 Open Access
Open Access
ARTICLE
Investigating the Cognitive Control of Social Media-Anxious Users Using a Psychological Experimental Approach
1
School of Psychology, Shaanxi Normal University, Xi’an, 710062, China
2
Shaanxi Provincial Key Laboratory of Behavior and Cognitive Neuroscience, Shaanxi Normal University, Xi’an, 710062, China
3
School of Psychology, Jiangxi Normal University, Nanchang, 330022, China
4
Laboratory of Psychology and Cognition Science of Jiangxi, Jiangxi Normal University, Nanchang, 330022, China
* Corresponding Author: Ling Xiang. Email:
International Journal of Mental Health Promotion 2023, 25(7), 863-871. https://doi.org/10.32604/ijmhp.2023.027303
Received 02 November 2022; Accepted 08 February 2023; Issue published 01 June 2023
Abstract
Social media has become increasingly popular and is now a significant tool for daily communication for many people. The use of social media can cause anxiety and have detrimental impacts on mental health. Cognitive impairment is more likely to affect individuals with anxiety. Investigating the cognitive abilities and mental health of social media users requires the development of new methodologies. This study employed the AX-Continuous Performance Test (AX-CPT) paradigm and the Stroop paradigm to study the cognitive control characteristics of trait anxiety, drawing on psychological experimental methods. Previous studies on whether trait anxiety impairs cognitive control remain controversial, possibly because cognitive control is viewed as a whole. It may also be due to the motivational effect of anxiety, which compensates for the impairment of cognitive control caused by anxiety through the recruitment of cognitive resources. Understanding the mental health and cognitive control traits of anxious social media users can be improved by using the Dual Mechanisms of Cognitive Control Account, which divides cognitive control into proactive and reactive control. The findings demonstrate that trait anxiety has an impact on both proactive and reactive control, while working memory load did not modulate the effect of trait anxiety on cognitive control. These results support the attentional control theory and provide a new approach to studying the mental health of social media users.Keywords
The rapid growth and widespread use of social media creates a media spectacle for individuals that is far larger than the real world. Users spend a lot of time using social media, and as a result, they share and publish more information than ever before. Social media broadens users’ online social capital, broadening their worldview and social network. However, it also has many unfavorable effects, such as social pressure, anxiety, network dependence, etc. College students use social networks online more frequently and in-depth, both in their academic and personal lives. It is obvious that college students also experience social media anxiety. Anxiety and depression can occur as a result of social media use [1]. What are the cognitive control characteristics of trait-anxious people under the influence of social media? An experimental psychological approach was used to study this question.
Trait anxiety is a personality tendency that results in individuals experiencing persistent, high-intensity anxiety and worry in a variety of situations [2]. Even in the absence of anxiety-inducing situations, people with high trait anxiety experience higher levels of anxiety. Trait anxiety can have an impact on several cognitive processing processes [3,4], particularly cognitive control. Cognitive control is a goal-oriented psychological process in which individuals recruit cognitive resources flexibly to regulate behavior in a changing environment [5], which plays a key role in selective attention, perception, memory, and problem-solving.
The results of earlier studies on the connection between trait anxiety and cognitive control, which largely focused on cognitive control as a whole, showed that trait anxiety has an impact on cognitive control. An oval-shaped object was presented on one side of the screen, and subjects were instructed to respond in the opposite direction of the target. They used an antisaccade paradigm to examine whether trait anxiety impairs dominant responses [6]. The results show that high-anxiety groups have longer correct antisaccade latencies than low-anxiety groups, while the two groups do not differ in terms of error rate, indicating that anxiety affects performance efficiency but not effectiveness. Their subsequent research also demonstrated that the prolonged latency of the antisaccade task in the high-anxiety group indicates an impairment in the inhibition of the dominant response [7]. Previous study used the flanker task to study the relation of trait anxiety and lateral inhibition and found that the high-anxiety group showed a greater interference effect (i.e., the difference between incongruent and congruent trials) than those in the low-anxiety group in reaction time, suggesting that trait anxiety modulates inhibition control. However, some studies found that trait anxiety does not affect cognitive control [8–10], and even higher trait anxiety results in better cognitive control [11]. For example, they used a modified version of the face-word Stroop task combined with the electroencephalographic method to study the relation between trait anxiety, conflict-processing, and dynamic adjustments in attentional allocation, and found a greater Stroop effect (difference between congruent and incongruent of face-word) as trait anxiety decreases [11].
The aforementioned dispute is explained by processing effectiveness [12,13] and attention control theory [14,15]. The idea clearly distinguishes between two concepts: processing efficiency and performance effectiveness. While processing effectiveness is the ratio of performance to effort, performance effectiveness measures how well a task is accomplished (an indicator typically is the error rate). Anxiety uses up cognitive resources, making them insufficient for the tasks at hand, but it can also increase motivation and encourage people to devote more time and resources to the work at hand, thereby improving performance. As a result, anxiety may not have a negative impact on performance effectiveness, but it may have a negative impact on processing efficiency. If the memory load is used to occupy the cognitive resources that subjects recruit through motivation, it is not clear whether trait anxiety affects cognitive control or not.
There is still a great deal of controversy surrounding the research on whether trait anxiety affects cognitive control. One aspect of the reason is that previous research on the relationship between trait anxiety and cognitive control has viewed cognitive control as a whole. Dual control theory holds that individuals have two cognitive strategies when resolving cognitive conflicts: one is proactive control, and the other is reactive control [16,17]. Proactive control is a cue-driven control that selectively processes and maintains task-related information before the task begins and then actively represents the cue in the subsequent time, thus forming response preparations. Thus, using cue information, the response to be made next is predicted. Reactive control is the unpreparedness for the response to be made and the flexible use of immediately available task-related information to resolve the conflict, and it consumes fewer cognitive resources than proactive control [17]. A previous study found that proactive control is thought to be a long-term mechanism that achieves top-down bias through sustained lateral prefrontal cortex-striatal activity [18]. Reactive control is thought to be a short-term mechanism with a bottom-up tendency, which is transiently activated in the prefrontal cortex based on a short period of conflict detected in the anterior cingulate cortex [19]. Researcher found that high-anxious participants had reduced sustained but increased transient activation in working memory areas, in comparison with low-anxious participants under inconsistent trials, suggesting that reduced cognitive control in high anxiety might be due to a transient, rather than sustained, pattern of working memory recruitment [20]. These studies imply that high-anxious participants may preferentially use reactive control to resolve conflict when detecting cognitive conflict compared to low trait-anxious participants and put in more effort and allocate more attentional resources to inhibit conflict. It is unclear whether trait anxiety impairs proactive control and reactive control if the memory load is used to occupy the cognitive resources that subjects recruit through motivation.
Proactive and reactive control are typically studied using the AX-CPT paradigm, which effectively separates these two types of control from cognitive control [16,21]. The paradigm consists of two types of stimuli: cues (A, B) and probes (X, Y), with a blank screen appearing in between the two. At the center of the screen, successive cue, blank screen, and probe stimuli are shown. The aim for the subjects is to react only to the probe (X) that appears after cue A (AY, BX, BY). Participants show a larger response propensity to the AX sequence because it accounts for 70% of the data while the other three sequences account for 10% each. In AX and BX sequences, cognitive conflict occurs between target response and non-target response. In the BX sequence, an individual bias toward proactive control prevents conflict by actively maintaining the representation of cue B before the presentation of probe X, while in the AY sequence, individual biased reactive control can enhance the processing of the immediately present probe Y, thus reducing the tendency of cue A to induce target responses. Proactive control is reflected by a reduction in response time or error rate for BX, and reactive control is shown by a reduction in response time or error rate for AY. Moreover, the color-word Stroop paradigm is used to study cognitive control. Manipulating the ratio of incongruent trials at mostly congruent (MC) and mostly incongruent (MI) contexts, respectively, in this paradigm can study reactive control [22–24]. Specifically, in the classic color-word Stroop task, the subjects’ task is to name the ink color and ignore the meaning of the word, and subjects have longer response time and higher error rates on incongruent trials (ink color does not match word meaning, such as “green” written in red ink) than on congruent trials (ink color matches the word’s meaning, such as “red” written in red ink), which is called the Stroop effect [25]. The Stroop effect is often used as a measure of cognitive control in studies of cognitive control [26–28]. Subsequently, the proportion of congruent and incongruent trials can be manipulated within a block, called list-wide manipulation, such as an MC block might be 75% congruent and 25% incongruent, and an MI block the reverse. Stroop effects are larger for MC than MI blocks, a finding termed the proportion congruent effect [23,29]. This phenomenon is explained by the conflict monitor theory, which claims that more conflict in the MI block results in more cognitive control and a decrease in the Stroop effect, whereas less conflict in the MC block results in less cognitive control and a boost in the Stroop effect [30]. Later researchers combined the MC and MI blocks with neutral trials, resulting in an equal number of congruent and incongruent trials [22,31–34]. The MC and MI blocks were counted separately, and the Stroop effect was still greater in the MC block than in the MI block, which is known as the item-specific proportion congruent effect (ISPC effect). The global cognitive control mechanism cannot explain this pattern of results because the proportion of congruent and incongruent trials is the same throughout the block. Rather, the ISPC effect is attributed to a local, item-specific mechanism that responds rapidly and arises to resolve conflicts after the onset of a stimulus. The ISPC effect measurement for reactive control is more sensitive [22–24]. We employed the AX-CPT and revised color-word Stroop tasks to measure whether under a load memory condition, trait anxiety affects proactive control and reactive control.
This study uses a psychological experimental approach to investigate the cognitive control characteristics of anxious individuals who use social media, providing new insights into the assessment and measurement of mental health and cognitive abilities related to social media use.
Firstly, 315 students from Jiangxi Normal University participated in a mass screening using the Chinese version of the trait anxiety portion of Spielberger’s State-Trait Anxiety Inventory [35], which was completed as part of a pretest. Participants with high (HTA group; upper 25th percentile of the distribution) or low (LTA group; lower 25th percentile of the distribution) scores were selected for further consideration. From these groups, 27 high-anxiety and low-anxiety subjects were recruited to participate in Experiment 1 and were asked to complete the same questionnaire again after the experiment (see Table 1 for details). Of the 54 participants (aged 18–25 years; M = 20.39, SD = 1.54), 42 were women. An independent sample t-test showed that the scores of the HTA group were higher than those of the LTA group in both the pre-test (t (52) = 12.07, p < 0.001, Cohen’s d = 3.29) and the post-test (t (52) = 18.14, p < 0.001, Cohen’s d = 4.94). All participants were right-handed, had normal or corrected vision, and had no color vision abnormalities.
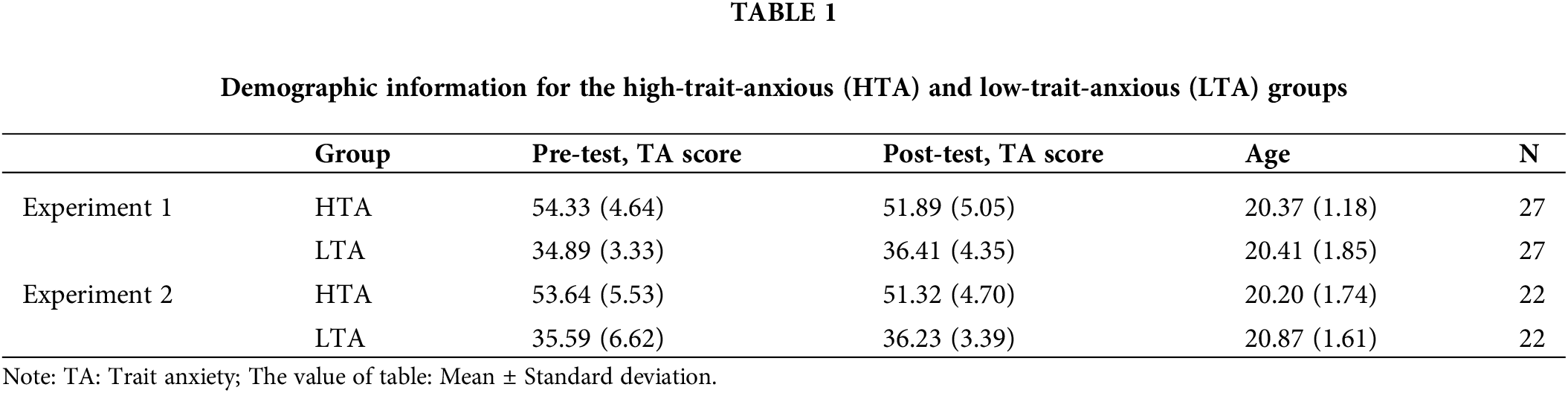
In the experiment, white capital letters A, E, G, P, R, S, and V are used as cues, and blue capital letters X, F, J, M, Q, and V are used as probes. The font of the letters was 36 Times New Roman. The AX, AY, BX, and BY sequences (“A”: A; “X”: X; “Y”: F, J, M, Q, U; “B”: E, G, P, R, S, V) accounted for 37.5%, 12.5%, 12.5%, and 37.5%, respectively. Compared to the traditional AX-CPT-70 [21,36], this manipulation has many advantages for dissociating proactive and reactive control. First, the frequency of the A- and B-cue types is equated (50/50 here vs. 80/20 in the traditional version), allowing us to control potential sources of variance related to the novelty/infrequency of both the B cue and the context for a nontarget response. B cues are less frequent than A cues in traditional AX-CPT, so subjects may process A and B cues differently because of differences in novelty and not because of the expected response to the subsequent probe [36]. Second, the proportion of probe stimuli X and Y in this experiment was both 50%, making subjects more susceptible to using cue information to anticipate upcoming responses. A and B cues were not only equated in the current version, but cue validities were also equalized through the manipulation. Cues can predict one of the trial types (X probe) with 75% accuracy, and B cues can predict one of the trial types (Y probe) with 75% accuracy. Subjects’ performance on the BX and AY sequences is a measure of their abilities for proactive and reactive control. The working memory load (WMload) is the same as in the previous study [37], in which the left and right two 50 s were presented first, and subjects were asked to add or subtract 2 from the left and right 50s according to the change in the presented asterisks during the experiment, with the calculated results entered at the end.
The procedure was designed using E-prime 2.0 software. All stimuli were presented on a 19-inch screen (refresh rate: 60 Hz; resolution: 1024 × 768) at a viewing distance of 60 cm. All participants were instructed to complete both the load and no-load tasks. Participants attended two experimental sessions, which were at least one day apart. An example of a load task/no-load trial sequence is shown in Fig. 1. Three white “x” were presented as fixation cross rows in the center of the screen, while either the left or the right ‘‘x’’ could change into a plus (+) or a minus (−) sign. In each block, participants were given the number ‘‘50’’ for both the ‘‘x’’ at the left and right position, and were asked to perform arithmetic operations on the left “x” with the first number in mind and on the right “x” with the second number in mind. Their task difficulty was increased by adding “2” to plus signs and subtracting “2” from minus signs. For instance, if a plus sign appears on the right side and the left and middle “x” remain unchanged, then the participant needs to add “2” to the second number in working memory while the first number remains unchanged from the previous trial. Note that each trial presented only one arithmetic stimulus. To perform arithmetic operations on working memory, participants always maintained two numbers. In the load task, participants needed to enter the final calculated results in the box on the monitor after each block (including five trials). In the no-load task, participants did not need to conduct arithmetic operations and were instructed to input “6666” in the box after each block.
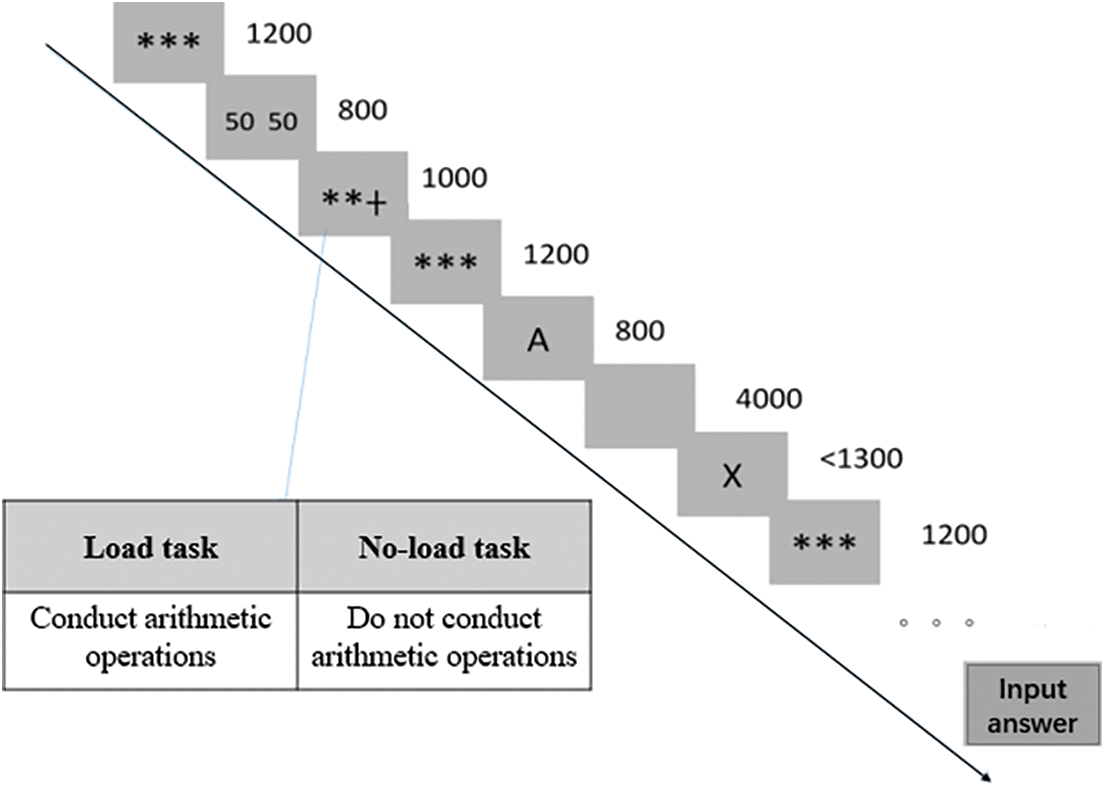
Figure 1: Procedure used in Experiment 1.
Twenty-two participants with high and low anxiety were invited to participate in Experiment 2, based on their scores on the questionnaire. After completing the experiment, trait anxiety was reassessed by having 44 participants (aged 18–25 years; M = 20.68, SD = 1.67) fill out the questionnaire again. The scores of the high trait anxiety (HTA) group were higher than those of the low trait anxiety (LTA) group in both the pre-test (t (52) = 12.80, p < 0.001, Cohen’s d = 3.86) and the post-test (t (52) = 12.20, p < 0.001, Cohen’s d = 3.68) according to independent sample t-tests. All participants were right-handed and had normal or corrected vision, as well as normal or corrected color vision.
Experiment 2 used the color-word Stroop task based on the item level [23,38]. Four Chinese characters indicating “blue”, “green”, “red” and “yellow”, and their corresponding colors were used. Based on the color (red and blue vs. yellow and green), the items were divided into two sets. One set of colors (e.g., red and blue) mostly contained congruent colors, which were presented 54 times with congruent Chinese words (e.g., “red” in red ink), as well as 6 times with each incongruent Chinese word (e.g., “red”, “blue” or “white” in red ink). The other set of colors (e.g., yellow and green) was mostly incongruent, with colors presented 54 times with incongruent Chinese words (e.g., 18 presentations of “red”, “blue”, and “green” in yellow ink) and 18 times with congruent words (e.g., “yellow” in yellow ink). The frequency of color-word combinations is shown in Table 2. In the mostly congruent (MC) set, the Chinese characters in color were 75% congruent and 25% incongruent, whereas in the mostly incongruent (MI) set, they were 25% congruent and 75% incongruent. In the example given above, the Chinese words “red” and “blue” were 56% congruent and 44% incongruent, whereas the Chinese terms “yellow” and “green” were 38% congruent and 62% incongruent. The assignment of colors to the item-set proportion congruency (ISPC) level was counterbalanced across participants. This experimental design effectively excludes contingency learning [31] from explaining ISPC effects, so that the resulting ISPC effect reflects reactive control [23,24,32,39].
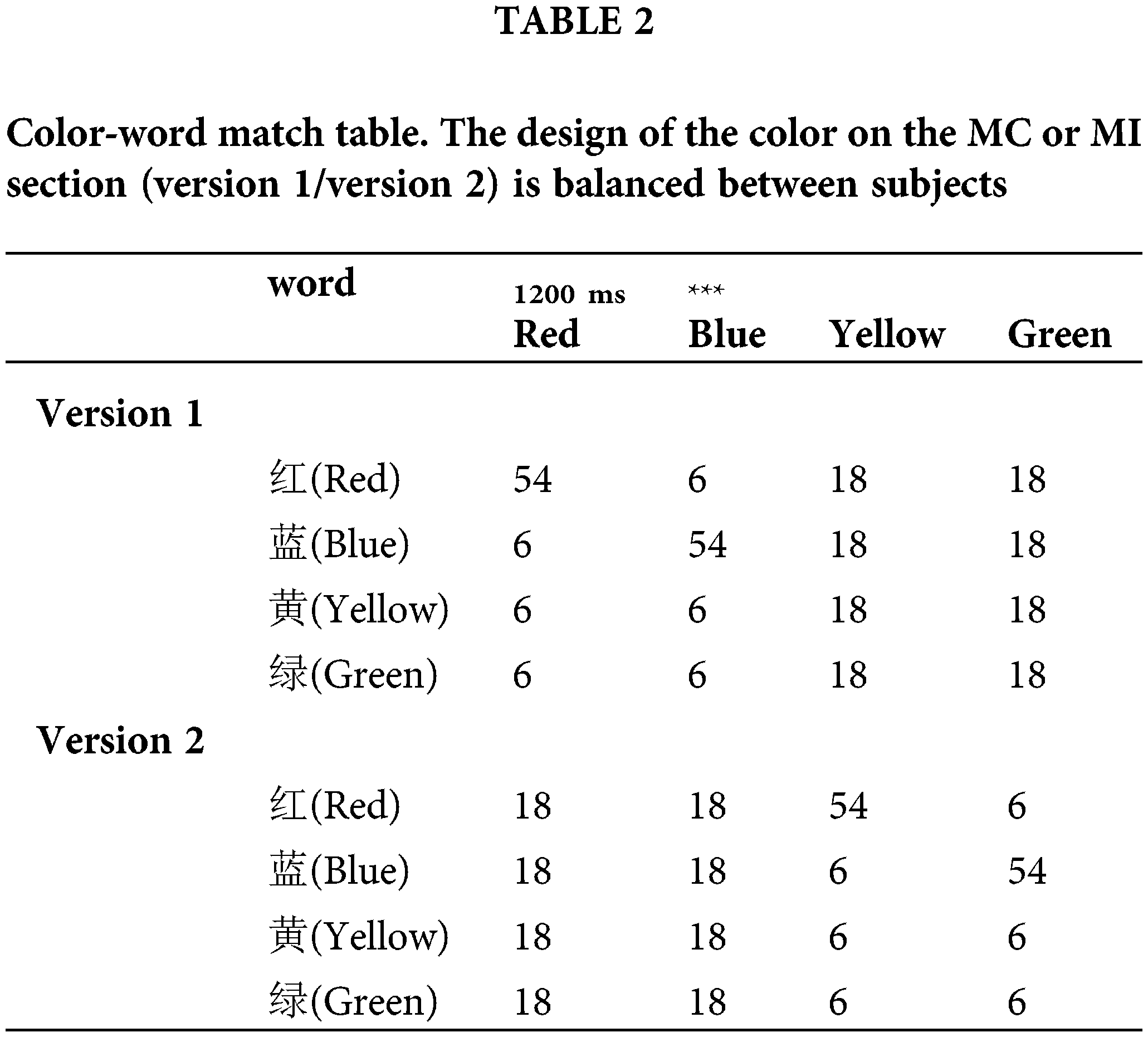
The procedure for Experiment 2 was the same as that of Experiment 1, except that the AX-CPT task was replaced by a color Stroop task, in which subjects needed to name the color of the word. As illustrated in Fig. 2, a load task/no-load trial sequence was used.
Figure 2: Procedure used in Experiment 2.
Reaction times of incorrect responses and reaction times that were more than 3 standard deviations from the mean were discarded. All statistical analyses were conducted using SPSS25 software. For Experiment 1, three-factor repeated-measures ANOVAs with trait anxiety (HTA group, LTA group), trial type (AX, AY, BX, BY), and WM load (load, no-load) were performed separately for RT and accuracy rate. For Experiment 2, four-factor repeated-measures ANOVAs with trait anxiety (HTA group, LTA group), item type (MC, MI), trial type (congruent, incongruent), and WM load (load, no-load) were conducted for RT and accuracy. The factor of trait anxiety was between-subjects for Experiment 1 and Experiment 2. For each ANOVA, the sphericity assumption was assessed using Mauchly’s test. The Greenhouse-Geisser adjustment for non-sphericity was applied as appropriate. Partial eta-squared (
The results of the repeated-measures ANOVAs showed that the main effect of WMload was significant (F (1,52) = 5.87, p < 0.05,
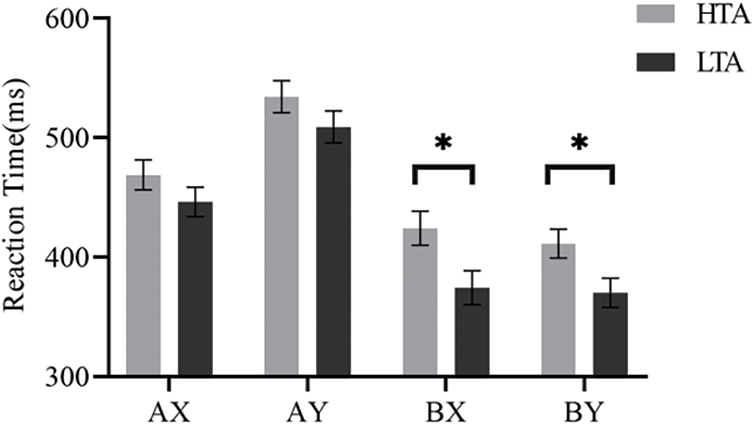
Figure 3: Reaction time in AX, AY, BX and BY sequences for each trait anxiety group. Error bars represent ±1 SE of the mean. * indicates p < 0.05.
The main effect of trial type was significant (F (3,156) = 42.19, p < 0.001,
Correct response latencies from 44 participants were entered into a four-way ANOVA with the WMload (load, no-load), item type (MC, MI), trial type (congruent, incongruent), and trait anxiety. Descriptive statistics of Experiment 2 are presented in Table 3. The analysis showed a main effect of WMload (F (1,42) = 62.34, p < 0.001,
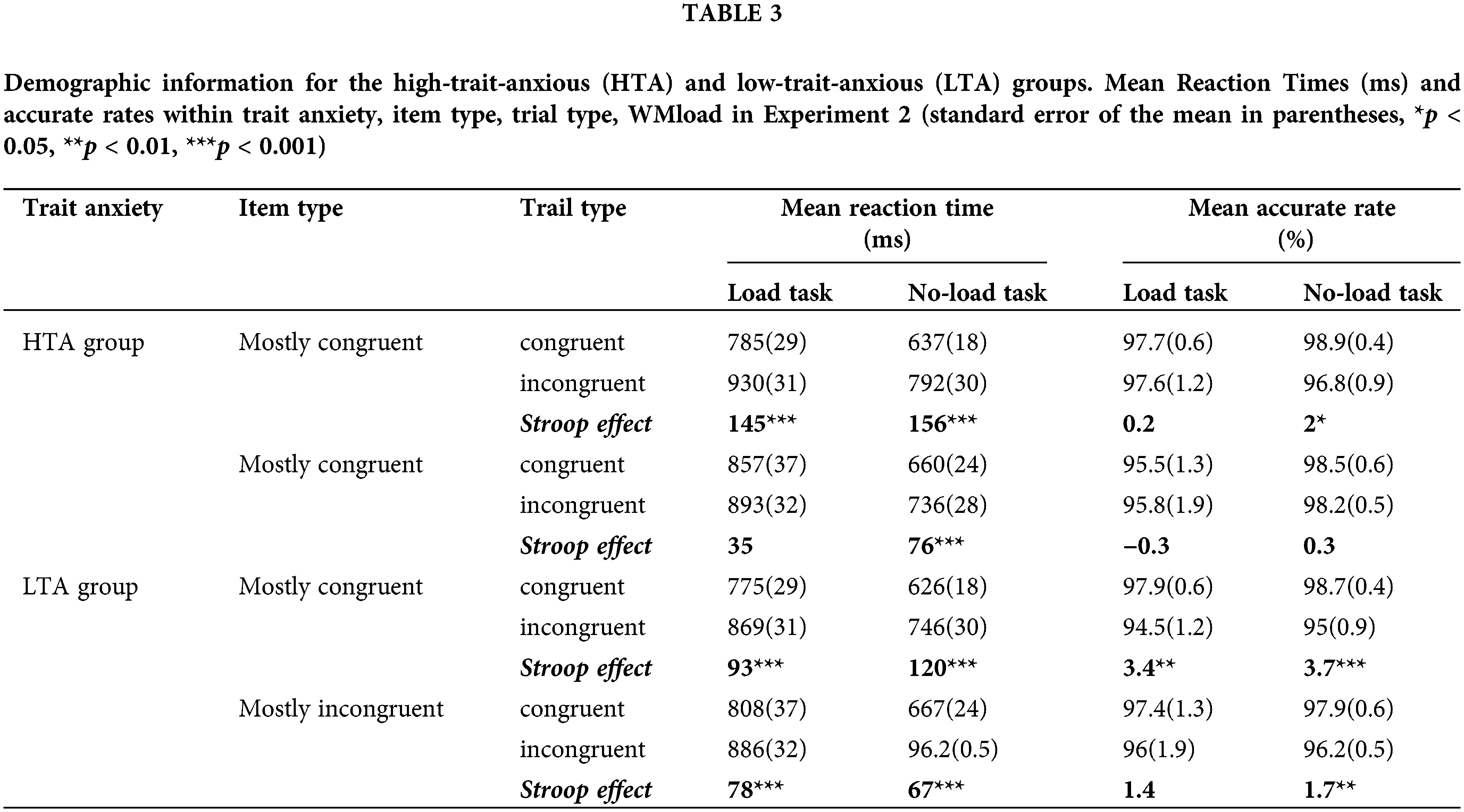
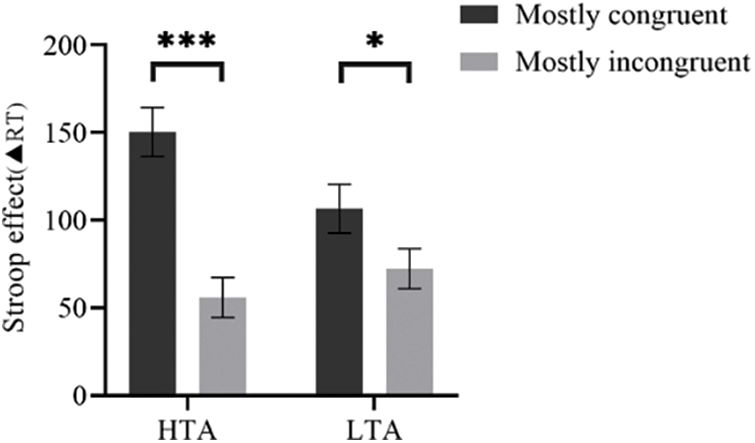
Figure 4: Stroop effect in Mostly congruent item and mostly incongruent item for each trait anxiety group, Error bars represent ±1 SE of the mean. *Indicates p < 0.05, ***Indicates p < 0.001.
There was also a main effect of trial type (F (1,42) = 7.01, p < 0.001,
To study the effect of trait anxiety on proactive and reactive control from the new perspective of dual control theory, the selection of a classic experimental paradigm and appropriate experimental materials is essential for the study. Many classic paradigms have been used to study the effect of trait anxiety on cognitive control, such as the flanker task [3,40], antisaccade task [6,41], Go/No-go task [42], and stop-signal task [43]. However, the aforementioned paradigms do not allow us to separate cognitive control from proactive and reactive control. It is more accurate to distinguish between proactive control and reactive control using the AX-CPT paradigm, which has been around for a while [44–47]. In contrast to reactive control, which is motivated by the probe, proactive control is defined by the AX-CPT as control initiated by the cue [16]. Because participants can plan and anticipate the following probe during the cue-probe period based on cue information, they are more likely to exercise proactive control on BX sequences. However, participants prefer to use reactive control on AY sequences because they do not need to prepare a response during the cue-probe delay, so they must reactivate the cue information when the probe appears. Participants’ performance on the BX and AY sequences reflects the use of proactive and reactive control. The color-word Stroop task at the item level is a common paradigm for the study of reactive control [24,33,39]. Jacoby suggested that ISPC effects may reflect rapid, online, stimulus-driven control over attentional filtering—a kind of oxymoronic “automatic control [22]. The ISPC effect reflects the subject’s ability to use reactive control [48]. Current research uses both paradigms to study the effect of trait anxiety on proactive and reactive control under a working memory load condition.
Cognitive control is a critical function in attentional control that can suppress irrelevant information or dominant responses, ensuring that individuals can effectively resolve cognitive conflicts. Trait anxiety mainly impairs cognitive control in terms of performance efficiency, such as reaction time [6,12]. Experiment 1 found that trait anxiety impaired cognitive control on performance efficiency. Specifically, the HTA group had a longer reaction time than the LTA group on BX sequences, but there was no difference between groups on AY sequences, indicating that trait anxiety impairs proactive control but does not affect reactive control. For accuracy rate, there was no difference between high and low trait anxiety for either the AY or BX sequences, indicating that trait anxiety does not affect proactive or reactive control in terms of performance effectiveness. Experiment 2 used the updated color-word Stroop task to assess how anxiety affects reactive control. According to other studies [49,50], a characteristic ISPC effect was detected, with higher interference seen for generally congruent items than for mostly incongruent items. The item-specific control account [38,51] can explain the ISPC effect, which is selectively activated when an item or feature of an item (such as color) is presented and linked to high levels of interference (i.e., conflict), suggesting high control demands. Experiment 2 also revealed that trait anxiety impaired reactive control. Specifically, the ISPC effect was larger for the HTA group than for the LTA group in reaction time. These results suggest that trait anxiety impairs the performance efficiency of cognitive control, i.e., proactive and reactive control, and provide new evidence for processing efficiency and attentional control theories.
The effect of trait anxiety on reactive control was not observed in Experiment 1, possibly because AX-CPT was not sensitive enough to measure reactive control. Furthermore, the color-word Stroop task in Experiment 2 excluded the possibility that subjects might use proactive control strategies, such as responding based on proportion congruence. Thus, the measure of reactive control in Experiment 2 was pure.
Qi found that trait anxiety only impairs cognitive control under working memory load because working memory load consumes limited cognitive resources and disputes the compensatory strategies that can recruit more cognitive resources of the HTA group [52]. Working memory load hampered the HTA group’s ability to use this compensatory technique to suppress conflict, indicating that trait anxiety reduces cognitive control. According to the attentional control theory, anxiety reduces processing speed more than performance effectiveness, and its negative effects on performance effectiveness grow as the demands placed on the working memory’s central executive increase [15]. Working memory load in this study uses resources for maintaining and updating working memory. Contrary to earlier findings, the working memory load did not affect the impact of trait anxiety on proactive control and reactive control in either Experiment 1 or Experiment 2. There may be two reasons for this. First, proactive control and reactive control were dissociated from cognitive control, so working memory load may not affect proactive and reactive control of trait anxiety. Second, due to the low working memory consumption in the experiment 1 load task (i.e., entering the final calculated results after five trials), it may not be enough to affect proactive and reactive control. In Experiment 2, reactive control of trait anxiety was studied, which has a low demand for cognitive resources, so working memory load does not affect reactive control of trait anxiety. There may be other explanations that need further exploration.
The present study provides a novel approach to investigating the cognitive characteristics and mental health of social media users. It specifically examined the cognitive control characteristics of individuals with social media anxiety, using the AX-CPT test and the updated color-word Stroop paradigm to differentiate proactive and reactive control components. The findings indicate that trait anxiety impairs both proactive and reactive control, and that working memory load does not affect the use of compensatory strategies in individuals with high trait anxiety, nor does it modulate the effect of trait anxiety on proactive or reactive control.
Acknowledgement: We would like to thank Editage (www.editage.cn) for English language editing.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Baoqiang Zhang and Ling Xiang; data collection: Baoqiang Zhang; analysis and interpretation of results: Baoqiang Zhang and Ling Xiang; draft manuscript preparation: Baoqiang Zhang. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Kolhar M, Kazi RNA, Alameen A. Effect of social media use on learning, social interactions, and sleep duration among university students. Saudi J Biol Sci [Internet]. 2021;28(4):2216–22. doi:https://doi.org/10.1016/j.sjbs.2021.01.010. [Google Scholar] [PubMed] [CrossRef]
2. Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait version: structure and content re-examined. Behav Res Ther [Internet]. 1998;36(7–8):777–88. doi:https://doi.org/10.1016/S0005-7967(98)00023-0. [Google Scholar] [PubMed] [CrossRef]
3. Pacheco-Unguetti AP, Acosta A, Callejas A, Lupianez J. Attention and anxiety: different attentional functioning under state and trait anxiety. Psychol Sci [Internet]. 2010;21(2):298–304. doi:https://doi.org/10.1177/0956797609359624. [Google Scholar] [PubMed] [CrossRef]
4. Du M, Peng Y, Li Y, Zhu Y, Yang S, Li J, et al. Effect of trait anxiety on cognitive flexibility: evidence from event-related potentials and resting-state EEG. Biol Psychol [Internet]. 2022;170(4):108319. doi:https://doi.org/10.1016/j.biopsycho.2022.108319. [Google Scholar] [PubMed] [CrossRef]
5. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci [Internet]. 2001;24(1):167–202. doi:https://doi.org/10.1146/annurev.neuro.24.1.167. [Google Scholar] [PubMed] [CrossRef]
6. Derakshan N, Ansari TL, Hansard M, Shoker L, Eysenck MW. Anxiety, inhibition, efficiency, and effectiveness an investigation using the antisaccade task. Exp Psychol [Internet]. 2009;56(1):48–55. doi:https://doi.org/10.1027/1618-3169.56.1.48. [Google Scholar] [PubMed] [CrossRef]
7. Ansari TL, Derakshan N. Anxiety impairs inhibitory control but not volitional action control. Cogn Emot [Internet]. 2010;24(2):241–54. doi:https://doi.org/10.1080/02699930903381531. [Google Scholar] [CrossRef]
8. Sadeh N, Bredemeier K. Individual differences at high perceptual load: the relation between trait anxiety and selective attention. Cogn Emot [Internet]. 2011;25(4):747–55. doi:https://doi.org/10.1080/02699931.2010.500566. [Google Scholar] [PubMed] [CrossRef]
9. Caparos S, Linnell KJ. Trait anxiety focuses spatial attention. Emot [Internet]. 2012;12(1):8–12. doi:https://doi.org/10.1037/a0026310. [Google Scholar] [PubMed] [CrossRef]
10. Stout DM, Shackman AJ, Larson CL. Failure to filter: anxious individuals show inefficient gating of threat from working memory of threat from working memory. Front Hum Neurosci [Internet]. 2013;7:1–10. doi:https://doi.org/10.3389/fnhum.2013.00058. [Google Scholar] [PubMed] [CrossRef]
11. Osinsky R, Gebhardt H, Alexander N, Hennig J. Trait anxiety and the dynamics of attentional control. Biol Psychol [Internet]. 2012;89(1):252–59. doi:https://doi.org/10.1016/j.biopsycho.2011.10.016. [Google Scholar] [PubMed] [CrossRef]
12. Eysenck MW, Calvo MG. Anxiety and performance: the processing efficiency theory. Cogn Emot [Internet]. 1992;6(6):409–34. doi:https://doi.org/10.1080/02699939208409696. [Google Scholar] [CrossRef]
13. Sun G, Zhang L. Processing efficiency theory to attentional control theory: new perspective for anxiety-performance relationship in sport psychology. Adv Cogn Psychol [Internet]. 2013;21(10):1851–64. doi:https://doi.org/10.3724/SP.J.1042.2013.01851. [Google Scholar] [CrossRef]
14. Derakshan N, Eysenck MW. Anxiety, processing efficiency, and cognitive performance new developments from attentional control theory. Eur Psychol [Internet]. 2009;14(2):168–76. doi:https://doi.org/10.1027/1016-9040.14.2.168. [Google Scholar] [CrossRef]
15. Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emot [Internet]. 2007;7(2):336–53. doi:https://doi.org/10.1037/1528-3542.7.2.336. [Google Scholar] [PubMed] [CrossRef]
16. Braver, TS, Gray, JR, Burgess, GC(2007). Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway, ARA, Jarrold, C, Kane, MJ, Miyake, A, Towse, JN, editors. Variation in Working Memory [Internet]. New York: Oxford University Press; 2007. p. 76–106. [Google Scholar]
17. Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci [Internet]. 2012;16(2):106–13. doi:https://doi.org/10.1016/j.tics.2011.12.010. [Google Scholar] [PubMed] [CrossRef]
18. Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp [Internet]. 2005;25(3):336–44. doi:https://doi.org/10.1002/(ISSN)1097-0193. [Google Scholar] [CrossRef]
19. Vink M, Zandbelt BB, Gladwin T, Hillegers M, Hoogendam JM, van den Wildenberg WPM et al. Frontostriatal activity and connectivity increase during proactive inhibition across adolescence and early adulthood. Human Brain Mapping [Internet]. 2014;35(9):4415–27. doi:https://doi.org/10.1002/hbm.22483. [Google Scholar] [PubMed] [CrossRef]
20. Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR et al. Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cogn Affect Behav Neurosci [Internet]. 2008;8(3):239–53. doi:https://doi.org/10.3758/CABN.8.3.239. [Google Scholar] [PubMed] [CrossRef]
21. Redick TS. Cognitive control in context: working memory capacity and proactive control. Acta Psychol [Internet]. 2014;145:1–9. doi:https://doi.org/10.1016/j.actpsy.2013.10.010. [Google Scholar] [PubMed] [CrossRef]
22. Jacoby LL, Lindsay DS, Hessels S. Item-specific control of automatic processes: stroop process dissociations. Psychon Bull Rev [Internet]. 2003;10(3):638–44. doi:https://doi.org/10.3758/BF03196526. [Google Scholar] [PubMed] [CrossRef]
23. Bugg JM, Crump MJC. In support of a distinction between voluntary and stimulus-driven control: a review of the literature on proportion congruent effects. Front Psychol [Internet]. 2012;3:1–16. doi:https://doi.org/10.3389/fpsyg.2012.00367. [Google Scholar] [PubMed] [CrossRef]
24. Bugg JM. Evidence for the sparing of reactive cognitive control with age. Psychol Aging [Internet]. 2014;29(1):115–27. doi:https://doi.org/10.1037/a0035270. [Google Scholar] [PubMed] [CrossRef]
25. MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull [Internet]. 1991;109(2):163–203. doi:https://doi.org/10.1037/0033-2909.109.2.163. [Google Scholar] [PubMed] [CrossRef]
26. Straub ER, Dames H, Kiesel A, Dignath D. Does body posture reduce the Stroop effect? Evidence from two conceptual replications and a meta-analysis. Acta Psychol [Internet]. 2022;224(4):103497. doi:https://doi.org/10.1016/j.actpsy.2022.103497. [Google Scholar] [PubMed] [CrossRef]
27. Straub ER, Schmidts C, Kunde W, Zhang J, Kiesel A, Dignath D, et al. Limitations of cognitive control on emotional distraction-Congruency in the Color Stroop task does not modulate the Emotional Stroop effect. Cogn Affect Behav Neurosci [Internet]. 2022;22(1):1–21. doi:https://doi.org/10.3758/s13415-021-00935-4. [Google Scholar] [PubMed] [CrossRef]
28. Luo X, Gu J, Zheng Y, Zhou X. Making a saccade enhances Stroop and Simon conflict control. Atten Percept Psychophys [Internet]. 2022;84(3):795–814. doi:https://doi.org/10.3758/s13414-022-02458-7. [Google Scholar] [PubMed] [CrossRef]
29. Lowe DG, Mitterer JO. Selective and divided attention in a stroop task. Can J Psychol [Internet]. 1982;36(4):684–700. doi:https://doi.org/10.1037/h0080661. [Google Scholar] [PubMed] [CrossRef]
30. Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev [Internet]. 2001;108(3):624–52. doi:https://doi.org/10.1037/0033-295X.108.3.624. [Google Scholar] [PubMed] [CrossRef]
31. Atalay NB, Misirlisoy M. Can contingency learning alone account for item-specific control? Evidence from within-and between-language ISPC effects. J Exp Psychol Learn Mem Cogn [Internet]. 2012;38(6):1578. [Google Scholar] [PubMed]
32. Bugg JM, Jacoby LL, Chanani S. Why it is too early to lose control in accounts of item-specific proportion congruency effects. J Exp Psychol Hum Percept Perform [Internet]. 2011;37(3):844–59. doi:https://doi.org/10.1037/a0019957. [Google Scholar] [PubMed] [CrossRef]
33. Grandjean J, D'Ostilio K, Fias W, Phillips C, Balteau E, Degueldre C, et al. Exploration of the mechanisms underlying the ISPC effect: evidence from behavioral and neuroimaging data. Neuropsychol [Internet]. 2013;51(6):1040–9. doi:https://doi.org/10.1016/j.neuropsychologia.2013.02.015. [Google Scholar] [PubMed] [CrossRef]
34. Schmidt JR, Besner D. The stroop effect: why proportion congruent has nothing to do with congruency and everything to do with contingency. J Exp Psychol Learn Mem Cogn [Internet]. 2008;34(3):514–23. doi:https://doi.org/10.1037/0278-7393.34.3.514. [Google Scholar] [PubMed] [CrossRef]
35. Shek DT. The Chinese version of the State-Trait Anxiety Inventory: its relationship to different measures of psychological well-being. J Clin Psychol [Internet]. 1993;49(3):349–58. doi:https://doi.org/10.1002/(ISSN)1097-4679. [Google Scholar] [CrossRef]
36. Chiew KS, Braver TS. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Front Psychol [Internet]. 2013;4:1–15. doi:https://doi.org/10.3389/fpsyg.2013.00015. [Google Scholar] [PubMed] [CrossRef]
37. Soutschek A, Strobach T, Schubert T. Working memory demands modulate cognitive control in the Stroop paradigm. Psychol Forsch [Internet]. 2013;77(3):333–47. doi:https://doi.org/10.1007/s00426-012-0429-9. [Google Scholar] [PubMed] [CrossRef]
38. Bugg JM, Hutchison KA. Converging evidence for control of color-word stroop interference at the item level. J Exp Psychol Hum Percept Perform [Internet]. 2013;39(2):433–49. doi:https://doi.org/10.1037/a0029145. [Google Scholar] [PubMed] [CrossRef]
39. Gonthier C, Braver TS, Bugg JM. Dissociating proactive and reactive control in the Stroop task. Mem Cogn [Internet]. 2016;44(5):778–88. doi:https://doi.org/10.3758/s13421-016-0591-1. [Google Scholar] [PubMed] [CrossRef]
40. Qi SQ, Ding C, Li H. Neural correlates of inefficient filtering of emotionally neutral distractors from working memory in trait anxiety. Cogn Affect Behav Neurosci [Internet]. 2014;14(1):253–65. doi:https://doi.org/10.3758/s13415-013-0203-5. [Google Scholar] [PubMed] [CrossRef]
41. Derakshan N, Smyth S, Eysenck MW. Effects of state anxiety on performance using a task-switching paradigm: an investigation of attentional control theory. Psychon Bull Rev [Internet]. 2009;16(6):1112–7. doi:https://doi.org/10.3758/PBR.16.6.1112. [Google Scholar] [PubMed] [CrossRef]
42. Righi S, Mecacci L, Viggiano MP. Anxiety, cognitive self-evaluation and performance: ERP correlates. J Anxiety Disord [Internet]. 2009;23(8):1132–38. doi:https://doi.org/10.1016/j.janxdis.2009.07.018. [Google Scholar] [PubMed] [CrossRef]
43. Savostyanov AN, Tsai AC, Liou M, Levin EA, Lee JD, Yurganov AV, et al. EEG-correlates of trait anxiety in the stop-signal paradigm. Neurosci Lett [Internet]. 2009;449(2):112–6. doi:https://doi.org/10.1016/j.neulet.2008.10.084. [Google Scholar] [PubMed] [CrossRef]
44. Richmond LL, Redick TS, Braver TS. Remembering to prepare: the benefits (and costs) of high working memory capacity. J Exp Psychol Learn Mem Cogn [Internet]. 2015;41(6):1764. [Google Scholar] [PubMed]
45. Frober K, Dreisbach G. How performance (non-)contingent reward modulates cognitive control. Acta Psychol [Internet]. 2016;168(9):65–77. doi:https://doi.org/10.1016/j.actpsy.2016.04.008. [Google Scholar] [PubMed] [CrossRef]
46. Salehinejad MA, Wischnewski M, Ghanavati E, Mosayebi-Samani M, Kuo MF, Nitsche MA. Cognitive functions and underlying parameters of human brain physiology are associated with chronotype. Nat Commun [Internet]. 2021;12(1):329. doi:https://doi.org/10.1038/s41467-021-24885-0. [Google Scholar] [PubMed] [CrossRef]
47. Eisma J, Rawls E, Long S, Mach R, Lamm C. Frontal midline theta differentiates separate cognitive control strategies while still generalizing the need for cognitive control. Sci Rep [Internet]. 2021;11(1):1–14. doi:https://doi.org/10.1038/s41598-021-94162-z. [Google Scholar] [PubMed] [CrossRef]
48. Hutchison KA, Bugg JM, Lim YB, Olsen MR. Congruency precues moderate item-specific proportion congruency effects. Atten Percept Psychophys [Internet]. 2016;78(4):1087–103. doi:https://doi.org/10.3758/s13414-016-1066-y. [Google Scholar] [PubMed] [CrossRef]
49. Blais C, Bunge S. Behavioral and neural evidence for item-specific performance monitoring. J Cogn Neurosci [Internet]. 2010;22(12):2758–67. doi:https://doi.org/10.1162/jocn.2009.21365. [Google Scholar] [PubMed] [CrossRef]
50. Bugg JM, Jacoby LL, Toth JP. Multiple levels of control in the Stroop task. Mem Cogn [Internet]. 2008;36(8):1484–94. doi:https://doi.org/10.3758/MC.36.8.1484. [Google Scholar] [PubMed] [CrossRef]
51. Bugg JM, McDaniel MA, Scullin MK, Braver TS. Revealing list-level control in the stroop task by uncovering its benefits and a cost. J Exp Psychol Hum Percept Perform [Internet]. 2011;37(5):1595–606. doi:https://doi.org/10.1037/a0024670. [Google Scholar] [PubMed] [CrossRef]
52. Qi SQ, Zeng QH, Luo YM, Duan HJ, Ding C, Hu WP et al. Impact of working memory load on cognitive control in trait anxiety: an ERP study. PLoS One [Internet]. 2014;9(11):e111791. doi:https://doi.org/10.1371/journal.pone.0111791. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools