 Open Access
Open Access
ARTICLE
The Relationship between Exercise and Psychotic Symptoms in College Students: A Cross-Sectional Analysis
Institute of Physical Education, Hunan University of Science and Technology, Xiangtan, 411201, China
* Corresponding Author: Haijun Tang. Email:
International Journal of Mental Health Promotion 2023, 25(7), 873-879. https://doi.org/10.32604/ijmhp.2023.028107
Received 30 November 2022; Accepted 12 January 2023; Issue published 01 June 2023
Abstract
An increasing number of studies have suggested that increased physical activity is associated with less mental illness. However, the relationship between exercise and psychotic experiences (PEs) is still unknown. The purpose of this study was to explore the relationship between exercise and PEs in college students in the United States. Data from the Health Mind Survey (2020–2021 round) were analyzed. Respondents included 137,916 college students who were asked about exercise and PEs (lifetime psychotic experiences, delusions, and hallucinations). A multivariate logistic regression analysis was used to investigate the relationship between exercise and PEs while controlling for demographic characteristics. There was a significant correlation between exercise and PEs among college students. Compared to students who exercised less than one hour per week, students who exercised five or more hours per week had fewer lifetime psychotic experiences. This same finding obtained for both male and female college students. The findings from the present study indicate that exercising for five or more hours each week is most correlated with decreased PEs among college students. However, experimental studies are required to extend and confirm our findings and determine the causality of this relationship.Keywords
Since the earliest study of the high incidence of schizophrenia in Chicago by Faris and Denham [1], many studies have confirmed that many adolescents with psychotic experiences (PEs) (one of the manifestations of mental abnormalities) have a greater risk of developing psychiatric disorders in adulthood [2]. Psychotic symptoms refer to subclinical symptoms such as hallucinations or delusions that are precursors to psychosis or mental disorders [3]. In the past decade, population complaints of PEs have attracted researchers’ attention. A meta-analysis of 61 studies estimated that 2.5% of young adults experienced PEs annually [4]. Evidence-based research has found that the prevalence of PEs youth ranges from 8% to 24% [5,6]. Recent studies have reported that the prevalence of PEs in college students is 16% in the U.S. [7], 24% in the United Kingdom, 13% in China, and 20% in Japan [6]. PEs are common in adolescence, often co-occur with mental disorders [8–11], and may increase the risk of depression, anxiety, and substance abuse during adolescence [12–14], and of psychosis later in life [15]. Some studies have found that PEs are correlated with various factors, such as sex, age, and mental health (depression, stress, and anxiety) [6,16]. College-aged students in particular are at the peak age of onset for mental problems, especially depression and anxiety [17–19]. They usually face a lot of social and academic pressure that can lead to psychopathology [20]. The stress from mental disorders can activate the hypothalamic pituitary adrenal axis that can result in psychosis [21,22].
Prior research has found that adolescents with psychosis are more likely to have comorbidities than those without psychosis [23]. Psychosis is also correlated with unhealthy lifestyles, such as poor diet, lack of exercise, and smoking [24,25]. Indeed, there is accumulating evidence suggesting that exercise is positively correlated with health outcomes (e.g., depression, cognitive preservation, reduced risk of chronic disease, and associated comorbidities) in different age groups [26–32]. More specifically, studies have also found positive correlations between physical activity (PA) and mental health (e.g., depression, anxiety, and stress) and quality of life in adolescents [33–35]. A handful of studies have studied the relationship between PA and psychotic disorders in young adults [15,36]. For example, Stubbs et al. found a negative correlation between PA and psychosis in young adults living in low- and middle-income countries (LIMC) [37,38]. Additionally, Eills et al. found that exercise lasting for 10 to 12 weeks was correlated with better mental health in young people with psychosis [25]. As PEs have been found to precede the development of psychotic disorders [17], it is necessary to develop a better understanding of how to decrease the risk of PEs in adolescents, particularly college students.
To date, some research has investigated the relationship between PA, or exercise, and PEs. Exercise, as a form of PA, is structured and planned to develop and enhance physical fitness [39]. Large-scale longitudinal studies have found that engaging in sports is negatively correlated with PEs in the general population. It is unclear whether the relationship between exercise and PEs exists in college students, and whether it generalizes to students in high-income countries (e.g., the United States). The present study will address these two gaps in the literature.
For this cross-sectional study, data from the Healthy Minds Study (HMS) were retrieved and analyzed by accessing the following link (https://healthymindsnetwork.org). This survey was administered using an internet-based method to university students at 79 universities in the United States between 2020 and 2021. A detailed description of the survey methods can be found elsewhere [3,40]. In brief, a random sampling method was employed to obtain a sample of 4,000 respondents aged 18 or greater from each university. If a university had fewer than 4,000 students, all recruited students from that university were included. The survey response rate was 16%. The Health Sciences and Behavioral Sciences Institutional Review Board at the University of Michigan approved this HMS protocol.
Participants answered one question about exercise: “In the past one month, how many hours weekly on average did you spend exercising? (Involving any moderate or vigorous exercise, where “moderate exercise” was roughly equivalent to brisk walking or biking)”. This question had the following answer options: “<1 h”, “2–3 h”, “3–4 h” and “5 h or more”. The amount of time spent exercising each week was considered a predictor variable, which was in line with the previous study [40].
Psychotic symptoms were considered the criterion variables in this study. Psychotic symptoms were assessed via the brief version of the World Health Organization Composite International Diagnostic Interview Psychosis Screen [3]. Participants were instructed to answer “yes” or “no” to the following four questions: (1) delusional mood: “A feeling something strange and unexplainable was going on that other people would find hard to believe?”; (2) delusion of reference and persecution: “A feeling that people were too interested in you or that there was a plot to harm you?”; (3) delusion of control: “A feeling that your thoughts were being directly interfered or controlled by another person, or your mind was being taken over by strange forces?”; and (4) hallucinations: “An experience of seeing visions or hearing voices that others could not see or hear when you were not half asleep, dreaming, or under the influence of alcohol or drugs?” The affirmation of any of these questions was coded as having experienced a psychotic experience in one’s lifetime, with the affirmation of questions (1), (2), or (3) coded as having experienced delusions, and the affirmation of question (4) coded as having experienced hallucinations. Each participant was also instructed to answer “yes” or “no” in response to whether they had experienced any of the above within the past year.
Control variables comprised the demographic characteristics of age, sex, race, and international student status (yes/no). Sex was coded as male, female, transgender, and other. Race was coded as African American/Black, American Indian or Alaskan Native, Asian American/Asian, Hispanic/Latino (a), Native Hawaiian or Pacific Islander, Middle Eastern, Arab, or Arab American, White, and other.
The statistical analyses in this cross-sectional study were done with SPSS 25.0 (IBM, Armonk, NY, USA). Values of p < 0.05 (two-tailed) were considered statistically significant. Descriptive statistics were used to explore the study respondents’ characteristics and prevalence of psychotic symptoms. A multivariate logistic regression analysis was used to investigate the relationship between exercise and psychotic symptoms, controlling for demographic characteristics (age, sex, race, and international student status). Less than one hour of exercise per week and no PEs were considered the reference groups in each logistic regression analysis. Odds ratios (OR) with 95% confidence intervals were considered as outcomes.
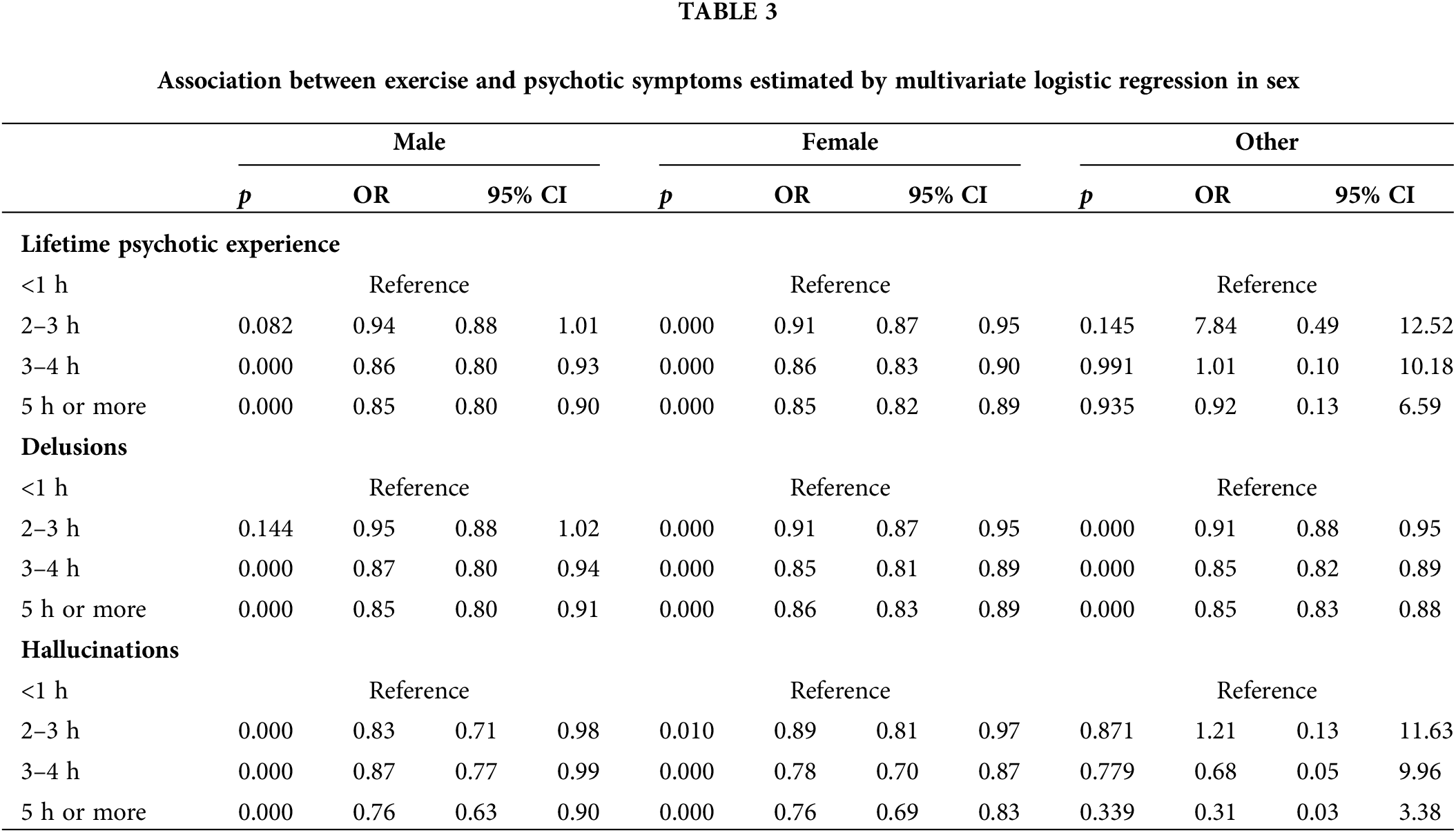
The study’s sample consisted of 137,916 college students with a mean age of 23.56 ± 7.23. The sample characteristics are presented in Table 1. Seventy one per cent of the students were female. With regard to exercise, 28% of the students exercised less than one hour a week, 18% exercised two to three hours a week, 12% exercised three to four hours a week, and 30% exercised five or more hours a week. With regard to lifetime psychotic experiences, 26% of students reported lifetime psychotic experiences, with 25% reporting delusions, and 4% reporting hallucinations.
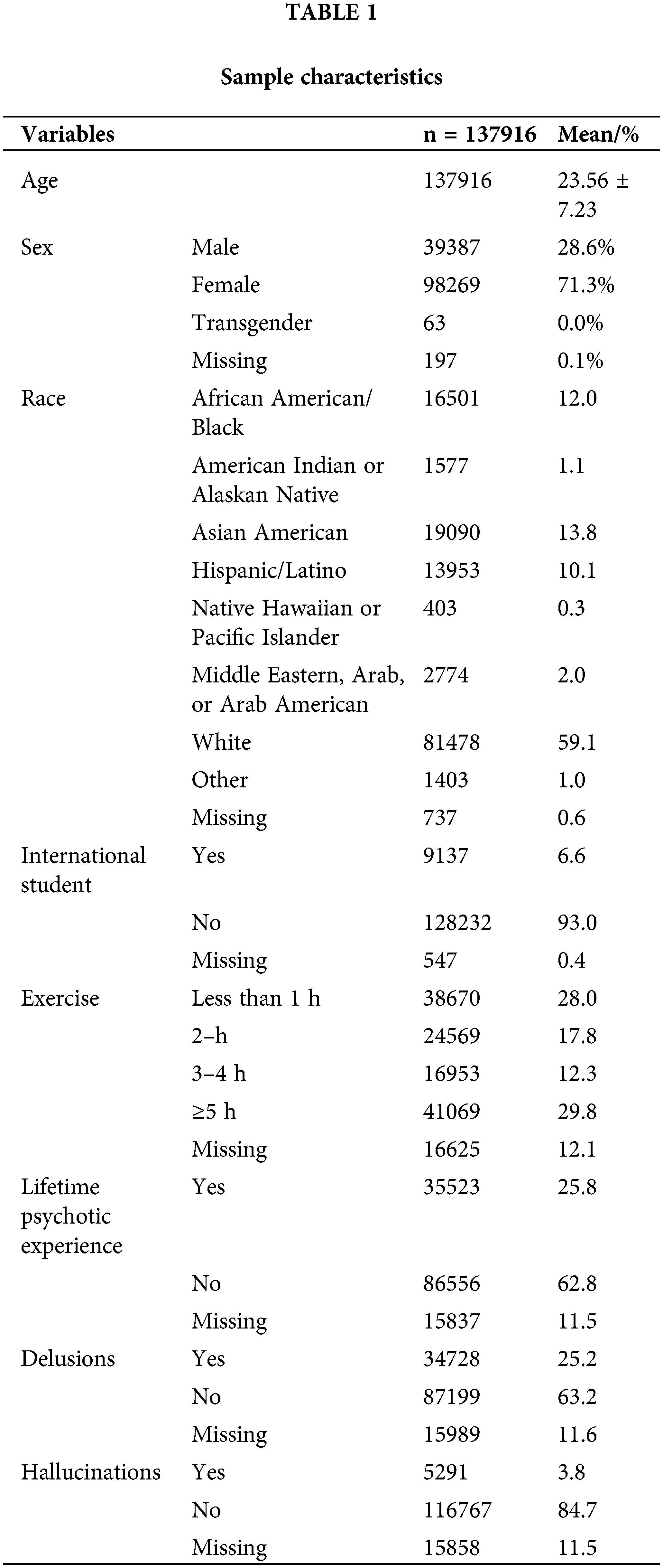
Table 2 depicts the results of the multivariate logistic regression analysis on the relationship between exercise and psychotic symptoms. Five or more hours of weekly exercise was significantly correlated with lower odds of lifetime psychotic experience (OR = 0.85, 95% CI [0.83, 0.88]) compared to less than one hour of weekly exercise, when controlling for all covariates. This correlation was consistent across the subtypes of psychotic experience: delusions (OR = 0.86, 95% CI [0.83, 0.89]) as well as hallucinations (OR = 0.77, 95% CI [0.71, 0.85]), controlling for all covariates.
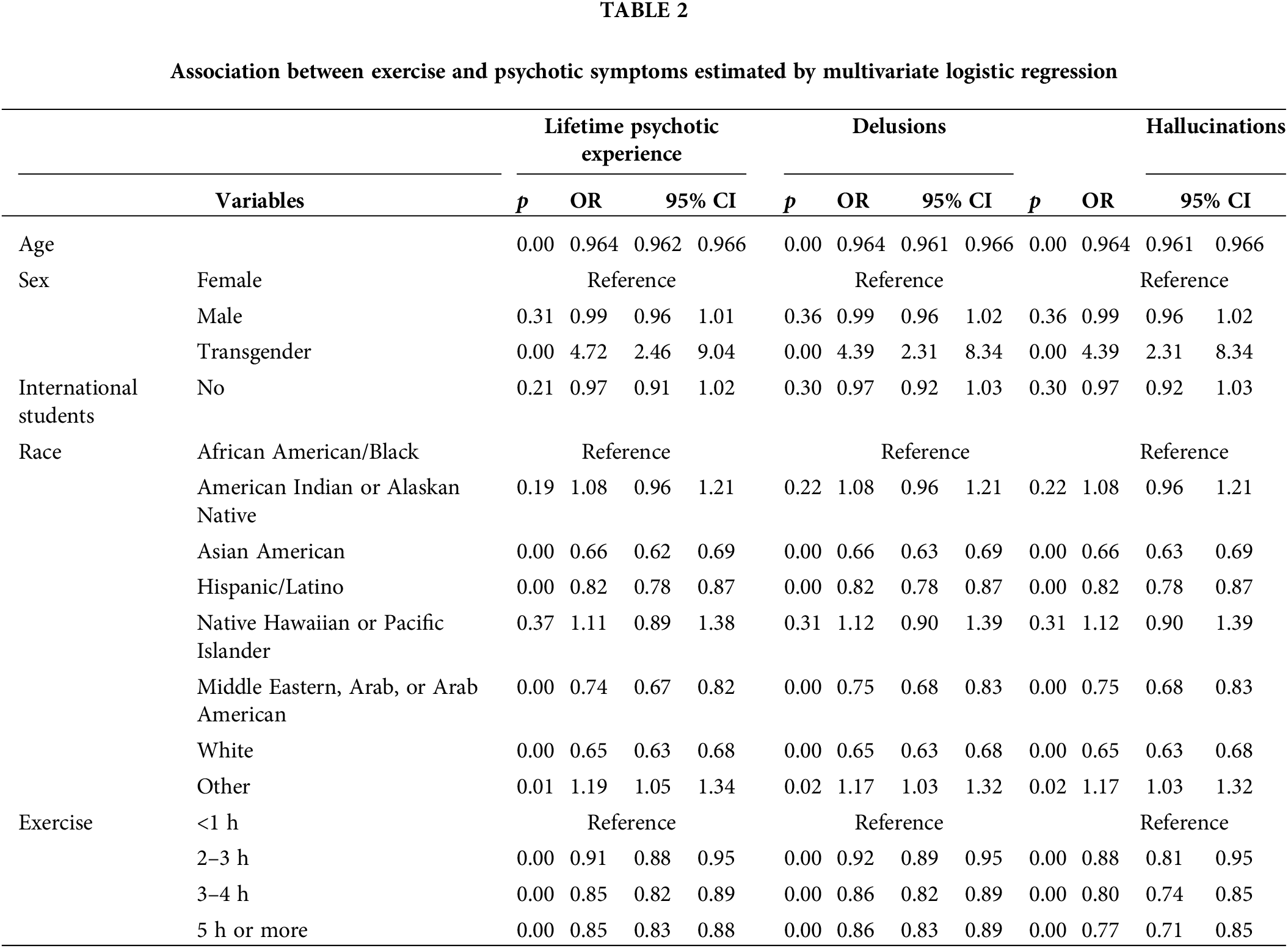
Table 3 displays the results of the multivariate logistic regression analysis on the relationship between exercise and psychotic symptoms by sex. Male students who exercised five or more hours per week had significantly lower odds of lifetime psychotic experience (OR = 0.85, 95% CI [0.80, 0.90]), including delusions (OR = 0.85, 95% CI [0.80, 0.91]) and hallucinations (OR = 0.76, 95% CI [0.63, 0.90]), compared to men who exercised less than one hour a week, when controlling for all covariates. Similarly, female students who exercised five or more hours a week also had significantly lower odds of lifetime psychotic experience (OR = 0.85, 95% CI [0.82, 0.89]), including delusions (OR = 0.86, 95% CI [0.83, 0.89]), and hallucinations (OR = 0.76, 95% CI [0.69, 0.83]), compared to women who exercised less than one hour a week, when controlling for all covariates.
To the best of our knowledge, this is the largest study to date to have explored the relationship between exercise and PEs among college students in the U.S. In summary, our study found that exercising five or more hours a week was inversely related to the risk of PEs (lifetime PE, delusions, and hallucinations) in college students compared to exercising less than one hour a week, when adjusting for all variables. This finding held when comparing male students to female students.
The finding of low lifetime PE risk in college students being strongly correlated with five or more hours of weekly exercise is in line with prior research [41]. For instance, a meta-analysis of 20 studies suggested that individuals with schizophrenia who engaged in 90 or more minutes of weekly exercise could significantly reduce their PE [41]. Firth et al. also found that 10-week of exercise produced a positive improvement in psychotic symptoms [42], and previous studies have reported that exercise alleviates hallucinations in the general population and in adults with psychiatric disorders [43,44]. Similarly, a cross-sectional study found that less exercise or being immobile was correlated with poor mental health and delusions in patients with psychotic disorders [45], and a longitudinal study by Suetani et al. also found that not exercising during adolescence was correlated with an increased risk of delusional ideation in the six subsequent years [46]. This study’s findings indicated that college students in particular who exercised for five or more hours a week had a low risk of experiencing delusions and hallucinations.
Despite the fact that the effective mechanism of exercise on psychosis is unknown, several explanations may be plausible. From a psychological perspective, previous studies have proposed that diverting attention from negative stimuli could enhance mental health during and after exercise [47,48]. Moreover, exercise is characterized by the development of close social relationships (e.g., communication opportunities and cooperation) and mutual support between individuals, which could play an essential role in the correlation between exercise and psychotic symptoms [49]. From a physiological perspective, neurobiological chemicals may be an important variable in psychotic symptoms. Some research has suggested that mental problems (e.g., stress and depression) are correlated with decreased brain derived neurotrophic factor (BDNF) levels [50,51]. In contrast, exercise can contribute to the regulation of BDNF levels to normal or pre-stress levels, although the stress-induced increase can result in the downregulation of BDNF levels. Furthermore, exercise has also been found to increase the release of endorphins that produce a relaxation effect and increase the availability of hormones to reduce psychotic symptoms [52], producing a possible explanation for this study’s finding that greater exercise is correlated with fewer psychotic symptoms.
The findings from the present study may raise concerns that college students are at high risk for psychotic experiences, and that college mental counselors should encourage greater weekly exercise to improve student mental health. Several weaknesses in this study should caution against such a causal recommendation. First, the correlational nature of this study prevents the identification of a causal relationship between exercise and psychotic symptoms. Second, this study focuses exclusively on a sample of college students in the U.S., thus further studies need to assess whether this correlation generalizes to older populations and those of other high-income countries. Third, the survey relied on self-report questionnaires that might under or overestimate the relationship between psychotic symptoms and exercise [53]. Fourth, the response rate was 16%, which may have resulted in sampling bias and generalization limitations.
This study adds to an emerging body of literature interested in identifying and reducing psychotic symptoms as precursors to the development of psychotic disorders, by showing that exercising five or more hours a week is correlated with fewer psychotic symptoms among college students.
Acknowledgement: We thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.
Funding Statement: The research is supported by: Hunan Provincial Department of Education Research Innovation General Project: Research on The Strategy of Improving College Students’ Health Literacy under the Background of Healthy China (CX20211027).
Author Contributions: The authors confirm their contribution to the paper as follows: YY and HT: conceptualization and methodology. YY: data curation and analysis, and writing—original draft preparation. YY and HT: writing—review, and editing. Both authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Faris R, Dunham H. Mental disorders in urban areas. An ecological study of schhizophrenia and other psychoses [Internet]. Oxford, England: University of Chicago Press; 1939. [Google Scholar]
2. Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM. Meta-analysis of the association of urbanicity with schizophrenia. Schizophr Bull [Internet]. 2012;38(6):1118–23. doi:https://doi.org/10.1093/schbul/sbs096. [Google Scholar] [PubMed] [CrossRef]
3. Narita Z, Banawa R, Zhou S, DeVylder J, Koyanagi A, Oh H. Loneliness and psychotic experiences among US university students: findings from the healthy minds study 2020. Psychiatry Res [Internet]. 2022;308:114362. doi:https://doi.org/10.1016/j.psychres.2021.114362. [Google Scholar] [PubMed] [CrossRef]
4. Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med [Internet]. 2013;43(6):1133–49. doi:https://doi.org/10.1017/S0033291712001626. [Google Scholar] [PubMed] [CrossRef]
5. Maijer K, Begemann MJH, Palmen SJMC, Leucht S, Sommer IEC. Auditory hallucinations across the lifespan: a systematic review and meta-analysis. Psychol Med [Internet]. 2018;48(6):879–88. doi:https://doi.org/10.1017/S0033291717002367. [Google Scholar] [PubMed] [CrossRef]
6. Fekih-Romdhane F, Pandi-Perumal SR, Conus P, Krebs MO, Cheour M, Seeman MV, et al. Prevalence and risk factors of self-reported psychotic experiences among high school and college students: a systematic review, meta-analysis, and meta-regression. Acta Psychiatr Scand [Internet]. 2022;146(6):492–514. doi:https://doi.org/10.1111/acps.13494. [Google Scholar] [PubMed] [CrossRef]
7. Oh HY, Davis EB, Klaunig M, Narita Z, Koyanagi A, Karcher NR. Religiousness and psychotic experiences among young adult college students in the United States. Int J Soc Psychiatry [Internet]. 2022. doi:https://doi.org/10.1177/00207640221135849. [Google Scholar] [PubMed] [CrossRef]
8. Kelleher I, Wigman JTW, Harley M, O’Hanlon E, Coughlan H, Rawdon C et al. Psychotic experiences in the population: association with functioning and mental distress. Schizophr Res [Internet]. 2015;165(1):9–14. doi:https://doi.org/10.1016/j.schres.2015.03.020. [Google Scholar] [PubMed] [CrossRef]
9. Healy C, Brannigan R, Dooley N, Coughlan H, Clarke M, Kelleher I, et al. Childhood and adolescent psychotic experiences and risk of mental disorder: a systematic review and meta-analysis. Psychol Med [Internet]. 2019;49(10):1589–99. doi:https://doi.org/10.1017/S0033291719000485. [Google Scholar] [PubMed] [CrossRef]
10. Chi XL, Liang KX, Chen ST, Huang QM, Huang LY, Yu Q, et al. Undefined mental health problems among Chinese adolescents during the COVID-19: the importance of nutrition and physical activity [Internet]. Elsevier; 2021. [Google Scholar]
11. Guo T, Zhang Z, Taylor A, Hall DL, Yeung AS, Kramer AF, et al. Association of social support with negative emotions among Chinese adolescents during Omicron-related lockdown of Shenzhen City: the roles of rumination and sleep quality. Front Psychiatry [Internet]. 2022;13:100218. doi:https://doi.org/10.3389/fpsyt.2022.957382. [Google Scholar] [PubMed] [CrossRef]
12. Bolhuis K, Koopman-Verhoeff ME, Blanken LME, Cibrev D, Jaddoe VWV, Verhulst FC, et al. Psychotic-like experiences in pre-adolescence: what precedes the antecedent symptoms of severe mental illness? Acta Psychiatr Scand [Internet]. 2018;138(1):15–25. doi:https://doi.org/10.1111/acps.12891. [Google Scholar] [PubMed] [CrossRef]
13. Hielscher E, Connell M, Lawrence D, Zubrick SR, Hafekost J, Scott JG. Prevalence and correlates of psychotic experiences in a nationally representative sample of Australian adolescents. Aust N Z J Psychiatry [Internet]. 2018;52(8):768–81. doi:https://doi.org/10.1177/0004867418785036. [Google Scholar] [PubMed] [CrossRef]
14. Chi X, Jiang W, Guo T, Hall DL, Luberto CM, Zou L. Relationship between adverse childhood experiences and anxiety symptoms among Chinese adolescents: the role of self-compassion and social support. Curr Psychol [Internet]. 2022;79(11):1482. doi:https://doi.org/10.1007/s12144-021-02534-5. [Google Scholar] [PubMed] [CrossRef]
15. Brokmeier LL, Firth J, Vancampfort D, Smith L, Deenik J, Rosenbaum S, et al. Does physical activity reduce the risk of psychosis? A systematic review and meta-analysis of prospective studies. Psychiatry Res [Internet]. 2020;284(2):112675. doi:https://doi.org/10.1016/j.psychres.2019.112675. [Google Scholar] [PubMed] [CrossRef]
16. Palacios-García V, León-del-Barco B, Mendo-Lázaro S, Saavedra-Macías J, Felipe-Castaño E. Mindfulness and psychotic experiences in college students. An Psicol Psychol [Internet]. 2018;34(2):233–40. doi:https://doi.org/10.6018/analesps.34.2.290171. [Google Scholar] [CrossRef]
17. Zou L, Wang T, Herold F, Ludyga S, Liu W, Zhang Y, et al. Associations between sedentary behavior and negative emotions in adolescents during home confinement: mediating role of social support and sleep quality. Int J Clin Heal Psychol [Internet]. 2023;23(1):100337. doi:https://doi.org/10.1016/j.ijchp.2022.100337. [Google Scholar] [PubMed] [CrossRef]
18. Kuang J, Zhong J, Yang P, Bai X, Liang Y, Cheval B, et al. Psychometric evaluation of the inventory of dimensions of emerging adulthood (IDEA) in China. Int J Clin Heal Psychol [Internet]. 2023;23(1):100331. doi:https://doi.org/10.1016/j.ijchp.2022.100331. [Google Scholar] [PubMed] [CrossRef]
19. Yu Q, Wong KK, Lei OK, Nie J, Shi Q, Zou L, et al. Comparative effectiveness of multiple exercise interventions in the treatment of mental health disorders: a systematic review and network meta-analysis. Sport Med-Open [Internet]. 2022;8(1):135. doi:https://doi.org/10.1186/s40798-022-00529-5. [Google Scholar] [PubMed] [CrossRef]
20. Mikolajczyk RT, Brzoska P, Maier C, Ottova V, Meier S, Dudziak U, et al. Factors associated with self-rated health status in university students: a cross-sectional study in three European countries. BMC Public Health [Internet]. 2008;8(1):1–10. doi:https://doi.org/10.1186/1471-2458-8-215. [Google Scholar] [PubMed] [CrossRef]
21. Maniam J, Antoniadis C, Morris MJ. Early-life stress, HPA axis adaptation, and mechanisms contributing to later health outcomes. Front Endocrinol (Lausanne) [Internet]. 2014;5(Suppl 3):374. doi:https://doi.org/10.3389/fendo.2014.00073. [Google Scholar] [PubMed] [CrossRef]
22. Li C, Zhang Y, Randhawa AK, Madigan DJ. Emotional exhaustion and sleep problems in university students: does mental toughness matter? Pers Individ Dif [Internet]. 2020;163(5):110046. doi:https://doi.org/10.1016/j.paid.2020.110046. [Google Scholar] [CrossRef]
23. Hua LL, Wilens TE, Martelon MK, Wong P, Wozniak J, Biederman J. Psychosocial functioning, familiality, and psychiatric comorbidity in bipolar youth with and without psychotic features. J Clin Psychiatry [Internet]. 2011;72(3):397–405. doi:https://doi.org/10.4088/JCP.10m06025yel. [Google Scholar] [PubMed] [CrossRef]
24. Zachariae C, Skov L. Obesity as a risk factor for psoriasis. J Eur Acad Dermatology Venereol [Internet]. 2020;34(5):915–6. doi:https://doi.org/10.1111/jdv.16434. [Google Scholar] [PubMed] [CrossRef]
25. Ellis N, Crone D, Davey R, Grogan S. Exercise interventions as an adjunct therapy for psychosis: a critical review. Br J Clin Psychol [Internet]. 2007;46(1):95–111. doi:https://doi.org/10.1348/014466506X122995. [Google Scholar] [PubMed] [CrossRef]
26. Zhao M, Chen S, You Y, Wang Y, Zhang Y. Effects of a therapeutic horseback riding program on social interaction and communication in children with autism. Int J Environ Res Public Health [Internet]. 2021;18(5):1–11. doi:https://doi.org/10.3390/ijerph18052656. [Google Scholar] [PubMed] [CrossRef]
27. Chen S, Zhang Y, Wang YT, Liu X, Song W, Du X. The effect of Qigong-based therapy on patients with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil [Internet]. 2020;34(12):1436–48. doi:https://doi.org/10.1177/0269215520946695. [Google Scholar] [PubMed] [CrossRef]
28. Xiao T, Jiao C, Yao J, Yang L, Zhang Y, Liu S, et al. Effects of basketball and baduanjin exercise interventions on problematic smartphone use and mental health among college students: a randomized controlled trial. eCAm [Internet]. 2021;2021(3):1–12. doi:https://doi.org/10.1155/2021/8880716. [Google Scholar] [PubMed] [CrossRef]
29. Zhou S, Chen S, Liu X, Zhang Y, Zhao M, Li W. Physical activity improves cognition and activities of daily living in adults with alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health [Internet]. 2022;19(3):1216. doi:https://doi.org/10.3390/ijerph19031216. [Google Scholar] [PubMed] [CrossRef]
30. Sun J, Buys N, Jayasinghe R. Effects of community-based meditative Tai Chi programme on improving quality of life, physical and mental health in chronic heart-failure participants. Aging Ment Heal [Internet]. 2014;18(3):289–95. doi:https://doi.org/10.1080/13607863.2013.875120. [Google Scholar] [PubMed] [CrossRef]
31. Santos-Lozano A, Barrán AT, Fernández-Navarro P, Valenzuela PL, Castillo-Garcia A, Ruilope LM, et al. Association between physical activity and cardiovascular risk factors: dose and sex matter. J Sport Heal Sci [Internet]. 2021;10(5):604–6. doi:https://doi.org/10.1016/j.jshs.2021.03.002. [Google Scholar] [PubMed] [CrossRef]
32. Scott D, Johansson J, Gandham A, Ebeling PR, Nordstrom P, Nordstrom A. Associations of accelerometer-determined physical activity and sedentary behavior with sarcopenia and incident falls over 12 months in community-dwelling Swedish older adults: “physical activity, sarcopenia, and falls”. J Sport Heal Sci [Internet]. 2021;10(5):577–84. doi:https://doi.org/10.1016/j.jshs.2020.01.006. [Google Scholar] [PubMed] [CrossRef]
33. Fluetsch N, Levy C, Tallon L. The relationship of physical activity to mental health: a 2015 behavioral risk factor surveillance system data analysis. J Affect Disord [Internet]. 2019;253(8):96–101. doi:https://doi.org/10.1016/j.jad.2019.04.086. [Google Scholar] [PubMed] [CrossRef]
34. Ozdemir F, Cansel N, Kizilay F, Guldogan E, Ucuz I, Sinanoglu B, et al. The role of physical activity on mental health and quality of life during COVID-19 outbreak: a cross-sectional study. Eur J Integr Med [Internet]. 2020;40(10223):101248. doi:https://doi.org/10.1016/j.eujim.2020.101248. [Google Scholar] [PubMed] [CrossRef]
35. Biddle SJH, Ciaccioni S, Thomas G, Vergeer I. Physical activity and mental health in children and adolescents: an updated review of reviews and an analysis of causality. Psychol Sport Exerc [Internet]. 2019;42(4):146–55. doi:https://doi.org/10.1016/j.psychsport.2018.08.011. [Google Scholar] [CrossRef]
36. Swora E, Boberska M, Kulis E, Knoll N, Keller J, Luszczynska A. Physical activity, positive and negative symptoms of psychosis, and general psychopathology among people with psychotic disorders: a meta-analysis. J Clin Med [Internet]. 2022;11(10):2719. doi:https://doi.org/10.3390/jcm11102719. [Google Scholar] [PubMed] [CrossRef]
37. Stubbs B, Koyanagi A, Schuch F, Firth J, Rosenbaum S, Gaughran F, et al. Physical activity levels and psychosis: a mediation analysis of factors influencing physical activity target achievement among 204 186 people across 46 low- and middle-income countries. Schizophr Bull [Internet]. 2017;43:536–45. doi:https://doi.org/10.1093/schbul/sbw111. [Google Scholar] [PubMed] [CrossRef]
38. Stubbs B, Vancampfort D, Firth J, Hallgren M, Schuch F, Veronese N, et al. Physical activity correlates among people with psychosis: data from 47 low- and middle-income countries. Schizophr Res [Internet]. 2018;193(9838):412–7. doi:https://doi.org/10.1016/j.schres.2017.06.025. [Google Scholar] [PubMed] [CrossRef]
39. Care D, Suppl SS. Facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes-2020. Diabetes Care [Internet]. 2020;43(Supplement_1):S48–65. doi:https://doi.org/10.2337/dc20-S005. [Google Scholar] [PubMed] [CrossRef]
40. Ning K, Yan C, Zhang Y, Chen S. Regular exercise with suicide ideation, suicide plan and suicide attempt in university students: data from the health minds survey 2018–2019. Int J Environ Res Public Health [Internet]. 2022;19(14):8856. doi:https://doi.org/10.3390/ijerph19148856. [Google Scholar] [PubMed] [CrossRef]
41. Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med [Internet]. 2015;45(7):1343–61. doi:https://doi.org/10.1017/S0033291714003110. [Google Scholar] [PubMed] [CrossRef]
42. Firth J, Carney R, Elliott R, French P, Parker S, McIntyre R, et al. Exercise as an intervention for first-episode psychosis: a feasibility study. Early Interv. Psychiatry [Internet]. 2018;12(3):307–15. doi:https://doi.org/10.1111/eip.12329. [Google Scholar] [PubMed] [CrossRef]
43. Faulkner G, Biddle S. Exercise as an adjunct treatment for schizophrenia: a review of the literature. J Ment Heal [Internet]. 1999;8(5):441–57. doi:https://doi.org/10.1080/09638239917157. [Google Scholar] [CrossRef]
44. Richardson CR, Faulkner G, McDevitt J, Skrinar GS, Hutchinson DS, Piette JD. Integrating physical activity into mental health services for persons with serious mental illness. Psychiatr Serv [Internet]. 2005;56(3):324–31. doi:https://doi.org/10.1176/appi.ps.56.3.324. [Google Scholar] [PubMed] [CrossRef]
45. Archie SM, Goldberg JO, Akhtar-Danesh N, Landeen J, McColl L, McNiven J. Psychotic disorders, eating habits, and physical activity: who is ready for lifestyle changes? Psychiatr Serv [Internet]. 2007;58(2):233–9. doi:https://doi.org/10.1176/ps.2007.58.2.233. [Google Scholar] [PubMed] [CrossRef]
46. Suetani S, Mamun A, Williams GM, Najman JM, McGrath JJ, Scott JG. Longitudinal association between physical activity engagement during adolescence and mental health outcomes in young adults: a 21-year birth cohort study. J Psychiatr Res [Internet]. 2017;94(11):116–23. doi:https://doi.org/10.1016/j.jpsychires.2017.06.013. [Google Scholar] [PubMed] [CrossRef]
47. Morgan WP. Affective beneficence of vigorous physical activity. Med Sci Sports Exerc [Internet]. 1985;17(1):94–100. doi:https://doi.org/10.1249/00005768-198502000-00015. [Google Scholar] [CrossRef]
48. Raedeke TD. The Relationship between enjoyment and affective responses to exercise. J Appl Sport Psychol [Internet]. 2007;19(1):105–15. doi:https://doi.org/10.1080/10413200601113638. [Google Scholar] [CrossRef]
49. van Woudenberg TJ, Bevelander KE, Burk WJ, Buijzen M. The reciprocal effects of physical activity and happiness in adolescents. Int J Behav Nutr Phys Act [Internet]. 2020;17:1–10. doi:https://doi.org/10.1186/s12966-020-01058-8. [Google Scholar] [PubMed] [CrossRef]
50. Hu S, Tucker L, Wu C, Yang L. Beneficial effects of exercise on depression and anxiety during the COVID-19 pandemic: a narrative review. Front Psychiatry [Internet]. 2020;11:4. doi:https://doi.org/10.3389/fpsyt.2020.587557. [Google Scholar] [PubMed] [CrossRef]
51. Toll A. Brain-derived neurotrophic factor levels in first episode of psychosis: a systematic review. World J Psychiatry [Internet]. 2015;5(1):154. doi:https://doi.org/10.5498/wjp.v5.i1.154. [Google Scholar] [PubMed] [CrossRef]
52. Shaphe MA, Chahal A. Relation of physical activity with the depression: a short review. J Lifestyle Med [Internet]. 2020;10(1):1–6. doi:https://doi.org/10.15280/jlm.2020.10.1.1. [Google Scholar] [PubMed] [CrossRef]
53. Rodriguez-Ayllon M, Acosta-Manzano P, Coll-Risco I, Romero-Gallardo L, Borges-Cosic M, Estévez-López F, et al. Associations of physical activity, sedentary time, and physical fitness with mental health during pregnancy: the GESTAFIT project. J Sport Heal Sci [Internet]. 2021;10(3):379–86. doi:https://doi.org/10.1016/j.jshs.2019.04.003. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools