 Open Access
Open Access
ARTICLE
The Effect of Sleep and Cognition Enhancement Multimodal Intervention for Mild Cognitive Impairment with Sleep Disturbance in the Community-Dwelling Elderly
Department of Nursing, Eulji University, Seongnam, 13135, South Korea
* Corresponding Author: Hae Kyoung Son. Email:
International Journal of Mental Health Promotion 2023, 25(11), 1197-1208. https://doi.org/10.32604/ijmhp.2023.041560
Received 27 April 2023; Accepted 17 July 2023; Issue published 08 December 2023
Abstract
Dementia prevalence has soared due to population aging. In Mild Cognitive Impairment (MCI) as a pre-dementia stage, sleep disturbances have raised much interest as a factor in a bidirectional relationship with cognitive decline. Thus, this study developed the Sleep and Cognition Enhancement Multimodal Intervention (SCEMI) based on Lazarus’ multimodal approach and conducted a randomized controlled experiment to investigate the effects of the novel program on sleep and cognition in MCI elderly. The participants were 55 MCI elderly with sleep disturbances at two dementia care centers located in S-city, Gyeonggi-do, South Korea (n = 25 in the experimental group and n = 30 in the control group). The study period was from November 01 to December 27, 2022. The experimental group received 8 sessions of SCEMI, 60 min per session once a week. The control group received general education and guidance using a simplified booklet on the sleep and cognitive improvement. For data collection, a self-reported questionnaire was used to investigate sleep quality, presleep arousal, cognitive function, stress, and depression. The results showed that, compared to the control group, the experimental group had significantly improved across all variables: sleep quality (U = 109.50, p < 0.001), presleep arousal (U = 11.50, p < 0.001), cognitive function (U = 72.00, p < 0.001), stress (U = 139.00, p < 0.001), and depression (U = 231.50, p = 0.015). Thus, the SCEMI appears to positively affect symptomatic improvement and delays the progression to dementia as an integrated intervention to enhance sleep and cognition in community-dwelling MCI elderly with sleep disturbances.Keywords
With increased average life expectancy, the elderly population has increased, accompanied by a global phenomenon of population aging. The rate of increase in the elderly population is particularly high in developing countries in Asia and South America [1]. As a result, the prevalence of dementia, a representative geriatric disease, has soared, while it is known to increase with increasing age to show a 2-fold increase in the number of patients every five years after the age of 65 years [2,3]. South Korea has been predicted to become a superaged society [1] as the number of dementia patients reaches 1 million by 2030 and 2 million by 2050, with a large increase in socioeconomic burdens [4].
Dementia causes irreversible pathological changes as well as psychobehavioral symptoms as the level of cognitive impairment becomes severe, which lowers the expected effects of the treatment. For dementia, therefore, prevention and early detection are critical [5]. In this regard, mild cognitive impairment (MCI) is viewed as a transitional stage from normal to dementia [6]. Hence, the probability of progression from MCI to dementia is relatively high, and MCI is considered a risk factor or a critical condition of dementia. The clinical significance is high as MCI offers insights into the pathogenesis of dementia at a pre-dementia stage [7]. Mild cognitive impairment should be differentiated from a cognitive decline caused by normal aging as the MCI-related cognitive decline does not negatively affect the activities of daily living (ADL) or instrumental ADL (iADL). At the same time, a preemptive interest should be taken toward preventing dementia as MCI causes functional decline at a rate averaging between healthy elderly and mild dementia patients [7,8].
The factors known to influence cognitive decline are negative mental health conditions such as depression, anxiety and stress, poor physical health conditions such as obesity and hypertension, and health risk factors such as smoking and inadequate physical activity [9]. Recently, sleep disturbances have gained attention as another critical risk factor in a bidirectional relationship with cognitive decline (i.e., sleep disturbances can significantly reduce the cognitive functioning of elderly individuals with MCI) [6,10–12]. The prevalence of sleep disturbances increases with age, suggesting that sleep is affected by aging itself and/or by aging-related conditions [13,14]. Notably, more than 60% of MCI patients experience sleep disturbances [8], which can induce cognitive dysfunction with a potential influence on the onset of dementia and reduce ADL to increase the burden of care [6,8,12–14].
Strategies should thus be developed to improve the sleep quality in MCI elderly. However, only a few protocols of integrated non-pharmacological interventions have been developed for sleep and cognition enhancement in MCI elderly, while much of the previous literature has focused on pharmacological interventions for sleep enhancement [15]. Notable disadvantages of this pharmacological approach in the elderly include the potential for side effects, interactions, and polypharmacy, and the side effects of sedative-hypnotics and benzodiazepine drugs include daytime sleepiness, nocturnal confusion, increased fall accidents, and dependency on drugs [16,17]. Sedative-hypnotics, in particular, may aggravate cognitive impairment in the elderly [16,17].
This has raised interest in non-pharmacological interventions for MCI patients [16]. The non-pharmacological interventions in previous studies include a moderate-intensity aerobic exercise program [18], mindfulness meditation [19], and the physical activity program [20]. These interventions had positive effects on sleep, cognition, depression, and anxiety; nevertheless, to maximize the optimal intervention effects on the enhancement of sleep and cognition in individuals with cognitive decline, Rodriguez et al. [21] highlighted the need to reduce the risk factors and enhance the protective factors through multi-component, multi-factorial, multi-level and customization strategies. In Lee et al. [22], a food art therapy of a multimodal approach positively affected the cognitive, emotional, and social functions in MCI patients. Bae et al. [23] also showed the effects of a multi-component intervention combining physical, cognitive, and social activities to enhance cognitive function. According to a systematic review of the interventions combining cognitive and physical domains [24], multi-component interventions positively affected the cognitive and motor abilities of the elderly with a risk of dementia. However, only a few multi-component interventions are available to enhance sleep and cognition in the MCI elderly [24,25]. Sleep disturbances in MCI are affected by multiple factors to imply a need for an integrated non-pharmacological approach for multi-component interventions. Thus, this study developed the Sleep and Cognition Enhancement Multimodal Intervention (SCEMI) based on Lazarus’ multimodal approach [26] for enhancing sleep and cognition and investigated the effects of the novel program on the sleep quality, pre-sleep arousal, cognitive function, depression, and stress.
The hypotheses in this study are as follows:
1. The SCEMI group will show a lower sleep quality score than will the control group.
2. The SCEMI group will show a lower score of presleep arousal than will the control group.
3. The SCEMI group will show a higher cognitive function score than will the control group.
4. The SCEMI group will show a lower score of depression than will the control group.
5. The SCEMI group will show a lower stress score than will the control group.
This randomized, controlled experimental study aimed at investigating the effects of a novel program SCEMI on sleep quality, presleep arousal, cognitive function, depression, and stress in MCI elderly with sleep disturbances.
The participants were recruited using an ad posted on the noticeboard at two dementia care centers located in S-city, Gyeonggi-do, South Korea. The participants were elderly patients diagnosed with MCI and experiencing sleep disturbances and who had never participated in a sleep-related intervention program. The diagnosis of MCI was based on the criteria of the International Working Group on Mild Cognitive Impairment.
To be specific, the inclusion criteria were (1) individuals aged ≥60 years and satisfying the MCI diagnostic criteria, (2) individuals with the score of Montreal Cognitive Assessment–Korean version (MoCA-K), a cognitive assessment tool developed for the screening of MCI, <24 points [27], (3) individuals with the score of Pittsburgh Sleep Quality Index (PSQI) >5 points, and (4) individuals able to communicate and provide responses to the questionnaire. The exclusion criteria were (1) individuals diagnosed with dementia, (2) individuals with a neurological disorder such as stroke, epilepsy, and Parkinson’s disease, (3) individuals with a major mental disorder (depression or anxiety disorder), and (4) individuals on sedative-hypnotics, anti-anxiety or anti-depression agents.
The sample size was estimated using G*Power 3.1 by Cohen’s formula in the following conditions: number of groups = 2, number of measurements = 2, testing power = 0.80, significance level = 0.05, and effect size = 0.5 [20]. The minimum sample size was n = 25 per group, and assuming a 20% dropout rate, 60 participants were recruited in total; n = 30 in the experimental group and n = 30 in the control group (Fig. 1). With the exclusion of 5 participants in the experimental group, who either discontinued the intervention due to a health-related (e.g., COVID-19) or personal reason or provided an incomplete questionnaire, the data of 55 participants (n = 25 in the experimental group and n = 30 in the control group) were analyzed.
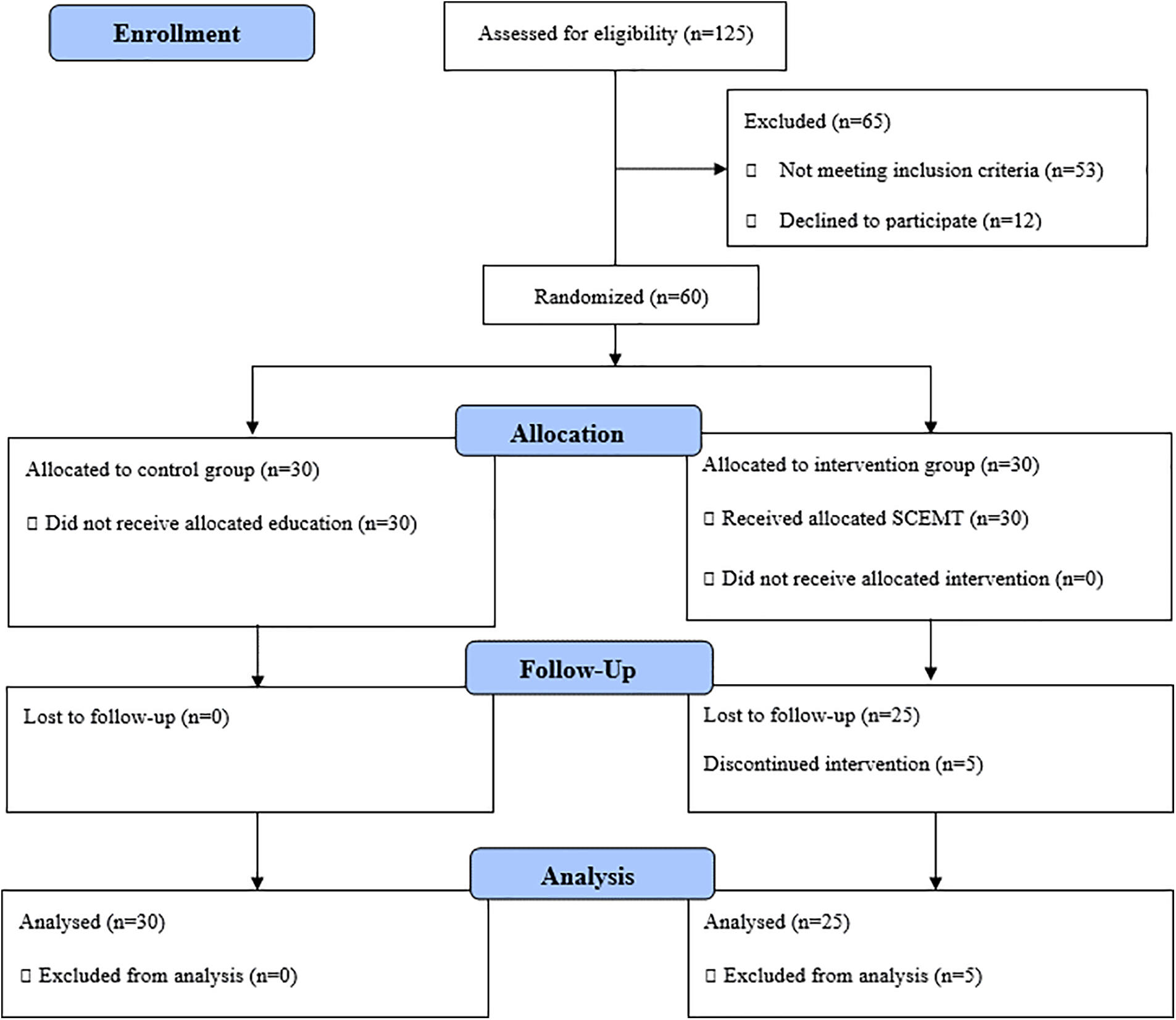
Figure 1: Consort flow diagram.
Through the ad, 125 participants were recruited. For the selection and enrollment of participants, the assessment for eligibility included two steps. First, two trained researchers conducted 1:1 interviews, each lasting within 10 min. Next, individual cognitive function tests, self-reported questionnaires, and InBody measurements were performed. Among the participants who agreed to participate, 60 participants were selected based on the inclusion and exclusion criteria and the study purpose. The participants were randomized into the experimental or control group by coin flipping (Fig. 1).
The general characteristics in this study included age, gender, marital status, education, living situation, sleeping condition, alcohol use, and smoking. To measure the height, weight, and body mass index (BMI), a body composition analyzer (InBody 770, InBody Co., Ltd., Korea) was used. For accurate measurements, participants were guided to wear as light as possible, and after removing any metallic materials from the body two hours before or after a meal, they were guided to stand straight on the electrodes with each hand lightly holding a palm electrode.
Sleep quality was measured using the PSQI developed by Buysse et al. [28]. The PSQI consists of 19 self-rated questions on subjective sleep quality, habitual sleep efficiency, sleep disturbances, sleep latency, sleep duration, use of sleep medication, and other sleep-related factors. Each item is rated on a scale from 0 (no difficulty) to 3 (severe difficulty), with the total score ranging between 0 and 21 and scores >5 points indicating poor sleep quality. Cronbach’s α was 0.98 in Buysse et al. [28] and 0.90 in this study.
Presleep arousal was measured using the Korean version of the Presleep Arousal Scale (PSAS), a tool developed by Nicassio et al. [29] and translated by Cho et al. [30]. The tool was developed to assess the physical and cognitive arousal levels before sleep using 16 questions. Each question is rated on a 5-point Likert scale, with higher scores indicating higher levels of presleep arousal. Cronbach’s α was 0.89 in Cho et al. [30] and 0.92 in this study.
Cognitive function was measured using the MoCA-K, a tool developed by Nasreddine et al. [31] for the screening of MCI and translated by Lee et al. [32]. The MoCA-K consists of 12 questions on visuoconstructional skills, language, attention, abstraction, delayed recall, and orientation, with the total score ranging from 0 to 30 and higher scores indicating higher levels of cognitive function. Cronbach’s α was 0.86 in Lee et al. [32] and 0.78 in this study.
Depression was measured using the Geriatric Depression Scale Short Form–Korean Version (GDSSF-K). The original tool Geriatric Depression Scale (GDS), was developed by Yesavage et al. [33] and modified by Kee [34] to suit the elderly in South Korea. The GDSSF-K consists of 15 questions, with the total score ranging from 0 to 15 and higher scores indicating higher levels of depression. Cronbach’s α was 0.88 in Kee [34] and 0.86 in this study.
Stress was measured using the Korean version of the Perceived Stress Scale (PSS), a tool developed by Cohen [35] and translated by Lee et al. [36]. The PSS measures the perceived stress level in situations that arise in daily life. The tool consists of 10 questions, including 5 on stress perception and 5 on stress management. Questions 4, 5, 7, and 8 were reverse-coded for scoring. Each question is rated on a 4-point Likert scale, with higher scores indicating higher stress. Cronbach’s α was 0.78 in Lee et al. [36] and 0.78 in this study.
SCEMI development and application
The SCEMI was constructed for MCI elderly with sleep disturbances, based on Lazarus’ multimodal approach [26] and through a systematic review of studies on non-pharmacological interventions for sleep enhancement in MCI and Alzheimer’s dementia [16].
The SCEMI involved 8 sessions, one session a week and each session lasting 60 min, and 8 sessions of telephone coaching, one session a week based on a previous study [25]. In particular, Naismith et al. [25] evaluated the efficacy of a four-session multicomponent group intervention for participants with MCI. The intervention consisted four fortnightly face-to-face sessions and fortnightly coaching phone calls [25]. In Lazarus’ multimodal theory [26], the Behavior component is a regular exercise combined with light exposure and behavioral strategies to practice sleep hygiene through sleep education. Based on a previous study where resetting the circadian rhythm led to increased sleep duration [37], a walking exercise (30 min) was included in this study. The Cognition component included cognitive training using a cognition calendar. In the Affect component, positive feedback and emotional support were given by an expert through sharing the experience of sleep and cognition activities of the past week. At the same time, negative feelings were neutralized and turned into positive language through education to focus on improving emotional factors. In the Sensation component, an aroma necklace and roll-on were used for olfactory stimulation, and breath meditation was performed in the Imagery component. Lastly, the Interpersonal-relations component included a weekly session of telephone coaching to assess the performance of sleep and cognitive training with support and encouragement and to discuss strategies for difficulties to raise self-confidence (Fig. 2). Table 1 presents the details of the SCEMI based on multimodal approach.

Figure 2: Sleep and cognition enhancement multimodal intervention.
A sleep expert provided sleep education with a doctorate degree and ≥15 years of clinical experience on patients regarding insomnia, MCI, and dementia, with the help of one research assistant. The education consisted of 8 weekly sessions, each lasting 60 min. Participants were given a booklet on sleep and a cognition calendar in the first session. The education comprised orientation, sharing the experience of sleep and cognition activities of the past week, sleep education, cognitive training, aroma inhalation training, and medication. The educational contents included the function of sleep, the relationship between insomnia and cognitive function, stimulation control, sleep and biorhythm, sleep inhibitors, and sleep hygiene, in reference to a previous study [13]. Except for the first session, each session began by sharing the experience, success, or barriers of practicing sleep and cognition activities of the past week. Considering that the participants are MCI patients, the education was repeatedly given via clear and detailed contents, which included stimulation control [38] and sleep hygiene education for the elderly. The sleep hygiene education applied in this study was as follows: (1) Try not too hard to sleep, (2) Stay active out of bed until feeling drowsy, (3) Wake up at a fixed time (and avoid staying in bed after that time even if the previous night’s sleep has been inadequate), (4) Avoid checking the clock upon waking up, (5) Perform abdominal breathing before sleep, and (6) Perform stretching after waking up in the morning.
Cognitive training was based on the MoCA [31] and used a cognition calendar. The components of the cognition calendar were pieces of training on (1) visuoconstructional skills (connecting numbers and letters in a given order, drawing cubes, drawing clocks, etc.), (2) naming (images of animals), (3) memory (memorizing and recalling five words), (4) attention (memorizing numbers in a given order, memorizing numbers in a reverse order, memorizing the days of the week in a reverse order, repeatedly subtracting 7 from 100, etc.), (5) language (repeating after stimuli, saying words beginning with a, etc.), (6) abstraction (finding similarities of two objects), (7) orientation (checking a weekly calendar and saying the day, month, year and the day of the week). The pieces of training (1)–(7) of the cognition calendar were revised each week to allow participants to be challenged with new words, numbers, and names weekly. In addition, a checklist was included in the cognition calendar to assess the performance regarding walking exercises and aroma inhalation.
Based on a previous study [39] reporting the positive effects of aroma inhalation in enhancing the quality of sleep in MCI and dementia patients, a set of essential oils with positive effects on sleep, depression, and anxiety were selected, and by consulting an expert with aroma therapist qualification, a 4:1:1 mixture of lavender oil, marjoram oil, and Roman chamomile oil was used. Lavender relaxes the mind and body, relieves stress, and reduces insomnia. Marjoram has effects of reducing anxiety and insomnia as well as calming effects, and Roman chamomile is effective for psychological stability and stress relief [40]. For simple and convenient daily use by MCI elderly with sleep disturbances, the inhalation was applied using an aroma necklace and roll-on. The aroma oil mixture was placed in a 0.20 mL glass vial to allow participants to wear it as a necklace so that the aromatic fragrance could be inhaled through the nose. At night before sleep, participants were guided to inhale the aroma before lying on the bed by applying the roll-on at a single pulse point of the radial artery behind the ear [41].
Breath meditation was used as an easy form of mindfulness meditation for the elderly based on a previous study reporting such effects as reduced sleep onset latency, enhanced sleep quality, and ameliorated insomnia symptoms [42]. Breath meditation focuses on the perception of the change of breath in line with emotion. Abdominal breathing to assist with relaxation was repeated five times, and if a thought emerged, the participant was guided to look into the thought and then refocus on breathing.
The contents of telephone coaching were based on the concept of verbal persuasion of Bandura [43]. The present researcher and a research assistant received telephone coaching training before the study. In this study, the telephone coaching proceeded as follows: Greeting, Evaluating the state of sleep of the past week, Assessing the sleep inhibitors and promotors, Intervention with support and encouragement, Assessing the performance regarding the cognitive training at home, and Discussing the strategies on difficulties, thereby providing social support as well as raising the self-confidence. For 8 weeks, the researcher and the research assistant conducted weekly telephone coaching (e.g., landline) on each participant. Each coaching session lasted 10–15 min.
Walking exercise was included in this study based on the reported effects to enhance sleep quality and reduce the sleep onset latency [44] in addition to the effects of enhanced cognitive function, reduced anxiety and depression, and enhanced quality of life. The daily goal of the walking exercise was 5,000 steps, set within the recommended range of 6,000–8,500 steps for adults aged ≥50 years [45]. In the first face-to-face education session, the participants were guided to download the Walk-on app and set a daily goal of 5,000 steps. The walking exercise intervention was applied for 8 weeks with 5 sessions a week, and the recorded number of steps of the past week was checked in the face-to-face education session. As a result, all 25 participants in experimental group completed ≥5,000 steps of walking, while 7 participants completed ≥10,000 steps.
Data collection and ethical considerations
This study was approved by the Institutional Review Board of E University in Seongnam-si, Gyeonggi-do, South Korea (IRB No. EU22-63). The consent for the experiment was obtained from the director and administrative officer of the data repository. The period of data collection was from November 01 to December 27, 2022. The experimental group received 8 sessions of SCEMI, while the control group received general education and guidance using a simplified booklet on the sleep and cognitive improvement. The participants in both groups completed the same set of structured questionnaires in the pre- and post-test. The participants were recruited through an ad posted with the consent of the administrative officer at the dementia care centers, and those voluntarily agreeing to participate were led to enrollment. Before the intervention, the researcher provided detailed verbal and written explanations of the study's purpose and procedures. The participants were informed through verbal and written explanations that the collected data would be anonymized, used solely for the study purpose, and discarded at the end of the study with full confidentiality on any personal information. They were also informed that they could withdraw from the study at any time, and signed consent was obtained from each participant.
Data was analyzed using SPSS version 21.0. For the homogeneity test before intervention on the general characteristics in the control and experimental groups, χ2-test and independent t-test were used. The Shapiro–Wilk test was used for the normality test on the dependent variables. For the homogeneity test before intervention and the test of intervention effects on the dependent variables, independent t-test, Mann–Whitney U test, and Wilcoxon signed ranks test were used.
Table 2 presents the general characteristics of the participants. The mean age of the participants was 71.35 years, and there were 22 female elderly (88.0%) in the experimental group and 24 female elderly (80.0%) in the control group. Regarding marital status, most participants in both groups were married (n = 15 [60.0%] in the experimental group and n = 18 [60.0%] in the control group), followed by the widowed (n = 8 (32.0%) in the experimental group and n = 10 [33.3%] in the control group). For education, most participants in both groups were high school graduates (n = 13 [52.0%] in the experimental group and n = 14 [46.7%] in the control group). With regards to the living situation, the number of those living with a spouse or others was 19 (76.0%) in the experimental group and 20 (66.7%) in the control group, which was greater than the number of those living alone at 6 (24.0%) and 10 (33.3%), respectively. Regarding the sleeping conditions, the number of those sleeping alone was greater than that of those not sleeping alone in both groups (n = 23 [92.0%] in the experimental group and n = 23 [76.7%] in the control group). Most participants replied that they did not use alcohol or smoke (n = 23 [92.0%] and n = 26 [86.7%] in the experimental group and n = 23 [92.0%] and n = 30 [100.0%] in the control group). On the other hand, a high percentage of participants in both groups replied that they drank caffeine (n = 20 [80.0%] in the experimental group and n = 18 [60.0%] in the control group). In both groups, the participants had at least one experience of nocturia. The mean BMI was 23.65 ± 3.30 in the experimental group to indicate a level of overweight and 22.63 ± 3.01 in the control group to indicate a normal weight level, although the difference was not statistically significant.
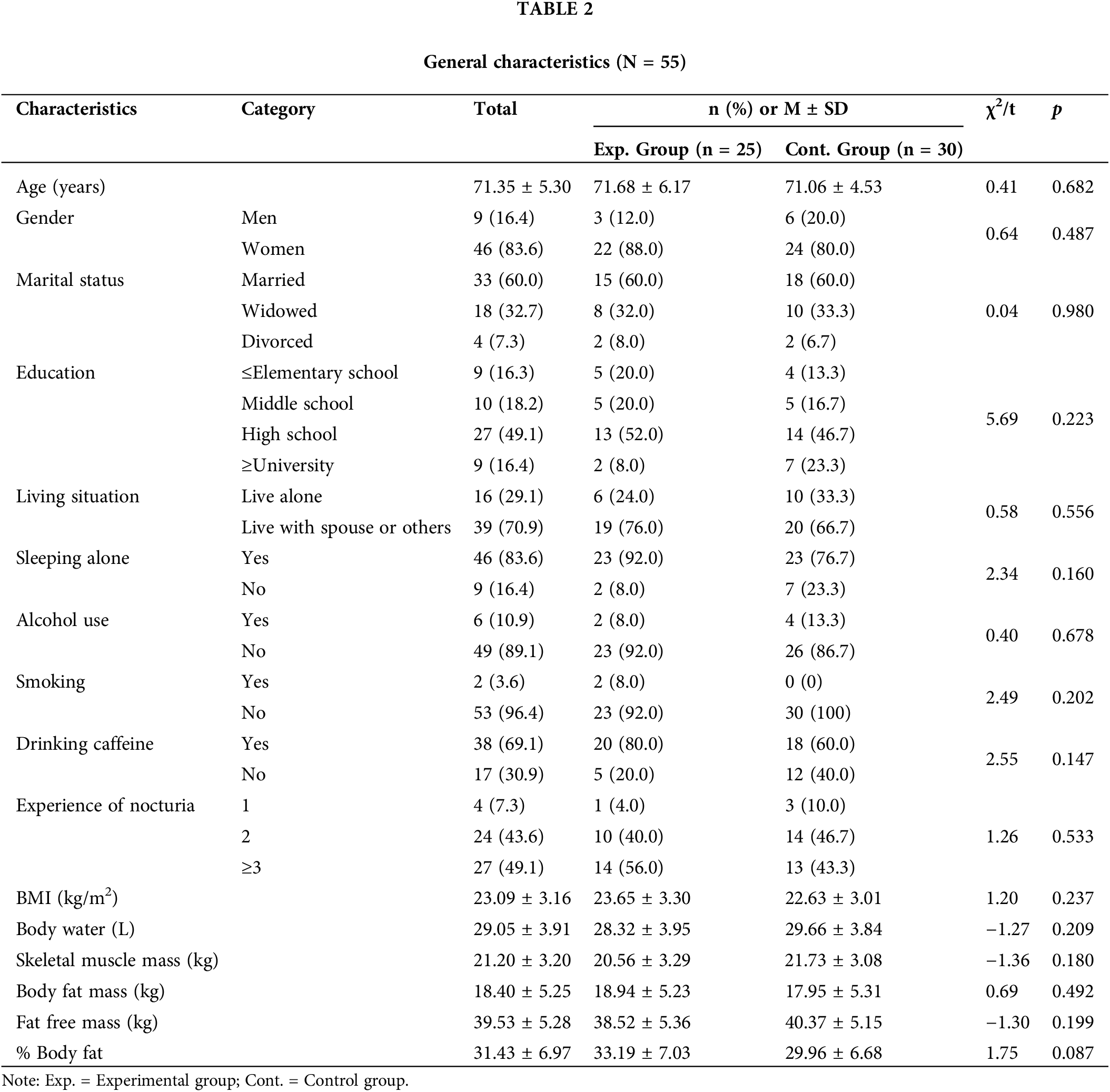
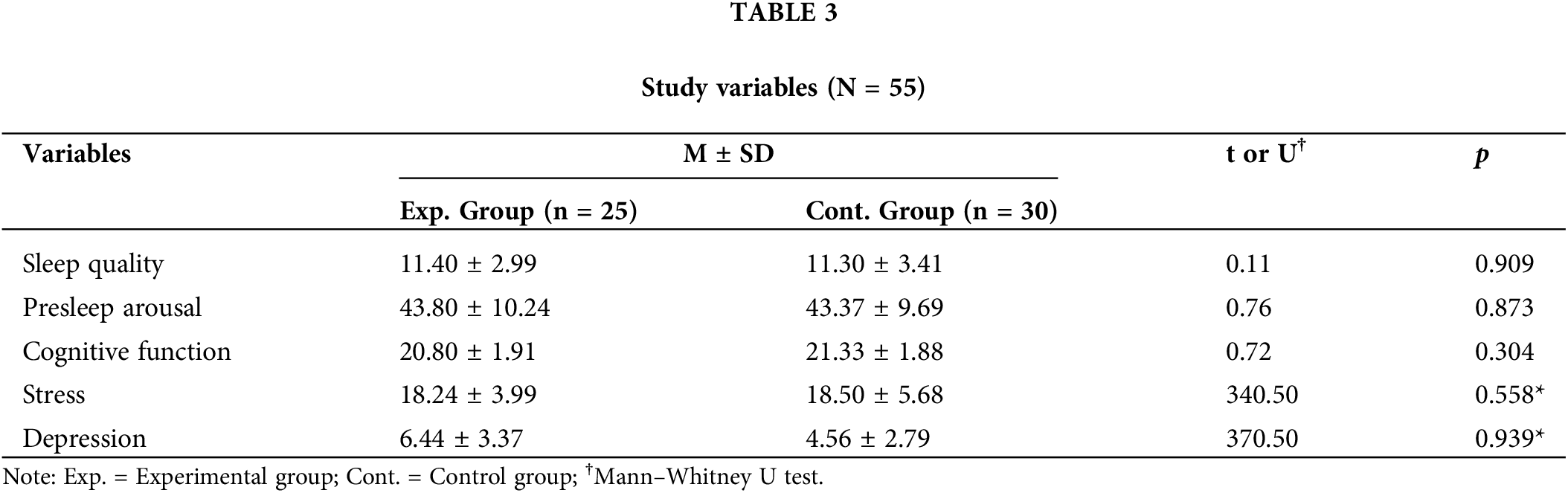
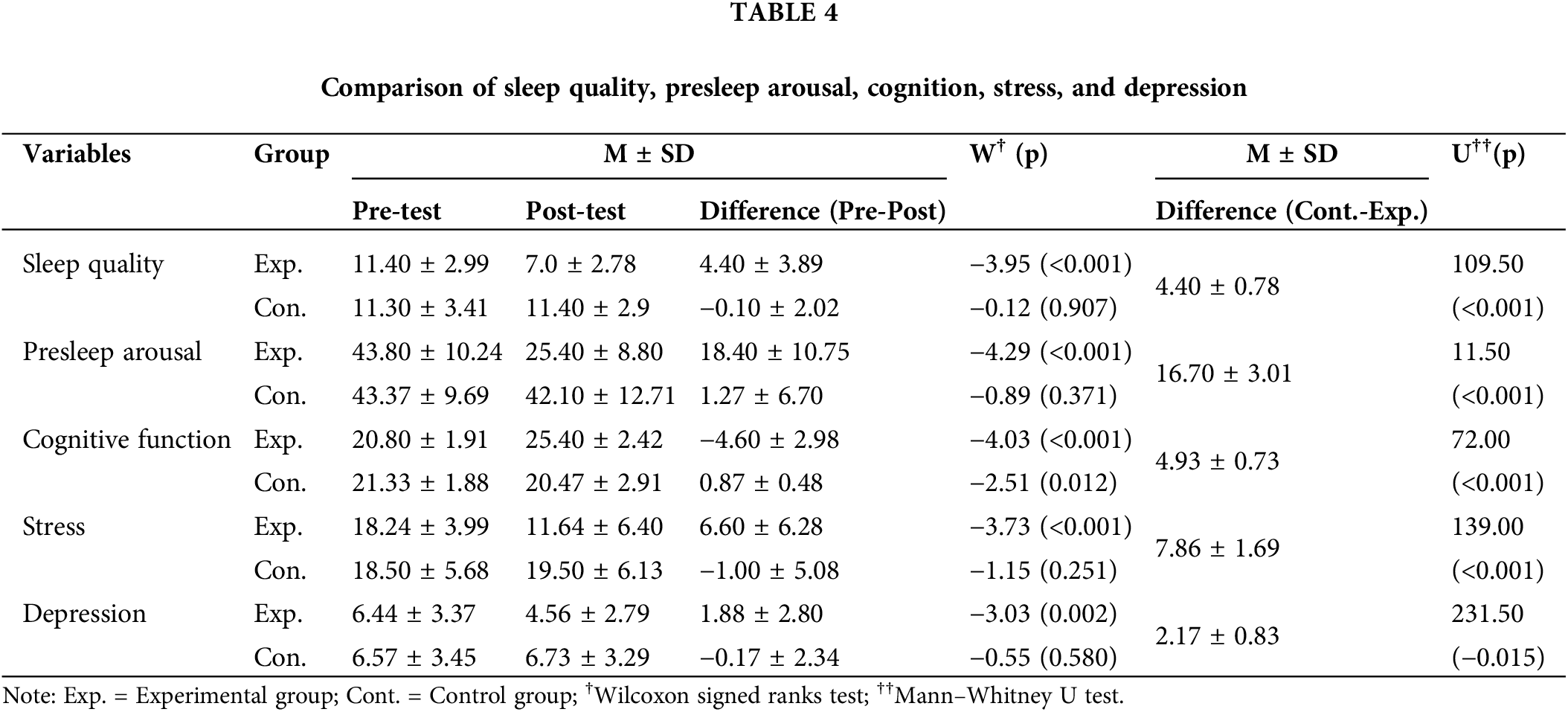
Table 3 presents the study variables. The Shapiro–Wilk test indicated that depression and stress did not satisfy normal distribution, and the Mann–Whitney U test analyzed the between-group homogeneity for the two variables. The results showed that sleep quality, presleep arousal, cognitive function, depression, and stress were homogeneous between the control and experimental groups.
The intervention effects (Table 4) indicated significant improvements in the experimental group compared to the control group as follows: sleep quality (U = 109.50, p < 0.001), presleep arousal (U = 11.50, p < 0.001), cognitive function (U = 72.00, p < 0.001), stress (U = 139.00, p < 0.001) and depression (U = 231.50, p = 0.015). The Wilcoxon signed ranks test was used to analyze the difference between pre- and post-test; the experimental group showed significant improvements in sleep quality (W = −3.95, p < 0.001), presleep arousal (W = −4.29, p < 0.001), cognitive function (W = −4.03, p < 0.001), stress (W = −3.73, p < 0.001) and depression (W = −3.03, p = 0.002).
This study developed and applied the SCEMI as an integrated non-pharmacological intervention incorporating multiple components to enhance sleep and cognition. The SCEMI significantly affected sleep quality, presleep arousal, cognitive function, depression, and stress in community-dwelling MCI elderly with sleep disturbances, which led to the following discussions.
First, the SCEMI was based on Lazarus’ multimodal approach [26] and was developed as a multi-component intervention in previous studies [13,16,25,32,38–42,44] with verified effects on sleep and cognition enhancement. For each component, Behavior focused on the behavioral strategies to practice sleep habits and sleep hygiene through sleep education; Affect focused on the emotional support and health information sharing by a health expert; Sensation involved aroma inhalation using an aroma necklace and roll-on; Cognition used cognitive training with a cognition calendar; Imagery applied breath meditation as a form of mindfulness meditation; Interpersonal-relations involved telephone-coaching by a researcher and research assistant as sleep partners. The effects of the SCEMI were shown to be similar to those of the single or multimodal interventions applied in previous studies. It is noteworthy that, in this study, the effects of single interventions were reviewed at a time when no standardized intervention for MCI elderly was available, based on which a multimodal intervention involving Behavior, Affect, Sensation, Cognition, and Interpersonal relations, was developed and validated for the sleep and cognition enhancement. Notably, for cognition-based therapies in patients with MCI, a systematic approach should be taken, and the report that a multimodal intervention is suitable for these patients [46] lent further support for the validity of the development of SCEMI. It is also significant that the program consists of such components as cognitive training using a cognition calendar, aroma inhalation, and walking exercise that could be easily performed by each MCI elderly with sleep disturbances without temporal or spatial constraints, with the benefit of simultaneous enhancement of sleep and cognition. Nevertheless, the synergistic effect through the combination of existing single interventions or the effect size of each intervention could not be directly compared. In light of this, repeated studies are necessary to standardize the program contents.
The results of this study revealed that sleep quality, presleep arousal, and cognitive function in MCI elderly were significantly improved after participation in the SCEMI. This agreed with a previous study on MCI patients with insomnia, which reported improvements in sleep quality, anxiety, and stress through an approach focused on emotional factors, such as a mindfulness meditation as a non-pharmacological intervention to enhance sleep and cognition [42]. The results also agreed with a study reporting enhanced cognitive functions in MCI elderly using a workbook through 10 sessions [47]. What should be noted, however, is that a cognition calendar was used in this study in lieu of a workbook or booklet to allow more convenient and familiar use by the elderly in daily life, and it is conjectured that the utility or effectiveness of the calendar matched those of the conventional educational materials to lead to similar results. The enhancement of sleep, in particular, is likely to have positively influenced the cognitive function of the MCI elderly in this study, based on the previous report on the close association of the health of MCI elderly with the prevention of physiological aging and neurodegenerative disease [34]. Nonetheless, it is necessary to check the extent and retention of intervention effects as only the short-term effects were verified in this study after a total of 8 weekly sessions of SCEMI in MCI elderly. Regarding this, a previous study using a multifactorial program for a 6-week intervention by weekly sessions [48] reported that the memory capacity in MCI patients was improved on the subjective memory test despite the lack of difference on the objective memory test. The study also reported that negative thoughts on the probability of cognitive decline were reduced and that the intervention effects were maintained after six months of follow-up monitoring. Thus, in the future, objective and subjective tests should be simultaneously used for the follow-up monitoring of effects and the test of short-term effects.
Furthermore, the levels of depression and stress in the MCI elderly in this study significantly decreased after the SCEMI. This coincided with previous studies showing reduced levels of depression and stress after aroma inhalation [39–41]. Aroma inhalation, in particular, as a type of aromatherapy using essential oils, is known to be effective for the relaxation of the body and mind through close association with emotion to reduce depression and stress and elicit positive moods [40]. The aroma inhalation applied in this study involved the inhalation via aroma necklace and roll-on containing a 4:1:1 mixture of lavender, marjoram, and Roman chamomile oil to reflect the efficacy of each essential oil. This had a beneficial effect on inducing emotional changes while being easily applied by MCI elderly. Of note is that, among the positive emotional changes, the reduction of depression could promote the enhancement of sleep and cognition, and the reduction of stress could assist with the body’s homeostasis and influence neurological behaviors [49,50]. Hence, a repeated study should be conducted on the interactions across each positive effect.
The participants in this study, throughout the SCEMI program, received counseling on ways to resolve individual difficulties via 8 sessions of telephone coaching. The participants could describe the personal situation to the coach to question and resolve the problem. The telephone coach assessed the adherence to sleep hygiene, walking exercise, and aroma inhalation and promoted the respective behaviors, and this is thought to have enhanced the sleep and cognitive function and reduced the levels of depression and stress in the participants. The interventions using telephone coaching were shown to have effects in various domains, from treatment adherence to lifestyle modification, pain control, and management of the chronic disease [51]. Renneberg et al. [52] claimed that tele-based health coaching programs were an effective intervention for the elderly with chronic disease regarding behavior planning, social support, and lifestyle improvement. Hence, telephone-coaching should be included in addition to face-to-face education in developing a multi-component intervention for MCI patients with sleep disturbances.
In this study, an intervention to enhance sleep and cognition in MCI elderly with sleep disturbances was tested. Despite the lack of relevant research, the significance lies in providing basic data on a multimodal intervention incorporating multiple components based on Lazarus’ multimodal approach [26]. The findings of this study are expected to contribute to maximizing the social interest and support for community-dwelling MCI elderly, and repeated studies must be conducted to establish a standardized intervention on the management of sleep and cognition in MCI elderly through the use of community-based resources such as dementia care centers. In addition, this study has evaluated the short-term effects of an intervention involving 8 sessions. In further studies, follow-up monitoring should be conducted to evaluate the long-term effects at 3, 6, and 12 months of intervention regarding sleep and cognition enhancement and the retention of positive effects in MCI elderly. The improvement for cognitive function in the MCI elderly after the SCEMI may partly be attributed to practice effects associated with the MoCA-based cognitive training. It is also recommended that polysomnography or actigraphy be performed in addition to the self-reported questionnaire for more objective results.
This study demonstrated the positive effects of an 8-week multimodal intervention SCEMI for sleep and cognition enhancement on sleep quality, presleep arousal, cognitive function, stress, and depression in MCI elderly with sleep disturbances. Notably, the SCEMI, as a readily applicable intervention for sleep and cognition enhancement in elderly patients, is predicted to be useful in the primary and secondary dementia prevention projects for community-dwelling MCI elderly in South Korea, with a rapid increase in the elderly population. Considering that MCI is a stage at which the progression to dementia could be delayed or prevented, an integrated intervention at an early stage would have positive effects on the incidence of dementia in MCI elderly as well as the reduction of the burden of support and care by the family. The intervention would also contribute to the reduction of the dementia-related medical cost.
Acknowledgement: The authors thank research participants for their participation in this study.
Funding Statement: This paper was supported by Eulji University in 2022.
Author Contributions: Study conception and design: E.K.H.; data collection: E.K.H.; analysis and interpretation of results: E.K.H., H.K.S.; draft manuscript preparation: E.K.H., H.K.S. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions, e.g., privacy or ethical.
Ethics Approval: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Eulji University (No. EU22-63). Informed consent was obtained from all the participants involved in the study.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Lee HO, Park JY. Relationship between oral health behavior and happiness index in elderly people. J Dent Hyg Sci. 2016;16(6):415–23. doi:https://doi.org/10.17135/jdhs.2016.16.6.415. [Google Scholar] [CrossRef]
2. Jorm AF. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 2000;46(4):219–27. doi:https://doi.org/10.1159/000022163. [Google Scholar] [PubMed] [CrossRef]
3. Jagger C, Andersen K, Breteler MM, Copeland JR, Helmer C, Baldereschi M. Prognosis with Parkinson’s disease in Europe: a collaborative study of population-based cohorts, neurologic diseases in the elderly research group. Neurology. 2000;54:516–20. [Google Scholar]
4. Kang IO, Lee SY, Kim SY, Park CY. Economic cost of dementia patients according to the limitation of the activities of daily living in Korea. Int J Geriatr Psychiatry. 2007;22(7):675–81. doi:https://doi.org/10.1002/gps.1729. [Google Scholar] [PubMed] [CrossRef]
5. Hori M, Kubota M, Kinoshita A. The support system for dementia patient and their caregiver with Skype and webcam. Gan To Kagaku Ryoho. 2008;35(Suppl 1):43–5. [Google Scholar] [PubMed]
6. da Silva RAPC. Sleep disturbances and mild cognitive impairment: a review. Sleep Sci. 2015;8(1):36–41. doi:https://doi.org/10.1016/j.slsci.2015.02.001. [Google Scholar] [PubMed] [CrossRef]
7. Shin KR, Kang Y, Jung D, Kim MY, Kim JS, Kim MJ, et al. Prevalence and characteristics of mild cognitive impairment in the community-dwelling elderly compared to elderly with normal cognitive function. Korean J Adult Nurs. 2011;23(1):40–9. [Google Scholar]
8. Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord. 2012;33(1):50–8. doi:https://doi.org/10.1159/000335363. [Google Scholar] [PubMed] [CrossRef]
9. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–94. doi:https://doi.org/10.1016/S1474-4422(14)70136-X. [Google Scholar] [PubMed] [CrossRef]
10. Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014;27(6):478–83. doi:https://doi.org/10.1097/YCO.0000000000000106. [Google Scholar] [PubMed] [CrossRef]
11. Gabryelewicz T, Styczynska M, Pfeffer A, Wasiak B, Barczak A, Luczywek E, et al. Prevalence of major and minor depression in elderly persons with mild cognitive impairment—MADRS factor analysis. Int J Geriatr Psychiatry. 2004;19(12):1168–72. doi:https://doi.org/10.1002/gps.1235. [Google Scholar] [PubMed] [CrossRef]
12. Shin I, Lim C, Shin H, Kim JM, Kim SW, Yoon JS. Relationship between sleep disturbance and cognitive dysfunction in patients with mild cognitive impairment. Alzheimers Dement. 2017;13(7S):1054. doi:https://doi.org/10.1016/j.jalz.2017.06.1504. [Google Scholar] [CrossRef]
13. Ancoli-Israel S, Ayalon L. Diagnosis and treatment of sleep disorders in older adults. Am J Geriatr Psychiatry. 2006;14(2):95–103. doi:https://doi.org/10.1097/01.JGP.0000196627.12010.d1. [Google Scholar] [PubMed] [CrossRef]
14. Stepnowsky CJ, Ancoli-Israel S. Sleep and its disorders in seniors. Sleep Med Clin. 2008;3(2):281–93. doi:https://doi.org/10.1016/j.jsmc.2008.01.011. [Google Scholar] [PubMed] [CrossRef]
15. McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev. 2016;11(12):CD009178. doi:https://doi.org/10.1002/14651858.CD009178.pub3. [Google Scholar] [PubMed] [CrossRef]
16. Blackman J, Swirski M, Clynes J, Harding S, Leng Y, Coulthard E. Pharmacological and nonpharmacological interventions to enhance sleep in mild cognitive impairment and mild Alzheimer’s disease: a systematic review. J Sleep Res. 2021;30(4):e13229. [Google Scholar] [PubMed]
17. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. doi:https://doi.org/10.1517/14740338.2013.827660. [Google Scholar] [PubMed] [CrossRef]
18. Song D, Yu DSF. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: a randomised controlled trial. Int J Nurs Stud. 2019;93(16):97–105. doi:https://doi.org/10.1016/j.ijnurstu.2019.02.019. [Google Scholar] [PubMed] [CrossRef]
19. Cai ZZ, Lin R, Wang XX, Yan YJ, Li H. Effects of mindfulness in patients with mild cognitive impairment with insomnia: a double-blind randomized controlled trial. Geriatr Nurs. 2022;47:239–46. doi:https://doi.org/10.1016/j.gerinurse.2022.08.001. [Google Scholar] [PubMed] [CrossRef]
20. Bademli K, Lok N, Canbaz M, Lok S. Effects of physical activity program on cognitive function and sleep quality in elderly with mild cognitive impairment: a randomized controlled trial. Perspect Psychiatr Care. 2019;55(3):401–8. doi:https://doi.org/10.1111/ppc.12324. [Google Scholar] [PubMed] [CrossRef]
21. Rodriguez (Then) FS, Jackson J, Ware C, Churchyard R, Hanseeuw B. Interdisciplinary and transdisciplinary perspectives: on the road to a holistic approach to dementia prevention and care. J Alzheimers Dis Rep. 2020;4(1):39–48. doi:https://doi.org/10.3233/ADR-180070. [Google Scholar] [PubMed] [CrossRef]
22. Lee H, Kim E, Yoon JY. Effects of a multimodal approach to food art therapy on people with mild cognitive impairment and mild dementia. Psychogeriatrics. 2022;22(3):360–72. doi:https://doi.org/10.1111/psyg.12822. [Google Scholar] [PubMed] [CrossRef]
23. Bae S, Lee S, Lee S, Jung S, Makino K, Harada K, et al. The effect of a multicomponent intervention to promote community activity on cognitive function in older adults with mild cognitive impairment: a randomized controlled trial. Complement Ther Med. 2019;42(640):164–9. doi:https://doi.org/10.1016/j.ctim.2018.11.011. [Google Scholar] [PubMed] [CrossRef]
24. Yang C, Moore A, Mpofu E, Dorstyn D, Li Q, Yin C. Effectiveness of combined cognitive and physical interventions to enhance functioning in older adults with mild cognitive impairment: a systematic review of randomized controlled trials. Gerontologist. 2020;60(8):633–42. doi:https://doi.org/10.1093/geront/gnz149. [Google Scholar] [PubMed] [CrossRef]
25. Naismith SL, Pye J, Terpening Z, Lewis S, Bartlett D. “Sleep Well, Think Well” group program for mild cognitive impairment: a randomized controlled pilot study. Behav Sleep Med. 2018;6:778–89. [Google Scholar]
26. Lazarus AA. Multimodal behavior therapy: treating the “basic id.” J Nerv Ment Dis. 1973;156(6):404–11. doi:https://doi.org/10.1097/00005053-197306000-00005. [Google Scholar] [PubMed] [CrossRef]
27. Park SH, Cheon JS, Park JY, Ko YJ, Oh BH. Usefulness of the montreal cognitive assessment in mild cognitive impairment. J Korean Soc Biol Ther Psychiatry. 2010;16(1):13–22. [Google Scholar]
28. Buysse DJ, Reynolds 3rd CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:https://doi.org/10.1016/0165-1781(89)90047-4. [Google Scholar] [PubMed] [CrossRef]
29. Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the presleep state: the development of the presleep arousal scale. Behav Res Ther. 1985;23(3):263–71. doi:https://doi.org/10.1016/0005-7967(85)90004-X. [Google Scholar] [PubMed] [CrossRef]
30. Cho Y, Kwon JH. Verification of the integrated model of insomnia including cognitive process and stress. Korean J Clin Psychol. 2012;31(1):135–50. doi:https://doi.org/10.15842/kjcp.2012.31.1.007. [Google Scholar] [CrossRef]
31. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment: Moca: a brief screening tool for MCI. J Am Geriatr Soc. 2005;53(4):695–9. doi:https://doi.org/10.1111/j.1532-5415.2005.53221.x. [Google Scholar] [PubMed] [CrossRef]
32. Lee JH, Lee KU, Lee DY, Kim KW, Jhoo JH, Kim JH, et al. Development of the Korean version of the consortium to establish a registry for Alzheimer’s disease assessment packet (CERAD-Kclinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci. 2002;57(1):47–53. doi:https://doi.org/10.1093/geronb/57.1.P47. [Google Scholar] [PubMed] [CrossRef]
33. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi:https://doi.org/10.1016/0022-3956(82)90033-4. [Google Scholar] [PubMed] [CrossRef]
34. Kee BS. A preliminary study for the standardization of geriatric depression scale short form-Korea version. J Korean Neuropsychiatr Assoc. 1996;35(2):298–307. [Google Scholar]
35. Cohen S. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park, CA: Sage Publications, Inc.; 1988. p. 31–67. [Google Scholar]
36. Lee JE, Lee MK. The effects of self-complexity and self-efficacy on depression and perceived stress. Korean Psychol Assoc. 2005:422–23. [Google Scholar]
37. McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Larson EB, Teri L. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer’s disease: results of a randomized, controlled trial: walking and light to improve sleep in ad. J Am Geriatr Soc. 2011;59(8):1393–402. doi:https://doi.org/10.1111/j.1532-5415.2011.03519.x. [Google Scholar] [PubMed] [CrossRef]
38. Bootzin RR, Epstein DR. Understanding and treating insomnia. Annu Rev Clin Psychol. 2011;7(1):435–58. doi:https://doi.org/10.1146/annurev.clinpsy.3.022806.091516. [Google Scholar] [PubMed] [CrossRef]
39. Kouzuki M, Kitao S, Kaju T, Urakami K. Evaluation of the effect of aroma oil as a bath salt on cognitive function. Psychogeriatrics. 2020;20(2):163–71. doi:https://doi.org/10.1111/psyg.12481. [Google Scholar] [PubMed] [CrossRef]
40. Oh HG. Handbook of aromatherapy. Seoul: Yangmoon; 2003. p. 73–117. [Google Scholar]
41. Kim IS, Kang SJ, Kim JO. Effects of the aroma inhalation method with a roll-on on life stress, salivary cortisol and fatigue in nursing student. J Korea Acad-Ind Coop Soc. 2014;15(12):7214–23. doi:https://doi.org/10.5762/kais.2014.15.12.7214. [Google Scholar] [CrossRef]
42. Shaif NAS, Doshi K, Lim J. Effects of mindfulness-based therapy for insomnia and a sleep hygiene/exercise programme on subjective-objective sleep discrepancy in older adults with sleep disturbances: exploratory secondary analysis of a randomised clinical trial. J Sleep Res. 2022;31(6):e13700. doi:https://doi.org/10.1111/jsr.13700. [Google Scholar] [PubMed] [CrossRef]
43. Bandura A. The explanatory and predictive scope of self-efficacy theory. J Soc Clin Psychol. 1986;4(3):359–73. doi:https://doi.org/10.1521/jscp.1986.4.3.359. [Google Scholar] [CrossRef]
44. Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother. 2012;58(3):157–63. doi:https://doi.org/10.1016/S1836-9553(12)70106-6. [Google Scholar] [PubMed] [CrossRef]
45. Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8(1):80. doi:https://doi.org/10.1186/1479-5868-8-80. [Google Scholar] [PubMed] [CrossRef]
46. Woods RT, Clare L. Cognition based therapies and mild cognitive impairment. In: Toukko DHAF, editor. Mild cognitive impairment: international perspectives. New York: Taylor & Francis; 2006. [Google Scholar]
47. Choi IH, Kim YK. Effects of a cognitive function enhancement program using a workbook for elderly with mild cognitive impairment. J Korean Soc Wellness. 2017;12(1):439. doi:https://doi.org/10.21097/ksw.2017.02.12.1.439. [Google Scholar] [CrossRef]
48. Han JH, Ko SG, Kwon JH, Jo IH, Ahn SM, Han CS, et al. Efficacy of a multifactorial cognitive ability enhancement program in MCI (mild cognitive impairment). Korean J Clin Psychol. 2008;27(4):805–21. doi:https://doi.org/10.15842/kjcp. [Google Scholar] [CrossRef]
49. Golinowska S, Groot W, Baji P, Pavlova M. Health promotion targeting older people. BMC Health Serv Res. 2016;16(S5):345. doi:https://doi.org/10.1186/s12913-016-1514-3. [Google Scholar] [PubMed] [CrossRef]
50. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi:https://doi.org/10.1152/physrev.00041.2006. [Google Scholar] [PubMed] [CrossRef]
51. Magill M, Apodaca TR, Borsari B, Gaume J, Hoadley A, Gordon REF, et al. A meta-analysis of motivational interviewing process: technical, relational, and conditional process models of change. J Consult Clin Psychol. 2018;86(2):140–57. doi:https://doi.org/10.1037/ccp0000250. [Google Scholar] [PubMed] [CrossRef]
52. Renneberg B, Schulze J, Böhme S, West SG, Schüz B. Effectiveness and equity evaluation of an insurance-wide telephone-counseling program for self-management of chronic diseases: the health coach study. Appl Psychol Health Well Being. 2022;14(2):606–25. doi:https://doi.org/10.1111/aphw.12322. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools