 Open Access
Open Access
ARTICLE
Evaluation of Perinatal and Developmental Outcomes in Neonates with Abstinence Syndrome Admitted to NICU
1
Department of Psychiatry, Shahid-Beheshti University of Medical Sciences, Tehran, 19839-63113, Iran
2
David Geffen School of Medicine, University of California, Los Angeles, CA, 90095, USA
3
Shahid-Beheshti University of Medical Sciences, Tehran, 19839-63113, Iran
4
Department of Pediatrics, Clinical Research Development Center, Mahdiyeh Educational Hospital, Shahid-Beheshti University of
Medical Sciences, Tehran, 19839-63113, Iran
* Corresponding Authors: Ali Naseh. Email: ;
International Journal of Mental Health Promotion 2023, 25(2), 265-274. https://doi.org/10.32604/ijmhp.2023.024773
Received 07 June 2022; Accepted 17 October 2022; Issue published 02 February 2023
Abstract
Drug abuse by pregnant women is one of the significant problems for mothers and their neonates. This study aimed to investigate the effects of maternal substance use disorder during pregnancy on neonatal developmental criteria. In a case-control study, clinical records of 90 neonates diagnosed with neonatal abstinence syndrome who were admitted to NICU in one of four hospitals affiliated with Shahid-Beheshti University of Medical Sciences in Tehran, Iran between 2017 and 2020 were compared to 90 neonates without neonatal abstinence syndrome (control group). Demographic information and data for neonatal developmental characteristics and complications were extracted from the clinical records of this convenience sample. Data for the type and method of maternal substance use during pregnancy were collected through a telephone call with mothers. Our data showed that the prevalence of drug addiction was 1.8% among pregnant women, and the most common drugs used by mothers were opium (n = 45%, 50%), amphetamine (n = 30%, 33%), and methadone (n = 14%, 16%). Neonates with abstinence syndrome had a higher prevalence of transient tachypnea of the newborn (TTN) (P = 0.004), and a prevalence of being admitted to NICU (P = 0.05) and for a longer duration (P < 0.001). Their mothers had a higher prevalence of having pre-eclampsia (P = 0.010). Using morphine vs. amphetamine showed no difference based on their effects on mothers and neonates. Substance use during pregnancy increased the prevalence of pregnancy complications (pre-eclampsia) and neonatal complications (TTN and prevalence and duration of hospitalization). Therefore, planning for the development of health policies to raise awareness among women and more broadly, all members of the community, is important to prevent the tendency to engage in this potentially high-risk behavior.Keywords
Substance use disorder and drug addiction among pregnant women is a real concern around the world and especially in Iran, where previous research has reported a prevalence of 0.69% to 1.4%, based on different regions in the country, among pregnant women [1,2]. Due to the essential role of women as half of the population, and their influence on the culture of the whole family as they are providers of care for the children, there is a need for increased attention to this important group. Substance use and drug dependence among pregnant women is a complex problem [3]. The causes of substance use among pregnant women are very diverse and many factors, including personal, psychological, social, economic, and cultural factors, are involved. Solving this problem is often more difficult than understanding its causes and factors. Failure to refer addicted pregnant women to health centers can be an obstacle to treatment, and threatening policies, such as the criminalization of substance use, discourage pregnant women from seeking medical treatment [3–5].
According to the World Health Organization, 5 to 10 percent of women use drugs during pregnancy, which makes the pregnant woman’s lifestyle harmful to herself and her baby. On the other hand, no medication intervention can change all the behaviors and psychiatric disorders associated with the use of illicit substances. Additionally, pregnant women who use drugs are less likely to receive prenatal care [6–9].
Infants who have been exposed to opioids during the prenatal period show signs of neonatal substance abstinence after birth. A national study by the U.S. Department of Drug Abuse and Mental Health found that illegal drug use was present in 7.4% of pregnant women aged 18 to 25 [6]. Symptoms associated with neonatal substance abstinence have been reported in 60% to 80% of infants who have been exposed to heroin or methadone during pregnancy [7]. Other studies have reported that from 2000 to 2009, neonatal substance abstinence syndrome increased from 1.2 to 3.9 per 1,000 live births, as well as opioid exposure increased from 1.19 to 5.63 per 1,000 births [8].
Babies born to addicted women may not only suffer from drug withdrawal syndrome, but also they may have several physical problems that keep them in the hospital and neonatal intensive care units. Newborns may show withdrawal symptoms during their first few days after birth until the substance leaves their system. Neonatal abstinence syndrome (NAS) is defined as a condition that may affect newborns who receive addictive substances during pregnancy through the placenta from their mothers. Symptoms may include high-pitched and excessive crying, being fussy or ill, vomiting and/or diarrhea, fever and sweating, fast breathing, frequent sucking, tremors, and diaper rash. Additionally, they may show hypertonia and overactive reflexes, difficulty gaining weight, trouble falling asleep and staying asleep, feeding difficulties, or skin mottling. These babies are more vulnerable and stay so during their lifetime. Later in life, these infants may experience mental disorders, problems in social communication skills, and an inability to problem-solving capabilities [7,10,11].
Given the importance of neonatal abstinence syndrome, which is an indicator of maternal substance use during pregnancy, this research investigates these cases to identify the effects of substance use on neonatal growth and delivery complications.
This case-control study included 90 neonates diagnosed with substance abstinence syndrome who were hospitalized at NICUs and were selected as the case group, and another 90 neonates who did not develop neonatal abstinence syndrome were selected as the control group. Study recruitment was based on convenience sample selection. Clinical records of both neonates and their mothers were collected from one of the four hospitals affiliated with Shahid-Beheshti University of Medical Sciences in Tehran, Iran, between 2017 and 2020.
Criteria for inclusion in this study included neonates from both male and female sex who had accessible clinical records. The exclusion criteria consisted of mothers who were required to follow a psychiatric medication regimen to treat their psychiatric illnesses, lack of records of essential information needed for this study, and neonate’s death during or up to seven days after delivery. If both mothers and their neonates met the inclusion criteria, after obtaining the necessary permits, the information required for this study was extracted from the clinical records of neonates and their mothers based on a checklist containing questions related to demographic information and their health complications, including information related to neonatal developmental characteristics.
To determine drug use in mothers during pregnancy, including its type and method of use, the researcher (a medical student) called the phone number listed in the patient’s clinical files, and after providing a description of the study to show the importance of conducting this research, and after acquiring verbal consent, information about mothers’ drug use during pregnancy was collected and recorded on the researcher’s checklist. The patient’s privacy was observed. The Finnegan chart was available in the neonate’s clinical records, and the final condition of the neonate was classified into three categories: normal, abnormal, and unfinished, based on its final registered score. The Finnegan Neonatal Abstinence Scoring System (FNASS), also known as the Finnegan score, is used to stratify the severity of opioid withdrawal in neonates. The scoring began within two hours of life. Each symptom and its associated degree of severity was assigned a score, and the total abstinence score was determined by adding the scores assigned to each symptom [12]. Records of the Finnegan scale assessed the severity of abstinence syndrome in drug-exposed neonates in terms of onset, progression, and symptom relief. This scale generally measures central nervous system disorders, metabolic disorders, vasomotor and respiratory disorders, and gastrointestinal and intestinal disorders.
On the Finnegan scale, a score above ten was considered a confirmation of substance withdrawal syndrome [12]. The Finnegan chart included the status of the baby at birth, which included scores starting from zero and increasing to the following numbers depending on each symptom’s increased severity: excessive crying or having a high-pitched cry: 3 points; sleep less than 2 h after weaning: 3 points; increased Moro reflex: 3 points; tremor: 3 points; increased muscle tone: 2 points; myoclonic jerk: 3 points; itching and cramping: 1 point; seizure: 3 points; fever: 3 points; mottled skin: 2 points; nasal congestion: 2 points; nasal flaring: 2 points; respiratory distress: 3 points; inadequate breastfeeding: 3 points; projectile vomiting: 3 points; and diarrhea: 3 points.
Finally, the results for neonates with NAS were evaluated and compared to the control group. Data were analyzed through descriptive statistical tests, t-test and its nonparametric equivalent Mann–Whitney U test for quantitative variables, and chi-square test and Fisher’s exact test for qualitative variables, using the version 23 SPSS software (Statistical Package for the Social Sciences software) and 2019 version of Stata software (Stata Corp., College Station, TX, USA). The confidence level of 95% and significance level of ≤0.05 were considered.
Our four-year data showed that the prevalence of drug addiction among pregnant women was 1.8%. Among the mothers of 90 neonates with substance abstinence syndrome, 45 (50%) had a history of opium use, one had a history of opium extract consumption, one had a history of heroin use, and one had a history of marijuana use. However, 14 (16%) mothers reported having a history of either methadone or crack use, and 30 (33%) mothers had a history of amphetamine use during pregnancy.
Neonates with NAS included 49% females, and mostly had a gestational age of 37 to 40 weeks (64%), with a birth weight of 2500 to 3500 grams (57%), with a head circumference measured at the birth of 33 to 37 cm (81%), were delivered through cesarean section (52%), had normal Apgar score (80%), had normal brain ultrasound (96%) and had a duration of hospitalization of one to two days in the NICU (80%). The average number of pregnancies in the mothers of these infants was 2.71 (±1.41).
For the control group (neonates without abstinence syndrome), most of them were female (51%), with a gestational age of 37 to 40 weeks (62%), with a birth weight of 2500 to 3500 grams (65%), with a head circumference of 33 to 37 cm (75%) at birth, delivered through cesarean section (62%), with less than one-day hospital stay in NICU (67%), with normal Apgar score (77%), and had normal brain ultrasound (96%). The average number of pregnancies in the mothers of these infants was 2.47 ( ±1.32).
Among neonates with abstinence syndrome, opioid/other drugs users compared to amphetamine users showed no significant differences based on delivery method and neonatal complications, including neonatal respiratory distress (P = 0.656), neonatal sepsis (P = 0.555), seizures (P = 0.204), transient tachypnea of the newborn or TTN (P = 0.718), meconium aspiration (P = 0.667), neonatal anomaly (P = 0.709), asphyxia (P = 0.547), neonatal jaundice (P = 0.456), cerebral hemorrhage (P = 0.709), or the results of Finnegan chart (P = 0.600) (Table 1).
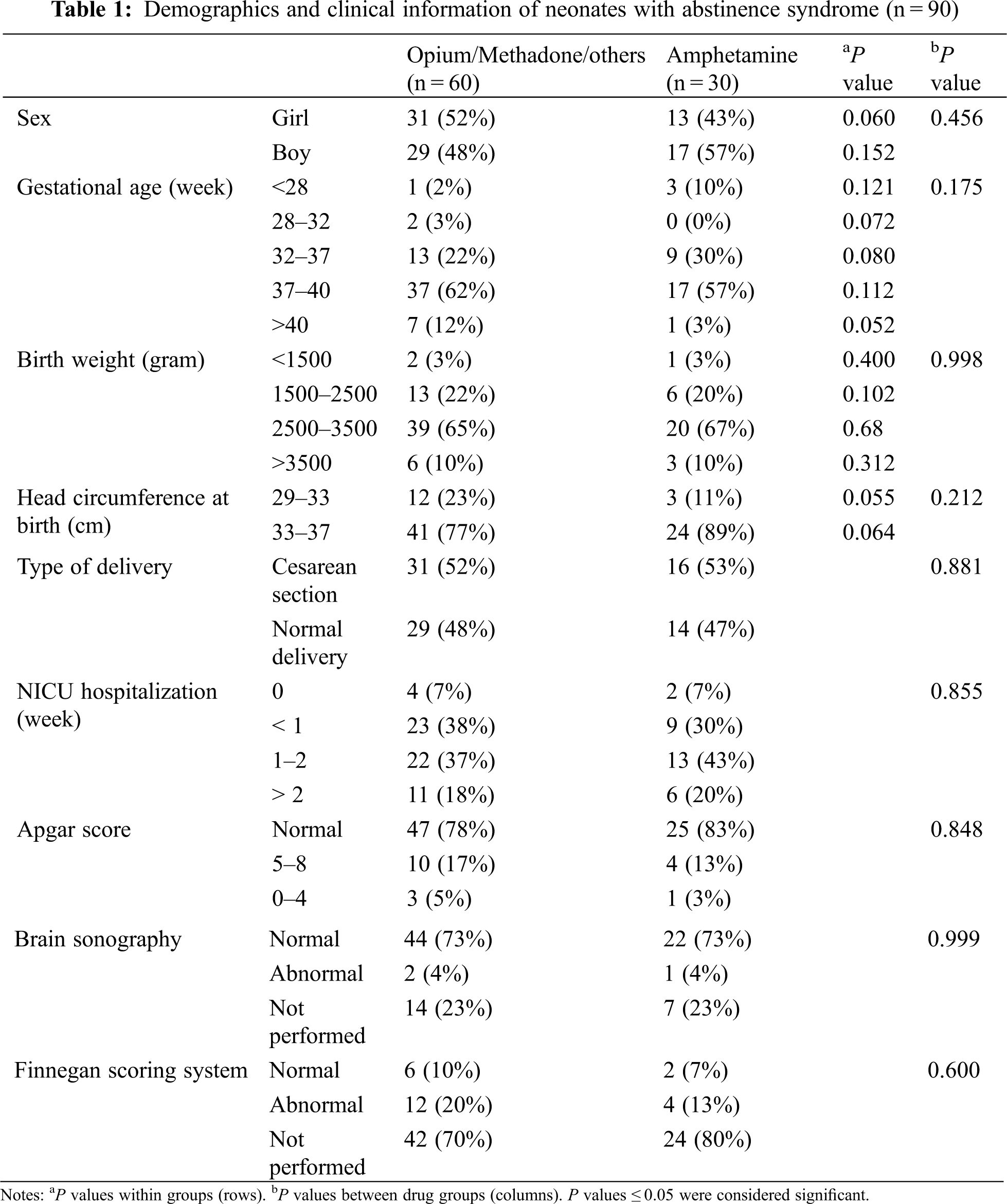
Also, among addicted mothers, opioid/other drugs users compared to amphetamine users did not show significant differences in complications of pregnancy, including intrauterine growth restriction (P = 0.213), pre-eclampsia (P = 0.555), gestational hypertension (P = 0.257), abortion (P = 0.626), and prenatal death (P = 0.593).
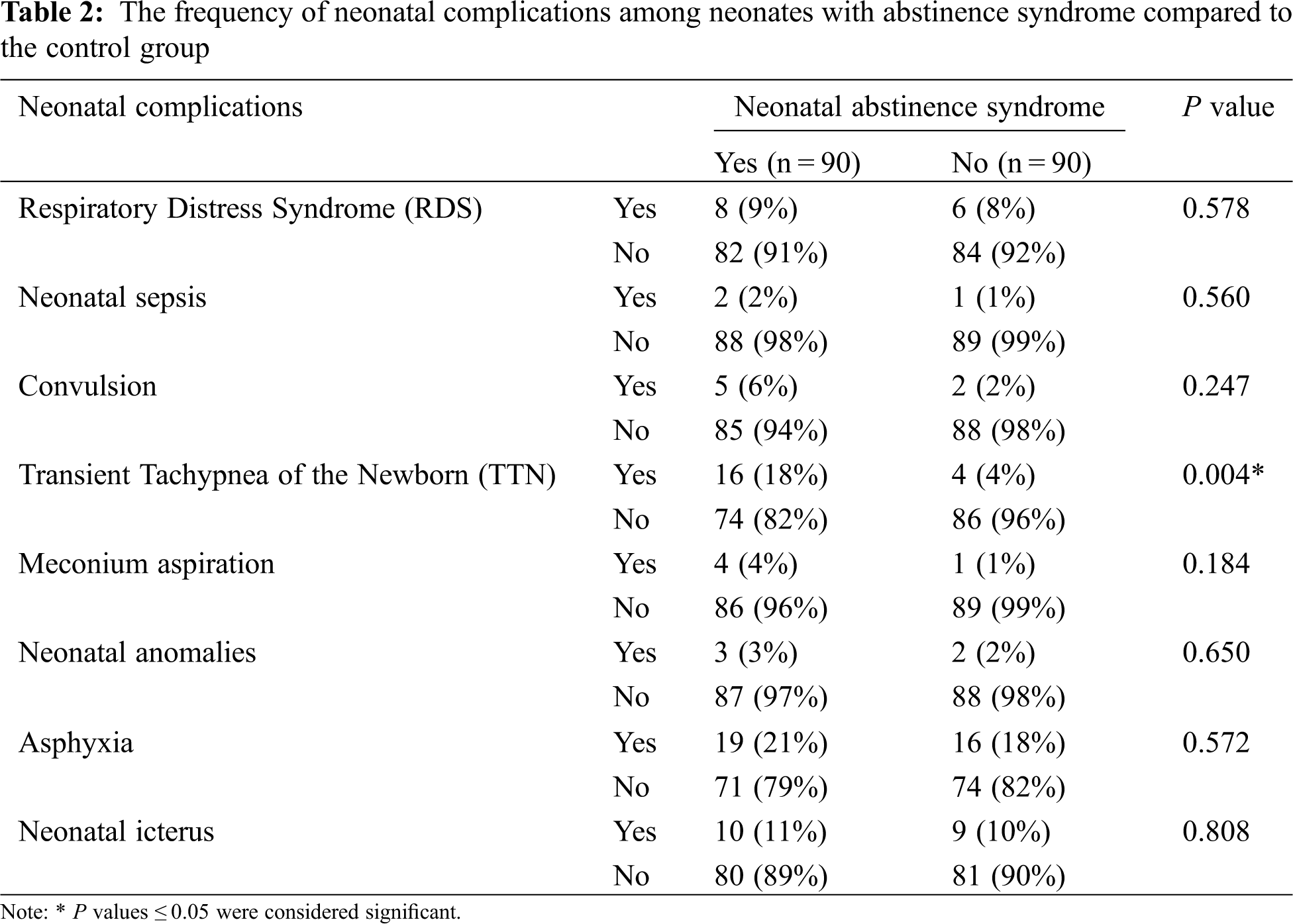
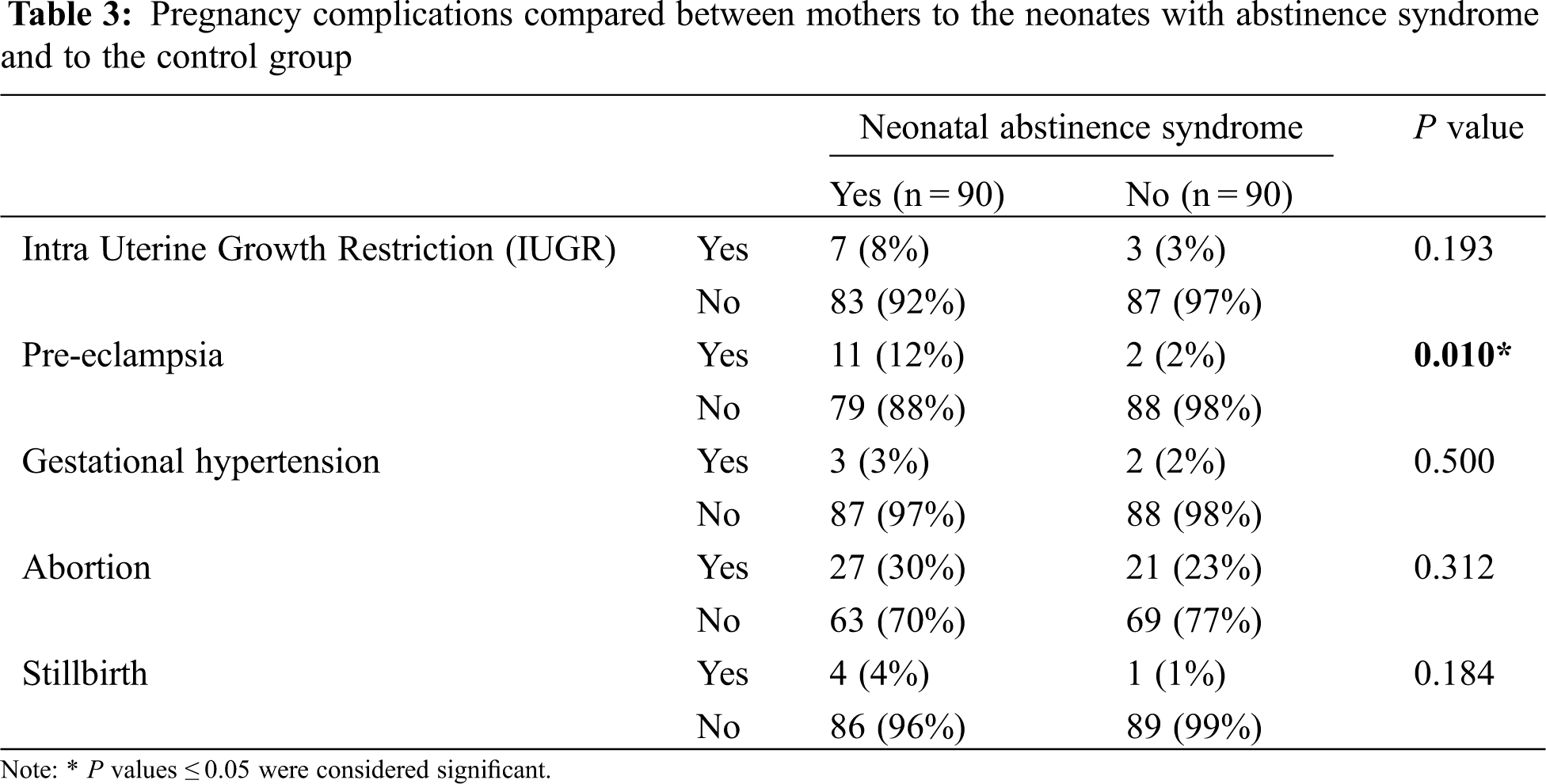
However, a comparison between the case and control groups showed that the prevalence of being admitted to NICU and the duration of NICU hospitalization in neonates with abstinence syndrome was significantly higher compared to the neonates in the control group (P = 0.05 and P < 0.001, respectively). Additionally, neonates with abstinence syndrome had a higher prevalence of TTN (P = 0.004) and a higher prevalence of pre-eclampsia in their mothers (P = 0.010) compared to the control group (Tables 2 and 3). Yet, between neonates with abstinence syndrome and the control group, there were no statistical differences based on gender (P = 0.766), gestational age (P = 0.480), birth weight (P = 0.752), birth head circumference (P = 0.372), number of pregnancies (P = 0.747), type of delivery (P = 0.175), and Apgar score (P = 0.466).
Our data showed the prevalence of drug addiction among pregnant women was 1.8% which shows an alarming rate of increase compared to the previous studies [1,2]. Our study indicated that the most drugs used among mothers included opium, amphetamine, and methadone, respectively. There have been limited studies on this topic in our region. For example, in a study by Gargari et al. in Tehran, they concluded that the most common drug used among pregnant women was opium [13] which aligns with our findings. However, another study by Vucinovic et al. in a 10-year study of pregnant women in Croatia found that the most common drug used by pregnant women in Croatia was heroin [14] which did not agree with our findings. The reason for this discrepancy may be due to differences in access to different drugs as well as cultural differences in different societies.
The majority of opiate-addict mothers suffer from other psychiatric comorbidities as well and may adopt a high-risk lifestyle that creates a higher incidence of sexually transmitted diseases, poor nutritional habits, and poor antenatal care [14].
However, apart from the type of drugs used in pregnancy, all of these are warnings for health officials and policymakers who investigate the prevalence, type, and causes of increased substance use during pregnancy. Hopefully, through the results of these studies, logical solutions can be found to prevent substance use during pregnancy.
Another finding of this study included the adverse effects of substance use in pregnancy in increasing the complications related to pregnancy. The prevalence of pre-eclampsia among mothers of infants with NAS was significantly higher than among mothers of the control group.
Also, Ramezanzadeh et al. in Tehran examined the consequences of smoking and drug use on maternal and fetal characteristics. Their study showed that maternal drug abuse during pregnancy increased the chances of adverse maternal outcomes (pre-eclampsia and hypertension) and adverse fetal outcomes [2]. These findings are consistent with the results of the present study.
The results of the study by Thaithumyanon et al. also showed that the prevalence of pre-eclampsia among mothers of infants with NAS was significantly higher than other pregnant women [15], which is similar to our results. In contrast, Alaee et al. examined the effects of opioid exposure on children during pregnancy and did not report a significant association between the prevalence of pre-eclampsia and drug use during pregnancy [16] which is inconsistent with the results of our study. The reason for this discrepancy may be the differences in the type of studies performed and the demographic differences between research sample populations.
The results of the present study showed that among the neonatal complications, the prevalence of TTN in neonates with NAS was significantly higher than in the control neonates. A study published by Mashmoul et al. [17] examined the prevalence of symptoms of drug withdrawal syndrome in infants and evaluated the severity of symptoms according to the mother’s job and the order of birth. Their study showed that infants with NAS often suffer from respiratory distress at the beginning of their birth and are more likely to become hospitalized compared to other infants, which is consistent with the results of our study.
A study by Torshizi et al. [1] examined the prevalence of drug use and its complications in pregnant women. It showed that 37.6% of neonates with NAS had fetal distress and the prevalence of neonatal respiratory disorders was significantly higher among neonates born to addicted mothers. Finally, the authors concluded that although the number of addicted pregnant women was not high, they are considered high-risk groups due to perinatal outcomes. As a result, preventive training programs during pregnancy should emphasize the discontinuation of drug use or their replacement with less harmful drugs.
Additionally, in their various studies, Smith et al. as well as James et al. concluded that drug use in pregnant women might increase the chances for the development of neonatal respiratory disorders, especially an increase in TTN [18,19], which were also in line with the results of our study. In contrast, Azizmohammadi et al. examined the relationship between substance use in pregnant women and pregnancy complications. They concluded that there is no significant relationship between neonatal respiratory disorders and maternal drug use during pregnancy [20]. This finding was contrary to our results. This discrepancy may be due to the differences in the sample size and the duration of follow-up for the two studies.
Our study showed that the duration of hospital stay in the NICU for neonates with NAS was longer than the control group. The study of Alaee et al. also showed that substance use during pregnancy leads to several negative consequences for the baby, such as increasing the likelihood of premature birth and birth defects. On the other hand, by damaging the central nervous system of the fetus, it affects children’s cognitive development, such as memory and learning, attention, language, problem-solving skills, executive activities, and creates behavioral damage, such as the development of depression or hyperactivity disorder [15].
Exposure to opioids before birth delays the growth and development of the brain and nerve structures in the embryonic or postnatal period and even increases the chances of brain damage in cases of ultrasound examination of the baby’s brain [15]. However, our results for brain sonography were inconclusive due to missing data for several neonates.
The results of our study showed that except for TTN, prevalence and length of hospitalization, and pre-eclampsia, there was no significant difference between neonates with NAS and control neonates for other fetal and neonatal complications. However, since most drugs of abuse easily cross the placenta, the results of other studies have shown the effects of such substances in creating adverse effects on the mother and fetus [21–23]. Given the sensitivity of the issue, more research with a larger sample size is warranted.
Due to our small sample size and incomplete data, this study could not show statistical significance for some of the possible adverse maternal and fetal outcomes compared to the control group. However, further research with larger sample size and long-term follow-up to investigate those effects is needed.
Additionally, since substance use is stigmatized in Iranian society, despite assuring mothers regarding the confidentiality of their information by the interviewer (researcher), the study participants may not have disclosed their substance use accurately, either intentionally or due to recall bias and have been mistakenly included in the control group. As a result, we could not obtain reliable data on substance use dosages and duration of consumption. While our study did not measure the degree of a dose-response relationship, it did evaluate the association between substance use and various pregnancy and neonatal complications. Therefore, further descriptive studies, such as examining the blood levels of drugs in suspected pregnant women, may be necessary to collect more accurate data.
Although brain sonography results were available for some of the patients, the retrospective structure of the study prevented us from collecting missing data for the rest of the patients.
Our data showed that substance use during pregnancy increases the prevalence of pregnancy complications (pre-eclampsia) and neonatal complications such as TTN and the prevalence and length of hospital stay in the NICU. Therefore, due to the increasing prevalence of substance use among pregnant women, it is important to develop evidence-based plans and health policies in the future aimed at raising awareness among women and, more broadly, members of the community to prevent mothers from engaging in this potentially risky behavior. For successful treatment of addicted mothers, which is measured by abstinence, the provision of support services is very important.
Acknowledgement: The authors are thankful to the personnel in the four hospitals affiliated with Shahid-Beheshti University of Medical Sciences (Taleghani, Imam Hossein, Loghman, and Mahdieh) in Tehran, Iran, and to the patients for enabling this research possible.
Article Contribution Statement: The authors confirm their contribution to the paper as follows: study conception and design: A. K., S. A., A. N.; data collection: F. R., S. A., A. N.; analysis, and interpretation of results: S. A., F. R; draft manuscript preparation: S. A., S. A. F. R. All authors reviewed the results and approved the final version of this manuscript.
Funding Statement: The authors received no funding for this study.
Conflicts of Interest: The authors declare they have no conflicts of interest to report regarding the present study.
References
1. Torshizi, M., Saadatjoo, S., Farabi, M. (2011). Prevalence of narcotic substance abuse and the maternal and fetal outcomes in pregnant women. Journal of Jahrom University of Medical Sciences, 9(3), 14–19. DOI 10.29252/jmj.9.3.3. [Google Scholar] [CrossRef]
2. Ramezanzadeh, F., Tavafian, S., Vahdaninia, M., Shariat, M., Montazeri, A. (2007). Maternal and fetal outcomes of narcotic substance abuse, cigarette smoking, and unsafe drugs. Hakim Research Journal, 10(3), 9–16. [Google Scholar]
3. Stone, R. (2015). Pregnant women and substance use: Fear, stigma, and barriers to care. Health Justice, 3, 2. DOI 10.1186/s40352-015-0015-5. [Google Scholar] [CrossRef]
4. Forray, A. (2016). Substance use during pregnancy. F1000 Research, 5, 887. DOI 10.12688/f1000research.7645.1. [Google Scholar] [CrossRef]
5. Hudak, M. L., Tan, R. C. (2012). Committee on drugs; committee on fetus and newborn; American academy of pediatrics. Neonatal drug withdrawal. Pediatrics, 129(2), e540–60. DOI 10.1542/peds.2011-3212. [Google Scholar] [CrossRef]
6. Substance Abuse and Mental Health Administration. Office of Applied Studies (2011). Results from the 2010 national survey on drug use and health: Summary of national findings. https://www.samhsa.gov/data/sites/default/files/NSDUHNationalFindingsResults2010-web/2k10ResultsRev/NSDUHresultsRev2010.pdf. [Google Scholar]
7. Roberts, S. C. M., Pies, C. (2011). Complex calculations: How drug use during pregnancy becomes a barrier to prenatal care. Maternal and Child Health Journal, 15, 333–341. DOI 10.1007/s10995-010-0594-7. [Google Scholar] [CrossRef]
8. Patrick, S. W., Schumacher, R. E., Benneyworth, B. D., Krans, E. E., McAllister, J. M. et al. (2012). Neonatal abstinence syndrome and associated health care expenditures United States 2000–2009. JAMA, 307(18), 1934–1940. [Google Scholar]
9. National Institute on Drug Abuse (2020). Substance use while pregnant and breastfeeding. https://nida.nih.gov/publications/research-reports/substance-use-in-women/substance-use-while-pregnant-breastfeeding. [Google Scholar]
10. Madgula, R. M., Groshkova, T., Mayet, S. (2011). Illicit drug use in pregnancy: Effects and management. Expert Review of Obstetrics & Gynecology, 6(2), 179–192. DOI 10.1586/eog.10.54. [Google Scholar] [CrossRef]
11. Goettler, S. M., Tschudin, S. (2014). Care of drug-addicted pregnant women: Current concepts and future strategies–An overview. Women’s Health, 10(2), 167–177. DOI 10.2217/WHE.14.7. [Google Scholar] [CrossRef]
12. McQueen, K. A., Murphy-Oikonen, J., Gerlach, K., Montelpare, W. (2011). The impact of infant feeding method on neonatal abstinence scores of methadone-exposed infants. Advances in Neonatal Care, 11(4), 282–90. DOI 10.1097/ANC.0b013e318225a30c. [Google Scholar] [CrossRef]
13. Gargari, S. S., Fallahian, M., Haghighi, L., Hosseinnezhad Yazdi, M., Dashti, E. et al. (2011). Perinatal complications in substance using in pregnancy. Razi Journal of Medical Sciences, 18(86), 22–30. [Google Scholar]
14. Vucinovic, M., Roje, D., Vucinovic, Z., Capkun, V., Bucat, M. et al. (2008). Maternal and neonatal effects of substance abuse during pregnancy: Our ten-year experience. Yonsei Medical Journal, 49(5), 705–713. DOI 10.3349/ymj.2008.49.5.705. [Google Scholar] [CrossRef]
15. Thaithumyanon, P., Limpongsanurak, S., Praisuwanna, P., Punnahitanon, S. (2005). Perinatal effects of amphetamine and heroin use during pregnancy on the mother and infant. Journal of Medical Association of Thailand, 88(11), 1506–1513. [Google Scholar]
16. Alaee, E., Azizi, H., Semnanian, S., Rashidy-pour, A. (2020). The effects of opioid exposure during pregnancy on offspring: Review article. Malaysian Journal of Medical Sciences, 23(4), 39–53. [Google Scholar]
17. Mashmoul, A., Bakhshani, N. M. (2015). Prevalence of drug withdrawal symptoms in infants and comparison symptoms according to mothers’ job and infants birth order in Zahedan. Journal of Research in Behavioural Sciences, 13(1), 22–28. [Google Scholar]
18. Smith, L. M., LaGasse, L. L., Derauf, C., Grant, P., Shah, R. et al. (2006). The infant development, environment, and lifestyle study: Effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics, 118(3), 1149–1156. DOI 10.1542/peds.2005-2564. [Google Scholar] [CrossRef]
19. James, D. K., Steer, P. J., Weinner, C. P., Gonik, B. (2006). High risk pregnancy management options. 3rd editionPhiladelphia: Sanders/Elsevier. [Google Scholar]
20. Azizmohammadi, S., Alavi, H. (2009). The relationship between drug abuse in pregnant women and pregnancy complications in Shahid Akbarabadi treatment-training center. Journal of Nurse and Physician within War, 23, 29–33. [Google Scholar]
21. Arntzen, A., Samuelsen, S. O., Bakketeig, L. S., Stoltenberg, C. (2004). Socioeconomic status and risk of infant death. A population-based study of trends in Norway. International Journal of Epidemiology, 33, 279–288. DOI 10.1093/ije/dyh054. [Google Scholar] [CrossRef]
22. Ross, E. J., Graham, D. L., Money, K. M., Stanwood, G. D. (2015). Developmental consequences of fetal exposure to drugs: What we know and what we still must learn. Neuropsychopharmacol, 40, 61–87. DOI 10.1038/npp.2014.147. [Google Scholar] [CrossRef]
23. Latuskie, K. A., Andrews, N. C. Z., Motz, M., Leibson, T., Austin, Z. et al. (2019). Reasons for substance use continuation and discontinuation during pregnancy: A qualitative study. Women and Birth, 32(1), e57–e64. DOI 10.1016/j.wombi.2018.04.001. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools