervention is a process of focused attention that may be well connected to specific brain areas [63]. Future research can reveal the mechanism of MBIs on mTBI symptoms from aspects such as telomerase activity, telomere length, and specific brain regions.
MBIs have specific effects on mental fatigue and cognitive deficits in patients with mTBI. However, the effect on psychological symptoms and the sustained effectiveness across all symptoms still need further exploration. Considering the particularity of military personnel suffering from mTBI, more studies targeting mTBI military personnel should be conducted. Therefore, it is necessary to continue to verify the effectiveness of intervention measures such as MBSR, ACT, and GOALS on mTBI patients in the future and to explore their mechanism. The design of future MBIs trials for mTBI patients needs to consider all factors, such as different populations and severity of TBI, to provide high-quality evidence that will better evaluate the rehabilitation effect of MBIs on mTBI patients.
Acknowledgement: The authors express their gratitude to Hairou Ren for her assistance in obtaining English literature and to Wencen Lan for her help revising the article.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Qiqi Feng, Bin Wang; data collection: Qiqi Feng, Yanqiu Wang; analysis and interpretation of results: Qiqi Feng, Zhijian Huang, Yanqiu Wang; draft manuscript preparation: Qiqi Feng, Zhijian Huang, Yanqiu Wang, Bin Wang. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The original contributions presented in the study are included in the supplementary material, further inquiries can be directed to the corresponding author.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/ijmhp.2024.049010.
References
1. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2019;130(4):1080–97. doi:10.3171/2017.10.JNS17352. [Google Scholar] [PubMed] [CrossRef]
2. Silverberg ND, Iverson GL, Arciniegas DB, Bayley MT, Bazarian JJ, Bell KR, et al. Expert panel survey to update the American congress of rehabilitation medicine definition of mild traumatic brain injury. Arch Phys Med Rehabil. 2021;102(1):76–86. doi:10.1016/j.apmr.2020.08.022. [Google Scholar] [PubMed] [CrossRef]
3. Baker MT, Moring JC, Hale WJ, Mintz J, Young-McCaughan S, Bryant RA, et al. Acute assessment of traumatic brain injury and post-traumatic stress after exposure to a deployment-related explosive blast. Mil Med. 2018;183(11–12):e555–63. doi:10.1093/milmed/usy100. [Google Scholar] [PubMed] [CrossRef]
4. Li Y, Li B. Research progress in diagnosis and treatment of mild traumatic brain injury and posttraumatic stress disorder comorbidity in the soldiers. Chin J Trauma. 2020;36(8):729–35.(In Chinese). doi:10.3760/cma.j.issn.1001-8050.2020.08.011. [Google Scholar] [CrossRef]
5. Hardy M, Kennedy J, Reid M, Cooper D. Differences in posttraumatic stress disorder, depression, and attribution of symptoms in service members with combat versus noncombat mild traumatic brain injury. J Head Trauma Rehabil. 2020;35(1):37–45. doi:10.1097/HTR.0000000000000486. [Google Scholar] [PubMed] [CrossRef]
6. Bogdanova Y, Verfaellie M. Cognitive sequelae of blast-induced traumatic brain injury: recovery and rehabilitation. Neuropsychol Rev. 2012;22(1):4–20. doi:10.1007/s11065-012-9192-3. [Google Scholar] [PubMed] [CrossRef]
7. Nakajima Y. A five-year model project for supporting persons with higher brain dysfunctions. High Brain Funct Res. 2006;26(3):263–73. doi:10.2496/hbfr.26.263. [Google Scholar] [CrossRef]
8. Stuss DT, Stethem LL, Hugenholtz H, Picton T, Pivik J, Richard MT. Reaction time after head injury: fatigue, divided and focused attention, and consistency of performance. J Neurol Neurosurg Psychiat. 1989;52(6):742–8. doi:10.1136/jnnp.52.6.742. [Google Scholar] [PubMed] [CrossRef]
9. Wylie GR, Flashman LA. Understanding the interplay between mild traumatic brain injury and cognitive fatigue: models and treatments. Concussion. 2017;2(4):CNC50. doi:10.2217/cnc-2017-0003. [Google Scholar] [PubMed] [CrossRef]
10. Osborn AJ, Mathias JL, Fairweather-Schmidt AK. Prevalence of anxiety following adult traumatic brain injury: a meta-analysis comparing measures, samples and postinjury intervals. Neuropsychology. 2016;30(2):247–61. doi:10.1037/neu0000221. [Google Scholar] [PubMed] [CrossRef]
11. Osborn AJ, Mathias JL, Fairweather-Schmidt AK. Depression following adult, non-penetrating traumatic brain injury: a meta-analysis examining methodological variables and sample characteristics. Neurosci Biobehav Rev. 2014;47:1–15. doi:10.1016/j.neubiorev.2014.07.007. [Google Scholar] [PubMed] [CrossRef]
12. Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA. 2010;303(19):1938. doi:10.1001/jama.2010.599. [Google Scholar] [PubMed] [CrossRef]
13. Ashman TA, Spielman LA, Hibbard MR, Silver JM, Chandna T, Gordon WA. Psychiatric challenges in the first 6 years after traumatic brain injury: cross-sequential analyses of axis I disorders. Arch Phys Med Rehabil. 2004;85(Suppl 2):S36–42. doi:10.1016/j.apmr.2003.08.117. [Google Scholar] [PubMed] [CrossRef]
14. Meachen SJ, Hanks RA, Millis SR, Rapport LJ. The reliability and validity of the brief symptom inventory-18 in persons with traumatic brain injury. Arch Phys Med Rehabil. 2008;89(5):958–65. doi:10.1016/j.apmr.2007.12.028. [Google Scholar] [PubMed] [CrossRef]
15. Mac Donald CL, Barber J, Jordan M, Johnson AM, Dikmen S, Fann JR, et al. Early clinical predictors of 5-year outcome after concussive blast traumatic brain injury. JAMA Neurol. 2017;74(7):821. doi:10.1001/jamaneurol.2017.0143. [Google Scholar] [PubMed] [CrossRef]
16. Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiat. 1982;4(1):33–47. doi:10.1016/0163-8343(82)90026-3. [Google Scholar] [PubMed] [CrossRef]
17. Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA. 2008;300(11):1350–2. doi:10.1001/jama.300.11.1350. [Google Scholar] [PubMed] [CrossRef]
18. Janssen M, Heerkens Y, Kuijer W, Van Der Heijden B, Engels J. Effects of mindfulness-based stress reduction on employees’ mental health: a systematic review. PLoS One. 2018;13(1):e0191332. doi:10.1371/journal.pone.0191332. [Google Scholar] [PubMed] [CrossRef]
19. Cicerone KD, Kalmar K. Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J Head Trauma Rehabil. 1995;10(3):1–17. doi:10.1097/00001199-199510030-00002. [Google Scholar] [CrossRef]
20. Lumba-Brown A, Ghajar J, Cornwell J, Bloom OJ, Chesnutt J, Clugston JR, et al. Representation of concussion subtypes in common postconcussion symptom-rating scales. Concussion. 2019;4(3):CNC65. doi:10.2217/cnc-2019-0005. [Google Scholar] [PubMed] [CrossRef]
21. Lao SA, Kissane D, Meadows G. Cognitive effects of MBSR/MBCT: a systematic review of neuropsychological outcomes. Conscious Cogn. 2016;45:109–23. doi:10.1016/j.concog.2016.08.017. [Google Scholar] [PubMed] [CrossRef]
22. Zhang Q, Zhao H, Zheng Y. Effectiveness of mindfulness-based stress reduction (MBSR) on symptom variables and health-related quality of life in breast cancer patients—A systematic review and meta-analysis. Support Care Cancer. 2019;27(3):771–81. doi:10.1007/s00520-018-4570-x. [Google Scholar] [PubMed] [CrossRef]
23. McHugh L, Simpson A, Reed P. Mindfulness as a potential intervention for stimulus over-selectivity in older adults. Res Dev Disabil. 2010;31(1):178–84. doi:10.1016/j.ridd.2009.08.009. [Google Scholar] [PubMed] [CrossRef]
24. Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. J Clin Psychol. 2006;62(3):373–86. doi:10.1002/jclp.20237. [Google Scholar] [PubMed] [CrossRef]
25. Novakovic-Agopian T, Posecion L, Kornblith E, Abrams G, McQuaid JR, Neylan TC, et al. Goal-oriented attention self-regulation training improves executive functioning in veterans with post-traumatic stress disorder and mild traumatic brain injury. J Neurotraum. 2021;38(5):582–92. doi:10.1089/neu.2019.6806. [Google Scholar] [PubMed] [CrossRef]
26. Ponsford J, Velikonja D, Janzen S, Harnett A, McIntyre A, Wiseman-Hakes C, et al. INCOG 2.0 guidelines for cognitive rehabilitation following traumatic brain injury, part II: attention and information processing speed. J Head Trauma Rehabil. 2023;38(1):38–51. doi:10.1097/HTR.0000000000000839. [Google Scholar] [PubMed] [CrossRef]
27. Shirvani S, Davoudi M, Shirvani M, Koleini P, Hojat Panah S, Shoshtari F, et al. Comparison of the effects of transcranial direct current stimulation and mindfulness-based stress reduction on mental fatigue, quality of life and aggression in mild traumatic brain injury patients: a randomized clinical trial. Ann Gen Psychiat. 2021;20(1):33. doi:10.1186/s12991-021-00355-1. [Google Scholar] [PubMed] [CrossRef]
28. Johansson B, Bjuhr H, Rönnbäck L. Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Inj. 2012;26(13–14):1621–8. doi:10.3109/02699052.2012.700082. [Google Scholar] [PubMed] [CrossRef]
29. Sander AM, Clark AN, Arciniegas DB, Tran K, Leon-Novelo L, Ngan E, et al. A randomized controlled trial of acceptance and commitment therapy for psychological distress among persons with traumatic brain injury. Neuropsychol Rehabil. 2021;31(7):1105–29. doi:10.1080/09602011.2020.1762670. [Google Scholar] [PubMed] [CrossRef]
30. Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the physiotherapy evidence database (PEDro). Aust J Physiother. 2002;48(1):43–9. doi:10.1016/S0004-9514(14)60281-6. [Google Scholar] [PubMed] [CrossRef]
31. Hara T, Shanmugalingam A, McIntyre A, Burhan AM. The effect of non-invasive brain stimulation (NIBS) on executive functioning, attention and memory in rehabilitation patients with traumatic brain injury: a systematic review. Diagnostics. 2021;11(4):627. doi:10.3390/diagnostics11040627. [Google Scholar] [PubMed] [CrossRef]
32. Cole MA, Muir JJ, Gans JJ, Shin LM, D’Esposito M, Harel BT, et al. Simultaneous treatment of neurocognitive and psychiatric symptoms in veterans with post-traumatic stress disorder and history of mild traumatic brain injury: a pilot study of mindfulness-based stress reduction. Mil Med. 2015;180(9):956–63. doi:10.7205/MILMED-D-14-00581. [Google Scholar] [PubMed] [CrossRef]
33. Bédard M, Felteau M, Marshall S, Cullen N, Gibbons C, Dubois S, et al. Mindfulness-based cognitive therapy reduces symptoms of depression in people with a traumatic brain injury: results from a randomized controlled trial. J Head Trauma Rehabil. 2014;29(4):E13–22. doi:10.1097/HTR.0b013e3182a615a0. [Google Scholar] [PubMed] [CrossRef]
34. McMillan TM, Robertson IH, Brock D, Chorlton L. Brief mindfulness training for attentional problems after traumatic brain injury: a randomised control treatment trial. Neuropsychol Rehabil. 2002;12(2):117–25. doi:10.1080/09602010143000202. [Google Scholar] [CrossRef]
35. Mitchell T, du Preez E, Theadom A. An intervention to improve coping strategies in adult male prisoners with a history of traumatic brain injury: a pilot randomised clinical trial. Clin Rehabil. 2021;35(8):1185–95. doi:10.1177/0269215521998535. [Google Scholar] [PubMed] [CrossRef]
36. Dindo L, Johnson AL, Lang B, Rodrigues M, Martin L, Jorge R. Development and evaluation of an 1-day acceptance and commitment therapy workshop for veterans with comorbid chronic pain, TBI, and psychological distress: outcomes from a pilot study. Contemp Clin Trials. 2020;90:105954. [Google Scholar] [PubMed]
37. McHugh L, Wood R. Stimulus over-selectivity in temporal brain injury: mindfulness as a potential intervention. Brain Inj. 2013;27(13–14):1595–9. [Google Scholar] [PubMed]
38. Bay E, Chan RR. Mindfulness-based versus health promotion group therapy after traumatic brain injury. J Psychosoc Nurs Ment Health Serv. 2019;57(1):26–33. [Google Scholar] [PubMed]
39. Novakovic-Agopian T, Kornblith E, Abrams G, Burciaga-Rosales J, Loya F, D’Esposito M, et al. Training in goal-oriented attention self-regulation improves executive functioning in veterans with chronic traumatic brain injury. J Neurotrauma. 2018;35(23):2784–95. [Google Scholar] [PubMed]
40. Bomyea J, Lang AJ, Schnurr PP. TBI and treatment response in a randomized trial of acceptance and commitment therapy. J Head Trauma Rehabil. 2017;32(5):E35–43. [Google Scholar] [PubMed]
41. Johansson B, Bjuhr H, Karlsson M, Karlsson JO, Rönnbäck L. Mindfulness-based stress reduction (MBSR) delivered live on the Internet to individuals suffering from mental fatigue after an acquired brain injury. Mindfulness. 2015;6(6):1356–65. doi:10.1007/s12671-015-0406-7. [Google Scholar] [CrossRef]
42. Kryza-Lacombe M, Santiago R, Hwang A, Raptentsetsang S, Maruyama BA, Chen J, et al. Resting-state connectivity changes after goal-oriented attentional self-regulation training in veterans with mild traumatic brain injury: preliminary findings from a randomized controlled trial. Neurotrauma Rep. 2023;4(1):420–32. doi:10.1089/neur.2022.0074. [Google Scholar] [PubMed] [CrossRef]
43. McMillan TM, Jongen EL, Greenwood RJ. Assessment of post-traumatic amnesia after severe closed head injury: retrospective or prospective? J Neurol Neurosurg Psychiatry. 1996;60(4):422–7. doi:10.1136/jnnp.60.4.422. [Google Scholar] [PubMed] [CrossRef]
44. Surawy C, Roberts J, Silver A. The effect of mindfulness training on mood and measures of fatigue, activity, and quality of life in patients with chronic fatigue syndrome on a hospital waiting list: a series of exploratory studies. Behav Cogn Psychother. 2005;33(1):103–9. doi:10.1017/S135246580400181X. [Google Scholar] [CrossRef]
45. Oman D, Bormann JE, Kane JJ. Mantram repetition as a portable mindfulness practice: applications during the COVID-19 pandemic. Mindfulness. 2022;13(6):1418–29. doi:10.1007/s12671-020-01545-w. [Google Scholar] [PubMed] [CrossRef]
46. Felicity LB, Whittingham K, Roslyn NB, McKinlay L, Sofronoff K. Does stepping stones triple P plus acceptance and commitment therapy improve parent, couple, and family adjustment following paediatric acquired brain injury? A randomised controlled trial. Behav Res Ther. 2015;73:58–66. doi:10.1016/j.brat.2015.07.001. [Google Scholar] [PubMed] [CrossRef]
47. Geary C, Rosenthal SL. Sustained impact of MBSR on stress, well-being, and daily spiritual experiences for 1 year in academic health care employees. J Altern Complement Med. 2011;17(10):939–44. doi:10.1089/acm.2010.0335. [Google Scholar] [PubMed] [CrossRef]
48. Grossman P, Tiefenthaler-Gilmer U, Raysz A, Kesper U. Mindfulness training as an intervention for fibromyalgia: evidence of postintervention and 3-year follow-up benefits in well-being. Psychother Psychosom. 2007;76(4):226–33. doi:10.1159/000101501. [Google Scholar] [PubMed] [CrossRef]
49. Reibel DK, Greeson JM, Brainard GC, Rosenzweig S. Mindfulness-based stress reduction and health-related quality of life in a heterogeneous patient population. Gen Hosp Psychiatry. 2001;23(4):183–92. doi:10.1016/S0163-8343(01)00149-9. [Google Scholar] [PubMed] [CrossRef]
50. Hartmann M, Kopf S, Kircher C, Faude-Lang V, Djuric Z, Augstein F, et al. Sustained effects of a mindfulness-based stress-reduction intervention in type 2 diabetic patients: design and first results of a randomized controlled trial (the heidelberger diabetes and stress-study). Diabetes Care. 2012;35(5):945–7. doi:10.2337/dc11-1343. [Google Scholar] [PubMed] [CrossRef]
51. Miller JJ, Fletcher K, Kabat-Zinn J. Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. Gen Hosp Psychiat. 1995;17(3):192–200. [Google Scholar]
52. Bédard M, Felteau M, Gibbons C, Klein RG, Mazmanian D, Fedyk K, et al. A mindfulness-based intervention to improve quality of life among individuals who sustained traumatic brain injuries: one-year follow-up. J Cogn Rehabil. 2005;23(1):8–13. [Google Scholar]
53. Rauwenhoff JC, Bol Y, van Heugten CM, Batink T, Geusgens CA, van den Hout AJ, et al. Acceptance and commitment therapy for people with acquired brain injury: rationale and description of the BrainACT treatment. Clin Rehabil. 2023;37(8):1011–25. [Google Scholar] [PubMed]
54. Rauwenhoff JC, Bol Y, Peeters F, van Heugten CM. Acceptance and commitment therapy is feasible for people with acquired brain injury: a process evaluation of the BrainACT treatment. Clin Rehabil. 2024;38(4):530–42. [Google Scholar] [PubMed]
55. Beit Yosef A, Jacobs JM, Shames J, Schwartz I, Gilboa Y. A performance-based teleintervention for adults in the chronic stage after acquired brain injury: an exploratory pilot randomized controlled crossover study. Brain Sci. 2022;12(2):213. [Google Scholar] [PubMed]
56. Schutte NS, Malouff JM. A meta-analytic review of the effects of mindfulness meditation on telomerase activity. Psychoneuroendocrino. 2014;42:45–8. doi:10.1016/j.psyneuen.2013.12.017. [Google Scholar] [PubMed] [CrossRef]
57. Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22(4):600–5. doi:10.1016/j.bbi.2007.12.004. [Google Scholar] [PubMed] [CrossRef]
58. Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: roles in cellular aging. Mutat Res. 2012;730(1–2):85–9. doi:10.1016/j.mrfmmm.2011.08.003. [Google Scholar] [PubMed] [CrossRef]
59. Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–78. doi:10.1016/j.biopsycho.2008.10.004. [Google Scholar] [PubMed] [CrossRef]
60. Puterman E, Epel E. An intricate dance: life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc Personal Psychol Compass. 2012;6(11):807–25. doi:10.1111/j.1751-9004.2012.00465.x. [Google Scholar] [PubMed] [CrossRef]
61. Hoge EA, Chen MM, Orr E, Metcalf CA, Fischer LE, Pollack MH, et al. Loving-kindness meditation practice associated with longer telomeres in women. Brain Behav Immun. 2013;32:159–63. doi:10.1016/j.bbi.2013.04.005. [Google Scholar] [PubMed] [CrossRef]
62. Moore AL, Carpenter DM, James RL, Miller TM, Moore JJ, Disbrow EA, et al. Neuroimaging and neuropsychological outcomes following clinician-delivered cognitive training for six patients with mild brain injury: a multiple case study. Front Hum Neurosci. 2020;14:229. doi:10.3389/fnhum.2020.00229. [Google Scholar] [PubMed] [CrossRef]
63. Sedlmeier P, Eberth J, Schwarz M, Zimmermann D, Haarig F, Jaeger S, et al. The psychological effects of meditation: a meta-analysis. Psychol Bull. 2012;138(6):1139–71. doi:10.1037/a0028168. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools